Abstract
We hypothesized cattle that differed in BW gain had different digestive tract microbiota. Two experiments were conducted. In both experiments, steers received a diet that consisted of 8.0% chopped alfalfa hay, 20% wet distillers grain with solubles, 67.75% dry-rolled corn, and 4.25% vitamin/mineral mix (including monensin) on a dry matter basis. Steers had ad libitum access to feed and water. In experiment 1, 144 steers (age = 310 ± 1.5 d; BW = 503 ± 37.2 kg) were individually fed for 105 d. Ruminal digesta samples were collected from eight steers with the greatest (1.96 ± 0.02 kg/d) and eight steers with the least ADG (1.57 ± 0.02 kg/d) that were within ±0.32 SD of the mean (10.1 ± 0.05 kg/d) dry matter. In experiment 2, 66 steers (age = 396 ± 1 d; BW = 456 ± 5 kg) were individually fed for 84 d. Rumen, duodenum, jejunum, ileum, cecum, and colon digesta samples were collected from eight steers with the greatest (2.39 ± 0.06 kg/d) and eight steers with the least ADG (1.85 ± 0.06 kg/d) that were within ±0.55 SD of the mean dry matter intake (11.9 ± 0.1 kg/d). In both studies, DNA was isolated and the V1 to V3 regions of the 16S rRNA gene were sequenced. Operational taxonomic units were classified using 0.03 dissimilarity and identified using the Greengenes 16S rRNA gene database. In experiment 1, there were no differences in the Chao1, Shannon, Simpson, and InvSimpson diversity indexes or the permutation multivariate analysis of variance (PERMANOVA; P = 0.57). The hierarchical test returned six clades as being differentially abundant between steer classifications (P < 0.05). In experiment 2, Chao1, Shannon, Simpson, and InvSimpson diversity indexes and PERMANOVA between steer classified as less or greater ADG did not differ (P > 0.05) for the rumen, duodenum, ileum, cecum, and colon. In the jejunum, there tended to be a difference in the Chao1 (P = 0.09) and Simpson diversity (P = 0.09) indexes between steer classifications, but there was no difference in the Shannon (P = 0.14) and InvSimpson (P = 0.14) diversity indexes. Classification groups for the jejunum differed (P = 0.006) in the PERMANOVA. The hierarchical dependence false discovery rate procedure returned 11 clades as being differentially abundant between steer classifications in the jejunum (P < 0.05). The majority of the OTU were in the Families Corynebacteriaceae and Coriobacteriaceae. This study suggests that intestinal differences in the microbiota of ruminants may be associated with animal performance.
Keywords: cattle, feed efficiency, microbiota
Introduction
Bacteria have the first opportunity to utilize nutrients consumed by cattle. Pre-gastric fermentation in the rumen results in the modification of nutrients before the animal can utilize them. This modification can have positive outcomes such as allowing ruminants to use the complex structural carbohydrates in plants via bacterial fermentation and can have negative outcomes when high-quality nutrients are modified to lower quality nutrients through fermentation. A potential cause of variation in feed efficiency could be a consequence of differences in nutrient utilization by bacteria amongst cattle. One of the difficulties in describing the relationship between the microbiota of the gastrointestinal (GI) tract and feed efficiency is the multiple definitions of feed efficiency (Guan et al., 2008; Myer et al. 2015a; Paz et al., 2018). A considerable amount of research conducted in this area has defined feed efficiency by classifying cattle as having less or greater residual feed intake (RFI, Koch et al., 1963). Most of the time cattle that are classified as having a less RFI also have less feed intake. A function of lower intakes is often slower passage rates (Colucci1 et al., 1982; Okine and Mathison, 1991) which may drive differences in the microbiota. Other models have studied extremes in intake and average daily gain (ADG) in a factorial design (Myer et al., 2015a, 2015b, 2015c, 2016). Because of the rumen’s known contribution to nutrient availability and methane production, many of the studies have concentrated on it and less research has been conducted on the remainder of the GI tract. The objective of the study was to characterize the microbiota differences throughout the digestive tract of finishing beef cattle that differed in ADG with a similar feed intake. We hypothesized that the microbiota of the digestive tract of cattle that differed in ADG at fixed feed intake differed.
Materials and Methods
Research protocols were approved and monitored by the U.S. Meat Animal Research Center Institutional and Animal Care Committee (#43.2) in accordance with the Guide for the Care and Use of Agricultural Animals in Agricultural Research and Teaching (2010).
Cattle
Experiment 1
Management of the steers has been previously described by Artegoitia et al. (2017) and Reynolds et al. (2017). One hundred forty-four Angus steers (age = 310 ± 1.5 d; body weight [BW] = 503 ± 37.2 kg) were individually fed for 105 d. Steers received a ration that as dry matter (DM) consisted of 8.0% chopped alfalfa hay, 20% wet distillers grain with solubles, 67.75% dry rolled corn, and 4.25% vitamin/mineral mix. The vitamin/mineral mix included 772 mg/kg monensin (as fed). Individual feed intake was measured using a Calan Broadbent Feeding System (Northwood, NH, USA). Steers had ad libitum access to feed and water. Feed was offered daily and feed refusals were measured weekly. Steer BW was measured on days 0, 1, 21, 42, 63, 84, 104, and 105. A quadratic regression was fit to individual animals for BW against days on study. Initial and final BW were estimated from the regression and ADG was calculated as the difference between final and initial BW divided by days on the study (105 d). The eight steers with the greatest (1.96 ± 0.02 kg/d) and eight steers with the least ADG (1.57 ± 0.02 kg/d) that were within ±0.32 SD of the mean (10.1 ± 0.05 kg/d) dry matter intake (DMI) were selected for the study.
Experiment 2
Cross-bred steers (n = 66, age = 396 ± 1 d; BW = 456 ± 5 kg) were individually fed for 84 d. Cattle were fed the same ration as in experiment 1 and managed the same. Steers had ad libitum access to feed and water. Feed was offered daily and feed refusals were measured weekly. Steer BW was measured on days 0, 1, 21, 42, 63, 83, and 84. A quadratic regression was fit to individual animals for BW on time. Initial and final BW were estimated from the regression, and ADG was calculated as the difference between final and initial BW divided by days on the study. The eight steers with the greatest (2.39 ± 0.06 kg/d) and eight steers with the least ADG (1.85 ± 0.06 kg/d) that were within ±0.55 SD of the mean (11.9 ± 0.1 kg/d) DMI were selected for the study.
Sample collection
On the day of slaughter, cattle were transported from their pens before the morning feed was offered to the abattoir (1.1 Km). Digesta samples (5 to 30 mL) were collected immediately following slaughter. Rumen fluid was mixed in the rumen and was collected. Duodenal digesta was collected approximately 5 cm caudal of the cranial duodenal flexure. Digesta from the jejunum was sampled approximately 1 m cranial to the ileocecal fold. Digesta in the ileum was sampled approximately 5 cm cranial to the ileocecal junction. Digesta from the cecum was sampled approximately 10 cm from the terminal end of the cecum. Digesta from the colon was sampled from the midpoint of the colon. Digesta was removed from the tract and placed in 50 mL screw top conical tubes. The tubes were flash frozen in liquid nitrogen and the samples were stored at −80 °C. Due to an absence of digesta in the jejunum seven less an six greater ADG steers were collected, in the ileum six less an five greater ADG steers were collected, and in the colon seven less an five greater ADG steers were collected.
Deoxyribonucleic acid extraction, amplification, and sequencing
Deoxyribonucleic acid (DNA) was extracted from digestive samples using a combination of bead beating and a DNA isolation spin column (Yu and Morrison, 2004). Briefly, cell lysis was achieved by bead beating 0.2 g of the sample in ZR BashingBead Lysis Tubes (Zymo Research Corp, Santa Ana, CA, USA) for 3 min at 21 Hz in the presence of 4% (wt/vol) SDS, 500 mM NaCl, and 50 mM EDTA using the TissueLyser II system (Qiagen, Hilden, Germany). After mechanical and chemical cell lysis, 10 M ammonium acetate (260 μL) was used to precipitate and remove the impurities and SDS followed by equal volume isopropanol precipitation for the recovery of the nucleic acids. Supernatants were treated with 2 μL ribonuclease (10 mg/mL) and proteinase K (QIAamp DNA Stool Mini Kit; Qiagen) followed by the use of QIAamp columns from the Qiagen DNA Stool Mini Kit (Qiagen).
The total DNA concentration was determined using a NanoDrop One Spectrophotometer (Thermo Scientific, Waltham, MA, USA). A total of 15 ng of total DNA from each sample obtained from the rumen, duodenum, jejunum, ileum, colon, and cecum were used in 10 μL polymerase chain reactions (PCRs). Amplicon library preparation was performed by PCR amplification of the V1 to V3 region of the 16S rRNA gene, using modified universal primers 27F (5′-Adaptor/AGAGTTTGATCCTGGCTCAG) and 519R (5′-Adaptor/GTATTACCGCGGCTGCTG). Each PCR reaction included the following: 1 μL of 0.1 μM each of 16S forward and reverse oligonucleotides, 1 μL of 1.85 μM oligonucleotide with 12 base pair index sequence and flow cell sequence, 0.2 μM oligonucleotide with the forward Illumina flow cell sequence, 1X HotStar buffer, 0.2 μL of 25 mM dNTP, 0.65 μL of 25 mM MgCl2, 1U HotStar Taq (Qiagen, Germantown, MD, USA), and adjusted to 10 μL with sterile water. Amplification was performed using a Bio-Rad Dyad thermal cycler with a 96-well head. Thermal cycling conditions were 95 °C for 15 min followed by 9 cycles of 94 °C for 1 min, 50 °C for 30 s, and 72 °C for 45 s, and 20 cycles of 94 °C for 20 s, 62 °C for 30 s, and 72 °C for 30 s, with a final extension of 72 °C for 5 min. Upon completion of amplification, 4 μL from each well was pooled and dNTPs and small primers were removed from 100 μL of the pooled product with the QiaQuick PCR purification kit. Amplicons were eluted in 25 μL of Qiagen elution buffer.
Larger primers and nonspecific PCR products under 200 bp in size were removed using the Zymo Research Select-A-Size kit (Zymo Research, Irvine, CA, USA). Amplicons (3 μg) >200 bp were isolated using 30 μL of 95% ethanol according to the manufacturer’s protocol. DNA fragments were eluted in 25 μL of DNA elution buffer.
The amplicon libraries were quantified with the NanoDrop One and diluted to approximately 4 nM. These diluted libraries were then quantified using the NEBNext Library quantification kit (New England Biolabs, Ipswich, MA, USA) and adjusted as needed to 4 nM. Libraries were sequenced with a 600 cycle V2 kit with 300 bp, paired-end reads on an Illumina MiSeq next-generation sequencer.
Sequence read processing and analysis
All sequences were processed using the Microbial Community Analysis (MICCA; Stable version: 1.7.0,) software package (Albanese et al., 2015). Paired sequences were merged with a minimum overlap of 20 and a maximum mismatch of 5 using VSEARCH (Rognes et al., 2016) within MICCA. Primer sequence was trimmed using PyNAST (Caporaso et al., 2010) within MICCA. Low-quality reads, singletons, and chimeric sequences were identified and removed using VSEARCH, and each operational taxonomic unit (OTU) was determined using Swarm v2 (Mahé et al., 2015) with 0.03 dissimilarity cutoff in MICCA. The OTUs were classified within MICCA using VSEARCH and the Greengenes 16S rRNA gene database (McDonald et al., 2012), and a phylogenetic tree was constructed with FastTree 2 (Price et al., 2010) within MICCA.
The biome file from MICCA was imported into the Phyloseq package (McMurdie and Holmes, 2013) of R. Chao1, Shannon, Simpson, and InvSimpson indexes were calculated from rarefied OTU count data. Group differences in these indexes were tested using an analysis of variance (ANOVA). After testing for diversity, OTUs not classified at the phylum level were removed from the data set as were OTUs not present in at least two copies in 20% or more of the libraries. The filtered, raw (not rarefied) data were log-transformed and a permutation multivariate analysis of variance (PERMANOVA) using a weighted UniFrac was conducted to determine if there were global shifts in the bacterial communities between treatments (Tang et al., 2016). Principal coordinates analysis (PCoA) using the weighted UniFrac distance matrix was conducted across sample sites to determine structural differences in bacterial communities across sites, and within sites to determine differences in bacterial communities associated with ADG classification.
To test for difference in OTU clades between steers classified as having less or greater ADG, a DESeq2 (Love et al., 2014) file was created from the filtered Phyloseq file. Data were adjusted with geometric means and variance stabilized (Callahan et al., 2016), and the hierarchical dependence false discovery rate procedure was implemented in structSSI (Sankaran and Holmes, 2014).
Results
Experiment 1
Rumen
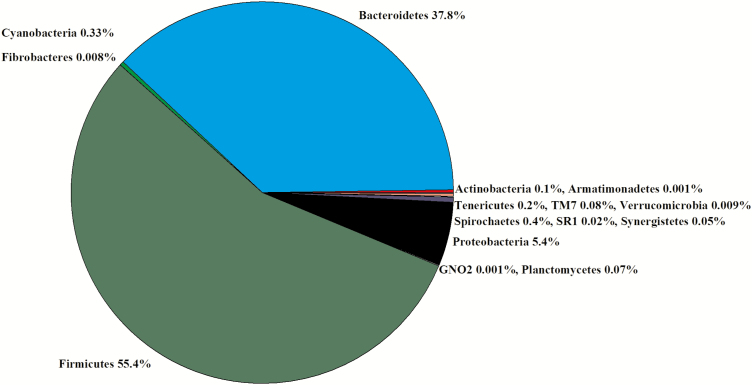
Ruminal digesta were the only samples collected in experiment 1. In the 16 libraries, there were 915,024 sequences that were binned to 967 OTUs at the 3% dissimilatory level. After the removal of OTUs that were not classified at the phylum level, there were 877,808 sequences binned to 894 OTUs; after filtering for low prevalence sequences, there were 871,697 sequences binned classified to 520 OTUs for analyses. The majority of the filtered OTUs belonged to either Firmicutes (55%) or Bacteroidetes (38%, Figure 1). Within Firmicutes, Lachnospiraceae (41%), Veillonellaceae (28%), and Ruminococcaceae (16%) were the predominant families. Within Bacteroidetes, Prevotellaceae (50%) and p-2534-18B5 (34%) were the predominant families. Within Proteobacteria, Succinivibrionaceae (95%) was the predominant family. There were no differences in the Chao1, Shannon, Simpson, and InvSimpson diversity indexes between steer classifications (Table 1). The weighted UniFrac PCoA demonstrated that there was no clustering between the ADG classifications (data not presented). Classification groups did not differ (P = 0.57) in the PERMANOVA. The hierarchical test returned six clades as being differentially abundant between steer classifications (P < 0.05; Table 2). Four of the clades contained OTU classified in the order Clostridiales. The other two clades contained phyla consisting of TM7 and Planctomycetes.
Figure 1.
Experiment 1: percent of OTU by phyla in the rumen of experiment 1 (n = 16).
Table 1.
Diversity indexes for OTU in the alimentary tract of steers classified as having a greater or less ADG at the average feed intake
| Location | Chao1 | Shannon | Simpson | InvSimpson |
|---|---|---|---|---|
| Experiment 1 | ||||
| Rumen | ||||
| Less ADG (n = 8) | 514 ± 29 | 3.49 ± 0.10 | 0.91 ± 0.01 | 14.9 ± 1.8 |
| Greater ADG (n = 8) | 551 ± 29 | 3.29 ± 0.10 | 0.92 ± 0.01 | 11.3 ± 1.8 |
| PTreatment | 0.38 | 0.17 | 0.21 | 0.18 |
| Experiment 2 | ||||
| Rumen | ||||
| Less ADG (n = 8) | 411 ± 27 | 3.32 ± 0.11 | 0.90 ± 0.01 | 10.7 ± 1.1 |
| Greater ADG (n = 8) | 410 ± 27 | 3.20 ± 0.11 | 0.89 ± 0.01 | 9.6 ± 1.1 |
| PTreatment | 0.98 | 0.48 | 0.45 | 0.49 |
| Duodenum | ||||
| Less ADG (n = 8) | 447 ± 52 | 3.68 ± 0.13 | 0.93 ± 0.01 | 17.5 ± 1.1 |
| Greater ADG (n = 8) | 399 ± 52 | 3.79 ± 0.13 | 0.95 ± 0.01 | 20.3 ± 1.1 |
| PTreatment | 0.53 | 0.55 | 0.25 | 0.47 |
| Jejunum | ||||
| Less ADG (n = 7) | 266 ± 9 | 2.20 ± 0.08 | 0.80 ± 0.02 | 5.1 ± 0.4 |
| Greater ADG (n = 6) | 291 ± 10 | 2.02 ± 0.08 | 0.75 ± 0.02 | 4.2 ± 0.4 |
| PTreatment | 0.09 | 0.14 | 0.09 | 0.14 |
| Ileum | ||||
| Less ADG (n =6) | 291 ± 20 | 2.13 ± 0.08 | 0.77 ± 0.02 | 4.7 ± 0.5 |
| Greater ADG (n =5) | 340 ± 22 | 2.14 ± 0.09 | 0.77 ± 0.02 | 4.5 ± 0.5 |
| PTreatment | 0.13 | 0.94 | 0.98 | 0.80 |
| Cecum | ||||
| Less ADG (n = 8) | 1,120 ± 104 | 4.71 ± 0.13 | 0.96 ± 0.01 | 29.3 ± 4.1 |
| Greater ADG (n = 8) | 1,202 ± 104 | 2.85 ± 0.13 | 0.97 ± 0.01 | 32.6 ± 4.1 |
| PTreatment | 0.99 | 0.45 | 0.43 | 0.58 |
| Colon | ||||
| Less ADG (n = 7) | 514 ± 29 | 3.49 ± 0.10 | 0.92 ± 0.01 | 14.9 ± 1.8 |
| Greater ADG (n = 5) | 551 ± 29 | 3.29 ± 0.10 | 0.91 ± 0.01 | 11.3 ± 1.8 |
| PTreatment | 0.38 | 0.17 | 0.21 | 0.18 |
Table 2.
Experiment 1—shift in rumen OTU in steers classified as having less (n = 8) or greater (n = 8) ADG
| Clade | P-value | Phylum | Class | Order | Family | Genus | Species | ADG |
|---|---|---|---|---|---|---|---|---|
| 1 | 0.048 | Firmicutes | Clostridia | Clostridiales | Lachnospiraceae | Coprococcus | NA | ↓ |
| Firmicutes | Clostridia | Clostridiales | Lachnospiraceae | NA | NA | ↓ | ||
| 2 | 0.040 | Firmicutes | Clostridia | Clostridiales | NA | NA | NA | ↑ |
| Firmicutes | Clostridia | Clostridiales | NA | NA | NA | ↑ | ||
| Firmicutes | Clostridia | Clostridiales | NA | NA | NA | ↑ | ||
| 3 | 0.002 | Firmicutes | Clostridia | Clostridiales | NA | NA | NA | ↓ |
| 4 | 0.025 | Firmicutes | Clostridia | Clostridiales | Ruminococcaceae | NA | NA | ↓ |
| 5 | 0.019 | TM7 | TM7-3 | CW040 | F16 | NA | NA | ↓ |
| TM7 | TM7-3 | CW040 | F16 | NA | NA | ↓ | ||
| 6 | 0.039 | Planctomycetes | Planctomycetia | Pirellulales | Pirellulaceae | NA | NA | ↓ |
NA, OTU not assigned; ↓, OTU directionally greater in less ADG; ↑, OTU directionally greater in greater ADG.
Experiment 2
Anatomical site
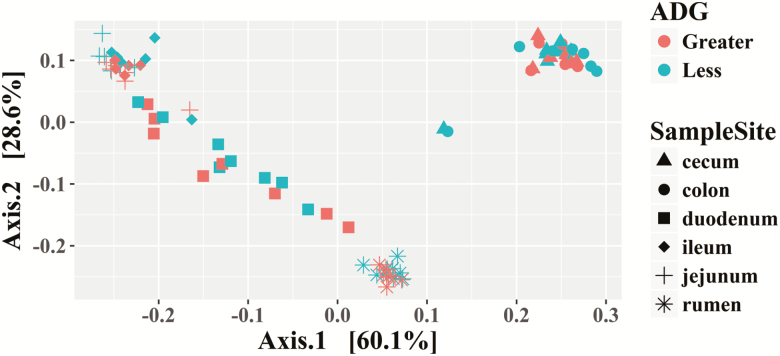
The PCoA demonstrates that the rumen microbiota differs from other sample sites (Figure 2). In general, the jejunum and ileum cluster together; while the duodenum is disperse, it clusters between the rumen and the remaining small intestine (Figure 2). The cecum and colon cluster together (Figure 2).
Figure 2.
Experiment 2: principal coordinate analysis using weighted UniFrac for the digestive tract of steers classified as having less or greater ADG at a common feed intake.
Rumen
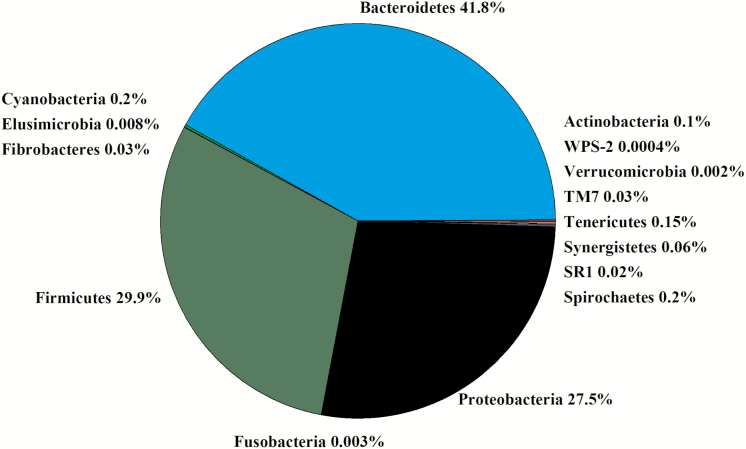
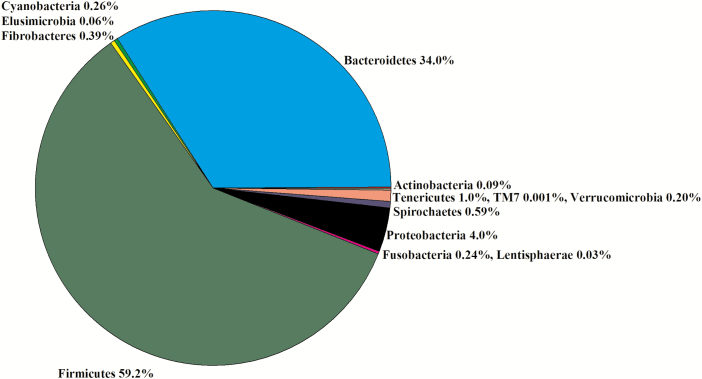
In the 16 libraries, there were 1,145,393 sequences that were binned to 819 OTUs at the 3% dissimilatory level. After the removal of OTUs that were not classified at the phylum level, there were 1,061,183 sequences binned to sequences in 750 OTUs; after filtering for low prevalence sequences, there were 1,051,469 sequences that binned to sequences in 465 OTUs for analyses. The majority of the filtered OTUs belonged to Bacteroidetes (42%), Firmicutes (30%), or Proteobacteria (28%; Figure 3). Within Firmicutes, Lachnospiraceae (29%), Veillonellaceae (45%), and Ruminococcaceae (7%) were the predominant families (Figure 4). Within Bacteroidetes, Prevotellaceae (83%), p-2534-18B5 (7%), and S24-7 (7%) were the predominant families (Figure 4). Within Proteobacteria, Succinivibrionaceae (99%) was the predominant family. There was no difference in the Chao1, Shannon, Simpson, and InvSimpson diversity indexes between steer classifications (Table 1). Classification groups did not differ (P = 0.13) in the PERMANOVA. The weighted UniFrac PCoA demonstrated that there was no clustering between the ADG classifications (data not presented). The hierarchical test returned five clades as being differentially abundant between steer classifications (P < 0.05; Table 3). Four of the clades contained OTU classified in the order Clostridiales and one clade was assigned to the phylum Bacteroidetes.
Figure 3.
Experiment 2: percent of OTU by phyla in the rumen of experiment 2 (n = 16).
Figure 4.
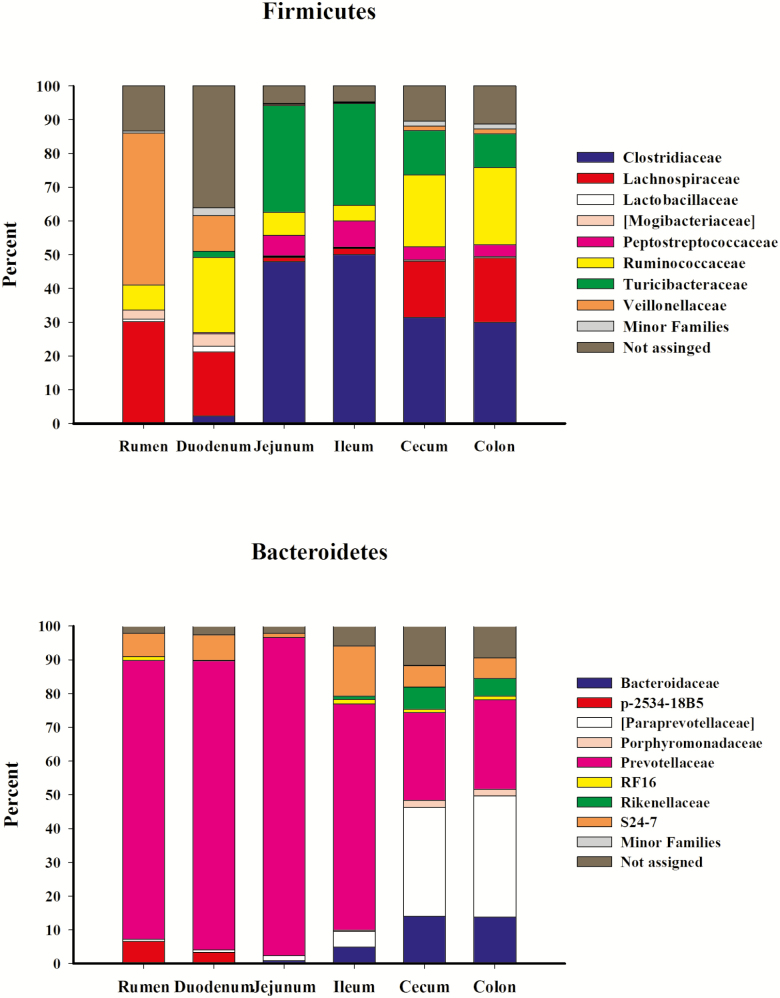
Experiment 2: relative abundance of (a) Firmicutes. Minor families include [Acidaminobacteraceae], Aerococcaceae, Alicyclobacillaceae, Anaerobrancaceae, Bacillaceae, Carnobacteriaceae, Christensenellaceae, Dehalobacteriaceae, Enterococcaceae, Erysipelotrichaceae, Eubacteriaceae, Lactobacillaceae, Leuconostocaceae, Paenibacillaceae, Peptococcaceae, Planococcaceae, Staphylococcaceae, Streptococcaceae, [Tissierellaceae], and Thermoactinomycetaceae. Not assigned OTUs were classified as Firmicutes but were not assigned a family. (b) Bacteroidetes by family. Minor families include: [Barnesiellaceae] and BS11. Not assigned OTUs were classified as Bacteroidetes but were not assigned a family.
Table 3.
Experiment 2–shift in Rumen OTU in steers classified as having less (n = 8) or greater (n = 8) ADG
| Clade | P-value | Phylum | Class | Order | Family | Genus | Species | ADG |
|---|---|---|---|---|---|---|---|---|
| 1 | 0.008 | Firmicutes | Clostridia | Clostridiales | NA | NA | NA | ↓ |
| 2 | 0.006 | Firmicutes | Clostridia | Clostridiales | [Mogibacteriaceae] | NA | NA | ↓ |
| Firmicutes | Clostridia | Clostridiales | [Mogibacteriaceae] | NA | NA | ↑ | ||
| 3 | 0.010 | Firmicutes | Clostridia | Clostridiales | Ruminococcaceae | Ruminococcus | NA | ↑ |
| 4 | 0.045 | Firmicutes | Clostridia | Clostridiales | Ruminococcaceae | NA | NA | ↓ |
| 5 | 0.048 | Bacteroidetes | Bacteroidia | Bacteroidales | Prevotellaceae | Prevotella | NA | ↓ |
NA, OTU not assigned; ↓, OTU directionally greater in less ADG; ↑, OTU directionally greater in greater ADG.
Duodenum
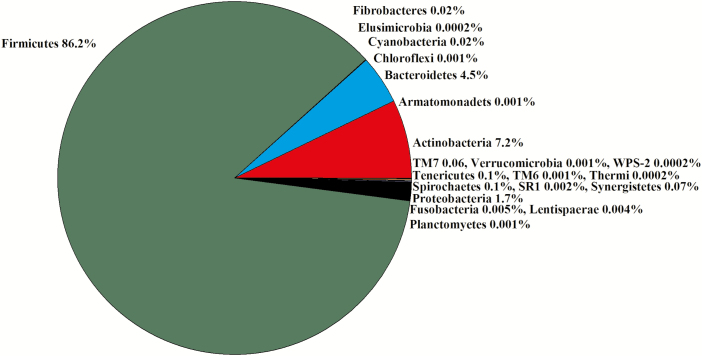
In the 16 libraries, there were 1,433,040 sequences that were binned to 1,170 OTUs at the 3% dissimilatory level. After the removal of OTUs that were not classified at the phylum level, there were 1,334,891 sequences binned to sequences in 1,094 OTUs; after filtering for low prevalence sequence, there were 1,323,083 sequences binned to sequences in 582 OTUs that remained for analyses. The majority of the OTUs belonged to Firmicutes (86%) and Actinobacteria (7.2%; Figure 5). Thirty-six percent of the OTUs within Firmicutes were not assigned at the family level. The predominant Firmicutes families were Ruminococcaceae (22%), Lachnospiraceae (19%), and Veillonellaceae (11%; Figure 4). The predominant Bacteroidetes family was Prevotellaceae (86%; Figure 4). There was no difference in the Chao1, Shannon, Simpson, and InvSimpson diversity indexes between steer classifications (Table 1). Classification groups did not differ (P = 0.81) in the PERMANOVA. The weighted UniFrac PCoA demonstrated that there was no clustering between the ADG classifications (data not presented). The hierarchical test returned seven clades as being differentially abundant between steer classifications (P < 0.05; Table 4). Four clades contained OTU assigned to the order Clostridiales and two clades contained OTU assigned to Bacillales. The seventh clade contained OTU assigned to the genus Prevotella.
Figure 5.
Experiment 2: percent of OTU by phyla in the duodenum (n = 16).
Table 4.
Experiment 2—shift in duodenum OTU in steers classified as having less (n = 8) or greater (n = 8) ADG
| Clade | P-value | Phylum | Class | Order | Family | Genus | Species | ADG |
|---|---|---|---|---|---|---|---|---|
| 1 | 0.036 | Firmicutes | Clostridia | Clostridiales | Lachnospiraceae | NA | NA | ↓ |
| Firmicutes | Clostridia | Clostridiales | Lachnospiraceae | NA | NA | ↓ | ||
| 2 | 0.043 | Firmicutes | Clostridia | Clostridiales | Lachnospiraceae | NA | NA | ↑ |
| 3 | 0.046 | Firmicutes | Clostridia | Clostridiales | Lachnospiraceae | NA | NA | ↓ |
| Firmicutes | Clostridia | Clostridiales | Lachnospiraceae | NA | NA | ↓ | ||
| 4 | 0.044 | Firmicutes | Clostridia | Clostridiales | [Mogibacteriaceae] | NA | NA | ↓ |
| 5 | 0.036 | Firmicutes | Bacilli | Bacillales | Planococcaceae | Ureibacillus | NA | ↓ |
| Firmicutes | Bacilli | Bacillales | Planococcaceae | Ureibacillus | NA | ↓ | ||
| 6 | 0.020 | Firmicutes | Bacilli | Bacillales | Bacillaceae | Bacillus | NA | ↓ |
| Firmicutes | Bacilli | Bacillales | NA | NA | NA | ↓ | ||
| Firmicutes | Bacilli | Bacillales | Bacillaceae | Bacillus | thermoamylovorans | ↓ | ||
| Firmicutes | Bacilli | Bacillales | Bacillaceae | Bacillus | NA | ↓ | ||
| 7 | 0.043 | Bacteroidetes | Bacteroidia | Bacteroidales | Prevotellaceae | Prevotella | NA | ↓ |
NA, OTU not assigned; ↓, OTU directionally greater in less ADG; ↑, OTU directionally greater in greater ADG.
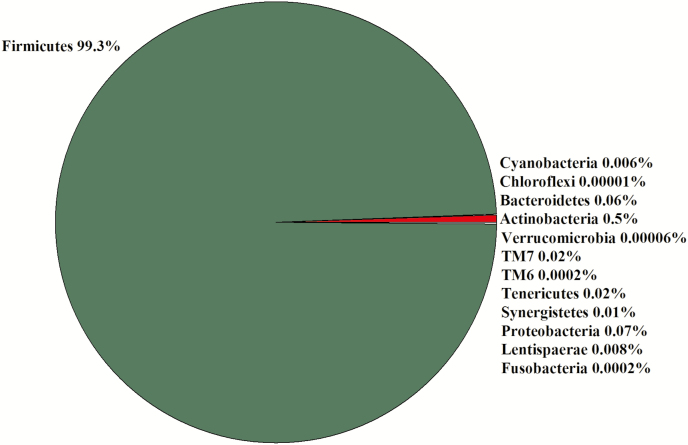
Jejunum
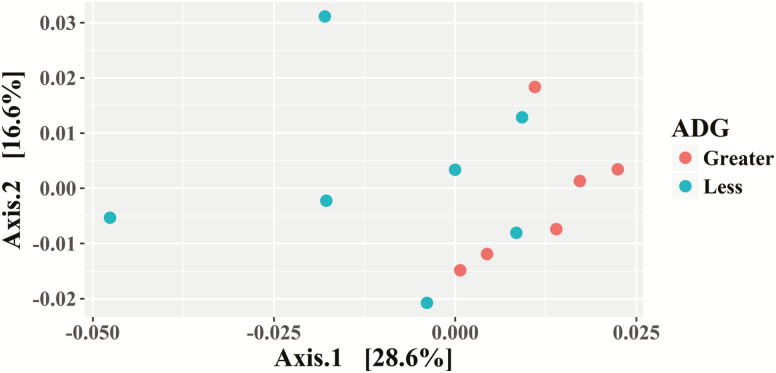
Due to a lack of digesta in some samples, seven libraries for less ADG animals and six libraries for greater ADG steers were constructed for a total of 13 libraries. In the 13 libraries, there were 2,705,918 sequences that were binned to 533 OTUs at the 3% dissimilatory level. After the removal of OTUs that were not classified at the phylum level, there were 2,692,465 sequences binned to sequences in 504 OTUs; after filtering for low prevalence sequence, there were 2,689,697 sequences binned to sequences in 298 OTUs remaining for analyses. The majority of the OTU belonged to Firmicutes (99%; Figure 6). The predominant Firmicutes families were Clostridiaceae (48%), Turicibacteraceae (32%), Ruminococcaceae (7%), and Peptostreptococcaceae (6%; Figure 4). The predominant Bacteroidetes family was Prevotellaceae (94%; Figure 4). There tended to be a difference (0.05 ≤ P < 0.1) in the Chao1 and Simpson diversity indexes between steer classifications, but there was no difference in the Shannon and InvSimpson diversity indexes between steer classifications (Table 1). Classification groups differed (P = 0.006) in the PERMANOVA. The weighted UniFrac PCoA suggests that the ADG classifications clustered (Figure 7). The hierarchical test returned 11 clades as being differentially abundant between steer classifications (P < 0.05; Table 5). Six clades were in the phylum Firmicutes, five of those were in the order Clostridiales, and one was in the genus Lactobacillus. One clade consisted of OTUs in the Family Corynebacteriaceae and two clades consisted of OTU in the Family Coriobacteriaceae. Another clade contained OTU in the phyla Cyanobacteria and Proteobacteria. One clade contained OTU classified as order Rhizobiales.
Figure 6.
Experiment 2: percent of OTU by phyla in the jejunum (n = 13).
Figure 7.
Experiment 2: principal coordinate analysis using weighted UniFrac for the jejunum of steers classified as having less or greater ADG at a common feed intake.
Table 5.
Experiment 2-shift in jejunum OTU in steers classified as having less (n = 7) or greater (n = 6) ADG
| Clade | P-value | Phylum | Class | Order | Family | Genus | Species | ADG |
|---|---|---|---|---|---|---|---|---|
| 1 | 0.049 | Firmicutes | Clostridia | Clostridiales | [Mogibacteriaaceae] | Mogibacterium | NA | ↓ |
| Firmicutes | Clostridia | Clostridiales | [Mogibacteriaaceae] | Mogibacterium | NA | ↓ | ||
| Firmicutes | Clostridia | Clostridiales | [Mogibacteriaaceae] | Mogibacterium | NA | ↓ | ||
| 2 | 0.028 | Firmicutes | Clostridia | Clostridiales | Lachnospiraceae | Shuttleworthia | NA | ↓ |
| 3 | 0.035 | Firmicutes | Clostridia | Clostridiales | Lachnospiraceae | Butyrivibrio | NA | ↑ |
| 4 | 0.021 | Firmicutes | Clostridia | Clostridiales | Veillonellaceae | Dialister | NA | ↑ |
| 5 | 0.031 | Firmicutes | Clostridia | Clostridiales | NA | NA | NA | ↑ |
| 6 | 0.023 | Firmicutes | Bacilli | Lactobacillales | Lactobacillaceae | Lactobacillus | NA | ↓ |
| 7 | 0.048 | Actinobacteria | Actinobacteria | Actinomycetales | Corynebacteriaceae | Corynebacterium | variable | ↑ |
| Actinobacteria | Actinobacteria | Actinomycetales | Corynebacteriaceae | Corynebacterium | NA | ↓ | ||
| Actinobacteria | Actinobacteria | Actinomycetales | Corynebacteriaceae | Corynebacterium | NA | ↓ | ||
| Actinobacteria | Actinobacteria | Actinomycetales | Corynebacteriaceae | Corynebacterium | NA | ↓ | ||
| Actinobacteria | Actinobacteria | Actinomycetales | Corynebacteriaceae | Corynebacterium | NA | ↓ | ||
| Actinobacteria | Actinobacteria | Actinomycetales | Corynebacteriaceae | Corynebacterium | NA | ↓ | ||
| 8 | 0.014 | Actinobacteria | Coriobacteriia | Coriobacteriales | Coriobacteriaceae | NA | NA | ↓ |
| Actinobacteria | Coriobacteriia | Coriobacteriales | Coriobacteriaceae | NA | NA | ↓ | ||
| Actinobacteria | Coriobacteriia | Coriobacteriales | Coriobacteriaceae | NA | NA | ↓ | ||
| Actinobacteria | Coriobacteriia | Coriobacteriales | Coriobacteriaceae | Atopobium | NA | ↓ | ||
| Actinobacteria | Coriobacteriia | Coriobacteriales | Coriobacteriaceae | NA | NA | ↓ | ||
| Actinobacteria | Coriobacteriia | Coriobacteriales | Coriobacteriaceae | NA | NA | ↓ | ||
| Actinobacteria | Coriobacteriia | Coriobacteriales | Coriobacteriaceae | NA | NA | ↑ | ||
| Actinobacteria | Coriobacteriia | Coriobacteriales | Coriobacteriaceae | NA | NA | ↑ | ||
| Actinobacteria | Coriobacteriia | Coriobacteriales | Coriobacteriaceae | Atopobium | NA | ↓ | ||
| Actinobacteria | Coriobacteriia | Coriobacteriales | Coriobacteriaceae | NA | NA | ↑ | ||
| 9 | 0.0004 | Actinobacteria | Coriobacteriia | Coriobacteriales | Coriobacteriaceae | NA | NA | ↑ |
| 10 | 0.044 | Cyanobacteria | 4C0d-2 | YS2 | NA | NA | NA | ↑ |
| Proteobacteria | Deltaproteobacteria | Desulfovibrionales | Desulfovibrionaceae | Desulfovibrio | D168 | ↑ | ||
| Proteobacteria | Deltaproteobacteria | Desulfovibrionales | Desulfovibrionaceae | Desulfovibrio | NA | ↑ | ||
| 11 | 0.005 | Proteobacteria | Alphaproteobacteria | Rhizobiales | Rhizobiaceae | Agrobacterium | NA | ↑ |
| Proteobacteria | Alphaproteobacteria | Rhizobiales | Brucellaceae | NA | NA | ↑ | ||
| Proteobacteria | Alphaproteobacteria | Rhizobiales | Brucellaceae | Ochrobactrum | NA | ↑ |
NA, OTU not assigned; ↓, OTU directionally greater in less ADG; ↑, OTU directionally greater in greater ADG.
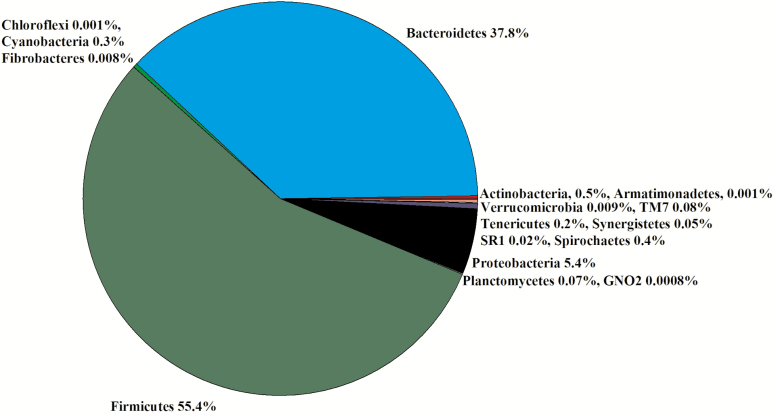
Ileum
Due to a lack of digesta in some samples, six libraries for less ADG animals and five libraries for greater ADG steers were constructed for a total of 11 libraries. In the 11 libraries, there were 2,117,019 sequences that were binned to 540 OTUs at the 3% dissimilatory level. After the removal of OTUs that were not classified at the phylum level, there were 2,110,582 sequences binned to 517 OTUs; after filtering for low prevalence sequence, there were 2,105,214 sequences that binned to 296 OTUs that were used for the analyses. The majority of the OTUs belonged to Firmicutes (55%) and Bacteroidetes (38%; Figure 8). Within Firmicutes, the Families Clostridiaceae (50%), Turicibacteraceae (30%), and Turicibacteraceae (8%) were predominant (Figure 4). Within Bacteroidetes, the Families Prevotellaceae (67%) and S24-7 (15%) were predominant (Figure 4). There was no difference in the Chao1, Shannon, Simpson, and InvSimpson diversity indexes between steer classifications (Table 1). Classification groups did not differ (P = 0.23) in the PERMANOVA. The weighted UniFrac PCoA demonstrated that there was no clustering between the ADG classifications (data not presented). The hierarchical test returned five clades as being differentially abundant between steer classifications (P < 0.05; Table 6). Three clades were classified in the phylum Firmicutes and two clades were classified in the phylum Actinobacteria.
Figure 8.
Experiment 2: percent of OTU by phyla in the ileum (n = 11).
Table 6.
Experiment 2—shift in ileum OTU in steers classified as having less (n = 6) or greater (n = 5) ADG
| Clade | P-value | Phylum | Class | Order | Family | Genus | Species | ADG |
|---|---|---|---|---|---|---|---|---|
| 1 | 0.047 | Firmicutes | Clostridia | Clostridiales | [Mogibacteriaceae] | NA | NA | ↑ |
| 2 | 0.039 | Firmicutes | Erysipelotrichia | Erysipelotrichales | Erysipelotrichaceae | Bulleidia | NA | ↑ |
| 3 | 0.042 | Firmicutes | Bacilli | Bacillales | Bacillaceae | Bacillus | NA | ↓ |
| 4 | 0.032 | Actinobacteria | Actinobacteria | Actinomycetales | Pseudonocardiaceae | Saccharopolyspora | NA | ↑ |
| 5 | 0.043 | Actinobacteria | Coriobacteriia | Coriobacteriales | Coriobacteriaceae | NA | NA | ↑ |
NA, OTU not assigned; ↓, OTU directionally greater in less ADG; ↑, OTU directionally greater in greater ADG.
Cecum
In the 16 libraries, there were 717,523 sequences that were binned to 2,272 OTUs at the 3% dissimilatory level. After the removal of OTUs that were not classified at the phylum level, there were 711,955 sequences binned to 2,185 OTUs; after filtering for low prevalence sequences, there were 696,026 sequences there were binned to 1,440 OTUs remaining for analyses. The majority of the OTUs belonged to Firmicutes (63%) and Bacteroidetes (30%; Figure 9). Within Firmicutes, the Families Clostridiaceae (31%), Ruminococcaceae (22%), and Lachnospiraceae (17%) were the most predominant (Figure 4). Within Bacteroidetes, Families [Paraprevotellaceae] (32%), Prevotellaceae (26%), and Bacteroidaceae (14%) were the most predominant (Figure 4). There was no difference in the Chao1, Shannon, Simpson, and InvSimpson diversity indexes between steer classifications (Table 1). Classification groups did not differ (P = 0.82) in the PERMANOVA. The weighted UniFrac PCoA demonstrated that there was no clustering between the ADG classifications (data not presented). The hierarchical test returned 29 clades as being differentially abundant between steer classifications (P < 0.05; Table 7). Eighteen of the clades contained OTU assigned to the order Clostridiales, and two clades contained OTU assigned to the order Erysipelotrichales. Two clades contained OTU assigned to the phylum Bacteroidetes, one clade contained an OTU assigned to phylum Proteobacteria, and the remaining clades contained OTU assigned to phylum Tenericutes.
Figure 9.
Experiment 2: percent of OTU by phyla in the cecum (n = 16).
Table 7.
Experiment 2—shift in cecum OTU in steers classified as having less (n = 8) or greater (n = 8) ADG
| Clade | P-value | Phylum | Class | Order | Family | Genus | Species | ADG |
|---|---|---|---|---|---|---|---|---|
| 1 | 0.010 | Firmicutes | Clostridia | Clostridiales | Lachnospiraceae | Anaerostipes | NA | ↓ |
| 2 | 0.039 | Firmicutes | Clostridia | Clostridiales | Lachnospiraceae | NA | NA | ↑ |
| Firmicutes | Clostridia | Clostridiales | Lachnospiraceae | NA | NA | ↑ | ||
| 3 | 0.047 | Firmicutes | Clostridia | Clostridiales | Lachnospiraceae | Dorea | NA | ↑ |
| Firmicutes | Clostridia | Clostridiales | NA | NA | NA | ↑ | ||
| 4 | 0.045 | Firmicutes | Clostridia | Clostridiales | Lachnospiraceae | Coprococcus | NA | ↑ |
| Firmicutes | Clostridia | Clostridiales | Lachnospiraceae | Coprococcus | NA | ↑ | ||
| 5 | 0.018 | Firmicutes | Clostridia | Clostridiales | Lachnospiraceae | NA | NA | ↑ |
| Firmicutes | Clostridia | Clostridiales | Lachnospiraceae | Butyrivibrio | NA | ↑ | ||
| Firmicutes | Clostridia | Clostridiales | Lachnospiraceae | NA | NA | ↑ | ||
| Firmicutes | Clostridia | Clostridiales | Lachnospiraceae | Lachnospira | NA | ↑ | ||
| Firmicutes | Clostridia | Clostridiales | Lachnospiraceae | Lachnospira | NA | ↓ | ||
| Firmicutes | Clostridia | Clostridiales | Lachnospiraceae | NA | NA | ↓ | ||
| Firmicutes | Clostridia | Clostridiales | Lachnospiraceae | NA | NA | ↑ | ||
| Firmicutes | Clostridia | Clostridiales | Lachnospiraceae | NA | NA | ↑ | ||
| Firmicutes | Clostridia | Clostridiales | Lachnospiraceae | NA | NA | ↓ | ||
| Firmicutes | Clostridia | Clostridiales | Lachnospiraceae | Coprococcus | NA | ↑ | ||
| Firmicutes | Clostridia | Clostridiales | Lachnospiraceae | NA | NA | ↑ | ||
| Firmicutes | Clostridia | Clostridiales | Lachnospiraceae | NA | NA | ↑ | ||
| 6 | 0.053 | Firmicutes | Clostridia | Clostridiales | Lachnospiraceae | NA | NA | ↑ |
| Firmicutes | Clostridia | Clostridiales | NA | NA | NA | ↓ | ||
| 7 | 0.021 | Firmicutes | Clostridia | Clostridiales | Ruminococcaceae | NA | NA | ↑ |
| 8 | 0.035 | Firmicutes | Clostridia | Clostridiales | Ruminococcaceae | NA | NA | ↓ |
| 9 | 0.037 | Firmicutes | Clostridia | Clostridiales | Ruminococcaceae | NA | NA | ↑ |
| Firmicutes | Clostridia | Clostridiales | Ruminococcaceae | NA | NA | ↓ | ||
| 10 | 0.018 | Firmicutes | Clostridia | Clostridiales | Ruminococcaceae | NA | NA | ↑ |
| 11 | 0.013 | Firmicutes | Clostridia | Clostridiales | Ruminococcaceae | NA | NA | ↓ |
| 12 | 0.005 | Firmicutes | Clostridia | Clostridiales | Ruminococcaceae | NA | NA | ↑ |
| 13 | 0.032 | Firmicutes | Clostridia | Clostridiales | Ruminococcaceae | NA | NA | ↑ |
| 14 | 0.016 | Firmicutes | Clostridia | Clostridiales | Ruminococcaceae | NA | NA | ↑ |
| 15 | 0.038 | Firmicutes | Clostridia | Clostridiales | NA | NA | NA | ↑ |
| Firmicutes | Clostridia | Clostridiales | NA | NA | NA | ↑ | ||
| Firmicutes | Clostridia | Clostridiales | NA | NA | NA | ↑ | ||
| 16 | 0.043 | Firmicutes | Clostridia | Clostridiales | NA | NA | NA | ↑ |
| 17 | 0.033 | Firmicutes | Clostridia | Clostridiales | NA | NA | NA | ↑ |
| 18 | 0.023 | Firmicutes | Clostridia | Clostridiales | NA | NA | NA | ↑ |
| 19 | 0.005 | Firmicutes | Erysipelotrichia | Erysipelotrichales | Erysipelotrichaceae | RFN20 | NA | ↓ |
| 20 | 0.049 | Firmicutes | Erysipelotrichia | Erysipelotrichales | Erysipelotrichaceae | NA | NA | ↑ |
| Firmicutes | Erysipelotrichia | Erysipelotrichales | Erysipelotrichaceae | NA | NA | ↓ | ||
| Firmicutes | Erysipelotrichia | Erysipelotrichales | Erysipelotrichaceae | NA | NA | ↑ | ||
| Firmicutes | Erysipelotrichia | Erysipelotrichales | Erysipelotrichaceae | NA | NA | ↑ | ||
| Firmicutes | Erysipelotrichia | Erysipelotrichales | Erysipelotrichaceae | NA | NA | ↓ | ||
| 21 | 0.032 | Bacteroidetes | Bacteroidia | Bacteroidales | S24-7 | NA | NA | ↑ |
| 22 | 0.039 | Bacteroidetes | Bacteroidia | Bacteroidales | NA | NA | NA | ↓ |
| 23 | 0.010 | Proteobacteria | Betaproteobacteria | Burkholderiales | Alcaligenaceae | Sutterella | NA | ↑ |
| 24 | 0.021 | Tenericutes | Mollicutes | RF39 | NA | NA | NA | ↑ |
| 25 | 0.039 | Tenericutes | Mollicutes | RF39 | NA | NA | NA | ↑ |
| Tenericutes | Mollicutes | RF39 | NA | NA | NA | ↑ | ||
| Tenericutes | Mollicutes | RF39 | NA | NA | NA | ↑ | ||
| 26 | 0.031 | Tenericutes | Mollicutes | RF39 | NA | NA | NA | ↑ |
| 27 | 0.032 | Tenericutes | Mollicutes | RF39 | NA | NA | NA | ↓ |
| 28 | 0.032 | Tenericutes | Mollicutes | RF39 | NA | NA | NA | ↑ |
| 29 | 0.031 | Tenericutes | Mollicutes | Anaeroplasmatales | Anaeroplasmataceae | Anaeroplasma | NA | ↑ |
NA, OTU not assigned; ↓, OTU directionally greater in less ADG; ↑, OTU directionally greater in greater ADG.
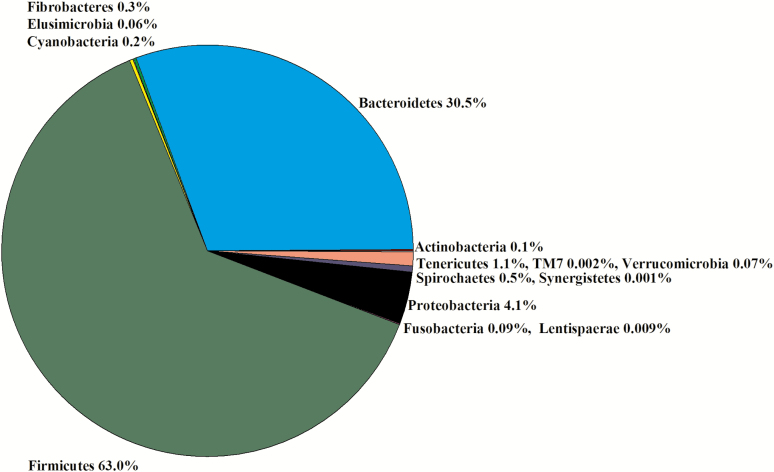
Colon
Due to a lack of digesta in some samples, seven libraries for less ADG animals and five libraries for greater ADG steers were constructed for a total of 12 libraries. In the 12 libraries, there were 915,024 sequences that were binned to 967 OTUs at the 3% dissimilatory level. After the removal of OTUs that were not classified at the phylum level, there were 877,808 sequences binned to 894 OTUs; after filtering for low prevalence sequences, there were 871,697 sequences binned to 520 OTUs that were used for analyses. The majority of the OTUs belonged to Firmicutes (59%) and Bacteroidetes (34%; Figure 10). Within Firmicutes, the Families Clostridiaceae (30%), Ruminococcaceae (23%), and Lachnospiraceae (19%) were the most predominant (Figure 4). Within Bacteroidetes, Families [Paraprevotellaceae] (36%), Prevotellaceae (27%), and Bacteroidaceae (14%) were the most predominant (Figure 4). There was no difference in the Chao1, Shannon, Simpson, and InvSimpson diversity indexes between steer classifications (Table 1). Classification groups did not differ (P = 0.57) in the PERMANOVA. The hierarchical test returned six clades as being differentially abundant between steer classifications (P < 0.05; Table 8). The weighted UniFrac PCoA demonstrated that there was no clustering between the ADG classifications (data not presented). Five clades contained OTU assigned to the phylum Firmicutes. Of those clades, five contained OTU in the order Clostridiales, and one clade contained an OTU in the order Planctomycetia. The sixth clade contained OTU in the phylum TM7.
Figure 10.
Experiment 2: percent of OTU by phyla in the colon (n = 12).
Table 8.
Experiment 2—shift in colon OTU in steers classified as having less (n = 7) or greater (n = 5) ADG
| Clade | P-value | Phylum | Class | Order | Family | Genus | Species | ADG |
|---|---|---|---|---|---|---|---|---|
| 1 | 0.048 | Firmicutes | Clostridia | Clostridiales | Lachnospiraceae | Coprococcus | NA | ↓ |
| Firmicutes | Clostridia | Clostridiales | Lachnospiraceae | NA | NA | ↓ | ||
| 2 | 0.040 | Firmicutes | Clostridia | Clostridiales | NA | NA | NA | ↑ |
| Firmicutes | Clostridia | Clostridiales | NA | NA | NA | ↑ | ||
| Firmicutes | Clostridia | Clostridiales | NA | NA | NA | ↑ | ||
| 3 | 0.002 | Firmicutes | Clostridia | Clostridiales | NA | NA | NA | ↓ |
| 4 | 0.025 | Firmicutes | Clostridia | Clostridiales | Ruminococcaceae | NA | NA | ↓ |
| 5 | 0.039 | Firmicutes | Planctomycetes | Planctomycetia | Pirellulales | Pirellulaceae | NA | ↓ |
| 6 | 0.019 | TM7 | TM7-3 | CW040 | F16 | NA | NA | ↓ |
| TM7 | TM7-3 | CW040 | F16 | NA | NA | ↓ |
NA, OTU not assigned; ↓, OTU directionally greater in less ADG; ↑, OTU directionally greater in greater ADG.
Discussion
A number of studies have addressed the relationship between feed efficiency for growth in beef cattle and digestive tract microbiota (Guan et al., 2008; Carberry et al., 2012; Hernandez-Sanabria et al., 2012; McCann et al., 2014; Myer et al., 2015a, 2015b, 2016; McGovern et al., 2018; Paz et al., 2018). One of the difficulties in interpreting these studies has been the inconsistent definition of feed efficiency. Different efficiency estimates are mathematically calculated differently which may result in differences in how cattle rank. Many of the studies have defined feed efficiency using RFI (Koch et al., 1963) as the metric (Guan et al., 2008; Carberry et al., 2012; Hernandez-Sanabria et al., 2012; McCann et al., 2014; McGovern et al., 2018). In these studies, cattle are typically classified into a greater and less RFI, and, by definition, the two classifications will differ in feed intake. Feed intake will influence passage rate in the digestive tract making it difficult to distinguish if differences in the microbiota are a function of host passage rate or are differences inherent to the core microbiome. Myer et al. (2015a, 2015b, 2015c, 2016) used an alternative model using extremes in BW gain and feed intake to parse the relationship of each variable to the microbiota. Like Myer et al. (2015a, 2015b, 2015c, 2016), Paz et al. (2018) associated the microbiota with feed intake and ADG. In addition, they associated the microbiota with the Gain to Feed ratio(G:F) ratio, but as noted previously, G:F has limitations as a measure of feed efficiency (Meyer and Garrett, 1967).
The role of the bacterial fermentation in ruminant nutrition has been well documented. Relationships between changes in the microbial community and diet have been extensively studied. Consequently, many of the studies have concentrated solely on the relationship between rumen microbiota and feed efficiency (Guan et al., 2008; Carberry et al., 2012; McCann et al., 2014; McGovern et al., 2018; Paz et al., 2018) with the exception of Myer et al. (2015b, 2015c, 2016). In the current study, we chose to study steers that differed in ADG at a common feed intake through the digestive tract. By fixing feed intake, we are attempting to study the changes in the core microbiome that contribute to the differences in animal ADG and investigate the role of other important GI tract sites in addition to the rumen.
The majority of the OTUs in the rumen of experiment 1 were assigned to the phyla Firmicutes, Bacteroidetes, and Proteobacteria. These three phyla predominate the rumen across different dietary situations. However, their relative abundance to each other differ in non-weaned calves (Malmuthuge et al., 2014), dairy cows (Mao et al., 2015), corn silage fed cattle (Nagem et al., 2013), and cattle fed high corn diets (Myer et al., 2015a). Diversity indexes in the current study suggest that there is not a major shift between the ADG classifications in the ruminal bacterial communities and population structures. A lack of diversity in the rumen has been observed regardless of the model for feed efficiency (McCann et al., 2014; Myer et al., 2015a; Li and Guan, 2017). In the current study, there was not a difference in diversity between cattle classified with different ADG, and this lack of difference in diversity is born out by only six clades being differential observed between ADG classifications in experiment 1. With the exception of one clade, the OTUs were more numerous in the least ADG steers. Four of the clades consisted of Firmicutes and all of the OTUs in those clades were classified as Clostridiales. One clade consisted of two OTUs classified as Lachnospiraceae that were more numerous in the least ADG steers. Shabat et al. (2016) found that dairy cows with the least feed efficiency (greater RFI) had greater Lachnospiraceae. Li and Guan (2017) found that Lachnospiraceae was associated with growing cattle with greater RFI (less efficient); however, these observations are reversed from those observed by Myer et al. (2015a) who found Lachnospiraceae was more abundant in steers with the greatest ADG. One clade contained three OTUs that were not classified below the order level (Clostridiales) and that were more numerous in the greater ADG steers. Given they were opposite to the Lachnospiraceae, it suggest that these OTU may have belonged to a different subtaxa and/or were physiologically different than the Lachnospiraceae. The fourth Firmicutes clade contained a single OTU classified as Ruminococcaceae that was greater in the least ADG steers. Gagen et al. (2015) determined there are acetogens that can be found in both the Families Lachnospiraceae and Ruminococcaceae. Acetogens can serve as a hydrogen sink and may increase if methane production is reduced. There are alternative arguments for the relationship between methane production and feed efficiency. One hypothesis is that energy not lost as methane can be captured in BW gain making the animal more efficient. Alternatively, methane is a product of digestion and increased methane may be associated with a more complete digestion resulting in greater nutrient availability of the feed. In an in vitro study using the same experimental model as this study, we did not observe a difference in methane production between steers classified as having different ADG (Freetly et al., 2015). One clade contained two OTUs classified as F16 in Phylum TM7 that were greater in least ADG steers. The TM7 phylum appears to be ubiquitous in the intestinal tracts of mammals, but it has never been cultured and its physiology is poorly understood. The final clade that differentiated with ADG classifications contained a single OTU in the Family Pirellulaceae and it was elevated in the least ADG steers.
In a study of the metabolome of the rumen of steers in experiment 1, Artegoitia et al. (2017) reported that the concentrations of alpha-Linolenic acid and linoleic acid were less in steers with greater ADG. Butyrivibrio and Ruminococcus are associated with the biohydrogenation of linoleic acid in the rumen (Polan et al., 1964; Maia et al., 2007; Paillard et al., 2007). Directional changes of vaccenic acid in the Artegoitia et al. (2017) study were the same as those for linoleic acid suggesting that differences might be driven by Butyrivibrio spp. In the current study, we did not observe a difference in OTUs classified to the genus Butyrivibrio; however, Butyrivibrio belongs to the order Clostridiales and an OTU of this same order not assigned a genus was greater in the least ADG classification. The directional increase for OTU in the Family Ruminococcaceae was mixed.
Artegoitia et al. (2017) reported that steers classified with greater ADG had a greater rumen phenylalanine, tyrosine, and tryptophan biosynthesis. Allison and Robinson (1967) found that Rhodospirillum rubrum could synthesize phenylalanine in the rumen. The synthesis appeared to occur via a transamination of phenylacetate. Phenylalanine catabolism has been demonstrated to occur in both ruminal bacterial and protozoan incubations, and tyrosine has been shown to be produced from phenylalanine (Amin and Onodera, 1997). Scheifinger et al. (1976) reported that Selenomonas ruminantium, Megasphaera, Streptococcus, Butyrivibrio, and Eubacterium are all capable of degrading phenylalanine. Except for Selenomonas ruminantium and Streptococcus, they also degrade tyrosine and tryptophan, but the ability of Selenomonas ruminantium to degrade tryptophan may be subspecies specific. Chen and Russell (1988) reported that Peptostreptococcus was capable of degrading phenylalanine, tryptophan, and tyrosine. The specific strains reported to be involved in phenylalanine metabolism did not include OTUs assigned in this study; however, Butyrivibrio, Eubacterium, Peptostreptococcus, and Selenomonas ruminantium belong to the order Clostridiales and they may be represented in one of the OTUs assigned at this level but not assigned to lower levels.
As in experiment 1, the predominant phyla in the rumen in experiment 2 were Bacteroidetes, Firmicutes, and Proteobacteria; however, there was a shift in the relative abundance of Families assigned to Firmicutes and Proteobacteria. A greater percentage of the Firmicutes OTUs were in the Family Lachnospiraceae in experiment 1 but a higher percentage were in the Family Veillonellaceae in experiment 2. There was also a difference in the Bacteroidetes with the differences in percentages of Prevotellaceae and p-2534-18B5 between the two experiments. With the exception of an OTU classified as Ruminococcaceae that was greater in the least ADG steers, there was no overlap between experiments 1 and 2 of clades that differed with ADG classification. Fewer OTUs were differentially observed in experiment 2 but most of the differentially observed OTUs were Firmicutes. Five of the six were assigned to the order Clostridiales and one belonged to the genus Prevotella.
Cattle in both experiments received the same diet. The experiments differed in the days on feed and the average DM intake. Collectively the two experiments do not support the hypothesis that changes in the rumen microbiota are associated with differences in ADG in this experimental model.
There was a shift in the relative abundance of phyla moving from the rumen to the lower track where Firmicutes become the predominant phyla. While Firmicutes are the predominant phyla, the family structure within the phyla changes across the digestive tract. The rumen and duodenum each have unique family structures. The family structures in the jejunum and ileum follow a similar pattern as does the family structures in the cecum and colon. This observation is consistent with what was observed in the PCoA where the rumen and duodenum had unique clusters and the jejunum and ileum clustered together as did the cecum and colon.
Nearly 40% of the Firmicutes in the duodenum were unassigned to a family level, indicating the lack of understanding and information regarding the microbial populations in the upper small intestine of the bovine. The predominance of Lachnospiraceae decreases in the remainder of the small intestine compared with the duodenum. The higher levels in the duodenum may be associated with washout from the rumen where it is one of the predominant families. There are several members of the Family Ruminococcaceae that are associated with fiber digestion (Fontes and Gilbert, 2010). Like Lachnospiraceae, it is not clear what fraction of the Ruminococcaceae are colonized in the duodenum and what fraction is a result of washout from the rumen. Volatile fatty acid production, in particular acetate from Bifidobacteria, has been proposed to have antimicrobial activity (Binda et al., 2018). Bifidobacteria have also been associated with lactate production which has been proposed to be a substrate for butyrate synthesis by other bacteria. Butyrate may be associated with promoting the synthesis of the mucosal layer (Binda et al., 2018); however, no OTUs belonging to the Actinobacteria phylum were differentially observed in these samples. Relative to those 13 OTUs that were differentially observed in the duodenum, 92% of the OTUs were greater in the least ADG steers. Three of the clades differentially observed between ADG classifications contained OTUs classified in Lachnospiraceae, and four out of five were greater in the least ADG steers, which was the same directional shift observed in the rumen of experiment 1. The remaining clades consisted of OTUs that were not classified in predominant families. Three of the clades consisted of OTU classified in the order Bacillales, which were not assigned to any of the predominant families, and all of these OTUs were greater in the least ADG animals. One clade included two OTUs in the genus Ureibacillus; however, it is not clear how their presence would be associated with differences in ADG. One clade contained three OTUs in the genus Bacillus, one of which was classified as the species Bacillus thermoamylovorans. Bacillus are thought to be part of the core human gut microbiome (Hong et al., 2009) and have been found in cattle feces (Wu et al., 2007). Bacillus have been shown to have antimicrobial activity including B. thermoamylovorans against Staphylococcus aureus (Hoyles et al., 2012). There have not been any causal relationships established between Bacillus and feed efficiency, but a potential mechanism would be for Bacillus to have antimicrobial activity against microbes that promote nutrient absorption by the animal. One clade contained a single OTU in the genus Prevotella. Like the rumen, Prevotella in the duodenum were greater in the steers with least ADG. This elevation in the duodenum may be a function of washout from the rumen.
The jejunum is the principal site of absorption for amino acids, simple carbohydrates, and fats. Firmicutes are the predominant phylum in the jejunum which is consistent with other studies (Malmuthuge and Griebel, 2014; Myer et al., 2016). Malmuthuge et al. (2014) study was in non-weaned calves and this study and Myer et al. (2016) steers were fed high corn diets. The calves would have received a diet high in sugars and the other two studies most likely had a high bypass starch content leading to high jejunum levels of Firmicutes in the jejunum. The current research is in contrast to Mao et al. (2015) who reported that most jejunum digesta OTUs were classified into the phyla Proteobacteria and Actinobacteria, but observed differences in the studies are likely a function of different diets. There was a shift in the families of Firmicutes moving from the duodenum with Clostridiaceae and Turicibacteraceae being the predominant families in the jejunum. The relative high abundance of Clostridiaceae in the jejunum is consistent with our previous findings from steers fed concentrate diets (Myer et al., 2016) and in preweaned calves (Dias et al., 2018). Clostridiaceae are anaerobic, spore-forming bacteria, and their presence in the digestive tract of ruminants is well documented. The combination of spore-forming biology and an application of ribosomal DNA methodology invites caution when interpreting the data since the presence of DNA does not necessarily equate to viable vegetative cells. However, these OTUs are different than those observed in the rumen and numerous Clostridiaceae are colonized in the jejunum. Most Clostridiaceae are commensal and important in the digestion of carbohydrates and proteins, and numerous species are involved with bile acid metabolism (Lopetuso et al., 2013). Some Clostridiaceae, such as Clostridium perfringens, are associated with disease (Songer, 1996). Turicibacteraceae have been associated with changes in diet (Howe et al., 2016); however, the diet in this current study was the same for both groups. Most of the studies have compared Turicibacteraceae in fecal or colon samples. Our study suggests that the relative levels of Turicibacteraceae are greater in the jejunum and duodenum than in the large intestine. Fecal Turicibacteraceae have been associated with diets high in protein in humans (Lippert et al., 2017) and relative protein concentrations in the digesta of the jejunum and ileum compared with the large intestine would be expected to be greater. While Clostridiaceae and Turicibacteraceae made up the predominant families in the jejunum, they were not contained in the clades that were differentially observed between ADG classifications.
The diversity indexes and PERANOVA suggest that steers with different ADG classifications had different jejunum microbiota community structures. The hierarchical dependence false discovery rate analyses identified 11 clades that differed between ADG classifications, and the number of OTUs differentially associated with greater and less ADG were similar. Six of the clades differentially observed consisted of OTUs predominately classified as Firmicutes. One of the clades consisted of OTUs classified in the genus Mogibacterium, and all of the OTUs were associated with cattle with a lesser ADG. This contrasts with our previous study where cattle with the extreme greatest ADG and least ADFI had a greater percentage of sequences belonging to the Family Mogibacteriaceae (Myer et al., 2016). The remaining Firmicutes clades consisted of a single OTU each. There was one OTU classified in the genus Shuttleworthia, and like Mogibacteriaceae, it was higher in the least ADG steers. One clade consisted of a single OTU of the genus Lactobacillus in the jejunum digesta and was greater in the cattle classified in the least ADG group. Lactobacillus is generally regarded in human health as being beneficial. Species of this genus has been associated with a deconjugation of bile acids which could lead to reduced lipid absorption (Moser and Savage, 2001) and, in production animals, this could be detrimental to lipid absorption and animal growth. In chickens, an increase in weight gain is associated with a decline in ileal Lactobacillus salivarius (Guban et al., 2006). One of the OTUs was classified as Butyrivibrio. Butyrivibrio is typically associated with hemicellulose breakdown in the rumen and the end product of fermentation is butyrate. It is not clear the extent that fiber digestion is occurring in the jejunum, but Butyrivibrio can use sugars as a substrate. Myer et al. (2016) did not observe an association with jejunum Butyrivibrio and weight gain but did see a relationship with feed intake.
Three of the clades that differed in the jejunum contained Actinobacteria. One of those clades consisted of the Family Corynebacteriaceae and the other clades of OTUs were in the Family Coriobacteriaceae. In the clade containing Corynebacteriaceae, all of the OTUs were classified within the genus Corynebacterium and, with the exception of one OTU, were elevated in the steers classified with the lesser ADG. Myer et al. (2016) found mixed results with respect to Corynebacteriaceae and BW gain, but it was elevated in steers with less average daily feed intake (ADFI). Fecal Coriobacteriaceae in hamsters has been positively correlated with cholesterol absorption (Martínez et al., 2009, 2013) and fecal Coriobacteriaceae in mice (Claus et al., 2011) and is positively correlated with elevated hepatic triglycerides. Collectively, these studies suggest that Coriobacteriaceae may modulate lipid metabolism in the host animal. The increased ADG is usually associated with increased protein gain. If Coriobacteriaceae are associated with more of the nutrients being used for adipose synthesis than protein, this may be a mechanism contributing to less ADG. Bouron et al. (2017) reported higher cholesterol levels in their least efficient cattle using an RFI model, suggesting a possible change in lipid metabolism.
In the jejunal digesta, a clade had an OTU in the phylum Cyanobacteria and two OTUs in the phylum Proteobacteria, and all three OTUs were elevated in the greater ADG steers. It is unlikely to have a clade containing OTU from different phyla. Based on a comparison of the sequence between the OTUs in the Cyanobacteria phylum, those in the Proteobacteria there are approximately 70% similarity in sequence between the OTUs (Blast; https://blast.ncbi.nlm.nih.gov/Blast.cgi). These finds suggest a possible taxonomic assignment error. The Cyanobacteria OTU were in the order YS2 which are unique in that they are non-photosynthetic Cyanobacteria, and it has been proposed they may belong to a separate phylum (Di Rienzi et al., 2013). Fecal YS2 has been observed to be greater in rabbits classified with greater rates of BW gain (Zeng et al., 2015); however, mechanisms for the relationship between its presence and growth have not been elucidated.
Another clade consisted of three OTUs classified in the Order Rhizobiales. Two OTUs were assigned in the Family Brucellaceae and one OTU to Rhizobiaceae. These OTUs were greater in the steers with greater ADG. The bacterial members of Family Brucellaceae are typically observed in soils and the environment; however, in animals, these bacteria can be pathogenic. Ochrobactrum is observed as a normal inhabitant of the intestine, it is not clear that there is an underlying mechanism associated with this relationship.
Like the jejunum, the ileum has the majority of the OTUs classified as Firmicutes. The second most abundant phylum was Bacteroidetes. Within the Firmicutes, the OTU assignment to families follows a similar pattern to that of the jejunum. Although morphologically different, the jejunum and ileum do not form discrete structures which might explain why the Firmicutes do not differ in composition. Like the rumen, the predominant Bacteroidetes family in the duodenum and jejunum is Prevotellaceae, a genus of bacteria known to utilize protein and carbohydrates for energy and growth (Zorec et al., 2014). Diversity indexes and PERMANOVA suggest that there were not any shifts in diversity with regard to ADG classification. However, the hierarchical analyses identified five clades that differed between ADG classifications. The OTUs contained in these clades were not within the most abundant families. Each clade contained a single OTU and three of these were Firmicutes. The OTUs classified as Mogibacteriaceae were associated with the greater ADG steers, which is reverse of what was observed at the family level in the jejunum. One clade contained Bulleidia and this OTU was also associated with the greater ADG steers. Greater abundances of Bulleidia in the duodenum is associated with obesity in humans (Angelakis et al., 2015). The third Firmicutes clade contained Bacillus, which was associated with the least ADG steers. Members of this genus are thought to have potential probiotic activity while others might be pathogenic (Elshaghabee et al., 2017). The remaining two clades were in the phylum Actinobacteria. One clade contained Saccharopolyspora. The final clade contained an OTU in the Family Coriobacteriaceae and like the majority of the OTUs from this family in the jejunum, it was elevated in the greater ADG steers.
Following the rumen, the cecum is the second major site of fermentation in ruminants. Firmicutes, Bacteroidetes, and Proteobacteria were predominant, and these observations agree with those of Myer et al. (2015c) and de Oliveira et al. (2013), and the phyla level follows a pattern similar to that observed in the ileum. However, there are shifts in the relative predominant families within the phyla Firmicutes and Bacteroidetes. Within the Firmicutes, Clostridiaceae and Ruminococcaceae are the primary families followed by Lachnospiraceae and Turicibacteraceae. The increase in Ruminococcaceae, Lachnospiraceae, and Turicibacteraceae most likely reflect the shift from absorption in the small intestine to fermentation of nutrients that escaped the rumen and small intestine. Within Bacteroidetes, [Paraprevotellaceae] and Prevotellaceae are the primary families followed by Bacteroidaceae. The increase in relative abundance compared with the small intestine also most likely reflects the longer nutrient residence time and fermentation of nutrients in the cecum compared with the small intestine. While the diversity indexes and PERMANOVA did not suggest a difference between the ADG classifications, the hierarchal test identified 27 clades that differed. In the cecum, 81% of the observed OTUs that associated with differences in cattle ADG were associated with positive ADG. Nine of the clades consisted of OTUs classified in the Family Lachnospiraceae or binned into OTU at the order Clostridiales. Li and Guan (2017) reported elevated levels of Lachnospiraceae in the rumen of cattle with lower feed efficiency as determined by RFI. In a previous study in the cecum, OTUs within Lachnospiraceae were mixed with their relationship to ADG and ADFI (Myer et al., 2015c). Quan et al. (2018) reported elevated levels of Lachnospiraceae in the cecum of pigs with a greater feed efficiency as measured by feed conversion ratio. The elevated levels of Lachnospiraceae may indicate a more active fermentation in the cecum resulting in increased nutrients via absorbable volatile fatty acids available to the animal. Many Lachnospiraceae produce butyrate, which is a nutrient for the gut. Two clades consisted of OTUs classified as Erysipelotrichaceae at the family level; however, OTU levels were mixed with regard to which ADG classification had a greater abundance. Myer et al. (2015c) reported Erysipelotrichaceae was greater in the cecum of steers with the greatest ADG and least ADFI. Erysipelotrichaceae has been associated with lipid metabolism and inflammation (Kaakoush, 2015). Five clades contained OTU classified as RF39 at the order level, and these were greater in steers classified with greater ADG. Nine clades contained Ruminococcaceae, most had a single OTU, and most were greater in the greater ADG steers. Like Lachnospiraceae, it is likely that elevated levels of Ruminococcaceae indicate a more complete fermentation and increased absorbable nutrients available to the animal.
At the phylum and family levels, the colon follows a similar pattern as the cecum; however, there were fewer clades that differed between ADG classifications. Three clades contained OTUs classified as Firmicutes and one clade contained OTUs classified as TM7. One clade contained two OTUs classified as Lachnospiraceae that were directionally opposite of what was observed in the cecum, being higher in the least ADG steers. One clade contained three OTUs classified as Clostridiales that were greater in the greater ADG steers. The third Firmicutes clade contained three OTUs that were all elevated in the least ADG steers. The TM7 clade had two OTUs classified as F16 that was greater in the least ADG steers.
This study demonstrates the diversity of bacteria down the digestive tract of ruminants. The jejunum which is a major sight for digestion and absorption had a large number of bacterial OTUs that were associated with animal gain, and these associations were nearly equal between the ADG classifications. The cecum had the largest number of bacterial associations with ADG of all the sites evaluated. Approximately 70% of the differentially observed OTUs in the cecum were associated with greater ADG, which might indicate that it has a greater role in animal performance than previously realized.
Acknowledgments
We acknowledge the technical support of Chris Haussler. The mention of a trade name, proprietary product, or specific equipment does not constitute a guarantee or warranty by the USDA and does not imply approval to the exclusion of other products that may be suitable. USDA is an equal opportunity provider and employer.
Glossary
Abbreviations
- GI
gastrointestinal
- MICCA
microbial community analysis
- OTU
operational taxonomic unit
- PCoA
principal coordinates analysis
- PERMANOVA
permutation multivariate analysis of variance
- RFI
residual feed intake
Conflict of interest statement
The authors did not have any conflicts of interest.
Literature cited
- Albanese D., Fontana P., De Filippo C., Cavalieri D., and Donati C.. . 2015. MICCA: a complete and accurate software for taxonomic profiling of metagenomic data. Sci. Rep. 5:9743. doi: 10.1038/srep09743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allison M. J., and Robinson I. M.. . 1967. Biosynthesis of phenylalanine from phenylacetate by Chromatium and Rhodospirillum rubrum. J. Bacteriol. 93:1269–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amin M. R., and Onodera R.. . 1997. In vitro metabolism of phenylalanine by ruminal bacteria, protozoa, and their mixture. J. Gen. Appl. Microbiol. 43:1–7. doi: 10.2323/jgam.43.1 [DOI] [PubMed] [Google Scholar]
- Angelakis E., Armougom F., Carrière F., Bachar D., Laugier R., Lagier J. C., Robert C., Michelle C., Henrissat B., and Raoult D.. . 2015. A metagenomic investigation of the duodenal microbiota reveals links with obesity. Plos One 10:e0137784. doi: 10.1371/journal.pone.0137784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artegoitia V. M., Foote A. P., Lewis R. M., and Freetly H. C.. . 2017. Rumen fluid metabolomics analysis associated with feed efficiency on crossbred steers. Sci. Rep. 7:2864. doi: 10.1038/s41598-017-02856-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binda C., Lopetusoa L. R., Rizzattia G., Gibiinoa G., Cennamob V., and Gasbarrini A.. . 2018. Actinobacteria: a relevant minority for the maintenance of guthomeostasis. Dig. Liver Dis. 50:421–428. doi: 10.1016/j.dld.2018.02.012 [DOI] [PubMed] [Google Scholar]
- Bouron S. L., Diel de Amorim M., Miller S. P., and Montanholi Y. R.. . 2017. Associations of blood parameters with age, feed efficiency and sampling routine in young beef bulls. Livestock Sci. 195:27–37. doi: 10.1016/j.livsci.2016.11.003 [DOI] [Google Scholar]
- Callahan B. J., Sankaran K., Fukuyama J. A., McMurdie P. J., and Holmes S. P.. . 2016. Bioconductor Workflow for Microbiome Data Analysis: from raw reads to community analyses. F1000Res. 5:1492. doi: 10.12688/f1000research.8986.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caporaso J. G., Bittinger K., Bushman F. D., DeSantis T. Z., Andersen G. L., and Knight R.. . 2010. PyNAST: a flexible tool for aligning sequences to a template alignment. Bioinformatics 26:266–267. doi: 10.1093/bioinformatics/btp636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carberry C. A., Kenny D. A., Han S., McCabe M. S., and Waters S. M.. . 2012. Effect of phenotypic residual feed intake and dietary forage content on the rumen microbial community of beef cattle. Appl. Environ. Microbiol. 78:4949–4958. doi: 10.1128/AEM.07759-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G. J., and Russell J. B.. . 1988. Fermentation of peptides and amino acids by a monensin-sensitive ruminal Peptostreptococcus. Appl. Environ. Microbiol. 54:2742–2749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claus S. P., Ellero S. L., Berger B., Krause L., Bruttin A., Molina J., Paris A., Want E. J., de Waziers I., Cloarec O., . et al. 2011. Colonization-induced host-gut microbial metabolic interaction. mBio. 2:e00271–e00210. doi: 10.1128/mBio.00271-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colucci1 P. E., Chase L. E., Van Soest P. J.. . 1982. Feed intake, apparent diet digestibility, and rate of particulate passage in dairy cattle. J. Dairy Sci. 65:1445–1456. doi: 10.3168/jds.S0022-0302(82)82367-9 [DOI] [Google Scholar]
- Dias J., Marcondes M. I., Motta de Souza S., Cardoso da Mata E Silva B., Fontes Noronha M., Tassinari Resende R., Machado F., Cuquetto Mantovani H., Dill-McFarland K. A., and Suen G.. . 2018. Bacterial community dynamics across the gastrointestinal tracts of dairy calves during preweaning development. Appl. Environ. Microbiol. 16:84. pii: e02675-17. doi: 10.1128/AEM.02675-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Rienzi S. C., Sharon I., Wrighton K. C., Koren O., Hug L. A., Thomas B. C., Goodrich J. K., Bell J. T., Spector T. D., Banfield J. F., . et al. 2013. The human gut and groundwater harbor non-photosynthetic bacteria belonging to a new candidate phylum sibling to Cyanobacteria. Elife. 2:e01102. doi: 10.7554/eLife.01102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elshaghabee F. M. F., Rokana N., Gulhane R. D., Sharma C. and Panwar H.. . 2017. Bacillus as potential probiotics: status, concerns, and future perspectives. Front. Microbiol. 10:1490. doi: 10.3389/fmicb.2017.01490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontes C. M., and Gilbert H. J.. . 2010. Cellulosomes: highly efficient nanomachines designed to deconstruct plant cell wall complex carbohydrates. Annu. Rev. Biochem. 79:655–681. doi: 10.1146/annurev-biochem-091208-085603 [DOI] [PubMed] [Google Scholar]
- Freetly H. C., Lindholm-Perry A. K., Hales K. E., Brown-Brandl T. M., Kim M., Myer P. R., and Wells J. E.. . 2015. Methane production and methanogen levels in steers that differ in residual gain. J. Anim. Sci. 93:2375–2381. doi: 10.2527/jas.2014-8721 [DOI] [PubMed] [Google Scholar]
- Gagen E. J., Padmanabha J., Denman S. E., and McSweeney C. S.. . 2015. Hydrogenotrophic culture enrichment reveals rumen Lachnospiraceae and Ruminococcaceae acetogens and hydrogen-responsive Bacteroidetes from pasture-fed cattle. FEMS Microbiol. Lett. 362:1. fnv104. doi: 10.1093/femsle/fnv104 [DOI] [PubMed] [Google Scholar]
- Guan L. L., Nkrumah J. D., Basarab J. A., and Moore S. S.. . 2008. Linkage of microbial ecology to phenotype: correlation of rumen microbial ecology to cattle’s feed efficiency. FEMS Microbiol. Lett. 288:85–91. doi: 10.1111/j.1574-6968.2008.01343.x [DOI] [PubMed] [Google Scholar]
- Guban J., Korver D. R., Allison G. E., and Tannock G. W.. . 2006. Relationship of dietary antimicrobial drug administration with broiler performance, decreased population levels of Lactobacillus salivarius, and reduced bile salt deconjugation in the ileum of broiler chickens. Poult. Sci. 85:2186–2194. doi: 10.1093/ps/85.12.2186 [DOI] [PubMed] [Google Scholar]
- American Dairy Science Association, American Society of Animal Science and Poultry Science Association. 2010. Guide for the care and use of agricultural animals in research and teaching. 3rd ed. Savoy (IL):Fed. Anim. Sci. Soc. [Google Scholar]
- Hernandez-Sanabria E., Goonewardene L. A., Wang Z., Durunna O. N., Moore S. S., and Guan L. L.. . 2012. Impact of feed efficiency and diet on adaptive variations in the bacterial community in the rumen fluid of cattle. Appl. Environ. Microbiol. 78:1203–1214. doi: 10.1128/AEM.05114-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong H. A., To E., Fakhry S., Baccigalupi L., Ricca E., and Cutting S. M.. . 2009. Defining the natural habitat of Bacillus spore-formers. Res. Microbiol. 160:375–379. doi: 10.1016/j.resmic.2009.06.006 [DOI] [PubMed] [Google Scholar]
- Howe A., Ringus D. L., Williams R. J., Choo Z. N., Greenwald S. M., Owens S. M., Coleman M. L., Meyer F., and Chang E. B.. . 2016. Divergent responses of viral and bacterial communities in the gut microbiome to dietary disturbances in mice. ISME J. 10:1217–1227. doi: 10.1038/ismej.2015.183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyles L., Honda H., Logan N. A., Halket G., La Ragione R. M., and McCartney A. L.. . 2012. Recognition of greater diversity of Bacillus species and related bacteria in human faeces. Res. Microbiol. 163:3–13. doi: 10.1016/j.resmic.2011.10.004 [DOI] [PubMed] [Google Scholar]
- Kaakoush N. O. 2015. Insights into the role of Erysipelotrichaceae in the human host. Front. Cell. Infect. Microbiol. 5:84. doi: 10.3389/fcimb.2015.00084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch R. M., Swiger L. A., Chambers D., and Gregory K. E.. . 1963. Efficiency of feed use in beef cattle. J. Anim. Sci. 22:486–494. doi: 10.2527/jas1963.222486x [DOI] [Google Scholar]
- Li F., and Guan L. L.. . 2017. Metatranscriptomic profiling reveals linkages between the active rumen microbiome and feed efficiency in beef cattle. Appl. Environ. Microbiol. 83:e00061-17. doi: 10.1128/AEM.00061-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippert K., Kedenko L., Antonielli L., Kedenko I., Gemeier C., Leitner M., Kautzky-Willer A., Paulweber B., and Hackl E.. . 2017. Gut microbiota dysbiosis associated with glucose metabolism disorders and the metabolic syndrome in older adults. Benef. Microbes 8:545–556. doi: 10.3920/BM2016.0184 [DOI] [PubMed] [Google Scholar]
- Lopetuso L. R., Scaldaferri F., Petito V., and Gasbarrini A.. . 2013. Commensal Clostridia: leading players in the maintenance of gut homeostasis. Gut Pathog. 5:23. doi: 10.1186/1757-4749-5-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love M. I., Huber W., and Anders S.. . 2014. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15:550. doi: 10.1186/s13059-014-0550-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahé F., Rognes T., Quince C., de Vargas C., and Dunthorn M.. . 2015. Swarm v2: highly-scalable and high-resolution amplicon clustering. PeerJ. 3:e1420. doi: 10.7717/peerj.1420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maia M. R., Chaudhary L. C., Figueres L., and Wallace R. J.. . 2007. Metabolism of polyunsaturated fatty acids and their toxicity to the microflora of the rumen. Antonie Van Leeuwenhoek 91:303–314. doi: 10.1007/s10482-006-9118-2 [DOI] [PubMed] [Google Scholar]
- Malmuthuge N., Griebel P. J., and Guan le L.. . 2014. Taxonomic identification of commensal bacteria associated with the mucosa and digesta throughout the gastrointestinal tracts of preweaned calves. Appl. Environ. Microbiol. 80:2021–2028. doi: 10.1128/AEM.03864-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao S., Zhang M., Liu J., and Zhu W.. . 2015. Characterising the bacterial microbiota across the gastrointestinal tracts of dairy cattle: membership and potential function. Sci. Rep. 5:16116. doi: 10.1038/srep16116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez I., Perdicaro D. J., Brown A. W., Hammons S., Carden T. J., Carr T. P., Eskridge K. M., and Walter J.. . 2013. Diet-induced alterations of host cholesterol metabolism are likely to affect the gut microbiota composition in hamsters. Appl. Environ. Microbiol. 79:516–524. doi: 10.1128/AEM.03046-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez I., Wallace G., Zhang C., Legge R., Benson A. K., Carr T. P., Moriyama E. N., and Walter J.. . 2009. Diet-induced metabolic improvements in a hamster model of hypercholesterolemia are strongly linked to alterations of the gut microbiota. Appl. Environ. Microbiol. 75:4175–4184. doi: 10.1128/AEM.00380-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCann J. C., Wiley L. M., Forbes T. D., Rouquette F. M. Jr, and Tedeschi L. O.. . 2014. Relationship between the rumen microbiome and residual feed intake-efficiency of Brahman bulls stocked on bermudagrass pastures. Plos One 9:e91864. doi: 10.1371/journal.pone.0091864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald D., Price M. N., Goodrich J., Nawrocki E. P., DeSantis T. Z., Probst A., Andersen G. L., Knight R., and Hugenholtz P.. . 2012. An improved Greengenes taxonomy with explicit ranks for ecological and evolutionary analyses of bacteria and archaea. ISME J. 6:610–618. doi: 10.1038/ismej.2011.139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGovern E., Kenny D. A., McCabe M. S., Fitzsimons C., McGee M., Kelly A. K., and Waters S. M.. . 2018. 16S rRNA sequencing reveals relationship between potent cellulolytic genera and feed efficiency in the rumen of bulls. Front. Microbiol. 9:1842. doi: 10.3389/fmicb.2018.01842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMurdie P. J., and Holmes S.. . 2013. phyloseq: an R package for reproducible interactive analysis and graphics of microbiome census data. Plos One 8:e61217. doi: 10.1371/journal.pone.0061217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer J. H., and Garrett W. N.. . 1967. Efficiency of feed utilization. J. Anim. Sci. 26:638–646. doi: 10.2527/jas1967.263638x [DOI] [Google Scholar]
- Moser S. A., and Savage D. C.. . 2001. Bile salt hydrolase activity and resistance to toxicity of conjugated bile salts are unrelated properties in lactobacilli. Appl. Environ. Microbiol. 67:3476–3480. doi: 10.1128/AEM.67.8.3476-3480.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myer P. R., Smith T. P., Wells J. E., Kuehn L. A., and Freetly H. C.. . 2015a. Rumen microbiome from steers differing in feed efficiency. Plos One 10:e0129174. doi: 10.1371/journal.pone.0129174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myer P. R., Wells J. E., Smith T. P., Kuehn L. A., and Freetly H. C.. . 2015b. Microbial community profiles of the colon from steers differing in feed efficiency. Springerplus 4:454. doi: 10.1186/s40064-015-1201-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myer P. R., Wells J. E., Smith T. P., Kuehn L. A., and Freetly H. C.. . 2015c. Cecum microbial communities from steers differing in feed efficiency. J. Anim. Sci. 93:5327–5340. doi: 10.2527/jas.2015-9415 [DOI] [PubMed] [Google Scholar]
- Myer P. R., Wells J. E., Smith T. P., Kuehn L. A., and Freetly H. C.. . 2016. Microbial community profiles of the jejunum from steers differing in feed efficiency. J. Anim. Sci. 94:327–338. doi: 10.2527/jas.2015-9839 [DOI] [PubMed] [Google Scholar]
- Nagem M., Oliveira V., Jewell K. A., Freitas F. S., Benjamin L. A., Tótola M. R., Borges A. C., Moraes C. A., and Suen G.. . 2013. Characterizing the microbiota across the gastrointestinal tract of a Brazilian Nelore steer. Vet. Microbiol. 164:307–331. doi: 10.1016/j.vetmic.2013.02.013 [DOI] [PubMed] [Google Scholar]
- Okine E. K., and Mathison G. W.. . 1991. Effects of feed intake on particle distribution, passage of digesta, and extent of digestion in the gastrointestinal tract of cattle. J. Anim. Sci. 69:3435–3445. doi: 10.2527/1991.6983435x [DOI] [PubMed] [Google Scholar]
- de Oliveira M. N., Jewell K. A., Freitas F. S., Benjamin L. A., Tótola M. R., Borges A. C., Moraes C. A., and Suen G.. . 2013. Characterizing the microbiota across the gastrointestinal tract of a Brazilian Nelore steer. Vet. Microbiol. 164:307–314. doi: 10.1016/j.vetmic.2013.02.013 [DOI] [PubMed] [Google Scholar]
- Paillard D., McKain N., Chaudhary L. C., Walker N. D., Pizette F., Koppova I., McEwan N. R., Kopecný J., Vercoe P. E., Louis P., . et al. 2007. Relation between phylogenetic position, lipid metabolism and butyrate production by different Butyrivibrio-like bacteria from the rumen. Antonie Van Leeuwenhoek 91:417–422. doi: 10.1007/s10482-006-9121-7 [DOI] [PubMed] [Google Scholar]
- Paz H. A., Hales K. E., Wells J. E., Kuehn L. A., Freetly H. C., Berry E. D., Flythe M. D., Spangler M. L., and Fernando S. C.. . 2018. Rumen bacterial community structure impacts feed efficiency in beef cattle. J. Anim. Sci. 96:1045–1058. doi: 10.1093/jas/skx081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polan C. E., McNeill J. J., and Tove S. B.. . 1964. Biohydrogenation of unsaturated fatty acids by rumen bacteria. J. Bacteriol. 88:1056–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price M. N., Dehal P. S., and Arkin A. P.. . 2010. FastTree 2–approximately maximum-likelihood trees for large alignments. Plos One 5:e9490. doi: 10.1371/journal.pone.0009490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan J., Cai G., Ye J., Yang M., Ding R., Wang X., Zheng E., Fu D., Li S., Zhou S., . et al. 2018. A global comparison of the microbiome compositions of three gut locations in commercial pigs with extreme feed conversion ratios. Sci. Rep. 8:4536. doi: 10.1038/s41598-018-22692-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds J. G., Foote A. P., Freetly H. C., Oliver W. T., and Lindholm-Perry A. K.. . 2017. Relationships between inflammation- and immunity-related transcript abundance in the rumen and jejunum of beef steers with divergent average daily gain. Anim. Genet. 48:447–449. doi: 10.1111/age.12546 [DOI] [PubMed] [Google Scholar]
- Rognes T., Flouri T., Nichols B., Quince C., and Mahé F.. . 2016. VSEARCH: a versatile open source tool for metagenomics. PeerJ. 4:e2584. doi: 10.7717/peerj.2584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankaran K., and Holmes S.. . 2014. structSSI: simultaneous and selective inference for grouped or hierarchically structured data. J. Stat. Softw. 59:1–21. doi: 10.18637/jss.v059.i13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheifinger C., Russell N., and Chalupa W.. . 1976. Degradation of amino acids by pure cultures of rumen bacteria. J. Anim. Sci. 43:821–827. doi: 10.2527/jas1976.434821x [DOI] [PubMed] [Google Scholar]
- Shabat S. K., Sasson G., Doron-Faigenboim A., Durman T., Yaacoby S., Berg Miller M. E., White B. A., Shterzer N., and Mizrahi I.. . 2016. Specific microbiome-dependent mechanisms underlie the energy harvest efficiency of ruminants. ISME J. 10:2958–2972. doi: 10.1038/ismej.2016.62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Songer J. G. 1996. Clostridial enteric diseases of domestic animals. Clin. Microbiol. Rev. 9:216–234. doi: 10.1128/CMR.9.2.216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Z. Z., Chen G., and Alekseyenko A. V.. . 2016. PERMANOVA-S: association test for microbial community composition that accommodates confounders and multiple distances. Bioinformatics 32:2618–2625. doi: 10.1093/bioinformatics/btw311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X. Y., Walker M., Vanselow B., Chao R. L., and Chin J.. . 2007. Characterization of mesophilic bacilli in faeces of feedlot cattle. J. Appl. Microbiol. 102:872–879. doi: 10.1111/j.1365-2672.2006.03106.x [DOI] [PubMed] [Google Scholar]
- Yu Z., and Morrison M.. . 2004. Improved extraction of PCR-quality community DNA from digesta and fecal samples. Biotechniques 36:808–812. doi: 10.2144/04365ST04 [DOI] [PubMed] [Google Scholar]
- Zeng B., Han S., Wang P., Wen B., Jian W., Guo W., Yu Z., Du D., Fu X., Kong F., . et al. 2015. The bacterial communities associated with fecal types and body weight of rex rabbits. Sci. Rep. 5:9342. doi: 10.1038/srep09342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorec M., Vodovnik M., and Marinšek-Logar R.. . 2014. Potential of selected rumen bacteria for cellulose and hemicellulose degradation. Food Technol. Biotechnol. 52:210–221. [Google Scholar]