Abstract
Background
Intestinal parasitic infections (IPIs) are major health problems in many developing countries. School children between the ages of 5 and 15 years suffer the highest infection rate and parasitic burden that are attributed to poor sanitation and hygiene. In Ethiopia, the prevalence of IPIs among school children is high (ranging from 66.7% to 83.8%).
Methods
School-based cross-sectional study was conducted in two primary schools at Harbu Town, Northeast Ethiopia from February to May, 2018. Systematic random sampling technique was employed to select study participants from the two school compounds. The sample size was determined by a single population proportion statistical formula and the minimum numbers of study participants defined were 400 school children. Socio-demographic and risk factor-related information were collected using structured questionnaire. Data about detection and identification of intestinal parasites were obtained from laboratory examination of stool specimen by using wet mount and formol-ether concentration techniques. Stool specimen from each study participant was collected using clean, properly labeled and leak-proof stool cup. The data were processed and analyzed using SPSS version 20 software.
Results
Out of a total of 400 study participants, 86 (21.5%) were found with one or more IPIs. Six different types of intestinal parasites were identified, Entamoeba histolytica was the most 33 (8.3%) detected parasite followed by Hymenolopis nana 19 (4.8%) and Schistosoma mansoni 19 (4.8%). The least identified parasite was Giardia lamblia, detected only from four study participants. Male study participants showed 2.42 times risk (AOR = 2.42, 95% CI = 1.25–4.7, P = 0.009) of acquiring parasitic infection than female. Presence of water body near to home and having contact with water bodies showed 7.64 (AOR= 7.64, 95% CI= 3.3–17.8, P= 0.000) and 4.6 (AOR=4.6, 95% CI: 2.04–10.57, P= 0.000) times risk of infection with parasitic infection among school children, respectively.
Conclusion
IPIs were highly prevalent health problem among the two primary school children in Harbu Town. Sex, availability of water bodies near to house and contact with water bodies were having significant association with the prevalence of IPIs.
Keywords: intestinal parasites, Harbu, school children, Ethiopia
Background
Intestinal parasitic infections (IPIs) are major health problems in many developing countries, particularly among pre-schooled and schooled children.1,2 Intestinal helminths and protozoans are among the commonest infections in humans living in developing countries.3 In tropical and sub-tropical regions, Soil-transmitted helminth (STHs) and parasitic intestinal protozoa are causes for an immeasurable amount of morbidity, discomfort and often mortality.4 Those living in poverty are more vulnerable to infection and almost 2 billion people are infected with STHs worldwide.5 According to the World Health Organization (WHO) fact sheet on STHs, globally over 568 million school-age children live in intestinal helminth prevalent area.6
A high prevalence of Schistosoma mansoni infection with light, moderate and heavy infection intensity was reported among school children in Ethiopia.7,8 Particularly, In Amhara region, a considerable amount of prevalence [6.9%, ranges from 0.5% to 40.1%] of S. mansoni among school children was reported. A high proportion of STHs [36.4] was also indicated in the region.9 Schistosoma mansoni infection is significantly higher among school children who practice swimming in the river.8 In warm, tropical environments, where STHs are endemic and where sanitation is inadequate, parasite eggs are excreted in the faeces of infected individuals and contaminate the soil. Humans become infected through ingestion of eggs or larvae that are passed in the faeces of infected people.5,10
School children between the ages of 5 and 15 years suffer the highest infection rate and parasitic burden that are attributed to poor sanitation and hygiene. Of the estimated 181 million school children in Sub-Saharan Africa (SSA), almost one-half are infected with hookworm, ascariasis, trichuriasis, or some combination of these infections.11,12 The adverse effects of IPIs among school children are diverse and alarming. Among the most important are growth stunting and malnutrition, including iron deficiency anemia, fatigue and diminished physical fitness, and impaired school attendance and cognitive performance.13,14
Intestinal parasite infections are highly prevalent among children in Ethiopia. Although IPIs are distributed across each regional states of the country, Southern and Amhara regional states carry the highest burden.1 In Ethiopia, the prevalence of IPIs among school children is high (ranging from 66.7% to 83.8%) and has been associated with adverse outcomes including anemia, malnutrition, stunting and thinness.15–19 The high prevalence of these infections and outcomes are associated with socio-economic status, poor in school deworming, inadequate hygiene and sanitary conditions, indicating the need for school-based anti-parasitic treatment and preventive interventions to prevent infection and re-infection.17,18,20
Several studies have been conducted on the prevalence and associated factors of IPIs among school children in Ethiopia. But in many parts of the country at large and specifically in Amhara region there are still localities with no epidemiological information on the prevalence of IPIs among vulnerable groups like school children. Among those localities Harbu Town which is located in south wollo zone, Amhara region, northeast Ethiopia is the one. Therefore, the objective of the present study was to assess the prevalence of IPIs (both protozoan and helminth) and to find out possible associated risk factors among school children in the area.
Methods
Study Design, Period and Area
From February to May, 2018 school-based cross-sectional study was conducted among two primary schools students at Harbu Town, Ethiopia (Harbu and Addis Mender Primary Schools). Harbu Town is found in Kalu woreda, South Wollo zone, Amhara region. The town is located 353 km northeast of Addis Ababa, which is the capital of the country. According to the Central Statistics Agency report 2007, the total population of Harbu was 28,903. Out of these, 13,146 and 15,757 were males and females, respectively. Agriculture is the main source of income for inhabitants in the area, where the farming system is characterized by small-scale production of mixed crops and livestock. The town gets water mainly from spring source and there is a massive production of sugar cane in the area.
Study Population and Sample Size Determination
The study participants were students attending at the two primary schools from grade 1 to grade 8. A total of 43 classes were available in the selected schools and each class contains an average of 59 students during the study period. The total numbers of students attending in the two primary schools were 2516 during study period. Students were selected by stratified sampling technique based on their educational level (grade 1 to grade 8) and then quota was allocated to each grade based on the total number of students. The target students were selected by systematic random sampling from each class based on their class roster. The study populations were determined by a single population proportion statistical formula used for sample size determination: n=Z2xP (1-P)/d2 where: Z at 95% confidence interval, P at 50%, d at 5% marginal error and 1-p (non-observed value). In line with these conditions the minimum numbers of study participants defined were 384. By considering a nonresponse rate, we were adding 4% of primary school children as a contingency and the final sample size became 400.
Inclusion and Exclusion Criteria
The study participants who had no history of anti-intestinal parasite drugs in the last two weeks prior to screening were included in this study. Those school children who were not willing to give stool samples were excluded from the study.
Stool Specimen Collection and Examination
For specimen collection, a single well labeled, clean, dry, disinfectant free, wide-mouthed plastic container were distributed to each study participant with instruction requesting them to bring stool sample immediately. The containers were labeled with children’s name, code number and date of collection. Stool samples were collected from each student along with questionnaire. After receiving about 10-g single stool specimen the laboratory personnel (investigators) prepared two slides from each stool specimen for wet mount in saline. Each slide was microscopically examined initially under low power (10×) bright field then under high power (40×) bright field examined at Harbu health center Laboratory. Simultaneously, samples were emulsified in a 10% formalin solution and transported to Wollo University College of Medicine and Health Sciences, Medical laboratory. From the emulsified sample 1 g (pea size) of feces was taken in about 4 mL of 10% formal water and then mixed and sieved in another tube. Then 3–4 mL of ether was added and centrifuged immediately at 750–1000 g (~3000 rpm) for 1 min. Finally, the supernatant was discarded, and then small portion of the sediment was transferred to a slide and covered with cover slip and examined first with 10X and then 40 X objectives and examined microscopically.21
Data Collection, Processing and Analysis
An interview-based structured questionnaire was used to collect socio-demographic and behavioral related data from each study participants. The data (both questionnaire-based and laboratory-based data) were collected by investigators. Data quality was checked and entered to Microsoft Excel and exported to SPSS version 20 software and analysed. Binary logistic regression was done to investigate the association between the dependent and independent variables by taking P < 0.05 as a cut off for statistical significant association. Variables that show significance at P-value of 0.3 during univariate analysis were selected for multivariable analysis. Adjusted odds ratios (AOR) and their 95% confidence intervals (CIs) were used as indicators of the strength of association.
Results
Socio-Demographic-Related Information
A total of 400 primary school children whose age ranges from 7 to 14 years were participated in this study. Out of the total study participants, 249 (62.25%) were female, 243 (60.75) study participants were in the age category of 10–12 years old, more than half of (62.5%) of the children’s mothers were uneducated, 236 (59%) of the study participant were living in urban areas of the town (Table 1).
Table 1.
Socio-Demographic Characteristics of Primary School Children and Their Parent/Guardian in Harbu Town, from February to May, 2018
| S. No. | Parameter | Intestinal Parasite Result | Total (No.) | |
|---|---|---|---|---|
| Negative No. (%) | Positive No. (%) | |||
| 1. | Sex | |||
| Male | 104 (33.1) | 47 (54.7) | 151 (37.75) | |
| Female | 210 (66.9) | 39 (45.3) | 249 (62.25) | |
| 2. | Age | |||
| 7–9 | 87 (27.7) | 25 (29.1) | 112 (28) | |
| 10–12 | 194 (61.8) | 49 (57) | 243 (60.75) | |
| ≥13 | 33 (10.5) | 12 (13.9) | 45 (11.25) | |
| 3. | Mother’s educational status | |||
| Uneducated | 194 (61.8) | 56 (65.1) | 250 (62.5) | |
| Primary | 94 (30) | 23 (26.7) | 117 (29.25) | |
| Secondary | 18 (5.7) | 5 (5.8) | 23 (5.75) | |
| College | 8 (2.5) | 2 (2.3) | 10 (2.5) | |
| 4. | Residence | |||
| Urban | 178 (56.7) | 58 (67.4) | 236 (59) | |
| Rural | 136 (43.3) | 28 (32.6) | 164 (41) | |
| 5. | Family size | |||
| 3–5 | 37 (11.8) | 5 (5.8) | 42 (10.5) | |
| 6–8 | 251 (79.9) | 75 (87.2) | 326 (81.5) | |
| ≥9 | 26 (8.3) | 6 (7) | 32 (8) | |
The majority of the study participants were having sugar cane eating habits on straight, almost all participants respond that there were no any domestic animals, particularly cattle, sheep and goats that are living together with the human home, 375 (93.75) study participants did not have the habit of walking on bare foot (Table 2).
Table 2.
Predisposing Factors for Prevalence of Intestinal Parasites Among Primary School Children in Harbu Town, from February to May, 2018
| S. No. | Parameter | Intestinal Parasite Result | Total No. (%) | |
|---|---|---|---|---|
| Negative No. (%) | Positive No. (%) | |||
| 1. | Good hygienic condition of finger nails | |||
| Yes | 281 (89.4) | 67 (77.9) | 354 (88.5) | |
| No | 27 (8.6) | 19 (22.1) | 46 (11.5) | |
| 2. | Sugarcane eating habit | |||
| Yes | 233 (74.2) | 48 (55.8) | 281 (70.25) | |
| No | 81 (25.8) | 38 (44.2) | 119 (29.75) | |
| 3. | Availability of water body near to home | |||
| Yes | 59 (18.8) | 71 (82.6) | 130 (32.5) | |
| No | 255 (81.2) | 15 (17.4) | 270 (67.5) | |
| 4. | Contact with water body | |||
| Yes | 23 (7.3) | 57 (66.3) | 80 (20) | |
| No | 291 (92.7) | 29 (33.7) | 320 (80) | |
| 5. | Domestic animal living with human at home | |||
| Yes | 8 (2.5) | 8 (9.3) | 16 (4) | |
| No | 306 (97.5) | 78 (90.7) | 384 (96) | |
| 6. | Habit of walking on bare foot | |||
| Yes | 15 (4.8) | 10 (11.6) | 25 (6.25) | |
| No | 299 (95.2) | 76 (88.4) | 375 (93.75) | |
| 7. | BMI | |||
| <18.5 | 200 (63.7) | 54 (62.8) | 254 (63.5) | |
| ≥18.5 | 114 (36.3) | 32 (37.2) | 146 (36.5) | |
Prevalence of Intestinal Parasites and Associated Factors
Out of the total 400 study participants, 86 (21.5%) were infected with intestinal parasites. School children in the age category of 10–12 years were harboring 49/86 (57%) of the total burden of IPIs in the study area (Table 1).
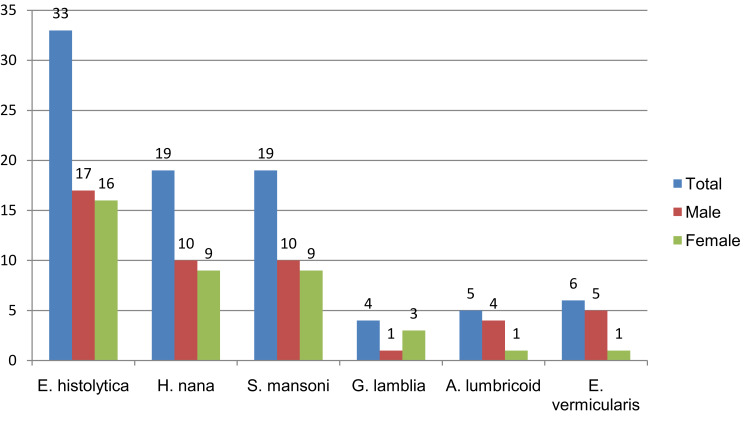
Six different types of intestinal parasites were identified. About 57% of the identified parasites were intestinal helminth whereas the rest were intestinal protozoan. Entamoeba histolytica was the most 33 (8.3%) prevalent parasite of all, followed by Hymenolopis nana 19 (4.8%) and Schistosoma mansoni 19 (4.8%). The least identified parasite was Giardia lamblia that was detected from four school children. Except G. lamblia, all identified parasites were more prevalent among male study participants (Figure 1).
Figure 1.
Types of detected parasites with distribution based on sex of study participants in Harbu Town Primary Schools from February to May, 2018.
We have assessed more than 15 variables in the presence or absence of association with IPIs. In the univariate analysis, at a P-value of 0.3, there were ten variables that showed an association with the infection. These variables were sex, nail hygiene, sugar cane utilization on street, residence, hand washing after toilet, presence of water bodies near the house, contact with water body, animals living at human home, habit of walking on bare foot and family size. But in the multivariate analysis, only three variables remain significantly associated factors for the prevalence of IPIs among school children.
The prevalence of IPIs was 31.1% and 15.7% for males and females, respectively. Male study participants showed 2.42 times risk of acquiring IPIs than female primary school children (AOR = 2.42, 95% CI = 1.25–4.7, P = 0.009). Presence of water body near to home and having contact with water bodies have shown 7.64 and 4.6 times risk of infection with IPIs among school children, respectively (Table 3).
Table 3.
Bivariable and Multivariable Analysis of Intestinal Parasitic Infections and Potential Risk Factors Among Primary School Children in Harbu Town, From February to May, 2018
| S. No. | Parameter | P–value | COR [95% CI] | P–value | AOR [95% CI] |
|---|---|---|---|---|---|
| 1. | Sex | ||||
| Male | 0.0003 | 2.4 [1.5–4.0] | 0.009 | *2.42 [1.25–4.7] | |
| Female | Ref. | Ref. | |||
| 2. | Good finger nail hygiene | ||||
| Yes | 0.001 | 0.33 [0.17–0.63] | – | – | |
| No | Ref. | ||||
| 3. | Sugarcane eating habit | ||||
| Yes | 0.001 | 0.44 [0.27–0.72] | – | – | |
| No | Ref. | ||||
| 4. | Residence | ||||
| Urban | 0.074 | 1.58 [0.96–2.62] | – | – | |
| Rural | |||||
| 5. | Availability of water body | ||||
| Yes | 0.000 | 20.4 [11.0–38.0] | 0.000 | *7.64 [3.3–17.8] | |
| No | Ref. | Ref. | |||
| 6. | Having contact with water bodies | ||||
| Yes | 0.000 | 24.9 [13.4–46.1] | 0.000 | *4.6 [2.04–10.57] | |
| No | Ref. | Ref. | |||
| 7. | Animals living with human home | ||||
| Yes | 0.008 | 3.92 [1.43–10.8] | – | – | |
| No | Ref. | ||||
| 8. | Habit of walking on barefoot | ||||
| Yes No |
0.024 | 2.62 [1.13–6.07] | – | – | |
Notes: Only significant values (p-value) are indicated by an asterisk indicator (*).
Except to Enterobius vermicularis, all the five detected parasite type predominantly identified from the school children whose age range was from 10 to 12 years. The majority of school children who were affected by the six parasite types were live in an urban area of the town. Schistosoma mansoni was a prevalent parasite among school children who did not have contact with a domestic animal, who did not walk on barefoot and among school children whose BMI was under 18.5. It is also more prevalent among school children who had contact with water bodies (Table 4).
Table 4.
Types of Detected Parasites with Distribution Based on Different Variables Among Study Participants in Harbu Town Primary Schools from February to May, 2018
| S. No. | Variables | Types of Parasite Detected | |||||
|---|---|---|---|---|---|---|---|
| H. nana | E. histolytica | A. lumbricoides | G. lamblia | S. mansoni | E. vermicularis | ||
| 1. | Water body near to home | ||||||
| Yes | 16 | 27 | 4 | 3 | 17 | 4 | |
| No | 3 | 6 | 1 | 1 | 2 | 2 | |
| 2. | Contact with water bodies | ||||||
| Yes | 12 | 22 | 4 | 3 | 13 | 3 | |
| No | 7 | 11 | 1 | 1 | 6 | 3 | |
| 3. | Age category | ||||||
| 7–9 | 6 | 8 | 1 | 0 | 5 | 5 | |
| 10–12 | 11 | 22 | 3 | 2 | 11 | 0 | |
| ≥13 | 2 | 3 | 1 | 2 | 3 | 1 | |
| 4. | Residence | ||||||
| Urban | 11 | 22 | 3 | 3 | 14 | 5 | |
| Rural | 8 | 11 | 2 | 1 | 5 | 1 | |
| 5. | Animal live at human home | ||||||
| Yes | 1 | 4 | 0 | 0 | 3 | 0 | |
| No | 18 | 29 | 5 | 4 | 16 | 6 | |
| 6. | Walking on bare foot | ||||||
| Yes | 3 | 4 | 1 | 0 | 1 | 1 | |
| No | 16 | 29 | 4 | 4 | 18 | 5 | |
| 7. | BMI | ||||||
| <18.5 | 12 | 21 | 2 | 3 | 15 | 1 | |
| ≥18.5 | 7 | 12 | 3 | 1 | 4 | 5 | |
| 8. | Family member | ||||||
| 3–5 | 0 | 4 | 0 | 0 | 0 | 1 | |
| 6–8 | 19 | 27 | 5 | 3 | 17 | 4 | |
| ≥9 | 0 | 2 | 0 | 1 | 2 | 1 | |
The second most parasites identified (H. nana and S. mansoni) have assessed for the presence or absence of any association with the independent variables. Having of water body near to home has showed significant association with Schistosoma mansoni infection [AOR: 11.5; 95% CI: 2.01–65.8; P-value: 0.006] (Table 5). Infection with H. nana had shown significant association with having of water bodies near to home [AOR: 7.69; 95% CI: 1.63–36.2; P-value: 0.01] of school children (Table 6).
Table 5.
Bivariable and Multivariable Analysis of S. mansoni Infections and Potential Risk Factors Among Primary School Children in Harbu Town, from February to May, 2018
| S. No. | Parameter | P-value | COR [95% CI] | P-value | AOR [95% CI] |
|---|---|---|---|---|---|
| 1 | Sex Male Female |
0.18 | 1.9 [0.75–4.77] | 0.59 | 1.33 [0.47–3.72] |
| 2 | Sugarcane eating habit Yes No |
0.031 | 0.36 [0.14–0.9] | 0.57 | 0.57 [0.21–1.55] |
| 3 | Water body near to home Yes No |
0.000 | 20.16 [4.58–88.7] | *0.006 | 11.5 [2.01–65.8] |
| 4. | Contact with water bodies Yes No |
0.000 | 10.15 [3.73–27.68] | 0.31 | 1.91 [0.54–6.7] |
| 5 | BMI <18.5 ≥18.5 |
0.16 | 2.23 [0.72–6.8] | 0.15 | 2.37 [0.73–7.69] |
Note: Only significant values (p-value) are indicated by an asterisk indicator (*).
Table 6.
Bivariable and Multivariable Analysis of H. nana Infections and Potential Risk Factors Among Primary School Children in Harbu Town, from February to May, 2018
| S. No. | Parameter | P-value | COR [95% CI] | P-value | AOR [95% CI] |
|---|---|---|---|---|---|
| 1 | Sex Male Female |
0.18 | 1.89 [0.75–4.77] | 0.59 | 1.32 [0.47–3.7] |
| 2 | Sugarcane eating habit Yes No |
0.001 | 0.18 [0.07–0.48] | *0.012 | 0.26 [0.09–0.75] |
| 3 | Water body near to home Yes No |
0.000 | 12.49 [3.57–43.7] | *0.01 | 7.69 [1.63–36.2] |
| 4. | Contact with water bodies Yes No |
0.000 | 7.89 [3–20.78] | 0.64 | 1.36 [0.38–4.86] |
| 5 | Bare foot walking habit Yes No |
0.093 | 3.06 [0.83–11.3] | 0.51 | 1.63 [0.38–6.94] |
Note: Only significant values (p-value) are indicated by an asterisk indicator (*).
Discussion
To identify a high-risk community (group) and to formulate appropriate intervention approaches it is better to have good epidemiological data on the prevalence and associated factors of IPs among school children in different localities. The current study was conducted in two primary Schools in Harbu Town that has a warm temperature condition and other predisposing factors for IPIs. Intestinal parasite is the major cause of morbidity among suspected cases at Harbu Town as it was indicated in the laboratory log book of Harbu health center.
In this study the overall prevalence of IPIs, including protozoan and helminth, was 21.5%. This was a comparable result with studies conducted in Southern part of Ethiopia (26.2%),22 (27.1%).23 The result was slightly higher than a study conducted in Nepal which was conducted among private and public schools.24 This difference might be due to variation of study groups in the two studies.
In the current study, the prevalence was lower in comparison with studies in various areas of Ethiopia such as Wukro town (60.7%),25 Amhara national regional state (77.9%),26 Ochollo (56.8%),27 Bahir-dar town (52.4%),28 and Haike town (30.5%).29 A higher overall prevalence of IPI was also reported from different parts of Africa.30,31 This might be due to differences in environmental and socioeconomic conditions, variations in method of diagnosis and community awareness. A huge variation in the sensitivity of wet mount, formol-ether concentration and Kato Katz tests in the diagnosis of intestinal parasites was documented.32
In the current study, E. histolytica was the predominant parasite with a prevalence rate of 8.3% (33/400) followed by H. nana (4.8%, 19/400) and S. mansoni (4.8%, 19/400). A study that has been conducted at North East Ethiopia indicated that H. nana infection was the most identified species and followed by E. histolytica.33 Hymenolepis nana was the most commonly identified and E. histolytica was the second common pathogenic intestinal protozoan parasites in a study conducted among primary school children in Tajikistan, where there was unimproved sources of drinking water.34 Another study in southeastern Ethiopia indicated S. mansoni (12.6%) was the most prevalent parasites followed by E. histolytica/dispar (5%).22 Like our study finding, some studies in Ethiopia reported that E. histolytica as the most identified parasite,25,28 whereas some others revealed it was the least identified parasites.27,29
Ascaris lumbricoides was one of the least identified parasites in the current study and this is in difference with various studies which indicates it is the commonest identified species. It was one of the dominant parasites in north,35,36 and southern Ethiopia.27 A study conducted in Nigeria and Nepal also revealed that A. lumbricoides was one of the dominant species.24,30 In the study area, there was no significant sanitation and personal hygiene problem that could be probable reasons for the low prevalence of Ascaris infections. Most children did not defecate in open field since they had sufficient latrine facilities in their home.
Even though the difference is slight, in the current study the prevalence of helminth (12.25%) was more than protozoan parasites (9.25%). In contrast, the study conducted in Ethiopia and Nepal indicated that the proportion of protozoan was higher than helminth.24,25 In another way, a much higher prevalence rate of helminth than that of protozoan parasites was indicated in studies conducted in Ethiopia.23,37 Giardia lamblia was one of the least identified parasites with a prevalence rate of 1% (Figure 1). Similarly, two studies in southern Ethiopia indicated that the species was one of the least identified parasites among school children.22,27 On the contrary to this finding, some other studies indicated that the species was the commonest parasite with a higher prevalence.25,26
In our study, S. mansoni was the second most prevalent parasite, whereas a study conducted in Northwest Ethiopia indicated the parasite was one of the least identified species,33 and the species was not even detected in a study conducted in Nigeria.30 This might be due to in our study most of the students with parasitic infection had a history of contact with water bodies and it was strongly associated with the infection. In the current study, the prevalence of Hookworm infection, which is the common STHs, was zero. On the contrary, there were some studies in Ethiopia, which showed hookworm infection was one of the identified health problems among school children.28,33,38 High chance of infection with intestinal parasite, particularly with hookworm, among children who used protective shoes irregularly compared to used shoes regularly was demonstrated.28,36 In the present study, primary school children were used protective shoe in a regular manner. So, this might be the possible reason why Hookworm infection was not detected.
In the bivariable analysis, there were various factors that had an association with the prevalence of IPIs. But in the multi-variable analysis only three variables [sex, availability of water bodies near to the house, having contact with water bodies] had shown a statistically significant association (Table 3). Male school children showed 2.42 times likelihood risk of acquiring IPIs in reference to female students [AOR = 2.42, 95% CI = 1.25–4.7, P = 0.009], which is in agreement with a study conducted in Mizan – Aman town.37 In comparison with female school children male children usually play outdoors and engage in outdoor activities like playing football, which may predispose them to higher risks of IPI. Whereas a study conducted in North West Ethiopia indicated female students were more likely to acquire infection with intestinal parasite.19
School children who had contact with water bodies were 4.6 times more likely to be infected with intestinal parasites [AOR=4.6, 95% CI = 2.04–10.57, P = 0.000]. Similarly, a study conducted in northeast Ethiopia showed swimming habits of the student had shown a strong association with parasitic infection.29 Unlike the current study, research work in Northwest Ethiopia did not show a significant association between swimming habits of school children with a prevalence of IPIs.33
The major limitation of the current study is that infection with intestinal parasites was determined by examination of single stool specimen of each study participant. Moreover, it was conducted only by using wet mount and formol-ether concentration techniques. Kato-Katz and modified AFB technique did not apply and in order to determine the intensity of the infection, we did not perform parasites egg count method.
Conclusion
Intestinal parasitic infections were highly prevalent health problem among the two primary school children in Harbu Town. What’s more, helminth was found slightly higher prevalent than protozoa. The present study revealed that the prevalence of IPIs in school children is significantly associated with sex of the study participants and having contact with water bodies. In addition, the presence of water bodies near to home had significant association with the overall prevalence of IPIs and with specific parasite infection of S. mansoni and H. nana. Health awareness programs should be provided especially to the parents and to school compound workers. The subsequent deworming program should be considered for the school children. Additional studies should be conducted in the study area with advanced microscopic and molecular techniques that can be helpful for a better diagnosis of intestinal parasites and to take appropriate prevention and control measures.
Acknowledgments
We deeply express our gratefulness to staff members and stakeholders of Harbu and Addis Mender Primary Schools without which this project would have not been possible. The authors would like to thank children’s guardians/parents. The authors also would like to acknowledge all laboratory personnel and other staff members of Harbu Health Center for their cooperation for the accomplishment of this study.
Funding Statement
No external funds were obtained; only institutional support from Wollo University.
Abbreviations
IPIs, intestinal parasitic infections; STHs, soil transmitted helminth; BMI, body mass index.
Data Sharing Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the manuscript.
Ethics Approval and Consent to Participate
The study was evaluated and approved by the Department Research Ethics Review Committee (MLS/0136/2018) of Wollo University, College of Medicine and Health Sciences, department of medical laboratory science and ethical clearance was obtained. A formal communication between the department and the schools higher officials was held through letter. A parent or legal guardian provided written informed consent since the study participants were under the age of 18 years. Any patient who was not willing to take part in the study had full right to do so and confidentiality of the study participants was also strictly maintained. Participants with positive IP result were communicated with the stakeholders to provide treatment. This study was fully conducted in accordance with the ethical consideration Declaration of Helsinki.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; gave final approval of the version to be published; and agree to be accountable for all aspects of this research work.
Disclosure
The authors declare that they have no competing interests.
References
- 1.Chelkeba L, Mekonnen Z, Alemu Y, Emana D. Epidemiology of intestinal parasitic infections in preschool and school-aged Ethiopian children: a systematic review and meta-analysis. BMC Public Health. 2020;20(1):117. doi: 10.1186/s12889-020-8222-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gizaw Z, Addisu A, Dagne H. Effects of water, sanitation and hygiene (WASH) education on childhood intestinal parasitic infections in rural Dembiya, northwest Ethiopia: an uncontrolled before-and-after intervention study. Environ Health Prev Med. 2019;24(1):16. doi: 10.1186/s12199-019-0774-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Houweling TA, Karim-Kos HE, Kulik MC, et al. Socioeconomic inequalities in neglected tropical diseases: a systematic review. PLoS Negl Trop Dis. 2016;10(5):e0004546. doi: 10.1371/journal.pntd.0004546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khanal L, Choudhury D, Rai S, et al. Prevalence of intestinal worm infestations among school children in Kathmandu, Nepal. Nepal Med Coll J. 2011;13(4):272–274. [PubMed] [Google Scholar]
- 5.WHO Guideline: Preventive Chemotherapy to Control Soil-Transmitted Helminth Infections in At-Risk Population Groups. World Health Organization; 2017. [PubMed] [Google Scholar]
- 6.Rashid M, Joshi M, Joshi H, Fatemi K. Prevalence of intestinal parasites among school going children in Bareilly District. Natl J Integr Res Med. 2011;2(1). [Google Scholar]
- 7.Mekonnen Z, Meka S, Zeynudin A, Suleman S. Schistosoma mansoni infection and undernutrition among school age children in Fincha’a sugar estate, rural part of West Ethiopia. BMC Res Notes. 2014;7(1):763. doi: 10.1186/1756-0500-7-763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alemu A, Atnafu A, Addis Z, et al. Soil transmitted helminths and Schistosoma mansoni infections among school children in Zarima town, northwest Ethiopia. BMC Infect Dis. 2011;11(1):189. doi: 10.1186/1471-2334-11-189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nute AW, Endeshaw T, Stewart AE, et al. Prevalence of soil-transmitted helminths and Schistosoma mansoni among a population-based sample of school-age children in Amhara region, Ethiopia. Parasit Vectors. 2018;11(1):431. doi: 10.1186/s13071-018-3008-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mondiale de la Santé O; Organization WH. Schistosomiasis and soil-transmitted helminthiases: number of people treated in 2016–Schistosomiase et géohelminthiases: nombre de personnes traitées en 2016. Wkly Epidemiol Rec. 2017;92(49):749–760. [PubMed] [Google Scholar]
- 11.Hotez PJ, Kamath A. Neglected tropical diseases in sub-Saharan Africa: review of their prevalence, distribution, and disease burden. PLoS Negl Trop Dis. 2009;3(8):e412. doi: 10.1371/journal.pntd.0000412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brooker S, Clements AC, Bundy DA. Global epidemiology, ecology and control of soil-transmitted helminth infections. Adv Parasitol. 2006;62:221–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bethony J, Brooker S, Albonico M, et al. Soil-transmitted helminth infections: ascariasis, trichuriasis, and hookworm. Lancet. 2006;367(9521):1521–1532. doi: 10.1016/S0140-6736(06)68653-4 [DOI] [PubMed] [Google Scholar]
- 14.Mbuh J, Nembu N. Malnutrition and intestinal helminth infections in schoolchildren from Dibanda, Cameroon. J Helminthol. 2013;87(1):46–51. doi: 10.1017/S0022149X12000016 [DOI] [PubMed] [Google Scholar]
- 15.Mathewos B, Alemu A, Woldeyohannes D, et al. Current status of soil transmitted helminths and Schistosoma mansoni infection among children in two primary schools in North Gondar, Northwest Ethiopia: a cross sectional study. BMC Res Notes. 2014;7(1):88. doi: 10.1186/1756-0500-7-88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Legesse M, Erko B. Prevalence of intestinal parasites among schoolchildren in a rural area close to the southeast of Lake Langano, Ethiopia. Ethiop J Health Dev. 2004;18(2). [Google Scholar]
- 17.Mahmud MA, Spigt M, Mulugeta Bezabih A, Lopez Pavon I, Dinant G-J, Blanco Velasco R. Risk factors for intestinal parasitosis, anaemia, and malnutrition among school children in Ethiopia. Pathog Glob Health. 2013;107(2):58–65. doi: 10.1179/2047773213Y.0000000074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alelign T, Degarege A, Erko B. Prevalence and factors associated with undernutrition and anaemia among school children in Durbete Town, northwest Ethiopia. Arch Public Health. 2015;73(1):34. doi: 10.1186/s13690-015-0084-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abdi M, Nibret E, Munshea A. Prevalence of intestinal helminthic infections and malnutrition among schoolchildren of the Zegie Peninsula, northwestern Ethiopia. J Infect Public Health. 2017;10(1):84–92. doi: 10.1016/j.jiph.2016.02.009 [DOI] [PubMed] [Google Scholar]
- 20.Yimam Y, Degarege A, Erko B. Effect of anthelminthic treatment on helminth infection and related anaemia among school-age children in northwestern Ethiopia. BMC Infect Dis. 2016;16(1):613. doi: 10.1186/s12879-016-1956-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cheesbrough M. District Laboratory Practice in Tropical Countries. Cambridge university press; 2006. [Google Scholar]
- 22.Tulu B, Taye S, Amsalu E. Prevalence and its associated risk factors of intestinal parasitic infections among Yadot primary school children of South Eastern Ethiopia: a cross-sectional study. BMC Res Notes. 2014;7(1):848. doi: 10.1186/1756-0500-7-848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alemu G, Abossie A, Yohannes Z. Current status of intestinal parasitic infections and associated factors among primary school children in Birbir town, Southern Ethiopia. BMC Infect Dis. 2019;19(1):270. doi: 10.1186/s12879-019-3879-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shrestha J, Bhattachan B, Rai G, Park EY, Rai SK. Intestinal parasitic infections among public and private schoolchildren of Kathmandu, Nepal: prevalence and associated risk factors. BMC Res Notes. 2019;12(1):192. doi: 10.1186/s13104-019-4225-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kidane E, Menkir S, Kebede A, Desta M. Prevalence of intestinal parasitic infections and their associations with anthropometric measurements of school children in selected primary schools, Wukro Town, Eastern Tigray, Ethiopia. Int J Curr Microbiol Appl Sci. 2014;3(3):11–29. [Google Scholar]
- 26.Alamir M, Awoke W, Feleke A. Intestinal parasites infection and associated factors among school children in Dagi primary school, Amhara National Regional State, Ethiopia. Health. 2013;2013. [Google Scholar]
- 27.Bugssa G, Dessalegn B, Alemu M, Desta H, Kahsay T. A survey of intestinal parasitic infections among Dega Ochollo primary school children Ochollo South Ethiopia. SJPH. 2015;3(1):56–60. doi: 10.11648/j.sjph.20150301.20 [DOI] [Google Scholar]
- 28.Hailegebriel T. Undernutrition, intestinal parasitic infection and associated risk factors among selected primary school children in Bahir Dar, Ethiopia. BMC Infect Dis. 2018;18(1):394. doi: 10.1186/s12879-018-3306-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Feleke DG, Arega S, Tekleweini M, Kindie K, Gedefie A. Schistosoma mansoni and other helminthes infections at Haike primary school children, North-East, Ethiopia: a cross-sectional study. BMC Res Notes. 2017;10(1):609. doi: 10.1186/s13104-017-2942-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gyang VP, Chuang T-W, Liao C-W, et al. Intestinal parasitic infections: current status and associated risk factors among school aged children in an archetypal African urban slum in Nigeria. J Microbiol Immunol Infect. 2019;52(1):106–113. doi: 10.1016/j.jmii.2016.09.005 [DOI] [PubMed] [Google Scholar]
- 31.Erismann S, Diagbouga S, Odermatt P, et al. Prevalence of intestinal parasitic infections and associated risk factors among schoolchildren in the Plateau Central and Centre-Ouest regions of Burkina Faso. Parasit Vectors. 2016;9(1):554. doi: 10.1186/s13071-016-1835-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yimer M, Hailu T, Mulu W, Abera B. Evaluation performance of diagnostic methods of intestinal parasitosis in school age children in Ethiopia. BMC Res Notes. 2015;8(1):820. doi: 10.1186/s13104-015-1822-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gelaw A, Anagaw B, Nigussie B, et al. Prevalence of intestinal parasitic infections and risk factors among schoolchildren at the University of Gondar Community School, Northwest Ethiopia: a cross-sectional study. BMC Public Health. 2013;13(1):304. doi: 10.1186/1471-2458-13-304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Matthys B, Bobieva M, Karimova G, et al. Prevalence and risk factors of helminths and intestinal protozoa infections among children from primary schools in western Tajikistan. Parasit Vectors. 2011;4(1):195. doi: 10.1186/1756-3305-4-195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alamneh Abera EN. Prevalence of gastrointestinal helminthic infections and associated risk factors among schoolchildren in Tilili town, northwest Ethiopia. Asian Pac J Trop Med. 2014;525:530. [DOI] [PubMed] [Google Scholar]
- 36.Dessie A, Gebrehiwot TG, Kiros B, Wami SD, Chercos DH. Intestinal parasitic infections and determinant factors among school-age children in Ethiopia: a cross-sectional study. BMC Res Notes. 2019;12(1):777. doi: 10.1186/s13104-019-4759-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jejaw A, Zemene E, Alemu Y, Mengistie Z. High prevalence of Schistosoma mansoni and other intestinal parasites among elementary school children in Southwest Ethiopia: a cross-sectional study. BMC Public Health. 2015;15(1):600. doi: 10.1186/s12889-015-1952-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alemayehu B, Tomass Z, Wadilo F, Leja D, Liang S, Erko B. Epidemiology of intestinal helminthiasis among school children with emphasis on Schistosoma mansoni infection in Wolaita zone, Southern Ethiopia. BMC Public Health. 2017;17(1):587. doi: 10.1186/s12889-017-4499-x [DOI] [PMC free article] [PubMed] [Google Scholar]