Abstract
Development of highly effective non-viral gene delivery vectors for transfection of diverse cell populations remains a challenge despite utilization of both rational and combinatorial driven approaches to nanoparticle engineering. In this work, multifunctional polyesters are synthesized with well-defined branching structures via A2 + B2/B3 + C1 Michael addition reactions from small molecule acrylate and amine monomers and then end-capped with amine-containing small molecules to assess the influence of polymer branching structure on transfection. These Branched poly(Ester Amine) Quadpolymers (BEAQs) are highly effective for delivery of plasmid DNA to retinal pigment epithelial cells and demonstrate multiple improvements over previously reported leading linear poly(beta-amino ester)s, particularly for volume-limited applications where improved efficiency is required. BEAQs with moderate degrees of branching are demonstrated to be optimal for delivery under high serum conditions and low nanoparticle doses further relevant for therapeutic gene delivery applications. Defined structural properties of each polymer in the series, including tertiary amine content, correlated with cellular transfection efficacy and viability. Trends that can be applied to the rational design of future generations of biodegradable polymers are elucidated.
Keywords: non-viral, gene delivery; polymeric nanoparticle; transfection; branched polymer, plasmid DNA
Graphical Abstract:
1. Introduction
Safe and effective gene delivery to specific cell populations has the potential to revolutionize medicine by enabling gene expression to be turned on or off precisely with the delivery of DNA or RNA. While viral vectors, particularly adeno-associated virus (AAV), have shown gains in the therapeutic delivery of DNA in some diseases, clinical level production of AAV remains an enormous challenge,1–2 nucleic acid carrying capacity is limited, and patient existing immunity can limit eligible patient populations.3–4 In contrast, non-viral nanoparticle based gene delivery methods have the potential to be both less expensive to produce, less immunogenic, and enable greater nucleic acid cargo capacity than AAV. However, non-viral gene delivery systems have suffered from low delivery efficacy to many cell types due to both systemic and intracellular delivery inefficiencies, which prevent translation to the clinic.5 While non-viral vectors have been demonstrated capable for effective delivery in vivo, there remains a need to develop enhanced nanoparticles that are more efficient, particularly for applications in which the administration route limits the dose.
Polyesters are a class of polymers that have been utilized for non-viral gene delivery with high efficacy both in vitro and in vivo to a variety of cell types.6–9 Synthesis of poly(beta-amino ester)s (PBAEs) in particular via Michael addition reactions is relatively easy to achieve and vast libraries of linear polymers have been synthesized to explore the solution space of possible polymer structures for purposes of gene delivery.10–12 Until recently, however, only linear PBAEs have been explored for their ability to deliver nucleic acids to mammalian cells, despite the demonstration that branching polymers are often more effective than their linear counterparts for delivery of plasmid DNA in a variety of polymer systems such as polyethyleneimine (PEI),13 and poly(2-dimethylaminoethyl methacrylate) (PDMAEMA).14, 15 Recent advances in the use of triacrylate monomers to synthesize branched polymers by Michael addition reaction have yielded polymers highly effective for delivery of nucleic acids to a variety of cell types, including cancer cells14–15, skin cells16, neural cells17 and mesenchymal stem cells.17 Much of this prior work in the synthesis of branched PBAEs has either failed to assess the efficacy of branched polymers against linear polymers across the entire range of possible w/w ratios or has only utilized linear polymer structures of insufficiently high molecular weight and cationicity to achieve effective gene delivery.16,19
Polyesters with beta-amino groups are rapidly biodegradable and finely tunable for properties such as hydrophobicity, molecular weight and cationic charge by selection of constituent monomers. These features enable certain structures to be highly effective for gene delivery but often require large empirical screens to identify effective structures. The biodegradability of PBAEs in aqueous solution is uncharacteristically short for polyesters with typical bond half-lives of 4–6h for the backbone ester bonds,18 enabling the polymers to degrade to non-toxic, hydrophilic oligomers within 24 hours. Hydrophobicity can be modulated for transfection of different cell types19 and molecular weight can be modulated by tuning the overall vinyl to amine ratio.11, 20 Linear acrylate-terminated PBAE polymers can also be end-capped with a variety of small molecule primary amines that increase the cationic charge of the polymer by adding secondary as well as primary amines to the polymer.21
Whereas with polyethylenimine (PEI), branching structure changes the cationic character of the polymer (linear polymers contain mostly secondary amines while branched polymers contain a tertiary amine at each branch point and a primary amine at each new terminal group), branching in a PBAE synthesis scheme does not dramatically change tertiary amines present in polymer structures of the same molecular weight. On the other hand, for PBAEs, branching structure can increase the density of end-capping functional groups, and these molecules have been shown previously to greatly enhance the transfection efficacy of linear polymers.18, 21 Branching in other polymeric systems has been further hypothesized to enhance the “needle effect” of endosomal escape mediated by polymer swelling, which could help explain this increase in efficacy.22–24
Here we present the synthesis and characterization of a new polymer series, Branched poly(Ester Amine) Quadpolymers (BEAQs). They are composed of four constituent monomers in ratios that influence the cationic character and hydrophobicity of the polymer species in a predictable manner. This work builds on the successes of poly(ester amine) materials such as linear PBAEs,12 poly(amine-co-ester) (PACE) terpolymers,25 and poly(alkylene maleate mercaptamines) (PAMA)s26 that have demonstrated the utility of amines to bind nucleic acids, ester linkages to facilitate nucleic acid release and reduce toxicity as well as the ability to modulate cation density and hydrophobicity. We utilized A2+B2/B3 Michael addition reactions to synthesize primarily acrylate terminated polymers with well-defined degrees of branching that were then end-capped with a C monomer to explore the influence of branching structure on transfection efficacy and nanoparticle properties. This further enabled us to incorporate fine control of small amine-containing molecule end-groups for engineering of polymer and nanoparticle surface properties and hypothesized cell-specific delivery.18, 27–29 Thus, the four components of the quadpolymers control degradability, hydrophobicity, branching, and cationicity which have large effects on delivery efficacy and cytotoxicity.30 We assessed each polymer quantitatively for plasmid DNA binding under various conditions to demonstrate that increased DNA binding is attributable to increased cationicity resulting from multiple end-caps as well as branching structure. Branching was further shown to improve DNA binding and transfection efficacy under conditions that normally destabilize polyplex nanoparticles.
2. Experimental
2.1. Materials:
Trimethylolpropane triacrylate (TMPTA/B8, CAS 15625895), Bisphenol A glycerolate (1 glycerol/phenol) diacrylate (BGDA/B7), CAS 4687–94-9) and 2-(3-Aminopropylamino)ethanol (E6, CAS 4461–39-6) were purchased from Sigma Aldrich and used without further purification. 4-amino-1-butanol (S4, CAS 13325–10-05) was purchased from Alfa Aesar. Acrylate monomers were stored with desiccant at 4°C, while amine monomers were stored with desiccant at room temperature. Plasmid peGFP-N1 (Addgene 2491) was used for transfection efficacy screens. Cy5-amine (230C0) was purchased from Lumiprobe (Hallandale Beach, FL), dissolved in DMSO at a concentration of 10 μg/μL and stored at −20°C in small aliquots. Plasmid DNA (eGFP-N1) was labeled as previously described using NHS-Psoralen with the fluorophore Cy5-amine at a density of approximately 1 fluorophore/50 base pairs DNA.31
2.2. Polymer synthesis:
BEAQs were synthesized according to the ratios in Table S1 at an overall vinyl:amine ratio of 2.2:1 and monomer concentration of 200 mg/mL in anhydrous DMF. The diacrylate monomer (B7) was first weighed out to a 20 mL scintillation vial, after which triacrylate monomer (B8) was added. Anhydrous DMF was added to the vial and monomers were fully vortexed into solution and heated to 90°C before adding primary amine monomer S4. Monomer purity was accounted for in synthesis calculations based on the vendor characterization of each lot. Monomer B7 was assumed to be 90% pure in the absence of any reported purity information. Monomer solutions were then stirred at 90°C for 24h, after which polymers were removed from the oven and mixed with a solution of monomer E6 (2-(3-Aminopropylamino)ethanol) in anhydrous DMF (final concentration 0.2 M) in the dark at room temperature for 1h. End-capped polymer solutions were then precipitated twice in diethyl ether (10x volume followed by 5x volume) and dried under vacuum for three days. Polymers were finally re-dissolved in anhydrous DMSO at 100 mg/mL and stored at −20°C in small volume aliquots. Polymers were named according to the triacrylate mole fraction; thus B8–50% corresponds to the 50% triacrylate mole fraction polymer formed between the diacrylate (B7), triacrylate (B8), amino (S4) and diamino (E6) monomers with the triacrylate (B8) monomer accounting for 50% of the vinyl moieties in the initial monomer mixture.
2.3. Polymer characterization:
Acrylate terminated polymers were sampled from reaction vials prior to end-capping reactions and precipitated twice in 10x volumes of diethyl ether to recover neat polymer. Acrylate terminated polymers were then dried under vacuum for 2h and analyzed via 1H NMR in CDCl3 (Bruker 500 MHz) to confirm the presence of acrylate peaks and quantify degree of branching. End-capped polymer likewise was characterized via 1H NMR in CDCl3 to confirm complete reaction of end-cap monomer with acrylate terminated polymers. End-capped polymer was also characterized via gel permeation chromatography (GPC) using a Waters system with autosampler, styragel column and refractive index detector to determine MN, MW and polydispersity index (PDI) relative to linear polystyrene standards. GPC measurements were performed as previously described with minor changes of flow rate (0.5 mL/min) and increase in sample run time to 75 minutes per sample.32
2.4. Polymer buffering capacity:
End-capped polymer buffering capacity as a function of polymer structure was assessed by titrating 10 mg (100 μL at 100 mg/mL) of polymer dissolved in 10 mL of acidified, 100 mM NaCl from pH 3.0 to pH 11.18 For titrations, pH was determined using a SevenEasy pH Meter (Mettler Toledo) with pH assessed after stepwise addition of 100 mM sodium hydroxide.
2.5. Polymer solubility limit:
Polymers were dissolved in pH 7.4, 150 mM PBS or pH 5.0, 25 mM NaAc at the specified maximum concentration and aliquoted (50 μL) to a round bottom 96 well plate (n=3 wells). Polymers were then diluted stepwise in their respective buffers and absorbance measurements were acquired with a plate reader (Biotek Synergy 2) at 600 nm (for opacity indicative of solubility limit). Absorbance measurements of 0.5 were defined as the maximum solubility point for purposes of plotting polymer solubility (Figure S2).
2.6. DNA binding assays:
Yo-Pro-1 iodide binding assays were run similarly to previously published results,33 where DNA and Yo-Pro-1 iodide (Thermo Fisher) were both diluted to a concentration of 1 μM (3.1 μg/mL plasmid) in either 25 mM NaAc, pH 5.0 or 150 mM PBS, pH 7.4 then mixed with polymer to give a 100 μL well volume in opaque black well plates. Green channel fluorescence was then measured using a plate reader after 30 minutes of incubation (Biotek Synergy 2). Gel electrophoresis binding experiments were run as previously described9 with nanoparticles prepared in either 25 mM NaAc buffer, pH 5.0 or 150 mM PBS, pH 7.4, diluted with 30% glycerol for loading into a 1% agarose gel.
2.7. Nanoparticle characterization:
Three samples were independently prepared for each nanoparticle formulation at the same concentrations as outlined in the transfection methods section. Nanoparticle hydrodynamic diameters in 25 mM NaAc, pH 5.0 were then determined by dynamic light scattering (DLS) in disposable micro-cuvettes using a Malvern Zetasizer NanoZS (Malvern Instruments, Marlvern, UK) with a detection angle of 173°. Samples were then diluted in 150 mM PBS at a dilution factor of 6 and measured again to determine nanoparticle hydrodynamic diameter in neutral, isotonic buffer followed by determination of zeta potential by electrophoretic light scattering in disposable zeta cuvettes at 25°C using the same Malvern Zetasizer NanoZS.
Transmission electron microscopy (TEM) images were acquired using a Philips CM120 (Philips Research, BriarcliffsManor, New York) on 400 square mesh carbon coated TEM grids. Samples were prepared at a DNA concentration of 0.045 μg/μL and polymer 40 w/w ratio in 25 mM NaAc, pH 5.0 after which 30 μL were allowed to coat TEM grids for 20 minutes. Grids were then dipped briefly in ultrapure water to remove excess dried salt, wicked dry and allowed to fully dry under vacuum before imaging.
2.8. Cell culture:
HEK293T and ARPE-19 cells were purchased from ATCC (Manassas, VA) and cultured in high glucose DMEM or DMEM/F12 respectively, supplemented with 10% heat inactivated fetal bovine serum and 1% penicillin/streptomycin. For noted 96-well plate transfection efficacy experiments, cells were plated in CytoOne 96-well tissue culture plates (USA Scientific, Ocala, FL) 24h prior to transfection with 12,000 cells/well in 100 μL complete media. For noted 384-well plate transfection experiments, cells were plated at 2,500 cells/well in 25 μL complete media in 384 well tissue culture plates (Santa Cruz, sc-206081) 24h prior to transfection. Cells were confirmed periodically to be mycoplasma negative via MycoAlert test (Lonza).
2.9. Transfection and cell uptake:
For 96 well plate transfections, nanoparticles were formed by dissolving synthesized polymers and eGFP-N1 plasmid DNA in 25 mM sodium acetate (NaAc) pH 5.0 then mixing in a 1:1 volume ratio. Nanoparticles were incubated at room temperature for five minutes, then 20 μL of the nanoparticle solution were added to each well of cells containing 100 μL of complete media and allowed to incubate for two hours, at which point the media was replaced. Transfection efficacy was assessed for percent-transfected cells and geometric mean expression approximately 48h following transfection using flow cytometry with a BD Accuri C6 flow cytometer with HyperCyt autosampler and gated in 2D against untreated cells in FlowJo (Figure S19). Cell viability was assessed using MTS Celltiter 96 Aqueous One (Promega, Madison, WI) cell proliferation assay approximately 24h following transfection. For 384 well plate transfection of low doses of nanoparticles, synthesized polymers in DMSO were dissolved in 25 mM NaAc buffer to a concentration of 7.5 μg/μL then mixed with DNA dissolved in 25 mM NaAc buffer in a 384 polypropylene nanoparticle source plate. Nanoparticles were then dispensed to plates of cells at low volumes using an Echo 550 liquid handler. After two days to allow for reporter expression, plates were scanned and analyzed using Cellomics Arrayscan VTI with live cell imaging module following staining with Hoechst 33342. Flow cytometry based cell uptake studies were performed in 96 well plates using 20% Cy5 labeled DNA as previously described.32. To remove associated nanoparticles that were extracellular membrane associated but had not undergone endocytosis, cells were washed once with 50 μg/mL heparin sulfate in 150 mM PBS following trypsinization and transfer to round bottom 96 well plates.32
2.10. Confocal microscopy.
Cells were plated on Nunc Lab-Tek 8 chambered borosilicate coverglass well plates (155411; Thermo Fisher) at 50,000 cells/well (ARPE-19) or 25,000 cells/well (HEK293T) two days prior to transfection in 250 μL phenol red free DMEM supplemented with 10% FBS and 1% penicillin/streptomycin. Nanoparticles were prepared as described above at 20 or 40 w/w ratios using Cy5 labeled plasmid DNA and eGFP-N1 plasmid DNA at an 0.8/0.2 mass ratio, then added to cells at a total dose of 1500 ng DNA/well and incubated for two hours. For imaging, cells were stained for 30 minutes with Hoechst 33342 at a 1:5000 dilution (H3570; Thermo Fisher) for nuclei visualization, as well as Cell Navigator Lysosome Staining dye with pKa 4.6 at a 1:2500 dilution (AAT Bio-quest, 22658) in phenol red free DMEM. Cells were then washed twice with phenol red free DMEM and imaged at 37°C in a 5% CO2 atmosphere. Images were acquired using a Zeiss LSM 780 microscope with Zen Blue software and 63x oil immersion lens. Specific laser channels used were 405 nm diode, 488 nm argon, 561 nm solid-state, and 639 nm diode lasers. Laser intensity and detector gain settings were maintained across all image acquisition. All Z-stacks were acquired for entire cell volume over scan area of 140 μm at Nyquist limit resolution.
2.11. Data analysis and figures:
FlowJo was used for flow cytometry analysis and Cellomics HCS Studio (Thermo Fisher) was used for image acquisition based transfection analysis. Polymer structures were characterized in ChemDraw (Perkin Elmer, Boston, MA) and Marvin (ChemAxon, Cambridge, MA) to determine logP and logD values. Calculation of normalized 50% serum transfection efficacy was performed by dividing the percent transfection or geometric mean transfection efficacy achieved in 50% serum media by the same nanoparticle (B8% and w/w ratio) formulation percent transfection or geometric mean transfection efficacy achieved in 10% serum. Confocal microscopy colocalization of plasmid DNA with lysosomes was assessed as intensity weighted colocalization in Zen Blue, then normalized by individual image area of plasmid DNA per image for statistical quantification.
2.12. Statistics:
Prism 8 (Graphpad, La Jolla, CA) was used for all statistical analyses and curve plotting. Unless otherwise specified, statistical tests were performed with a global alpha value of 0.05. Unless otherwise stated, absence of statistical significance markings where a test was stated to have been performed signified no statistical significance. Statistical significance was denoted as follows: *p<0.05; **p<0.01; ***p<0.001; ****p<0.0001.
3. Results
3.1. Branched poly(Ester Amine) Quadpolymer Synthesis and Characterization
3.1.1. Synthesis of Acrylate Terminated Polymers
A series of Branched poly(Ester Amine) Quadpolymers (BEAQ) with differential degrees of branching was synthesized via step-growth A2 + B2/B3 Michael addition reactions from a small molecule diacrylate (BGDA/B7), triacrylate (TMPTA/B8) and amino-alcohol (S4) monomers (Figure 1 and Table S1). In the synthesis scheme of A2 + B2/B3 + C, A2 corresponds to the primary amine monomer (S4) that can react twice, B2 corresponds to the diacrylate monomer (termed B7) that can react twice, B3 corresponds to the triacrylate monomer (termed B8) that can react three times and C refers to the end-cap monomer, which reacts once due to its presence in excess. We confirmed that each polymer was primarily acrylate terminated after 24 hours of synthesis via 1H NMR (Figure S1) by the presence of acrylate peaks between 5.5–6.5 ppm. Analysis of the acrylate terminated polymer structures with 1H NMR also enabled determination of polymer properties including the actual triacrylate mole-fraction of each polymer as well as number of end-cap moieties per polymer molecule (Table 1). By precisely varying the triacrylate monomer mole fraction, while maintaining the same 2.2:1 vinyl to amine mole ratio, the degree of branching was able to be carefully modulated in the resulting polymers as assessed by 1H NMR. Further, by synthesizing the polymers in each series at the same purity-accounted overall vinyl to amine ratio, the number average (MN) molecular weights within each series of polymers were all very close to 4 kDa as shown by gel permeation chromatography (GPC) (Table 1).
Figure 1.
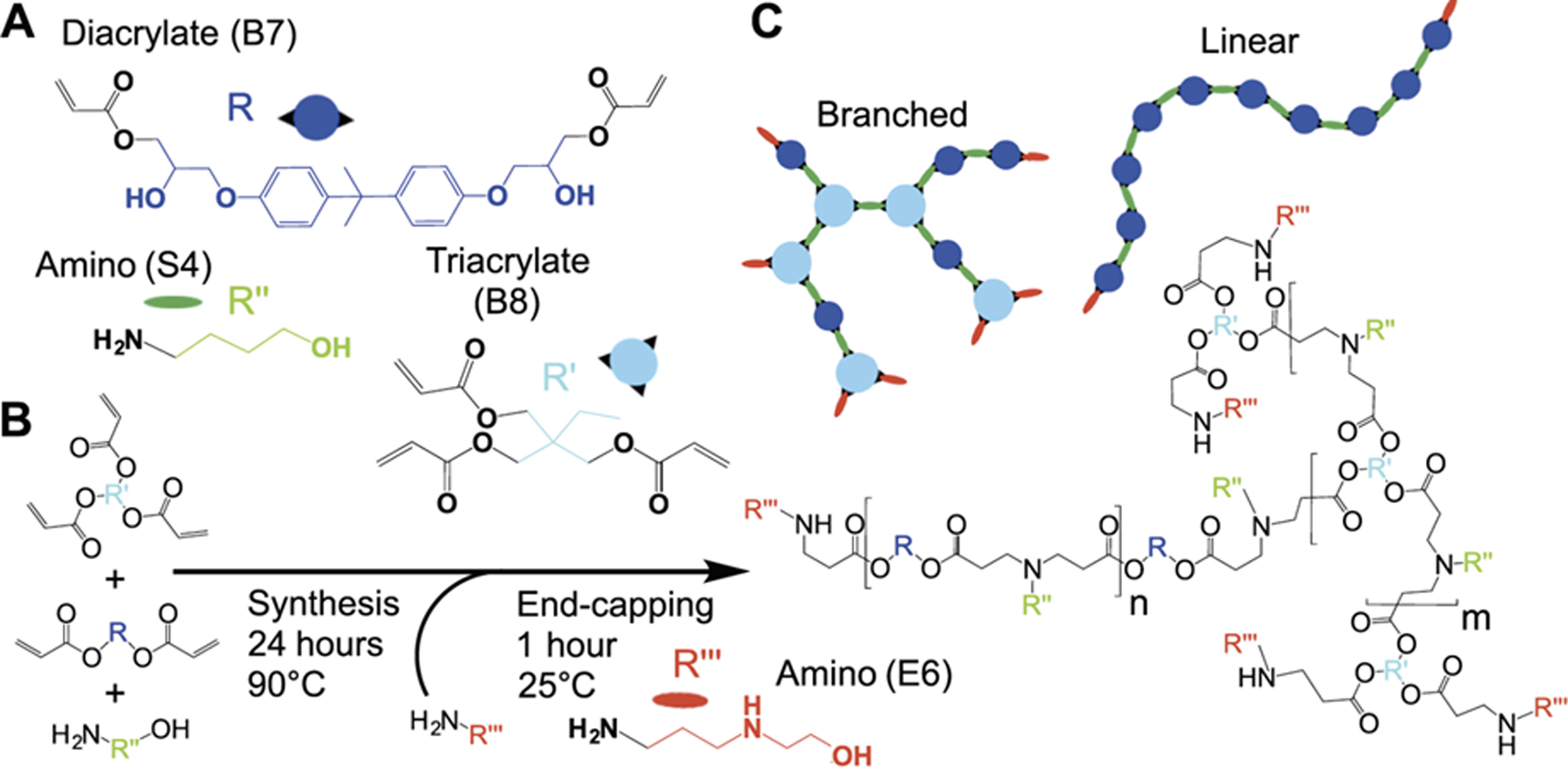
Synthesis of Branched poly(Ester Amine) Quadpolymers (BEAQ). A) Diacrylate monomer B7 and triacrylate monomer B8 were mixed with side-chain monomer S4 to synthesize a series of BEAQs with increasing triacrylate mole fraction and degree of branching. B) Linear polymers possess two end-cap structures per molecule (red), while each triacrylate monomer in branched polymers results in an additional end-cap moiety for every branch point. C) One-pot synthesis of acrylate terminated base polymers was performed at 90°C and 200 mg/mL in DMF for 24 hours. Polymers were then end-capped with monomer E6 at room temperature for one hour to yield the final product.
Table 1.
Structural properties of synthesized polymers
| Triacrylate Mole Fraction (%) | Theoretical End-caps per | End-cap Polymer Mass | GPC MN | GPC MW | GPC PDI | |
|---|---|---|---|---|---|---|
| Theoretical | Actual | Molecule | Fraction (%) | (Da) | (Da) | |
| 0 | 0.0 | 2.0 | 5.7 | 4700 | 5700 | 1.203 |
| 10 | 15.1 | 3.0 | 9.1 | 4700 | 5700 | 1.216 |
| 20 | 22.8 | 3.4 | 11.5 | 4200 | 5200 | 1.258 |
| 40 | 34.8 | 3.9 | 15.0 | 4200 | 5100 | 1.223 |
| 50 | 47.1 | 4.9 | 17.1 | 4200 | 5800 | 1.369 |
| 60 | 58.5 | 4.5 | 22.0 | 4200 | 5900 | 1.411 |
| 80 | 83.3 | 5.5 | 27.5 | 4800 | 18300 | 3.849 |
| 90 | 91.7 | 6.5 | 28.2 | 3100 | 21600 | 6.952 |
3.1.2. End-cap Modification of Polymers
PBAEs have been “end-capped” with small molecule monomers possessing secondary and tertiary amines that increase the overall polymer amine density, resulting in linear polymers with tertiary amines along the polymer backbone and greater amine density at just the two ends of the linear polymers.12, 21, 34–35 Most of the small molecule end-caps shown previously to increase transfection efficacy with linear PBAE structures21 increase the cationicity of the polymer at both pH 5 and 7 due to the fact that end-capping with primary amine monomers adds at minimum two secondary amines to linear PBAEs. Here, we utilized monomer 2-(3-aminopropylamino)ethanol (termed E6) for end-capping purposes, as it has been shown to be effective as an end-capping group with linear polymers and non-cytotoxic to multiple cell lines.33,38 In contrast to previously reported branched polymer schemes, including branched PBAE schemes, this end-capping molecule exclusively increases the secondary amine content of the polymer. All BEAQs were confirmed to be completely end-capped by 1H NMR and the number average of end-cap moieties per polymer molecule as estimated from NMR spectra ranged from two for the linear polymer to seven for the 90% triacrylate mole fraction polymer (Table 1). Notably, end-cap molecular mass fraction contribution in these polymers reaches near 30% for the high triacrylate mole fraction polymers, whereas linear PBAEs have an end-cap monomer mass fraction of approximately 5%, which reduces further for higher molecular weight linear polymers (Table 1). Polydispersity in moderately branched BEAQs was minimized by synthesizing at a dilute concentration, while high polydispersity of hyperbranched BEAQs with triacrylate mole fraction >60% is consistent with other hyperbranched polymer synthesis schemes.36
3.1.3. Polymer Series Hydrophobicity
The chemical properties of each polymer in the series with known Mn and monomer composition were predicted in silico to assess the influence of branching with TMPTA on polymer hydrophobicity. Hydrophobicity was assessed as predicted partition coefficient (logP) and ionization influenced distribution coefficient (logD) at neutral and acidic pH values (Figure 2a, S2), demonstrating that branching increases BEAQ hydrophilicity for the monomers utilized here, and that pH sensitive ionization plays an important role in polymer solubility. Branching was hypothesized to reduce both polymer logP and logD values as a greater number of E6 monomer end-cap moieties in branched structures increased the prevalence of hydrophilic hydroxyl groups and charged secondary amines; polymers with a high degree of branching were further subject to reduction in hydrophobicity due to the fact that the mass fraction of the diacrylate monomer B7, which contains a bisphenol group, was likewise reduced. We confirmed this predicted reduction in hydrophobicity experimentally via an absorbance based assay, to show that BEAQs with at least 40% triacrylate mole fraction were over twice as soluble as the linear B8–0% polymer under both low pH and physiological pH conditions (Figure S2).
Figure 2.
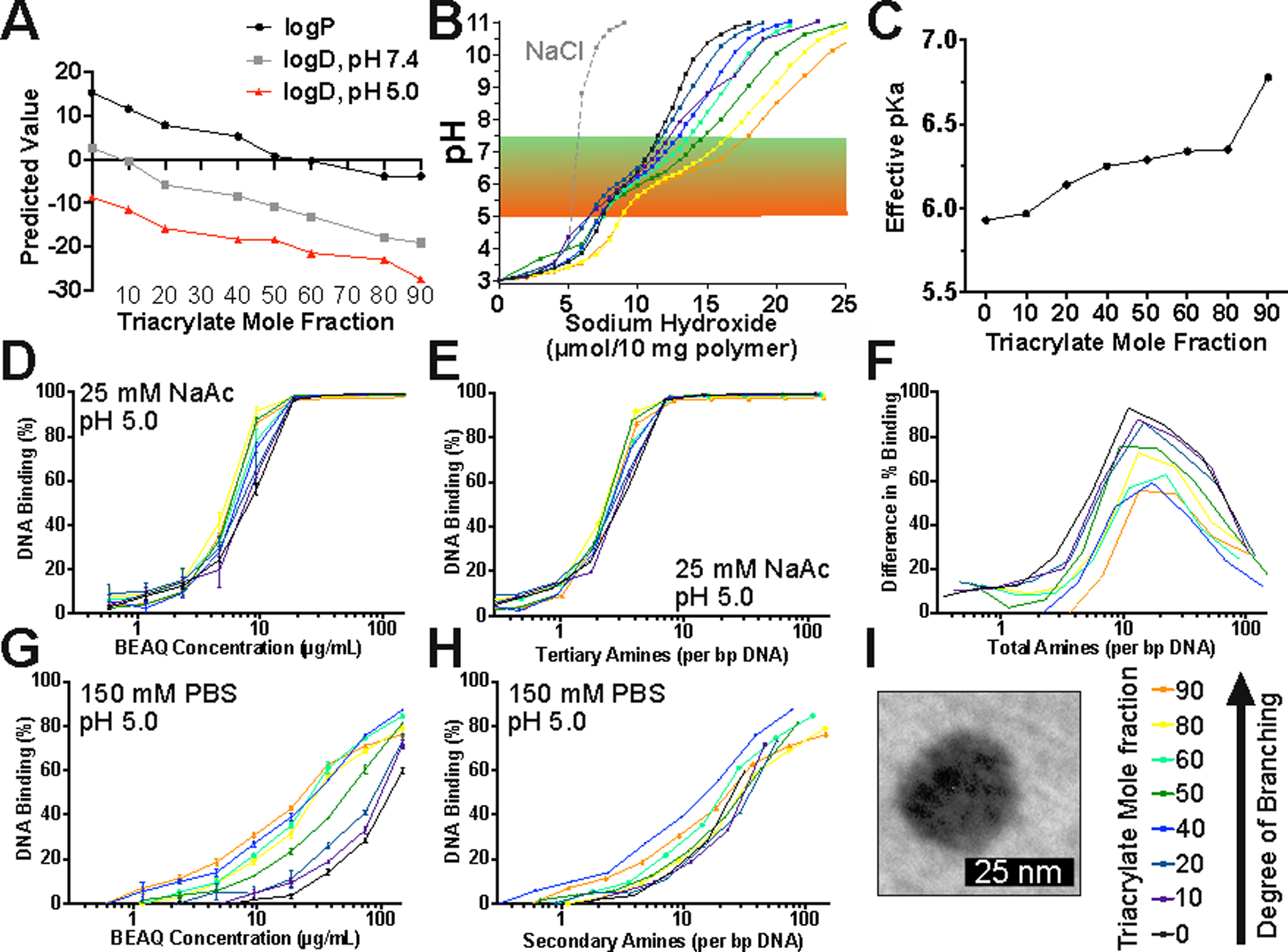
Polymer properties influenced by triacrylate mole fraction. A) Predicted properties of partition coefficient (logP) and distribution coefficient (logD) for differentially branched polyesters. B) Titration of BEAQs. C) Effective pKa value of maximum buffering point between pH 4.5–8.5 of differentially branched PBAEs. D) Competition binding assay of polymer and Yo-Pro-1 iodide at low pH. (n=3 wells, mean ± SEM) E) DNA binding in low pH buffer normalized to PBAE tertiary amine content. F) Difference in degree of binding between pH 5 and pH 7.4 calculated as a function of total amines per bp DNA. G) Competition DNA binding assay in isotonic, neutral buffer. (n=3 wells, mean ± SEM) H) DNA binding in isotonic, neutral buffer normalized to secondary amine content. I) TEM image of 20% triacrylate mole fraction polymer nanoparticles.
3.1.4. Polymer Series Buffering Capacity
Titration of the polymers demonstrated buffering capacity in the physiological pH range for hypothesized endosomal escape properties (5 to 7.4), as BEAQs with greater triacrylate mole fraction possessed a larger buffering capacity in this range (Figure 2B). Effective pKa in the pH range from 5 to 8 was calculated as the pH at the maximum normalized buffering capacity of the derivative of the titration curves defined as Δ(-OH)/Δ(pH) (Figure S2B). Effective pKa was demonstrated to increase moderately with increased branching from approximately 6.0 to 6.75 (Figure 2C). These results are due to the combined effects of additional tertiary amine density in the polymer backbone and the presence of additional secondary amines in end-groups as the branching increases.18 Tertiary amine density calculated relative to the base polymer structures (Table S4) shows that diacrylate B7+S4 polymer repeat units have much lower tertiary amine density than triacrylate B8+S4*2 repeat units and physical spacing of the tertiary amines in high diacrylate B7 content polymers is greater than for high triacrylate B8 content polymers. But following end-capping with monomer E6, tertiary amine density is similar amongst all synthesized polymers while secondary amine density increased substantially with triacrylate mole fraction from 0.851 to 4.194 mMol per gram polymer for B8–0% and B8–90% respectively (Table S5).
3.1.5. Polymer Series DNA Binding
Assessment of BEAQ/DNA binding strength interactions via Yo-Pro-1 iodide competition binding assays further demonstrated the influence of branching in polymer structure (Figure 2D,F). At pH 5, linear and branched polymers were equally effective at binding plasmid DNA, while in isotonic, neutral buffer at pH 7.4, branched polymers statistically outperformed linear polymers for DNA binding (Table S6). To assess if increases in DNA binding strength of the BEAQs were attributable primarily to branched structure or changes in amine content, we calculated Yo-Pro-1 iodide quenching as a function of secondary, tertiary, and total amine content per base-pair DNA from known structural characteristics of each polymer (Figure S3). DNA binding normalized to tertiary amine content effectively condensed the binding assay results at pH 5, while normalization of DNA binding in neutral, isotonic buffer to secondary amine content most effectively condensed the results to fit one curve (Figure 2E,G). Gel electrophoresis DNA retention assays were similarly in agreement with these results, demonstrating that branching improved DNA binding particularly in neutral, isotonic buffer (Figure S4). These results indicate that BEAQ backbone tertiary amines play an important role in polymer complexation with DNA at low pH, but secondary amines in BEAQ end-cap structures are primarily responsible for binding plasmid DNA following dilution into neutral solutions.
Further analysis of the difference between binding at low pH and neutral pH do, however, reveal that the increase in end-cap density of branched polymers was not exclusively responsible for increased binding at neutral pH. Scaling the difference in binding efficacy as a function of total amines per base pair DNA revealed that branched polymers were more effective at maintaining DNA binding in a manner that is attributable to structural changes instead of increases in amine content (Figure 2H).
3.2. Nanoparticle Properties
Dynamic light scattering (DLS) measurements of polymer/DNA polyplex nanoparticles to assess hydrodynamic diameter demonstrated effective independence of nanoparticle properties with regards to branching. DLS measurements of polymeric nanoparticles formed in 25 mM NaAc, pH 5.0 at a 40 w/w ratio to DNA showed that all polymers formed nanoparticles with a hydrodynamic diameter of approximately 50–100 nm that maintained a diameter of approximately 100 nm following a 6-fold dilution into 150 mM PBS (Figure S5A). All nanoparticle formulations showed similar zeta potential values of approximately +15 mV (Figure S5B). Select formulations were analyzed via TEM, which showed dried nanoparticle diameters between 30–60 nm (Figure S5C). Notably, the linear 0% triacrylate mole fraction (B8–0%) particles were the smallest when assessed by TEM at 32±3 nm, compared to a mean of 54±6 nm for B8–50% nanoparticles, which may be attributable to slightly stronger intermolecular polymer interactions driven by increased hydrophobic effect for the less branched polymers with higher B7 fraction / lower triacrylate monomer B8 fraction.
3.3. Cellular Transfection
3.3.1. Nanoparticle Uptake was Not Influenced by Branching Structure
We hypothesized that the increased number of end-cap moieties per polymer molecule would result in increased cellular uptake, as end-capping linear PBAEs has been demonstrated to improve cellular uptake compared to acrylate terminated and side-chain monomer terminated linear PBAEs.21 Further, end-cap structures have been shown to convey cell type specificity,21, 27 as well as partially contribute buffering capacity of PBAEs in the physiologically relevant pH range.18 To assess whether the increased number of end-cap moieties per polymer molecule for BEAQs would yield greater cell uptake relative to linear PBAEs, we assessed cellular uptake by flow cytometry of nanoparticles formed with Cy5 labeled plasmid DNA in HEK293T and ARPE-19 cells at moderate fluorophore labeling density. All polymers were generally effective for mediating cellular uptake of plasmid DNA, with above 95% of cells testing as positive for DNA uptake gated against the untreated cells (Figure S6). These branched polymers showed no significant improvement in cellular uptake at equivalent w/w ratios to the linear polymer. Thus, an increased number of 2-(3-Aminopropylamino) ethanol end-cap moieties per polymer molecule did not mediate higher cellular uptake as hypothesized.
3.3.2. BEAQ Nanoparticles Mediate High Transfection Efficacy
To assess the ability of BEAQs to effectively deliver plasmid DNA to both easier-to-transfect and difficult-to-transfect cell types, HEK293T cells and ARPE-19 retinal pigment epithelial cells were chosen for transfection studies with the reporter gene eGFP-N1. In these two cell lines, the BEAQs nanoparticles achieved up to 99% and 77% transfection efficacy respectively in complete medium as assessed by flow cytometry, which is greater than any reported transfection efficacy using non-viral methods in either cell line to the best of our knowledge (Figure 3).
Figure 3.
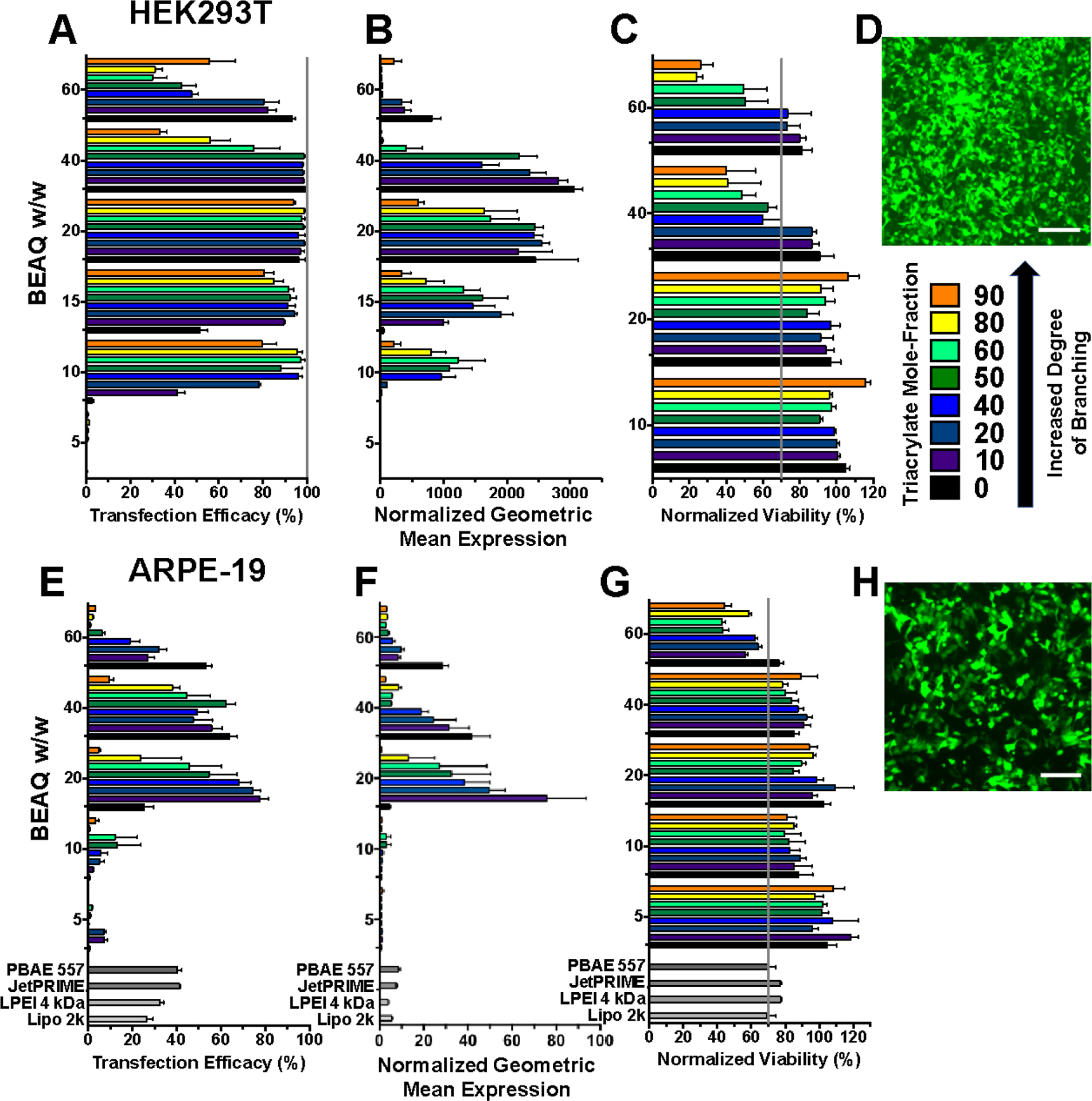
In vitro transfection with BEAQs in 10% serum media. HEK239T cells A) percent transfection efficacy, B) normalized geometric mean expression C) viability and D) fluorescence micrograph of cells transfected to express eGFP with the 20 w/w ratio, 50% triacrylate mole-fraction BEAQ. ARPE-19 cells E) percent transfection efficacy, F) normalized geometric mean expression, G) viability and H) fluorescence micrograph of cells transfected to express eGFP with 20 w/w, B8–20% triacrylate mole-fraction BEAQ. (Scale bars 200 μm. n = 4 wells, mean ± SEM).
Among commercial reagents we fully tested and optimized, including 25 kDa branched polyethlyenimine (BPEI), 4 kDa linear polyethylenimine (LPEI), JetPRIME® and Lipofectamine 2000® (Figure S7 and S8), JetPRIME gave the highest level of transfection in ARPE-19 cells at approximately 40% transfection with tolerable viability. Linear PEI gave slightly higher transfection, but at the cost of substantial cytotoxicity. The maximum level of transfection achieved in ARPE-19 cells with the reported BEAQ polymers is likewise higher than our previously optimized top linear PBAE 557 formulation, which we found transfected only 40–45% of these cells with keeping cytotoxicity <30%.37 This formulation was previously shown to lead to transfection in vivo following subretinal injection in mice, making it likely for these BEAQ nanoparticles to function in a similar manner in vivo.37
3.3.3. Moderate Branching in BEAQs Improves Stability in Physiological Serum Conditions
Effective delivery under physiological serum conditions remains a challenge for cationic nanoparticle based gene delivery, due to the shielding and aggregation effects of serum proteins.38 To assess nanoparticle performance under these conditions, the BEAQs were evaluated for transfection in HEK293T and ARPE-19 cells incubated in 50% serum medium during a two hour nanoparticle incubation (Figure S9). Under these challenging transfection conditions, which more closely model an in vivo systemic administration, BEAQs demonstrated remarkably statistically improved transfection efficacy compared to their linear counterparts, which was particularly pronounced at low w/w ratios in both cell lines (Figure 4A,B). The optimal BEAQ-50 branched polymer was capable of transfecting 98% and 65% of HEK293T and ARPE-19 cells under 50% serum conditions. After normalizing transfection efficacy results in 50% serum to matched results in 10% serum conditions, BEAQ nanoparticles reported here maintain 80% and 70% geometric mean expression in HEK293T cells and ARPE-19 cells with no reduction in percentage of cells transfected (Figure S10).
Figure 4.
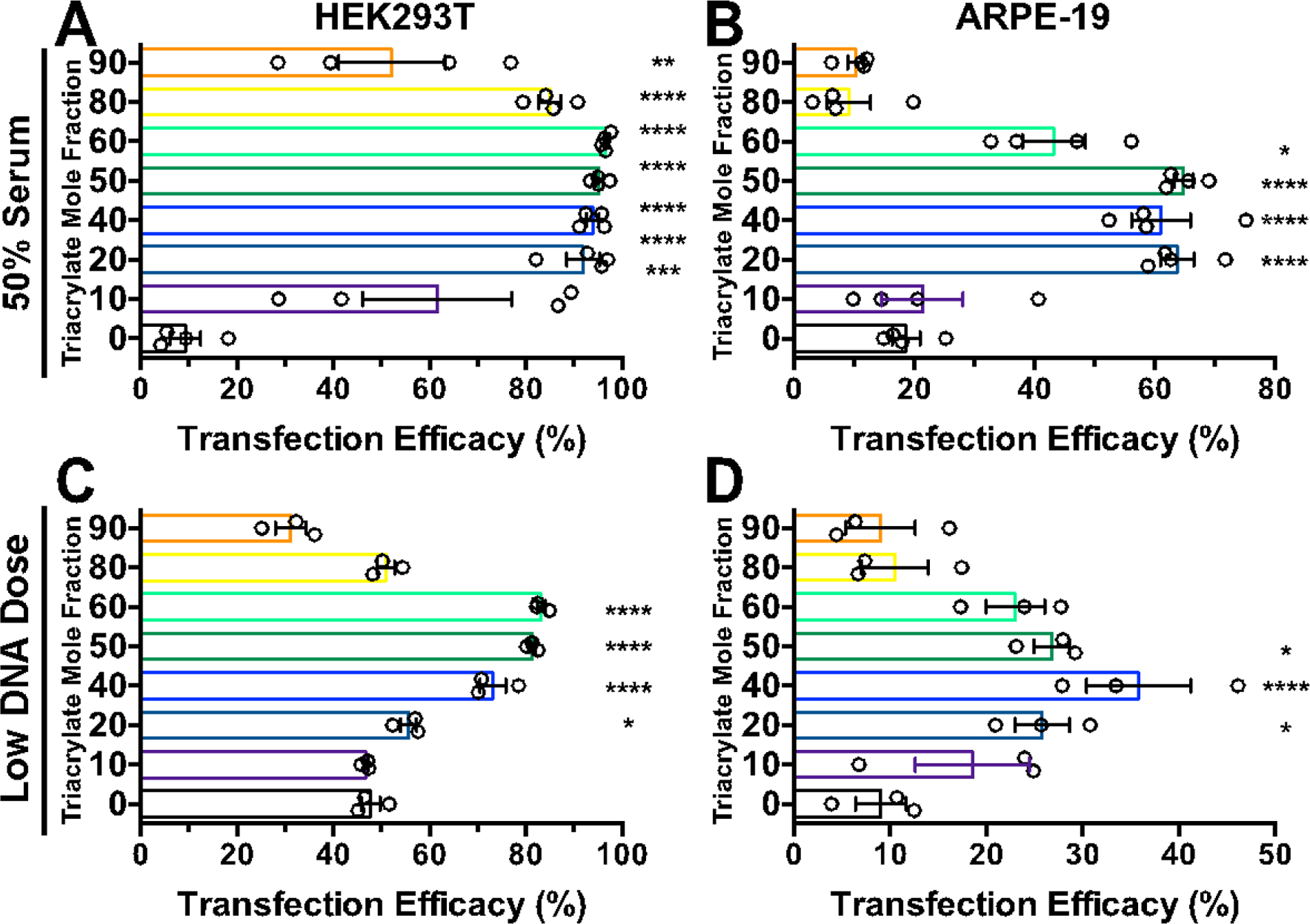
The effect of transfection conditions on BEAQs. High serum (50%) transfection of A) HEK293T and B) ARPE-19 cells with 20 w/w nanoparticles (normal DNA dose of 600 ng/well in 96 well plates). Low nanoparticle dose transfection with 40 w/w nanoparticles of C) HEK293T (5 ng) and D) ARPE-19 (10 ng) doses in 384 well plates. (n = 4 wells, mean ± SEM, statistical markings show results of one-way ANOVA with Dunnett corrected multiple comparisons tests to the linear polymer)
3.3.4. Moderate Branching Improves Transfection at Low Plasmid Doses
Transfection at low nanoparticle doses likewise better mimics conditions encountered in vivo following administration and dilution into biological fluids. At very low nanoparticle doses, plasmid concentrations between 16–256 pM (0.25–4 pg/cell) in 384 well plates, moderately branched triacrylate mole fraction BEAQs showed statistically higher transfection compared to the optimized corresponding linear PBAE in both cell lines (Figures S11,S12). Overall with statistical assessment at all w/w ratios tested, B8–40% and B8–50% performed the best in both cell lines. Optimal w/w ratio was notably shifted for low DNA dose transfections, such that 60 w/w BEAQ nanoparticles showed better transfection than 20 w/w particles at very low doses (≤5 ng/well). Cell viability was not strongly affected under any of the conditions.
3.4. Branching reduces degree of lysosomal accumulation following uptake
Transfection of HEK293T and ARPE-19 cells with Cy5-labeled plasmid DNA followed by assessment of lysosome colocalization with confocal microscopy at 4 and 24 hours following nanoparticle treatment demonstrated that less internalized DNA was colocalized with lysosomes when delivered by B8–50% BEAQs compared to the linear B8–0% polymer (Figure 5A). For accurate quantification of lysosomal colocalization throughout the entire cell volume, Z-stacks were acquired at both time-points and nanoparticle area per slice was used to scale the respective contribution to calculated z-stack lysosome correlation coefficient (Figure S13). Representative uncropped maximum intensity projection images of acquired Z-stacks for each condition show a high level of Cy5-DNA uptake with limited lysosome colocalization for all conditions (Figures S14 and S15). All nanoparticle formulations tested demonstrated a statistically significant increase in lysosome colocalization between 4 and 24 hours following nanoparticle treatment (Figure 5C), however, the degree of change in lysosome accumulation was lower with the B8–50% BEAQ nanoparticles, specifically for the higher 40 w/w ratio tested, which yielded less than 20% of internalized DNA as detectable in lysosomes at 24 hours in either cell type. The degree of lysosome colocalization for the linear B8–0% polymer at 24 hours (0.4) was still far below the colocalization we have previously measured for PLL (0.78) and BPEI (0.7), despite the ability of BPEI to much more effectively buffer protons on a per-unit basis.39 This result supports the notion that amphiphilic polyesters mediate lysosomal in a different manner than polyethylenimine, as their degree of lysosomal avoidance is not proportional to their buffering capacity. At 24 hours following nanoparticle treatment, cells expressing eGFP from the 20% unlabeled fraction of plasmid DNA were visible for all conditions (Figures S16 and S17). Cy5-labeled plasmid DNA was also detectable in the nucleus of some cells that typically were also strongly expressing eGFP at the 24-hour time point (Figure 6). Analysis of single slices from z-stacks did, however, reveal that most plasmid DNA internalized had not localized to the nucleus at 24 hours post-treatment, even when it avoided lysosomal degradation.
Figure 5.
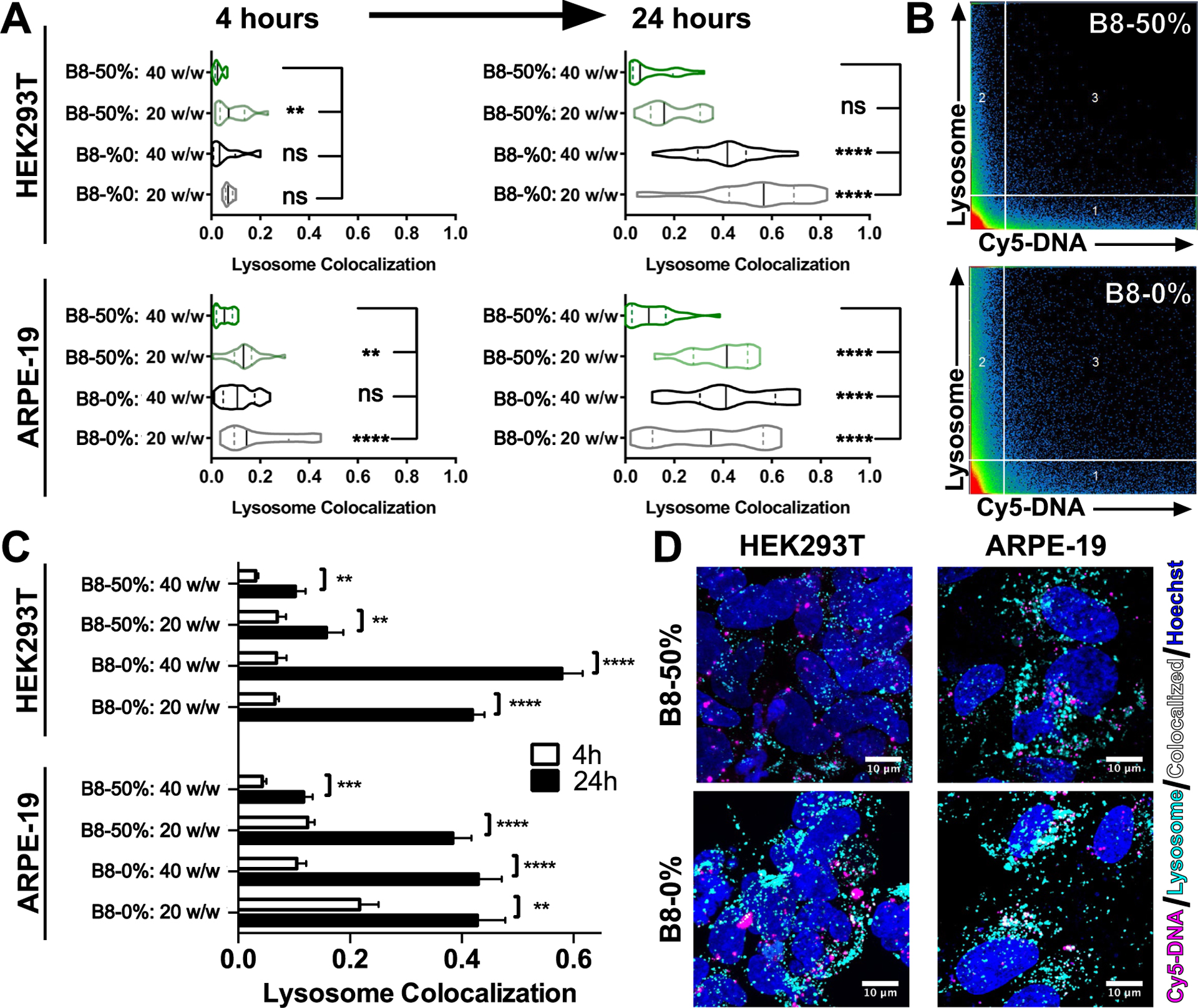
Lysosome colocalization assessment with confocal microscopy. A) Cells transfected with B8–0% and B8–50% at low (20 w/w) and high (40 w/w) nanoparticles and assessed by confocal microscopy show statistically significant differences in the degree of lysosome colocalization. Assessed by one-way ANOVA with Dunnett corrected multiple comparisons to the B8–50%: 40 w/w conditions. B) Representative 2D scattergrams of HEK293T cells at 24 hours post-treatment using 20 w/w nanoparticles. Region 3 represents colocalized pixel intensities. C) All conditions in both cell lines showed statistically significant (Holm-Sidak corrected multiple t-tests) increases in the degree of lysosome colocalization between 4 hours and 24 hours following transfection. (Bars show mean ± SEM of n>100 cells.) D) Representative maximum intensity projection images of cells transfected with 20 w/w nanoparticles 24 hours following transfection, showing lysosome colocalization in white.
Figure 6.
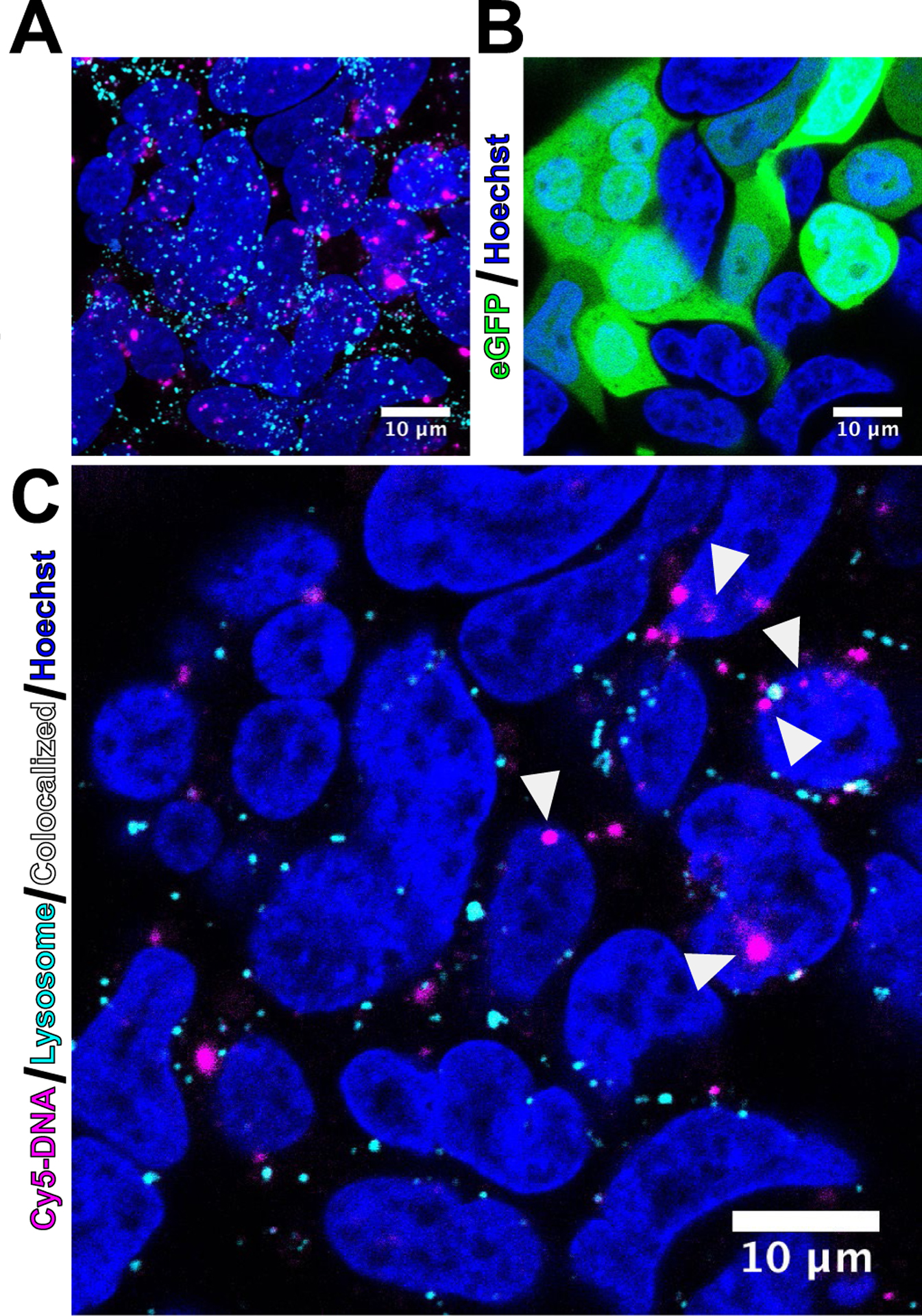
Nuclear localization of plasmid DNA and expression of eGFP assessed by confocal microscopy. HEK293T cells were transfected 24 hours prior with B8–50%: 20 w/w nanoparticles containing 80% non-coding, Cy5-labeled plasmid DNA and 20% coding eGFP-N1 plasmid DNA. A) Maximum intensity projection demonstrating high level of labeled plasmid DNA remaining in the cells with minimal lysosome colocalization. B) Strong eGFP expression from the 20% of unlabeled plasmid DNA. C) A single z-slice shows Cy5-labeled plasmid DNA localized to the nucleus in select cells (white arrows).
3.5. Trends in Transfection from Differentially Branched BEAQs
We analyzed transfection efficacy of each polymer over the multiple w/w ratios tested as functions of polymer concentration and known specific buffering capacity as well as secondary, tertiary and total amine content. To account for overall population expression and effects of polymers on viability, we scaled geometric mean expression values by viability and normalized to the maximum geometric mean expression value of each polymer structure to give viability normalized expression. Viability normalized expression was then plotted against each variable of interest (Figure 7). All BEAQs demonstrated clear biphasic trends in normalized geometric mean expression. Upon fitting a single quadratic curve to the data from all polymers, tertiary amine density as a function of tertiary amines per base pair DNA was revealed to be the most important chemical property for predicting optimal w/w ratio for transfection efficacy. Particularly, a single curve quadratic fit for all polymer data across all structures for HEK293T and ARPE-19 cells gave R2 values of 0.761 and 0.615 respectively.
Figure 7.
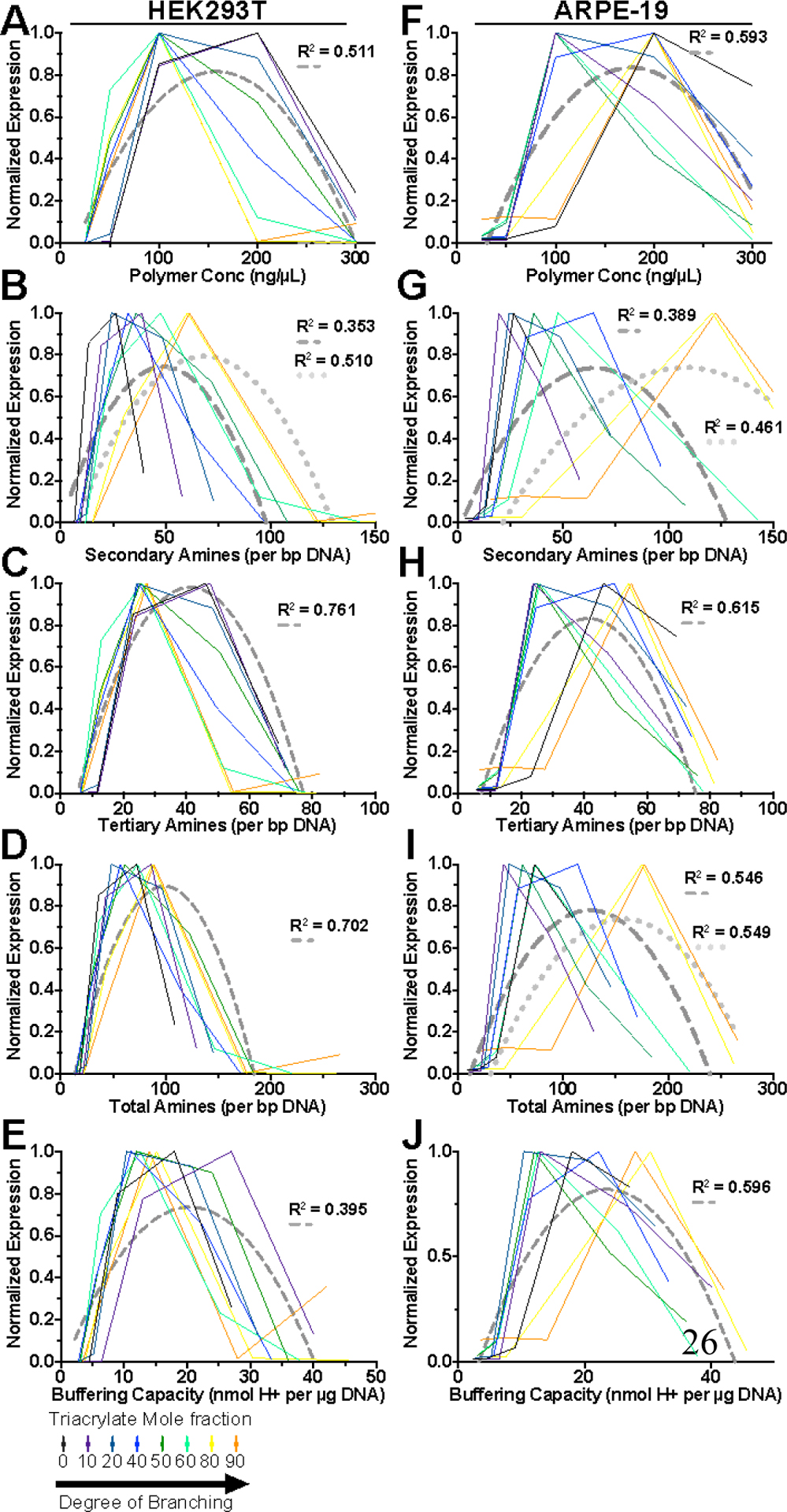
Correlation between BEAQ properties and viability normalized geometric mean expression. Geometric mean expression plots were normalized to the maximum expression for each polymer and scaled by viability at that w/w ratio for A-E) HEK293T cells and F-J) ARPE-19 cells. Dashed-grey curves show a single quadratic fit of all data points for that cell line with calculated R2. Plots showing dotted-grey curves in addition to dashed-grey curves were statistically determined to require two fitted quadratic curves to adequately describe the data.
Polyethylenimine did not exhibit the same biphasic trends between amine content and geometric mean expression as BEAQs, but did demonstrate optimal amine content of approximately 30 secondary amines which may be attributable to the greater cytotoxicity encountered with using PEI that limits utilization of high w/w ratios (Figure S18). Interestingly, highly branched 25 kDa BPEI had a much higher optimal total amines per bp DNA similar to the synthesized BEAQs, which may be attributable to the level of interaction between amines in linear polymers compared to branched polymers. Spatial accessibility of amines in polymer structures and steric hindrance in branched polymers may necessitate greater overall amine content.
4. Discussion
Branching has been demonstrated to yield enhanced transfection in many cationic polymer systems and studied in PBAEs through the use of monomers with trifunctional amine monomers40 or trifunctional triacrylate monomers for generation of branched polymers.17 Here, we sought to explore the exact nature by which branching can improve transfection efficacy of these polymers through a fair comparison of fully effective linear PBAEs to equivalent branched species. For this purpose, we synthesized a series of polymers with well-defined degrees of branching that was quantified via NMR and GPC. These BEAQs are notable in part due to the manner in which end-capping with the chosen E6 monomer affected amine density, particularly through adding secondary amines to the polymer structure. We hypothesized that the branching structure and high end-cap moiety mass fraction in BEAQs would show improved DNA binding at neutral pH and would be more effective for delivery at lower w/w ratios as compared to linear PBAEs due to their increased secondary amine cationicity.
BEAQs were shown via computational and experimental methods to be more water soluble due to the increased prevalence of hydrophilic end-cap moieties and more effective at buffering in the physiological pH range. We further calculated the effective pKa value of each polymer to demonstrate that branching influenced the pH point of maximal buffering capacity. Given the long-standing hypothesis that titration capability of polycations in the pH 5–7.4 range is responsible for “proton sponge hypothesis” driven endosomal escape,39, 41–44 direct variation of the buffering capacity and effective pKa allowed evaluation of the importance of buffering in gene delivery with these polymers. Through quantitative competition DNA binding assays we demonstrated that branching improved DNA binding as a function of both increased secondary amine content via additional end-cap monomers as well as branching structure by normalized binding efficacy to specific amine content of each polymer. Importantly, BEAQs were much more effective at binding nucleic acids compared to the linear polymer following dilution into neutral, isotonic buffer.
Using the two well characterized cell lines human embryonic kidney HEK293T and human retinal pigment epithelium ARPE-19, these polymers demonstrated extremely high transfection efficacy (up to 99% and 77% respectively) with no notable cytotoxicity at utilized doses. BEAQs did not demonstrate greater nanoparticle uptake compared to the linear polymer but did improve transfection efficacy and reduce the necessary w/w ratio, effectively improving polymer efficiency of transfection at a given polymer mass. As the highly branched B8–80% and B8–90% polymers possessed the greatest buffering capacity and the most relevant effective pKa values (nearer to pH 7) but the lowest transfection efficacy, our results further indicate that buffering capability and endosomal escape is likely not the rate-limiting step to mediating successful transfection in this polymer system. These results reinforce findings from other groups in alternative polymer systems that polymer buffering capacity between pH 4–7.4 is a necessary, but not on its own a sufficient property for transfection.45
Under more challenging transfection conditions of extremely low nanoparticle doses or under physiological serum conditions, moderately branched BEAQs were statistically shown to outperform the equivalent linear PBAE and possess extremely high transfection efficacy for the reported conditions. At ultra-low plasmid DNA doses, the efficiency of plasmid DNA delivery was rather remarkable compared to previously reported optimal nanoparticles, including PBAE terpolymers that include alkyl side chains for improved colloidal stability that were shown to require roughly 3x the DNA dose used here to transfect HeLa cells with similar efficacy.46 Further, in physiological serum conditions these BEAQ nanoparticles demonstrated an impressive degree of transfection compared to what has been reported in the literature. Fluorinated PAMAM dendrimers were reported to have their transfection efficacy reduced to 30% of what it was in 10% serum when the nanoparticles were added to cells in 50% serum.47 In contrast, BEAQ nanoparticles maintained >70% geometric mean expression under matched conditions to 10% serum transfections. Other non-viral transfection reagents have similarly been reported to facilitate transfection under physiological serum conditions, but often yield only 30–40% the mean expression level of the same particles in 10% serum.48
That being said, even at this relatively high level of efficacy of non-viral transfection, much room is left for improvement in non-viral vector efficiency as compared to viral vectors that have evolved for over a billion years for efficient transduction. At the low doses tested of 5–10 ng plasmid DNA/well, there were approximately 200,000–400,000 plasmids available for every cell in the well (Calculation S1). Based on recent estimates of approximately 10 plasmids per polyplex nanoparticle31, 49 there could still be over 20,000 nanoparticles added per cell at this dose, which is a high multiplicity of infection (MOI). With an estimated number of 5,000 plasmids from polyplex nanoparticles being internalized per cell under higher dose transfection conditions and an estimated 1/5 of those plasmids reaching the nuclear envelope, uptake of nanoparticles appears to be a significant hurdle to effective transfection in vitro.32 In comparison to efficient viruses, the low nanoparticle doses tested here are far above the order of magnitude MOI used for adenovirus (1–1,000) and various lentiviruses (1–200) to yield similar levels of expression.50–51 In contrast, naturally occurring AAVs are often used at a much higher MOI of up to 100,000 to achieve similarly detectable reporter gene based levels of transfection in hard-to-transduce cell lines.52–53 Spark Therapeutics recently completed a successful phase III clinical trial using subretinal delivery of AAV for the first FDA approved gene therapy, voretigene neparvovec-rzyl, demonstrating the clinical potential of non-integrating gene therapy.54 Given the similar level of MOI for BEAQ and AAV and coupled with challenges in scaling production of AAV for clinical utilization1–2 and the limitations of AAV cargo capacity, non-viral delivery of episomal plasmid DNA with this BEAQ system may be a viable strategy for clinical delivery of DNA to RPE cells.
Escape from endosomes and avoidance of lysosomal degradation remains a significant hurdle to nanomaterials aiming to achieve cytosolic delivery. Estimates of endosomal escape of lipid nanoparticles for siRNA has revealed that less than 2% of cargo internalized to endosomes typically reaches the cytosol55–56, which has been improved by some recent lipid nanoparticle formulations yielding up to 15% escape in HeLa cells.57 Polyplex nanoparticles similarly suffer from low endosomal escape efficacy, with the classic materials such as polyethylenimine and polylysine almost exclusively remaining in acidified vesicles and undergoing lysosomal degradation despite the ability of the former material in particular to buffer hydrogen ions.41 Transport to acidic lysosomes occurs rapidly following internalization, with nanoparticles typically reaching a lysosomal compartment within one hour following internalization.58 In contrast to these findings for most other polymeric materials, we demonstrated that BEAQs largely avoid lysosomal degradation with <20% of labeled plasmid DNA being detectable in acidified vesicles at 24 hours post-treatment compared to 40–50% DNA delivered with the linear polymer detected in acidified vesicles. These results are promising in that they demonstrate that branching may improve the ability of these polymers to achieve endosomal escape, which remains a primary hurdle to effective gene delivery.
Finally, we demonstrated how polymer structure, as a function of hydrophobicity and cationicity, related directly to optimal polymer/DNA mass ratio and to transfection efficacy as these variables have been shown repeatedly to be crucial to yielding robust transfection in other polymer systems.30 To identify structure-function relationships between these polymers and transfection efficacy, we analyzed viability and geometric mean expression as a function of individual polymer properties including buffering capacity, secondary, tertiary and total amine content per bp plasmid DNA. This is the first reported analysis of this type reported to our knowledge and yielded insights into the features of polycations that make them effective for transfection. In particular, we demonstrated that the optimal number of tertiary amines per bp plasmid DNA was near constant across the entire range of branching, while optimal numbers of secondary amines increased with degree of branching. With further knowledge of precise desired polymer structures, solid phase synthesis of alternating copolymers is an option which has been utilized in the synthesis of precisely defined polymers for gene delivery.59 Degradation rate of polymers could also play a role in the differences in transfection, as differences in constituent monomers can affect the specific degradation rate.18
The total possible solution space for BEAQs that may be highly effective for gene delivery is vast, as there are many diacrylate monomers, side-chain amino and end-capping amino monomers available that have been shown to yield linear polymers effective for transfection of diverse cell types. Synthesis of BEAQs via the guidelines outlined here and in previous publications14 will enable the rapid prototyping of diverse polymers that may yield further gains to efficient nucleic acid delivery as well as insights into polymeric structure/function relationships. The presented method for generating BEAQs can likewise be easily expanded to include utilization of branching monomers with other triacrylate monomer use as well as quaternary or greater functionality such as pentaerythritol tetraacrylate or dipentaerythritol penta-/hexa-acrylate to further increase structural diversity.
5. Conclusion
Branched poly(ester amine) quadpolymers (BEAQs) were successfully synthesized and characterized and were demonstrated to have multiple enhancements over leading non-viral gene delivery materials including optimized linear PBAEs, BPEI, JetPRIME, and Lipofectamine 2000. BEAQs with a moderate degree of branching were shown to more tightly bind plasmid DNA, maintain DNA binding following dilution in neutral, isotonic buffer, and possess higher solubility in aqueous media compared to linear analogs. Branched polymers formed from diacrylate (B7) and triacrylate (B8) monomers were highly effective for plasmid DNA delivery, and moderately branched BEAQs best maintained efficacy at physiologically relevant high serum concentrations. Analysis of chemical structure highlighted the importance of the ability to buffer pH at approximately 20 nmol H+/ μg DNA as well as the key parameter of tertiary amine content at approximately 40 tertiary amines per base pair of DNA. Through differential control of polymer branching, BEAQs were found to be efficient for non-viral gene delivery to difficult-to-transfect human cells. BEAQs are promising as therapeutic gene delivery vehicles and these findings have implications for the design, identification, and optimization of next-generation polymeric materials for nucleic acid delivery.
Supplementary Material
Acknowledgements
The authors thank the lab of Dr. Donald Zack and the Wilmer Equipment Core for use of the Echo 550 liquid handler and Cellomics Arrayscan for low dose transfection experiments.
Funding.
The authors thank the following sources for funding support: NSF Graduate Research Fellowships DGE-0707427 to DRW and DGE-1232825 to YR; the Bloomberg~Kimmel Institute for Cancer Immunotherapy; the NIH (R01EB016721, R01EB022148, R01CA195503, S10 OD016374 and the Wilmer Core Grant P30 EY001765); and a Research to Prevent Blindness / Dr. H. James and Carole Free Catalyst Award for Innovative Research Approaches for Age-Related Macular Degeneration to JJG.
Footnotes
Associated Content
The Supporting Information is available free of charge on the ACS Publications website. Supporting figures include chemical and binding properties, nanoparticle characteristics and uptake, optimization of transfection conditions and additional confocal microscopy images. Supporting tables include ratios of monomers and calculated statistical analyses.
References:
- 1.Kotin RM, Large-scale recombinant adeno-associated virus production. Hum. Mol. Genet 2011, 20 (R1), R2–R6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wright JF, Manufacturing and characterizing AAV-based vectors for use in clinical studies. Gene Ther. 2008, 15 (11), 840. [DOI] [PubMed] [Google Scholar]
- 3.Kotterman MA; Schaffer DV, Engineering adeno-associated viruses for clinical gene therapy. Nat. Rev. Genet 2014, 15, 445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Asokan A; Schaffer DV; Samulski RJ, The AAV vector toolkit: poised at the clinical crossroads. Mol. Ther 2012, 20 (4), 699–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yin H; Kanasty RL; Eltoukhy AA; Vegas AJ; Dorkin JR; Anderson DG, Non-viral vectors for gene-based therapy. Nat Rev Genet 2014, 15 (8), 541–555. [DOI] [PubMed] [Google Scholar]
- 6.Mangraviti A; Tzeng SY; Kozielski KL; Wang Y; Jin Y; Gullotti D; Pedone M; Buaron N; Liu A; Wilson DR; Hansen SK; Rodriguez FJ; Gao G-D; DiMeco F; Brem H; Olivi A; Tyler B; Green JJ, Polymeric Nanoparticles for Nonviral Gene Therapy Extend Brain Tumor Survival in Vivo. ACS Nano 2015, 9 (2), 1236–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Keeney M; Ong S-G; Padilla A; Yao Z; Goodman S; Wu JC; Yang F, Development of poly(β-amino ester)-based biodegradable nanoparticles for nonviral delivery of minicircle DNA. ACS nano 2013, 7 (8), 7241–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cho S-W; Yang F; Son SM; Park H-J; Green JJ; Bogatyrev S; Mei Y; Park S; Langer R; Anderson DG, Therapeutic angiogenesis using genetically engineered human endothelial cells. J. Control. Release 2012, 160 (3), 515–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tzeng SY; Wilson DR; Hansen SK; Quiñones‐Hinojosa A; Green JJ, Polymeric nanoparticle‐based delivery of TRAIL DNA for cancer‐specific killing. Bioeng. Transl. Med 2016, 1 (2), 149–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anderson DG; Lynn DM; Langer R, Semi-automated synthesis and screening of a large library of degradable cationic polymers for gene delivery. Angewandte Chemie (International ed. in English) 2003, 42 (27), 3153–8. [DOI] [PubMed] [Google Scholar]
- 11.Akinc A; Anderson DG; Lynn DM; Langer R, Synthesis of poly (β-amino ester) s optimized for highly effective gene delivery. Bioconjug. Chem 2003, 14 (5), 979–988. [DOI] [PubMed] [Google Scholar]
- 12.Green JJ; Langer R; Anderson DG, A combinatorial polymer library approach yields insight into nonviral gene delivery. Acc. Chem. Res 2008, 41 (6), 749–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Intra J; Salem AK, Characterization of the transgene expression generated by branched and linear polyethylenimine-plasmid DNA nanoparticles in vitro and after intraperitoneal injection in vivo. J. Control. Release 2008, 130 (2), 129–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cutlar L; Zhou D; Gao Y; Zhao T; Greiser U; Wang W; Wang W, Highly branched poly (β-amino esters): Synthesis and application in gene delivery. Biomacromolecules 2015, 16 (9), 2609–2617. [DOI] [PubMed] [Google Scholar]
- 15.Liu S; Gao Y; Zhou D; Greiser U; Guo T; Guo R; Wang W, Biodegradable Highly Branched Poly (β-Amino Ester) s for Targeted Cancer Cell Gene Transfection. ACS Biomaterials Science & Engineering 2016. [DOI] [PubMed] [Google Scholar]
- 16.Zhou D; Gao Y; Aied A; Cutlar L; Igoucheva O; Newland B; Alexeeve V; Greiser U; Uitto J; Wang W, Highly branched poly (β-amino ester) s for skin gene therapy. J. Control. Release 2016, 244, 336–346. [DOI] [PubMed] [Google Scholar]
- 17.Zhou D; Cutlar L; Gao Y; Wang W; O’Keeffe-Ahern J; McMahon S; Duarte B; Larcher F; Rodriguez BJ; Greiser U, The transition from linear to highly branched poly (β-amino ester) s: Branching matters for gene delivery. Science advances 2016, 2 (6), e1600102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sunshine JC; Peng DY; Green JJ, Uptake and transfection with polymeric nanoparticles are dependent on polymer end-group structure, but largely independent of nanoparticle physical and chemical properties. Mol. Pharm 2012, 9 (11), 3375–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bishop CJ; Ketola T.-m.; Tzeng SY; Sunshine JC; Urtti A; Lemmetyinen H; Vuorimaa-Laukkanen E; Yliperttula M; Green JJ, The effect and role of carbon atoms in poly(β-amino ester)s for DNA binding and gene delivery. J. Am. Chem. Soc 2013, 135 (18), 6951–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eltoukhy AA; Siegwart DJ; Alabi CA; Rajan JS; Langer R; Anderson DG, Effect of molecular weight of amine end-modified poly(β-amino ester)s on gene delivery efficiency and toxicity. Biomaterials 2012, 33 (13), 3594–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sunshine J; Green JJ; Mahon KP; Yang F; Eltoukhy A. a.; Nguyen DN; Langer R; Anderson DG, Small-Molecule End-Groups of Linear Polymer Determine Cell-type Gene-Delivery Efficacy. Advanced Materials 2009, 21 (48), 4947–4951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cho YW; Kim JD; Park K, Polycation gene delivery systems: escape from endosomes to cytosol. J. Pharm. Pharmacol 2003, 55 (6), 721–734. [DOI] [PubMed] [Google Scholar]
- 23.Lim Y.-b.; Kim S.-m.; Suh H; Park J.-s., Biodegradable, endosome disruptive, and cationic network-type polymer as a highly efficient and nontoxic gene delivery carrier. Bioconjug. Chem 2002, 13 (5), 952–957. [DOI] [PubMed] [Google Scholar]
- 24.Hall A; Lächelt U; Bartek J; Wagner E; Moghimi SM, Polyplex evolution: Understanding biology, optimizing performance. Mol. Ther 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou J; Liu J; Cheng CJ; Patel TR; Weller CE; Piepmeier JM; Jiang Z; Saltzman WM, Biodegradable poly(amine-co-ester) terpolymers for targeted gene delivery. Nat Mater 2012, 11 (1), 82–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yan H; Zhu D; Zhou Z; Liu X; Piao Y; Zhang Z; Liu X; Tang J; Shen Y, Facile synthesis of semi-library of low charge density cationic polyesters from poly (alkylene maleate) s for efficient local gene delivery. Biomaterials 2018. [DOI] [PubMed] [Google Scholar]
- 27.Kim J; Sunshine JC; Green JJ, Differential polymer structure tunes mechanism of cellular uptake and transfection routes of poly(β-amino ester) polyplexes in human breast cancer cells. Bioconjug. Chem 2014, 25 (1), 43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guerrero-Cázares H; Tzeng SY; Young NP; Abutaleb AO; Quiñones-Hinojosa A; Green JJ, Biodegradable polymeric nanoparticles show high efficacy and specificity at DNA delivery to human glioblastoma in vitro and in vivo. ACS nano 2014, 8 (5), 5141–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tzeng SY; Higgins LJ; Pomper MG; Green JJ, Student award winner in the Ph.D. category for the 2013 society for biomaterials annual meeting and exposition, april 10–13, 2013, Boston, Massachusetts : biomaterial-mediated cancer-specific DNA delivery to liver cell cultures using synthetic poly(beta-a. Journal of biomedical materials research. Part A 2013, 101 (7), 1837–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou Z; Liu X; Zhu D; Wang Y; Zhang Z; Zhou X; Qiu N; Chen X; Shen Y, Nonviral cancer gene therapy: Delivery cascade and vector nanoproperty integration. Advanced drug delivery reviews 2017, 115, 115–154. [DOI] [PubMed] [Google Scholar]
- 31.Wilson DR; Mosenia A; Suprenant MP; Upadhya R; Routkevitch D; Meyer RA; Quinones-Hinojosa A; Green JJ, Continuous microfluidic assembly of biodegradable poly(beta-amino ester)/DNA nanoparticles for enhanced gene delivery. J Biomed Mater Res A 2017, 105 (6), 1813–1825. [DOI] [PubMed] [Google Scholar]
- 32.Bishop CJ; Majewski RL; Guiriba T-RM; Wilson DR; Bhise NS; Quiñones-Hinojosa A; Green JJ, Quantification of cellular and nuclear uptake rates of polymeric gene delivery nanoparticles and DNA plasmids via flow cytometry. Acta Biomaterialia 2016, 37, 120–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tzeng SY; Green JJ, Subtle changes to polymer structure and degradation mechanism enable highly effective nanoparticles for siRNA and DNA delivery to human brain cancer. Advanced healthcare materials 2013, 2 (3), 468–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sunshine JC; Akanda MI; Li D; Kozielski KL; Green JJ, Effects of base polymer hydrophobicity and end-group modification on polymeric gene delivery. Biomacromolecules 2011, 12 (10), 3592–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bhise NS; Gray RS; Sunshine JC; Htet S; Ewald AJ; Green JJ, The relationship between terminal functionalization and molecular weight of a gene delivery polymer and transfection efficacy in mammary epithelial 2-D cultures and 3-D organotypic cultures. Biomaterials 2010, 31 (31), 8088–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Voit B, New developments in hyperbranched polymers. Journal of Polymer Science Part A: Polymer Chemistry 2000, 38 (14), 2505–2525. [Google Scholar]
- 37.Sunshine JC; Sunshine SB; Bhutto I; Handa JT; Green JJ, Poly(β-amino ester)-nanoparticle mediated transfection of retinal pigment epithelial cells in vitro and in vivo. PLoS One 2012, 7 (5), e37543–e37543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guo W; Lee RJ, Efficient gene delivery via non-covalent complexes of folic acid and polyethylenimine. J. Control. Release 2001, 77 (1), 131–138. [DOI] [PubMed] [Google Scholar]
- 39.Wilson DR; Routkevitch D; Rui Y; Mosenia A; Wahlin KJ; Quinones-Hinojosa A; Zack DJ; Green JJ, A Triple-Fluorophore-Labeled Nucleic Acid pH Nanosensor to Investigate Non-viral Gene Delivery. Mol. Ther 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhong Z; Song Y; Engbersen JFJ; Lok MC; Hennink WE; Feijen J, A versatile family of degradable non-viral gene carriers based on hyperbranched poly (ester amine) s. J. Control. Release 2005, 109 (1–3), 317–329. [DOI] [PubMed] [Google Scholar]
- 41.Benjaminsen RV; Mattebjerg MA; Henriksen JR; Moghimi SM; Andresen TL, The possible “proton sponge” effect of polyethylenimine (PEI) does not include change in lysosomal pH. Mol. Ther 2013, 21 (1), 149–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Behr J-P, The proton sponge: a trick to enter cells the viruses did not exploit. CHIMIA International Journal for Chemistry 1997, 51 (1–2), 34–36. [Google Scholar]
- 43.Boussif O; Lezoualc’h F; Zanta M. a.; Mergny MD; Scherman D; Demeneix B; Behr JP, A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: polyethylenimine. Proc. Natl. Acad. Sci. U. S. A 1995, 92 (16), 7297–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lächelt U; Kos P; Mickler FM; Herrmann A; Salcher EE; Rödl W; Badgujar N; Bräuchle C; Wagner E, Fine-tuning of proton sponges by precise diaminoethanes and histidines in pDNA polyplexes. Nanomedicine : nanotechnology, biology, and medicine 2014, 10 (1), 35–44. [DOI] [PubMed] [Google Scholar]
- 45.Funhoff AM; van Nostrum CF; Koning GA; Schuurmans-Nieuwenbroek NME; Crommelin DJA; Hennink WE, Endosomal Escape of Polymeric Gene Delivery Complexes Is Not Always Enhanced by Polymers Buffering at Low pH. Biomacromolecules 2004, 5 (1), 32–39. [DOI] [PubMed] [Google Scholar]
- 46.Eltoukhy AA; Chen D; Alabi CA; Langer R; Anderson DG, Degradable terpolymers with alkyl side chains demonstrate enhanced gene delivery potency and nanoparticle stability. Advanced Materials 2013, 25 (10), 1487–1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu H; Wang Y; Wang M; Xiao J; Cheng Y, Fluorinated poly (propylenimine) dendrimers as gene vectors. Biomaterials 2014, 35 (20), 5407–5413. [DOI] [PubMed] [Google Scholar]
- 48.Wen Y; Zhang Z; Li J, Highly Efficient Multifunctional Supramolecular Gene Carrier System Self‐Assembled from Redox‐Sensitive and Zwitterionic Polymer Blocks. Advanced Functional Materials 2014, 24 (25), 3874–3884. [Google Scholar]
- 49.Beh CW; Pan D; Lee J; Jiang X; Liu KJ; Mao H-Q; Wang T. h., Direct interrogation of DNA content distribution in nanoparticles by a novel microfluidics-based single-particle analysis. Nano letters 2014, 14 (8), 4729–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Green JJ; Zugates GT; Tedford NC; Huang YH; Griffith LG; Lauffenburger D. a.; Sawicki J. a.; Langer R; Anderson DG, Combinatorial Modification of Degradable Polymers Enables Transfection of Human Cells Comparable to Adenovirus. Advanced Materials 2007, 19 (19), 2836–2842. [Google Scholar]
- 51.Hoyng SA; De Winter F; Gnavi S; Van Egmond L; Attwell CL; Tannemaat MR; Verhaagen J; Malessy MJA, Gene delivery to rat and human Schwann cells and nerve segments: a comparison of AAV 1–9 and lentiviral vectors. Gene Ther. 2015, 22 (10), 767. [DOI] [PubMed] [Google Scholar]
- 52.Ellis BL; Hirsch ML; Barker JC; Connelly JP; Steininger RJ; Porteus MH, A survey of ex vivo/in vitro transduction efficiency of mammalian primary cells and cell lines with Nine natural adeno-associated virus (AAV1–9) and one engineered adeno-associated virus serotype. Virol J. 2013, 10 (1), 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gaj T; Staahl BT; Rodrigues GMC; Limsirichai P; Ekman FK; Doudna JA; Schaffer DV, Targeted gene knock-in by homology-directed genome editing using Cas9 ribonucleoprotein and AAV donor delivery. Nucleic Acids Res. 2017, gkx154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Russell S; Bennett J; Wellman JA; Chung DC; Yu Z-F; Tillman A; Wittes J; Pappas J; Elci O; McCague S, Efficacy and safety of voretigene neparvovec (AAV2-hRPE65v2) in patients with RPE65-mediated inherited retinal dystrophy: a randomised, controlled, open-label, phase 3 trial. The Lancet 2017, 390 (10097), 849–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gilleron J; Querbes W; Zeigerer A; Borodovsky A; Marsico G; Schubert U; Manygoats K; Seifert S; Andree C; Stöter M; Epstein-Barash H; Zhang L; Koteliansky V; Fitzgerald K; Fava E; Bickle M; Kalaidzidis Y; Akinc A; Maier M; Zerial M, Image-based analysis of lipid nanoparticle-mediated siRNA delivery, intracellular trafficking and endosomal escape. Nat. Biotechnol 2013, 31 (7), 638–46. [DOI] [PubMed] [Google Scholar]
- 56.Wittrup A; Ai A; Liu X; Hamar P; Trifonova R; Charisse K; Manoharan M; Kirchhausen T; Lieberman J, Visualizing lipid-formulated siRNA release from endosomes and target gene knockdown. Nat. Biotechnol 2015, 33 (8), 870–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sabnis S; Kumarasinghe ES; Salerno T; Mihai C; Ketova T; Senn JJ; Lynn A; Bulychev A; McFadyen I; Chan J, A Novel Amino Lipid Series for mRNA Delivery: Improved Endosomal Escape and Sustained Pharmacology and Safety in Non-human Primates. Mol. Ther 2018, 26 (6), 1509–1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stewart MP; Lorenz A; Dahlman J; Sahay G, Challenges in carrier‐mediated intracellular delivery: moving beyond endosomal barriers. Wiley Interdisciplinary Reviews: Nanomedicine and Nanobiotechnology 2016, 8 (3), 465–478. [DOI] [PubMed] [Google Scholar]
- 59.Levacic AK; Morys S; Wagner E, Solid-phase supported design of carriers for therapeutic nucleic acid delivery. Biosci. Rep 2017, 37 (5), BSR20160617. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


