Abstract
Purinergic receptors play important roles in central nervous system (CNS). These receptors are involved in cellular neuroinflammatory responses that regulate functions of neurons, microglial and astrocytes. Based on their endogenous ligands, purinergic receptors are classified into P1 or adenosine, P2X and P2Y receptors. During brain injury or under pathological conditions, rapid diffusion of extracellular adenosine triphosphate (ATP) or uridine triphosphate (UTP) from the damaged cells, promote microglial activation that result in the changes in expression of several of these receptors in the brain.
Imaging of the purinergic receptors with selective Positron Emission Tomography (PET) radioligands has advanced our understanding of the functional roles of some of these receptors in healthy and diseased brains. In this review, we have accumulated a list of currently available PET radioligands of the purinergic receptors that are used to elucidate the receptor functions and participations in CNS disorders. We have also reviewed receptors lacking radiotracer, laying the foundation for future discoveries of novel PET radioligands to reveal these receptors roles in CNS disorders.
Keywords: purinergic receptors, central nervous system, PET ligands, biology, neuroinflammation
Introduction
The cell surface purinergic receptors (purinoceptors) are plasma membrane proteins found in nearly all mammalian tissues including the central nervous system (CNS).1 The history of the purinergic receptors goes back to early 20th century when, for the first time, an observation was made that purines effected cardiovascular physiology.2 Almost half a century later, these receptors were classified based on their endogenous ligands into P1 and P2 categories.3
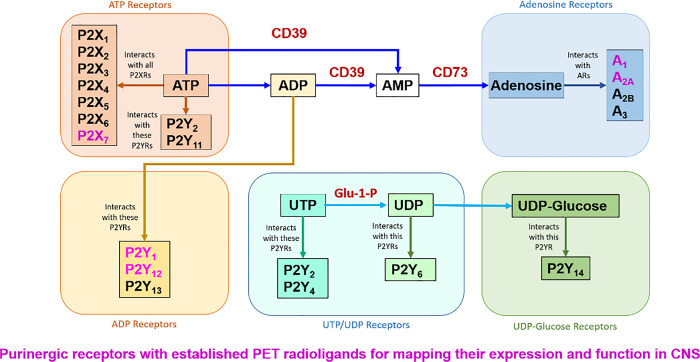
P1 or adenosine receptors (ARs) are a family of G protein–coupled receptors (GPCRs) with 4 subtypes: A1, A2A, A2B, and A3. P2 receptors are subgrouped into the ligand-gated ion channel receptors P2X with 7 receptor subtypes: P2X1, P2X2, P2X3, P2X4, P2X5, P2X6, P2X7, and P2Y, which are G protein-coupled metabotropic receptors with 8 subtypes: P2Y1, P2Y2, P2Y4, P2Y6, P2Y11, P2Y12, P2Y13, and P2Y14 (Figure 1).4 Burnstock has recently published an excellent review article on purinergic receptors, their distributions, and functions revealing the importance of these receptors in physiological system.5 Purinergic receptors play major roles in CNS disorders including Alzheimer disease (AD), Parkinson disease (PD), Huntington disease (HD), frontotemporal dementia (FD), amyotrophic lateral sclerosis (ALS), multiple scleroses (MS), traumatic brain injury (TBI), stroke, cerebral ischemia, epilepsy, psychiatric diseases, sleep disorder, and neuropathic pain.1,3,6,7
Figure 1.
Purinergic receptor subfamilies and their endogenous ligands.
In the CNS, adenosine 5′-triphosphate (ATP), an energy source for neurons and glial cells, also acts as an extracellular purinergic signaling molecule that controls communication between brain cells.8 The steady state concentration of cytosolic ATP is high, ranging between 5 and 10 mM, and very low (nM) in the extracellular space.9 Under pathological conditions and CNS insults such as trauma, ischemic stroke, epileptogenic seizures, cellular stress, neuroinflammation, and neurodegenerative disorders, high concentration of ATP is released to the extracellular region as a danger signal creating a cascade of events that eventually damages the neurons.10,11
High level of extracellular ATP from the damaged cells enforces microglia to undergo chemotaxis to the site of injury in order to remove cell debris from these sites.12 Microglial activation13 results in upregulation of P2X4 and P2X7 14,15 and downregulation of P2Y12 receptor expression.16 This balance between the expression of P2X4, P2X7, and P2Y12 receptors dictate the destiny of microglia.17 A relative expression levels of P2X4 and P2X7 receptors are positive indicator of microglial activation, while P2Y12 receptor is a negative predictor.18 Additionally, upon release of the large amount of ATP (hundreds of μmol), P2Y1 and P2X7 receptors facilitate movement of ramified microglia to the damage site, while P2Y6 receptor, a normally expressed receptor on the activated microglia, intervenes the process of phagocytosis.3,19 Furthermore, extracellular ATP can be converted to adenosine via ectonucleotidases CD39 and CD73 that are present in microglia20 and in turn activates ARs as well.21,22
While novel ligands of some purinergic receptors are currently used as pharmacological tools to define and modify actions of these receptor subtypes in the CNS,23 there is still a growing need to clearly understand these receptors’ roles in the brain, specifically as it relates to neuroinflammation and neurodegeneration. Positron emission tomography (PET) imaging has advanced our understanding of the functions of purinergic receptors in healthy and pathological brains.24,25 Herein, we have reviewed the significance of the purinergic receptors in the CNS and accumulated a comprehensive list of the existing PET radioligands that have been used as tools for understanding the functions of these receptors.
Adenosine, Receptors and Functions in the CNS
Adenosine has been widely recognized as an inhibitory modulator of the CNS.26 It acts as a homeostatic modulator at synapses26,27 and participates in neurotransmitter release,28 neuronal excitability, synaptic plasticity,29 and local inflammatory processes.30,31 Adenosine is complicated in neurobiology of learning and memory29,32,33 by overstimulating the N-methyl-d-aspartic acid (NMDA) receptors34,35 that influence long-term potentiation (LTP) and long-term depression (LTD).29 Additionally, adenosine participates in modulation of neurotransmissions exerted by dopamine (DA) and acetylcholine (Ach).36-48 Consumption of drugs of abuse and psychostimulants, either acutely or chronically, has shown to modify adenosine level in the brain.49 As such, a more clear understanding of the involvement of adenosine signaling pathway during addiction might help to explore potential treatments for substance use dependence.50 Several reports have indicated the involvement of adenosine in neuropathological conditions including stroke,51,52 epilepsy,53 PD,54-57 and other neurodegeneration disorders.31
Extracellular adenosine binds to its 4 receptor subtypes A1, A2A, A2B, and A3 to exert its effect in the CNS.30 A1R and A2AR have high affinities of 70 and 150 nM, respectively, while A2BR and A3R have a distinctly lower affinities of 5100 and 6500 nM, respectively, for adenosine.58 All ARs are present on neurons,52 astrocytes,59 oligodendrocytes,60 and microglia.61
In the brain, A1 and A2A are the major ARs.58 A1 receptor, the most abundant subtype, is widely distributed in the cortex, hippocampus, and cerebellum, while A2A receptor is mainly localized in the striatum and olfactory bulb.30 Presynaptically, A1 and A2A interact with adenosine to modulate the release of neurotransmitters.62 Postsynaptically, adenosine decreases cellular excitability through activation of A1Rs or inhibition of A2ARs.63 Thus, A1Rs impose an inhibitory brake on excitatory transmission, while A2A receptors engage in promoting excitatory effect.64 In general, A1R is considered a neuroprotective and A2A receptor is designated as a neurodegenerative receptor.65 Consequently, adenosine mainly effects brain functions through interaction with these 2 receptors, A1 and A2A,64 and a fine balance between inhibitory action of A1 and excitatory function of A2A receptors influences the neuromodulatory effect of adenosine.
Adenosine receptors undergo different activities during neurodegeneration progression.66 While both A1 and A2A receptors have shown upregulation in the frontal cortex,67 the A1R expression was reduced in hippocampus, specifically in dentate gyrus, and in CA1, but not in CA3 region.68 Additionally, studies of brain of patients with AD have revealed reduction of striatal A1Rs in this population.31 Several studies have shown that both A1R and A2AR may be involved in metabolism of amyloid β (Aβ) protein and A1 agonists and A2A antagonists might serve as an effective therapy for treating patients with AD.69,70 Moreover, there are some evidences that support the cross talk between A1Rs and A2ARs in other age-related disorders.71-73
Both A1 and A2A receptors are also expressed in endothelial cells of the primary human brain, suggesting that modulation of these receptors can alter blood–brain barrier (BBB) and result in abnormal brain permeability that could interfere with drug delivery into the CNS.74
Provided the roles of A1 and A2A receptors in brain pathologies, the availability of scientific tools such as specific PET radioligands for evaluation of these receptors functions under normal and pathophysiological conditions would be desirable and could help elucidate novel therapeutic strategies.75
Adenosine A1R and Functions in the CNS
A1 is the most abundant AR subtype in the brain with broad distribution in neurons of the cortex, hippocampus, and cerebellum.31,58 Several studies have shown that activation of adenosine A1R promoted neuroprotection, induced sedation, reduced anxiety, inhibited seizures,76 and reduced A1R exacerbated neuronal damage.58 Significant reduction in A1R expression was detected in layers of the dentate gyrus in the brain of AD subjects,68,77 providing evidence that A1R agonists might be an effective therapy for treatment of AD even at late stages of the disease.78 Additionally, A1R agonists or adenosine reuptake inhibitors have shown to decrease the extent of brain damage in most brain injuries.78,79 There are evidences of increased microglial proliferation; enhanced matrix metalloproteinase 12 (MMP-12) expression, inducible nitric oxide synthase, and pro-inflammatory interleukin-1β (IL-1β); and exacerbated demyelination in MS and neuronal injury in A1R knockdown animal models.80,81 The positive effect of A1Rs activation in the CNS suggests that this receptor could be one of the most promising targets for the development of novel drugs with neuroprotective effect for the treatment of neurological and psychiatric disorders.69
Several A1R agonist have been reported to date; most of them have only minimal brain penetration. A nonselective agonist MRS5474 has shown antidepressant and anticonvulsant activities.82 While not optimum to fully map all the functions of the A1R in the brain, several 11 C and 18 F PET radioligands of the receptor have been evaluated for imaging of the A1R in the brain as described herein.
[ 11 C] PET radioligands of adenosine A1R
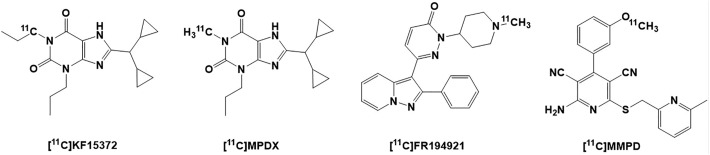
As shown in Figure 2, several 11 C PET radioligands of the A1R have been developed and tested thus far. The first PET radiotracer was developed by 11 C radiolabeling of the xanthene-derived A1R antagonist KF15372, [11 C]KF15372, and showed a specific and reversible brain uptake but an unacceptable high fraction of nonspecific binding that limited its use in preclinical evaluation of the A1R.83,84 [11 C]MPDX, another analog of A1R antagonist KF15372, was developed to measure regional A1R densities in the brain of rodent and in patients with diffuse axonal injury, a model for TBI.85 It detected an increase in A1R expression in areas surrounding the injuries in the brain, emphasizing on neuroprotective and neuromodulatory effects of A1R in TBI.85 Moreover, [11 C]MPDX was also used to investigate the cerebral density of A1Rs in early stages of PD and showed a higher binding potential in the temporal lobe of the patients with PD compared to the healthy controls.86 Similarly, [11 C]MPDX was used for mapping of the A1Rs in the brain of aged human compared to the young subjects and showed a significantly lower BPND in the frontal, temporal, occipital, parietal cortices, and thalamus of aged subjects.87 [11 C]MPDX is currently the most widely used PET agent for imaging the A1Rs in human brain.83,85 Interestingly, [11 C]MPDX was employed to identify the selective antagonists (DPCPX and caffeine) and agonist (N6-cyclopentyladenosine [CPA]) binding sites on the A1Rs and suggested that different ligands (agonists and/or antagonists) bind to A1Rs allosterically.88
Figure 2.
Structures of the adenosine A1 receptor [11C] PET radioligands: [11C]KF15372, [11C]MPDX, [11C]FR194921, and [11C]MMPD. PET indicates positron emission tomography.
The first nonxanthine 11C PET ligand of A1 R was [11C]FR194921, an analog of a potent A1R antagonist FR194921 (Ki = 2.9 nM).89,90 The PET imaging with [11C]FR194921 showed selective accumulation of A1Rs in the hippocampus, cerebral cortex, striatum, thalamus, and cerebellum of the rat brain.89 However, the specific binding of [11C]FR194921 was not as high as expected.89 Recently, a highly potent partial A1R agonist 2-amino-4-(3-methoxyphenyl)-6-(((6-methylpyridine-2-yl)methyl)thio)pyridine-3,5-dicarbonitrile was labeled with 11C to produce [11C]MMPD and showed brain uptake that was consistent with A1R.91 [11C]MMPD is currently under further evaluation for participation of A1R in sleep mechanisms.91
[18F] PET radioligands of adenosine A1R
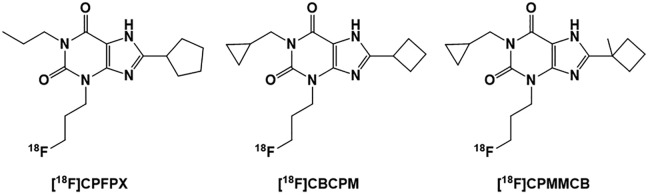
Few 18F PET radioligands have been developed and evaluated for imaging of the A1R as shown in Figure 3. Of these, [18F]CPFPX has shown high affinity and selectivity for A1R6,92; however, due to the high in vivo metabolism, this radiotracer exhibited a short biological half-life of only about 10 minutes.84 Despite this fact, [18F]CPFPX has been used for imaging of A1Rs in the human brain93 and is currently a standard PET radioligand for evaluation of the A1R density in CNS disorders such as sleep–wake research.84,94-96
Figure 3.
Structures of the adenosine A1 receptor [18F] PET radioligands: [18F]CPFPX, [18F]CBCPM, and [18F]CPMMCB. PET indicates positron emission tomography.
In order to improve metabolic stability inherent in [18F]CPFPX, two additional fluorinated PET analogs [18F]CBCPM and [18F]CPMMCB were developed and tested.84 In vitro autoradiographic studies of rat brain slices with [18F]CBCPM and [18F]CPMMCB revealed accumulation of both compounds in regions known to have a high A1R expression. However, in vitro metabolite studies using human liver microsomes identified comparable metabolic instabilities for these radioligands, similar to that of the parent ligand [18F]CPFPX.84
Importantly, both [11C]MPDX or [18F]CPFPX are inverse agonist of the A1R. [11C]MPDX did not compete with either endogenous or exogenous agonist in receptor binding but did show an increased binding potential without enhanced tracer delivery to the brain.88 Despite stated limitations, these tracers85 have presented promising imaging tools for mapping of A1R in the brain86,87,96. A list of all aforementioned A1R PET radioligands is presented in Table 1.
Table 1.
Adenosine A1 Receptor PET Ligands for CNS Studies.
| Receptor | PET ligand | Affinity (nM) | Status | References |
|---|---|---|---|---|
| A1R | [11C]KF15372 | 3.0 (Ki) | Exhibited high fraction of nonspecific binding that limited its use in preclinical evaluation of the A1 receptor. | 83 |
| A1R | [11C]MPDX | 4.2 (Ki, r) | Used to study A1R function in patients with AD. Studied in patients with TBI and in patients with early stages of PD. Currently, the most widely used PET agent for imaging the A1R in human brain. | 85-87 |
| A1R | [11C]FR194921 | 4.96 (Ki, r) 2.91 (Ki, h) |
Showed acceptable BBB permeability, but relatively low specific binding in the rodent brain that limited its further use. | 89,90 |
| A1R | [11C]MMPD | 0.49 (Ki, r) 0.8 (Ki, h) |
Exhibited an A1R partial agonist activity. Showed BBB permeability. Currently under evaluation in sleep mechanisms. | 91 |
| A1R | [18F]CPFPX | 3.49 (Ki, r) 0.18 (Ki, h) 4.4 (Kd, r) 1.26 (Kd, h) |
Used to study sleep deprivation in humans. Fast metabolic degradation. An inverse agonist of the A1 receptor. |
84,93-96
|
| A1R | [18F]CBCPM | 8.86 (Ki) | Exhibited low nonspecific binding. Metabolic degradation rate similar to [18]CPFPX. | 84 |
| A1R | [18F]CPMMCB | 3.73 (Ki) | Exhibited low nonspecific binding. Metabolic degradation rate similar to [18]CPFPX. | 84 |
Abbreviations: AD, Alzheimer disease; A1R, adenosine A1 receptor; BBB, brain–blood barrier; CNS, central nervous system; h, human; m, mice; PD, Parkinson disease; PET, positron emission tomography; r, rat.
Adenosine A2AR and Functions in the CNS
Highly expressed in the basal ganglia, A2ARs specially reside on GABAergic neurons of the striatum.58 These receptors are also expressed at low level in hippocampus, cortex, and other brain regions, and the extrastriatal increase in A2ARs has been detected in pathological challenge models and animal models of neuroinflammation.97 Several studies have revealed an increased level of A2AR expression in hippocampal neurons of patients with AD and in animal models of cognition.98,99 The same studies reported that inhibition or genetic deletion of A2A receptors enhanced memory function in the brain.100 A2A receptors are also expressed in areas of the brain that is rich in DA,101 providing a possibility of being considered as a target for developing drugs that prevent addiction.102
Inhibition of A2AR has resulted in a complete shift of LTD to LTP, supporting a major role of A2ARs in cognitive deficits.103 Inhibition of A2AR has also been promising in reduction of excitotoxicity in neurons104,105 and in movement diminished motor symptoms in PD.54,55,106-111 Additionally, in vitro studies of A2AR antagonists have shown to prevent Aβ-induced neurotoxicity and synaptotoxicity,99,100 while A2A receptor agonists increased Aβ production.100 However, study of APP/PS1 mice treated with A2A receptor antagonist istradefylline, an anti-Parkinson drug, showed an increase in Aβ42 accumulation in cortical, but not in the hippocampal neurons.112 The underlying relationship between amyloid deposition, AD progression, and adenosine remains unclear and require more clarification.97,113,114 Nevertheless, there is an indication that activation A2A receptor can result in microglia activation and antagonists of A2A receptor can reverse this process.115 Some studies have suggested that A2A receptor inhibition might also contribute to control of astrogliosis as well,116 and selective elimination of A2A receptors from astrocytes has resulted in memory improvement in animal models of AD.113 Therefore, in addition to microglia, astrocytes might also be a responsible culprit, associating A2A receptor with neuroinflammatory and neurodegenerative diseases.
Interestingly, excitotoxicity prevention by the A2A receptor antagonist appears to be time dependent, and while A2A receptor antagonist SCH58261 completely blocked the induced glutamate release in rat striatum,117 its effect was reversed 2 weeks after the treatment.105 Remarkably, this spontaneous glutamate release in response to SCH58261 treatment was different in young rats compared to the aged ones.104 Additionally, recent study suggested that, although A2A receptor antagonists initially protected against transient ischemic injury, this protective effect disappeared 7 days after ischemia and despite continued treatment with the antagonist.118
Application of pharmacological tools of the A2A receptors have shown a significant benefit in treating several CNS disorders,119 and thus, PET imaging of the A2A receptors has been useful to study in vivo expression of A2A receptors in normal and under pathophysiological brains.
[11C] PET radio ligands of adenosine A2AR
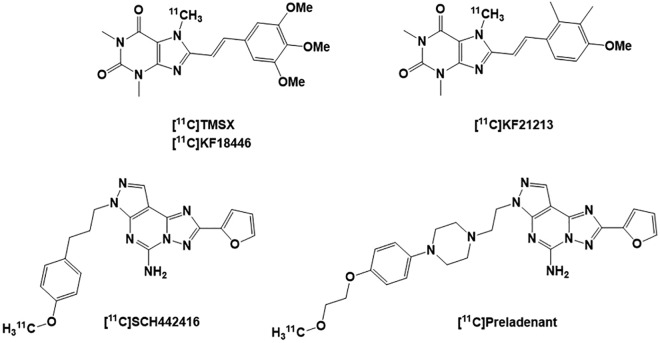
Several 11C radioligands of the A2R have been developed for PET imaging of this receptor as shown in Figure 4. These most studied ligands are 2 xanthine-derived compounds, [11C]TMSX ([11C]KF18446) and [11C]KF21213, and 2 nonxanthene compounds, [11C]SCH442416 and [11C]Preladenant.25 Within the xanthene-based PETs, [11C]TMSX has been successfully evaluated in vivo in rodent (mice and rat) and in nonhuman primate (monkeys) and has detected A2ARs in the brain with good striatum/cerebellum uptake ratio in the above animal species.25,120-122 Another xanthine PET ligand [11C]KF21213 also displayed good striatal/cerebellum uptake ratio in rodent (10.5 at 60 minutes) but showed a lower signal to noise ratio in nonhuman primate brain.121,123 Among the latter 2 xanthene-derived PET radioligands, [11C]TMSX has been the most suitable radiotracer for mapping the A2ARs and exhibited the highest binding potential in the striatum.120 Currently, [11C]TMSX is the most broadly used PET imaging radioligand for visualization of A2A receptor in the brain and therefore is considered the gold standard for brain imaging of the A2A receptors.121,122 A major consideration when using this tracer is the fact that dosing and blood sampling need to be performed under dimmed light due to [11C]TMSX photoisomerization.
Figure 4.
Structures of the adenosine A2A receptor [11C] PET radioligands: [11C]TMSX, [11C]KF21213, [11C]SCH442416, and [11C]Preladenant. PET indicates positron emission tomography.
To overcome the photoisomerization issue inherent with xanthene radiotracer [11C]TMSX, a potent, selective, and reversible nonxanthene A2AR antagonist SCH442416 was radiolabeled to produce [11C]SCH442416 and exhibited a good striatum/cerebellum uptake ratio with slow rate of metabolism in rat.124 In rhesus monkeys, [11C]SCH442416 was rapidly accumulated in the brain, with twice as much radioactivity concentration in the striatum than in the cerebellum, but it showed a high nonspecific binding activity in monkey brain.124 [11C]SCH442416 has been used to study receptor occupancy and involvement of striatal A2ARs in the brain of PD patients with dyskinesia.125,126 Both A2ARs antagonists [11C]TMSX and [11C]SCH442416 have already been used in multiple studies in human.122,124 Preladenant, a PD drug, was also radiolabeled with 11C to produce [11C]Preladenant.126-128 Studies of this PET tracer in the brain of monkey showed an uptake that is consistent with the distribution of A2ARs with highest uptake in the putamen and the caudate, respectively.128 The lowest uptake of [11C]Preladenant was observed in the cerebellum. Estimated binding potential values of [11C]Preladenant with different scan durations were similar (4.3-5.3 in A2AR-rich regions).128 Preinjection with nonradiolabeled Preladenant reduced the tracer uptake in regions rich in A2AR and pretreatment with caffeine reduced tracer uptake in the striatum in a dose-dependent manner. [11C]Preladenant PET is a suitable tool to study A2AR occupancy in the brain.128 The regional distribution of [11C]Preladenant PET is consistent with known A2AR densities in the brain.126
[18F] PET radio ligands of adenosine A2AR
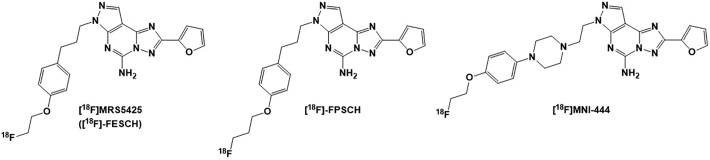
Few 18F PET radioligand derivatives of potent and selective A2AR antagonist SCH442416 were developed for imaging of the A2ARs. These PET ligands include [18F]MRS5425 ([18F]-FESCH),129,130 [18F]-FPSCH,130 and [18F]MNI-444131 as shown in Figure 5. The A2AR-mediated uptake of [18F]MRS5425 was higher in the striatum of the 6-OHDA lesion-induced rats compared to that of the normal rats, making [18F]MRS5425 a suitable PET radiotracer for imaging of PD patients.129
Figure 5.
Structures of the adenosine A2A receptor [18F] PET radioligands: [18F]MRS5425 ([18F]-FESCH), [18F]-FPSCH, and [18F]MNI-444. PET indicates positron emission tomography.
A fluoropropyl analog [18F]-FPSCH was also developed and studied for mapping of the A2A receptors expression in rat brain.130 Both [18F]-FESCH and [18F]-FPSCH showed similar striatum/cerebellum ratios post injection as well as reversible binding in the brains of rat.130 However, dynamic PET imaging for 60 minutes, under baseline and blocking conditions, demonstrated [18F]MRS5425 ([18F]-FESCH) to be the most suitable 18F PET radioligand for quantifying A2A receptor expression in rat brain.130
Another highly potent nonxanthene 18F PET radioligand analog of SCH442416, [18F]MNI-444 (Ki = 2.8 nM, human recombinant A2ARs) was developed to noninvasively monitor A2A receptor densities and functions in the brain of patients with PD.131 [18F]MNI-444 radioligand has shown high uptake, rapid kinetics, and high target/nontarget ratios in the brain, consistent with A2A receptor distribution.131,132 Thus far, [18F]MNI-444 has turned out to be a superior imaging tracer among all the 18F PET radioligands for studying and mapping the A2AR in the brain.133 A list of all A2A receptor PET radioligands is presented in Table 2.
Table 2.
Adenosine A2A Receptor PET Ligands for CNS Studies.
| Receptor | PET ligand | Affinity (nM) | Status | References |
|---|---|---|---|---|
| A2AR | [11C]TMSX ([11C]KF18446) |
5.9 (Ki, r) | Used widely and considered a gold standard PET ligand for mapping A2R. Has been studied in human subjects and in patients with PD, HD, and MS. | 25,121,122 |
| A2AR | [11C]KF21213 | 3.0 (Ki, r) | Possessed high in vitro selectivity (A2A/A1 >3300). Good striatal/cerebellum uptake ratio in rodent, but lower signal to noise ratio in nonhuman primate brain. |
25,123
|
| A2AR | [11C]SCH442416 | 0.048 (Ki, h) 0.5 (Ki, r) |
Studied in patients with PD who suffer from the levodopa-induced dyskinesia. The first suitable nonxanthine A2AR PET ligand. |
25,124
|
| A2AR | [11C]Preladenant | 1.1 (Ki, h) 2.5 (Ki, r) |
Studied in rat, rhesus monkeys, and human with PD. First human study was published in 2017. | 25,125,126,128 |
| A2AR | [18F]MRS5425 ([18F]-FESCH) |
12.4 (Ki) | Used for quantifying A2A receptor expression in the rat brain and showed higher concentration in the striatum of the 6-OHDA lesion induced in rats, possibly a suitable PET radiotracer for imaging of PD. |
129,130
|
| A2AR | [18F]-FPSCH | 53.6 (Ki) | Propyl analog of [18F]FESCH and very similar in property, but less suitable PET. | 130 |
| A2AR | [18F]MNI-444 | 2.8 (Ki, h) | Exhibited superior property for studying and mapping the A2AR in the brain. Used as PET and SPECT radiopharmaceutical to study human brain. Showed high uptake, rapid kinetics, and high target/nontarget ratios in the brain, consistent with A2A receptor distribution. | 25,131-133 |
Abbreviations: A2AR, adenosine 2A receptor; CNS, central nervous system; h, human; HD, Huntington disease; m, mice; MS, multiple sclerosis; 6-OHDA, 6-hydroxydopamine; PD, Parkinson disease; PET, positron emission tomography; r, rat.
P2X Receptors and Functions in the CNS
P2X receptors (P2XRs) are a family of 7 fast-acting subreceptors P2X1 to P2X7. These nonselective cation-gated channels receptors exhibit high Ca2+ permeability upon activation by extracellular ATP.134,135. P2X receptors are widely distributed on non-neuronal and neuronal cells and participate in numerous physiological as well as pathophysiological processes.15 Several studies have suggested the change in P2XRs expression under neuroinflammatory, nerve transmission, and pain sensation conditions.136 Activation of some P2XRs has been associated with various pathological disorders of CNS including neuroinflammation and neurodegeneration.36
With the exception of P2X7R that is only activated by high concentration of ATP (hundreds of μM), other P2X receptor subtypes are usually activated at high nM to low μM ATP concentration.10,11. In the CNS, P2XRs participate in modulation of neurotransmission, neuron-glia communication, inflammation, and apoptosis.136-139 Adenosine 5′-triphosphate released under physiological conditions modulate synaptic plasticity by acting on P2X receptors via Ca2+-dependent interaction with the NMDA receptors that facilitate LTP in the hippocampus.140 In general, overexpression of the P2X3, P2X4, and P2X7 receptors have been detected in CNS disorders and their antagonists could potentially be useful therapies for the treatment of CNS diseases including neurodegeneration and brain injuries.6,136,141 Among subtypes of the P2XRs, P2X7R has been the focus of many studies as a therapeutic target for treating brain disorders.142 Herein, we focus on 3, P2X3, P2X4 and P2X7, receptors and review their existing PET radioligands.
P2X3 Receptor and Functions in the CNS
P2X3 receptors, either as a homomeric P2X3 or a combination of P2X2-P2X3 receptors, are primarily expressed on nociceptive sensory neurons143 and mediate the ATP nociceptive signaling.144 In the spinal cord, released ATP from injured cells facilitates glutamate release from primary afferent neurons by its action at the presynaptic P2X3 receptors.144,145 P2X3 knockout animals have shown to exhibit a reduction of activity of afferent nerves and nociceptive signaling,146 and P2X3 receptor expression downregulation by antagonist A-317491 has resulted in reduced mechanical hyperalgesia and neuropathic pain,147,148 supporting the effect of ATP on peripheral nerve afferents.146
Thus far, few antagonists of P2X3 and P2X2/3 have been identified. One of them, A-317491, has shown to reduce mechanical allodynia and thermal hyperalgesia following chronic nerve constriction.149,150 AF-353 is another P2X3 receptor antagonist that has shown similar potency for human and rat recombinant P2X3 homotrimers (IC50 = 8.7 and IC50 = 8.9 nM, respectively).151 A prodrug version of AF-353, (RO-51), has been developed to treat urological dysfunction and chronic pain.152 A recently marketed P2X3R antagonist, gefapixant (AF-219, MK-7264), is used for reduction of exaggerated, persistent, and frequent urge to cough as a result of hypersensitized sensory neurons, triggered by injury or infection.153-155 Recently, a series of 5-hydroxy pyridine derivatives were synthesized and evaluated for their activities at hP2X3 receptors.156 One of the compounds in this series, prodrug 28, has shown antiallodynic activity in spinal nerve ligation and chemotherapy-induced peripheral neuropathy in rats.156 This and other data on the P2X3R antagonists indicate that targeting the P2X3 receptors could be a promising treatment for neuropathic pain. Thus far, there is no identified PET radioligand for evaluation of the P2X3 receptors, to the best of our knowledge.
P2X4 Receptor and Functions in the CNS:
P2X4 receptor, the first identified P2X receptor, is widely expressed in peripheral nervous system and CNS.157 P2X4 receptors are one of the most abundantly expressed functional purinergic receptors found on glial cells and most neurons17,158 and are upregulated on activated microglia after brain and spinal cord injuries.159 Similar to P2X7R, P2X4R facilitate ion efflux through cell membrane and induces activation of inflammasomes.137 Supporting evidences indicate that P2X4 receptors physically couple with GABAA receptors as well as with the P2X2 receptors and this cross talk may play a role in regulating synaptic signaling and plasticity of neurons.160 Alcohol abuse is known to enhance neuroinflammation through P2X4Rs activation161 and there are suggestions of implication of P2X4Rs in tolerance to morphine and hyperalgesia induction by morphine.161,162 P2X4 receptors are upregulated in TBI6, in acute experimental encephalomyelitis (EAE) rodent model of multiple sclerosis17 and following hypoxia and ischemia events.163 In neurons, P2X4R has shown to stimulate activation of the inflammasome caspase-1 resulting in cytokines IL-18 and IL-β release, and in P2X4R knockout mice, impaired inflammasome signaling was reported to couple to the reduction of IL-β level.164 Inhibition of P2X4 receptors by antagonists prior to cerebral ischemia has resulted in an attenuation of the neuroinflammation response and health of neuronal tissue.165 Additionally, P2X4 receptor upregulation has been reported in several rodent models including mechanical allodynia,14 superoxide dismutase 1-mutation models of ALS,166 EAE model of multiple sclerosis,17 post spinal cord injury,164 formalin-induced inflammatory pain,167 TBI,168 and ischemia.165 These data support the central role that P2X4 receptors play in coordinating the microglial response to cellular injuries and/or diseases.
Therefore, P2X4 receptor antagonists might have potentials for the treatment of neuropathic pain,23 epilepsy, stroke, multiple sclerosis, and neurodegenerative diseases such as PD and AD.159,169 Paroxetine, a selective serotonin reuptake inhibitor, has shown to behave as an allosteric antagonist of P2X4Rs at high concentrations (IC50 = 2.45 μM, rat, and IC50 = 1.87 μM, human).170 Thus far, attempts to identify potent and selective antagonist of P2X4Rs have resulted in the discovery of allosteric ligands with low potency and poor aqueous solubility. Among these antagonists is the benzodiazepine derivative 5-BDBD (IC50 = 0.5 μM)171 and its analogs172 that possessed allosteric antagonism, but low potency at P2X4R.172 The urea derivative BX-430 was another allosteric P2X4 receptor antagonist with low potency (IC50 = 0.54 μM).173 An additional allosteric P2X4Rs antagonist is the high lipophilic and poor soluble carbamate PSB-12054 with good selectivity and reasonable potency at human P2X4Rs but much less potency at rat and mice P2X4Rs.174 An analog of PSB-12054, PSB-12062 with better solubility, was developed later and showed equal potency at human, rat, and mouse and good selectivity for P2X4R versus P2X1, P2X3, and P2X7 receptors.175 Recently, a new diazepine antagonist NP-1815-PX with reasonable potency and selectivity at P2X4Rs (IC50 = 0.26 μM, hP2X4R, concentration dependent)176 has shown an antiallodynic effect and suppression of mechanical allodynia in mice with traumatic nerve damage without affecting acute nociceptive pain and motor function, suggesting that microglial P2X4Rs could potentially act as an important target for treating chronic pain.176 Nippon Chemiphar has reported the discovery of yet another potent antagonist of the P2X4Rs, NC-2600 for the treatment of neuropathic pain. Phase I evaluation of NC-2600 has been completed and phase II evaluation is underway. NC-2600 is believed to be the first-in-class candidate to control pain by targeting glial cells. NC-2600 is currently under safety/tolerability studies. To our best knowledge, lack of highly potent P2X4Rs ligands has limited efforts to develop PET ligand for this receptor.
P2X7 Receptor and Functions in the CNS
P2X7R is regarded as an important silent receptor as its expression is only upregulated when ATP concentration increases to a high level,177 suggesting the high relevance of P2X7Rs in pathological conditions.178 P2X7Rs are expressed on presynaptic neurons, astrocytes, and oligodendrocytes, but its highest concentrations is expressed on microglia where it releases pro-inflammatory cytokine IL-1β, a key mediator of chronic inflammation and chronic pain.6,178 Several studies of the P2X7 receptors have shown involvement of this receptor in animal models of neuroinflammatory diseases including AD,137,179,180 PD,181 HD,182 FD,1 ALS,183 MS,184 TBI,15 cerebral ischemia,6 epilepsy,185 depression,186,187 anxiety, and bipolar disorders.178 Astrocytic P2X7Rs expression has also shown to be involved in the neurotoxic phenotype model of ALS.188
Stimulation of P2X7Rs by high level of ATP (hundreds of μM) produces a large transmembrane pores, permeable to large molecular sizes of up to 900 Da, promoting further increase in extracellular ATP release that can lead to activation of caspases and result in cell death.189 P2X7 receptor expression in the CNS could be increased with systemic administration of bacterial lipopolysaccharide (LPS), providing a realistic mechanism similar to systemic infection in the brain.6 Genetic deficiency and pharmacological inhibition of P2X7 receptors have shown to attenuate hyperactivity induced by amphetamine in the model of manic bipolar disorder.190 Mood stabilizer drugs such as lithium and valproate reversed ATP-induced cell death in the hippocampus, an action that is probably mediated by P2X7 receptors.191,192
Discovery of a number of potent and selective P2X7Rs antagonists has been instrumental in studying the receptor in human and rodent. Some of these ligands including AZD9056193 and CE-224535194 were developed for the treatment of inflammation but failed to exhibit benefits in patients.194 Other existing and understudy ligands of the P2X7 receptors include A438079, A740003, A804598, A839977, AZ1060612, AZ11645373, GSK1482160, and GW791343.9,195
Some P2X7 receptor antagonists were specifically developed to study disorders of the CNS. These are the brain penetrant benzamides GSK1482160,196 JNJ-42253432,197 JNJ-47965567,198 triazoles JNJ-54232334 and JNJ-54140515,199 JNJ-54166060,200 JNJ-54173717,201 JNJ-54175446,202 and JNJ-55308942.203 These molecules have demonstrated P2X7 receptor antagonist activities in rodent and human. Three of these molecules, GSK1482160, JNJ-54175446, and JNJ-55308942, have already moved into clinical trials for evaluation of the disorders of CNS.187
Association of P2X7R activation with pro-inflammatory phenotype of microglia in CNS diseases makes P2X7R an interesting and valuable biomarker of inflammation.9 Development of useful PET radioligands for imaging the P2X7Rs in CNS can potentially enable studies of the pharmacology and functional role of this receptor in neuroinflammation and evaluate the effect of therapeutic agents in treating neuroinflammatory and neurodegenerative diseases. Fortunately, an ample number of potent and selective P2X7R ligands has presented opportunities to develop a few 11C and 18F PET radioligands of the receptor as described herein.24,204
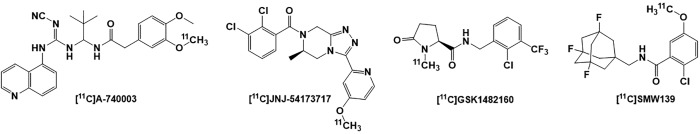
[11C] PET radioligands of P2X7R
Several antagonists of the P2X7R have been radiolabeled with 11C for evaluation of the receptor expression and function as shown in Figure 6. The selective P2X7R antagonist A-740003 (IC50 = 18 nM, rP2X7R and IC50 = 40 nM, hP2X7R) was radiolabeled with 11C to produce [11C]A-740003,205 but showed low biodistribution and poor brain permeability.205 The first brain penetrable 11C PET radioligand for quantification of P2X7R expression in the brain was [11C]JNJ-54173717. This tracer showed high potency in humanized rat P2X7R (IC50 = 4.2 nM, hP2X7R)206 and excellent uptake in the hP2X7R overexpressing striatum area that was reduced by pretreatment with nonradioactive antagonists JNJ-54173717 and JNJ-42253432, suggesting selective P2X7R binding of this radiotracer in the brain.24 Additionally, [11C]JNJ-54173717 displayed high brain uptake in rhesus monkey, an indication of BBB penetrability to study receptor expression levels in neurodegenerative disorders in humans.206 Another potent P2X7 receptor antagonist, benzamide GSK1482160 was also radiolabeled with 11C to produce PET radioligand [11C]GSK1482160 (Ki = 2.63 nM, IC50 = 3 nM, hP2X7 and Kd = 1.15 ± 0.12 nM, hP2X7R).207-209 Evaluation of [11C]GSK1482160 in mouse model of LPS-induced neuroinflammation showed increased uptake of 3.6-fold compared with saline-treated mice in all studied organs (2.9- to 5.7-fold).208 In the EAE rat model of MS, [11C]GSK1482160 uptake was high in rat lumbar spinal cord and the highest uptake was measured at the EAE peak stage.209 Micro-PET studies of [11C]GSK1482160 in rhesus monkey has shown high tracer retention and a homogeneous brain distribution.209 All of these studies strongly correlated the [11C]GSK1482160 uptake with the P2X7 R overexpression on activated microglia and its participation in neuroinflammation.209 Another 11C PET radioligand of P2X7 R antagonist was developed by radiolabeling of the SMW139 (Ki = 32 nM, hP2X7R)210 and was evaluated in a humanized rat model to study the expression of P2X7R in striatum.210 Even though [11C]SMW139 did not detect overexpression of the P2X7 R in postmortem brain of patients with AD,210,211 this PET radioligand has entered clinical evaluation in patients with MS and is currently the first in human to study neuroinflammation in patients with MS.210,212
Figure 6.
Structures of the P2X7 receptor [11C] PET radioligands: [11C]A-740003, [11C]JNJ-54173717, [11C]GSK1482160, and [11C]SMW139. PET indicates positron emission tomography.
[18F] PET radioligands of P2X7R
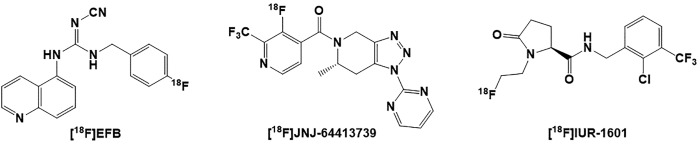
Thus far, there are reports of 3 known 18F radioligands for evaluation of the P2X7R expression as shown in Figure 7. An analog of a potent P2X7R antagonist A-804598 was radiolabeled with 18F to yield [18F]EFB that showed high affinity for human and rat P2X7R.213 However, this PET tracer suffered from a low brain uptake in both healthy and LPS-treated rats that limited its application for brain imaging of the receptor.213 Another PET radioligand [18F]JNJ-64413739 was developed by 18F radiolabeling of a potent and selective P2X7 R antagonist JNJ-64413739 (Ki = 2.7 nM, rat cortex, Ki = 15.9 nM, hP2X7R). [18F]JNJ-64413739 has shown to be an effective PET ligand for mapping of P2X7R in human brain.214 The PET imaging studies with [18F]JNJ-64413739 in nonhuman primate showed engagement of the tracer with the P2X7R. In vitro blocking experiments of [18F]JNJ-64413739 with 2 known P2X7R antagonists demonstrated inhibition of the tracer binding to rat brain tissue sections in a dose-dependent manner.214-216 While [18F]JNJ-64413739 may be a useful tool for imaging of neuroinflammation, lack of a reference region in image analysis (ie, similar to TSPO) might hinder its use as an optimum PET radiotracer for detection of neuroinflammation.214,217 Most recently, our team has synthesized a novel 18F radioligand [18F]IUR-1601, the fluoroethyl analog of GSK1482160.218 [18F]IUR-1601 has been successfully evaluated in vitro and is currently under evaluation in 5XFAD animal model of AD. A list of all P2X7 receptor PET radioligands is presented in Table 3.
Figure 7.
Structures of the P2X7 receptor [18F] PET radioligands: [18F]EFB, [18F]JNJ-64413739, and [18F]IUR-1601. PET indicates positron emission tomography.
Table 3.
P2X7 Receptor PET Ligands for CNS Studies.
| Receptor | PET ligand | Affinity (nM) | Status | References |
|---|---|---|---|---|
| P2X7R | [11C]A-740003 | 18 (IC50, r) 40 (IC50, h) |
Showed low brain uptake and a moderate metabolic rate. | 205 |
| P2X7R | [11C]JNJ-54173717 | 1.6 ± 0.1 (Ki, r) 4.2 (IC50, h) 7.6 (IC50, r) |
Entered rat brain and showed excellent uptake in the human P2X7R overexpressing striatum. Showed high initial brain uptake in nonhuman primates. | 201,206 |
| P2X7R | [11C]GSK1482160 | 2.63 ± 0.6 (Ki) 1.15 ± 0.12 (Kd) 3 (IC50, h) |
Possessed high P2X7 selectivity and good blood–brain barrier permeability. Studied in mouse model of LPS-induced neuroinflammation and EAE rat model of MS. Showed a high tracer retention and a homogeneous brain distribution in rhesus monkey. In preclinical evaluation. | 196,207-209 |
| P2X7R | [11C]SMW139 | 32 ± 5 (Ki, h) 24.5 ± 5.5 (IC50, h) 158 ± 44 (IC50, m) |
Entered clinical trial to study neuroinflammation in patients with MS. | 210-212 |
| P2X7R | [18F]EFB |
2.88 (Ki, h) 36.1 (Ki, r) 547 (Ki, m) |
Exhibited low brain uptake due to limited compatibility of the cyanoguanidine moiety with BBB entry in rats. Limited solubility. | 213 |
| P2X7R | [18F]JNJ-64413739 | 15.9 ± 2.0 (Ki, h) 2.7 ± 1.1 (Ki, r) 1.0 ± 0.2 (IC50, h) 2.0 ± 0.6 (IC50, r) |
Showed dose-dependent competitive binding with JNJ-54175446 in monkey PET studies. A potential imaging biomarker of central neuroinflammation. Entered clinical trial in 2017. |
214-217
|
| P2X7R | [18F]IUR-1601 | 4.31 (Ki) 7.86 (IC50) |
Evaluated in in vitro assays and is currently under evaluation in 5XFAD animal model of AD. |
218
|
Abbreviations: AD, Alzheimer disease; BBB, brain–blood barrier; CNS, central nervous system; h, human; LPS, lipopolysaccharides; m, mice; MS, multiple sclerosis; PET, positron emission tomography; P2X7R, P2X7 receptor; r, rat.
P2Y Receptors and Functions in the CNS
The metabotropic P2Y receptors are a family of GPCRs with 8 subtypes: P2Y1, P2Y2, P2Y4, P2Y6, P2Y11, P2Y12, P2Y13, and P2Y14, with ubiquitous expression and effect in body.18,219 In the CNS, P2Y receptors are localized on neurons, microglia, astrocytes, and oligodendrocytes where they have important physiological roles in glial-cell communication, neurotransmission, and neurogenesis.220,221 The hippocampus expresses P2Y1, P2Y2, P2Y4, P2Y6, and P2Y12 receptors in addition to all the P2X receptor subtypes.67
In contrast to the ion channel P2X receptors, P2YRs are activated by several endogenous ligands including the adenine nucleotides: ADP (acting on P2Y1, P2Y12, and P2Y13) and ATP (acting on P2Y2 and P2Y11), and the uridine nucleotides UTP (acting on P2Y2 and P2Y4), UDP (acting on P2Y6), and the UDP-glucose (acting on P2Y14).222
Several studies have revealed that during brain injury and under pathological conditions, neurons,223 astrocytes,189 and microglia224 release high concentration of ATP that acts as a neuromodulator of the P2Y receptors.134,225 P2Y receptor activation then induces fast synaptic transmission through postsynaptic P2X receptors in the brain.135 Therefore, P2Y receptors affect the release of number of neurotransmitters225 through actions on calcium influx.226
The P2Y receptors, individually or in combination, participate in many biological conditions. P2Y1R has a complex role in modulation of DA release, even though there is no evidence of its existence in the dopaminergic terminals of the prefrontal cortex.227,228 P2Y1, P2Y12, and P2Y13 receptors specifically block the release of noradrenaline in the spinal cord,229 the hippocampus,230 and in the cortex,228 while these same receptors inhibit the release of serotonin in the cortex.231 P2Y1, P2Y2, P2Y4, P2Y12, and P2Y13 receptors have also shown to inhibit the release of glutamate in the spinal cord,226 the hippocampus synapses, and the cerebral cortex.221 P2Y12 receptor is known as a protective receptor that stimulates microglial migration toward neuronal damage.16 Functional studies have demonstrated the involvement of P2Y receptors in seizure pathology, as well.232
Some of the P2Y receptors have prominent roles in neurodegenerative diseases. For example, during neuronal injuries, P2Y2, P2Y4, and P2Y6 receptors regulate the phagocytic activity of microglia upon leaked UTP and UDP from injured hippocampal cells.233 Microglia execute the uptake of cellular debris specifically through P2Y6 receptor.233 P2Y1, P2Y4, and P2Y12 are prominent P2YRs in the brain and represent favorable targets for treating neuroinflammatory diseases and neurodegenerative disorders including AD.46,226
Activation of some P2YRs has shown to inhibit the excitatory transmission mediated by postsynaptic NMDA receptors and increase the inhibitory action of the GABAA receptors prompting LTP.226,234 In the CA1 region of hippocampus, released ATP from astrocytes has shown to result in LTD of synapses from neighboring neurons via activation of the presynaptic P2Y receptors, indicating participation of ATP from activated astrocytes in this form of plasticity.140 In a specific region of the brain, the medial habenular nucleus that is involved in depression, stress, and nicotine withdrawal234,235 an application of UTP or UDP resulted in LTP of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor-mediated currents, apparently through activation of presynaptic P2Y4R.226
There has been suggestions that activation of P2Y2 and P2Y4 receptors may be useful in treating neurodegenerative diseases.18,221 Studies of rat primary cerebellar neurons has provided evidence that P2Y13 receptor activation protected neurons against oxidative stress-induced death.236 P2Y1 receptor has specifically emerged as a new target for treating cognitive dysfunction in CNS.226,237,238
Overall, investigations of the P2Y family receptors have been challenging due to the lack of potent, selective, and high-specific-radioactivity PET radioligands for these receptors. Herein, we present the subfamily of P2Y receptors and their ligands that are known to have important functions in the CNS.
P2Y1 Receptor and Functions in the CNS
P2Y1 receptor is one of the most abundant P2Y receptor subtype in brain tissues with large expression on neurons of the cerebellum,237 cerebral cortex, and ischemia-sensitive regions of the hippocampus that is predominantly implicated in AD.239 P2Y1R is also expressed on oligodendrocytes and astrocytes in the brain and optic nerves.240,241 Human P2Y1R is activated by ADP (EC50 = 10 nM),220,221 and ADP activation of the receptor induces platelet activation making this receptor as an important antithrombotic drug target.242 Like P2X7 receptor, P2Y1 receptor also mediates activation of microglia after brain injuries and insult.243
There are reports of P2Y1 receptor upregulation in CNS under pathological conditions such as mechanical injury,244 ischemia,245 and neurodegeneration.246 Additionally, hyperactivity of astrocytic P2Y1 receptors have been detected in animal models of AD246,247 and increased expression of the receptor has been observed in hippocampus and cortex of postmortem brain sections in patients with AD.248 P2Y1R is also upregulated after stroke and TBI and inhibition of the receptor has been shown to reduce cognition deficit resulted from these conditions.249 Indeed, antagonists of the P2Y1 receptor have shown to reduce neuronal injury and improve spatial memory in rat model of TBI.250,251
Inhibition of astrocytic P2Y1R has resulted in cytokine and chemokine transcriptional suppression and brain protection.247,250 Blocking of hippocampal P2Y1 receptors has shown to enhance synaptic signaling and might be responsible for promotion of antioxidant mechanism that consequently results in pro-survival pathways.249,252 P2Y1R antagonists have also shown to mediate and upregulate the oxidoreductase enzymes by increasing tolerance to hydrogen peroxide.253 A recent study has shown that P2Y1 agonist MRS2365 initiated nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) release after stroke and enhanced neuroinflammatory responses, while P2Y1 receptor antagonist MRS2179 attenuated inflammation and reduced the infarct size.250,251 Furthermore, P2Y1 antagonist has shown to help patients with schizophrenia to experience reduction in unnecessary information and noise entering their brain.254
Ironically, there is an evidence that P2Y1Rs may also promote axonal elongation to offset the neurotoxic effects of neurofibrillary tangles and have a neuroprotective effect in patients with AD.255 Nevertheless, there are still more supporting data that P2Y1R antagonist could potentially be appropriate candidates for the treatment of neurodegenerative diseases.247-249
PET radioligand of P2Y1R
Overall, investigation of the P2Y family receptors has been challenging due to the absence of potent, selective, and high-specific-radioactivity PET radioligands. Recently, a highly potent (IC50 = 10 nM) P2Y1R antagonist (compound 18) was identified and radiolabeled with [18F] ([18F]18) as shown in Figure 8. Although [18F]18 exhibited fast in vivo metabolism, its high potency and unique allosteric binding mode has provided an opportunity to investigate it as a potential PET tracer for mapping the P2Y1 receptor.256 Additionally, highly potent, selective, and high specific radioligand [32P]MRS2500 has been used successfully to measure human P2Y1 receptor expression in Sf9 insect cell membrane.257
Figure 8.
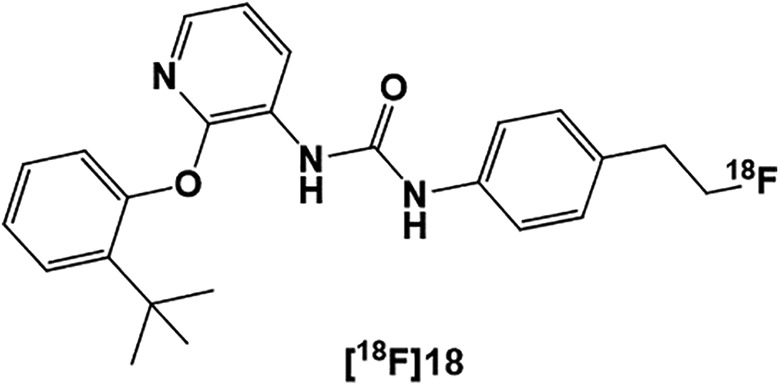
[18F]Radiolabeled P2Y1R PET ligand [18F]18. PET indicates positron emission tomography.
P2Y2 Receptor and Functions in the CNS
One of the most studied receptors in this family is the P2Y2R, with a wide distribution in all cells in human body and particularly in immune cells.258 In the brain, P2Y2 receptor is expressed on neurons, microglia, and astrocytes.12,259 Under normal brain conditions, there is a low expression of P2Y2R on neurons, but it can be upregulated to exert neuroprotective effects against the release of pro-inflammatory cytokine IL-1β as a result of P2X7R expression on activated microglia.260 In the AD mouse model TgCRND8, genetic deletion of P2Y2 receptor has shown to enhance early AD pathology, while activation of the receptor enhanced phagocytosis and degradation of the Aβ peptide.261 Furthermore, activation of P2Y2R has proven to result in degradation of amyloid precursor protein by α-secretase, yielding to soluble APPα peptide that prevented production and accumulation of the neurotoxic Aβ1-42. 262,263 In studies that compared brain neocortex and parietal cortex of postmortem patients with AD to those of the normal aged controls, the low level of P2Y2R expression was associated with neuropathology and synapse loss in patients with AD,263 presenting additional support for neuroprotective function of P2Y2R in AD pathology.264 Additionally, activation of the P2Y2R has shown to promote neurite outgrowth.265 These studies suggest that loss of neuroprotective functions of P2Y2R might contribute to disease pathogenesis in AD, and therefore, targeting the P2Y2Rs with agonist might be a promising strategy to boost neuroprotection in neurodegenerative diseases.
P2Y2R is equally activated by ATP and UTP (EC50 = 0.3-3 μM),221 suggesting its close proximity to conditions such as inflammation and apoptosis.258 All known agonists of P2Y2R are derivatives of ATP and UDP (UDPpS, MRS2698, INS37217, INS48823, α,β-methylene-UDP, 5-bromo-UTP, PSB-1114). One such agonist INS365 (diquafosol, EC50 = 100 nM) has been approved as an ophthalmic solution for the treatment of dry eye syndrome.266 PSB1114 is another known P2Y2R agonist that possesses 60-fold selectivity over the P2Y4R or the P2Y6R.267 Two of the P2Y2R agonists INS37217 and MRS2698 are currently in clinical trials for treating cystic fibrosis.258 Thus far, there are no reports of any PET radioligand for imaging of this receptors.
P2Y4 Receptor and Functions in the CNS
The P2Y4R is present in all cells of the brain, including neurons,268 astrocytes,269 and microglia.270 However, the functional role of the receptor is still ambiguous. It is believed that P2Y4 R might complement the P2Y2R since both receptors are present in glial end feet in vicinity of the blood vessel walls.271 Human P2Y4R is stimulated by UTP (EC50 = 73 nM), but not by ATP.272 However, both nucleotides activate the rat and mouse P2Y4 receptors. In microglia, P2Y4 receptors are involved in ATP triggered pinocytosis that results in the uptake of soluble Aβ1-42, and either P2Y4 knockdown or ATP deficiency has shown to decrease this process.273 Hence, in addition to the P2Y12 receptor-mediated “find me” signal16 and the P2Y6 receptor-mediated “eat me” signal,233 P2Y4 receptors facilitate “drink me” signal that enables uptake of soluble Aβ by microglia.273 Therefore, activation of P2Y4 receptor in AD may have a neuroprotective effect possibly through uptake of Aβ1-42. 273,274
Thus far, there has been no report of a selective P2Y4R agonists or antagonists. Nonselective P2Y agonists UTPγS,275 5-bromo-UTP,276 INS365, INS37217, and INS45973 also exhibit agonist activity for the P2Y4R.277,278 Recently, an anthraquinone derivative was synthesized and showed selective and noncompetitive antagonist activity at the hP2Y4Rs (IC50 = 233 nM).279 To the best of our knowledge, there has been no report of any PET radioligand for mapping of the P2Y4Rs thus far.
P2Y6 Receptor and Functions in the CNS
The P2Y6 receptor is distributed on both immune and nonimmune cells and plays an important role in mammalian innate immunity.280 It is preferentially activated by UDP (EC50 = 15 nM).221 Under conditions that cause neuronal damage or in response to LPS, UDP leakage from damaged cells facilitates uptake and removal of cellular debris by activation of the microglial P2Y6 receptors,6,281 especially in PD.221 Indeed, P2X6R is regarded as a potential clinical biomarker of PD and other neuroinflammatory diseases.282
Additionally, UDP has shown to promote feeding through activation of P2Y6 receptors in AgRP neurons. These neurons are known to be involved in systemic insulin resistance which is an onset of obesity-associated hyperphagia.283 Moreover, hypothalamic UDP concentrations have shown to be increased in obesity disorder.283
Inhibition of P2Y6R has proven to be a potential therapeutic strategy for treatment of neuroinflammation, PD,282 feeding disorders, and systemic insulin resistance in obesity condition.283 Potent and selective nonnucleotide P2Y6R antagonist MRS2578 (IC50 = 37 nM, hP2Y6 R and IC50 = 98 nM, rP2Y6R) has shown to inhibit UDP-induced phagocytosis and prevent LPS-induced neuronal loss in mixed neuronal/glial cultures.284 MRS2578 specifically lacks any antagonist activity at P2Y1,2,4,11 receptors.285,286 Recently, a novel selective hP2Y6R antagonist TIM-38 was reported with low potency (IC50 = 4.3 μM).287 TIM-38 could be a useful pharmacological tool and a starting point for the development of therapeutic agents against P2Y6 receptor-implicated disease. Activation of P2Y6R by either its endogenous ligand UDP or selective agonist MRS-2693 has shown to promote production of pro-inflammatory cytokines IL-6 and IL-8 and contribute to phagocytosis of neurons.288,289 To the best of our knowledge, there has been no report of any PET radioligand for mapping of the P2Y6Rs.
P2Y12 Receptor and Functions in the CNS
P2Y12 receptor is activated by endogenous agonist ADP (EC50 = 60 nM).221 It acts as a regulator of blood clotting; therefore, it is targeted for the treatment of thromboembolisms.290 In normal brain, P2Y12R expression level is high on M2 type microglia291 but downregulates under pathological conditions or after LPS treatment.291,292 Indeed, expression of P2Y12 in microglia was undetectable 24 hours after injury.16 During microglial transition from highly ramified to an amoeboid state, low level of P2Y12 receptors is an indication of the receptor role in early responses of microglia to the brain injury.16 Immunohistochemical studies of postmortem brains from patients with AD and MS have shown reduction of P2Y12 receptor expression on microglia near the injury sites.291 Therefore, P2Y12 receptor could potentially act as a valuable biomarker for detecting the activity of human microglia during CNS pathologies in neurodegenerative diseases.291 P2Y12 is also expressed on astrocytes of the rat cortex and hippocampal pyramidal neurons and on oligodendrocytes where is involved in myelination.271,293
Within the P2Y receptor family, both P2Y12 and P2Y6 receptors233 control microglia activation and migration to the injury site; however, P2Y12R expression is decreased, while P2Y6R expression is increased.294,295 P2Y12 receptor also participates in a crosstalk with A3R to perform the process extension of microglia,296 suggesting the nucleotides action on P2Y12 as a primary target to induce microglial chemotaxis early on in response to CNS injury. Therefore, P2Y12R can potentially be targeted for the treatment of neurodegenerative diseases.16
A wide variety of antithrombotic P2Y12R antagonists such as ticlopidine (Ticlid), clopidogrel (Plavix), ticagrelor (Brilinta), prasugrel (Effient), ticagrelor (AR-C 69931),297 and MRS-2395298 have been developed for the treatment of platelet aggregation.269,299 Inhibition of the P2Y12R through knockdown of expression or by pharmacological inhibitor has resulted in less neuronal injury.294 The direct effects of the P2Y12 antagonists on cardiovascular system may indirectly heal neural injury and CNS diseases.16 Therefore, inhibition of both P2Y6 and P2Y12 receptors with their antagonists may prevent phagocytosis of salvageable cells and could be a promising path in treating neuroinflammation-induced neurodegeneration.251
PET Radioligand of P2Y12R
Since P2Y12 receptors are the only identified target exclusively expressed on M2-type microglia, PET imaging of this receptor could help detect the precise role of microglial phenotype in each stage of neuroinflammation and identify stages of the neurodegeneration diseases. Thus, an antagonist of P2Y12R (sulfonylureas compound 5, with IC50 = 6 nM)300 was radiolabeled with 11C to produce [11C]5, as shown in Figure 9 and used as a PET tracer for evaluation of the P2Y12 receptor301 function in MS disease progression.24,302 Unfortunately, [11C]5 was shown to be an unstable tracer that metabolized rapidly in plasma and in an ex vivo biodistribution study in rats, and only very low brain uptake of this radioligand was detected in this study.302 Therefore, its use for PET imaging of the P2Y12 receptor is not favored.
Figure 9.
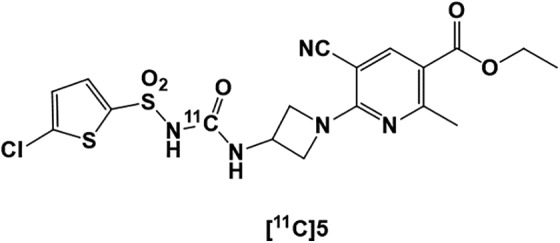
[11C]Radiolabeled P2Y12R PET ligand [11C]5. PET indicates positron emission tomography.
P2Y13 Receptor and Functions in the CNS
P2Y13 receptor is one of the most recently identified nucleotide receptor on neurons.303 Like P2Y1 and P2Y12, P2Y13 receptor belongs to a group of P2Y receptors responding to endogenous nucleotides ADP.304 P2Y13Rs are specifically present in cerebellar astrocytes, microglia, and granule neurons where they, and not the P2Y1 receptors, participate in the ADP-evoked calcium responses with P2Y13 expression higher in microglia than in the astrocytes.305 In granule neurons, P2Y13 receptors have been coupled to PI3K/Akt pathway that prevents neuronal death.304 Additionally, P2Y13-mediated ERK1/2 signaling has shown to trigger activation of CREB, suggesting an antiapoptotic act of the P2Y13 receptor against glutamate neurotransmitter toxicity.304 P2Y13 receptors are implicated in the release of acetylcholine from synapses and play key roles in neuronal cell differentiation and axonal elongation.305,306
Remarkably, activation of microglial P2Y12 and P2Y13 receptors following inflammation induces the release of paracrine mediators via upregulation of the P2Y1 and P2Y12 receptors on proliferated astroglia, and upon reduction of inflammation and microglia phenotype change, both P2Y12 and P2Y13 have shown to be downregulated on astrocytes.305
While ADP is the known endogenous agonist of P2Y13 (EC50 = 60 nM),221 2-MeSADP, a nonselective P2Y12/13 agonist, is even more potent at this receptor.271 However, inosine 5′-diphosphate sodium salt (IDP) is the preferential selective P2Y13 agonist with 5-fold more potency for hP2Y13 over the P2Y12 receptor.306 Furthermore, IDP with EC50 = 9.2 nM is more potent at murine P2Y13 than at human P2Y13 (EC50 = 552 nM).306 Inosine 5′-diphosphate sodium salt is currently considered as a potent P2Y13 receptor agonist.306
Among the P2Y13 receptor antagonists, there are some nonselective P2Y12/13 antagonist including a highly potent P2Y12 antagonist AR-C69931 (IC50 = 0.4 nM) and 2-MeSAMP.221 However, nonnucleoside MRS-2211 is a selective antagonist of P2Y13 and displays high selectivity over P2Y1 and P2Y12 receptors.307
P2Y14 Receptor and Functions in the CNS
The P2Y14 receptor is preferentially expressed in hematopoietic stem cells of both humans and mice.308 While physiological functions of this receptor remain to be established, expression of the P2Y14 receptor has been detected in immune cells, suggesting its connotation with inflammation.309 Most of the data on P2Y14 is associated with its peripheral effects, but there are indications of its expression in human astrocytes310 and rat cortical and cerebellar astrocytes.311 Increased P2Y14 receptor expression in LPS-mediated microglial activation also suggests its role in CNS inflammatory responses.312 In mice, P2Y14 deficiency has not shown to carry a noticeable CNS effect under homeostatic conditions, but showed reduced tolerance to glucose and insulin secretion deficiency.313 A variety of factors including aging, radiation therapy, consecutive exposure to chemotherapy, and repeated bone marrow transplantation have shown to increase senescence in animals lacking P2Y14 receptor.314
Therapeutic effect of the P2Y14R activation on CNS diseases are not fully elucidated yet. The P2Y14R is activated by UDP-glucose (EC50 = 80 nM).221 This endogenous ligand is not prone to hydrolysis and acts as an extracellular pro-inflammatory mediators.315 UDP also acts as a P2Y14 R agonist, overlapping with the P2Y6R. Several analogs of UDP including MRS2802 and MRS2905 have exhibited high potency and selectivity at the P2Y14 over the P2Y6 and other P2Y receptors.316 Releases of nucleotide-sugars in astrocytes play an important role in maintaining the normal status of the cell via P2Y14 receptors.317
Potential P2Y14R antagonists are dihydropyridopyrimidine base compound with analogs acting as noncompetitive antagonists of the receptor.318 Another set of P2Y14 R antagonists are naphthoic acid and derivatives that inhibited [3H]UDP binding to the P2Y14R, suggesting orthosteric antagonism for P2Y14 receptors.319 A selective and highly potent competitive antagonist PPTN that was converted to a prodrug has shown to increase bioavailability allowing further studies of this receptor.320 PPTN has shown to inhibit chemotaxis of human neutrophils in cell line expressing P2Y14 receptor.320 An analog of Alexa Fluor 488 (AF488), MRS4174 has also exhibited selectivity and a remarkably high binding activity of 80 pM at the P2Y14R.320 There has been no report of any PET radioligand for mapping of the P2Y14Rs.
Concluding Remarks
Existing evidences indicate that chronic inflammation mediated by modulation of neurons and activation of microglia and astrocytes plays significant roles in CNS disorders and specifically in neurodegenerative diseases. Decades of research toward the discovery and development of treatments for these diseases, especially the neurodegeneration, while successful to some extent, still faces hurdles. The probability that some failed therapies have engaged wrong targets might be a possible explanation. Preclinical findings suggest that elucidation of target engagement of drugs in CNS disorders via PET imaging of the known brain biomarkers can assist to track disease progression, guide drug development, and monitor therapies for the treatment of these disorders. This task requires having access to the number of receptor-selective molecular probes. Especially in early stage of neurodegenerative diseases, in addition to evaluation of cerebrospinal fluid and plasma samples of an individual, PET imaging of pro-inflammatory biomarker of the same individual may help identify the causes of inflammation and potentially assist developing an efficient translational application of relevant therapeutic interventions. Purinergic receptors present promising potential for PET imaging of the neurological disorder biomarkers. These receptors have experienced an exciting journey since the discovery of their first member in early 20th century. Currently, a number of 11C and 18F PET radioligands of the adenosine, particularly the A1 and A2A receptors, and the fast synaptic P2X receptor subtypes, in particular, the P2X7 receptor have helped to elucidate the expression and functions of these purinergic receptors in CNS disorders. Despite emerging facts regarding participation of the P2Y signaling in the brain, their functions are not fully recognized. This is largely due to lack of availability of selective nonnucleotide and brain penetrable ligands to be radiolabeled as PET radiotracer for evaluation of their expression and functions in the brain. However, a list of P2Y receptor ligands have been mentioned in this review to enlighten and guide interested scientists in discovering novel PET ligand for noninvasive approach to evaluate the P2Y receptor contribution in the brain disorders and especially the neurodegeneration diseases.
Footnotes
Authors’ Note: H. Zarrinmayeh has over 20 years of research experience as a medicinal chemist in pharmaceutical industry where she designed and discovered lead drug candidates for the treatment of various disorders including cancer and especially the diseases and disorders of the CNS. Upon joining Indiana University Radiology and Imaging Sciences Department, Dr. Zarrinmayeh resumed her research in the area of the design and development of novel P2X7 receptor PET radioligand for evaluation of neuroinflammation and assessment of neurodegeneration. Her contribution has yielded to the discovery of a novel 18F PET radioligands for evaluation of the P2X7, a biomarker of neuroinflammation in CNS disorders. Dr. Territo has more than 20 years of experience in physiology, pharmacology, medical imaging, and biomarker development in support of phenotyping and therapeutic response in both pharmaceutical industry (10 years) and academia (+10 years), where his experiences led to the development of translational imaging biomarkers in the area of neuroscience, oncology, and cardiovascular diseases. At IUSM, Dr. Territo’s research has incorporated both Tracer Development and Validation and Pre-Clinical Imaging techniques. The Tracer Development and Validation Lab was established to support development of novel imaging tracers by integration of molecular methods, physiology, pharmacology, imaging, and analysis modeling. Dr. Territo oversees the in vitro, in vivo, and ex vivo imaging studies of 11C and 18F PET radioligands and is involved in study analysis and statistical modeling of the data from these studies.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Hamideh Zarrinmayeh, PhD  https://orcid.org/0000-0002-3604-4924
https://orcid.org/0000-0002-3604-4924
References
- 1. Saitoh HT, Tsuda M, Inoue K. Role of purinergic receptors in CNS function and neuroprotection. Adv Pharmacol. 2011;61:495–528. doi:10.1016/B978-0-12-385526-8.00015-1 [DOI] [PubMed] [Google Scholar]
- 2. Bennet DW, Drury AN. Further observations relating to the physiological activity of adenine compounds. J Physiol. 1931;72(3):288–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Burnstock G. Purinergic signalling and disorders of the central nervous system. Nat Rev Drug Discov. 2008;7(7):575–590. doi:10.1038/nrd2605 [DOI] [PubMed] [Google Scholar]
- 4. Burnstock G. Editor’s note. Purinerg Signal. 2018;14:213 doi:10.1007/s11302-018-9613-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Burnstock G. Purine and purinergic receptors. Brain Neurosci Advances. 2018;2:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Beamer E, Goloncser F, Horvath G, et al. Purinergic mechanisms in neuroinflammation: an update from molecules to behavior. Neuropharmacology. 2016;104:94–104. doi:10.1016/j.neuropharm.2015.09.019 [DOI] [PubMed] [Google Scholar]
- 7. Burnstock G. An introduction to the roles of purinergic signalling in neurodegeneration, neuroprotection and neuroregeneration. Neuropharmacology. 2016;104:4–17. doi:10.1016/j.neuropharm.2015.05.031 [DOI] [PubMed] [Google Scholar]
- 8. Burnstock G. Historical review: ATP as a neurotransmitter. Trends Pharmacol Sci. 2006;27(3):166–176. doi:10.1016/j.tips.2006.01.005 [DOI] [PubMed] [Google Scholar]
- 9. Bhattacharya A, Biber K. The microglial ATP-gated ion channel P2X7 as a CNS drug target. Glia 2016;64(10):1772–1787. doi:10.1002/glia.23001 [DOI] [PubMed] [Google Scholar]
- 10. Roszek K, Czarnecka J. Is ecto-nucleoside triphosphate diphosphohydrolase (NTPDase)-based therapy of central nervous system disorders possible? Mini-Rev Med Chem. 2015;15(1):5–20. doi:10.2174/1389557515666150219114416 [DOI] [PubMed] [Google Scholar]
- 11. Yegutkin GG. Nucleotide- and nucleoside-converting ectoenzymes: important modulators of purinergic signalling cascade. Biochim Biophys Acta. 2008;1783(5):673–694. doi:10.1016/j.bbamcr.2008.01.024 [DOI] [PubMed] [Google Scholar]
- 12. Inoue K. Purinergic systems in microglia. Cell Mol Life Sci. 2008;65(19):3074–3080. doi:10.1007/s00018-008-8210-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Domercq M, Villoldo NV, Matute C. Neurotransmitter signaling in the pathophysiology of microglia. Front Cell Neurosci. 2013;7:49 doi:10.3389/fncel.2013.00049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ulmann L, Hatcher JP, Hughes JP, et al. Up-regulation of P2X4 receptors in spinal microglia after peripheral nerve injury mediates BDNF release and neuropathic pain. J neurosci. 2008;28(44):11263–11268. doi:10.1523/JNEUROSCI.2308-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Di Virgilio F, Dal Ben D, Sarti AC, Giuliani AL, Falzoni S. The P2X7 receptor in infection and inflammation. Immunity. 2017;47(1):15–31. doi:10.1016/j.immuni.2017.06.020 [DOI] [PubMed] [Google Scholar]
- 16. Haynes SE, Hollopeter G, Yang G, et al. The P2Y12 receptor regulates microglial activation by extracellular nucleotides. Nat Neurosci. 2006;9(12):1512–1519. doi:10.1038/nn1805 [DOI] [PubMed] [Google Scholar]
- 17. Villoldo NV, Domercq M, Martin A, Llop J, Vallejo VG, Matute C. P2X4 receptors control the fate and survival of activated microglia. Glia. 2014;62(2):171–184. doi:10.1002/glia.22596 [DOI] [PubMed] [Google Scholar]
- 18. Burnstock G. Physiology and pathophysiology of purinergic neurotransmission. Physiol Rev. 2007;87(2):659–797. doi:10.1152/physrev.00043.2006 [DOI] [PubMed] [Google Scholar]
- 19. Grabot EB, Pankratov Y. Modulation of central synapses by astrocyte-released ATP and postsynaptic P2X Receptors. Neural Plast. 2017;2017:9454275 doi:10.1155/2017/9454275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Braun N, Sevigny J, Robson SC, et al. Assignment of ecto-nucleoside triphosphate diphosphohydrolase-1/cd39 expression to microglia and vasculature of the brain. Eur j neurosci. 2000;12(12):4357–4366. [PubMed] [Google Scholar]
- 21. Fredholm BB, IJzerman AP IJ, Jacobson KA, Klotz KN, Linden J. International Union of Pharmacology. XXV. Nomenclature and classification of adenosine receptors. Pharmacol Rev. 2001;53(4):527–552. [PMC free article] [PubMed] [Google Scholar]
- 22. Choi IS, Cho JH, Lee MG, Jang IS. Enzymatic conversion of ATP to adenosine contributes to ATP-induced inhibition of glutamate release in rat medullary dorsal horn neurons. Neuropharmacology. 2015;93:94–102. doi:10.1016/j.neuropharm.2015.01.020 [DOI] [PubMed] [Google Scholar]
- 23. Jacobson KA, Muller CE. Medicinal chemistry of adenosine, P2Y and P2X receptors. Neuropharmacology. 2016;104:31–49. doi:10.1016/j.neuropharm.2015.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Narayanaswami V, Dahl K, Gauthier VB, Josephson L, Cumming P, Vasdev N. Emerging PET radiotracers and targets for imaging of neuroinflammation in neurodegenerative diseases: outlook beyond TSPO. Mol Imaging. 2018;17:1536012118792317 doi:10.1177/1536012118792317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Vuorimaa A, Rissanen E, Airas L. In vivo PET imaging of adenosine 2A receptors in neuroinflammatory and neurodegenerative disease. Contrast Media Mol Imaging. 2017;2017:6975841 doi:10.1155/2017/6975841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Boison D. Adenosine as a modulator of brain activity. Drug News Perspect. 2007;20(10):607–611. doi:10.1358/dnp.2007.20.10.1181353 [DOI] [PubMed] [Google Scholar]
- 27. Schmidt J, Ferk P. Safety issues of compounds acting on adenosinergic signalling. J Pharm Pharmacol. 2017;69(7):790–806. doi:10.1111/jphp.12720 [DOI] [PubMed] [Google Scholar]
- 28. Sebastiao AM, Ribeiro JA. Fine-tuning neuromodulation by adenosine. Trends Pharmacol Sci. 2000;21:341–346. [DOI] [PubMed] [Google Scholar]
- 29. de Mendoncca A, Ribeiro JA. Adenosine and synaptic plasticity. Drug Dev Res. 2001;52:283–290. doi:10.1002/ddr.1125 [Google Scholar]
- 30. Sebastiao AM, Ribeiro JA. Adenosine receptors and the central nervous system. Handb Exp Pharmacol. 2009;193:471–534. doi:10.1007/978-3-540-89615-9_16 [DOI] [PubMed] [Google Scholar]
- 31. Stone TW, Ceruti S, Abbracchio MP. Adenosine receptors and neurological disease: neuroprotection and neurodegeneration. Handb Exp Pharmacol. 2009;(193):535–587. doi:10.1007/978-3-540-89615-9_17 [DOI] [PubMed] [Google Scholar]
- 32. Costenla AR, Cunha RA, de Mendonca A. Caffeine, adenosine receptors, and synaptic plasticity. J Alzheimers Dis. 2010;20(suppl 1):S25–S34. doi:10.3233/JAD-2010-091384 [DOI] [PubMed] [Google Scholar]
- 33. Costenla AR, Diogenes MJ, Canas PM, et al. Enhanced role of adenosine A(2A) receptors in the modulation of LTP in the rat hippocampus upon ageing. Eur J Neurosci. 2011;34(1):12–21. doi:10.1111/j.1460-9568.2011.07719.x [DOI] [PubMed] [Google Scholar]
- 34. Leon Navarro DA, Albasanz JL, Martin M. Functional cross-talk between adenosine and metabotropic glutamate receptors. Curr Neuropharmacol. 2019;17(5):422–437. doi:10.2174/1570159X16666180416093717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nakanishi S. Molecular diversity of glutamate receptors and implications for brain function. Science 1992;258(5082):597–603. [DOI] [PubMed] [Google Scholar]
- 36. Burnstock G. Purinergic signalling and neurological diseases: an update. CNS Neurol Disord Drug Targets. 2017;16:257–265. doi:10.2174/1871527315666160922104848 [DOI] [PubMed] [Google Scholar]
- 37. Lewis MH, Primiani C, Muehlmann AM. Targeting dopamine D2, adenosine A2A, and glutamate mGlu5 receptors to reduce repetitive behaviors in deer mice. J Pharmacol Exp Ther. 2019;369(1):88–97. doi:10.1124/jpet.118.256081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ciruela F, Soler MG, Guidolin D, et al. Adenosine receptor containing oligomers: their role in the control of dopamine and glutamate neurotransmission in the brain. Biochim Biophys Acta. 2011;1808(5):1245–1255. doi:10.1016/j.bbamem.2011.02.007 [DOI] [PubMed] [Google Scholar]
- 39. Piomelli D, Pilon C, Giros B, Sokoloff P, Martres MP, Schwartz JC. Dopamine activation of the arachidonic acid cascade as a basis for D1/D2 receptor synergism. Nature. 1991;353(6340):164–167. doi:10.1038/353164a0 [DOI] [PubMed] [Google Scholar]
- 40. Krugel U, Koles L, Illes P. Integration of neuronal and glial signalling by pyramidal cells of the rat prefrontal cortex; control of cognitive functions and addictive behaviour by purinergic mechanisms. Neuropsychopharmacol Hung. 2013;15(4):206–213. [PubMed] [Google Scholar]
- 41. Burnstock G. Introduction to purinergic signalling in the brain. Adv Exp Med Biol. 2013;986:1–12. doi:10.1007/978-94-007-4719-7_1 [DOI] [PubMed] [Google Scholar]
- 42. Koles L, Kato E, Hanuska A, et al. Modulation of excitatory neurotransmission by neuronal/glial signalling molecules: interplay between purinergic and glutamatergic systems. Purinergic Signal. 2016;12(1):1–24. doi:10.1007/s11302-015-9480-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Delic J, Zimmermann H. Nucleotides affect neurogenesis and dopaminergic differentiation of mouse fetal midbrain-derived neural precursor cells. Purinergic Signal. 2010;6(4):417–428. doi:10.1007/s11302-010-9206-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hempel C, Norenberg W, Sobottka H, et al. The phenothiazine-class antipsychotic drugs prochlorperazine and trifluoperazine are potent allosteric modulators of the human P2X7 receptor. Neuropharmacology. 2013;75:365–379. doi:10.1016/j.neuropharm.2013.07.027 [DOI] [PubMed] [Google Scholar]
- 45. Othman T, Legare D, Sadri P, Lautt WW, Parkinson FE. A preliminary investigation of the effects of maternal ethanol intake during gestation and lactation on brain adenosine A(1) receptor expression in rat offspring. Neurotoxicol Teratol. 2002;24(2):275–279 [DOI] [PubMed] [Google Scholar]
- 46. Burnstock G, Krugel U, Abbracchio MP, Illes P. Purinergic signalling: from normal behaviour to pathological brain function. Prog Neurobiol. 2011;95(2):229–274. doi:10.1016/j.pneurobio.2011.08.006 [DOI] [PubMed] [Google Scholar]
- 47. Aliagas E, Menendez IV, Sevigny J, et al. Reduced striatal ecto-nucleotidase activity in schizophrenia patients supports the “adenosine hypothesis”. Purinergic Signal. 2013;9(4):599–608. doi:10.1007/s11302-013-9370-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Rebola N, Oliveira CR, Cunha RA. Transducing system operated by adenosine A(2A) receptors to facilitate acetylcholine release in the rat hippocampus. Eur J Pharmacol. 2002;454(1):31–38 [DOI] [PubMed] [Google Scholar]
- 49. Brown RM, Short JL. Adenosine A(2A) receptors and their role in drug addiction. J Pharm Pharmacol. 2008;60(11):1409–1430. doi:10.1211/jpp/60.11.0001 [DOI] [PubMed] [Google Scholar]
- 50. Salem A, Hope W. Role of endogenous adenosine in the expression of opiate withdrawal in rats. Eur J Pharmacol. 1999;369(1):39–42 [DOI] [PubMed] [Google Scholar]
- 51. Choi JH, Cha JK, Huh JT. Adenosine diphosphate-induced platelet aggregation might contribute to poor outcomes in atrial fibrillation-related ischemic stroke. J Stroke Cerebrovasc Dis, 2014;23(3):e215–e220. doi:10.1016/j.jstrokecerebrovasdis.2013.10.011 [DOI] [PubMed] [Google Scholar]
- 52. Manwani B, McCullough LD. Function of the master energy regulator adenosine monophosphate-activated protein kinase in stroke. J Neurosci Res. 2013;91(8):1018–1029. doi:10.1002/jnr.23207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Boison D. Adenosine and epilepsy: from therapeutic rationale to new therapeutic strategies. Neuroscientist. 2005;11(1):25–36. doi:10.1177/1073858404269112 [DOI] [PubMed] [Google Scholar]
- 54. Chen JF. Adenosine A2A receptors and Parkinson’s disease: benefits and challenges. Purinerg Signal. 2018;14:S71–S71. [Google Scholar]
- 55. Duenas VF, Ferre S, Ciruela F. Adenosine A(2A)-dopamine D-2 receptor heteromers operate striatal function: impact on Parkinson’s disease pharmacotherapeutics. Neural Regen Res. 2018;13(2):241–243. doi:10.4103/1673-5374.226388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Soliman AM, Fathalla AM, Moustafa AA. Adenosine role in brain functions: pathophysiological influence on Parkinson’s disease and other brain disorders. Pharmacol Rep 2018;70(4):661–667. doi:10.1016/j.pharep.2018.02.003 [DOI] [PubMed] [Google Scholar]
- 57. Pinna A, Serra M, Morelli M, Simola N. Role of adenosine A(2A) receptors in motor control: relevance to Parkinson’s disease and dyskinesia. J Neural Transm. 2018;125(8):1273–1286. doi:10.1007/s00702-018-1848-6 [DOI] [PubMed] [Google Scholar]
- 58. Stockwell J, Jakova E, Cayabyab FS. Adenosine A1 and A2A receptors in the brain: current research and their role in neurodegeneration. Molecules. 2017;22(4):pii: E676 doi:10.3390/molecules22040676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Biber K, Klotz KN, Berger M, Gebicke Härter PJ, van Calker D. Adenosine A1 receptor-mediated activation of phospholipase C in cultured astrocytes depends on the level of receptor expression. J neurosci. 1997;17(2):4956–4964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Othman T, Yan HL, Rrvkees SA. Oligodendrocytes express functional A1 adenosine receptors that stimulate cellular migration. Glia. 2003;44(2):166–172. doi:10.1002/glia.10281 [DOI] [PubMed] [Google Scholar]
- 61. GebickeHaerter PJ, Christoffel F, Timmer J, Northoff H, Berger M, Van Calker D. Both adenosine A1- and A2-receptors are required to stimulate microglial proliferation. Neurochem Int. 1996;29(1):37–42. doi:10.1016/0197-0186(95)00137-9 [PubMed] [Google Scholar]
- 62. Dunwiddie TV, Haas HL. Adenosine increases synaptic facilitation in the in vitro rat hippocampus: evidence for a presynaptic site of action. J Physiol. 1985;369:365–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Thompson SM, Haas HL, Gahwiler BH. Comparison of the actions of adenosine at pre- and postsynaptic receptors in the rat hippocampus in vitro. J Physiol. 1992;451:347–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Gomes CV, Kaster MP, Tome AR, Agostinho PM, Cunha RA. Adenosine receptors and brain diseases: neuroprotection and neurodegeneration. Biochim Biophys Acta. 2011;1808(5):1380–1399. doi:10.1016/j.bbamem.2010.12.001 [DOI] [PubMed] [Google Scholar]
- 65. Cunha RA. Neuroprotection by adenosine in the brain: from A(1) receptor activation to A (2A) receptor blockade. Purinergic Signal. 2005;1(2):111–134. doi:10.1007/s11302-005-0649-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Albasanz JL, Perez S, Barrachina M, et al. Up-regulation of adenosine receptors in the frontal cortex in Alzheimer’s disease. Brain Pathol. 2008;18(2):211–219. doi:10.1111/j.1750-3639.2007.00112.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Burnstock G, Krugel U, Abbracchio MP, Illes P. Purinergic signalling: from normal behaviour to pathological brain function. Progress Neurobiol. 2011;95(2):229–274. doi:10.1016/j.pneurobio.2011.08.006 [DOI] [PubMed] [Google Scholar]
- 68. Kalaria RN Sromek S Wilcox BJ, Unnerstall JR. Hippocampal adenosine A1 receptors are decreased in Alzheimer’s disease. Neurosci Lett. 1990;118(2):257–260 [DOI] [PubMed] [Google Scholar]
- 69. Cieslak M, Wojtczak A. Role of purinergic receptors in the Alzheimer’s disease. Purinerg Signal. 2018;14(4):331–344. doi:10.1007/s11302-018-9629-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Cieslak M, Wojtczak A. Role of purinergic receptors in the Alzheimer’s disease. Purinergic Signal. 2018;14(4):331–344. doi:10.1007/s11302-018-9629-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Chen Z, Stockwell J, Cayabyab FS. Adenosine A1 receptor-mediated endocytosis of AMPA receptors contributes to impairments in long-term potentiation (LTP) in the middle-aged Rat hippocampus. Neurochem Res. 2016;41(5):1085–1097. doi:10.1007/s11064-015-1799-3 [DOI] [PubMed] [Google Scholar]
- 72. Chen Z, Xiong C, Pancyr C, Stockwell J, Walz W, Cayabyab FS. Prolonged adenosine A1 receptor activation in hypoxia and pial vessel disruption focal cortical ischemia facilitates clathrin-mediated AMPA receptor endocytosis and long-lasting synaptic inhibition in rat hippocampal CA3-CA1 synapses: differential regulation of GluA2 and GluA1 subunits by p38 MAPK and JNK. J Neurosci. 2014;34(29):9621–9643. doi:0.1523/JNEUROSCI.3991-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Stockwell J, Chen Z, Niazi M, Nosib S, Cayabyab FS. Protein phosphatase role in adenosine A1 receptor-induced AMPA receptor trafficking and rat hippocampal neuronal damage in hypoxia/reperfusion injury. Neuropharmacology. 2016;102:254–265. doi:10.1016/j.neuropharm.2015.11.018 [DOI] [PubMed] [Google Scholar]
- 74. Carman AJ, Mills JH, Krenz A, Kim DG, Bynoe MS. Adenosine receptor signaling modulates permeability of the blood-brain barrier. J neurosci. 2011;31(37):13272–13280. doi:10.1523/JNEUROSCI.3337-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Mishina M, Ishiwata K. Adenosine receptor PET imaging in human brain. Int Rev Neurobiol. 2014;119:51–69. doi:10.1016/B978-0-12-801022-8.00002-7 [DOI] [PubMed] [Google Scholar]
- 76. Paul S, Khanapur S, Rybczynska AA, et al. Small-animal PET study of adenosine A(1) receptors in rat brain: blocking receptors and raising extracellular adenosine. J Nucl Med. 2011;52(8):1293–1300. doi:10.2967/jnumed.111.088005 [DOI] [PubMed] [Google Scholar]
- 77. Rahman A. The role of adenosine in Alzheimer’s disease. Curr Neuropharmacol. 2009;7(3):207–216. doi:10.2174/157015909789152119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Giunta S, Andriolo V, Castorina A. Dual blockade of the A1 and A2A adenosine receptor prevents amyloid beta toxicity in neuroblastoma cells exposed to aluminum chloride. Int J Biochem Cell Biol. 2014;54:122–136. doi:10.1016/j.biocel.2014.07.009 [DOI] [PubMed] [Google Scholar]
- 79. de Mendonca A, Sebastiao AM, Ribeiro JA. Adenosine: does it have a neuroprotective role after all? Brain Res Rev. 2000;33(2-3):258–274. doi:10.1016/S0165-0173(00)00033-3 [DOI] [PubMed] [Google Scholar]
- 80. Kashfi S, Ghaedi K, Baharvand H, Nasr Esfahani MH, Javan M. A1 Adenosine receptor activation modulates central nervous system development and repair. Mol Neurobiol. 2017;54(10):8128–8139. doi:10.1007/s12035-016-0292-6 [DOI] [PubMed] [Google Scholar]
- 81. Tsutsui S, Schnermann J, Noorbakhsh F, et al. A1 adenosine receptor upregulation and activation attenuates neuroinflammation and demyelination in a model of multiple sclerosis. J neurosci. 2004;24(6):1521–1529. doi:10.1523/JNEUROSCI.4271-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Jacobson KA, Tosh DK, Jain S, Gao ZG. Historical and current adenosine receptor agonists in preclinical and clinical development. Front Cell Neurosci. 2019;13:124 doi:10.3389/fncel.2019.00124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Noguchi J, Ishiwata K, Furuta R, et al. Evaluation of carbon-11 labeled KF15372 and its ethyl and methyl derivatives as a potential CNS adenosine A1 receptor ligand. Nucl Med Biol. 1997;24(1):53–59 [DOI] [PubMed] [Google Scholar]
- 84. Kreft S, Bier D, Holschbach MH, Schulze A, Coenen HH. New potent A1 adenosine receptor radioligands for positron emission tomography. Nucl Med Biol. 2017;44:69–77. doi:10.1016/j.nucmedbio.2016.09.004 [DOI] [PubMed] [Google Scholar]
- 85. Hayashi S, Inaji M, Nariai T, et al. Increased binding potential of brain adenosine a1 receptor in chronic stages of patients with diffuse axonal injury measured with [1-methyl-(11)C] 8-dicyclopropylmethyl-1-methyl-3-propylxanthine positron emission tomography imaging. J Neurotrauma. 2018;35(1):25–31. doi:10.1089/neu.2017.5006 [DOI] [PubMed] [Google Scholar]
- 86. Mishina M, Ishii K, Kimura Y, et al. Adenosine A1 receptors measured with (11) C-MPDX PET in early Parkinson’s disease. Synapse. 2017;71(8). doi:10.1002/syn.21979 [DOI] [PubMed] [Google Scholar]
- 87. Mishina M, Kimura Y, Sakata M, et al. Age-related decrease in male extra-striatal adenosine A1 receptors measured using(11)C-MPDX PET. Front Pharmacol. 2017;8:903 doi:10.3389/fphar.2017.00903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Paul S, Khanapur S, Sijbesma JW, et al. Use of 11C-MPDX and PET to study adenosine A1 receptor occupancy by nonradioactive agonists and antagonists. J Nucl Med. 2014;55(2):315–320. doi:10.2967/jnumed.113.130294 [DOI] [PubMed] [Google Scholar]
- 89. Matsuya T, Takamatsu H, Murakami Y, Noda A. Synthesis and evaluation of [C-11]FR194921 as a nonxanthine-type PET tracer for adenosine A(1) receptors in the brain. Nuclear Med Biol. 2005;32(8):837–844. doi:10.1016/j.nucmedbio.2005.06.008 [DOI] [PubMed] [Google Scholar]
- 90. Maemoto T, Tada M, Mihara T, et al. Pharmacological characterization of FR194921, a new potent, selective, and orally active antagonist for central adenosine A1 receptors. J Pharmacol Sci. 2004;96(1):42–52. doi:10.1254/jphs.fp0040359 [DOI] [PubMed] [Google Scholar]
- 91. Guo M, Gao ZG, Tyler R, et al. Preclinical evaluation of the first adenosine A1 receptor partial agonist radioligand for positron emission tomography imaging. J Med Chem. 2018;61(22):9966–9975. doi:10.1021/acs.jmedchem.8b01009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Elmenhorst D, Meyer PT, Matusch A, Winz OH, Zilles K, Bauer A. Test-retest stability of cerebral A1 adenosine receptor quantification using [18F]CPFPX and PET. Eur J Nucl Med Mol Imaging. 2007;34(7):1061–1070. doi:10.1007/s00259-006-0309-x [DOI] [PubMed] [Google Scholar]
- 93. Meyer PT, Elmenhorst D, Bier D, et al. Quantification of cerebral A1 adenosine receptors in humans using [18F]CPFPX and PET: an equilibrium approach. Neuroimage. 2005;24(4):1192–1204. doi:10.1016/j.neuroimage.2004.10.029 [DOI] [PubMed] [Google Scholar]
- 94. Elmenhorst D, Elmenhorst EM, Hennecke E, et al. Recovery sleep after extended wakefulness restores elevated A1 adenosine receptor availability in the human brain. Proc Natl Acad Sci U S A. 2017;114(16):4243–4248. doi:10.1073/pnas.1614677114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Herzog H, Elmenhorst D, Winz O, Bauer A. Biodistribution and radiation dosimetry of the A1 adenosine receptor ligand 18F-CPFPX determined from human whole-body PET. Eur J Nucl Med Mol Imaging. 2008;35(8):1499–1506. doi:10.1007/s00259-008-0753-x [DOI] [PubMed] [Google Scholar]
- 96. Bauer A, Holschbach MH, Cremer M, et al. Evaluation of 18F-CPFPX, a novel adenosine A1 receptor ligand: in vitro autoradiography and high-resolution small animal PET. J Nucl Med. 2003;44(10):1682–1689. [PubMed] [Google Scholar]
- 97. Ingwersen J, Wingerath B, Graf J, et al. Dual roles of the adenosine A2a receptor in autoimmune neuroinflammation. J Neuroinflamm. 2016;13:48 doi:10.1186/s12974-016-0512-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Cunha RA, Constantino MC, Sebastiao AM, Ribeiro JA. Modification of A1 and A2a adenosine receptor binding in aged striatum, hippocampus and cortex of the rat. Neuroreport. 1995;6(11):1583–1588. [DOI] [PubMed] [Google Scholar]
- 99. Viana da Silva S, Haberl MG, Zhang P, et al. Early synaptic deficits in the APP/PS1 mouse model of Alzheimer’s disease involve neuronal adenosine A2A receptors. Nat Commun. 2016;7:11915 doi:10.1038/ncomms11915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Canas PM, Porciuncula LO, Cunha GM, et al. Adenosine A2A receptor blockade prevents synaptotoxicity and memory dysfunction caused by beta-amyloid peptides via p38 mitogen-activated protein kinase pathway. J neurosci. 2009;29(47):14741–14751. doi:10.1523/JNEUROSCI.3728-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Peterson JD, Goldberg JA, Surmeier DJ. Adenosine A2a receptor antagonists attenuate striatal adaptations following dopamine depletion. Neurobiol Dis. 2012;45:409–416. doi:10.1016/j.nbd.2011.08.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Ferre S, Quiroz C, Woods AS, et al. An update on adenosine A2A-dopamine D2 receptor interactions: implications for the function of G protein-coupled receptors. Curr Pharm Des. 2008;14(15):1468–1474. doi:10.2174/138161208784480108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Ferreira MT, Ferreira DG, Batalha VL, et al. Age-related shift in LTD is dependent on neuronal adenosine A2A receptors interplay with mGluR5 and NMDA receptors. Mol Psychiatry. 2018. doi:10.1038/s41380-018-0110-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Corsi C, Melani A, Bianchi L, Pedata F. Striatal A2A adenosine receptor antagonism differentially modifies striatal glutamate outflow in vivo in young and aged rats. Neuroreport. 2000;11(11):2591–2595. [DOI] [PubMed] [Google Scholar]
- 105. Corsi C, Pinna A, Gianfriddo M, Melani A, Morelli M, Pedata F. Adenosine A2A receptor antagonism increases striatal glutamate outflow in dopamine-denervated rats. Eur J Pharmacol. 2003;464(1):33–38. [DOI] [PubMed] [Google Scholar]
- 106. Uchida S, Tashiro T, Uchida MK, Mori A, Jenner P, Kanda T. Adenosine A(2)A-receptor antagonist istradefylline enhances the motor response of L-DOPA without worsening dyskinesia in MPTP-treated common marmosets. J Pharmacol Sci. 2014;124(4):480–485. [DOI] [PubMed] [Google Scholar]
- 107. Horita TK, Kobayashi M, Mori A, Jenner P, Kanda T. Effects of the adenosine A2A antagonist istradefylline on cognitive performance in rats with a 6-OHDA lesion in prefrontal cortex. Psychopharmacology (Berl). 2013;230:345–352. doi:10.1007/s00213-013-3158-x [DOI] [PubMed] [Google Scholar]
- 108. Kondo T, Mizuno Y, Japanese Istradefylline Study G. A long-term study of istradefylline safety and efficacy in patients with Parkinson disease. Clin Neuropharmacol. 2015;38(2):41–46. doi:10.1097/WNF.0000000000000073 [DOI] [PubMed] [Google Scholar]
- 109. Pugliese AM, Traini C, Cipriani S, et al. The adenosine A2A receptor antagonist ZM241385 enhances neuronal survival after oxygen-glucose deprivation in rat CA1 hippocampal slices. Br J Pharmacol. 2009;157(5):818–830. doi:10.1111/j.1476-5381.2009.00218.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Uchida S, Soshiroda K, Okita E, et al. The adenosine A2A receptor antagonist, istradefylline enhances the anti-parkinsonian activity of low doses of dopamine agonists in MPTP-treated common marmosets. Eur J Pharmacol. 2015;747:160–165. doi:10.1016/j.ejphar.2014.11.038 [DOI] [PubMed] [Google Scholar]
- 111. Yuzlenko O, Kiec-Kononowicz K. Potent adenosine A1 and A2A receptors antagonists: recent developments. Curr Med Chem. 2006;13(30):3609–3625. [DOI] [PubMed] [Google Scholar]
- 112. Lu J, Cui J, Li X, et al. An anti-Parkinson’s disease drug via targeting adenosine A2A receptor enhances amyloid-beta generation and gamma-secretase activity. PLoS One. 2016;11(11):e0166415 doi:10.1371/journal.pone.0166415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Orr AG, Hsiao EC, Wang MM, et al. Astrocytic adenosine receptor A2A and Gs-coupled signaling regulate memory. Nat Neurosci. 2015;18(3):423–434. doi:10.1038/nn.3930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Franco R, Navarro G. Adenosine A2A receptor antagonists in neurodegenerative diseases: huge potential and huge challenges. Front Psychiatry. 2018;9:68 doi:10.3389/fpsyt.2018.00068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Orr AG, Orr AL, Li XJ, Gross RE, Traynelis SF. Adenosine A(2A) receptor mediates microglial process retraction. Nat Neurosci. 2009;12(7):U872–U884. doi:10.1038/nn.2341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Brambilla R, Cottini L, Fumagalli M, Ceruti S, Abbracchio MP. Blockade of A2A adenosine receptors prevents basic fibroblast growth factor-induced reactive astrogliosis in rat striatal primary astrocytes. Glia. 2003;43(2):190–194. doi:10.1002/glia.10243 [DOI] [PubMed] [Google Scholar]
- 117. Popoli P, Pintor A, Domenici MR, et al. Blockade of striatal adenosine A2A receptor reduces, through a presynaptic mechanism, quinolinic acid-induced excitotoxicity: possible relevance to neuroprotective interventions in neurodegenerative diseases of the striatum. J Neurosci. 2002;22(5):1967–1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Melani A, Dettori I, Corti F, Cellai L, Pedata F. Time-course of protection by the selective A2A receptor antagonist SCH58261 after transient focal cerebral ischemia. Neurol Sci. 2015;36(8):1441–1448. doi:10.1007/s10072-015-2160-y [DOI] [PubMed] [Google Scholar]
- 119. Chen JF, Sonsalla PK, Pedata F, et al. Adenosine A2A receptors and brain injury: broad spectrum of neuroprotection, multifaceted actions and “fine tuning” modulation. Prog Neurobiol. 2007;83(5):310–331. doi:10.1016/j.pneurobio.2007.09.002 [DOI] [PubMed] [Google Scholar]
- 120. Ishiwata K, Kawamura K, Kimura Y, Oda K, Ishii K. Potential of an adenosine A(2A) receptor antagonist [C-11]TMSX for myocardial imaging by positron emission tomography: a first human study. Ann Nucl Med. 2003;17(6):457–462. doi:10.1007/Bf03006434 [DOI] [PubMed] [Google Scholar]
- 121. Ishiwata K, Mishina M, Kimura Y, Keiichi O, Toru S, Kenji I. First visualization of adenosine A(2A) receptors in the human brain by positron emission tomography with [11C]TMSX. Synapse. 2005;55(2):133–136. doi:10.1002/syn.20099 [DOI] [PubMed] [Google Scholar]
- 122. Mishina M, Ishiwata K, Kimura Y, et al. Evaluation of distribution of adenosine A2A receptors in normal human brain measured with [11C]TMSX PET. Synapse. 2007;61(9):778–784. doi:10.1002/syn.20423 [DOI] [PubMed] [Google Scholar]
- 123. Wang WF, Ishiwata K, Nonaka H, et al. Carbon-11-labeled KF21213: a highly selective ligand for mapping CNS adenosine A(2A) receptors with positron emission tomography. Nucl Med Biol. 2000;27(6):541–546. doi:10.1016/s0969-8051(00)00126-8 [DOI] [PubMed] [Google Scholar]
- 124. Moresco RM, Todde S, Belloli S, et al. In vivo imaging of adenosine A2A receptors in rat and primate brain using [11C]SCH442416. Eur J Nucl Med Mol Imaging. 2005;32:405–413. doi:10.1007/s00259-004-1688-5 [DOI] [PubMed] [Google Scholar]
- 125. Ramlackhansingh AF, Bose SK, Ahmed I, et al. Adenosine 2A receptor availability in dyskinetic and nondyskinetic patients with Parkinson disease. Neurology. 2011;76(21):1811–1816. doi:10.1212/WNL.0b013e31821ccce4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Sakata M, Ishibashi K, Imai M, et al. Initial evaluation of an adenosine A2A receptor ligand, (11)C-Preladenant, in healthy human subjects. J Nucl Med. 2017;58(9):1464–1470. doi:10.2967/jnumed.116.188474 [DOI] [PubMed] [Google Scholar]
- 127. Neustadt BR, Hao J, Lindo N, et al. Potent, selective, and orally active adenosine A2A receptor antagonists: arylpiperazine derivatives of pyrazolo[4,3-e]-1,2,4-triazolo[1,5-c]pyrimidines. Bioorg Med Chem Lett. 2007;17(5):1376–1380. doi:10.1016/j.bmcl.2006.11.083 [DOI] [PubMed] [Google Scholar]
- 128. Zhou X, Boellaard R, Ishiwata K, et al. In vivo evaluation of (11)C-preladenant for PET imaging of adenosine A2A receptors in the conscious monkey. J Nucl Med. 2017;58(5):762–767. doi:10.2967/jnumed.116.182410 [DOI] [PubMed] [Google Scholar]
- 129. Bhattacharjee AK, Lang L, Jacobson O, et al. Striatal adenosine A(2A) receptor-mediated positron emission tomographic imaging in 6-hydroxydopamine-lesioned rats using [(18)F]-MRS5425. Nucl Med Biol 2011; 38(6): 897–906. doi:10.1016/j.nucmedbio.2011.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Khanapur S, van Waarde A, Dierckx RA, Elsinga PH, Koole MJ. Preclinical evaluation and quantification of (18)F-fluoroethyl and (18)F-fluoropropyl analogs of SCH442416 as radioligands for PET imaging of the adenosine A2A receptor in rat brain. J Nucl Med. 2017;58(3):466–472. doi:10.2967/jnumed.116.178103 [DOI] [PubMed] [Google Scholar]
- 131. Barret O, Hannestad J, Vala C, et al. Characterization in humans of 18F-MNI-444, a PET radiotracer for brain adenosine 2A receptors. J Nucl Med. 2015;56(4):586–591. doi:10.2967/jnumed.114.152546 [DOI] [PubMed] [Google Scholar]
- 132. Vala C, Morley TJ, Zhang XC, et al. Synthesis and in vivo evaluation of Fluorine-18 and Iodine-123 Pyrazolo[4,3-e]-1,2,4-triazolo[1,5-c]pyrimidine derivatives as PET and SPECT radiotracers for mapping A(2A) receptors. Chemmedchem. 2016;11(17):1936–1943. doi:10.1002/cmdc.201600219 [DOI] [PubMed] [Google Scholar]
- 133. Barret O, Hannestad J, Alagille D, et al. Adenosine 2A receptor occupancy by tozadenant and preladenant in rhesus monkeys. J Nucl Med 2014;55(10):1712–1718. doi:10.2967/jnumed.114.142067 [DOI] [PubMed] [Google Scholar]
- 134. Khakh BS, North RA. Neuromodulation by extracellular ATP and P2X receptors in the CNS. Neuron. 2012;76(1):51–69. doi:10.1016/j.neuron.2012.09.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. North RA. P2X receptors. Philos Trans R Soc Lond B Biol Sci. 2016;371(1700):20150427 doi:10.1098/rstb.2015.0427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Burnstock G. Physiopathological roles of P2X receptors in the central nervous system. Curr Med Chem. 2015;22(7):819–844. [DOI] [PubMed] [Google Scholar]
- 137. Burnstock G. P2X ion channel receptors and inflammation. Purinergic Signal. 2016;12(1):59–67. doi:10.1007/s11302-015-9493-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Burnstock G. Pathophysiology and therapeutic potential of purinergic signaling. Pharmacological Rev. 2006;58(1):58–86. doi:10.1124/pr.58.1.5 [DOI] [PubMed] [Google Scholar]
- 139. Ichinohe S, Ishii T, Takahashi H, Kaneda M. Physiological contribution of P2X receptors in postreceptoral signal processing in the mouse retina. Neurosci Res. 2017;115:5–12. doi:10.1016/j.neures.2016.09.012 [DOI] [PubMed] [Google Scholar]
- 140. Lalo U, Palygin O, Verkhratsky A, Grant SG, Pankratov Y. ATP from synaptic terminals and astrocytes regulates NMDA receptors and synaptic plasticity through PSD-95 multi-protein complex. Sci Rep. 2016;6:33609 doi:10.1038/srep33609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Coddou C, Yan Z, Obsil T, Huidobro Toro JP, Stojilkovic SS. Activation and regulation of purinergic P2X receptor channels. Pharmacol Rev. 2011;63(3):641–683. doi:10.1124/pr.110.003129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Rech JC, Bhattacharya A, Letavic MA, Savall BM. The evolution of P2X7 antagonists with a focus on CNS indications. Bioorg Med Chem Lett. 2016;26(16):3838–3845. doi:10.1016/j.bmcl.2016.06.048 [DOI] [PubMed] [Google Scholar]
- 143. Lewis C, Neidhart S, Holy C, North RA, Buell G, Surprenant A. Coexpression of P2X2 and P2X3 receptor subunits can account for ATP-gated currents in sensory neurons. Nature. 1995;377(3548):432–435. doi:10.1038/377432a0 [DOI] [PubMed] [Google Scholar]
- 144. Fabbretti E. ATP P2X3 receptors and neuronal sensitization. Front Cell Neurosci. 2013;7:236 doi:10.3389/fncel.2013.00236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Barclay J, Patel S, Dorn G, et al. Functional downregulation of P2X3 receptor subunit in rat sensory neurons reveals a significant role in chronic neuropathic and inflammatory pain. J neurosci. 2002;22(18):8139–8147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Kuan YH, Shyu BC. Nociceptive transmission and modulation via P2X receptors in central pain syndrome. Mol Brain. 2016;9(1):58 doi:10.1186/s13041-016-0240-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. McGaraughty S, Wismer CT, Zhu CZ, et al. Effects of A-317491, a novel and selective P2X3/P2X2/3 receptor antagonist, on neuropathic, inflammatory and chemogenic nociception following intrathecal and intraplantar administration. Br J Pharmacol. 2003;140(8):1381–1388. doi:10.1038/sj.bjp.0705574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Gum RJ, Wakefield B, Jarvis MF. P2X receptor antagonists for pain management: examination of binding and physicochemical properties. Purinergic Signal. 2012;8(suppl 1):41–56. doi:10.1007/s11302-011-9272-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Jarvis MF, Burgard EC, McGaraughty S, et al. A-317491, a novel potent and selective non-nucleotide antagonist of P2X3 and P2X2/3 receptors, reduces chronic inflammatory and neuropathic pain in the rat. Proc Natl Acad Sci U S A. 2002;99(26):17179–17184. doi:10.1073/pnas.252537299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Oliveira MC, Pelegrini-da-Silva A, Tambeli CH, Parada CA. Peripheral mechanisms underlying the essential role of P2X3,2/3 receptors in the development of inflammatory hyperalgesia. Pain. 2009;141(1-2):127–134. doi:10.1016/j.pain.2008.10.024 [DOI] [PubMed] [Google Scholar]
- 151. Gever JR, Soto R, Henningsen RA, et al. AF-353, a novel, potent and orally bioavailable P2X3/P2X2/3 receptor antagonist. Br J Pharmacol. 2010;160(6):1387–1398. doi:10.1111/j.1476-5381.2010.00796.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Kaan TK, Yip PK, Grist J, et al. Endogenous purinergic control of bladder activity via presynaptic P2X3 and P2X2/3 receptors in the spinal cord. J Neurosci. 2010;30(12):4503–4507. doi:10.1523/JNEUROSCI.6132-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Ryan NM, Vertigan AE, Birring SS. An update and systematic review on drug therapies for the treatment of refractory chronic cough. Expert Opin Pharmacother. 2018;19(7):687–711. doi:10.1080/14656566.2018.1462795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Merck. Merck Announces Presentation of Phase 2 Results for MK-7264, an Investigational, P2X3 Receptor Antagonist, Being Evaluated for the Treatment of Chronic Cough (2017, 2019). https://investors.merck.com/news/press-release-details/2017/Merck-Announces-Presentation-of-Phase-2-Results-for-MK-7264-an-Investigational-P2X3-Receptor-Antagonist-Being-Evaluated-for-the-Treatment-of-Chronic-Cough/default.aspx
- 155. NIH. Phase 3 Study of Gefapixant (MK-7264) in Adult Participants With Chronic Cough (MK-7264-027). ICH GCP|Clinical Trials Registry; 2018. [Google Scholar]
- 156. Jung YH, Kim YO, Lin H, et al. Discovery of potent antiallodynic agents for neuropathic pain targeting P2X3 receptors. ACS Chem Neurosci. 2017;8(7):1465–1478. doi:10.1021/acschemneuro.6b00401 [DOI] [PubMed] [Google Scholar]
- 157. Soto F, Garcia Guzman M, Gomez Hernandez JM, Hollmann M, Karschin C, Stühmer W. P2X4: an ATP-activated ionotropic receptor cloned from rat brain. Proc Natl Acad Sci U S A. 1996;93(8):3684–3688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Ohsawa K, Irino Y, Nakamura Y, Chihiro A, Kazuhide I, Shinichi K. Involvement of P2X4 and P2Y12 receptors in ATP-induced microglial chemotaxis. Glia. 2007;55(6):604–616. doi:10.1002/glia.20489 [DOI] [PubMed] [Google Scholar]
- 159. Stokes L, Layhadi JA, Bibic L, Dhuna K, Fountain SJ. P2X4 receptor function in the nervous system and current breakthroughs in pharmacology. Front Pharmacol. 2017;8:291 doi:10.3389/fphar.2017.00291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Jo YH, Donier E, Martinez A, Maurice G, Estelle T, Eric Boué G. Cross-talk between P2X4 and gamma-aminobutyric acid, type a receptors determines synaptic efficacy at a central synapse. J Biol Chem. 2011;286(22):19993–20004. doi:10.1074/jbc.M111.231324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Gofman L, Fernandes NC, Potula R. Relative role of Akt, ERK and CREB in alcohol-induced microglia P2X4R receptor expression. Alcohol Alcohol. 2016; 51(6): 647–654. doi:10.1093/alcalc/agw009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Horvath RJ, Romero Sandoval EA, De Leo JA. Inhibition of microglial P2X4 receptors attenuates morphine tolerance, Iba1, GFAP and mu opioid receptor protein expression while enhancing perivascular microglial ED2. Pain. 2010;150(3):401–413. doi:10.1016/j.pain.2010.02.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Li F, Wang L, Li JW, et al. Hypoxia induced amoeboid microglial cell activation in postnatal rat brain is mediated by ATP receptor P2X4. BMC Neurosci. 2011;12:111 doi:10.1186/1471-2202-12-111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. de Rivero Vaccari JP, Bastien D, Yurcisin G, et al. P2X4 receptors influence inflammasome activation after spinal cord injury. J Neurosci. 2012;32(9):3058–3066. doi:10.1523/JNEUROSCI.4930-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Cavaliere F, Florenzano F, Amadio S, et al. Up-regulation of P2X2, P2X4 receptor and ischemic cell death: prevention by P2 antagonists. Neuroscience. 2003;120(1):85–98. [DOI] [PubMed] [Google Scholar]
- 166. D’Ambrosi N, Finocchi P, Apolloni S, et al. The proinflammatory action of microglial P2 receptors is enhanced in SOD1 models for amyotrophic lateral sclerosis. J Immunol. 2009;183(7):4648–4656. doi:10.4049/jimmunol.0901212 [DOI] [PubMed] [Google Scholar]
- 167. Guo LH, Trautmann K, Schluesener HJ. Expression of P2X4 receptor by lesional activated microglia during formalin-induced inflammatory pain. J Neuroimmunol. 2005;163(1-2):120–127. doi:10.1016/j.jneuroim.2005.03.007 [DOI] [PubMed] [Google Scholar]
- 168. Zhang Z, Artelt M, Burnet M, Trautmann K, Schluesener HJ. Lesional accumulation of P2X4 receptor+ monocytes following experimental traumatic brain injury. Exp Neurol. 2006;197(1):252–257. doi:10.1016/j.expneurol.2005.09.015 [DOI] [PubMed] [Google Scholar]
- 169. Burnstock G. Purinergic signalling: therapeutic developments. Front Pharmacol. 2017;8:661 doi:10.3389/fphar.2017.00661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Nagata K, Imai T, Yamashita T, Tsuda M, Saitoh HT, Inoue K. Antidepressants inhibit P2X4 receptor function: a possible involvement in neuropathic pain relief. Mol Pain. 2009;5:20 doi:10.1186/1744-8069-5-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Balazs B, Danko T, Kovacs G, Köles L, Hediger MA, Zsembery A. Investigation of the inhibitory effects of the benzodiazepine derivative, 5-BDBD on P2X4 purinergic receptors by two complementary methods. Cell Physiol Biochem. 2013;32(1):11–24. doi:10.1159/000350119 [DOI] [PubMed] [Google Scholar]
- 172. Wang M, Gao MZ, Meyer JA, et al. Synthesis and preliminary biological evaluation of radiolabeled 5-BDBD analogs as new candidate PET radioligands for P2X4 receptor. Bioorgan Med Chem. 2017;25(14):3835–3844. doi:10.1016/j.bmc.2017.05.031 [DOI] [PubMed] [Google Scholar]
- 173. Tian MQ, Abdelrahman A, Weinhausen S, et al. Carbamazepine derivatives with P2X4 receptor-blocking activity. Bioorgan Med Chem. 2014;22(3):1077–1088. doi:10.1016/j.bmc.2013.12.035 [DOI] [PubMed] [Google Scholar]
- 174. Olmos VH, Abdelrahman A, El-Tayeb A, Freudendahl D, Weinhausen S, Müller CE. N-substituted phenoxazine and acridone derivatives: structure-activity relationships of potent P2X4 receptor antagonists. J Med Chem. 2012;55(22):9576–9588. doi:10.1021/jm300845v [DOI] [PubMed] [Google Scholar]
- 175. Hernandez-Olmos V, Abdelrahman A, El-Tayeb A, Freudendahl D, Weinhausen S, Müller CE. N-Substituted phenoxazine and acridone derivatives: structure-activity relationships of potent P2X4 receptor antagonists. J Med Chem. 2012;55(22):9576–9588. doi:10.1021/jm300845v [DOI] [PubMed] [Google Scholar]
- 176. Matsumura Y, Yamashita T, Sasaki A, et al. A novel P2X4 receptor-selective antagonist produces anti-allodynic effect in a mouse model of herpetic pain. Sci Rep. 2016;6:32461 doi:10.1038/srep32461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Di Virgilio F, Ceruti S, Bramanti P, Abbracchio MP. Purinergic signalling in inflammation of the central nervous system. Trends Neurosci. 2009;32(2):79–87. doi:10.1016/j.tins.2008.11.003 [DOI] [PubMed] [Google Scholar]
- 178. Bartlett R, Stokes L, Sluyter R. The P2X7 receptor channel: recent developments and the use of P2X7 antagonists in models of disease. Pharmacol Rev. 2014;66(3):638–675. doi:10.1124/pr.113.008003 [DOI] [PubMed] [Google Scholar]
- 179. Diaz Hernandez JI, Villafuertes RG, Otegui ML, et al. In vivo P2X7 inhibition reduces amyloid plaques in Alzheimer’s disease through GSK3beta and secretases. Neurobiol Aging. 2012;33(8):1816–1828. doi:10.1016/j.neurobiolaging.2011.09.040 [DOI] [PubMed] [Google Scholar]
- 180. Woods LT, Ajit D, Camden JM, Erb L, Weisman GA. Purinergic receptors as potential therapeutic targets in Alzheimer’s disease. Neuropharmacology. 2016;104:169–179. doi:10.1016/j.neuropharm.2015.10.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Marcellino D, Boomgaard DS, Sanchez Reina MD, et al. On the role of P2X(7) receptors in dopamine nerve cell degeneration in a rat model of Parkinson’s disease: studies with the P2X(7) receptor antagonist A-438079. J Neural Transm (Vienna). 2010;117(6):681–687. doi:10.1007/s00702-010-0400-0 [DOI] [PubMed] [Google Scholar]
- 182. Hernandez MD, Zaera MD, Nogueiro JS, et al. Altered P2X7-receptor level and function in mouse models of Huntington’s disease and therapeutic efficacy of antagonist administration. FASEB J. 2009;23(6):1893–1906. doi:10.1096/fj.08-122275 [DOI] [PubMed] [Google Scholar]
- 183. Gandelman M, Peluffo H, Beckman JS, Cassina P, Barbeito L. Extracellular ATP and the P2X7 receptor in astrocyte-mediated motor neuron death: implications for amyotrophic lateral sclerosis. J Neuroinflammation. 2010;7:33 doi:10.1186/1742-2094-7-33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Amadio S, Parisi C, Piras E, et al. Modulation of P2X7 receptor during inflammation in multiple sclerosis. Front Immunol. 2017;8:1529 doi:10.3389/fimmu.2017.01529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Engel T, Pacheco AJ, Miras Portugal MT, et al. P2X7 receptor in epilepsy; role in pathophysiology and potential targeting for seizure control. Int J Physiol Pathophysiol Pharmacol. 2012;4(4):174–187 [PMC free article] [PubMed] [Google Scholar]
- 186. Deussing JM, Arzt E. P2X7 receptor: a potential therapeutic target for depression? Trends Mol Med. 2018;24(9):736–747. doi:10.1016/j.molmed.2018.07.005 [DOI] [PubMed] [Google Scholar]
- 187. Bhattacharya A. Recent advances in CNS P2X7 physiology and pharmacology: focus on neuropsychiatric disorders. Front Pharmacol. 2018;9:30 doi:10.3389/fphar.2018.00030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Cieslak M, Roszek K, Wujak M. Purinergic implication in amyotrophic lateral sclerosis-from pathological mechanisms to therapeutic perspectives. Purinergic Signal. 2019;15:1–15. doi:10.1007/s11302-018-9633-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Franke H, Verkhratsky A, Burnstock G, et al. Pathophysiology of astroglial purinergic signalling. Purinergic Signal. 2012;8(3):629–657. doi:10.1007/s11302-012-9300-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Gubert C, Fries GR, Pfaffenseller B, et al. Role of P2X7 Receptor in an Animal Model of Mania Induced by D-Amphetamine. Mol Neurobiol. 2016;53(1):611–620. doi:10.1007/s12035-014-9031-z [DOI] [PubMed] [Google Scholar]
- 191. Wilot LC, Bernardi A, Frozza RL, et al. Lithium and valproate protect hippocampal slices against ATP-induced cell death. Neurochem Res. 2007;32(9):1539–1546. doi:10.1007/s11064-007-9348-3 [DOI] [PubMed] [Google Scholar]
- 192. Stokes L, Spencer SJ, Jenkins T. Understanding the role of P2X7 in affective disorders—are glial cells the major players? Front Cell Neurosci. 2015;9:258 doi:10.3389/fncel.2015.00258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Eser A, Colombel JF, Rutgeerts P, et al. Safety and efficacy of an oral inhibitor of the purinergic receptor P2X7 in adult patients with moderately to severely active Crohn’s disease: a randomized placebo-controlled, double-blind, phase IIa study. Inflamm Bowel Dis. 2015;21(7):2247–2253. doi:10.1097/MIB.0000000000000514 [DOI] [PubMed] [Google Scholar]
- 194. Stock TC, Bloom BJ, Wei N, et al. Efficacy and Safety of CE-224,535, an Antagonist of P2X(7) receptor, in treatment of patients with rheumatoid arthritis inadequately controlled by methotrexate. J Rheumatol. 2012;39(5):720–727. doi:10.3899/jrheum.110874 [DOI] [PubMed] [Google Scholar]
- 195. Baudelet D, Lipka E, Millet R, et al. Involvement of the P2X7 purinergic receptor in inflammation: an update of antagonists series since 2009 and their promising therapeutic potential. Curr Med Chem. 2015;22(7):713–729. [DOI] [PubMed] [Google Scholar]
- 196. Ali Z, Laurijssens B, Ostenfeld T, et al. Pharmacokinetic and pharmacodynamic profiling of a P2X7 receptor allosteric modulator GSK1482160 in healthy human subjects. Br J Clin Pharmacol. 2013;75:197–207. doi:10.1111/j.1365-2125.2012.04320.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Lord B, Aluisio L, Shoblock JR, et al. Pharmacology of a novel central nervous system-penetrant P2X7 antagonist JNJ-42253432. J Pharmacol Exp Ther 2014;351(4):628–641. doi:10.1124/jpet.114.218487 [DOI] [PubMed] [Google Scholar]
- 198. Bhattacharya A, Wang Q, Ao H, et al. Pharmacological characterization of a novel centrally permeable P2X7 receptor antagonist: JNJ-47965567. Br J Pharmacol. 2013;170(4):624–640. doi:10.1111/bph.12314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Lord B, Ameriks MK, Wang Q, et al. A novel radioligand for the ATP-gated ion channel P2X7: [3 H] JNJ-54232334. Eur J Pharmacol. 2015;76(5):551–559. doi:10.1016/j.ejphar.2015.09.026 [DOI] [PubMed] [Google Scholar]
- 200. Swanson DM, Savall BM, Coe KJ, et al. Identification of (R)-(2-Chloro-3-(trifluoromethyl)phenyl)(1-(5-fluoropyridin-2-yl)-4-methyl-6,7-di hydro-1H-imidazo[4,5-c]pyridin-5(4 H)-yl)methanone (JNJ 54166060), a small molecule antagonist of the P2X7 receptor. J Med Chem. 2016;59(5):8535–8548. doi:10.1021/acs.jmedchem.6b00989 [DOI] [PubMed] [Google Scholar]
- 201. Rudolph DA, Alcazar J, Ameriks MK, et al. Novel methyl substituted 1-(5,6-dihydro-[1,2,4]triazolo[4,3-a]pyrazin-7(8 H)-yl)methanones are P2X7 antagonists. Bioorg Med Chem Lett. 2015;25:3157–3163. doi:10.1016/j.bmcl.2015.06.004 [DOI] [PubMed] [Google Scholar]
- 202. Timmers M, Ravenstijn P, Xi L, et al. Clinical pharmacokinetics, pharmacodynamics, safety, and tolerability of JNJ-54175446, a brain permeable P2X7 antagonist, in a randomised single-ascending dose study in healthy participants. J Psychopharmacol. 2018;32(3):1341–1350. doi:10.1177/0269881118800067 [DOI] [PubMed] [Google Scholar]
- 203. Bhattacharya A, Lord B, Grigoleit JS, et al. Neuropsychopharmacology of JNJ-55308942: evaluation of a clinical candidate targeting P2X7 ion channels in animal models of neuroinflammation and anhedonia. Neuropsychopharmacology. 2018;43(5):2586–2596. doi:10.1038/s41386-018-0141-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Janssen B, Vugts DJ, Windhorst AD, et al. PET imaging of microglial activation-beyond targeting TSPO. Molecules. 2018:23 doi:10.3390/molecules23030607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Janssen B, Vugts DJ, Funke U, et al. Synthesis and initial preclinical evaluation of the P2X7 receptor antagonist [(1)(1)C]A-740003 as a novel tracer of neuroinflammation. J Labelled Comp Radiopharm. 2014;57(3):509–516. doi:10.1002/jlcr.3206 [DOI] [PubMed] [Google Scholar]
- 206. Ory D, Celen S, Gijsbers R, et al. Preclinical evaluation of a P2X7 receptor-selective radiotracer: PET studies in a rat model with local overexpression of the human P2X7 receptor and in nonhuman primates. J Nucl Med. 2016;57(2):1436–1441. doi:10.2967/jnumed.115.169995 [DOI] [PubMed] [Google Scholar]
- 207. Gao MZ, Wang M, Green MA, et al. Synthesis of [C-11]GSK1482160 as a new PET agent for targeting P2X(7) receptor. Bioorgan Medl Chem Lett. 2015;25(1):1965–1970. doi:10.1016/j.bmcl.2015.03.021 [DOI] [PubMed] [Google Scholar]
- 208. Territo PR, Meyer JA, Peters JS, et al. Characterization of C-11-GSK1482160 for targeting the P2X7 receptor as a biomarker for neuroinflammation. J Nucl Med. 2017;58(1):458–465. doi:10.2967/jnumed.116.181354 [DOI] [PubMed] [Google Scholar]
- 209. Han J, Liu H, Liu C, et al. Pharmacologic characterizations of a P2X7 receptor-specific radioligand, [11C]GSK1482160 for neuroinflammatory response. Nucl Med Commun. 2017;38(2):372–382. doi:10.1097/MNM.0000000000000660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Janssen B, Vugts DJ, Wilkinson SM, et al. Identification of the allosteric P2X7 receptor antagonist [(11)C]SMW139 as a PET tracer of microglial activation. Sci Rep. 2018;8(2):6580 doi:10.1038/s41598-018-24814-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Wilkinson SM, Barron ML, O’Brien-Brown J, et al. Pharmacological evaluation of novel bioisosteres of an adamantanyl benzamide P2X7 receptor antagonist. ACS Chem Neurosci. 2017;8(2):2374–2380. doi:10.1021/acschemneuro.7b00272 [DOI] [PubMed] [Google Scholar]
- 212. Hagens MHJ, Golla SSV, Janssen B, et al. The P2X7 receptor tracer [(11)C]SMW139 as an in vivo marker of neuroinflammation in multiple sclerosis: a first-in man study. Eur J Nucl Med Mol Imaging. 2020;47(3):379–389. doi:10.1007/s00259-019-04550-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Fantoni ER, Dal Ben D, Falzoni S, et al. Design, synthesis and evaluation in an LPS rodent model of neuroinflammation of a novel (18)F-labelled PET tracer targeting P2X7. EJNMMI Res. 2017;7(1):31 doi:10.1186/s13550-017-0275-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Koole M, Schmidt M, Hijzen A, et al. (18)F-JNJ-64413739, a novel PET ligand for the P2X7 ion channel: radiation dosimetry, kinetic modeling, test-retest variability and occupancy of the P2X7 antagonist JNJ-54175446. J Nucl Med. 2018:9 doi:10.2967/jnumed.118.216747 [DOI] [PubMed] [Google Scholar]
- 215. Kolb HC, Barret O, Bhattacharya A, et al. Preclinical evaluation and nonhuman primate receptor occupancy study of (18)F-JNJ-64413739, a PET radioligand for P2X7 receptors. J Nucl Med. 2019;60(4):1154–1159. doi:10.2967/jnumed.118.212696 [DOI] [PubMed] [Google Scholar]
- 216. Berdyyeva T, Xia C, Taylor N, et al. PET Imaging of the P2X7 Ion channel with a novel tracer [(18)F]JNJ-64413739 in a rat model of neuroinflammation. Mol Imaging Biol. 2019;21(3):871–878. doi:10.1007/s11307-018-01313-2 [DOI] [PubMed] [Google Scholar]
- 217. Koole M, Schmidt ME, Hijzen A, et al. (18)F-JNJ-64413739, a novel PET ligand for the P2X7 Ion channel: radiation dosimetry, kinetic modeling, test-retest variability, and occupancy of the p2x7 antagonist JNJ-54175446. J Nucl Med. 2019;60(5):683–690. doi:10.2967/jnumed.118.216747 [DOI] [PubMed] [Google Scholar]
- 218. Gao M, Wang M, Glick-Wilson BE, et al. Synthesis and preliminary biological evaluation of a novel P2X7 R radioligand [(18)F]IUR-1601. Bioorg Med Chem Lett. 2018;28(4):1603–1609. doi:10.1016/j.bmcl.2018.03.044 [DOI] [PubMed] [Google Scholar]
- 219. Weisman GA, Camden JM, Peterson TS, et al. P2 receptors for extracellular nucleotides in the central nervous system: role of P2X7 and P2Y(2) receptor interactions in neuroinflammation. Mol Neurobiol. 2012;46(5):96–113. doi:10.1007/s12035-012-8263-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Del Puerto A, Wandosell F, Garrido JJ. Neuronal and glial purinergic receptors functions in neuron development and brain disease. Front Cell Neurosci. 2013;7(3):197 doi:10.3389/fncel.2013.00197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Weisman GA, Woods LT, Erb L, et al. P2Y receptors in the mammalian nervous system: pharmacology, ligands and therapeutic potential. CNS Neurol Disord Drug Targets. 2012;11(6):722–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Jacobson KA, Paoletta S, Katritch V, et al. Nucleotides acting at P2Y receptors: connecting structure and function. Mol Pharmacol. 2015;88(4):220–230. doi:10.1124/mol.114.095711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Fields RD. Nonsynaptic and nonvesicular ATP release from neurons and relevance to neuron-glia signaling. Semin Cell Dev Biol. 2011;22(1):214–219. doi:10.1016/j.semcdb.2011.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Imura Y, Morizawa Y, Komatsu R, et al. Microglia release ATP by exocytosis. Glia. 2013;61(7):1320–1330. doi:10.1002/glia.22517 [DOI] [PubMed] [Google Scholar]
- 225. Sperlagh B, Heinrich A, Csolle C. P2 receptor-mediated modulation of neurotransmitter release-an update. Purinergic Sign. 2007;3(5):269–284. doi:10.1007/s11302-007-9080-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Guzman SJ, Gerevich Z. P2Y receptors in synaptic transmission and plasticity: therapeutic potential in cognitive dysfunction. Neural Plast. 2016;2016(1):12073 93. doi:10.1155/2016/1207393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Krugel U, Kittner H, Franke H, et al. Stimulation of P2 receptors in the ventral tegmental area enhances dopaminergic mechanisms in vivo. Neuropharmacology. 2001;40(3):1084–1093. [DOI] [PubMed] [Google Scholar]
- 228. Koch H, Bespalov A, Drescher K, et al. Impaired cognition after stimulation of P2Y1 receptors in the rat medial prefrontal cortex. Neuropsychopharmacology. 2015;40(2):305–314. doi:10.1038/npp.2014.173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Heinrich A, Kittel A, Csolle C, et al. Modulation of neurotransmitter release by P2X and P2Y receptors in the rat spinal cord. Neuropharmacology. 2008;54(8):375–386. doi:10.1016/j.neuropharm.2007.10.013 [DOI] [PubMed] [Google Scholar]
- 230. Csolle C, Heinrich A, Kittel A, et al. P2Y receptor mediated inhibitory modulation of noradrenaline release in response to electrical field stimulation and ischemic conditions in superfused rat hippocampus slices. J Neurochem. 2008;106:347–360. doi:10.1111/j.1471-4159.2008.05391.x [DOI] [PubMed] [Google Scholar]
- 231. Von Kugelgen I, Kurz K, Starke K. P2-purinoceptor-mediated autoinhibition of sympathetic transmitter release in mouse and rat vas deferens. Naunyn Schmiedebergs Arch Pharmacol. 1994;349(10):125–132. [DOI] [PubMed] [Google Scholar]
- 232. Alves M, Beamer E, Engel T. The metabotropic purinergic p2y receptor family as novel drug target in epilepsy. Front Pharm. 2018:9 doi:10.3389/fphar.2018.00193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233. Inoue K. UDP facilitates microglial phagocytosis through P2Y6 receptors. Cell Adhes Migr. 2007;1(4):131–132. doi:10.4161/cam.1.3.4937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234. Saitow F, Murakoshi T, Suzuki H, et al. Metabotropic P2Y purinoceptor-mediated presynaptic and postsynaptic enhancement of cerebellar GABAergic transmission. J Neurosci. 2005;25(6):2108–2116. doi:10.1523/JNEUROSCI.4254-04.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235. Viswanath H, Carter AQ, Baldwin PR, et al. The medial habenula: still neglected. Front Hum Neurosci. 2013;7(3):931 doi:10.3389/fnhum.2013.00931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236. Espada S, Ortega F, Molina-Jijon E, et al. The purinergic P2Y(13) receptor activates the Nrf2/HO-1 axis and protects against oxidative stress-induced neuronal death. Free Radic Biol Med. 2010;49(6):416–426. doi:10.1016/j.freeradbiomed.2010.04.031 [DOI] [PubMed] [Google Scholar]
- 237. Fujita T, Tozaki-Saitoh H, Inoue K. P2Y1 receptor signaling enhances neuroprotection by astrocytes against oxidative stress via IL-6 release in hippocampal cultures. Glia. 2009;57(2):244–257. doi:10.1002/glia.20749 [DOI] [PubMed] [Google Scholar]
- 238. Zhang X, Lu F, Chen YK, et al. Discovery of potential orthosteric and allosteric antagonists of P2Y1 R from Chinese herbs by molecular simulation methods. Evid Based Complement Alternat Med. 2016;2016(1):4320201 doi:10.1155/2016/4320201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239. Moore D, Chambers J, Waldvogel H, et al. Regional and cellular distribution of the P2Y(1) purinergic receptor in the human brain: striking neuronal localisation. J Comp Neurol. 2000;42(1):374–384. [DOI] [PubMed] [Google Scholar]
- 240. Fumagalli M, Brambilla R, D’Ambrosi N, et al. Nucleotide-mediated calcium signaling in rat cortical astrocytes: Role of P2X and P2Y receptors. Glia. 2003;43(2):218–203. doi:10.1002/glia.10248 [DOI] [PubMed] [Google Scholar]
- 241. Fries JE, Wheeler-Schilling TH, Guenther E, et al. Expression of P2Y1, P2Y2, P2Y4, and P2Y6 receptor subtypes in the rat retina. Invest Ophthalmol Vis Sci. 2004;45(3):3410–3417. doi:10.1167/iovs.04-0141 [DOI] [PubMed] [Google Scholar]
- 242. Savi P, Beauverger P, Labouret C, et al. Role of P2Y1 purinoceptor in ADP-induced platelet activation. FEBS Lett. 1998;42(2):291–295. [DOI] [PubMed] [Google Scholar]
- 243. Franke H, Schepper C, Illes P, et al. Involvement of P2X and P2Y receptors in microglial activation in vivo. Purin Sign. 2007;3(1):435–445. doi:10.1007/s11302-007-9082-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244. Franke H, Krugel U, Schmidt R, et al. P2 receptor-types involved in astrogliosis in vivo. Br J Pharmacol. 2001;134(5):1180–1189. doi:10.1038/sj.bjp.0704353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245. Sun JJ, Liu Y, Ye ZR. Effects of P2Y1 receptor on glial fibrillary acidic protein and glial cell line-derived neurotrophic factor production of astrocytes under ischemic condition and the related signaling pathways. Neurosci Bull. 2008;24(3):231–243. doi:10.1007/s12264-008-0430-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246. Delekate A, Fuchtemeier M, Schumacher T, Cordula U, Marco F, Gabor CP. Metabotropic P2Y1 receptor signalling mediates astrocytic hyperactivity in vivo in an Alzheimer’s disease mouse model. Nat Commun. 2014;5(1):5422 doi:10.1038/ncomms6422 [DOI] [PubMed] [Google Scholar]
- 247. Reichenbach N, Delekate A, Breithausen B, et al. P2Y1 receptor blockade normalizes network dysfunction and cognition in an Alzheimer’s disease model. J Exp Med. 2018;215(22):1649–1663. doi:10.1084/jem.20171487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248. Moore D, Iritani S, Chambers J, et al. Immunohistochemical localization of the P2Y1 purinergic receptor in Alzheimer’s disease. Neuroreport. 2000;11(5):3799–3803. [DOI] [PubMed] [Google Scholar]
- 249. Choo AM, Miller WJ, Chen YC, et al. Antagonism of purinergic signalling improves recovery from traumatic brain injury. Brain. 2013;136(3):65–80. doi:10.1093/brain/aws286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250. Kuboyama K, Harada H, Tozaki-Saitoh H, et al. Astrocytic P2Y(1) receptor is involved in the regulation of cytokine/chemokine transcription an cerebral damage in a rat model of cerebral ischemia. J Cerebr Blood F Met. 2011;31:1930–1941. doi:10.1038/jcbfm.2011.49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251. Forster D, Reiser G. Supportive or detrimental roles of P2Y receptors in brain pathology?—the two faces of P2Y receptors in stroke and neurodegeneration detected in neural cell and in animal model studies. Purin Sign. 2015;11(4):441–454. doi:10.1007/s11302-015-9471-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252. Brown SG, King BF, Kim YC, et al. Activity of novel adenine nucleotide derivatives as agonists and antagonists at recombinant rat P2X receptors. Drug Develop Res. 2000;49(3):253–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253. Shinozaki Y, Koizumi S, Ishida S, et al. Cytoprotection against oxidative stress-induced damage of astrocytes by extracellular ATP via P2Y1 receptors. Glia. 2005;49:288–300. doi:10.1002/glia.20118 [DOI] [PubMed] [Google Scholar]
- 254. Krugel U. Purinergic receptors in psychiatric disorders. Neuropharmacology. 2016;104(3):212–225. doi:10.1016/j.neuropharm.2015.10.032 [DOI] [PubMed] [Google Scholar]
- 255. del Puerto A, Diaz-Hernandez JI, Tapia M, et al. Adenylate cyclase 5 coordinates the action of ADP, P2Y1, P2Y13 and ATP-gated P2X7 receptors on axonal elongation. J Cell Sci. 2012;125(3):176–188. doi:10.1242/jcs.091736 [DOI] [PubMed] [Google Scholar]
- 256. Moldovan RP, Wenzel B, Teodoro R, et al. Studies towards the development of a PET radiotracer for imaging of the P2Y1 receptors in the brain: synthesis, (18)F-labeling and preliminary biological evaluation. Eur J Med Chem. 2019;165(4):142–159. doi:10.1016/j.ejmech.2019.01.006 [DOI] [PubMed] [Google Scholar]
- 257. Houston D, Ohno M, Nicholas RA, et al. [32P]2-iodo-N6-methyl-(N)-methanocarba-2’-deoxyadenosine-3’,5’-bisphosphate ([32P]MRS2500), a novel radioligand for quantification of native P2Y1 receptors. Br J Pharmacol. 2006;147(2):459–467. doi:10.1038/sj.bjp.0706453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258. Xu P, Feng X, Luan H, et al. Current knowledge on the nucleotide agonists for the P2Y2 receptor. Bioorg Med Chem. 2018;26(5):366–375. doi:10.1016/j.bmc.2017.11.043 [DOI] [PubMed] [Google Scholar]
- 259. Inoue K, Tsuda M. Purinergic systems, neuropathic pain and the role of microglia. Exp Neurol. 2012;234(3):293–301. doi:10.1016/j.expneurol.2011.09.016 [DOI] [PubMed] [Google Scholar]
- 260. Kong Q, Peterson TS, Baker O, et al. Interleukin-1beta enhances nucleotide-induced and alpha-secretase-dependent amyloid precursor protein processing in rat primary cortical neurons via up-regulation of the P2Y(2) receptor. J Neurochem. 2009;109(3):1300–1310. doi:10.1111/j.1471-4159.2009.06048.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261. Weisman GA, Ajit D, Lucas WT, et al. Loss of P2Y2 nucleotide receptors enhances early pathology in the TgCRND8 mouse model of Alzheimer’s disease. J Neurochem. 2013;125(3):257–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262. Kim HJ, Ajit D, Peterson TS, et al. Nucleotides released from Abeta(1)(-)(4)(2) -treated microglial cells increase cell migration and Abeta(1)(-)(4)(2) uptake through P2Y(2) receptor activation. J Neurochem. 2012;121(4):228–238. doi:10.1111/j.1471-4159.2012.07700.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263. Lai MK, Tan MG, Kirvell S, et al. Selective loss of P2Y2 nucleotide receptor immunoreactivity is associated with Alzheimer’s disease neuropathology. J Neural Transm (Vienna). 2008;115(4):1165–1172. doi:10.1007/s00702-008-0067-y [DOI] [PubMed] [Google Scholar]
- 264. Erb L, Cao C, Ajit D, et al. P2Y receptors in Alzheimer’s disease. Biol Cell. 2015;107(6):1–21. doi:10.1111/boc.201400043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265. Peterson TS, Thebeau CN, Ajit D, et al. Up-regulation and activation of the P2Y(2) nucleotide receptor mediate neurite extension in IL-1beta-treated mouse primary cortical neurons. J Neurochem. 2013;125(5):885–896. doi:10.1111/jnc.12252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266. Yamane M, Ogawa Y, Fukui M, et al. Long-term rebamipide and diquafosol in two cases of immune-mediated dry eye. Optom Vis Sci. 2015;92(3):S25–S32. doi:10.1097/OPX.0000000000000523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267. El-Tayeb A, Qi A, Nicholas RA, et al. Structural modifications of UMP, UDP, and UTP leading to subtype-selective agonists for P2Y2, P2Y4, and P2Y6 receptors. J Med Chem. 2011;54(2):2878–2890. doi:10.1021/jm1016297 [DOI] [PubMed] [Google Scholar]
- 268. Song X, Guo W, Yu Q, et al. Regional expression of P2Y(4) receptors in the rat central nervous system. Purinergic Sign. 2011;7(1):469–488. doi:10.1007/s11302-011-9246-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269. Burnstock G. The therapeutic potential of purinergic signalling. Biochem Pharmacol. 2018;15(1):157–165. doi:10.1016/j.bcp.2017.07.016 [DOI] [PubMed] [Google Scholar]
- 270. Abbracchio MP, Burnstock G, Boeynaems JM, et al. International union of pharmacology LVIII: update on the P2Y G protein-coupled nucleotide receptors: from molecular mechanisms and pathophysiology to therapy. Pharm Rev. 2006;58(6):281–341. doi:10.1124/pr.58.3.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271. Weisman GA, Woods LT, Erb L, et al. P2Y receptors in the mammalian nervous system: pharmacology, ligands and therapeutic potential. CNS Neurol Disord Drug Targets. 2012;11(4):722–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272. Brinson AE, Harden TK. Differential regulation of the uridine nucleotide-activated P2Y4 and P2Y6 receptors. SER-333 and SER-334 in the carboxyl terminus are involved in agonist-dependent phosphorylation desensitization and internalization of the P2Y4 receptor. J Biol Chem. 2001;27(6):11939–11948. doi:10.1074/jbc.M009909200 [DOI] [PubMed] [Google Scholar]
- 273. Li HQ, Chen C, Dou Y, et al. P2Y4 receptor-mediated pinocytosis contributes to amyloid beta-induced self-uptake by microglia. Mol Cell Biol. 2013;33(3):4282–4293. doi:10.1128/MCB.00544-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274. Rafehi M, Malik EM, Neumann A, et al. Development of potent and selective antagonists for the UTP-activated P2Y4 receptor. J Med Chem. 2017;60(1):3020–3038. doi:10.1021/acs.jmedchem.7b00030 [DOI] [PubMed] [Google Scholar]
- 275. Jacobson KA, Jarvis MF, Williams M. Purine and pyrimidine (P2) receptors as drug targets. J Med Chem. 2002;45(2):4057–4093. doi:10.1021/jm020046y [DOI] [PubMed] [Google Scholar]
- 276. Nguyen T, Erb L, Weisman GA, et al. Cloning, expression, and chromosomal localization of the human uridine nucleotide receptor gene. J Biol Chem. 1995;270(6):30845–30848. [DOI] [PubMed] [Google Scholar]
- 277. Yerxa BR, Sabater JR, Davis CW, et al. Pharmacology of INS37217 [P(1)-(uridine 5’)-P(4)- (2’-deoxycytidine 5’)tetraphosphate, tetrasodium salt], a next-generation P2Y(2) receptor agonist for the treatment of cystic fibrosis. J Pharmacol Exp Ther. 2002;302(2):871–880. doi:10.1124/jpet.102.035485 [DOI] [PubMed] [Google Scholar]
- 278. Communi D, Motte S, Boeynaems JM, et al. Pharmacological characterization of the human P2Y4 receptor. Eur J Pharmacol 1996;317(5):383–389. [DOI] [PubMed] [Google Scholar]
- 279. Rafehi M, Malik EM, Neumann A, et al. Development of potent and selective antagonists for the UTP-activated P2Y(4) receptor. J Med Chem. 2017;60(5):3020–3038. doi:10.1021/acs.jmedchem.7b00030 [DOI] [PubMed] [Google Scholar]
- 280. Communi D, Parmentier M, Boeynaems JM. Cloning, functional expression and tissue distribution of the human P2Y6 receptor. Biochem Biophys Res Commun. 1996;222(2):303–308. doi:10.1006/bbrc.1996.0739 [DOI] [PubMed] [Google Scholar]
- 281. Bianco F, Fumagalli M, Pravettoni E, et al. Pathophysiological roles of extracellular nucleotides in glial cells: differential expression of purinergic receptors in resting and activated microglia. Brain Res Brain Res Rev. 2005;48(2):144–156. doi:10.1016/j.brainresrev.2004.12.004 [DOI] [PubMed] [Google Scholar]
- 282. Yang XD, Lou Y, Liu GD, et al. Microglia P2Y6 receptor is related to Parkinson’s disease through neuroinflammatory process. J Neuroinflamm. 2017;14(1). doi:10.1186/s12974-017-0795-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283. Steculorum SM, Timper K, Engstrom Ruud L, et al. Inhibition of P2Y6 signaling in AgRP neurons reduces food intake and improves systemic insulin sensitivity in obesity. Cell Rep. 2017;18(7):1587–1597. doi:10.1016/j.celrep.2017.01.047 [DOI] [PubMed] [Google Scholar]
- 284. Sil P, Hayes CP, Reaves BJ, et al. P2Y6 receptor antagonist MRS2578 inhibits neutrophil activation and aggregated neutrophil extracellular trap formation induced by gout-associated monosodium urate crystals. Journal of immunology. 2017;198(1):428–442. doi:10.4049/jimmunol.1600766 [DOI] [PubMed] [Google Scholar]
- 285. Mamedova LK, Joshi BV, Gao ZG, et al. Diisothiocyanate derivatives as potent, insurmountable antagonists of P2y(6) nucleotide receptors. Biochemical Pharmacology. 2004;67(9):1763–1770. Doi:10.1016/j.bcp.2004.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286. Burnstock G, Knight GE. Cellular distribution and functions of P2 receptor subtypes in different systems. Int Rev Cytol. 2004;240:31–304. doi:10.1016/S0074-7696(04)40002-3 [DOI] [PubMed] [Google Scholar]
- 287. Ito M, Egashira SI, Yoshida K, et al. Identification of novel selective P2Y6 receptor antagonists by high-throughput screening assay. Life Sci. 2017;180:137–142. doi:10.1016/j.lfs.2017.05.017 [DOI] [PubMed] [Google Scholar]
- 288. Hao Y, Liang JF, Chow AW, Cheung W-T, Ko W-H. P2Y6 receptor-mediated proinflammatory signaling in human bronchial epithelia. PLoS One. 2014;9(9):e10623 5. doi:10.1371/journal.pone.0106235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289. Neher JJ, Neniskyte U, Hornik T, Brown GC. Inhibition of UDP/P2Y6 purinergic signaling prevents phagocytosis of viable neurons by activated microglia in vitro and in vivo. Glia. 2014;62(9):1463–1475. doi:10.1002/glia.22693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290. Dorsam RT, Kunapuli SP. Central role of the P2Y12 receptor in platelet activation. J Clin Invest. 2004;113(3):340–345. doi:10.1172/JCI20986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291. Mildner A, Huang H, Radke J, Stenzel W, Priller J. P2Y(12) receptor is expressed on human microglia under physiological conditions throughout development and is sensitive to neuroinflammatory diseases. Glia. 2017;65(2):375–387. doi:10.1002/glia.23097 [DOI] [PubMed] [Google Scholar]
- 292. Amadio S, Parisi C, Montilli C, Carrubba AS, Apolloni S, Volonté C. P2Y(12) receptor on the verge of a neuroinflammatory breakdown. Mediat Inflamm 2014;2014:975849 doi:10.1155/2014/975849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293. Amadio S, Parisi C, Montilli C, Carrubba AS, Apolloni S, Volonté C. P2Y(12) receptor on the verge of a neuroinflammatory breakdown. Mediators Inflamm 2014; 2014:975849 doi:10.1155/2014/975849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294. Webster CM, Hokari M, McManus A, et al. Microglial P2Y12 deficiency/inhibition protects against brain ischemia. PLoS One. 2013;8(8):e70927 doi:10.1371/journal.pone.0070927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295. Koizumi S, Ohsawa K, Inoue K, Kohsaka S. Purinergic receptors in microglia: functional modal shifts of microglia mediated by P2 and P1 receptors. Glia. 2013;61(1):47–54. doi:10.1002/glia.22358 [DOI] [PubMed] [Google Scholar]
- 296. Ohsawa K, Sanagi T, Nakamura Y, Suzuki E, Inoue K, Kohsaka S. Adenosine A3 receptor is involved in ADP-induced microglial process extension and migration. J Neurochem. 2012;121(2):217–227. doi:10.1111/j.1471-4159.2012.07693.x [DOI] [PubMed] [Google Scholar]
- 297. Bhatt DL, Stone GW, Mahaffey KW, et al. Effect of platelet inhibition with cangrelor during PCI on ischemic events. N Engl J Med. 2013;368(14):1303–1313. doi:10.1056/NEJMoa1300815 [DOI] [PubMed] [Google Scholar]
- 298. Mitrugno A, Rigg RA, Laschober NB, et al. Potentiation of TRAP-6-induced platelet dense granule release by blockade of P2Y12 signaling with MRS2395. Platelets. 2018;29(4):383–394. doi:10.1080/09537104.2017.1316482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299. Wallentin L. P2Y(12) inhibitors: differences in properties and mechanisms of action and potential consequences for clinical use. Eur Heart J. 2009;30(16):1964–1977. doi:10.1093/eurheartj/ehp296 [DOI] [PubMed] [Google Scholar]
- 300. Bach P, Bostrom J, Brickmann K, et al. Synthesis, structure-property relationships and pharmacokinetic evaluation of ethyl 6-aminonicotinate sulfonylureas as antagonists of the P2Y(1)(2) receptor. Eur J Med Chem. 2013;65:360–375. doi:10.1016/j.ejmech.2013.04.007 [DOI] [PubMed] [Google Scholar]
- 301. Villa A, Klein B, Janssen B, et al. Identification of new molecular targets for PET imaging of the microglial anti-inflammatory activation state. Theranostics. 2018;8(19):5400–5418. doi:10.7150/thno.25572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302. Beaino W, Janssen B, Kooij G, et al. Purinergic receptors P2Y12 R and P2X7 R: potential targets for PET imaging of microglia phenotypes in multiple sclerosis. J Neuroinflammation. 2017;14(1):259 doi:10.1186/s12974-017-1034-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303. Erb L, Cao C, Ajit D, Weisman GA. P2Y receptors in Alzheimer’s disease. Biol Cell. 2015;107(1):1–21. doi:10.1111/boc.201400043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304. Perez-Sen R, Queipo MJ, Morente V, Ortega F, Delicado EG, Miras-Portugal MT. Neuroprotection mediated by P2Y13 nucleotide receptors in neurons. Comput Struct Biotechnol J. 2015;13:160–168. doi:10.1016/j.csbj.2015.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305. Quintas C, Vale N, Goncalves J, Queiroz G. Microglia P2Y13 receptors prevent astrocyte proliferation mediated by P2Y1 receptors. Front Pharmacol. 2018;9:418 doi:10.3389/fphar.2018.00418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306. Guarracino JF, Cinalli AR, Fernandez V, Roquel LI, Losavio AS. P2Y13 receptors mediate presynaptic inhibition of acetylcholine release induced by adenine nucleotides at the mouse neuromuscular junction. Neuroscience. 2016;326:31–44. doi:10.1016/j.neuroscience.2016.03.066 [DOI] [PubMed] [Google Scholar]
- 307. Kim YC, Lee JS, Sak K, et al. Synthesis of pyridoxal phosphate derivatives with antagonist activity at the P2Y13 receptor. Biochem Pharmacol. 2005;70(2):266–274. doi:10.1016/j.bcp.2005.04.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308. Lee BC, Cheng T, Adams GB, et al. P2Y-like receptor, GPR105 (P2Y14), identifies and mediates chemotaxis of bone-marrow hematopoietic stem cells. Genes Dev. 2003;17(13):1592–1604. doi:10.1101/gad.1071503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309. Skelton L, Cooper M, Murphy M, Platt A. Human immature monocyte-derived dendritic cells express the G protein-coupled receptor GPR105 (KIAA0001, P2Y14) and increase intracellular calcium in response to its agonist, uridine diphosphoglucose. J Immunol. 2003;171:1941–1949. [DOI] [PubMed] [Google Scholar]
- 310. Moore DJ, Murdock PR, Watson JM, et al. GPR105, a novel G(i/o)-coupled UDP-glucose receptor expressed on brain glia and peripheral immune cells, is regulated by immunologic challenge: possible role in neuroimmune function. Mol Brain Res. 2003;118(1-2):10–23. doi:10.1016/S0169-328x(03)00330-9 [DOI] [PubMed] [Google Scholar]
- 311. Carrasquero LM, Delicado EG, Jimenez AI, Pérez-Sen R, Miras-Portugal MT. Cerebellar astrocytes co-express several ADP receptors. Presence of functional P2Y(13)-like receptors. Purinergic Signal. 2005;1(2):153–159. doi:10.1007/s11302-005-6211-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312. Lazarowski ER, Harden TK. UDP-sugars as extracellular signaling molecules: cellular and physiologic consequences of P2Y14 receptor activation. Mol Pharmacol. 2015;88(1):151–160. doi:10.1124/mol.115.098756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313. Meister J, Le Duc D, Ricken A, et al. The G protein-coupled receptor P2Y14 influences insulin release and smooth muscle function in mice. J Biol Chem. 2014;289(34):23353–23366. doi:10.1074/jbc.M114.580803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314. Cho J, Yusuf R, Kook S, et al. Purinergic P2Y(1)(4) receptor modulates stress-induced hematopoietic stem/progenitor cell senescence. J Clin Invest. 2014;124(7):3159–3171. doi:10.1172/JCI61636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315. Gao ZG, Ding Y, Jacobson KA. UDP-glucose acting at P2Y14 receptors is a mediator of mast cell degranulation. Biochem Pharmacol. 2010;79(6):873–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316. Das A, Ko H, Burianek LE, Barrett MO, Harden TK, Jacobson kA. Human P2Y(14) receptor agonists: truncation of the hexose moiety of uridine-5’-diphosphoglucose and its replacement with alkyl and aryl groups. J Med Chem. 2010;53(1):471–480. doi:10.1021/jm901432g [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317. Kinoshita M, Nasu-Tada K, Fujishita K, Sato k, Koizumi S. Secretion of matrix metalloproteinase-9 from astrocytes by inhibition of tonic P2Y14-receptor-mediated signal(s). Cell Mol Neurobiol. 2013;33(1):47–58. doi:10.1007/s10571-012-9869-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318. Guay D, Beaulieu C, Belley M, et al. Synthesis and SAR of pyrimidine-based, non-nucleotide P2Y14 receptor antagonists. Bioorg Med Chem Lett. 2011;21(10):2832–2835. doi10.1016/j.bmcl.2011.03.084 [DOI] [PubMed] [Google Scholar]
- 319. Gauthier JY, Belley M, Deschenes D, et al. The identification of 4,7-disubstituted naphthoic acid derivatives as UDP-competitive antagonists of P2Y(14). Bioorg Med Chem Lett. 2011;21(10):2836–2839. doi:10.1016/j.bmcl.2011.03.081 [DOI] [PubMed] [Google Scholar]
- 320. Kiselev E, Barrett MO, Katritch V, et al. Exploring a 2-naphthoic acid template for the structure-based design of P2Y14 receptor antagonist molecular probes. ACS Chem Biol. 2014;9(12):2833–2842. doi:10.1021/cb500614p [DOI] [PMC free article] [PubMed] [Google Scholar]