Abstract
Scope
Urolithins, gut microbial metabolites derived from ellagic acid (EA), reach significant concentrations in the human colon. Urolithin-A (Uro-A) exerts anti-inflammatory activity in animal models of inflammatory bowel diseases (IBDs). We hypothesized that urolithins could modulate the biosynthesis of leukocyte-derived inflammatory eicosanoids from the 5-lipoxygenase (5-LOX), cyclooxygenase-2 (COX-2) and 5-LOX/COX-2 pathways, relevant in the onset and progression of IBDs, including 5-hydroxyeicosatetraenoic acids (5-HETEs), leukotriene-B4 (LTB4), prostaglandin E2 (PGE2) and hemiketals (HKE2 and HKD2).
Methods and results
Leukocytes, obtained from six healthy donors, were stimulated with lipopolysaccharide and calcium ionophore A23187. Urolithins, at concentrations found in the human colon (1−15 μM), decreased eicosanoid biosynthesis and COX-2 levels in the activated leukocytes. In contrast, EA and conjugated urolithins (glucuronides and sulfates) were inactive. Uro-A and isourolithin-A (IsoUro-A) reduced the formation of the 5-LOX/COX-2 products HKE2 and HKD2 through the COX-2 pathway (down-regulation of COX-2 and prostaglandin E2), whereas urolithin C reduced 5-HETE and LTB4 via inhibition of 5-LOX.
Conclusions
Our results show that physiologically relevant colonic urolithins target eicosanoid biosynthetic pathways. The effect on HKs and LTB4 formation is unprecedented and expands the knowledge on anti-inflammatory mechanisms of urolithins against IBDs.
Keywords: Urolithin, COX-2, 5-LOX, inflammatory bowel disease, eicosanoid
1. Introduction
The term inflammatory bowel disease (IBD) mainly refers to ulcerative colitis (UC) and Crohn’s disease (CD), characterized by chronic inflammation of the gastrointestinal tract. After decades of investigation, the etiology of IBDs is still not fully understood [1]. An accepted model of IBD pathogenesis considers alteration of the host-microbiota interaction as a triggering factor. Microbial dysbiosis and gut barrier alteration allow the interaction of microbes with immune cells leading to dysregulated activation of leukocytes and enhanced synthesis of a plethora of molecules (i.e., cytokines and eicosanoids), resulting in constant leukocyte trafficking and massive accumulation of lymphocytes, neutrophils, and (or) macrophages [2]. This disturbs the ratio of pro-/anti-inflammatory molecules, which generates long-lasting inflammation and contributes to IBD complications such as ulcer formation, fibrosis, and cancer [3, 4].
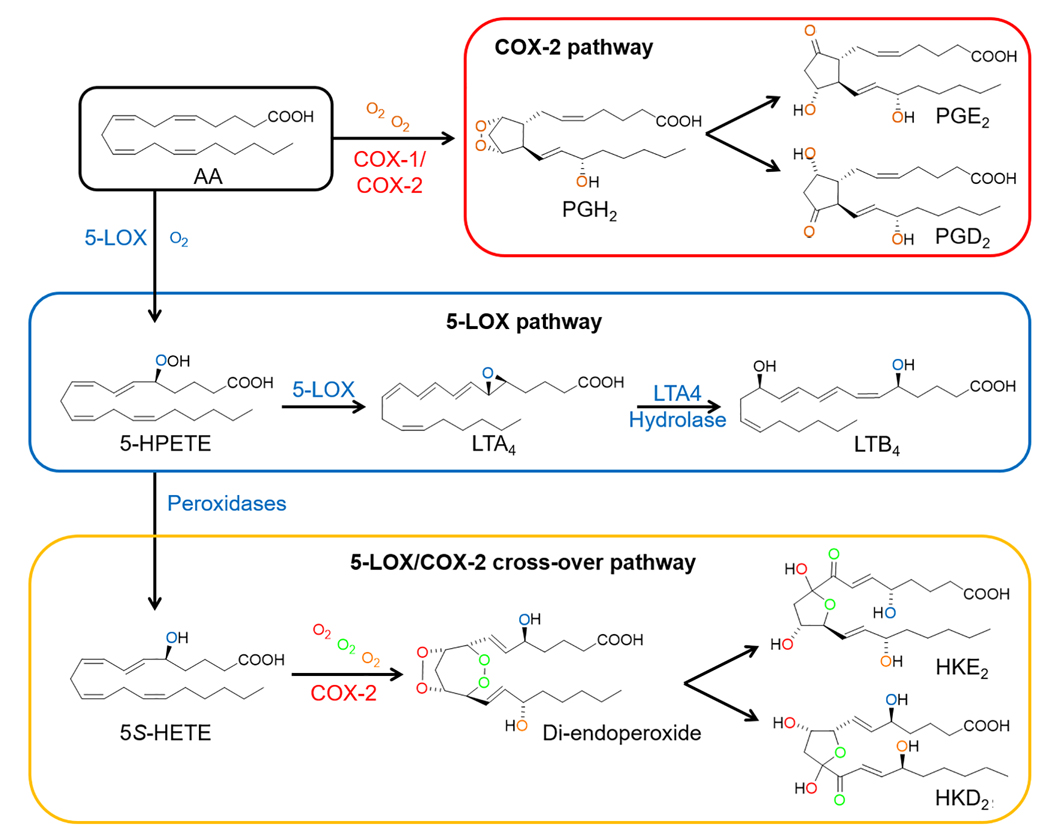
5-Lipoxygenase (5-LOX) and cyclooxygenase-2 (COX-2) are two crucial enzymes in inflammatory disorders and cancer development through the formation of eicosanoids [5]. Biosynthesis of prostaglandins (PGs) is initiated with the oxygenation of arachidonic acid (AA) by COX-2 to form the endoperoxide PGH2, which is the precursor of effector prostaglandins like PGE2 and PGD2. 5-Lipoxygenase (5-LOX) oxygenates AA forming 5S-hydroxyperoxyeicosatetraenoic acid (5S-HPETE), which is dehydrated to leukotriene (LT)-A4 as the precursor to all LTs or reduced to 5S-HETE. 5S-HETE can serve as a substrate of COX-2, establishing a link between the 5-LOX and COX-2 pathways. The reaction of 5S-HETE with COX-2 generates an unstable di-endoperoxide (equivalent to PGH2), which is subsequently transformed to two hemiketal (HK) eicosanoids, HKE2 and HKD2 [6–9] (Figure 1). PGs and LTs are well-investigated molecules in IBD. COX-2 expressing macrophages synthesize PGE2, which regulates the immune response, induces epithelial cell proliferation, and increases vascular permeability [10]. LTB4 is a potent chemoattractant agent that recruits neutrophils to the inflamed intestine, where it can promote epithelial injury through the production of reactive oxygen species [11]. Less is known about the biological activity of HKE2 and HKD2, which require the cross-over of 5-LOX- and COX-2 activities to be synthesized. These eicosanoids promote migration and tubulogenesis of endothelial cells [6] and inhibit platelet aggregation [12]. A common anti-inflammatory strategy involves the reduction of the biosynthesis of PGs and LTs via inhibition of the COX-2 and 5-LOX pathways. Aspirin is a paradigmatic inhibitor of PGs synthesis through blocking COX-2 activity [13], while drugs such as zileuton or montelukast target the 5-LOX pathway [14]. However, the side effects associated with these drugs [15, 16] indicate the importance of searching for alternative therapeutic and preventive strategies, including the use of natural products.
Figure 1.
Key reactions in the biosynthesis of eicosanoids by 5-lipoxygenase (5-LOX) and cyclooxygenase-2 (COX-2). Arachidonic acid (AA) is the common substrate for the synthesis of PGs (COX-2 pathway) and LTs (5-LOX pathway). In the 5-LOX/COX-2 crossover pathway, both enzymes act sequentially to synthesize HKE2 and HKD2 as final products.
Urolithins, gut microbiota metabolites derived from ellagitannins (ETs) and ellagic acid (EA), are considered to be responsible for the health effects associated with the consumption of ellagitannin-rich food products (pomegranate, walnuts or berries) [17]. Urolithins are absorbed from the intestinal lumen where they reach relevant concentrations [18] and undergo enterohepatic circulation [19] with extensive phase-II metabolism yielding mainly glucuronide and sulfate conjugates, which have been detected in the bloodstream (0.2–20 μM) and systemic tissues such as prostate or mammary tissue [20, 21]. In vitro and in vivo studies have reported that urolithins (Uro-A as the most active metabolite) exert anti-inflammatory effects through the preservation of the colonic architecture, attenuation of DSS-induced microbiota changes, inhibition of NF-κB and, COX-2 expression, and reduction of PGE2 formation in intestinal tissues and cells [22–24]. Targeting the synthesis of soluble mediators by immune cells has emerged as an exciting approach in IBDs therapy [2, 4]. The 5-LOX/COX-2 pathway (and its HKE2 and HKD2 products) offers a new option to advance in the understanding of the molecular mechanisms underlying the effect of urolithins against IBDs. In this study, we have studied whether urolithins, including Uro-A, IsoUro-A, Uro-B, and Uro-C, and their most relevant phase-II conjugates (glucuronides and sulfates) modulate the formation of 5-LOX (5-HETE and LTB4), COX-2 (PGE2), and 5-LOX/COX-2 (HKE2 and HKD2) products in a human isolated leukocyte model. We have also investigated the effect of these metabolites on 5-LOX and COX-2 protein levels and the enzymatic activity of COX-2.
2. Material and Methods
2.1. Materials
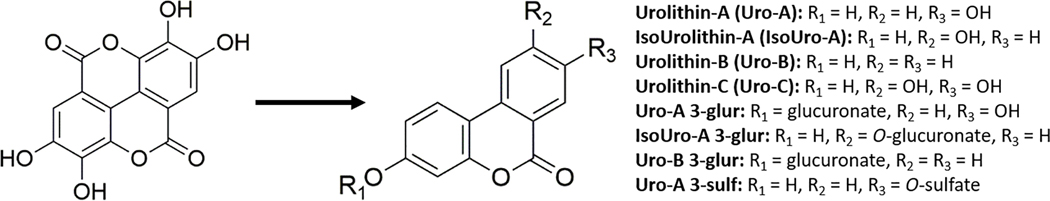
Ellagic acid (EA), dimethylsulfoxide (DMSO), lipopolysaccharide (LPS) from Escherichia coli (0111:B4), and RIPA buffer were purchased from Sigma (St. Louis, MO, USA). Phosphatase and protease inhibitors were obtained from ROCHE (USA). Calcium ionophore A23187 and d4-PGE2 were obtained from Cayman Chemical (Ann Arbor, MI, USA). Urolithins (Uro) metabolites Uro-A, Uro-B, and isourolithin A (IsoUro-A), and the conjugates Uro-A glucuronide (Uro-A-glur), Uro-B glucuronide (Uro-B-glur), isourolithin- glucuronide (IsoUro-A-glur) and Uro-A sulfate (Uro-A-sulf) conjugates, were obtained from Villapharma Research S.L. (Parque Tecnológico de Fuente Alamo, Murcia, Spain) (Figure 2). Uro-C was obtained from Toronto Research Chemical (Toronto, ON, Canada).
Figure 2.
Chemical structures of ellagic acid and its free and conjugated urolithin metabolites
2.2. Dosage Information
EA and urolithins were diluted in DMSO. The cells were treated with these compounds at concentrations ranging from 15 to 1 μM (≤0.5% DMSO, v/v). These concentrations are similar to those detected in vivo, and no toxic effects have been previously reported under the conditions of our study [17].
2.3. Leukocytes isolation and eicosanoids biosynthesis
A mixture of leukocytes, including neutrophils, lymphocytes, monocytes, eosinophils, and basophils were obtained from healthy donorś blood. The study was approved by the Vanderbilt University Medical Center Institutional Review Board (091243), and written informed consent was signed by the volunteers (n=6) before blood samples were obtained. Blood (45 mL) was collected in a syringe containing 6% dextran solution (10 mL) and sodium citrate (4.5 mL). The syringe was placed upright 60 min to separate red cells from leukocytes. The upper layer rich in leukocytes was collected in a 50 mL tube, centrifuged (317×g; 15 min at 10 °C), and the cells obtained were washed with sterile PBS and centrifuged again (188×g; 10 min at 10 °C). Next, for red cells lysis, 9 mL deionized water was added to 2 mL of cell suspension for 30 s, and then the tonicity was immediately reestablished with 1 mL 10X PBS. The leukocytes were centrifuged again (543×g; 10 min at 10 °C) and diluted in Roswell Park Memorial Institute (RPMI) medium (2 g/L glucose, 0.1% fetal bovine serum (FBS), 2 mM L-glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin) to a final concentration of 107 cells/mL. The cell solution, obtained from each volunteer, was distributed in a 24-well plate (1 mL per well) and pre-treated with 15 μM EA or urolithins and their conjugated metabolites, respectively, for 1 h. Next, the cells were treated with 10 μg/mL LPS for 5 h to stimulate COX-2 expression while cells were maintained in an incubator at 37°C and 5% CO2. In the last 15 min of the LPS incubation, A23187 (5 μM) was added for 5-LOX activation. After the incubation, the cells were pelleted by centrifugation (545×g for 5 min), and the supernatant (1 mL) was mixed with an equal volume of 0.1% acetic acid (pH 3.5). As internal standard d4-PGE2 was added to the samples prior to extraction using Waters HLB cartridges (Waters, Milford, MA, USA). The samples were eluted in methanol (MeOH), evaporated, and AMPP-derivatized, as previously described [25]. For protein analysis, the pellet obtained was washed in cold PBS, followed by the addition of RIPA buffer supplemented with protease and phosphatase inhibitors. The sample was incubated on ice for 30 min, centrifuged at 13,300×g for 15 min, and the supernatant kept at −80 °C until analysis.
2.4. Cell culture conditions and treatments
Murine RAW264.7 macrophages were purchased from the American Type Culture Collection (ATCC, Rockville, USA) and cultured as recommended by ATCC. Cells were routinely grown in Dulbecco’s Modified Eagle Medium (DMEM) medium (4.5 g/L glucose, 10% FBS, 2 mM L-glutamine, 1 mM Na-pyruvate, 25 mM HEPES, 100 U/mL penicillin, and 100 mg/mL streptomycin) until reaching 80–90% confluence. For the experiments, the cells were seeded at 300,000 cells/cm2 in a 12-well plate and incubated under the same conditions described for leukocytes (see above) for 24 h. The medium was removed, the cells washed with PBS and incubated in DMEM containing 0.1% FBS for 4 h to minimize the effect of FBS on COX-2 expression [26]. Next, the cells were treated with Uro-A, IsoUro-A, and Uro-C at 15 μM for 1 h, followed by LPS treatment (10 μg/mL) for 5 h and 5 μM A23187 for the last 15 min. Control cells (0.5% DMSO) were run in parallel. Protein extraction was performed in RIPA buffer supplemented with protease and phosphatase inhibitors. The experiment was repeated 3 times. Passages 4–5 and population doubling level (PDL) between 8 and 9 were used for all the experiments.
2.5. Effect of urolithins on COX-2 activity
Human recombinant COX-2 was expressed in Sf9 insect cells as previously described [27] and incubated in 100 μL ammonium acetate (100 mM, pH 8.2; containing 2 μM hematin and 500 μM phenol) for 2 min at room temperature, followed by the addition of 15 μM Uro-A or IsoUro-A, and incubated for 15 additional min. The reaction was initiated by the addition of 3 μg arachidonic acid (AA), conducted for 15 min at room temperature, and stopped by adding 4.25 μL HCl (1N) together with 20 ng d4-PGE2 as an internal standard. Samples were extracted using activated Waters 30 mg HLB cartridges, eluted in 500 mM MeOH, evaporated under a stream of N2, diluted in 50 μL mobile phase, filtered using 0.22 μm Spin-X centrifuge tube filters, and analyzed in the negative ionization mode by LC-MS. Assays were done at least in triplicate for each treatment.
2.6. Western Blot analysis
The changes in 5-LOX and COX-2 protein content were evaluated by using the same protein amount of total lysates (20 μg protein/lane), which were quantified using the DC protein assay kit (Bio-Rad, Hercules, CA, USA). Proteins were separated by 10% SDS-polyacrylamide gel and transferred to a PVDF membrane. After washing, the membranes were incubated with polyclonal 5-LOX (78 KDa, BD Bioscience, CA, USA) or COX-2 (72 KDa, Cayman Chemical, MI, USA) primary antibody at 1:1000 dilution, followed by anti-mouse IgG incubation at 1:3000 (Cell Signaling, MA, USA) and exposed to X-ray film. 5-LOX and COX-2 intensities were normalized to GAPDH. As COX-2 positive control, we included 2 μg cell lysate obtained from RAW264.7 macrophages treated with 100 ng/mL LPS for 5 h [28]. Western blot (WB) analyses were done at least in triplicate for each treatment.
2.7. LC-MS analysis
Eicosanoid separation and quntification in positive ion mode was carried out as previously described [25]. Eicosanoid formation in the COX-2 reaction with AA was analyzed in negative ion mode (underivatized samples) using chromatographic separation by a Zorbax Eclipse Plus C18 1.8-μm column (2.1 × 50 mm; Agilent Technologies). A linear gradient started with 100% of water:acetonitrile (95:5, v/v), reaching 100% of water:acetonitrile (5:95, v/v) at 5 min, and kept for 1 min. The initial conditions were re-established at 6.01 min and maintained for 1 min. The transitions recorded in the selected reaction monitoring (SRM) in negative and positive ion mode (AMPP-derivatized compounds) for LTB4, 5-HETE, PGE2, d4-PGE2, HKE2, and HKD2 were reported elsewhere [25]. The ion transition recorded for PGD2 in negative ion mode was: m/z 351 to 271 (+15 eV).
2.8. Statistical analysis
Statistical differences were analyzed using Prism 5 (La Jolla, CA, USA). For normally distributed data, one-way ANOVA followed by Newman Keuls’ post-hoc test was used. Results with p values <0.05 were considered statistically significant.
3. Results
3.1. Urolithins decrease eicosanoids formed by the 5-LOX, COX-2 and 5-LOX/COX-2 pathways
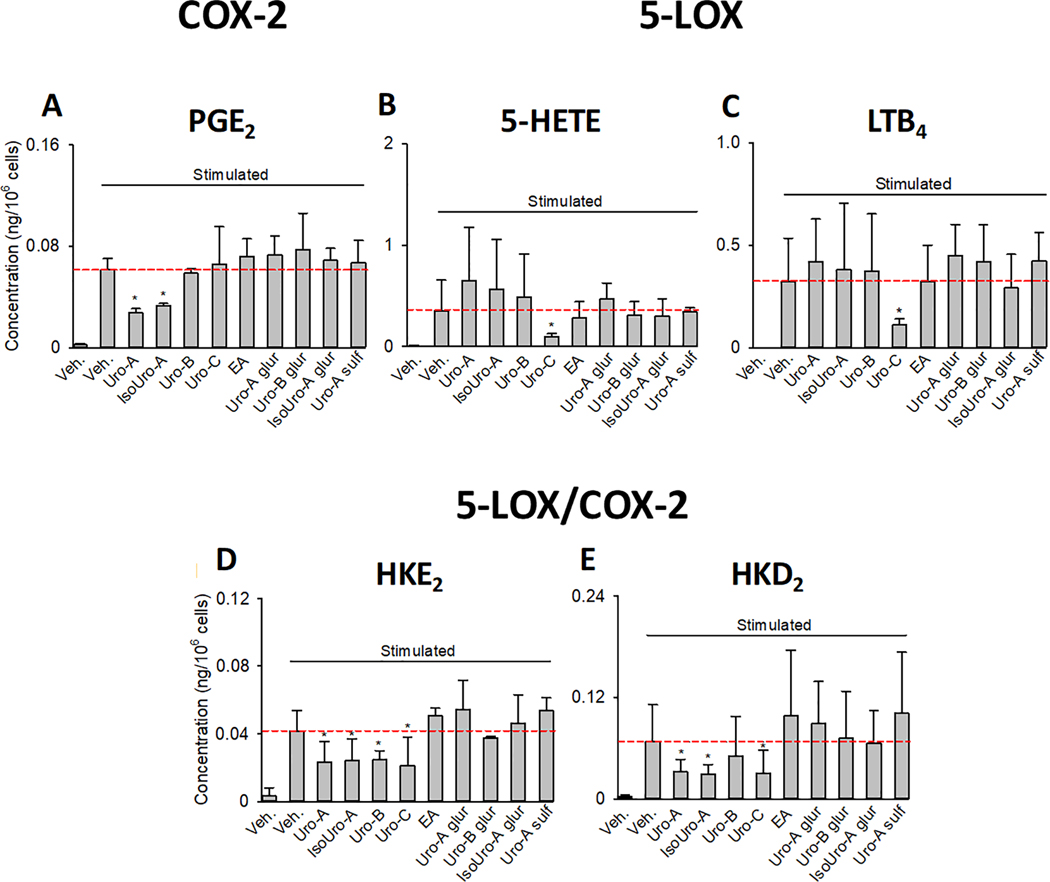
Treatment of leukocytes with 15 μM Uro-A and IsoUro-A decreased formation of PGE2 (Fig. 3A). Uro-C, at the same concentration, was the only compound tested to decrease formation of 5-HETE and LTB4 in human leukocytes stimulated with LPS and A23187 (Fig. 3B,C). The two hemiketals, which require both 5-LOX and COX-2 for their formation, were inhibited by Uro-A, IsoUro-A, and Uro-C (Fig. 3D,E), consistent with the effects of these urolithins on the individual enzymes. Uro-B (15 μM), unexpectedly, inhibited formation of HKE2 although not of HKD2. This could indicate an inhibitory effect on a – so far uncharacterized – enzyme that transforms the diendoperoxide intermediate specifically to HKE2 or it may be due to variability in the non-enzymatic transformation of the diendoperoxide to the HKs. EA and the conjugated metabolites had no effect on eicosanoid formation. Representative LC-MS chromatograms illustrating the effect of Uro-A, IsoUro-A, and Uro-C on eicosanoids formation are shown in Supporting Information Figure S1. Quantitative analysis showed that Uro-A and IsoUro-A decreased biosynthesis of HKE2 and HKD2 by ~43% and ~55%, respectively, as well as PGE2 by 46–55%. Uro-C decreased HKE2 and HKD2 by 40–60% and the 5-LOX products 5-HETE and LTB4 by 73% and 65%, respectively, when compared to control activated leukocytes.
Figure 3.
Effect of EA and urolithins (conjugated and free forms) on eicosanoid biosynthesis in stimulated human leukocytes. The bar charts show the level of PGE2 (A), 5-HETE (B), LTB4 (C), HKE2 (D), and HKD2 (E) quantified using LC-MS/MS analysis after AMPP-derivatization. The dashed red horizontal line indicates the concentration quantified in stimulated leukocytes. The results are shown as average ± standard deviation (SD) of six volunteers. Each treatment was analyzed in duplicate.
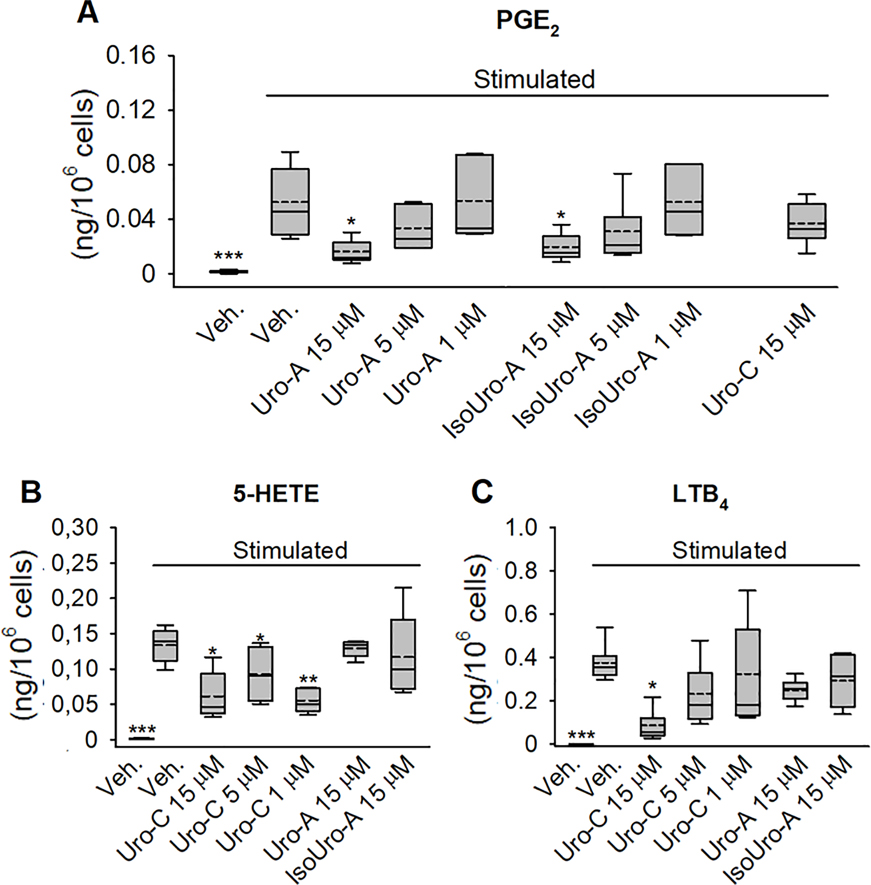
Based on these results, we selected Uro-A, IsoUro-A, and Uro-C for a dose-response analysis (15, 5, and 1 μM). In line with the initial screening, Uro-A and IsoUro-A decreased LPS-induced PGE2 formation dose-dependently, although the effect was only statistically significant (p<0.05) at 15 μM (Figure 4A). Consistent with the previous analysis, no effect was observed on the formation of 5-LOX products (5-HETE and LTB4) by Uro-A and IsoUro-A (Figure 4B and 4C), and likewise, Uro-C did not inhibit PGE2 production (Figure 4A). Reduction of 5-HETE formation was observed in the samples treated with Uro-C at concentrations from 15 to 1 μM (~32–58%; p<0.05) (Figure 4B), but not at concentrations below 1 μM (data not shown). Uro-C exerted a dose-dependent inhibition on the biosynthesis of LTB4, reaching 77% reduction at 15 μM (p<0.05) (Figure 4C).
Figure 4.
Dose-dependent effect of Uro-A, IsoUro-A, and Uro-C on COX-2 and 5-LOX product formation in stimulated human leukocytes. PGE2 (A), 5-HETE (B), and LTB4 (C) were quantified using LC-MS/MS analysis after AMPP derivatization. The effect of Uro-C on PGE2 as well as Uro-A and IsoUro-A on 5-HETE and LTB4 were included as controls. The box plots show the range of data between the 25th and 75th percentiles. Veh., vehicle. Solid horizontal line within bars: median. Dash horizontal line: mean. from 6 volunteers (n = 6). Each treatment was analyzed in duplicate. *p<0.05, **p<0.01, ***p<0.001 compared to the stimulated/veh.-treated group.
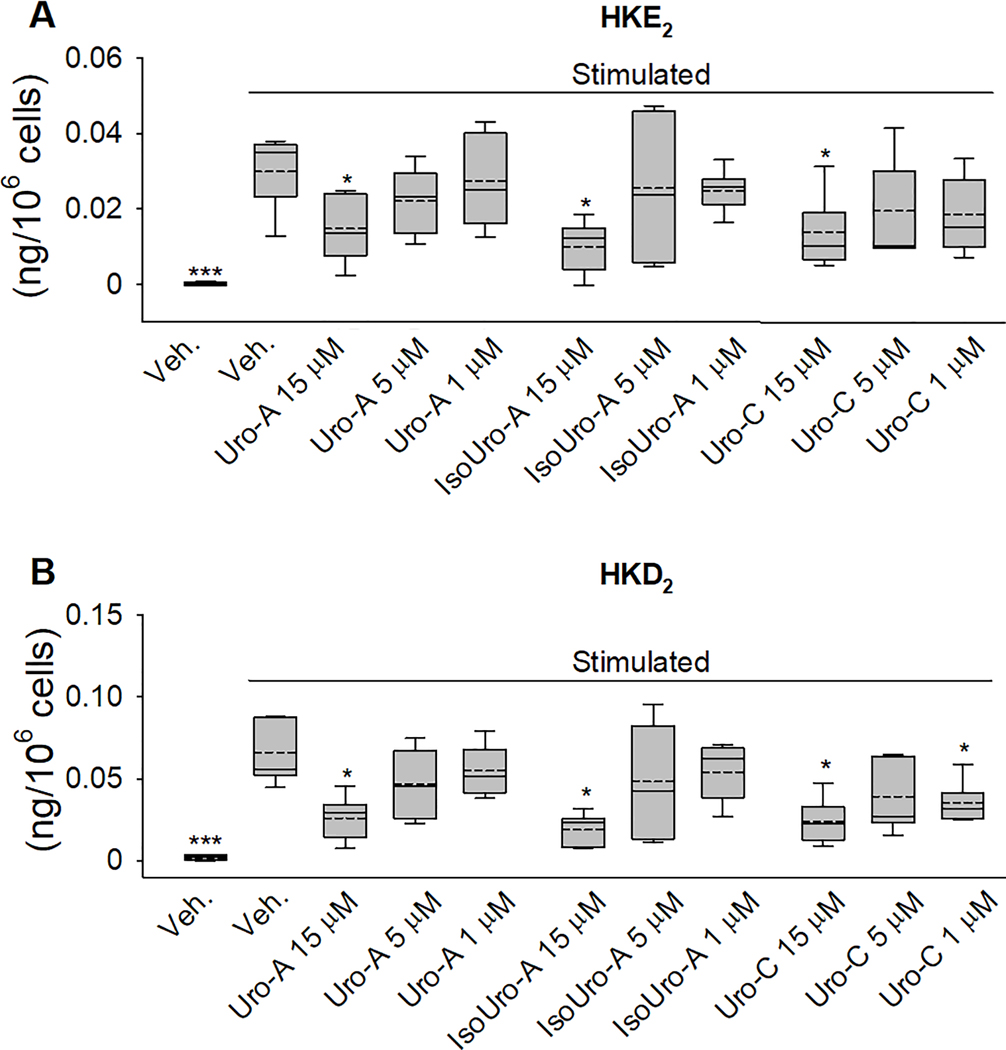
Biosynthesis of the 5-LOX/COX-2 cross-over eicosanoids (HKE2 and HKD2) was dose-dependently decrease by Uro-A and IsoUro-A treatments, although this was statistically significant (p<0.05) only at 15 μM (Figure 5). Uro-C, at 15 μM, also exerted a significant attenuation on the formation of HKE2 (54%; p<0.05) and HKD2 (63%; p<0.01). At lower concentrations, 5 and 1 μM, Uro-C exerted a non-significant reduction of HKE2 (35 and 38%, respectively). Unexpectedly, at 1μM, Uro-C exerted a significant reduction (46%; p<0.05) of HKD2, whereas at 5 μM the decrease observed (40%) was not significant, what could be related to the higher variability observed (Figure 5B).
Figure 5.
Dose-dependent effect of Uro-A, IsoUro-A, and Uro-C on hemiketal (HK) formation by the 5-LOX/COX-2 cross-over pathway in stimulated human leukocytes. HKE2 (A) and HKD2 (B) were quantified using LC-MS/MS analysis after AMPP-derivatization. The box plots show the range of data between the 25th and 75th percentiles. Veh., vehicle. Solid horizontal line within bars: median. Dash horizontal line: mean. (SD) from 6 volunteers. Each treatment was analyzed in duplicate. *p<0.05, ***p<0.001 compared to the stimulated/veh.-treated group.
3.2. Effect of Uro-A and IsoUro-A on COX-2 activity
Inhibition of PGE2 and HK formation by Uro-A and IsoUro-A in the leukocytes might be achieved via direct inhibition of the COX-2 enzyme or through downregulation of its expression. To test the former, we tested the effect of Uro-A and IsoUro-A on recombinant purified human COX-2 and analyzed enzymatic activity using LC-MS. In the absence of the urolithins, PGE2 was the major eicosanoid formed while the concentration of PGD2 was almost 20-fold lower. Uro-A and IsoUro-A failed to reduce formation of PGE2 and PGD2 by purified COX-2 (results not shown), suggesting inhibition in leukocytes was not due to a direct effect on the enzymatic activity of COX-2.
3.3. Effect of Uro-A, IsoUro-A, and Uro-C on COX-2 and 5-LOX expression levels
We next investigated whether the effect of Uro-A, IsoUro-A, and Uro-C on eicosanoid formation was mediated via downregulation of 5-LOX and COX-2 expression levels.
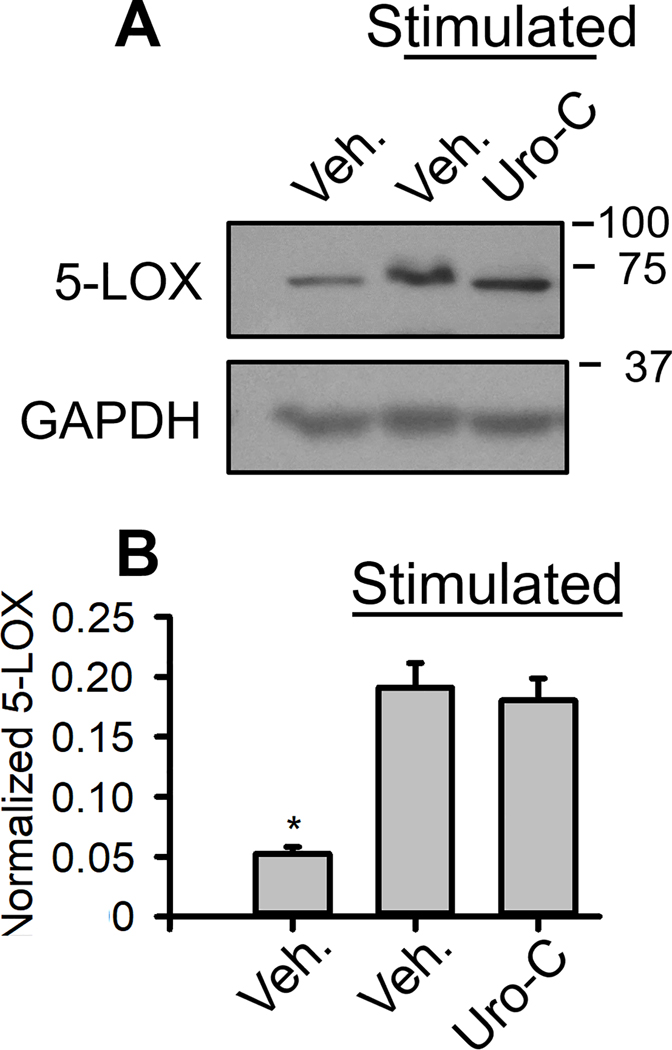
Downregulation of 5-LOX expression may explain the reduced 5-HETE and LTB4 levels in Uro-C-treated leukocytes. WB analysis showed that Uro-C did not change the expression level of 5-LOX in the leukocytes, suggesting the effect is through inhibition of the 5-LOX enzyme (Figure 6).
Figure 6.
Western blot analysis of 5-LOX expression in human leukocytes. 5-LOX expression levels were quantified by densitometry analysis using ImageJ software. Veh.: vehicle. The experiment was repeated in leukocytes from 6 volunteers. *p<0.05, compared to the stimulated/veh.-treated group.
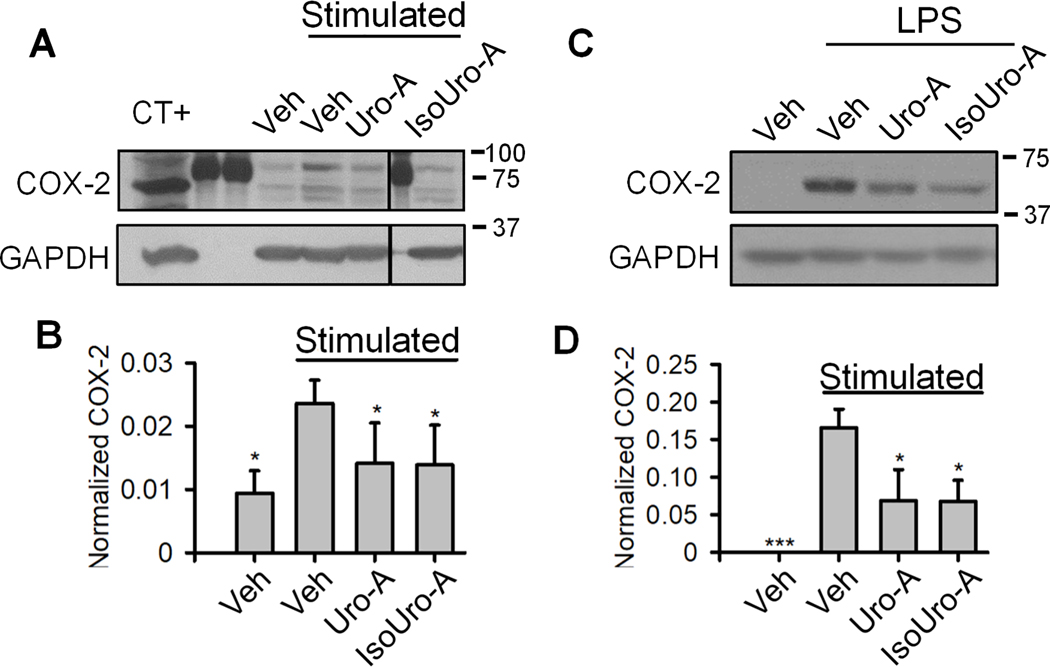
We next tested the effect of Uro-A and IsoUro-A on COX-2 expression levels in stimulated leukocytes. As expected, LPS treatment increased COX-2 levels in leukocytes, whereas in the presence of Uro-A or IsoUro-A (15 μM), this effect was attenuated (Figure 7A and B). A positive control (RAW264.7 cells protein extract) and molecular weight markers were included to ensure identification of the correct protein band representing COX-2. Uro-A and IsoUro-A (15 μM) had a similar inhibitory effect on COX-2 expression in LPS-treated RAW264.7 macrophages. (Figure 7C and D).
Figure 7.
Western blot analysis of COX-2 expression in stimulated human leukocytes (A) and murine RAW264.7 macrophages (C). The COX-2 expression levels in leukocytes (B) and macrophages (D) were quantified by densitometry analysis using ImageJ software. The lanes 2, 3, and 7 in (A) were loaded with protein molecular weight markers. The size of each marker (kDa) is given at the right of the figure. Veh.: vehicle. CT+: cell lysate obtained from LPS-treated RAW264.7 cells used as positive control. The vertical line in (A) indicates non-consecutive lanes in the same membrane. The experiment was performed using leukocytes from 6 volunteers. In RAW264.7 cells, the assay was repeated 3 times. *p<0.05 compared to the stimulated/veh.-treated group.
4. Discussion
Uro-A was first reported by our group 10 years ago as the molecule responsible for the anti-inflammatory effects associated with the consumption of ETs-rich foodstuff at the intestinal level [24]. Since then, the investigations have focused on elucidating the biological activity of urolithins (i.e., Uro-A, Uro-B, Uro-C, etc.) and EA, as well as the underlying molecular mechanisms [22, 23, 29]. A common point in these studies was the investigation of the anti-inflammatory effects of urolithins. However, the possible effect on some crucial inflammatory mediators produced by immune cells, such as eicosanoids, has been scarcely studied. Here we show that urolithins, at concentrations detected in human colonic tissue and lumen [18], might exert anti-inflammatory effects via modulation of 5-LOX and COX-2 metabolite formation in primary human leukocytes. Primary human leukocytes (monocytes, granulocytes and lymphocytes) is an interesting cellular model used in previous in vitro studies of intestinal inflammation [30]. We report effects of urolithins on traditional eicosanoids like COX-2 derived PGE2 and 5-LOX derived 5-HETE and LTB4 but also on the more recently discovered hemiketal eicosanoids, namely HKD2 and HKE2, formed by consecutive transformation of AA by 5-LOX and COX-2 [6].
The COX-2 metabolite PGE2 is a key mediator of intestinal inflammation. PGE2 promotes immune tolerance, epithelial proliferation and healing, as well as inhibition of apoptosis in intestinal epithelial cells [3]. However, a high level of PGE2 in animal models of colitis exacerbates the intestinal damage [31]. These studies indicate that the modulation of intestinal PGE2 level is an essential factor determining whether it is a disease-suppressive or pro-inflammatory molecule. Down-regulation of PGE2 in colitis animal models after consumption of pomegranate- and Uro-A-enriched diet was found to ameliorate intestinal inflammation [24], and has been associated with the effect of urolithins on intestinal cells under inflammatory conditions [22, 23]. In this study, similar to Uro-A, we found that its isomer IsoUro-A down-regulated PGE2 biosynthesis in activated leukocytes. These results suggest that the main colonic urolithins (Uro-A and IsoUro-A) could target PGE2 biosynthesis in vivo interacting with different cell types under inflammatory conditions.
5-LOX plays a unique role in IBD through the formation of leukotrienes. Massive accumulation of 5-LOX expressing neutrophils and marked high levels of LTB4 are hallmarks of IBD reported in mucosa from IBD patients [32] and colitis animal models [33]. In this regard, inhibition of LTB4 formation by natural products could be an important target for the treatment of IBD. We found that Uro-C exerted a dose-dependent reduction of LTB4 biosynthesis by stimulated leukocytes. This finding brings an additional mechanism to those reported by Piwowarski et al., who described lower levels of IL-8, ROS, and myeloperoxidase in stimulated neutrophils treated with Uro-C [34]. In addition to LT, 5-HETE is a major product of 5-LOX activity in leukocytes. In line with its effect on LTB4, Uro-C also interfered with the 5-LOX pathway reducing the formation of 5-HETE. The significance of 5-HETE formation in IBD and its potential role are unclear. 5-HETE may have limited activity by itself but may serve as a precursor to biologically active molecules [35, 36].
5S-HETE is an efficient substrate for COX-2 [7, 8]. The reaction of 5S-HETE with COX-2 generates an unstable di-endoperoxide, an intermediate that rearranges into the biologically active hemiketal eicosanoids, HKE2, and HKD2 [9]. Their effect on angiogenesis, including the promotion of endothelial cell migration and tubulogenesis [6], could have importance in IBD since the gut microvascular endothelium is a critical element in the onset and perpetuation of inflammation [37]. Thus, inhibition of HKE2 and HKD2 biosynthesis might be an attractive therapeutic antiangiogenic strategy by targeting neovascularization in IBD. Our results showed that free urolithins (Uro-A, IsoUro-A, and Uro-C), at the concentrations investigated, reduced the formation of HKE2 and HKD2. These effects were consistent with the effect of Uro-A and IsoUro-A on PGE2 (COX-2 product), and Uro-C on 5-HETE and LTB4 (5-LOX products). Uro-A and IsoUro-A reduced the formation of HKE2 and HKD2 (and PGE2) via down-regulation of COX-2 expression. The effect of Uro-A is in agreement with previous in vitro and animal studies that reported an anti-inflammatory effect via reduction of PGE2 levels together with a downregulation of COX-2 in colonic myofibroblasts and DSS-colitis animal model in F344 rats [22, 24]. Our data show the potential anti-inflammatory effect of the Uro-A isomer, IsoUro-A, that is specific for individuals belonging to the so-called “urolithin metabotype (UM)-B” (human subjects who produce Uro-A, IsoUro-A, and Uro-B as final urolithins) [38].
The effect of Uro-C on formation of HKs as well as 5-HETE and LTB4 most likely occurred through modulation of the 5-LOX activity, not the expression level of the enzyme. Uro-C decreased HK formation together with the 5-LOX products while sparing the level of the COX-2 metabolite PGE2. This result is comparable to that exerted on cancer cell models [39] and leukocytes [25]by the specific 5-LOX competitive inhibitor AA861, which blocked 5-LOX metabolites and HK biosynthesis without affecting PGE2 levels. This possible mechanism needs confirmation in future studies by incubation of the purified 5-LOX enzyme with Uro-C and specific inhibitors.
The structural differences between Uro-A, IsoUro-A, and Uro-C may help understand (at least partially) their different effects observed. Previous studies have described two hypotheses regarding the structure-activity of urolithins: i) their activity is related to the -OH group at C8 [40–42] or ii) to the number of -OH groups present in the molecule [43]. The effect of Uro-A on COX-2 pathway aligns with the first hypothesis, while the activity showed by Uro-C on 5-LOX pathway is in agreement with the second. Notably, these hypotheses fail to explain the effect of IsoUro-A on COX-2 pathway, what opens the door to investigate in future studies the structure-activity relationship of this molecule.
The intestine is not an isolated system, and IBD is frequently related to extra-intestinal manifestations, including colitis-associated musculoskeletal manifestations and hypertrophic osteoarthropathy [4, 44, 45]. Based on the anti-inflammatory activity reported for circulating phase-II metabolites of urolithins [46], we tested whether conjugated metabolites could modulate the formation of COX-2, 5-LOX, and 5-LOX/COX-2 metabolites. However, the treatment with Uro-A 3-glur, Uro-A 3-sulf, IsoUro-A 3-glur, and Uro-B 3-glur exerted no effect on eicosanoid production. This lack of anti-inflammatory activity was consistent with previous data observed for other activities of urolithins such as antiproliferative, vasorelaxant, and (or) estrogenic, suggesting that phase-II conjugation of urolithins dampens their biological activity in vivo and in vitro [47–49]. Although free urolithins are hardly detected in the bloodstream, a recent in vivo study has shown deconjugation of Uro-A glucuronide to Uro-A in a systemic LPS-induced inflammation rat model, suggesting presence of free Uro-A in microenvironments subjected to inflammatory stimuli [50]. Nevertheless, the capacity of leukocytes to hydrolyze urolithins has not been investigated, justifying future studies on the metabolism of urolithins by human leukocytes.
Clinical management of IBD using single 5-LOX or COX-2 inhibitors (i.e., analgesic and anti-inflammatory drugs) is inefficient [51–53]. An explanation for this failure resides on the possible shunting of the common substrate AA between 5-LOX and COX-2 when one pathway is inhibited [14]. Simultaneous inhibition of both enzymes has been proposed as an effective treatment in the prevention of intestinal inflammation and cancer [39, 54]. The dissimilar effects of Uro-A and IsoUro-A compared to Uro-C on 5-LOX and COX-2 offer an interesting strategy for dual inhibition of both enzymes. This could be relevant in human subjects belonging to the UM-A metabotype (80% Uro-A and 20% Uro-C), and UM-B (50% IsoUro-A, 20% Uro-B, 20% Uro-A, 10% Uro-C) compared to UM-0 (non-producers of urolithins) [18, 48, 49]. To determine whether these metabotypes are relevant in IBD carefully designed in vitro and in vivo studies are required.
Overall, this study describes, for the first time, the capacity of colonic urolithins (Uro-A, IsoUro-A, and Uro-C) to modulate well-defined branches of eicosanoid biosynthesis (i.e., 5-LOX and COX-2) as well as their cross-over in human leukocytes. We are aware that this model overlooks the interactions between immune and intestinal cells. However, it allows studying biosynthesis in a mixture of cells that can be found in the inflamed mucosa. Besides, this is the best model reported to study HK biosynthesis to date. Uro-A, IsoUro-A, and Uro-C showed the capacity to interfere with the 5-LOX/COX-2 pathway, reducing the formation of the two hemiketal eicosanoids HKE2 and HKD2 in a dose-dependent manner. This effect of urolithins (or any other phenolic compound) on the 5-LOX/COX-2 pathway is unprecedented. HK eicosanoids are novel mediators of inflammation that could be physiologically relevant in IBD (their specific role is yet to be determined), and will undoubtedly broaden our understanding of the mechanisms by which urolithins exert their anti-inflammatory effects, at the intestinal level, associated with the consumption of ETs-rich foodstuff.
Supplementary Material
Acknowledgments
This work was supported by U. S. National Institutes of Health (NIH) National Institute of General Medical Sciences (NIGMS) Grant R01GM076592 (to C.S.). J.A.G.-B. was supported by a postdoctoral award from the American Heart Association (16POST30690001) and a Juan de la Cierva contract (IJCI-2016-27633) from the Ministry of Science, Innovation and Universities (Spain). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIGMS or the NIH.
Footnotes
Conflict of interest
Authors declare not having any financial or personal interest, nor having an association with any individuals or organizations that could have influenced inappropriately the submitted work.
5. Bibliography
- [1].Zhang YZ, Li YY, Inflammatory bowel disease: pathogenesis. World J Gastroenterol 2014, 20, 91–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Perez-Jeldres T, Tyler CJ, Boyer JD, Karuppuchamy T, Bamias G, Dulai PS, Boland BS,Sandborn WJ Patel DR, Rivera-Nieves J, Cell Trafficking Interference in Inflammatory Bowel Disease: Therapeutic Interventions Based on Basic Pathogenesis Concepts. Inflamm Bowel Dis 2019, 25, 270–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Stenson WF, The universe of arachidonic acid metabolites in inflammatory bowel disease: can we tell the good from the bad? Curr Opin Gastroenterol 2014, 30, 347–351. [DOI] [PubMed] [Google Scholar]
- [4].Neurath MF, Targeting immune cell circuits and trafficking in inflammatory bowel disease. Nat Immunol 2019, 20, 970–979. [DOI] [PubMed] [Google Scholar]
- [5].Claria J, Romano M, Pharmacological intervention of cyclooxygenase-2 and 5-lipoxygenase pathways. Impact on inflammation and cancer. Curr Pharm Des 2005, 11, 3431–3447. [DOI] [PubMed] [Google Scholar]
- [6].Griesser M, Suzuki T, Tejera N, Mont S, Boeglin WE, Pozzi A, Schneider C, Biosynthesis of hemiketal eicosanoids by cross-over of the 5-lipoxygenase and cyclooxygenase-2 pathways. Proc Natl Acad Sci U S A 2011, 108, 6945–6950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Griesser M, Boeglin WE, Suzuki T, Schneider C, Convergence of the 5-LOX and COX-2 pathways: heme-catalyzed cleavage of the 5S-HETE-derived di-endoperoxide into aldehyde fragments. J Lipid Res 2009, 50, 2455–2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Schneider C, Boeglin WE, Yin H, Stec DF, Voehler M, Convergent oxygenation of arachidonic acid by 5-lipoxygenase and cyclooxygenase-2. J Am Chem Soc 2006, 128, 720–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Gimenez-Bastida JA, Suzuki T, Sprinkel KC, Boeglin WE, Schneider C, Biomimetic synthesis of hemiketal eicosanoids for biological testing. Prostaglandins Other Lipid Mediat 2017, 132, 41–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Montrose DC, Nakanishi M, Murphy RC, Zarini S, McAleer JP, Vella AT, Rosenberg DW, The role of PGE2 in intestinal inflammation and tumorigenesis. Prostaglandins Other Lipid Mediat 2015, 116–117, 26–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Lobos EA, Sharon P, Stenson WF, Chemotactic activity in inflammatory bowel disease. Role of leukotriene B4. Dig Dis Sci 1987, 32, 1380–1388. [DOI] [PubMed] [Google Scholar]
- [12].Boer RE, Gimenez-Bastida JA, Boutaud O, Jana S, Schneider C, Sulikowski GA, Total Synthesis and Biological Activity of the Arachidonic Acid Metabolite Hemiketal E2. Org Lett 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Gimenez-Bastida JA, Boeglin WE, Boutaud O, Malkowski MG, Schneider C, Residual cyclooxygenase activity of aspirin-acetylated COX-2 forms 15 R-prostaglandins that inhibit platelet aggregation. FASEB J 2019, 33, 1033–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Dennis EA, Norris PC, Eicosanoid storm in infection and inflammation. Nat Rev Immunol 2015, 15, 511–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Patrignani P, Patrono C, Cyclooxygenase inhibitors: From pharmacology to clinical read-outs. Biochim Biophys Acta 2015, 1851, 422–432. [DOI] [PubMed] [Google Scholar]
- [16].Temml V, Garscha U, Romp E, Schubert G, Gerstmeier J, Zsofia K, Matuszczak B, Waltenberger B, Stuppner H, Werz O, Schuster D, Discovery of the first dual inhibitor of the 5-lipoxygenase-activating protein and soluble epoxide hydrolase using pharmacophore-based virtual screening. Sci Rep 2017, 7, 42751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Tomas-Barberan FA, Gonzalez-Sarrias A, Garcia-Villalba R, Nunez-Sanchez MA, Selma MV, Garcia-Conesa MT, Espin JC, Urolithins, the rescue of “old” metabolites to understand a “new” concept: Metabotypes as a nexus among phenolic metabolism, microbiota dysbiosis, and host health status. Mol Nutr Food Res 2017, 61. [DOI] [PubMed] [Google Scholar]
- [18].Nuñez-Sanchez MA, Garcia-Villalba R, Monedero-Saiz T, Garcia-Talavera NV, Gomez-Sanchez MB, Sanchez-Alvarez C, Garcia-Albert AM, Rodriguez-Gil FJ, Ruiz-Marín M, Pastor-Quirante FA, Martínez-Diaz F, Yañez-Gascon MJ, Gonzalez-Sarrias A, Tomas-Barberan FA, Espín JC, Targeted metabolic profiling of pomegranate polyphenols and urolithins in plasma, urine and colon tissues from colorectal cancer patients. Mol Nutr Food Res 2014, 58, 1199–1211. [DOI] [PubMed] [Google Scholar]
- [19].Espín JC, Gonzalez-Barrio R, Cerda B, Lopez-Bote C, Rey AI, Tomas-Barberan FA, Iberian pig as a model to clarify obscure points in the bioavailability and metabolism of ellagitannins in humans. J Agric Food Chem 2007, 55, 10476–10485. [DOI] [PubMed] [Google Scholar]
- [20].Avila-Galvez MA, Garcia-Villalba R, Martinez-Diaz F, Ocana-Castillo B, Monedero-Saiz T, Torrecillas-Sanchez A, Abellan B, González-Sarrías A, Espín JC, Metabolic Profiling of Dietary Polyphenols and Methylxanthines in Normal and Malignant Mammary Tissues from Breast Cancer Patients. Mol Nutr Food Res 2019, 63, e1801239. [DOI] [PubMed] [Google Scholar]
- [21].González-Sarrías A, Gimenez-Bastida JA, García-Conesa MT, Gomez-Sánchez MB, García-Talavera NV, Gil-Izquierdo A., Sánchez-Alvarez C., Fontana-Compiano LO, Morga-Egea JP, Pastor-Quirante FA, Martínez-Díaz F Tomás-Barberán FA, Espín JC, Occurrence of urolithins, gut microbiota ellagic acid metabolites and proliferation markers expression response in the human prostate gland upon consumption of walnuts and pomegranate juice. Mol Nutr Food Res 2010, 54, 311–322. [DOI] [PubMed] [Google Scholar]
- [22].González-Sarrías A, Larrosa M, Tomás-Barberán FA, Dolara P., Espín JC, NF-kappaB-dependent anti-inflammatory activity of urolithins, gut microbiota ellagic acid-derived metabolites, in human colonic fibroblasts. Br J Nutr 2010, 104, 503–512. [DOI] [PubMed] [Google Scholar]
- [23].Gimenez-Bastida JA, Larrosa M, González-Sarrías A, Tomás-Barberán FA, Espín JC, García-Conesa MT, Intestinal ellagitannin metabolites ameliorate cytokine-induced inflammation and associated molecular markers in human colon fibroblasts. J Agric Food Chem 2012, 60, 8866–8876. [DOI] [PubMed] [Google Scholar]
- [24].Larrosa M, González-Sarrías A, Yañez-Gascon MJ, Selma MV, Azorín-Ortuño M., Toti S, Tomás-Barberán FA, Dolara P, Espín JC, Anti-inflammatory properties of a pomegranate extract and its metabolite urolithin-A in a colitis rat model and the effect of colon inflammation on phenolic metabolism. J Nutr Biochem 2010, 21, 717–725. [DOI] [PubMed] [Google Scholar]
- [25].Gimenez-Bastida JA, Shibata T, Uchida K, Schneider C, Roles of 5-lipoxygenase and cyclooxygenase-2 in the biosynthesis of hemiketals E2 and D2 by activated human leukocytes. FASEB J 2017, 31, 1867–1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Kim EC, Zhu Y, Andersen V, Sciaky D, Cao HJ, Meekins TJ, Smith TJ, Lance P, Cytokine-mediated PGE2 expression in human colonic fibroblasts. Am J Physiol 1998, 275, C988–994. [DOI] [PubMed] [Google Scholar]
- [27].Schneider C, Boeglin WE, Brash AR, Identification of two cyclooxygenase active site residues, Leucine 384 and Glycine 526, that control carbon ring cyclization in prostaglandin biosynthesis. J Biol Chem 2004, 279, 4404–4414. [DOI] [PubMed] [Google Scholar]
- [28].Yip-Schneider MT, Barnard DS, Billings SD, Cheng L, Heilman DK, Lin A, Marshall SJ, Crowell PL, Marshall MS, Sweeney CJ, Cyclooxygenase-2 expression in human pancreatic adenocarcinomas. Carcinogenesis 2000, 21, 139–146. [DOI] [PubMed] [Google Scholar]
- [29].Singh R, Chandrashekharappa S, Bodduluri SR, Baby BV, Hegde B, Kotla NG, Hiwale AA, Saiyed T, Patel P, Vijay-Kumar M, Langille MGI, Douglas GM, Cheng X, Rouchka EC, Waigel SJ, Dryden GW, Alatassi H, Zhang H-G, Haribabu B, Vemula PK, Jala VR, Enhancement of the gut barrier integrity by a microbial metabolite through the Nrf2 pathway. Nat Commun 2019, 10, 89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Kim HJ, Li H, Collins JJ, Ingber DE, Contributions of microbiome and mechanical deformation to intestinal bacterial overgrowth and inflammation in a human gut-on-a-chip. Proc Natl Acad Sci U S A 2016, 113, E7–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Sheibanie AF, Yen JH, Khayrullina T, Emig F, Zhang M, Tuma R, Ganea D, The proinflammatory effect of prostaglandin E2 in experimental inflammatory bowel disease is mediated through the IL-23-->IL-17 axis. J Immunol 2007, 178, 8138–8147. [DOI] [PubMed] [Google Scholar]
- [32].Sharon P, Stenson WF, Enhanced synthesis of leukotriene B4 by colonic mucosa in inflammatory bowel disease. Gastroenterology 1984, 86, 453–460. [PubMed] [Google Scholar]
- [33].Cuzzocrea S, Rossi A, Mazzon E, Di Paola R, Genovese T, Muia C, Achille PC, Sautebin L, 5-Lipoxygenase modulates colitis through the regulation of adhesion molecule expression and neutrophil migration. Lab Invest 2005, 85, 808–822. [DOI] [PubMed] [Google Scholar]
- [34].Piwowarski JP, Granica S, Kiss AK, Influence of gut microbiota-derived ellagitannins’ metabolites urolithins on pro-inflammatory activities of human neutrophils. Planta Med 2014, 80, 887–895. [DOI] [PubMed] [Google Scholar]
- [35].Powell WS, Rokach J, Biosynthesis, biological effects, and receptors of hydroxyeicosatetraenoic acids (HETEs) and oxoeicosatetraenoic acids (oxo-ETEs) derived from arachidonic acid. Biochim Biophys Acta 2015, 1851, 340–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].O’Flaherty JT, Jacobson D, Redman J, Mechanism involved in the mobilization of neutrophil calcium by 5-hydroxyeicosatetraenoate. J Immunol 1988, 140, 4323–4328. [PubMed] [Google Scholar]
- [37].Binion DG, Rafiee P, Is inflammatory bowel disease a vascular disease? Targeting angiogenesis improves chronic inflammation in inflammatory bowel disease. Gastroenterology 2009, 136, 400–403. [DOI] [PubMed] [Google Scholar]
- [38].Tomas-Barberan FA, Garcia-Villalba R, Gonzalez-Sarrias A, Selma MV, Espin JC, Ellagic acid metabolism by human gut microbiota: consistent observation of three urolithin phenotypes in intervention trials, independent of food source, age, and health status. J Agric Food Chem 2014, 62, 6535–6538. [DOI] [PubMed] [Google Scholar]
- [39].Ye YN, Wu WK, Shin VY, Bruce IC, Wong BCY, Cho CH, Dual inhibition of 5-LOX and COX-2 suppresses colon cancer formation promoted by cigarette smoke. Carcinogenesis 2005, 26, 827–834. [DOI] [PubMed] [Google Scholar]
- [40].Kang I, Kim Y, Tomas-Barberan FA, Espin JC, Chung S, Urolithin A C, and D, but not iso-urolithin A and urolithin B, attenuate triglyceride accumulation in human cultures of adipocytes and hepatocytes. Mol Nutr Food Res 2016, 60, 1129–1138. [DOI] [PubMed] [Google Scholar]
- [41].Dirimanov S, Hogger P, Screening of Inhibitory Effects of Polyphenols on Akt-Phosphorylation in Endothelial Cells and Determination of Structure-Activity Features. Biomolecules 2019, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Larrosa M, Gonzalez-Sarrias A, Garcia-Conesa MT, Tomas-Barberan FA, Espin JC, Urolithins, ellagic acid-derived metabolites produced by human colonic microflora, exhibit estrogenic and antiestrogenic activities. J Agric Food Chem 2006, 54, 1611–1620. [DOI] [PubMed] [Google Scholar]
- [43].Giorgio C, Mena P, Del Rio D, Brighenti F, Barocelli E, Hassan-Mohamed I, Callegari D, Lodola A, Tognolini M, The ellagitannin colonic metabolite urolithin D selectively inhibits EphA2 phosphorylation in prostate cancer cells. Mol Nutr Food Res 2015, 59, 2155–2167. [DOI] [PubMed] [Google Scholar]
- [44].Rhee SM, Park KJ, Ha YC, Hypertrophic osteoarthropathy in patient with Crohn’s disease: a case report. J Bone Metab 2014, 21, 151–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Ferreira S d. C., Oliveira B. B. M. d., Morsoletto AM, Ana de Lourdes CM, Troncon L. E. d. A., Extraintestinal manifestations of inflammatory bowel disease: Clinical aspects and pathogenesis. Journal of Gastroenterology and Digestive Diseases 2018, 3, 8. [Google Scholar]
- [46].Gimenez-Bastida JA, Gonzalez-Sarrias A, Larrosa M, Tomas-Barberan F, Espin JC, Garcia-Conesa MT, Ellagitannin metabolites, urolithin A glucuronide and its aglycone urolithin A, ameliorate TNF-alpha-induced inflammation and associated molecular markers in human aortic endothelial cells. Mol Nutr Food Res 2012, 56, 784–796. [DOI] [PubMed] [Google Scholar]
- [47].Gonzalez-Sarrias A, Gimenez-Bastida JA, Nunez-Sanchez MA, Larrosa M, Garcia-Conesa MT, Tomas-Barberan FA,, Espin JC, Phase-II metabolism limits the antiproliferative activity of urolithins in human colon cancer cells. Eur J Nutr 2014, 53, 853–864. [DOI] [PubMed] [Google Scholar]
- [48].Van Rymenant E, Grootaert C, Beerens K, Needs PW, Kroon PA, Kerimi A, Williamson G, Garcia-Villalba R, Gonzalez-Sarrias A, Tomas-Barberan FA, Van Camp J, Van de Boorde J , Vasorelaxant activity of twenty-one physiologically relevant (poly)phenolic metabolites on isolated mouse arteries. Food Funct 2017, 8, 4331–4335. [DOI] [PubMed] [Google Scholar]
- [49].Avila-Galvez MA, Espin JC, Gonzalez-Sarrias A, Physiological Relevance of the Antiproliferative and Estrogenic Effects of Dietary Polyphenol Aglycones versus Their Phase-II Metabolites on Breast Cancer Cells: A Call of Caution. J Agric Food Chem 2018, 66, 8547–8555. [DOI] [PubMed] [Google Scholar]
- [50].Avila-Galvez MA, Gimenez-Bastida JA, Gonzalez-Sarrias A, Espin JC, Tissue deconjugation of urolithin A glucuronide to free urolithin A in systemic inflammation. Food Funct 2019, 10, 3135–3141. [DOI] [PubMed] [Google Scholar]
- [51].Wallace JL, Eicosanoids in the gastrointestinal tract. Br J Pharmacol 2019, 176, 1000–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Roberts WG, Simon TJ, Berlin RG, Haggitt RC, Snyder ES, Stenson WF, Hanauer SB, Reagan JE, Cagliola A, Tanaka WK, Simon S, Berger ML, Leukotrienes in ulcerative colitis: results of a multicenter trial of a leukotriene biosynthesis inhibitor, MK-591. Gastroenterology 1997, 112, 725–732. [DOI] [PubMed] [Google Scholar]
- [53].Felder JB, Korelitz BI, Rajapakse R, Schwarz S, Horatagis AP, Gleim G, Effects of nonsteroidal antiinflammatory drugs on inflammatory bowel disease: a case-control study. Am J Gastroenterol 2000, 95, 1949–1954. [DOI] [PubMed] [Google Scholar]
- [54].Cianchi F, Cortesini C, Magnelli L, Fanti E, Papucci L, Schiavone N, Messerini L, Vannacci A, Capaccioli S, Perna F, Lulli M, Fabbroni V, Perigli G, Bechi P, Masini E, Inhibition of 5-lipoxygenase by MK886 augments the antitumor activity of celecoxib in human colon cancer cells. Mol Cancer Ther 2006, 5, 2716–2726. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.