Abstract
Airborne fungi are associated with upper and lower airway inflammatory diseases. Alternaria is commonly found in nasal secretions and induces the production of chemical mediators from sinonasal mucosa. This study aimed to establish an Alternaria-induced chronic rhinosinusitis (CRS) mouse model and determine the influence of host allergic background on the immunopathological characteristics of CRS. BALB/c mice were used for establishing the CRS model. Alternaria was intranasally instilled for 8 or 16 weeks with or without ovalbumin (OVA) presensitization. Total serum IgE and Alternaria-specific IgE levels were measured by enzyme-linked immunosorbent assay (ELISA). Interleukin (IL)-4, IL-10, interferon (IFN)-γ, and tumor necrosis factor (TNF)-α levels in nasal lavage fluid (NLF) and splenocytes were measured by ELISA and their mRNAs and levels of associated transcription factors in sinonasal mucosa were determined with quantitative reverse-transcriptase polymerase chain reaction (RT-PCR). Hematoxylin-eosin staining and periodic acid-Schiff staining were performed to evaluate histological changes. Total serum IgE was increased in both allergic and non-allergic CRS. IL-4 was strongly expressed in NLF in both allergic and non-allergic CRS at 16 weeks and not only eosinophils but also neutrophils were increased in NLF of non-allergic CRS mice. The levels of Th1, Th2, and Treg cytokines and transcription factor mRNAs were significantly increased in sinonasal mucosa of non-allergic CRS mice. Both inflammatory cell infiltration and goblet cell hyperplasia were increased in CRS mice. Repeated intranasal instillation of Alternaria results in sinonasal inflammation with inflammatory cell infiltration. The sinonasal mucosal immune responses against Alternaria were shown to differ depending on the host allergic background.
Introduction
Chronic rhinosinusitis (CRS) encompasses a heterogeneous group of diseases that can be classified as eosinophilic or non-eosinophilic CRS based on the dominant inflammatory cell types. CRS can also be divided into Th1, Th2, and Th17 dominant CRS based on the presence of lymphocyte effector cells in sinonasal tissues. [1] Although the etiology and pathogenesis of CRS are not fully understood, CRS is characterized by the chronic inflammation of sinonasal mucosa with a heterogeneous group of inflammatory responses against allergens, bacteria, fungi, and viruses. CRS had been considered an infectious disease with the focus being placed on identifying the pathogenic microbial organisms. However, recent studies have suggested that CRS may not be related to immune-mediated diseases, the involvement of which may cause the exacerbation of local inflammatory responses.
Fungi are ubiquitous in nature, but relatively few species have been implicated in human diseases. Alternaria, Aspergillus, Penicillium, and Cladosporium are commonly found in nasal secretions not only in CRS but also in healthy individuals. [2] Fungi are increasingly recognized as important pathogens in patients with sinusitis; however, their role in the pathogenesis of CRS remains controversial. Fungal components, such as proteins and enzymes, induce immune responses and result in the production of chemical mediators through the interaction of cell membrane receptors. [3, 4] Alternaria and Aspergillus extracts activate upper and lower airway epithelial cells and enhances the production of several inflammatory mediators; they are considered risk factors for the development of asthma, allergic rhinitis, and CRS. [5–8]
Alternaria alternata results in allergic airway inflammation with increased lung expression of type 2 cytokines and eosinophils. [9] In the presence of sinus mucosal trauma, the inoculation of Alternaria and Aspergillus was shown to induce sinonasal inflammation with inflammatory cell infiltration, epithelial thickening, and goblet cell hyperplasia. [10, 11] Intranasal challenge with Aspergillus plus ovalbumin can induce allergic inflammation in sinonasal mucosa with the development of an eosinophilic CRS model. [12] Compare with Aspergillus, Alternaria strongly enhanced chemical mediatory production from nasal epithelial cells and influenced Th immune responses. [13] Allergic rhinitis and CRS are an inflammatory condition within the sinonasal mucosa. The relationship between allergic rhinitis and CRS is controversial. Nasal allergy related inflammatory mediators have been postulated to develop CRS. [14] However, the evidence linking allergy to CRS is quite low. [15] The aim of this study was to evaluate whether Alternaria could induce inflammatory immune responses in sinonasal mucosa and to know if the allergic status of the host could affect the development of fungi induced mucosal inflammation. So we established a CRS mouse model by repeated intranasal instillation of Alternaria with or without presensitization by ovalbumin (OVA) and to compare their immunopathological characteristics.
Materials and methods
Alternaria-induced CRS mouse model
Female BALB/c mice (6 weeks old) were purchased from Hyosung Science Inc. (Daegu, South Korea). They were maintained under standard conditions in a pathogen-free cage. This study was conducted in accordance with the guidelines of the National Institute of Health and was approved by the Institutional Review Board of Animal Experiments of Daegu Catholic University Medical Center (DCIAFCR-180718-11).
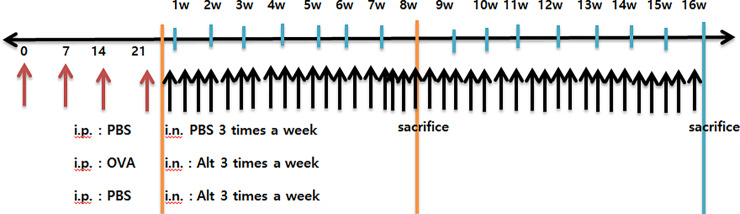
Intraperitoneal injection of ovalbumin (OVA, 75 μg) in 200 μL of phosphate-buffered saline (PBS) containing 2 mg of aluminum hydroxide (Sigma Aldrich, St. Louis, MO, USA) was performed on days 0, 7, 14, and 21 to develop an allergic predisposition. Then, mice were challenged intranasally with 50 μg/mL Alternaria alternata; culture filtrate extracts in PBS for 8 or 16 weeks to develop an allergic Alternaria CRS model. Alternaria alternata extracts (Lot#:312142, Greer Lab, Lenoir, NC, USA) were concentrated, dialyzed, and lyophilized from the medium liquid in which the fungi had been cultivated withextraction of proteins into the media. Intraperitoneal injection of PBS followed by intranasal instillation of Alternaria for 8 or 16 weeks was designed to create a non-allergic Alternaria CRS model. Intraperitoneal injection of PBS alone followed by intranasal instillation of PBS for 8 or 16 weeks was designed to create negative control mice. Experimental mice were sacrificed 24 hours after the final challenge, as shown in Fig 1.
Fig 1. Schematic diagram of Alternaria induced chronic rhinosinusitis mouse model.
Intraperitoneal injection of ovalbumin (OVA) then intranasal instillation of Alternaria for 8 or 16 weeks was designed as an allergic CRS model. Intraperitoneal injections of phosphate buffered saline (PBS) then intranasal instillation of Alternaria for 8 or 16 weeks were designed as non-allergic CRS model. Intraperitoneal injection of PBS then intranasal instillation of PBS for 8 or 16 weeks was designed as negative control mice.
Evaluation of chemical mediators in Nasal Lavage Fluid (NLF)
NLF was collected 24 hours after the last challenge at 8 or 16 weeks. A 21-gauge catheter was inserted through a partial tracheal resection site in the direction of the upper airway and into the nasopharynx. One mL of cold PBS was gently perfused into the sinonasal cavity and collected in a tube. NLF was centrifuged at 2,000 rpm for 7 minutes at 4°C. Supernatant was collected to determine the levels of interleukin (IL)-4, IL-10, interferon (IFN)-γ, and tumor necrosis factor (TNF)-α using enzyme-linked immunosorbent assay (ELISA) kits (R&D Systems, Minneapolis, MN, USA). The pellet was resuspended in PBS and stained with May-Grunwald-Giemsa stain and cells differentiated into eosinophils, neutrophils, lymphocytes, and other cells. The average number of cells in five high power field was determined by a well-trained researcher who did not have experimental information on the slides.
Measurement of serum total IgE
Blood samples were collected from the inferior vena cava 24 hours after the last intranasal challenge. Serum was obtained by centrifugation and total IgE was measured using ELISA (Pharmingen, San Diego, CA, USA).
Measurement of cytokines and transcription factor mRNA in sinonasal mucosa
Total RNA was extracted from the sinonasal mucosa using Trizol reagent (Invitrogen, Carlsbad, CA, USA). The RNA purity and concentration were measured using a spectrophotometer (Beckman, Mountain View, CA, USA). Complementary DNA was made from 1 μg of RNA using reverse-transcription polymerase chain reaction (RT-PCR) amplification with a PerkinElmer (Norwalk, CT, USA) thermal cycler. From the amplified cDNA, the quantitative polymerase chain reaction was performed using an SYBR Green PCR core kit (PE Applied Biosystems, Foster City, CA, USA). The expression levels of mRNA were measured by the cycle threshold (2-ΔΔCT) method and were normalized to β-actin. S1 Table shows the primers used in this study. Initial denaturation was performed at 95°C for 2 minutes, followed by 40 cycles consisting of denaturation at 94°C for 10 seconds, annealing at 60°C for 10 seconds, and elongation at 72°C for 45 seconds. Two technical replicates and 3–4 biological replications were prepared for mRNA study.
Activation of splenocytes with Alternaria
Spleen tissues were isolated from mice and separated into single cells using a 70 μm cell strainer. Red blood cells (RBCs) were removed with RBC lysis buffer (BioLegend, San Diego, CA, USA). The cells were incubated in Roswell Park Memorial Institute-1640 medium supplemented with 10% fetal bovine serum, 100 U/mL penicillin, and 100 μg/mL streptomycin (Gibco, Grand Island, NY, USA). After stimulation with 100 μg/mL Alternaria for 72 hours, the supernatant was collected and stored at -70°C until assay. IL-4, IL-10, IFN-γ, and TNF-α level in supernatants were measured using ELISA kits (R&D Systems).
Histological evaluation of sinonasal mucosa
Mice were painlessly sacrificed with a lethal dose of intraperitoneally administered sodium pentobarbital. Specimens were decalcified in ethylenediaminetetraacetic acid and embedded in paraffin. The tissue was cut into 5-μm-thick coronal sections. Three anatomically similar sections were chosen as in a previous study. [12]
Inflammatory cell infiltration and epithelial thickness were quantified in hematoxylin and eosin-stained sections. The degree of submucosal inflammatory cell infiltration was quantified into four categories as follows (0: none, 1: mild, occasional scattered inflammatory cells, 2: moderate, 3: severe, diffuse infiltration of inflammatory cells). Goblet cell numbers were quantified by Periodic acid-Schiff (PAS) staining at × 200 magnification and the average number of goblet cells was counted using an eyepiece reticle. Epithelial thickness was directly measured at × 400 magnification through a video camera (Olympus Optical Co. Ltd., Tokyo, Japan) and analyzed with DP controller software (ver. 2.2.1.227). All tissue sections were examined blindly with respect to the source of the tissue and counts were determined at three different mucosal areas for each of the three sections per mouse.
Statistical analysis
All measured parameters are expressed as mean ± standard deviation. One-way analysis of variance followed by Tukey’s test was performed for normally distributed data and the Kruskal-Wallis test with post-hoc Bonferroni-Dunn test was performed for non-normally distributed data (SPSS ver. 21, IBM Corp., Armonk, NY, USA). A probability value of less than 0.05 was considered to represent statistical significance.
Results
Total serum IgE level
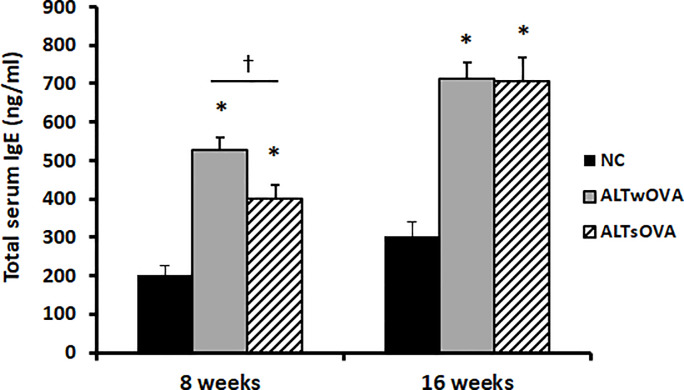
Repeated intranasal instillation of Alternaria significantly elevated total serum IgE level regardless of presensitization with OVA at 8 and 16 weeks. Total serum IgE level at 8 weeks was much higher in the presensitized mice (528.2 ± 287.4 ng/mL) than in the non-sensitized group (398.2 ± 160.6 ng/mL), but these groups’ levels became similar after 16 weeks (712.3 ± 171.3 ng/mL with presensitization, 704.3 ± 431.6 ng/mL without presensitization) (Fig 2).
Fig 2. Total serum IgE level at 8 and 16 weeks with repetitive instillation of Alternaria.
IgE level was significantly increased both allergic and non-allergic mouse model (n = 7 mice per group). NC: negative control, ALTwOVA: Alternaria instillation with ovalbumin (OVA) presensitization, ALTsOVA; Alternaria instillation without ovalbumin (OVA) presensitization. *p<0.05 vs NC group.
Chemical mediators and inflammatory cells in NLF
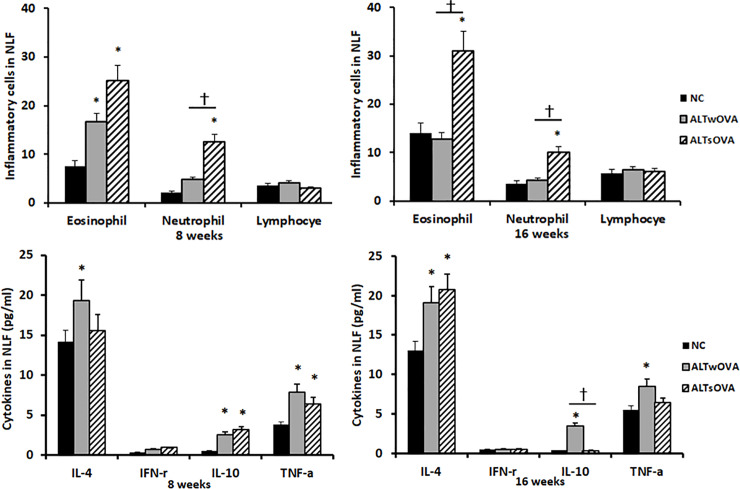
Repeated intranasal instillation of Alternaria elevated the level of IL-4, IL-10, and TNF-α in NLF of allergic CRS mice at 8 (IL-4, 19.4 ± 9.6 pg/mL; IL-10, 2.6 ± 2.3 pg/mL; TNF-α, 7.9 ± 5.9 pg/mL) and 16 weeks (IL-4, 19.1 ± 5.9 pg/mL; IL-10, 3.5 ± 1.7 pg/mL; TNF-α, 8.5 ± 5.2 pg/mL). Without presensitization with OVA, the elevated IL-10 level of 3.2 ± 2.5 pg/mL at 8 weeks became as low as 0.4 ± 0.2 pg/mL at 16 weeks. INF-γ level was not significantly influenced by the intranasal instillation of Alternaria (Fig 3).
Fig 3. Inflammatory cell differentials and chemical mediator levels in Nasal Lavage Fluid (NLF) of the Alternaira induced chronic rhinosinusitis mouse model.
Eosinophil and neutrophils were increased in NLF of non-allergic mouse model. Th2, Treg, and inflammatory cytokine revel were strongly expressed in NLF of allergic mouse model (n = 7 mice per group). NC: negative control, ALTwOVA: Alternaria instillation with ovalbumin (OVA) presensitization, ALTsOVA; Alternaria instillation without ovalbumin (OVA) presensitization. *p<0.05 vs NC group, †p<0.05 vs with or without OVA group.
When the mice were treated with Alternaria for 8 and 16 weeks, eosinophil and neutrophil counts were significantly increased in NLF of non-allergic CRS mice. Only the level of eosinophils was increased at 8 weeks in NLF of OVA-sensitized mice, compared with that in negative control mice. The levels of lymphocytes in NLF did not differ significantly among the three groups (Fig 3).
Chemical mediator production from splenocytes
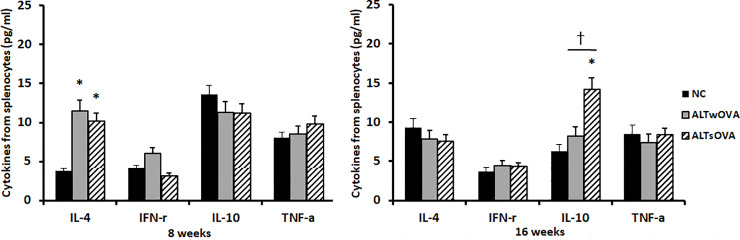
Mouse spleen cells were stimulated with Alternaria for 72 hours. IL-4 production was significantly increased in allergic (11.5 ± 4.7 pg/mL) and non-allergic (10.2 ± 5.6 pg/mL) CRS mice compared with that in the control group (3.8 ± 2.7 pg/mL) at 8 weeks. However, the levels of IL-10, IFN-γ, and TNF-α production did not differ significantly among the three groups. In intranasal instilled mice at 16 weeks, only IL-10 production was significantly increased in non-allergic CRS mice (14.3 ± 7.3 pg/mL) compared with that in the other groups (sensitized group, 8.21 ± 6.5 pg/mL; negative control group, 6.3 ± 3.7 pg/mL) (Fig 4).
Fig 4. Chemical mediator production from splenocyte after stimulated with 100 ug/mL of Alteranria for 72 hours.
IL-4 production was significantly increased in allergic and non-allergic mica at 8 weeks and IL-10 production was significantly increased in non-allergic mice (n = 7 mice per group). NC: negative control, ALTwOVA: Alternaria instillation with ovalbumin (OVA) presensitization, ALTsOVA; Alternaria instillation without ovalbumin (OVA) presensitization. *p<0.05 vs NC group, †p<0.05 vs with or without OVA group.
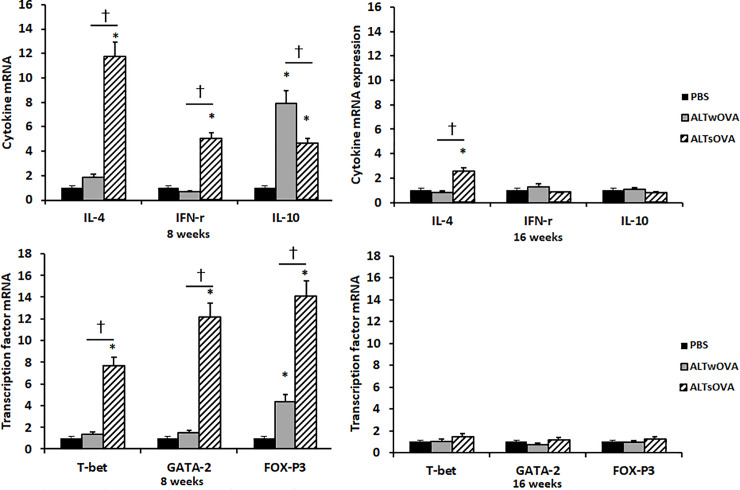
mRNA expression of sinonasal mucosal cytokines and transcription factors
We performed real-time RT-PCR to determine the effect of the intranasal instillation of Alternaria on the mRNA expression of Th-related cytokine and T-cell subset transcription factors in sinonasal mucosa. After 8 weeks of instillation with Alternaria, IL-10 and FOXp3 mRNA expression levels were significantly increased in both allergic and non-allergic CRS mice. The mRNA expression of IL-4, INF-γ, T-bet, and GATA3 was significantly increased by Alternaria in non-allergic CRS mice compared with that in allergic CRS and negative control mice. After 16 weeks of instillation with Alternaria, only IL-4 mRNA expression was significantly increased in the non-allergic group compared with that in the allergic and negative control groups (Fig 5).
Fig 5. Chemical mediator mRNA and transcription factor expression in sinonasal mucosa of Alternaria induced Chronic RhinoSinusitis (CRS) mouse model.
IL-4 and IFN-γ and their transcription factor mRNA expression were significantly increased non-allergic CRS group at 8 weeks. IL-10 and its transcription factor mRNA expression were significantly increased both allergic and non-allergic CRS group at 8 weeks (3–4 mice per group). NC: negative control, ALTwOVA: Alternaria instillation with ovalbumin (OVA) presensitization, ALTsOVA; Alternaria instillation without ovalbumin (OVA) presensitization. *p<0.05 vs NC group, †p<0.05 vs with or without OVA group.
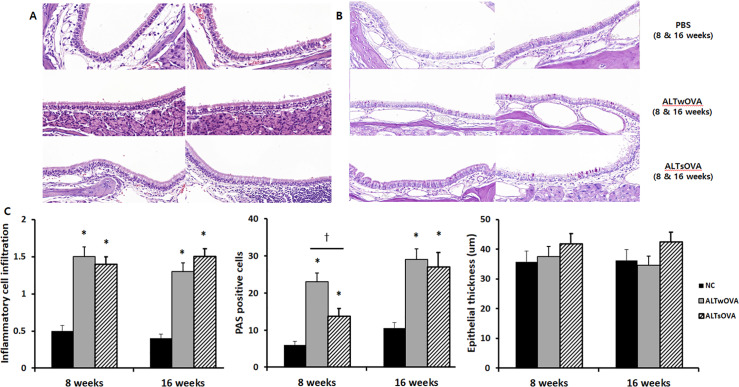
Histopathological characteristics
The intranasal instillation of Alternaria significantly increased inflammatory cell infiltration of the submucosal area with or without OVA sensitization at 8 (1.5 ± 0.6 and 1.4 ± 0.5 with or without sensitization) and 16 weeks (1.3 ± 0.3 and 1.5 ± 0.5 with or without sensitization), compared with that in the control group (0.5 ± 0.3 and 0.4 ± 0.3 at 8 and 16 weeks) (Fig 6).
Fig 6. Histologic characteristics of the Alternaira induced Chronic RhinoSinusitis (CRS) mouse model.
Inflammatory cell infiltration (A, x400) and periodic acid-Schiff (PAS) positive cells (B, x400) were significantly increased in sinonasal mucosa of allergic and non-allergic CRS mouse model of the Alternaira induced chronic rhinosinusitis mouse model (3–4 mice per group). NC: negative control, ALTwOVA: Alternaria instillation with ovalbumin (OVA) presensitization, ALTsOVA; Alternaria instillation without ovalbumin (OVA) presensitization. *p<0.05 vs NC group, †p<0.05 vs with or without OVA group.
PAS-positive goblet cells in sinonasal mucosa were significantly increased in both allergic and non-allergic CRS groups at 8 (23.0 ± 13.4 and 13.8 ± 7.5 respectively) and 16 weeks (29.0 ± 9.7 and 27.0 ± 13.3 respectively) compared with that in the control group (6.0 ± 2.5 and 10.5 ± 3.4 at 8 and 16 weeks). Although epithelial thickness tended to increase in the Alternaria-instilled groups, there was no significant difference among the three groups (Fig 6).
Discussion
Fungi are environmentally ubiquitous and commonly found in airway secretions of healthy and CRS patients. [1, 16] Protease activity form Alternaria induces proinflammatory cytokines and thymic stromal lymphopoietin (TSLP) production from respiratory epithelial cells and commonly associated with CRS. [17, 18] Epithelial cells derived TSLP and IL-33 secretion into the airway induce Th2-type inflammation. [17, 19] In this study, we tried to determine the immunologic effect of Alteranria alternata on sinonasal mucosa inflammation and the immunopathologic characteristics of Alternaria induced CRS mouse model. Repetitive intranasal stimulation with Alternaria results in prominent sinonasal inflammation regardless allergic predisposition. The association between allergy and CRS remains debatable and nearly an equal number of studies supported or refuted an association of allergy with both CRS with nasal polyps or CRS without nasal polyps. [20] According to our data, Alternaria could develop sinonasal inflammation regardless of allergic host background and Alternaria induced Th2 dominant immune response with allergic predisposition, whereas Th1 and Th2 immune responses without allergic predisposition. Fungal spores are continuously inhaled and deposit in sinonasal mucosal and influence mucosal immune responses. However, we used culture filtrated extracts of Alternaria to develop CRS mouse model, so we need further study to determine the immunopathologic characteristics of Alternaria spore induced CRS mouse.
Alternaria develops an allergic airway response characterized by increased Th2 cytokine and eosinophil infiltration and high level of serum IgE. [21] Intranasal instillation of Alternaria caused the high level of serum IgE in regardless of allergic status. Eosinophil count was increased in NLF by intranasal instillation of Alternaria at 8 weeks, in regardless of allergic status. However, at 16 weeks, eosinophils only increased in non-allergic CRS mouse. And neutrophils are only increased in non-allergic CRS mouse. Sinonasal mucosal inflammatory cell infiltration was not significantly influenced the allergic status of mice. These findings suggest that the intranasal instillation of Alternaira can induce allergic responses with high serum total IgE, tissue inflammatory cell infiltration, and mucus production regardless of allergic status. However, the eosinophilic inflammatory responses seem to more dominant in allergic CRS mouse and both eosinophilic and neutrophilic inflammatory responses seem to happen without allergic predisposition.
Intranasal instillation of Alternaria increased IL-4 concentration in NLF at 8 and 16 weeks in allergic CRS mouse. Although IL-4 was not statistically significantly increased at 8 weeks, the increasing pattern of IL-4 in NLF was similar in non-allergic CRS mouse. However, when the splenocytes were stimulated with Alternaria, IL-4 was significantly increased at 8 weeks but not 16 weeks. These results may be due to the intranasal installation induce early local and systemic Th2 inflammatory responses, and over time, the systemic Th2 inflammatory response was weakened with continuous Th2 local inflammatory responses. Treg cytokine, IL-10 was increased in NLF of allergic CRS mice at 8 and 16 weeks and from splenocytes in non-allergic CRS mouse at 16 weeks. These findings may indicate that intranasal instillation of Alternaria show different local and systemic immune responses. After 8 weeks of intranasal instillation developed local and systemic Th1 and/or Th2 inflammation. However, after 16 weeks, local inflammatory immune responses diminished with increased systemic immune tolerance with increased production of IL-10. According to the previous studies, CRS mouse models developed inflammatory immune response after 4 to 12 weeks of stimulation with single pathogens. [10, 12, 22] When the CRS mouse model was made with multiple airborne allergens, sinonasal inflammation maintained for more than 12 weeks. [23] We presume that regional exposure with single allergens may induce local and systemic inflammatory immune responses initially, then local inflammatory responses may decrease with increased systemic immune tolerance. To maintain or aggravate the local and systemic inflammation, another pathogenic stimulation may be needed. The role of fungi in pathogenesis in CRS is still controversial. However, if the sinonasal fungal inflammation goes with a bacterial or viral infection, the local and systemic inflammations may persist and aggravate for a longer period of time. Respiratory epithelial cells are the first mucosal cells exposed to the environmental stimuli, such as airborne allergens, fungi, viruses, and bacteria. If the innate immune defense against pathogens is destroyed by some reason, these pathogens may interact to aggravate local and systemic inflammation. To determine the differences in the immune responses against Alternaria, we need to evaluate local and systemic inflammatory patterns at different time intervals and compare the expressions of other inflammatory and suppressive cytokines in sinonasal mucosa.
Sinonasal mucosal cytokine and their transcription factor mRNA expression pattern was different from cytokine concentration in NLF. Th1 and Th2 cytokine and their transcription factor mRNA were significantly increased in non-allergic CRS mouse and Treg cytokine and transcription factor mRNA was significantly increased regardless of allergic predisposition. The exact reason could not explain with this study, but there are some possibilities. Sinonasal mucosal mRNA level could represent protein concentration of sinonasal mucosa but not the concentration in NLF. Unfortunately, we did not measure the protein level of Th cytokines and directly compare mRNA and protein levels in mucosa. The IL-4 and transcription factor mRNA expression was not statistically significant increase in allergic CRS mouse. However, IL-4 and its transcription factor mRNA expression tended to increase with significant increase of IL-4 in NLF. These results may associate with time interval difference between mRNA expression, protein production, and their secretion to nasal cavity.
Conclusion
Repetitive intranasal instillation of Alternaria results in Th2 and eosinophil dominant immune response in allergic predisposition state. Without allergic background, repetitive stimulation of Alternaria results in not only Th2 but also Th1 and Treg immune response with eosinophil and neutrophil dominant inflammation. Based on these results, intranasal instillation of Alternaria can induce sinonasal mucosa inflammation with Th immune responses. And the sinonasal mucosal immune responses against Alternaria were different based on the differences host allergic background and different time intervals.
Supporting information
(DOCX)
(XLS)
(XLS)
(XLS)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (Ministry of Science and ICT) (2019R1F1A1047757) to PI, SH Shin.
References
- 1.Cao PP, Wang ZC, Schleimer RP, Liu Z. Pathophysiologic mechanisms of chronic rhinosinusitis and their roles in emerging disease endotypes. Ann Allergy Asthma Immunol. 2019;122(1):33–40. 10.1016/j.anai.2018.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shin SH, Ye MK, Lee YH. Fungus culture of the nasal secretion of chronic rhinosinusitis patients: seasonal variations in Daegu, Korea. Am J Rhinol. 2007;21(5):556–9. 10.2500/ajr.2007.21.3065 [DOI] [PubMed] [Google Scholar]
- 3.Balenga NA, Klichinsky M, Xie Z, Chan EC, Zhao M, Jude J, et al. A fungal protease allergen provokes airway hyper-responsiveness in asthma. Nat Commun. 2015;6:6763 10.1038/ncomms7763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu D, Wei Y, Bleier BS. Emerging Role of Proteases in the Pathogenesis of Chronic Rhinosinusitis with Nasal Polyps. Front Cell Infect Microbiol. 2017;7:538 10.3389/fcimb.2017.00538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Murai H, Okazaki S, Hayashi H, Kawakita A, Hosoki K, Yasutomi M, et al. Alternaria extract activates autophagy that induces IL-18 release from airway epithelial cells. Biochem Biophys Res Commun. 2015;464(4):969–74. 10.1016/j.bbrc.2015.05.076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lehmann S, Sprunken A, Wagner N, Tenbrock K, Ott H. Clinical relevance of IgE-mediated sensitization against the mould Alternaria alternata in children with asthma. Ther Adv Respir Dis. 2017;11(1):30–9. 10.1177/1753465816680786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Katotomichelakis M, Nikolaidis C, Makris M, Proimos E, Aggelides X, Constantinidis TC, et al. Alternaria and Cladosporium calendar of Western Thrace: Relationship with allergic rhinitis symptoms. Laryngoscope. 2016;126(2):E51–6. 10.1002/lary.25594 [DOI] [PubMed] [Google Scholar]
- 8.Lawrence LA, Mulligan JK, Roach C, Pasquini WN, Soler ZM, Banglawala SM, et al. Superoxide dismutase reduces the inflammatory response to Aspergillus and Alternaria in human sinonasal epithelial cells derived from patients with chronic rhinosinusitis. Am J Rhinol Allergy. 2015;29(2):89–93. 10.2500/ajra.2015.29.4155 [DOI] [PubMed] [Google Scholar]
- 9.Kim HK, Baum R, Lund S, Khorram N, Yang SL, Chung KR, et al. Impaired induction of allergic lung inflammation by Alternaria alternata mutant MAPK homologue Fus3. Exp Lung Res. 2013;39(9):399–409. 10.3109/01902148.2013.835009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ahn BH, Park YH, Shin SH. Mouse model of Aspergillus and Alternaria induced rhinosinusitis. Auris Nasus Larynx. 2009;36(4):422–6. 10.1016/j.anl.2008.08.009 [DOI] [PubMed] [Google Scholar]
- 11.Alt JA, Lee WY, Davis BM, Savage JR, Kennedy TP, Prestwich GD, et al. A synthetic glycosaminoglycan reduces sinonasal inflammation in a murine model of chronic rhinosinusitis. PLoS One. 2018;13(9):e0204709 10.1371/journal.pone.0204709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim HC, Lim JY, Kim S, Kim JH, Jang YJ. Development of a mouse model of eosinophilic chronic rhinosinusitis with nasal polyp by nasal instillation of an Aspergillus protease and ovalbumin. Eur Arch Otorhinolaryngol. 2017;274(11):3899–906. 10.1007/s00405-017-4717-2 [DOI] [PubMed] [Google Scholar]
- 13.Shin SH, Ye MK, Lee DW, Chae MH, Han BD. Nasal Epithelial Cells Activated with Alternaria and House Dust Mite Induce Not Only Th2 but Also Th1 Immune Responses. Int J Mol Sci. 2020;21(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kennedy JL, Borish L. Chronic sinusitis pathophysiology: the role of allergy. Am J Rhinol Allergy. 2013;27(5):367–71. 10.2500/ajra.2013.27.3906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wise SK, Lin SY, Toskala E, Orlandi RR, Akdis CA, Alt JA, et al. International Consensus Statement on Allergy and Rhinology: Allergic Rhinitis. Int Forum Allergy Rhinol. 2018;8(2):108–352. 10.1002/alr.22073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hirotsu M, Shiozawa A, Ono N, Miwa M, Kikuchi K, Ikeda K. Fungal extracts detected in eosinophilic chronic rhinosinusitis induced cytokines from the nasal polyp cells. Laryngoscope. 2014;124(9):E347–53. 10.1002/lary.24655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kouzaki H, O'Grady SM, Lawrence CB, Kita H. Proteases induce production of thymic stromal lymphopoietin by airway epithelial cells through protease-activated receptor-2. J Immunol. 2009;183(2):1427–34. 10.4049/jimmunol.0900904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matsuwaki Y, Wada K, White T, Moriyama H, Kita H. Alternaria fungus induces the production of GM-CSF, interleukin-6 and interleukin-8 and calcium signaling in human airway epithelium through protease-activated receptor 2. Int Arch Allergy Immunol. 2012;158 Suppl 1:19–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nagata Y, Maruoka S, Gon Y, Mizumura K, Kishi H, Nomura Y, et al. Expression of IL-25, IL-33, and Thymic Stromal Lymphopoietin in Nasal Polyp Gland Duct Epithelium in Patients With Chronic Rhinosinusitis. Am J Rhinol Allergy. 2019;33(4):378–87. 10.1177/1945892419835333 [DOI] [PubMed] [Google Scholar]
- 20.Marcus S, Roland LT, DelGaudio JM, Wise SK. The relationship between allergy and chronic rhinosinusitis. Laryngoscope Investig Otolaryngol. 2019;4(1):13–7. 10.1002/lio2.236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Valladao AC, Frevert CW, Koch LK, Campbell DJ, Ziegler SF. STAT6 Regulates the Development of Eosinophilic versus Neutrophilic Asthma in Response to Alternaria alternata. J Immunol. 2016;197(12):4541–51. 10.4049/jimmunol.1600007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim DW, Khalmuratova R, Hur DG, Jeon SY, Kim SW, Shin HW, et al. Staphylococcus aureus enterotoxin B contributes to induction of nasal polypoid lesions in an allergic rhinosinusitis murine model. Am J Rhinol Allergy. 2011;25(6):e255–61. 10.2500/ajra.2011.25.3727 [DOI] [PubMed] [Google Scholar]
- 23.Park SC, Kim SI, Hwang CS, Cho HJ, Yoon JH, Kim CH. Multiple airborne allergen-induced eosinophilic chronic rhinosinusitis murine model. Eur Arch Otorhinolaryngol. 2019;276(8):2273–82. 10.1007/s00405-019-05465-x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(XLS)
(XLS)
(XLS)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.