Abstract
Background:
Today, cardiovascular disease is one of the main causes of mortality and disability in most developed and developing countries. The prediction of the major causes of deaths all over the world at all ages shows that 61% of deaths are due to chronic diseases, of which 30% is due to cardiovascular disease. The aim of this study was to assess the cost-utility analysis of atorvastatin for the prevention of cardiovascular diseases using the Markov model.
Methods:
Markov model with a lifetime horizon was developed to evaluate economic and health outcomes for atorvastatin drugs for the prevention of cardiovascular diseases for a cohort of 1,000 patients. The effectiveness indicator in this study was quality-adjusted life-years (QALYs); robustness of results was examined by one-way and probabilistic sensitivity analysis.
Results:
The results showed that the use of atorvastatin compared to no drug intervention was highly cost-effective with USD173 per additional QALY. The results of one-way and probabilistic sensitivity analysis confirmed the results of this study. The findings of this study also showed that the highest cost items were hospitalization costs in the cardiac care unit (CCU). Also, the highest cost items in para-clinical services were related to echocardiography costs, and troponin constituted the largest cost of laboratory tests.
Conclusions:
Based on the results of this study, it is recommended that cardiologists use atorvastatin in the prevention of cardiovascular disease.
Keywords: Atorvastatin, Markov model, cardiovascular diseases, cost-benefit analysis
Introduction
Cardiovascular disease is a major cause of death in most developed and developing countries. An estimation report on the most common causes of death throughout the globe, including all age groups, showed that 61% of death cases were due to chronic diseases, of which 30% were a result of cardiovascular diseases. Also, chronic diseases contribute to 48% of the burden of diseases, 10% of which are due to cardiovascular diseases. In our country, cardiovascular diseases account for 79% of the death of chronic diseases.[1,2] Preventive measures for cardiovascular diseases are applied at various levels. Interventions and preventive procedures can decrease the occurrence of cardiovascular diseases and brain strokes by approximately 20–30% along with a similar decrease in mortality rate and an increase in quality of life.[3] In addition to preventive interventions, a drug intervention can also be applied as a means of decreasing complications of cardiovascular diseases.[4] One of the most significant preventive drug interventions for the cardiovascular disease includes the use of statin drugs. Statins are most Wcommonly prescribed as a class of drugs for reducing blood cholesterol.[5] Statins block the liver's pathways for producing cholesterol, depleting the liver cells of any cholesterol, and finally allowing the liver to extract the cholesterol from the blood. Statins also aid the recapture of cholesterol from sediments in the artery wall, thus, eradicating coronary artery diseases.[6,7,8] Statins can be defined as a class of cholesterol-lowering agents mainly used in the prevention of cardiovascular diseases resulting from increased blood lipids. Statins work by inhibition of the HMG-CoA reductase enzyme. Various members of the drug class of statins are available in drug markets throughout the world, the most common type of which include atorvastatin, fluvastatin, lovastatin, pravastatin, and simvastatin.[9,10] One member of the statin class of medication with the highest impact in decreasing low-density lipoprotein (LDL) cholesterol and the lowest drug intervention is atorvastatin.[11,12] Atorvastatin is primarily used in treating high blood cholesterol and decreasing the risk of brain strokes, heart attacks or cardiac complications in type-2 diabetic patients, heart diseases, and other risk factors. Presently, atorvastatin is available in the form of 10, 20, and 40 mg tablets.[3,13,14,15] It is worth noting that despite the production of this drug in Iran, no economic studies have been conducted on the cost-effectiveness no cost-utility of this drug in Iran. This study aimed to assess the cost-utility of atorvastatin in preventing cardiovascular diseases using the Markov model.
Methods
We developed a five-state Markov model that is identical in structure for both alternatives. The study population included 1,000 hypothetical cohorts above 45 years of age. Individuals were distributed into various Markov states according to probability transition (the probability of a patient transferring between Markov states) for a period of 1 year. Markov states included healthy, myocardial infarction (MI) during the first year, MI during years after the first year, fatal MI, non-MI death Table 1. Based on Figure 1, individuals with perfect health may either remain healthy or enter different states including nonfatal MI, fatal MI, or non-MI death. Also, patients who have previously experienced nonfatal MI can experience fatal MI or non-MI death. The probability transition of post-MI to other states is similar to nonfatal MI. The competitor option for this study was selected as no intervention and quality-adjusted life-year (QALY) was used as an effectiveness index. The QALY measure is calculated according to the utility value of each Markov state, which is extracted from other studies.[16] The time horizon of the study was chosen as a lifetime. Given that the time horizon was more than 1 year, the cost and QALYs were discounted with an annual rate of 7.2% and 3%, respectively.[17] Required information included data on costs,[16] utility,[16] relative risks,[18] probability transition,[19,20] and mortality rate of cardiovascular diseases and other causes of death.[21,22] This study was performed from the patient's perspective. Direct medical costs including costs of drugs, laboratory tests, appointments, hospitalization, and para-clinical services were extracted from domestic studies and entered into the proposed Markov model. The proposed Markov model was illustrated using the TREE AGE PRO 2011 software. Incremental cost-effectiveness was later calculated by dividing the difference of cost to difference of effectiveness. One-way sensitivity analysis and probabilistic analysis performed to increase the robustness of results.
Table 1.
Relative risks for the use of atorvastatin
| Relative risk of using atorvastatin | Value | Reference |
|---|---|---|
| Healthy to nonfatal MI | 0.65 | 15 |
| Healthy to fatal MI | 0.74 | 15 |
MI: Myocardial infarction
Figure 1.
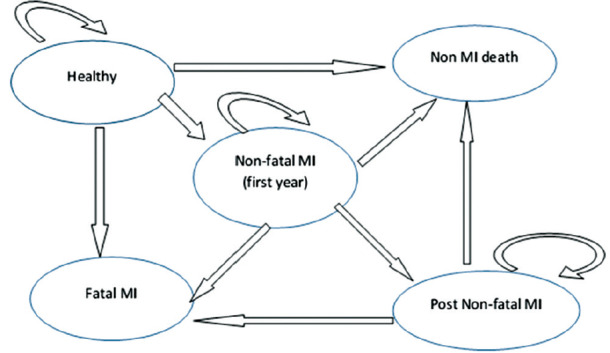
Markov model o atorvastatin drug
Results
Table 2, indicated that the cardiac care unit (CCU) hospitalization fee had the highest cost among all cost items. Also, echocardiography and troponin test had the highest costs among para-clinical services and laboratory tests, respectively. Streptokinase was the most expensive drug item. The results of Table 3 indicated that mean QALYs and costs were estimated to be USD 227 and 17 for atorvastatin and USD 154 and 16 for no action, respectively. Based on Figure 2, the cost and effectiveness of atorvastatin are higher compared to no intervention. For decision-making, the incremental cost-effectiveness ratio (ICER) must be calculated and compared with the threshold value. Calculation of ICER is shown as follows:
Table 2.
The cost components of atorvastatin
| Cost item | Cost (dollars) | Cost item | Cost (dollars) | Cost item | Cost (dollars) | Cost item | Cost (dollars) |
|---|---|---|---|---|---|---|---|
| CCU hospitalization fee | 78 | Fitness test | 19 | BS | 0.45 | CPK | 2.49 |
| General care units hospitalization fee | 61 | Laboratory tests | TG | 0.71 | SGOT | 0.63 | |
| Consultant visit fee | 3 | CBC Dif. | 0.74 | Cholesterol | 0.52 | SGPT | 0.63 |
| General practitioner visit fee | 3 | BUN | 0.41 | PTINR | 0.93 | ESR | 0.26 |
| Para-clinical services | Cr | 0.52 | PTT | 0.93 | |||
| Electrocardiograph | 3 | Na | 0.59 | Troponin | 2.45 | ||
| Echocardiography | 36 | K | LDH | 1.86 | |||
| Table 2 (continued) | |||||||
| Drug item | Cost (dollars) | Drug item | Cost (dollars) | Drug item | Cost (dollars) | ||
| 20 mg Rosuvastain | 0.148 | Enoxaparin | 3.72 | Captopril | 0.01 | ||
| ASA | 0.01 | Atorvastatin10 | 0.03 | Streptokinase | 9.29 | ||
| Clopidogrel | 0.29 | Ranitidine | 0.02 | Prescription costs | 0.2 | ||
| Metoprolol | 0.01 | Oxazepam | 0.01 | c | |||
CCU: Coronary care unit, CBC Dif: Complete blood count, BUN: Blood urea nitrogen, CR: Creatinine, NA: Sodium, K: Potassium, BS: Blood sugar, TG: Thyroglobulin, PTINR: Prothrombin time, PTT: Partial thromboplastin time, LDH: Lactate dehydrogenase, CPK: Creatine phosphokinase, SGOT: Serum glutamic-oxaloacetic transaminase, SGPT: Serum glutamic-pyruvic transaminase, ESR: Erythrocyte sedimentation rate
Table 3.
The results of cost-utility analysis
| Compared items | Cost | QALY | Cost difference | QALY difference | Result |
|---|---|---|---|---|---|
| Atorvastatin | USDv227 | 17 | USD 173 | 1 | Requires comparing ICER with threshold value |
| Non-intervention | USD 54 | 16 |
QALY: Quality-adjusted life year, USD: United States, Dollar, ICER: Incremental cost-effectiveness ratio
Figure 2.
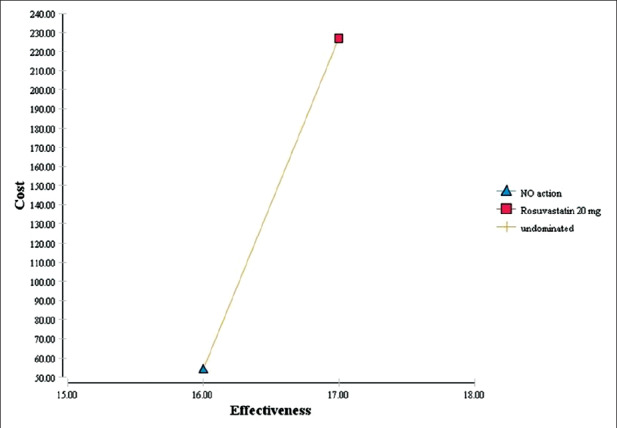
Cost-utility analysis of atorvastatin compared to non-intervention
Incremental cost-effectiveness ratio 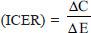
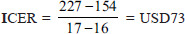
The threshold was calculated based on the WHO method (three times of GDP per capita, USD 5627).[23] Given that ICER was lower than the threshold; atorvastatin was cost-effective compared to no intervention.
Sensitivity analysis
Uncertainty is an inevitable factor in all economic assessments, therefore, this study assessed stability and generalization of results using sensitivity analysis.[24] In the one-way sensitivity analysis, the value of each variable increased by 20% and the tornado diagram was drawn [Figure 3]. The results of tornado maps [Figure 3] indicated that the study results had the highest sensitivity towards an increase in QALY value for no-intervention and the lowest sensitivity toward increased costs in no-intervention. [Figure 4] showed the results of the probabilistic sensitivity analysis using Monte Carlo simulation with 1,000 samples as input and a normal distribution for cost and QALY along with beta distribution for probabilities. The results showed that atorvastatin was more cost-effective than no intervention with maximum willingness to pay (threshold= 3* USD5627).
Figure 3.
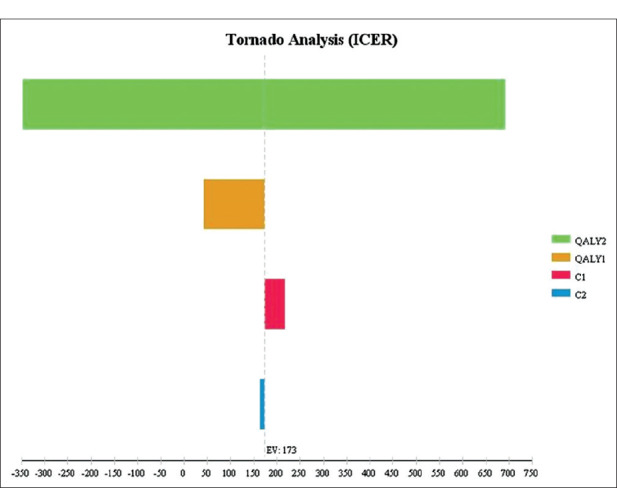
Tornado diagram for one-way sensitivity analysis
Figure 4.
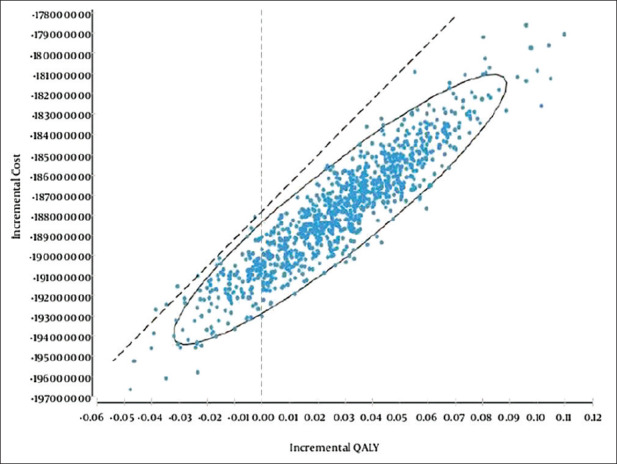
Probabilistic sensitivity analysis of using atorvastatin compared to no-intervention
Discussion
Economic assessments play a significant role in the optimal allocation of resources and accurate medical decision-making procedures within the health system. This study examined the cost-utility of atorvastatin in preventing cardiovascular diseases in Iran using the Markov Model. This is the first instance of such a study and the results indicated that atorvastatin, is more cost-effective in comparison with no intervention. Results were further verified by one-way and probabilistic sensitivity analysis. Study results also indicated the cost of hospitalization in CCU, constituted the highest cost among various items. The highest costs of para-clinical services and laboratory tests were from echocardiography and troponin tests, respectively. Streptokinase was also indicated as the most expensive drug among other drug items. Results of a study by Palmer et al. on the cost-effectiveness of atorvastatin in England showed that atorvastatin had a lower cost and higher effectiveness compared to fluvastatin.[25] The results of this study were consistent with those obtained by Palmer et al.
Research by Heirich et al. in England showed that atorvastatin in 20 and 40 mg dosages were more cost-effective than rosuvastatin, pravastatin, and simvastatin.[26] The results of the present study were also consistent with those obtained by Heircih et al. Another study by Casta et al. in England showed that atorvastatin was more cost-effective compared to rosuvastatin. The results of sensitivity analysis also indicated that this drug has a higher cost-effectiveness ratio for every clinical effectiveness unit for a wide spectrum of monetary values compared to other members of the statins class of medication.[27] Since atorvastatin is readily available and used for treating cardiovascular diseases in most hospitals in Iran, the results of this study can be generalized to other Iranian hospitals. However, considering the differences in cost coverage offered by insurance companies, the patients' willingness to pay, and the prevalence of cardiovascular diseases for different countries, the results of this study cannot be generalized to other countries.
Financial support and sponsorship
This study was supported by Vice Chancellor for Research and Technology, Isfahan, Iran.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
This study was supported by Vice Chancellor for Research and Technology, Isfahan, Iran (Grant Number 196201).
References
- 1.Fuster V, Kelly BB, Vedanthan R. Global cardiovascular health: Urgent need for an intersectoral approach. J Am Coll Cardiol. 2011;58:1208–10. doi: 10.1016/j.jacc.2011.05.038. [DOI] [PubMed] [Google Scholar]
- 2.Sadeghi M, Haghdoost AA, Bahrampour A, Dehghani M. Modeling the burden of cardiovascular diseases in Iran from 2005 to 2025: The impact of demographic changes. Iran J Public Health. 2017;46:506–16. [PMC free article] [PubMed] [Google Scholar]
- 3.Ahmed M, Husain NE, Almobarak A. Nonalcoholic fatty liver disease and risk of diabetes and cardiovascular disease: What is important for primary care physicians? J Family Med Prim Care. 2015;4:45–52. doi: 10.4103/2249-4863.152252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fakhrzadeh H, Bandarian F, Adibi H, Samavat T, Malekafzali H, Hodjatzadeh E, et al. Coronary heart disease and associated risk factors in Qazvin: A population-based study. East Mediterr Health J. 2008;14:33–41. [PubMed] [Google Scholar]
- 5.Shamsddin, Fazil M, Ansari S, Ali J. Atorvastatin solid dispersion for bioavailability enhancement. J Adv Pharm Technol Res. 2016;7:22–6. doi: 10.4103/2231-4040.169873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Okopien B, Buldak L, Boldys A. Benefits and risks of the treatment with fibrates-A comprehensive summary. Expert Rev Clin Pharmacol. 2018;11:1099–112. doi: 10.1080/17512433.2018.1537780. [DOI] [PubMed] [Google Scholar]
- 7.Dalugama C, Pathirage M, Kularatne SAM. Delayed presentation of severe rhabdomyolysis leading to acute kidney injury following atorvastatin-gemfibrozil combination therapy: A case report. J Med Case Rep. 2018;12:143. doi: 10.1186/s13256-018-1685-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gupta A, Thompson D, Whitehouse A, Collier T, Dahlof B, Poulter N, et al. Adverse events associated with unblinded, but not with blinded, statin therapy in the Anglo-Scandinavian cardiac outcomes trial-lipid-lowering arm (ASCOT-LLA): A randomised double-blind placebo-controlled trial and its non-randomised non-blind extension phase. Lancet (London, England) 2017;389:2473–81. doi: 10.1016/S0140-6736(17)31075-9. [DOI] [PubMed] [Google Scholar]
- 9.Nichols GA, Philip S, Reynolds K, Granowitz CB, Fazio S. Increased cardiovascular risk in hypertriglyceridemic patients with statin-controlled ldl cholesterol. J Clin Endocrinol Metab. 2018;103:3019–27. doi: 10.1210/jc.2018-00470. [DOI] [PubMed] [Google Scholar]
- 10.Reiner Z. Managing the residual cardiovascular disease risk associated with HDL-cholesterol and triglycerides in statin-treated patients: A clinical update. Nutr Metab Cardiovasc Dis. 2013;23:799–807. doi: 10.1016/j.numecd.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 11.Kapur NK, Musunuru K. Clinical efficacy and safety of statins in managing cardiovascular risk. Vasc Health Risk Manag. 2008;4:341–53. doi: 10.2147/vhrm.s1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Antony B, Merina B, Sheeba V. Amlamax in the management of dyslipidemia in humans. Indian J Pharm Sci. 2008;70:504–7. doi: 10.4103/0250-474X.44604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Solmi M, Correll CU, Carvalho AF, Ioannidis JPA. The role of meta-analyses and umbrella reviews in assessing the harms of psychotropic medications: Beyond qualitative synthesis. Epidemiol Psychiatr Sci. 2018;27:1–6. doi: 10.1017/S204579601800032X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Priti K, Agrawal A, Ranwa BL. High versus low dose statin therapy in Indian patients with acute ST-segment elevation myocardial infarction undergoing thrombolysis. Indian Heart J. 2017;69:453–7. doi: 10.1016/j.ihj.2017.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim J-S, Kim J, Choi D, Lee CJ, Lee SH, Ko Y-G, et al. Efficacy of high-dose atorvastatin loading before primary percutaneous coronary intervention in ST-segment elevation myocardial infarction: The STATIN STEMI trial. JACC Cardiovasc Interv. 2010;3:332–9. doi: 10.1016/j.jcin.2009.11.021. [DOI] [PubMed] [Google Scholar]
- 16.Amirsadri M, Hassani AJDJoPS. Cost-effectiveness and cost-utility analysis of OTC use of simvastatin 10 mg for the primary prevention of myocardial infarction in Iranian men. Daru. 2015;23:56. doi: 10.1186/s40199-015-0129-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nikfar S, Kebriaeezadeh A, Dinarvand R, Abdollahi M, Sahraian MA, Henry D, et al. Cost-effectiveness of different interferon beta products for relapsing-remitting and secondary progressive multiple sclerosis: Decision analysis based on long-term clinical data and switchable treatments. DARU J Pharm Sci. 2013;21:50. doi: 10.1186/2008-2231-21-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ward S, Jones ML, Pandor A, Holmes M, Ara R, Ryan A, et al. A systematic review and economic evaluation of statins for the prevention of coronary events. Health Technol Assess. 2007;11:1–160. doi: 10.3310/hta11140. [DOI] [PubMed] [Google Scholar]
- 19.Hafshejani A, Sarrafzadegan N, Attar Moghaddam H, Hosseini S, AsadiLari MJJIMS. Predictive factors of survival in patients with an acute myocardial infarction among genders in Iran. 2012;30:1611–21. [Google Scholar]
- 20.Farkhani EM. Survival rate and its related factors in patients with acute myocardial infarction. Med J Mashhad Univ Med Sci. 2014;57:636–46. [Google Scholar]
- 21.Statistical center of Iran. Selected Findings of the 2011 National Population and Housing Census. [Last accessed on 2020 Feb 12]. Available from: https://wwwamarorgir/english/Population-and-Housing-Censuses .
- 22.Ministry of Interior; National organization for civil registration; Statistical year book 1390. [Last accessed on 2020 Feb 12]. Available from: https://wwwsabteahvalir/en .
- 23.Philippines - National Demographic and Health Survey 2017. 2017. Available from: https://datacatalogworldbankorg/dataset/philippines-national-demographic-and-health-survey-2017 .
- 24.Ravangard R, Jafari A, Rahgoshai I, Zamirian M, Aghasadeghi K, Moarref A, et al. Comparison of the cost-effectiveness of transesophgeal and transthoracic echocardiographies to detect cardioembolic causes of stroke in non-selected patients. Int Cardiovasc Res J. 2018;12:48–52. [Google Scholar]
- 25.Palmer S, Brady A, Ratcliffe A. The cost-effectiveness of a new statin (rosuvastatin) in the UK NHS. Int J Clin Pract. 2003;57:792–800. [PubMed] [Google Scholar]
- 26.Hirsch M, O'donnell JC, Jones P. Rosuvastatin is cost-effective in treating patients to low-density lipoprotein-cholesterol goals compared with atorvastatin pravastatin and simvastatin: Analysis of the STELLAR trial. Eur J Cardiovasc Prev Rehabil. 2005;12:18–28. [PubMed] [Google Scholar]
- 27.Costa-Scharplatz M, Ramanathan K, Frial T, Beamer B, Gandhi S. Cost-effectiveness analysis of rosuvastatin versus atorvastatin, simvastatin, and pravastatin from a Canadian health system perspective. Clin Ther. 2008;30:1345–57. doi: 10.1016/s0149-2918(08)80061-6. [DOI] [PubMed] [Google Scholar]


