Supplemental Digital Content is available in the text.
Abstract
Objectives:
To establish the first regional quality improvement collaborative solely dedicated to follow-through care of high-risk infants after Neonatal intensive care unit (NICU) discharge and to characterize extremely low birth weight (ELBW) follow-up in New England.
Methods:
Eleven of 14 follow-up programs in New England partnered with the Vermont Oxford Network (VON) ELBW project for an initial data collection project. We collected information about the health status and developmental outcomes of infants born ≤1,000 g or younger than 28 weeks 2014–2016 at the 18–24 months corrected for gestational age (CGA) follow-up visit. VON collected and compiled the data.
Results:
Of 993 eligible infants, 516 (52.0%) had follow-up visits. The rehospitalization rate was 33.9%, mostly respiratory illness. Ninety-six children (19.3%) had weight less than 10th percentile and 44 (8.9%) had weight less than third percentile at 18–24 months. Only 170 (61.4%) children had recommended hearing screening after NICU discharge. Forty-six (9.1%) had cerebral palsy; 81 of the 441 infants that completed all 3 sections of the Bayley Scales of Infant Development, third edition (18.4%) had any composite score less than 70. Over half of the social and demographic data were missing.
Conclusion:
Most quality initiatives in neonatology stop at NICU discharge. This first project by the New England Follow-up Network showed a low rate for clinical follow-up. It demonstrated many opportunities to improve postdischarge follow-through specific to NICU-based care. Future projects will aim to improve the quality of follow-through services through collaborative learning, data sharing, and comparative outcomes.
INTRODUCTION
Comprehensive long-term follow-up of high-risk infants after neonatal intensive care unit (NICU) discharge has long been considered an essential part of neonatal care.1 Despite published recommendations on the timing, content,2 and quality metrics for3 medical and developmental assessments for preterm infants, there is significant variation in follow-up practices among centers.4,5 There are also critical knowledge gaps about high-risk infant follow-up, including referral and participation rates in follow-up programs, adherence rates for recommended screening protocols, and the impact of variation in practices on long-term outcomes.
One highly successful approach to understand practice variation and improve care is the quality improvement learning collaborative. The overarching principle is that deep understanding of between-institution practice variation—and associated differential outcomes—can be leveraged to inform collective improvement efforts.6–8 In pediatrics, state and regional collaboratives have addressed improvement goals such as narrowing variation in neonatal intensive care practices,9 reducing catheter-associated infections,10 increasing preterm infant breastfeeding rates,11 and improving the quality of the hospital discharge process.12
Noting the high degree of heterogeneity in clinical follow-up,4,5 academic medical centers in New England with high-risk follow-up programs established the New England Neonatal Follow-Up Network (NEFUN). NEFUN is the first regional collaborative solely dedicated to improving clinical follow-up care after NICU discharge. Our overall aims were to standardize process and outcomes measures across centers, to develop, implement, and evaluate novel interventions to improve follow-through service delivery and clinical outcomes.
To develop operational processes and protocols and to establish ourselves as a working collaborative, we began by partnering with the Vermont Oxford Network Extremely Low Birth Weight Follow-up Project (VON ELBW) for 2014, 2015, and 2016 data collection periods.13–15 This collaborative effort between NEFUN and VON provided an excellent opportunity to learn from VON proven expertise in data collection and data-sharing for quality improvement in newborn medicine. By the end of the 2019 academic year, we aimed for all member NICUs with established or affiliated follow-up programs to review retrospectively collected health and developmental data on surviving ELBW infants born between 2014 and 2016. Members complete predetermined data collection tools using an electronic submission process. With this first project, we established processes for and feasibility of collecting outcomes data at the regional level, characterized attributes of network members, described the 18–24 months corrected for gestational age (CGA) medical and developmental outcomes of infants participating in New England follow-up programs, and identified specific areas for targeted quality improvement intervention. We describe here the organizational approach, NEFUN partnership with VON, and initial findings.
METHODS
Setting
In 2016, representatives from 11 of the 15 high-risk infant follow-up programs in the 6-state New England region (Conn., Mass., Maine, N.H., R.I., Vt.) met for a 2-day conference held at Bretton Woods, N.H., to present their specific program procedures and protocols. Small working groups met to discuss challenges and opportunities in the following 3 areas: respiratory health, growth and nutrition, and neurodevelopment. The group outlined the planks of a mission statement and identified the next steps. Members then worked independently to develop local data collection processes. We convened periodic conference calls to discuss progress, identify challenges, and share solutions. NEFUN members also took advantage of regional and national conferences to meet in person.
Procedures
All except 1 of the NEFUN member institutions were existing members of the VON and contributed data annually to the VON VLBW database when the collaborative launched. This fact allowed NEFUN members to submit follow-up data to VON under existing membership agreements. The 1 NEFUN follow-up site that was not an existing VON member required permission from its local Institutional Review Board to participate.
VON provided each NEFUN member site with the list of their ELBW infants born in 2014, 2015, and 2016 and discharged alive from their center. Each NEFUN member site matched their VON ELBW list against their records of infants referred for follow-up at the time of NICU discharge, enrolled in follow-up, and seen for an 18- to 24-month CGA visit. Each NEFUN member site completed and returned the ELBW follow-up data forms to VON. VON processed the data and prepared reports for distribution.
Each center undertook this project as a Quality Improvement Initiative and did not seek supervision by the Institutional Review Board per their policies. Each NEFUN program adhered to local guidelines regarding data sharing with the VON ELBW Project to ensure secure data transfer and protection of patient privacy. There was no additional cost for existing VON members to participate.
Variables
Variables, as categorized in the VON ELBW follow-up data collection forms (Supplemental Digital Content, which displays VON Health Status Report, http://links.lww.com/PQ9/A178), were as follows: social and demographic information, health services, and developmental services. Sociodemographic data included family composition, maternal age at birth, parental education, and poverty status. Health services and supports after ultimate birth hospitalization discharge included the use of medical equipment by category, feeding, speech, or motor supports, hospital readmissions and reason for admission, and surgical procedures between discharge and the 18- to 24-month CGA visit, if any, categorized by type. We noted infant deaths between ultimate birth hospitalization discharge and 18- to 24-month CGA follow-up. VON tabulated the follow-up rate for each center and NEFUN as a whole by dividing the number of infants seen at 18–24 months CGA by the total number of infants alive and eligible for follow-up visits or infants with unknown status.
The developmental status report included anthropometric measures at the time of the follow-up visit. We categorized growth parameters as being below the third and 10th percentile, according to the National Center for Health Statistics, CDC Growth Charts.16 Neurosensory outcomes included vision or hearing impairment, presence and type of cerebral palsy, assessment of muscle tone, and attainment of gross motor milestones. We reported the Bayley Scales of Infant Development, third edition (BSID-3), cognitive, language, and motor composite scores.17 For this project, we defined vision impairment as blindness or need for prescription glasses; we defined hearing impairment as deafness or use of an amplification device. We defined severe disability as vision or hearing impairment, inability to walk without support, diagnosis of cerebral palsy, or a Bayley score 2 SDs below the mean on any subscale.
Analyses
After receiving the data, VON calculated the total number and the percentage of infants with each outcome, and the individual hospital rates at the 25th and 75th percentiles (Q1 and Q3). We combined data from birth years 2014–2016 and present summary results from the network as a whole, rather than individual institutions.
RESULTS
Network Description
There are 15 infant follow-up programs in New England (Fig. 1). Of these, 14 (93%) are members of the New England Follow-up Network. Eight (57%) member institutions participated in data collection for the 2014 and 2015 birth year cohorts. Participation increased to 11 (86%) centers for the 2016 birth year cohort.
Fig. 1.
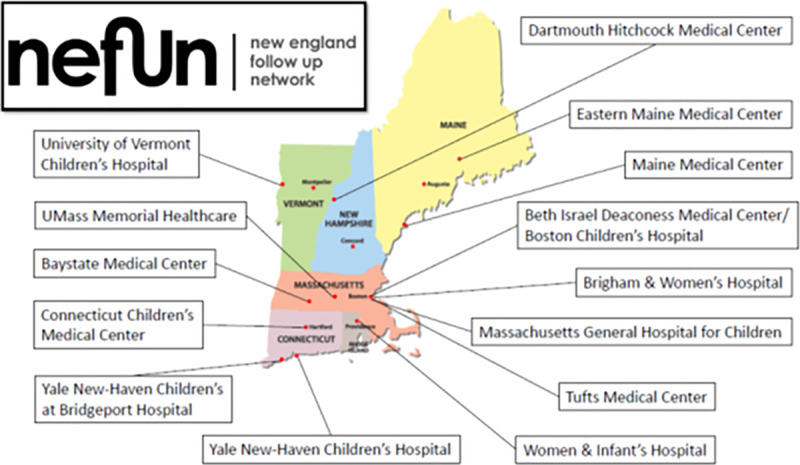
New England Follow-up Network member centers by location.
The NEFUN member programs serve overlapping, though not identical, patient populations. Gestational age at birth is the most common eligibility criterion, with all 14 programs enrolling infants born below 28-week gestation and late-preterm infants with medical complexity. One program enrolls late preterm infants with social complexity. A subgroup of NEFUN centers also provides follow-up for term-born infants with hypoxic-ischemic encephalopathy, congenital heart disease, neonatal abstinence syndrome, and those requiring ECMO.
The cadence, frequency, and duration of follow-up visits vary among NEFUN centers. One program sees infants according to a proscribed, age-based timing for assessments (eg, 6, 12, 24 months, etc.), and 1 program only plans visits according to individual clinical needs. Twelve follow-up programs see infants according to both schedule and clinical needs. Programs vary in the duration of program participation with follow-up ending between 2 and 5 years of age.
The provider type also varies by the member program. All programs employ medical doctors to perform physical examinations at each visit and utilize the BSID-3 to assess cognitive, language, and motor abilities after the 6 months CGA. Psychologists administer the cognitive and language domains in only four (29%) programs and a developmental pediatrician in 2 programs (14%). Physical or occupational therapists perform motor assessments in the majority of programs (71%). Of note, no 2 programs have the same configuration of staff.
The setting for each follow-up program varied among NEFUN members. Nine programs (62%) serve infants discharged from a single NICU, 2 (14%) receive referrals from multiple NICUs within a single health system, and 3 (21%) serve infants discharged from multiple NICUs across multiple health systems. Ten programs (71%) have affiliations with academic medical centers, and 8 (57%) serve as training sites for fellows in newborn medicine.
Follow-up Rate
Of infants alive at discharge, there were no deaths between discharge and follow-up for the 2014 birth year cohort, 1 death for 2015, and 7 deaths for 2016. There were 993 infants eligible for follow-up from the combined 2014–2016 birth year cohorts; 516 (52.0%; interquartile range [IQR] 41.0, 69.7) had evaluations between 18 and 24 months of age. Follow-up rates were similar for each birth year.
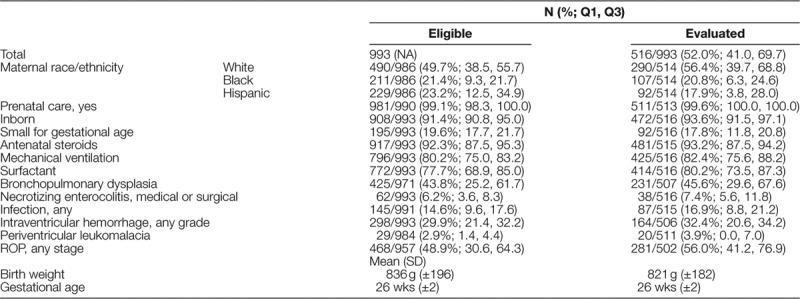
Infant Characteristics
Eligible infants had a mean birth weight of 836 g (±196) and mean gestation of 26 weeks (±2) which was similar to those infants completing the evaluation at 18–24 months [birth weight 821 g (±182); gestational age 26 weeks (±2)] (Table 1). The majority of eligible and evaluated infants were born at the hospital at which they received care (inborn) and received antenatal steroids and exogenous surfactant. Ninety-two (17.8%) evaluated infants had birth weights below their 10th percentile for gestational age (small for gestational age). The majority of infants followed received mechanical ventilation; 45.6% (IQR 29.6, 67.6) had a diagnosis of bronchopulmonary dysplasia. Thirty-two percent of infants had any grade of intraventricular hemorrhage, and 56% had any stage of retinopathy of prematurity (ROP).
Table 1.
Characteristics of Extremely Low Birth Weight Infants in the New England Follow-up Network, 2014–2016
Family Characteristics
The mean maternal age for the 2014–2016 cohort was 30.5 years (±5.6). The majority of infants resided in a 2-parent home (76.4%) and had primary caregivers whose first language was English (89.0%). Half of eligible infants’ mothers were white (49.7%; 38.5, 55.7), 23.2% (IQR 12.5, 34) Hispanic, and 21.4% (IQR 9.3, 21.7) black. A higher percentage of infants born to white mothers participated in an 18- to 24-month follow-up compared to infants born to black and Hispanic mothers. Close to 50% of data on parental education and family income level were missing and are therefore not reported.
Health Services and Supports
Eighty-five percent of children received some form of health service support after NICU discharge [439/511 (85.9%; IQR 80.0, 98.5)] (Table 2). The most common services were supports for speech and motor development, followed in descending frequency by respiratory medication, oral feeding support, and home oxygen therapy.
Table 2.
Health Services Utilization after NICU Discharge

The rate of rehospitalization was 33.9% (IQR 29.5, 40.0), most commonly for respiratory illness. Thirty-two percent of children had a surgical procedure after leaving the NICU. The most common procedure was the insertion of tympanostomy tubes, followed by feeding tube placement, and inguinal hernia repair.
Developmental Status
Half of the data on receipt of recommended vision and hearing screening by 18–24 months CGA were missing (response rate 50.5% and 53.7%, respectively). Of the children with nonmissing data, nearly all received recommended vision assessments and 170 (61.4%; IQR 41.4, 80.0) had a hearing screening. No children were blind; 4 children had hearing impairment in both ears (0.8%; IQR 0.0, 1.3) (Table 3).
Table 3.
Neurosensory and Neurodevelopmental Outcomes at 18- to 24-months Corrected Age
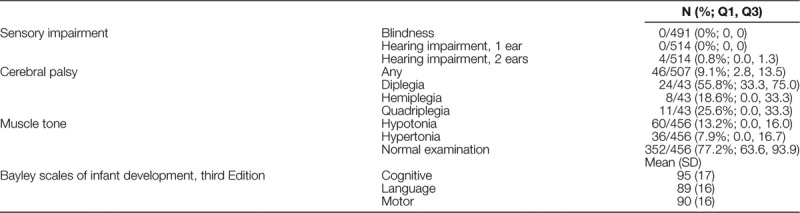
Less than 10 percent of the children had a diagnosis of cerebral palsy, 46/507 (9.1%; IQR 2.8, 13.5). The majority of children had normal neurologic examinations without evidence of hypertonia or hypotonia. BSID-3 scores were in the average–normal range for the majority of children. Eighty-one of the 441 infants that completed all 3 sections of the BSID-3 (18.4%) had any composite score <70. Of the 33 infants who did not have a complete BSID-3, 3 (9.1%) had severe delays that precluded testing.
Growth
There were 96 children (19.3%; IQR 13.3, 24.5) whose weight was below the 10th percentile at 18–24 months CGA and 44 (8.9%; IQR 5.7, 11.3), whose weight was below the third percentile (Table 4). The head circumference followed a similar pattern.
Table 4.
Growth Parameters at 18- to 24-months Corrected Age

DISCUSSION
The NEFUN successfully met the aim of its first collaborative project. Participation gradually increased each year, with 11 of 14 institutions submitting follow-up data for the 2016 birth year cohort. Barriers to participation in our first data collection project included difficulty identifying a local project champion (4 sites), delay in obtaining local institutional review board approval for participation (2 sites) and not having 18- to 24-month CGA data available due to only recently establishing a follow-up program (1 site).
To our knowledge, the NEFUN is the first multistate regional collaborative in the United States established with the sole purpose of improving the quality of clinical care provided to high-risk infants through NICU-associated follow-up programs. The California Perinatal Quality of Care Collaborative has partnered with the California Children’s Services follow-up program to improve access and participation. They report an 80% referral rate for 8,000 infants <1,500 g discharged home in 2010–2011.18 Although they note a considerable regional variation in referral rates, they did not report on specific infant outcomes. The NICHD Neonatal Research Network has contributed greatly to knowledge about neonatal health through high-quality multicenter clinical trials and observational studies. However, the network’s focus remains on clinical research and not quality improvement efforts for NICU or outpatient follow-up health services.19 The Canadian Neonatal Follow-up Network is a collaboration with the Canadian Neonatal Network that facilitates collaboration in research, integrated data collection, and knowledge translation. It improves the quality of care and long-term outcomes of children seen in their programs.20 The New England Follow-up Network aims to apply rigorous improvement science methods to the follow-through of NICU-associated health problems and the processes of infant follow-up. An advantage of our population-based approach is that it reflects the current state of clinical care as experienced by infants, families, and providers.
Program Heterogeneity
Our network is relatively small, with 14 programs in just 6 states. We note a very high level of variability in eligibility criteria, visit frequency, program duration, and staff composition among network centers. Even for the well-defined ELBW population, an infant may participate in follow-up for 2, 3, or 5 years, and variably have evaluations by a psychologist, occupational therapist, or developmental pediatrician depending on her state of residence. It is striking that no 2 programs in the network are exactly alike. Such heterogeneity makes comparing follow-up processes and infant outcomes difficult, if not impossible. A future aim of the network is to harmonize assessments and data collection across populations and sites to allow uniform tracking of quality improvement efforts.
Follow-up Rates
The rate of follow-up for high-risk infants in our network was 52.0% for eligible infants born between 2014 and 2016, with an IQR between 41.0% and 69.7%. Although there is debate about which infants require follow-up after NICU discharge, those born ≤1,000 g or younger than 28 weeks gestational age represent the population most vulnerable to health and developmental challenges over time. Most follow-up programs would expect to see these infants. The low rate of follow-up may be explained by infants having a visit outside the 18- to 24-month corrected age window or loss to follow-up due to families moving out of the region or changes to health insurance coverage. The cost of visit copays and family perception of the follow-up value may also contribute to loss to follow-up. The next steps will involve determining the key drivers of variability in follow-up rates within and between institutions across our network and instituting efforts to ensure access to follow-up for all infants in this highest of risk groups.
Screening
Rates of both retinal and ophthalmologic screening postdischarge were high, at least for the half of infants for whom we had data. The American Academy of Pediatrics (AAP) recommends that all infants born ≤1,500 g or ≤30 weeks’ gestation should receive dilated retinal examinations by a trained pediatric ophthalmologist to screen for ROP. Ophthalmologists should discontinue periodic assessments only in the presence of full retinal vascularization, zone III vascularization without prior ROP, postmenstrual age of 50 weeks and no prethreshold disease, or resolving ROP.21 Many infants are ready for NICU discharge before meeting these criteria and therefore receive outpatient ophthalmology examinations. Confirming complete follow-up to full retinal vascularization is a critical aspect of postdischarge care. Additionally, because all ELBW infants are at a higher risk for strabismus, amblyopia, high refractive errors, and cataracts compared to term-born peers, it is recommended that they receive examination within the first year of life, ideally within 4–6 months of discharge.21
Rates of hearing assessment postdischarge were lower than expected for the infants with complete data. The AAP recommends diagnostic hearing testing by 24–30 months of age for all infants after an NICU stay of 5 days or longer, even if they passed the initial hearing screen.22 Local practices vary, with many centers in our network recommending audiology screening for ELBW infants between 9 and 12 months of age. Moreover, the AAP currently recommends ongoing surveillance of communicative development for all infants beginning at 2 months of age regardless of previous hearing-screening outcomes.23 Confirming a complete follow-through for audiology screening is another critical aspect of postdischarge care. For both vision and hearing screening, our goal is 100% adherence to recommended guidelines. More detailed information about referral practices, availability of community-based follow-up services, and missing documentation is needed before we can implement improvement efforts.
Growth and Nutrition
The pediatric community defines growth failure as weight under the 10th percentile for corrected age on a standardized growth chart.24 Although many ELBW infants leave the NICU with weights below the 10th percentile,25 most catch-up to their term-born peers by early school age.26 Precise estimates of suboptimal growth after NICU discharge are lacking, in part due to variation in how growth is measured and reported.27 Poor growth reflects inadequate nutritional supports, presenting opportunities for dietary interventions before and after discharge.25 Furthermore, the literature shows a link between poor growth and poor health and development,28–30 making the optimization of growth a priority for improving global outcomes. The percentage of infants with weight below the 10th percentile at 18–24 months is slightly higher than the small for gestational age rate in the 2014–2016 birth year cohorts, suggesting both persistence of poor growth and growth failure of appropriately grown infants over time (ie, children crossing growth percentiles from discharge to follow-up). Mapping trajectories of growth from birth to discharge and then to later childhood and assessing nutrition practices across sites before and after discharge will help guide the next steps to improve this important outcome measure.
Social Determinants
Last, we found different follow-up rates among infants born to white, black, and Hispanic mothers. Potential explanations include programmatic and policy barriers, inadequate communication, and implicit bias among members of the healthcare team. Also, much of the social and economic data from our sample were missing. Unlike medication use and rehospitalization data that providers routinely record in the electronic medical record, a clinician needs to ask parents about income, education, and family structure. These questions are highly personal and not routinely asked during clinical encounters. The role of social determinants in shaping population patterns of service availability and health and developmental outcomes is clear.31 Developing robust and reliable measures of social and economic context will be crucial to our efforts in parsing the contributions of biologic and social risk factors, understanding the drivers of outcomes variation, and identifying targets for improvement efforts.
CONCLUSIONS
Quality improvement collaboratives are powerful tools for reducing variability in medical practice, in turn improving the safety and quality of the care we provide. To our knowledge, most US-based quality initiatives in neonatology to date stop at the NICU door. We have established the New England Follow-up Network to address the variation in postdischarge follow-up program practices at the regional level. Moving forward, NEFUN will strive to standardize process and outcomes measures across centers and to develop, implement, and evaluate novel interventions to improve service delivery and clinical outcomes. There is also the potential to evaluate the impact of organizationally based operations on clinical follow-up work, as well as state- and agency-based impacts on supportive services. We envision that this process will lead to best practices for high-risk infant follow-up, improve the continuum of care from the NICU to the community, and provide follow-through on the promise of optimal health and development of all high-risk infants in New England.
DISCLOSURE
The authors have no financial interest to declare in relation to the content of this article.
ACKNOWLEDGMENTS
This work would not be possible without the efforts of the following individuals from our member institutions, our steering committee, and partners at the Vermont Oxford Network.
Steering committee are Tyler Hartman, MD, Director; Jonathan S Litt, MD, MPH, ScD (jlitt@bidmc.harvard.edu), Operations Director; Charles Mercier, MD, Scientific Advisor; Lawrence Rhein, MD, MPH, Scientific Advisor; and Betty Vohr, MD, Scientific Advisor.
Member institutions (leads) are Baystate Medical Center, Springfield, Mass. (Laura Madore, MD); Beth Israel Deaconess Medical Center/Boston Children’s Hospital, Boston, Mass. (Jane Stewart, MD); Brigham and Women’s Hospital, Boston, Mass. (Jennifer Benjamin, MD); Connecticut Children’s Medical Center, Hartford, Conn. (Shabnam Lainwala, MD); Dartmouth Hitchcock Medical Center, Lebanon, N.H. (Tyler Hartman, MD); Eastern Maine Medical Center, Bangor, Maine (John Hagerty, MD); Massachusetts General Hospital for Children, Boston, Mass. (Melissa Woythaler, DO); Maine Medical Center, Portland, Maine (Alan Picarillo, MD); Tufts Medical Center, Boston, Mass. (Megan Reyes-Wang, DO); UMASS Memorial Medical Center, Worcester, Mass. (Lawrence Rhein, MD, MPH); University of Vermont Children’s Hospital, Burlington, Vt. (Deirdre O’Reilly, MD, MPH); Women and Infant’s Hospital, Providence, R.I. (Elizabeth McGowan, MD); Yale-New Haven Children’s Hospital, New Haven, Conn. (Angela Montgomery, MD, MSEd); and Yale-New Haven Children’s at Bridgeport Hospital, Bridgeport, Conn. (Christine Butler, MD).
VON Partners are Sharla Crowley; Erika Edwards, PhD, MPH; Karla Ferrelli, MS; Kate Morrow, MS; Roger Soll, MD; and VON ELBW Follow-up Steering Committee.
Supplementary Material
Footnotes
Published online May 5, 2020
Supplemental digital content is available for this article. Clickable URL citations appear in the text.
To cite: Litt JS, Edwards EM, Lainwala S, Mercier C, Montgomery A, O’Reilly D, Rhein L, Woythaler M, Hartman T, on behalf of the New England Follow-up Network. Optimizing High-risk Infant Follow-up in Nonresearch-based Paradigms: The New England Follow-up Network. Pediatr Qual Saf 2020;2:e287.
REFERENCES
- 1.McCormick MC. Long-term follow-up of infants discharged from neonatal intensive care units. JAMA. 1989;261:1767–1772. [PubMed] [Google Scholar]
- 2.American Academy of Pediatrics. Follow-up care of high-risk infants. Pediatrics. 2004;114(Suppl 5):1377–1397. [Google Scholar]
- 3.Wang CJ, McGlynn EA, Brook RH, et al. Quality-of-care indicators for the neurodevelopmental follow-up of very low birth weight children: results of an expert panel process. Pediatrics. 2006;117:2080–2092. [DOI] [PubMed] [Google Scholar]
- 4.Bockli K, Andrews B, Pellerite M, et al. Trends and challenges in United States neonatal intensive care units follow-up clinics. J Perinatol. 2014;34:71–74. [DOI] [PubMed] [Google Scholar]
- 5.Kuppala VS, Tabangin M, Haberman B, et al. Current state of high-risk infant follow-up care in the United States: results of a national survey of academic follow-up programs. J Perinatol. 2012;32:293–298. [DOI] [PubMed] [Google Scholar]
- 6.Lannon CM, Peterson LE. Pediatric collaborative networks for quality improvement and research. Acad Pediatr. 2013;13(6 Suppl):S69–S74. [DOI] [PubMed] [Google Scholar]
- 7.Nadeem E, Olin SS, Hill LC, et al. Understanding the components of quality improvement collaboratives: a systematic literature review. Milbank Q. 2013;91:354–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hulscher ME, Schouten LM, Grol RP, et al. Determinants of success of quality improvement collaboratives: what does the literature show? BMJ Qual Saf. 2013;22:19–31. [DOI] [PubMed] [Google Scholar]
- 9.Horbar JD, Rogowski J, Plsek PE, et al. Collaborative quality improvement for neonatal intensive care. NIC/Q Project Investigators of the Vermont Oxford Network. Pediatrics. 2001;107:14–22. [DOI] [PubMed] [Google Scholar]
- 10.Wheeler DS, Giaccone MJ, Hutchinson N, et al. A hospital-wide quality-improvement collaborative to reduce catheter-associated bloodstream infections. Pediatrics. 2011;128:e995–e1004; quiz e10041007. [DOI] [PubMed] [Google Scholar]
- 11.Lee HC, Kurtin PS, Wight NE, et al. A quality improvement project to increase breast milk use in very low birth weight infants. Pediatrics. 2012;130:e1679–e1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu S, Tyler A, Logsdon T, et al. A quality improvement collaborative to improve the discharge process for hospitalized children. Pediatrics. 2016;138:e20143604. [DOI] [PubMed] [Google Scholar]
- 13.Horbar JD. The Vermont Oxford network: evidence-based quality improvement for neonatology. Pediatrics. 1999;103(1 Suppl E):350–359. [PubMed] [Google Scholar]
- 14.Horbar JD, Soll RF, Edwards WH. The Vermont Oxford network: a community of practice. Clin Perinatol. 2010;37:29–47. [DOI] [PubMed] [Google Scholar]
- 15.Mercier CE, Dunn MS, Ferrelli KR, et al. ; Vermont Oxford Network ELBW Infant Follow-Up Study Group. Neurodevelopmental outcome of extremely low birth weight infants from the Vermont Oxford network: 1998–2003. Neonatology. 2010;97:329–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.National Center for Health Statistics. CDC Growth Charts. 2000; www.cdc.gov/growthcharts/percentile_data_files.htm. Accessed October 8, 2018.
- 17.Luttikhuizen dos Santos ES, de Kieviet JF, Königs M, et al. Predictive value of the Bayley scales of infant development on development of very preterm/very low birth weight children: a meta-analysis. Early Hum Dev. 2013;89:487–496. [DOI] [PubMed] [Google Scholar]
- 18.Hintz SR, Gould JB, Bennett MV, et al. Referral of very low birth weight infants to high-risk follow-up at neonatal intensive care unit discharge varies widely across California. J Pediatr. 2015;166:289–295. [DOI] [PubMed] [Google Scholar]
- 19.Higgins RD, Shankaran S. The neonatal research network: history since 2003, future directions and challenges. Semin Perinatol. 2016;40:337–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Canadian Neonatal Follow-up Network. Canadian Neonatal Follow-up Network. 2009; www.cnfun.ca. Accessed October 3, 2019.
- 21.American Academy of Pediatrics Section on Ophthalmology. Screening examination of premature infants for retinopathy of prematurity. Pediatrics. 2013;1311189–195. [DOI] [PubMed] [Google Scholar]
- 22.American Academy of Pediatrics Joint Committee on Infant Hearing. Year 2007 position statement: principles and guidelines for early hearing detection and intervention programs. Pediatrics. 2007;120:898–921. [DOI] [PubMed] [Google Scholar]
- 23.Committee on Practice and Ambulatory Medicine, Bright Futures Periodicity Schedule Workgroup. 2017 Recommendations for preventive pediatric health care. Pediatrics. 2017;139:e20170254. [DOI] [PubMed] [Google Scholar]
- 24.Martin CR, Brown YF, Ehrenkranz RA, et al. ; Extremely Low Gestational Age Newborns Study Investigators. Nutritional practices and growth velocity in the first month of life in extremely premature infants. Pediatrics. 2009;124:649–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Griffin IJ, Cooke RJ. Nutrition of preterm infants after hospital discharge. J Pediatr Gastroenterol Nutr. 2007;45 (Suppl 3):S195–S203. [DOI] [PubMed] [Google Scholar]
- 26.Hack M, Schluchter M, Cartar L, et al. Growth of very low birth weight infants to age 20 years. Pediatrics. 2003;112(1 Pt 1):e30–e38. [DOI] [PubMed] [Google Scholar]
- 27.Schehr LK, Johnson TS. Concept analysis of growth failure in preterm infants in the NICU. J Obstet Gynecol Neonatal Nurs. 2017;46:870–877. [DOI] [PubMed] [Google Scholar]
- 28.Belfort MB, Cohen RT, Rhein LM, et al. Preterm infant growth and asthma at age 8 years. Arch Dis Child Fetal Neonatal Ed. 2016;101:F230–F234. [DOI] [PubMed] [Google Scholar]
- 29.Kan E, Roberts G, Anderson PJ, et al. ; Victorian Infant Collaborative Study Group. The association of growth impairment with neurodevelopmental outcome at eight years of age in very preterm children. Early Hum Dev. 2008;84:409–416. [DOI] [PubMed] [Google Scholar]
- 30.Belfort MB, Gillman MW, Buka SL, et al. Preterm infant linear growth and adiposity gain: trade-offs for later weight status and intelligence quotient. J Pediatr. 2013;163:1564–1569 e1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oberg C, Colianni S, King-Schultz L. Child health disparities in the 21st century. Curr Probl Pediatr Adolesc Health Care. 2016;46:291–312. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


