Abstract
The reproductive performance of cattle can be suppressed by heat stress. Reproductive organ temperature, especially ovarian temperature, may affect follicle development and ovulation. The establishment of a technique for long-term measurement of ovarian temperature could prove useful in understanding the mechanisms underlying the temperature-dependent changes in follicular development and subsequent ovulation in cows. Here we report a novel method facilitating long-term and continuous recording of ovarian parenchymal temperature in cows. The method revealed that the ovarian temperature in the luteal phase was constantly maintained lower than the vaginal temperature, and that the diurnal temperature variation in the ovary was significantly greater than that in the vagina, suggesting that the ovaries may require a lower temperature than other organs to maintain their functions. This novel method could be used for the further understanding of ovarian functions during estrous cycles in cows.
Keywords: Japanese Black cow, Ovarian temperature, Ovary, Temperature gradients
Reproductive performance of livestock animals, such as beef and dairy cattle, is very important in calf production, which is one of the major critical factors affecting farmers’ income. Generally, heat stress suppresses reproductive performance in cattle [1,2,3,4,5,6]. Knowledge of the mechanism mediating the suppression of reproductive performance under heat stress is important to solve this problem. In previous studies, body temperature, ovarian temperature, and uterine and vaginal temperatures have been associated with fertility success, especially ovulation efficiency. The mean follicular fluid temperature in preovulatory follicles was lower than the temperatures of both the uterine surface and rectum in ovulating cows, whereas no such difference in temperature was detected in non-ovulating cows [7,8,9]. Similarly, temperature gradient variations in reproductive organs could affect the efficiency of ovulation and fertilization in female rabbits, swine, and humans [10]. Under heat stress conditions, development of the dominant follicle is suppressed [1], suggesting that suppression is due to a higher reproductive organ temperature [11]. Thus, reproductive organ temperature could be critical to ovulation and/or follicle development.
Measurement of ovarian temperature is challenging. Since daily ovarian temperature can be measured only during a certain time period [12, 13], it is difficult to analyze the maintenance of temperature gradients. Furthermore, the temperature changes periodically according to the hormonal change in the estrous cycle as well as the circadian rhythm [14].
The present study was undertaken to establish a method for long-term temperature measurement of the ovarian and reproductive organs, such as vagina, in cattle. We successfully established a method for the long-term (over one week) recording of in vivo ovarian tissue temperature in cattle and applied the method to three cows. To our knowledge, this is the first report of continuous prolonged recording of in vivo ovarian temperature. The three cows showed normal estrous behavior (standing behavior and mucus discharge), appetite, and vigor during and after the recording periods of ovarian temperature. The method required only a small incision made using local anesthesia and the wearable data logger imposed no physical restriction after the surgery. Moreover, the method could facilitate the recording of the ovarian temperature under a tie-stall condition, which allows the cows to access food and water ad libitum.
The findings indicate several advantages of the novel method. The first is the ability to measure ovarian temperature with minimal physical stress, including the short operation needed to install the hardware. In previous studies the methods for the direct recording of ovarian temperature required a large incision and laparotomy under general anesthesia [12, 15]. The difference between the present and previous methods indicates that the current technique could be more useful for in vivo ovarian temperature recording throughout the estrous cycle.
The other advantage of the current method is that it allows the observation of ovarian status using ultrasonography examination. This method, which only requires the insertion of a needle probe into the ovary, minimizes injury to the ovary. Thus, the morphological changes of the contralateral ovary can be observed through the estrous cycle. Figures 1A and 1B present images of the probe with the ovary parenchyma at the end of the experiment in the operated ovary (Fig. 1A) and the structures in the contralateral ovary (Fig. 1B). A Graafian follicle was evident in cow #1 and a corpus luteum in cow #2. Cow #3 showed no structure. The images could be used to diagnose the stage of the estrous cycle. The method allowed us to obtain physiological information, such as ovarian status and endocrine and behavioral changes, related to ovarian function during the long-term ovarian temperature recording.
Fig. 1.
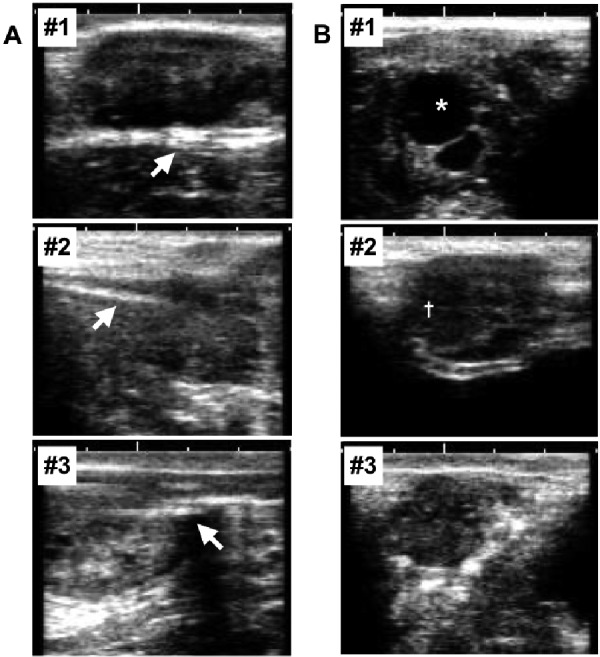
Ultrasonographic images indicating the probe (arrows) with the ovary parenchyma at the end of the experiment in the operated ovary (A) and the structures in the contralateral ovary (B) in each cow. Cow #1 showed a Graafian follicle (*), cow #2 showed a corpus luteum (†), and cow #3 showed no structure in the contralateral ovary. One unit of white scale bars denotes 10 mm.
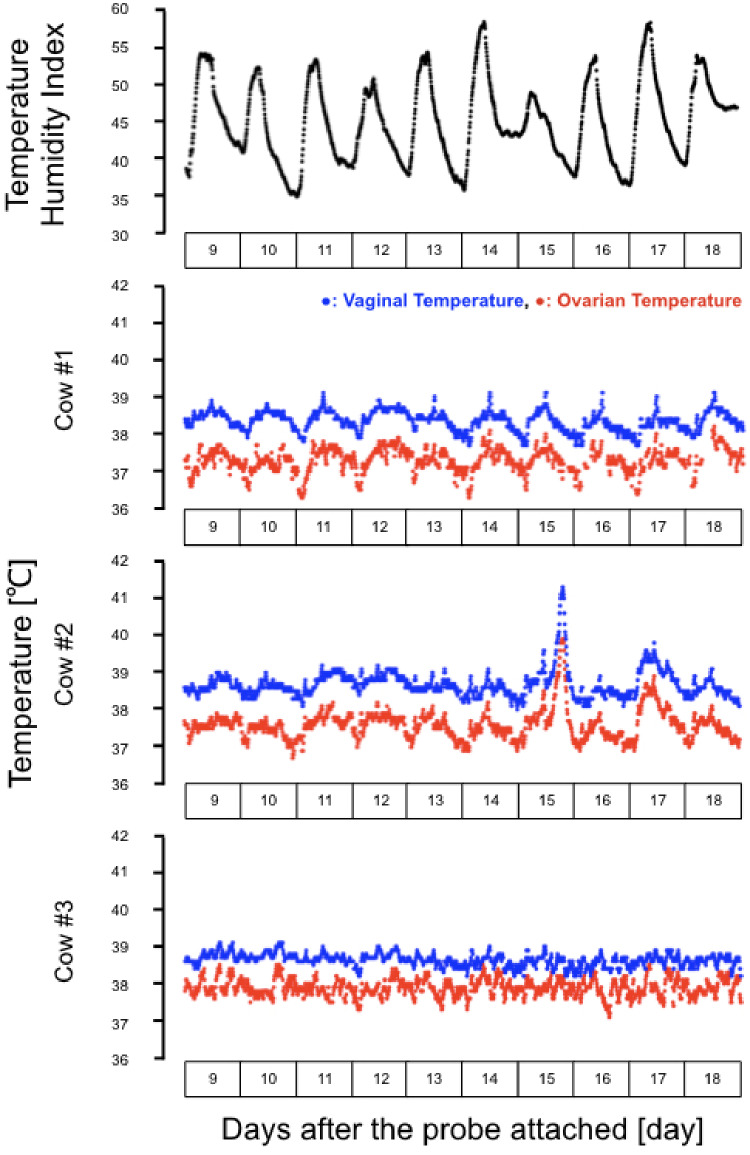
We observed that the circadian changes in temperature of the ovaries and vagina synchronized with the ambient condition, as evident by the circadian changes in the temperature-humidity index (THI). The synchronization of the ovarian temperature with the vaginal temperature (Fig. 2) suggested that the reproductive organs shared the same blood supply. Indeed, the abdominal aorta from the central bloodstream has many branches connecting the reproductive organs. Of note, the ovarian temperature was lower than the vaginal temperature throughout the experimental period in all cows (Fig. 2), as reported previously [10]. The lower temperature of the ovary as compared to the vagina could be due to the counter-current exchange of heat in the ovarian pedicle and uterine vein [10, 12]. Presently, the diurnal temperature variation in the ovary was significantly larger than that in the vagina (cow #1, P < 0.05; cows #2 and #3, P < 0.01; all cows, P < 0.01; Table 1). This is consistent with the fact that the blood supply for the vagina is downstream of the ovary. It is likely that the ovary may receive much of its blood supply directly from the abdominal aorta. The ovarian artery, which directly provides blood to the ovary, has arteries that branch to the uterus and vagina. The internal iliac artery also branches to the vagina [16].
Fig. 2.
The mean temperature-humidity index (THI) change (black circles), raw data of the changes in the ovarian (red circles), and the vaginal temperature (blue circles) in three cows at 9 to 18 days after the surgery (15 to 24 days after PGF2α treatment). THI range in this period represents a non-stress conditions for beef cows. The ovarian and vaginal temperature showed synchronized diurnal variation.
Table 1. THI and maximum (Tmax) and minimum (Tmin) temperatures of ovary and vagina in each cow.
| Entire period |
Diurnal change |
||||||
|---|---|---|---|---|---|---|---|
| Average | Max | Min | Max | Min | ΔTHI (max-min) | ||
| (mean ± SD) | (mean ± SD) | (mean ± SD) | (mean ± SD) | ||||
| THI | 45.07 ± 5.57 | 58.57 | 34.89 | 53.94 ± 2.96 | 36.89 ± 1.42 | 17.05 ± 3.75 | |
| TAverage (oC) | Tmax (oC) | Tmin (oC) | Tmax (oC) | Tmin (oC) | ΔTemperature (Tmax-Tmin)(oC) | ||
| (mean ± SD) | (mean ± SD) | (mean ± SD) | (mean ± SD) | ||||
| Ovary | Cow#1 | 37.29 ± 0.32 | 38.20 | 36.30 | 37.97 ± 0.22 | 36.44 ± 0.13 | 1.53 ± 0.26 a |
| Cow#2 | 37.60 ± 0.41 | 39.90 | 36.70 | 38.34 ± 0.64 | 36.92 ± 0.11 | 1.42 ± 0.62 A | |
| Cow#3 | 37.89 ± 0.23 | 38.50 | 37.10 | 38.41 ± 0.13 | 37.38 ± 0.14 | 1.03 ± 0.15 A | |
| mean (n = 3) | 37.60 ± 0.41 | 38.24 ± 0.43 | 36.91 ± 0.41 | 1.33 ± 0.44 A | |||
| Vagina | Cow#1 | 38.35 ± 0.27 | 39.10 | 37.70 | 39.01 ± 0.11 | 37.83 ± 0.16 | 1.18 ± 0.24 b |
| Cow#2 | 38.69 ± 0.38 | 41.30 | 38.00 | 39.37 ± 0.72 | 38.20 ± 0.13 | 1.17 ± 0.75 B | |
| Cow#3 | 38.64 ± 0.17 | 39.10 | 38.20 | 38.97 ± 0.09 | 38.31 ± 0.14 | 0.66 ± 0.12 B | |
| mean (n = 3) | 38.56 ± 0.32 | 39.12 ± 0.45 | 38.11 ± 0.25 | 1.00 ± 0.51 B | |||
a, b Different letters indicate significant difference in ovarian and vaginal temperature within the same cow and the mean level of the three cows (paired t-test, P < 0.05). A, B Different letters indicate significant difference in ovarian and vaginal temperature within the same cow and mean level of the three cows (paired t-test, P < 0.01)
Cow #2 showed a transient increase in both ovarian and vaginal temperatures. The highest temperatures (ovarian, 39.90ºC; vaginal, 41.30ºC) were recorded 15 days after the attachment of the probe (Fig. 2). It is possible that the cow might have been transiently infected at that time. The infection, if it existed, would not be due to the surgical damage, because the cow showed normal diurnal changes in the ovarian temperature for 2 weeks after the surgery and normal behavior and appetite after the surgery. Cow #3 showed indistinct circadian changes in the temperature, in terms of the average, maximum, and minimum temperature of the ovary and vagina, suggesting that the ovarian and vaginal temperatures may show individual differences.
In conclusion, the present study establishes a method for the long-term recording of the ovarian parenchyma temperature in tie-stall cows with minimized stress during both surgery and recording. The data show that the ovarian temperature was maintained constantly lower than the vaginal temperature, suggesting that the lower temperature would be required for normal folliculogenesis and ovulation. This novel method could be useful for the further understanding of ovarian function.
Methods
The experiment was conducted on the farm of the Field Science Center, Graduate School of Bioagricultural Sciences, Nagoya University (35°6’42”N, 137°4’57”E), Togo-town, Aichi, Japan, from January to February 2019. Three multiparous, non-lactating Japanese Black cows (Bos taurus) were used. Each was tied in an individual free stall barn bedded with straw and fed with mixed feeds (silages made from ryegrass straw and/or timothy grass) at 0900 h and 1600 h with free access to water. The diet was formulated to meet or exceed nutritional requirements for non-lactating Japanese Black cows (Japanese Feeding Standard for Beef Cattle, 2008). All animals displayed more than three estrous cycles after calving and had no disease and dysfunctions in the uterus and ovary as judged by the pre-examination ultrasonography diagnosis. All experimental procedures were approved by the Committee of the Care and Use of Experimental Animals at the Graduate School of Bioagricultural Sciences, Nagoya University (approval number 2018031366).
Before the experiment, estrous stage of all three cows was synchronized by a modified method based on the Ovsynch protocol with a progesterone device for Japanese Black cow [17] as follows. The cows received an intravaginal progesterone device (Ovapron V; Kyoritsu Seiyaku, Tokyo, Japan) for 7 days after treatment with estradiol benzoate (OVAHORMON®; Aska Animal Health, Tokyo, Japan). They were then treated with prostaglandin F2α (PGF2α) according to the Ovsynch protocol without a second administration of gonadotropin-releasing hormone (GnRH) [18]. Six days after the PGF2α treatment, all cows received a transrectal ultrasonography examination for the observation of ovarian status. The surgery to attach the probe in the ovary was then performed. The cows, which had a dominant follicle without a corpus luteum (CL) on the day of surgery, received GnRH to induce ovulation and CL formation.
The cows were placed in a standing position and were administered 0.05 mg/kg of xylazine hydrochloride (Selactar 0.2%; Bayer, Tokyo, Japan) intravenously for sedation. The hair of the left flank region and the tail head region was clipped, and the skin of these regions was cleaned and disinfected. Caudal epidural anesthesia was administered for colpotomy and local anesthesia in the flank region for celiotomy with procaine hydrochloride (Enpro injection, Kyoritsu Seiyaku). Procaine hydrochloride was administered in the first coccygeal space at 0.5 mg/kg and 50 ml/head in the left flank region with an inverted-L pattern method using an 18 g × 1 1/2 inch needle (Terumo, Tokyo, Japan), because the risk of tissue and organ damage is lower with a left flank incision than an approach from the right flank region [19].
The colpotomy was conducted first for the insertion of a probe into the pelvic cavity. After lavage of the vulva with saline and disinfection, the vaginal wall was incised approximately 2 cm from where it adjoins to the cervix with a scalpel blade and similarly for the ovariectomy via the vaginal wall [20, 21]. The vaginal incision was made at the two/ten o'clock position since it is easy to approach the right/left ovary. For this procedure, the right ovary was preferred for long-term temperature recording, because the rumen may obstruct the probe insertion and recording if the probe is attached to the left ovary. However, the probe insertion involved the left ovary in one cow, because the right ovary of that cow showed a dominant follicle, to observe the further folliculogenesis and CL formation. The thin flexible probe (Type K, Class 1 thermocouple probe, 0602 0493; Testo SE & Co. KGaA, Lenzkirch, Germany; Fig. 3A) was used for temperature recording. The diameter of a probe shaft was 0.25 mm. The probe was adjusted to the inner hole of a 21 g × 1 1/2 inch needle (Terumo) and was attached to the needle (Fig. 3B). The sterile probe was inserted through the incision into the pelvic cavity and was maintained temporarily. The celiotomy was performed to attach the probe to the ovary. The procedure was similar to an ovariectomy via the abdominal wall [22, 23]. An incision was made in the caudal one-third of the left fossa for an easy approach to the ovary. A probe was brought from the pelvic cavity via the abdominal wall through the vaginal wall. The tip of the probe with the 21G needle was bent in a J-shape and brought back into the abdomen. Either side of the ovary was hooked by the bent probe with the mesometrium from dorsal to ventral. The abdominal wall was closed conventionally and the incision in the vagina was not sutured. After the surgery, the probe was fixed with skin and connected to the data logger (176T4; Testo SE & Co. KGaA). The logger and the probes were kept on the loin area with an adhesive and an elastic bandage (Fig. 3C). After surgery, antibiotics (5 mg/kg of streptomycin and 4000 IU/kg of benzylpenicillin, Mycillin; Meiji-seika, Tokyo, Japan) were injected into the muscle for 3 days to avoid postsurgical infection. The recommended administration period of antibiotics is at least 3 days more because the current procedure is a kind of celiotomy.
Fig. 3.
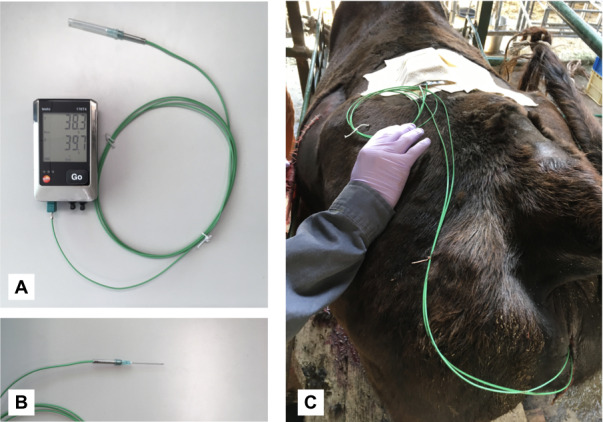
(A) The data logger with the flexible 0.25 mm diameter probe used for ovarian temperature measurement. (B) A 21 g × 1 1/2” needle attached the probe. (C) The logger and probes were kept on the loin area with an adhesive and an elastic bandage.
The ovarian temperature was recorded every 10 min using the data logger with the probe inserted into the unilateral ovary. In this study, the right ovary temperature was obtained in two of the three cows, with left ovary temperature monitored in cow #3. The vaginal temperature was also recorded with the intravaginal logger, which was prepared using modifications of a prior method [24] at 10-min intervals. Briefly, the logger had a thermal probe mounted on a progesterone-free controlled internal drug release device (pfCIDR, Eazi-Breed™ CIDR®; Zoetis, Parsippany-Troy Hills, NJ, USA), with the probe attached to the wing of the pfCIDR. The pfCIDR with the logger was inserted in the vaginal cavity approximately 20 cm from the vulva. After the temperature measurement, the probes were removed without any surgical procedure. In detail, all cows received only the rectal palpation and the probes were pressed and jerked gently backward from the ovary by a transrectal procedure. After the removal of the probe hook, the probe was removed through the vagina. The ovarian structure and estrous stage were confirmed with the transrectal ultrasonography examination in all cows after the temperature recording.
The ambient temperature and humidity in the experimental stalls were recorded every 2 min using a data recorder (174H; Testo SE & Co. KGaA) during the experimental period for assessment of the ambient condition. THI was calculated using the following formula: THI = (1.8T + 32) – (0.55 – 0.0055RH) (1.8T – 26), where T is a temperature in degrees Celsius and RH is relative humidity expressed as a percentage [25]. The mean THI during the experimental period was 45.07 ± 5.57 (the highest, 58.57; the lowest, 34.89; mean of diurnal variation, 17.05 ± 3.75; Table 1). The THI range is a non-stress condition for beef cows [26].
Acknowledgments
The authors thank the technical staff of the Field Science Center, Graduate School of Bioagricultural Sciences, Nagoya University. This research was supported in part by JSPS KAKENHI Grant Number 19K21167 to YM. The authors do not have any conflict of interest to declare.
References
- 1.Schüller LK, Michaelis I, Heuwieser W. Impact of heat stress on estrus expression and follicle size in estrus under field conditions in dairy cows. Theriogenology 2017; 102: 48–53. [DOI] [PubMed] [Google Scholar]
- 2.Silanikove N. Effects of heat stress on the welfare of extensively managed domestic ruminants. Livest Prod Sci 2000; 67: 1–18. [Google Scholar]
- 3.Bohmanova J, Misztal I, Cole JB. Temperature-humidity indices as indicators of milk production losses due to heat stress. J Dairy Sci 2007; 90: 1947–1956. [DOI] [PubMed] [Google Scholar]
- 4.Polsky L, von Keyserlingk MAG. Invited review: Effects of heat stress on dairy cattle welfare. J Dairy Sci 2017; 100: 8645–8657. [DOI] [PubMed] [Google Scholar]
- 5.De Rensis F, Scaramuzzi RJ. Heat stress and seasonal effects on reproduction in the dairy cow—a review. Theriogenology 2003; 60: 1139–1151. [DOI] [PubMed] [Google Scholar]
- 6.West JW. Effects of heat-stress on production in dairy cattle. J Dairy Sci 2003; 86: 2131–2144. [DOI] [PubMed] [Google Scholar]
- 7.López-Gatius F, Hunter RHF. Pre-ovulatory follicular temperature in bi-ovular cows. J Reprod Dev 2019; 65: 191–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hunter RHF, López-Gatius F, López-Albors O. Temperature gradients in vivo influence maturing male and female gametes in mammals: evidence from the cow. Reprod Fertil Dev 2017; 29: 2301–2304. [DOI] [PubMed] [Google Scholar]
- 9.El-Sheikh Ali H, Kitahara G, Tamura Y, Kobayashi I, Hemmi K, Torisu S, Sameshima H, Horii Y, Zaabel S, Kamimura S. Presence of a temperature gradient among genital tract portions and the thermal changes within these portions over the estrous cycle in beef cows. J Reprod Dev 2013; 59: 59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hunter RHF. Temperature gradients in female reproductive tissues. Reprod Biomed Online 2012; 24: 377–380. [DOI] [PubMed] [Google Scholar]
- 11.Kaufman JD, Saxton AM, Ríus AG. Short communication: Relationships among temperature-humidity index with rectal, udder surface, and vaginal temperatures in lactating dairy cows experiencing heat stress. J Dairy Sci 2018; 101: 6424–6429. [DOI] [PubMed] [Google Scholar]
- 12.Hunter RHF, Bøgh IB, Einer-Jensen N, Müller S, Greve T. Pre-ovulatory graafian follicles are cooler than neighbouring stroma in pig ovaries. Hum Reprod 2000; 15: 273–283. [DOI] [PubMed] [Google Scholar]
- 13.López-Gatius F, Hunter R. Clinical relevance of pre-ovulatory follicular temperature in heat-stressed lactating dairy cows. Reprod Domest Anim 2017; 52: 366–370. [DOI] [PubMed] [Google Scholar]
- 14.Hunter RHF. Temperature gradients in female reproductive tissues and their potential significance. Anim Reprod 2009; 6: 7–15. [Google Scholar]
- 15.Grinsted J, Blendstrup K, Andreasen MP, Byskov AG. Temperature measurements of rabbit antral follicles. J Reprod Fertil 1980; 60: 149–155. [DOI] [PubMed] [Google Scholar]
- 16.Nabors B, Linford R. Anatomy of the reproductive system of the cow. In: Hopper RM, (ed.). Bovine Reproduction. Hoboken, NJ: John Wiley and Sons, Inc.; 2014: 191–194. [Google Scholar]
- 17.Kawate N, Itami T, Choushi T, Saitoh T, Wada T, Matsuoka K, Uenaka K, Tanaka N, Yamanaka A, Sakase M, Tamada H, Inaba T, Sawada T. Improved conception in timed-artificial insemination using a progesterone-releasing intravaginal device and Ovsynch protocol in postpartum suckled Japanese Black beef cows. Theriogenology 2004; 61: 399–406. [DOI] [PubMed] [Google Scholar]
- 18.Pursley JR, Mee MO, Wiltbank MC. Synchronization of ovulation in dairy cows using PGF2α and GnRH. Theriogenology 1995; 44: 915–923. [DOI] [PubMed] [Google Scholar]
- 19.Wishart DF, Snowball JB. Endoscopy in cattle: observation of the ovary in situ. Vet Rec 1973; 92: 139–143. [DOI] [PubMed] [Google Scholar]
- 20.Habermehl NL. Heifer ovariectomy using the Willis spay instrument: Technique, morbidity and mortality. Can Vet J 1993; 34: 664–667. [PMC free article] [PubMed] [Google Scholar]
- 21.Fubini SL. Surgery of the ovary. In: Fubini SL, Ducharme N. (eds.), Farm Animal Surgery 1st ed. St. Louis: Saunders, Elsevier Health Sciences; 2004: 379–382. [Google Scholar]
- 22.Hofmeyr CFB. The female genitalia. In: Hofmeyr CFB. (ed.), Ruminant Urogenital Surgery. Ames: Iowa State University Press; 1987: 122–147. [Google Scholar]
- 23.Peiró JR, Nogueira GM, Nogueira GP, Perri SHV, Cardoso D. Ovariectomy by left flank approach in prepubertal Nelore (Bos indicus) heifers. Can J Vet Res 2009; 73: 237–240. [PMC free article] [PubMed] [Google Scholar]
- 24.Lea JM, Niemeyer DDO, Reed MT, Fisher AD, Ferguson DM. Development and validation of a simple technique for logging body temperature in free-ranging cattle. Aust J Exp Agric 2008; 48: 741–745. [Google Scholar]
- 25.Dikmen S, Hansen PJ. Is the temperature-humidity index the best indicator of heat stress in lactating dairy cows in a subtropical environment? J Dairy Sci 2009; 92: 109–116. [DOI] [PubMed] [Google Scholar]
- 26.Mader TL, Davis MS, Brown-Brandl T. Environmental factors influencing heat stress in feedlot cattle. J Anim Sci 2006; 84: 712–719. [DOI] [PubMed] [Google Scholar]