Abstract
Background
Noscapine is an opium alkaloid that has recently been shown to potentiate anti-cancer therapeutic effects by inducing apoptosis in various malignant cells without any detectable toxicity. However, the mechanism by which noscapine induces apoptosis in colon cancer cells remains unclear.
Materials and Methods
In this study, we explored the anti-cancer activity of noscapine in 5-fluorouracil (5-FU)-resistant human colon cancer cell lines HT29/5-FU and LoVo/5-FU and investigated the possible underlying mechanism. The apoptosis and mitochondrial morphology of cells were detected by TUNEL assay and transmission electron microscopy (TEM). The mitochondrial membrane potential (MMP) was determined using JC-1. The mitochondrial permeability transition pore (mPTP) opening was detected by the calcein-AM/cobalt assay. The levels of glucose, lactic, and ATP in cells were evaluated by ELISA kits. Relative protein expression levels were detected by Western blot.
Results
We verified that PTEN was involved in noscapine-induced apoptosis in HT29/5-FU and LoVo/5-FU cells. Noscapine greatly increased mitochondrial damage by altering mitochondrial morphology, inducing mitochondrial membrane potential depolarization, and enabling mitochondrial permeability transition pore opening in HT29/5-FU and LoVo/5-FU cells. In addition, noscapine inhibited the Warburg effect by decreasing the levels of glucose, lactic acid, and ATP and inhibiting the protein expression of glucose transporter 1, lactate dehydrogenase-B, hexokinase 2, and pyruvate kinase M2 in HT29/5-FU and LoVo/5-FU cells. However, PTEN interference counteracted the effect of noscapine on mitochondrial damage and the Warburg effect in HT29/5-FU and LoVo/5-FU cells by decreasing the activation of PI3K/mTOR signaling.
Conclusion
These results indicated that noscapine induced the apoptosis of HT29/5-FU and LoVo/5-FU human colon cancer cells by regulating mitochondria damage and the Warburg effect via PTEN, and the process is closely related to the PI3K/mTOR signaling pathway.
Keywords: colon cancer, noscapine, mitochondrial damage, PTEN, PI3K/mTOR
Introduction
Colon cancer is one of the most common malignancies and leading causes of cancer-related deaths worldwide.1 Though colon cancer diagnosis and treatment have been improved greatly, 25–30% of patients are diagnosed too late to be eligible for resection.2 Surgical resection and chemoradiotherapy remain the main methods of colon cancer treatment, but prognosis is still far from satisfactory and the incidence of colon cancer is increasing.3 Approximately 50% of colon cancer patients will experience recurrence and metastasis, and more than 90% of recurrences occur within 5 years after surgery.4 Challenges in colon cancer treatment include severe adverse reactions to traditional chemotherapy and metastasis of cancer cells to other organs or tissues. Therefore, there is great clinical demand for new and effective reagents to advance colon cancer treatment.
Noscapine is an opium phthalide isoquinoline alkaloid that has been used as an oral antitussive agent with very little toxic effect in animals and humans.5 It binds stoichiometrically to tubulin, alters its conformation, affects microtubule assembly, and arrests mammalian cells in mitosis.6 Noscapine has been shown to induce the apoptosis of many cell types and has demonstrated potent anti-tumor activity in a variety of solid tumors through mitochondrial pathways.7,8 Mitochondria are an important source of cell energy and metabolism, and changes in mitochondrial structure and function are the basis of the transformation from aerobic phosphorylation into aerobic glycolysis, or the Warburg effect.9 Aberrant metabolism in cancer cells is reflected by altered glucose metabolism. Energy metabolism in tumor cells is abnormal and proceeds via the Warburg effect, wherein cancer cells preferentially convert glucose into lactate.10 Studies have confirmed that the Warburg effect is related to changes in the tumor microenvironment, adaptation to hypoxic environments, activation of oncogenes, inactivation of tumor suppressor genes, influence of mitochondrial function, and abnormal expression of glucose metabolism-related enzymes. These phenomena provide tumor cells with growth advantages, helping them escape apoptosis, promoting tumor metastasis, and increasing drug resistance.11,12
Cancer cells become resistant via mechanisms of drug inactivation, alteration of specific drug targets, drug-induced damage, and evasion of apoptosis.13 5-fluorouracil (5-FU) is one of the common chemotherapeutic regimens for colon cancer, but long-term exposure of cancer cells to 5-FU may result in chemo-resistant phenotypes.14 It has been reported that the PTEN/PI3K/AKT signaling pathway, which mediates tumor occurrence, development, and drug resistance, is abnormally activated in tumor cells.15,16 Moreover, PI3K/AKT/mTOR plays an important role in the Warburg effect in tumor cells via regulation of the expression of glycolytic enzymes, including glucose transporter 1 (GLUT1), lactate dehydrogenase B (LDHB), hexokinase 2 (HK2), and pyruvate kinase M2 (PKM2).17,18
In the present study, we hypothesized that noscapine affects PTEN/PI3K/AKT-mediated cell apoptosis and proliferation by regulating the Warburg effect and mitochondrial morphology and function in tumor cells.
Materials and Methods
Chemicals and Cell Culture
Noscapine was purchased from abcam (ab215525, Cambridge, UK). 5-FU was purchased from Aladdin (F100149, Shanghai, China). The human colon cancer cell lines HT29 and LoVo were obtained from the China Center for Type Culture Collection (Wuhan, China). HT29 cells were cultured in McCoy’s medium (Gibco, Grand Island, USA) and LoVo cells were cultured in F12K medium (Gibco) supplemented with 10% fetal bovine serum and maintained in a humidified atmosphere containing 5% CO2 and 95% air at 37°C. 5-FU-resistant HT29 and LoVo cells (HT29/5-FU cells and LoVo/5-FU, respectively) were established by exposing parental cells to an initial dose of 0.5 μg/mL 5-FU. After three passages, the cells were exposed to 1.0 μg/mL 5-FU. Finally, the 5-FU concentration was increased to 2.0 μg/mL.
Cells were divided into five groups: control (Con, HT29/5-FU or LoVo/5-FU cells with no treatment); HT29/5-FU or LoVo/5-FU cells transfected with empty vectors (EV); HT29/5-FU or LoVo/5-FU cells treated with noscapine (Nos); HT29/5-FU or LoVo/5-FU cells transfected with PTEN interference vectors (si-PTEN); HT29/5-FU or LoVo/5-FU cells transfected with PTEN interference vectors and treated with noscapine (si-PTEN+Nos).
Plasmids and Transfection
pSIREN interference vectors were purchased from Addgene (Watertown, USA). The interference fragment (F: 5ʹ-GATACCTAGAACTTATCAAACCCTTTCTCGAGAAAGGGTTTGATAAGTTCTAGTTTTTG-3ʹ, R: 5ʹ-AATTAAAAACTAGAACTTATCAAACCCTTTCTCGAGAAAGGGTTTGATAAGTTCTAGG-3ʹ) was inserted into the pSIREN vector with BamHI and EcoRI restriction sites to produce pSIREN-PTEN. Cells were transfected with corresponding empty vectors (EV) or pSIREN-PTEN (si-PTEN) using Lipofectamine 2000 (11668-027, Invitrogen) according to the manufacturer’s instruction.
RNA Extraction and Quantitative Real-Time PCR
Cells were homogenized in Trizol reagent (TaKaRa, Japan) for total RNA isolation and then the RNA was quantified by an ultraviolet spectrophotometer and 1% agarose electrophoresis. 1 mg total RNA of each sample was reverse transcribed to obtain first-strand cDNA using the PrimeScript® RT reagent Kit with gDNA Eraser (TaKaRa, Japan) according to manufacturer’s instructions. Expression patterns of PTEN were analyzed using quantitative real-time PCR (qRT-PCR). Primers for the amplification were: PTEN-F (5ʹ- ACCATAACCCACCACAG-3ʹ) and PTEN-R (5ʹ- TACACCAGTTCGTCCCT-3ʹ). The qRT-PCR reaction was performed as the following step: pre-denaturation at 95 °C for 30 s, followed by 40 cycles of denaturation at 95 °C for 5 s, annealing at 58 °C for 20 s and elongation at 72 °C for 20 s. The transcriptional levels of PTEN were calculated using the ∆∆Ct method. For each group, the quantification was triplicated.
Western Blot
Cells were washed in phosphate-buffered saline (PBS) and lysed using radioimmunoprecipitation assay buffer (Invitrogen, Carlsbad, CA) supplemented with a protease inhibitor cocktail (Roche, Pleasanton, CA, USA). The protein concentration was evaluated using a bicinchoninic acid protein assay kit (Bioswamp, PAB180007, Wuhan, China). Equivalent amounts of proteins (30 μg) from each sample were subjected to sodium dodecyl-polyacrylamide electrophoresis, transferred to a polyvinylidene fluoride membrane, blocked in 5% fat-free milk for 2 hours at room temperature, and incubated with the following primary antibodies: PTEN (ab32199, 1:10,000, abcam), PI3K (ab191606, 1:10,000, abcam), p-PI3K (ab182651, 1:10,000, abcam), mTOR (ab32028, 1:10,000, abcam), p-mTOR (ab109268, 1:10,000, abcam), GLUT1 (ab652, 1:10,000, abcam), LDHB (ab85319, 1:10,000, abcam), HK2 (ab209847, 1:10,000, abcam), PKM2 (ab150377, 1:10,000, abcam), or GAPDH (PAB36264, 1:10,000, Bioswamp). Then, the membranes were washed with Tris-buffered saline and incubated with goat anti-rabbit IgG secondary antibody (SAB43711, 1:10,000, Bioswamp) for 2 h at room temperature. An enhanced chemiluminescence kit (Pierce) was used to detect specific bands and autoradiograms were quantified by densitometry (Quantity One software, Bio-Rad, Hercules, CA, USA) using GAPDH as a control. For each group, the quantification was triplicated.
Terminal Deoxynucleotidyl Transferase dUTP Nick End Labeling (TUNEL) Assay
Apoptotic HT29/5-FU and LoVo/5-FU cells were detected by an In Situ Cell Death Detection Kit, POD (PAB180028, Bioswamp). Cells were fixed in 4% paraformaldehyde at room temperature for 15 minutes. After washing with PBS, the adherent cell monolayer was permeabilized with 0.1% Triton X-100 in PBS and incubated with TUNEL reagent at 37 °C for 1 hour. The cells were then washed three times with PBS and the apoptotic features of cell death were characterized using fluorescence microscopy (Eclipse TS100; Nikon Corporation, Tokyo, Japan).
Enzyme-Linked Immunosorbent Assay (ELISA)
The levels of glucose, lactic, and ATP in cells were evaluated by ELISA kits. Glucose (F006), lactic (A019-2), and ATP (A095) ELISA kits were obtained from Nanjing Jiancheng Bioengineering Institute (Nanjing, China) and the assay was carried out according to the manufacturer’s protocol.
Transmission Electron Microscopy (TEM)
To observe mitochondrial morphology, HT29/5-FU and LoVo/5-FU cells were pretreated with glutaraldehyde. Take out the sample, cut it up and fixed with 4% glutaraldehyde and in 1% osmium tetroxide, dehydrated in graded ethanol, and embedded in epon. Cells sections were placed on a formvar carbon-coated 200-mesh copper electron microscopy grid, incubated for 5 min at room temperature, and subjected to standard uranyl acetate staining. The grid was washed three times with PBS and allowed to semi-dry at room temperature before TEM observation (Hitachi H7500, Tokyo, Japan).
Mitochondrial Membrane Potential (MMP) Measurement
MMP was determined using JC-1 (PAB180068, Bioswamp) according to the manufacturer’s instruction. Cells were incubated with 1 mL of JC-1 at 37°C for 20 min and washed twice with dyeing buffer. The fluorescence level was determined immediately using a fluorescence microscope (Eclipse TS100; Nikon Corporation, Tokyo, Japan).
Assessment of Mitochondrial Permeability Transition Pore (mPTP) Opening
mPTP opening was detected by the calcein-AM/cobalt assay. Cells were incubated with calcein-AM (5 μM) and CoCl2 (5 μM) for 15 min at 37 °C. The calcein loaded into the mitochondria was preserved, and cytosolic calcein was quenched by CoCl2. The results were analyzed by flow cytometry.
Statistical Analysis
Data are expressed as the mean ± standard deviation (SD). One-way analysis of variance with post hoc test was used to compare differences between multiple groups using SPSS 19.0 software (IBM Corp., Armonk, NY, USA). P < 0.05 was considered statistically significant.
Results
PTEN Is Involved in Noscapine-Induced HT29/5-FU and LoVo/5-FU Cell Apoptosis
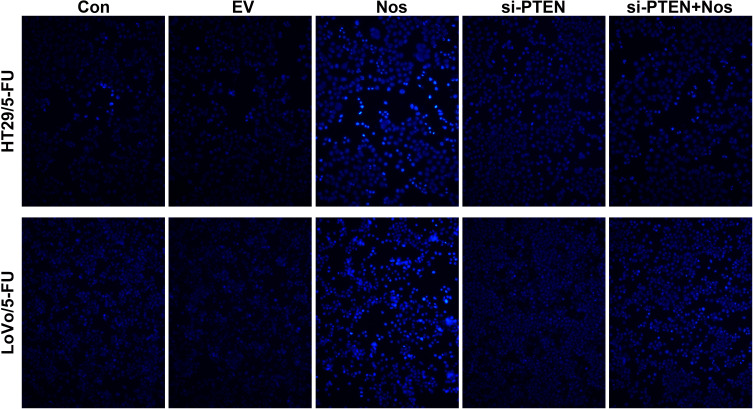
The apoptosis of HT29/5-FU and LoVo/5-FU cells was examined by TUNEL assay, and the results are shown in Figure 1. Noscapine increased the number of apoptotic HT29/5-FU and LoVo/5-FU cells, but PTEN interference weakened the noscapine-induced apoptosis of HT29/5-FU and LoVo/5-FU cells.
Figure 1.
Noscapine induced the apoptosis of HT29/5-FU and LoVo/5-FU cells. TUNEL-positive cells were observed under a fluorescence microscope (magnification, 200×). The TUNEL-positive cells in Noscapine group was higher than that in control group, indicating noscapine treatment induced the apoptosis of HT29/5-FU and LoVo/5-FU cells.
Effect of PTEN Interference on Noscapine-Induced Mitochondrial Injury and mPTP Opening in HT29/5-FU and LoVo/5-FU Cells
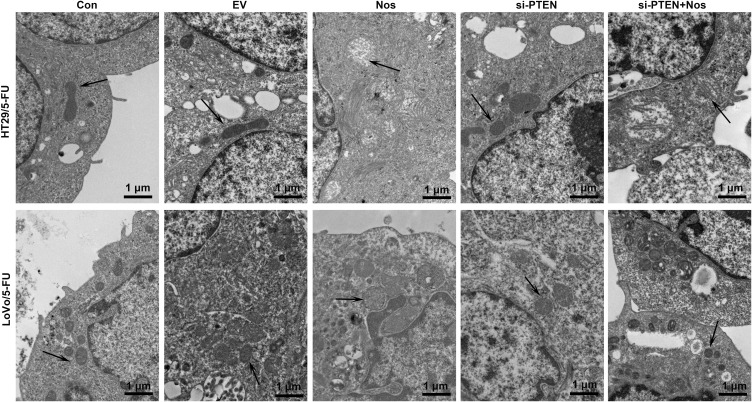
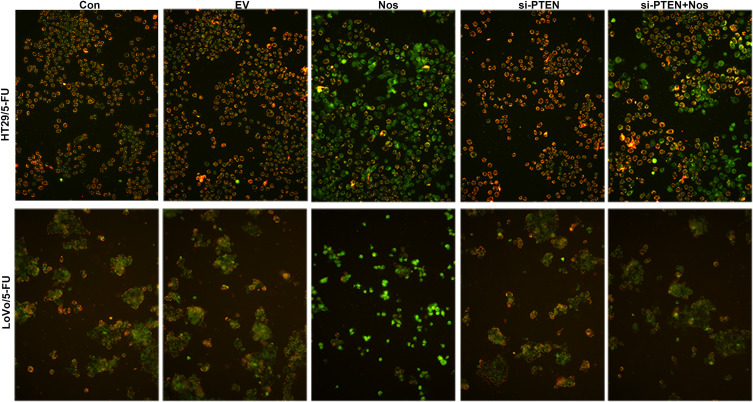
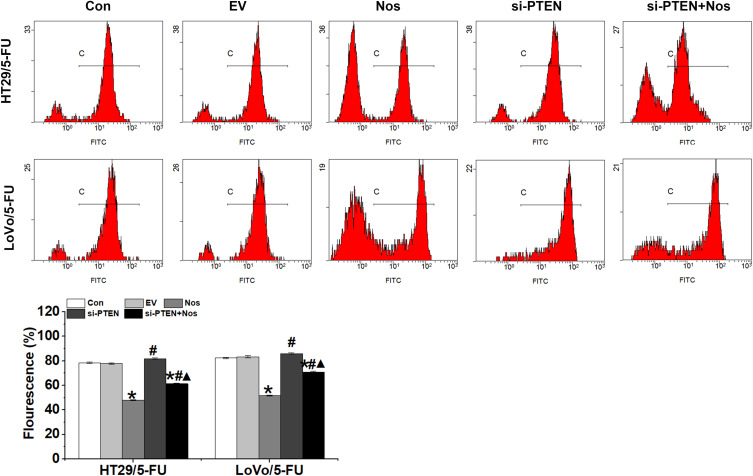
To observe noscapine-induced mitochondrial damage in HT29/5-FU and LoVo/5-FU cells, we used electron microscopy and specific reagent kits to study mitochondrial morphology, MMP, and mPTP opening. In noscapine-treated cells, mitochondrial morphology was evidently enlarged (black arrow, Figure 2), and the outer and inner mitochondrial membranes were hardly distinguishable. PTEN interference combined with noscapine administration markedly reduced mitochondrial enlargement and maintained mitochondrial morphology. Assays using the fluorescent JC-1 probes demonstrated that noscapine induced MMP depolarization, but PTEN interference ameliorated noscapine-induced MMP depolarization, as shown by high-content screening of JC-1 staining (Figure 3). We then measured the mPTP opening by the CoCl2-calcein fluorescence-quenching assay. In terms of mPTP opening, entrapped calcein was released from the mitochondrial matrix, and CoCl2 quenched cytosolic calcein but not that in the mitochondria, which does not transport cobalt. As shown in Figure 4, the fluorescence of mitochondrial calcein was decreased significantly in noscapine-treated HT29/5-FU and LoVo/5-FU cells, signifying mPTP opening. However, PTEN interference ameliorated noscapine-induced mPTP opening.
Figure 2.
PTEN interference alleviated noscapine-induced mitochondrial damage in HT29/5-FU and LoVo/5-FU cells. Mitochondrial morphology (arrows) was observed using electron microscopy, scale bar = 1 μm.
Figure 3.
MMP in HT29/5-FU and LoVo/5-FU cells was determined using JC-1. The fluorescence level was determined immediately using a fluorescence microscope (magnification, 200×).
Figure 4.
mPTP opening in HT29/5-FU and LoVo/5-FU cells were detected by the calcein-AM/cobalt assay. Data are presented as the mean ± SD (n = 3). *P < 0.05 compared with Con, #P < 0.05 compared with Nos, ▲P < 0.05 compared with siPTEN.
PTEN Interference Reversed the Impact of Noscapine on the Warburg Effect in HT29/5-FU and LoVo/5-FU Cells
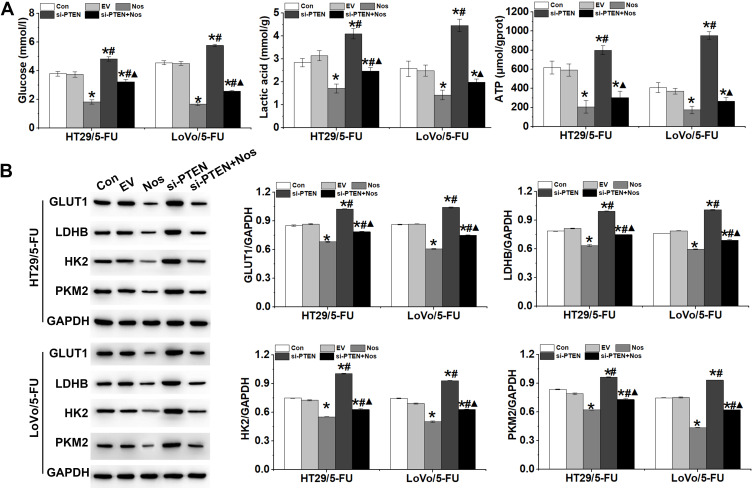
The Warburg effect is closely correlated with drug resistance in tumor cells. We hypothesized that PTEN may regulate the resistance of HT29/5-FU and LoVo/5-FU cells to noscapine via the Warburg effect. As shown in Figure 5A, glucose uptake, lactic acid level, and ATP production, which are hallmarks of glycolysis, were decreased significantly in noscapine-treated HT29/5-FU and LoVo/5-FU cells but increased significantly with PTEN interference. In addition, the protein expression of GLUT1, LDHB, HK2, and PKM2, which are rate-limited enzymes in the Warburg effect, was downregulated significantly in noscapine-treated HT29/5-FU and LoVo/5-FU cells but was upregulated significantly by PTEN interference (Figure 5B).
Figure 5.
PTEN interference reversed the effect of noscapine on the Warburg effect in HT29/5-FU and LoVo/5-FU cells. (A) Production of glucose, lactic acid, and ATP in HT29/5-FU and LoVo/5-FU cells was detected by ELISA. (B) Expression of glycometabolism-related proteins GLUT1, LDHB, HK2, and PKM2 was detected by Western blot. Data are presented as the mean ± SD (n = 3). *P < 0.05 compared with Con, #P < 0.05 compared with Nos, ▲P < 0.05 compared with siPTEN.
PTEN Interference Reversed the Effect of Noscapine on PI3K/mTOR Signaling Activation in HT29/5-FU and LoVo/5-FU Cells
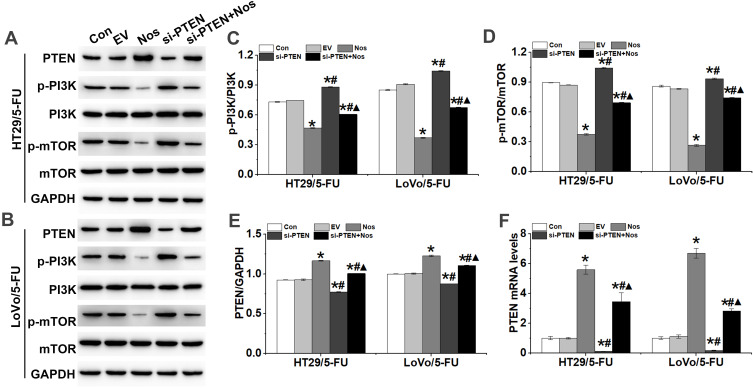
Noscapine inhibits metabolism-regulating molecules such as PI3K, AKT, and mTOR. Here, we found that noscapine inhibited PI3K and mTOR phosphorylation in HT29/5-FU and LoVo/5-FU cells (Figure 6). Previous studies have indicated that the activation of PI3K/AKT signaling triggered the Warburg effect and enhanced drug resistance in cancer cells.19 PTEN has been identified as a dual-specificity phosphatase and regulates cell growth, apoptosis, invasion, and differentiation by negatively regulating the PI3K/AKT signaling pathway. Furthermore, noscapine induced the protein expression of PTEN in HT29/5-FU and LoVo/5-FU cells, and PTEN interference inhibited the noscapine-decreased activation of the PI3K/mTOR signaling pathway (Figure 6).
Figure 6.
PTEN interference counteracted the effect of noscapine on the activation of PI3K/mTOR signaling in HT29/5-FU and LoVo/5-FU cells. (A–E) Western blot was used to detect the protein expression of PTEN, p-PI3K, PI3K, p-mTOR, and mTOR in HT29/5-FU and LoVo/5-FU cells. (F) PTEN mRNA levels were detected by RT-qPCR. Data are presented as the mean ± SD (n = 3). *P < 0.05 compared with Con, #P < 0.05 compared with Nos, ▲P < 0.05 compared with siPTEN.
Discussion
Colon cancer is one of the leading malignancies in the gastrointestinal system and the unsatisfactory prognosis needs to be greatly ameliorated. The major clinical treatments for colon cancer include chemotherapy and surgery. However, prognosis remains undesirable even though targeted therapy has been introduced to clinical settings.3 Challenges in cancer treatment include serious side effects of traditional chemotherapy drugs, tumor metastasis, and drug resistance. There is an urgent clinical need to explore effective drugs for the treatment of colon cancer. In the present study, we identified the anti-cancer activity of noscapine in HT29/5-FU and LoVo/5-FU colon cancer cells via the activation of PI3K/mTOR signaling through decreasing the expression of PTEN.
Noscapine is considered to be a safe anti-tumor agent and has demonstrated anti-tumor activity both in vitro and in vivo in various cancer cells that are resistant to conventional anti-tumor drugs.8 More importantly, noscapine did not exhibit severe side effects that are commonly seen with many chemotherapeutic agents.20 In this study, noscapine significantly induced the apoptosis of HT29/5-FU and LoVo/5-FU human colon cancer cells, suggesting that it acted as an anti-cancer agent for colon cancer treatment. In terms of the mechanism, various targets have been reported to be related to the anti-cancer activity of noscapine, such as the inhibition of NF/κB signaling and the induction of p53 and mitochondria-mediated apoptosis.7,21-23 So far, the specific molecular mechanism remains unclear, and thorough investigation needs to be carried out to unveil this process.
In the present study, we found that noscapine induced mitochondrial-mediated apoptosis and inhibited the Warburg effect in HT29/5-FU and LoVo/5-FU cells. It is well known that mitochondria play an important role in the regulation of cellular death.24 Through integration of diverse intracellular signals, mPTP serves as a gate that, once switched-on, triggers the apoptotic process.25 We identified that noscapine induced mitochondrial dysfunction by regulating MMP and mPTP, consequently activating apoptosis in HT29/5-FU and LoVo/5-FU cells. Reduced mitochondrial membrane potential and decreased cellular ATP are likely due to an increase in the permeability of the inner mitochondrial membrane that results from prolonged mPTP opening due to noscapine treatment.26 The above results suggested that noscapine induced apoptosis of HT29/5-FU and LoVo/5-FU cells via the mitochondrial pathway.
The Warburg effect, also known as aerobic glycolysis, is a metabolic phenomenon by which cancer cells produce lactate from glucose even under non-hypoxic conditions. Studies have reported that altered mitochondrial metabolism is associated with the Warburg effect.27 Data accumulated thus far have shown that the PI3K/AKT/mTOR signaling pathway is a classic pro-survival pathway in various types of cells.28,29 In addition, the PI3K/AKT/mTOR signaling pathway play a critical role in regulating the Warburg effect, mainly in tumor cells.30 Our results indicated that noscapine inhibits the Warburg effect by decreasing the activation of PI3K/mTOR in HT29/5-FU and LoVo/5-FU cells, which suggested that the PI3K/mTOR signaling pathway is involved in noscapine-induced HT29/5-FU and LoVo/5-FU cell apoptosis by promoting the Warburg effect. Moreover, enzymes that directly regulate glycolysis have been implicated in promoting a drug-resistant phenotype. Targeting key metabolic enzymes can enhance therapeutic efficacy or combat drug resistance by promoting drug-induced apoptosis of cancer cells.31 Based on our results, inhibiting the Warburg effect may contribute to increasing the sensitivity of HT29/5-FU and LoVo/5-FU cells to noscapine. Therefore, we speculated that targeting the Warburg effect improves the response to cancer therapeutics, and the combination of targeted drugs with cellular metabolism inhibitors may be a promising strategy to improve drug sensitivity in cancer therapy.
The tumor suppressor PTEN has been shown to be frequently mutated in many human cancers, including colon cancer.32 PTEN specifically catalyzes the dephosphorylation of the 3′ phosphate of the inositol ring in PIP3, resulting in the biphosphate product PIP2 and inhibiting the phosphorylation of PI3K, thereby blocking AKT and its downstream kinase activity to induce apoptosis.33 It is well known that the PTEN silencing contributes to the activation of the PI3K/AKT signaling pathway and consequent tumorigenesis.34 In this study, we found that noscapine induced PTEN expression in HT29/5-FU and LoVo/5-FU cells, and PTEN interference reversed the effect of noscapine on cell apoptosis via the activation of PI3K/mTOR signaling. Mutation of the PTEN gene can lead to abnormal activation of PIP3 and prevention of cell death. In agreement with previous studies, the expression of p-PI3K and p-mTOR was significantly increased in HT29/5-FU and LoVo/5-FU cells treated with noscapine after transfection with si-PTEN.
In conclusion, this is the first report revealing that PTEN is involved in noscapine-induced human colon cancer cell apoptosis. Noscapine induced the apoptosis of HT29/5-FU and LoVo/5-FU human colon cancer cells by regulating mitochondrial damage and the Warburg effect via PTEN, and the mechanism is closely related to PI3K/mTOR signaling. This study elucidated the molecular mechanism underlying the effect of noscapine on colon cancer cells and provides a new target for the therapy of colon cancer.
Acknowledgments
This study is supported by Hubei Province Natural Science Foundation of China (2018CFB725 and 2019CFB749), Project of Health and Family Planning Commission of Wuhan Municipality (WX17B05), and the Hubei Province Local Guidance Technological Development Project (2019ZYYD067).
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Siegel RL, Miller KD, Fedewa SA, et al. Colorectal cancer statistics, 2017. CA Cancer J Clin. 2017;67(3):177–193. doi: 10.3322/caac.21395 [DOI] [PubMed] [Google Scholar]
- 2.Van CE, Nordlinger B, Cervantes A. Advanced colorectal cancer: ESMO clinical practice guidelines for treatment. Ann Oncol. 2010;21(5):v93–v97. doi: 10.1093/annonc/mdq222 [DOI] [PubMed] [Google Scholar]
- 3.Karanikas M, Esebidis A. Increasing incidence of colon cancer in patients < 50 years old: a new entity? Ann Transl Med. 2016;4:164. doi: 10.21037/atm.2016.04.05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu Y, Song Y, Xiong Y, et al. MicroRNA-21 (Mir-21) promotes cell growth and invasion by repressing tumor suppressor PTEN in colorectal cancer. Cell Physiol Biochem. 2017;43(3):945–958. doi: 10.1159/000481648 [DOI] [PubMed] [Google Scholar]
- 5.Sebak S, Mirzaei M, Malhotra M, et al. Human serum albumin nanoparticles as an efficient noscapine drug delivery system for potential use in breast cancer: preparation and in vitro analysis. Int J Nanomedicine. 2010;5:525–532. doi: 10.2147/ijn.s10443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ye K, Ke Y, Keshava N, et al. Opium alkaloid noscapine is an antitumor agent that arrests metaphase and induces apoptosis in dividing cells. Proc Natl Acad Sci USA. 1998;95(4):1601–1606. doi: 10.1073/pnas.95.4.1601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang ZR, Liu M, Peng XL, et al. Noscapine induces mitochondria-mediated apoptosis in human colon cancer cells in vivo and in vitro. Biochem Biophys Res Commun. 2012;421:627–633. doi: 10.1016/j.bbrc.2012.04.079 [DOI] [PubMed] [Google Scholar]
- 8.Liu M, Luo XJ, Liao F, et al. Noscapine induces mitochondria-mediated apoptosis in gastric cancer cells in vitro and in vivo. Cancer Chemother Pharmacol. 2011;67:605–612. doi: 10.1007/s00280-010-1356-3 [DOI] [PubMed] [Google Scholar]
- 9.Samudio I, Fiegl M, Andreeff M. Mitochondrial uncoupling and the warburg effect: molecular basis for the reprogramming of cancer cell metabolism. Cancer Res. 2009;69:2163–2166. doi: 10.1158/0008-5472.CAN-08-3722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Epstein T, Gatenby RA, Brown JS. The warburg effect as an adaptation of cancer cells to rapid fluctuations in energy demand. PLoS One. 2017;12:e0185085. doi: 10.1371/journal.pone.0185085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jiang SH, Li J, Dong FY, et al. Increased serotonin signaling contributes to the warburg effect in pancreatic tumor cells under metabolic stress and promotes growth of pancreatic tumors in mice. Gastroenterology. 2017;153:277–291:e219. doi: 10.1053/j.gastro.2017.03.008 [DOI] [PubMed] [Google Scholar]
- 12.Bhattacharya B, Mohd MF, Soong R. The warburg effect and drug resistance. Br J Pharmacol. 2016;173:970–979. doi: 10.1111/bph.13422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mader RM, Schmidt WM, Steger GG, et al. Molecular mechanisms of drug resistance. Int J Clin Pharmacol Ther. 2009;47:49–50. doi: 10.5414/CPP47049 [DOI] [PubMed] [Google Scholar]
- 14.Tentes IK, Schmidt WM, Krupitza G, et al. Long-term persistence of acquired resistance to 5-fluorouracil in the colon cancer cell line SW620. Exp Cell Res. 2010;316(19):3172–3181. doi: 10.1016/j.yexcr.2010.09.003 [DOI] [PubMed] [Google Scholar]
- 15.Zheng L, Zhang Y, Liu Y, et al. MiR-106b induces cell radioresistance via the PTEN/PI3K/AKT pathways and p21 in colorectal cancer. J Transl Med. 2015;13(1):252. doi: 10.1186/s12967-015-0592-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ke TW, Wei PL, Yeh KT, et al. MiR-92a promotes cell metastasis of colorectal cancer through PTEN-mediated PI3K/AKT pathway. Ann Surg Oncol. 2015;22:2649–2655. doi: 10.1245/s10434-014-4305-2 [DOI] [PubMed] [Google Scholar]
- 17.Cui J, Quan M, Jiang W, et al. Suppressed expression of LDHB promotes pancreatic cancer progression via inducing glycolytic phenotype. Med Oncol. 2015;32(5):143. doi: 10.1007/s12032-015-0589-8 [DOI] [PubMed] [Google Scholar]
- 18.Zha X, Sun Q, Zhang H. mTOR upregulation of glycolytic enzymes promotes tumor development. Cell Cycle. 2011;10(7):1015–1016. doi: 10.4161/cc.10.7.15063 [DOI] [PubMed] [Google Scholar]
- 19.Liu J, Pan C, Guo L, et al. A new mechanism of trastuzumab resistance in gastric cancer: MACC1 promotes the warburg effect via activation of the PI3K/AKT signaling pathway. J Hematol Oncol. 2016;9(1):76. doi: 10.1186/s13045-016-0302-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Doddapaneni R, Patel K, Chowdhury N, et al. Reversal of drug-resistance by noscapine chemo-sensitization in docetaxel resistant triple negative breast cancer. Sci Rep. 2017;7:15824. doi: 10.1038/s41598-017-15531-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aneja R, Ghaleb AM, Zhou J, et al. P53 and p21 determine the sensitivity of noscapine-induced apoptosis in colon cancer cells. Cancer Res. 2007;67:3862–3870. doi: 10.1158/0008-5472.CAN-06-4282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Doddapaneni R, Patel K, Chowdhury N, et al. Noscapine chemosensitization enhances docetaxel anticancer activity and nanocarrier uptake in triple negative breast cancer. Exp Cell Res. 2016;346:65–73. doi: 10.1016/j.yexcr.2016.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Quisbert-Valenzuela EO, Calaf GM. Apoptotic effect of noscapine in breast cancer cell lines. Int J Oncol. 2016;48(6):2666–2674. doi: 10.3892/ijo.2016.3476 [DOI] [PubMed] [Google Scholar]
- 24.Jacquemin G, Margiotta D, Kasahara A, et al. Granzyme B-induced mitochondrial ROS are required for apoptosis. Cell Death Differ. 2015;22(5):862–874. doi: 10.1038/cdd.2014.180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gao J, Sana R, Calder V, et al. Mitochondrial permeability transition pore in inflammatory apoptosis of human conjunctival epithelial cells and T cells: effect of cyclosporin A. Invest Ophthalmol Vis Sci. 2013;54(7):4717–4733. doi: 10.1167/iovs.13-11681 [DOI] [PubMed] [Google Scholar]
- 26.Cione E, Tucci P, Senatore V, et al. Synthesized esters of ferulic acid induce release of cytochrome c from rat testes mitochondria. J Bioenerg Biomembr. 2008;40:19–26. doi: 10.1007/s10863-007-9097-7 [DOI] [PubMed] [Google Scholar]
- 27.Archer SL, Gomberg-Maitland M, Maitland ML, et al. Mitochondrial metabolism, redox signaling, and fusion: a mitochondria-ROS-HIF-1alpha-Kv1.5 O2-sensing pathway at the intersection of pulmonary hypertension and cancer. Am J Physiol Heart Circ Physiol. 2008;294:H570–H578. doi: 10.1152/ajpheart.01324.2007 [DOI] [PubMed] [Google Scholar]
- 28.Li L, Xu M, Li X, et al. Platelet-derived growth factor-B (PDGF-B) induced by hypoxia promotes the survival of pulmonary arterial endothelial cells through the PI3K/Akt/Stat3 pathway. Cell Physiol Biochem. 2015;35(2):441–451. doi: 10.1159/000369709 [DOI] [PubMed] [Google Scholar]
- 29.Yang X, Cheng Y, Li P, et al. A lentiviral sponge for miRNA-21 diminishes aerobic glycolysis in bladder cancer T24 cells via the PTEN/PI3K/AKT/mTOR axis. Tumour Biol. 2015;36(1):383–391. doi: 10.1007/s13277-014-2617-2 [DOI] [PubMed] [Google Scholar]
- 30.Xiao Y, Peng H, Hong C, et al. PDGF promotes the warburg effect in pulmonary arterial smooth muscle cells via activation of the PI3K/AKT/mTOR/HIF-1α signaling pathway. Cell Physiol Biochem. 2017;42(4):1603–1613. doi: 10.1159/000479401 [DOI] [PubMed] [Google Scholar]
- 31.Tamada M, Nagano O, Tateyama S, et al. Modulation of glucose metabolism by CD44 contributes to antioxidant status and drug resistance in cancer cells. Cancer Res. 2012;72(6):1438–1448. doi: 10.1158/0008-5472.CAN-11-3024 [DOI] [PubMed] [Google Scholar]
- 32.Sun Y, Tian H, Wang L. Effects of PTEN on the proliferation and apoptosis of colorectal cancer cells via the phosphoinositol-3-kinase/Akt pathway. Oncol Rep. 2015;33:1828–1836. doi: 10.3892/or.2015.3804 [DOI] [PubMed] [Google Scholar]
- 33.Stambolic V, Suzuki A, de la Pompa JL, et al. Negative regulation of PKB/Akt-dependent cell survival by the tumor suppressor PTEN. Cell. 1998;95(1):29–39. doi: 10.1016/S0092-8674(00)81780-8 [DOI] [PubMed] [Google Scholar]
- 34.Salmena L, Carracedo A, Pandolfi PP. Tenets of PTEN tumor suppression. Cell. 2008;133(3):403–414. doi: 10.1016/j.cell.2008.04.013 [DOI] [PubMed] [Google Scholar]