Abstract
Pulmonary MRI can now provide high-resolution images that are sensitive to early disease and specific to inflammation in cystic fibrosis (CF) lung disease. With specificity and function limited via CT, there are significant advantages to MRI. Many of the modern MRI techniques can be performed throughout life, and can be employed to understand changes over time, in addition to quantification of treatment response. Proton density and T1/T2 contrast images can be obtained within a single breath hold, providing depiction of structural abnormalities and active inflammation. Modern radial and/or spiral UTE techniques rival CT in resolution for depiction and quantification of structure, for both airway and parenchymal abnormalities. Contrast perfusion MRI techniques are now utilized routinely to visualize changes in pulmonary and bronchial circulation that routinely occur in CF lung disease, and non-contrast techniques are moving closer to clinical translation. Functional information can be obtained from non-contrast proton images alone, using techniques such as Fourier decomposition. Hyperpolarized-gas MRI, increasingly using 129Xe, is now becoming more widespread and has been demonstrated to have high sensitivity to early airway obstruction in CF via ventilation MRI. The sensitivity of 129Xe MRI promises future use in personalized medicine, management of early CF lung disease, and in future clinical trials. By combining structural and functional techniques, with or without hyperpolarized gases, regional structure-function relationships can be obtained, giving insight into pathophysiology of disease and improved clinical management. This article reviews the modern MRI techniques that can routinely be employed for CF lung disease in nearly any large medical center.
Keywords: magnetic resonance imaging, hyperpolarized, cystic fibrosis, pulmonary, lung, airways, longitudinal, xenon
INTRODUCTION
Cystic fibrosis (CF) is a fatal genetic disorder that produces disease in the lungs, pancreas, gastrointestinal tract, liver and male reproductive tract (1). It is the most common autosomal recessive disorder leading to premature death in Caucasians, and has a worldwide prevalence ≥70,000 (2). CF is caused by mutations in the Cystic Fibrosis Transmembrane conductance Regulator (CFTR) gene, which encodes the CFTR chloride and bicarbonate channel (1). Dysfunctional CFTR in the airway epithelium produces dehydrated and viscous mucus, leading to airway obstruction, inflammation, infection and ultimately progressive airway damage. CF lung disease manifests in early childhood, and the standard tool to detect and monitor pulmonary status is spirometry. The most commonly used spirometry measurement in CF is the forced expiratory volume in one second (FEV1, percent predicted) (3). This measurement is portable, well standardized and adaptable to clinic environments and clinical trials. However, there are clear limitations surrounding FEV1 use in CF to monitor lung function, including the effort dependence of the test, age limitations (it is difficult to obtain reliable data in toddlers before the age of 5–6 years, and it cannot be performed in infants without sedation), day to day test variability within subjects, and insensitivity to early or mild lung disease. Indeed, due to these limitations, the CF care and research community has sought to develop new tools for lung disease monitoring that are more sensitive and potentially adaptable to younger patients. The most advanced of these is the multiple breath washout (MBW), and the most common MBW measurement to quantify lung disease is the lung clearance index (LCI) (3). The LCI indirectly measures ventilation inhomogeneity, is effort independent, and can be performed in younger children with appropriate coaching. It has demonstrated increased sensitivity to detect early lung disease compared with FEV1, and has begun to be commonly used in clinical trials of CF therapies (2, 4–7). The test typically takes 30 – 60 minutes to complete, yet it has not been determined whether MBW/LCI can be used routinely in the clinic.
Imaging is also used to monitor CF lung disease, including chest X-ray, chest CT, and MRI. Chest X-ray is fast, simple, effort independent and easily standardized, with established scoring systems that correlate with disease progression (8, 9). Unfortunately, it lacks sensitivity to early disease, and its role in CF disease monitoring has declined. Chest CT is also a fast, simple and easily standardized tool to monitor CF lung disease; it remains the clinical gold standard, with established scoring systems that correlate with disease status (10, 11). Inspiratory and expiratory scans provide exquisite sensitivity to detect bronchiectasis, mucus plugging, and air trapping, and advances in automated scoring should help to standardize analysis (10, 12, 13). Tomographic imaging has the distinct advantage of localizing regional lung disease, which is not possible with pulmonary function tests. CT imaging, however, carries the risk of exposure to ionizing radiation, particularly concerning in younger pediatrics and in routine follow up. Despite the development of low dose scans, there continues to be reluctance across much of the CF care community to routinely use chest CT to serially monitor CF lung disease throughout childhood and adulthood. MRI (the focus of this review article) is emerging as a tool to monitor CF lung disease, and like CT can detect regional disease (10). Modern techniques have addressed past issues of low signal (from short T2*) and respiratory motion, with newer methods that capture lung function. This includes imaging of both perfusion and ventilation, with proton and hyperpolarized-gas methods. Indeed, with these advances, it is likely that structural and functional MR imaging will become a standard part of CF lung disease monitoring and could serve as the only common lung disease monitoring tool that spans diagnosis to adulthood.
Current clinical practice and the role of imaging
The current management of CF relies heavily on evidence-based therapies that have been demonstrated to be effective in well-controlled clinical trials (1). Standard pulmonary therapies include regular airway clearance (generally twice daily, using a variety of methods such as chest vest, positive expiratory pressure, chest physiotherapy), inhaled mucolytics (e.g. dornase alpha), inhaled mucus hydrators (e.g. hypertonic saline), oral anti-inflammatories (e.g.: ibuprofen, azithromycin), and inhaled antibiotics (e.g. aztreonam lysinate for inhalation, tobramycin inhalation solution). More recently drugs that help restore function to dysfunctional CFTR have become available to CF patients with discrete CFTR mutations, with profound benefits observed in some patient genotypes. Closely coupled with these daily therapies is disease monitoring, including regular CF clinic visits, blood monitoring (e.g.: vitamin levels, liver function tests, diabetes screening), sputum microbiology, and lung disease status (e.g.: pulmonary function tests and imaging). The burden of CF care is high, with daily therapies for adolescents and adults that can require 2 hours per day or more for completion. With the advent of highly effective CFTR modulators, a future goal in CF is the development of highly sensitive monitoring tools to more effectively personalize complex CF care regimens, helping patients to focus on therapies that are most beneficial to them and minimize therapies with little direct benefit. Tomographic imaging, particularly MRI that can be used for more routine assessment and follow up, has a strong potential to play a role in future clinical care.
A relatively novel algorithm in the treatment of CF lung disease is to start treatment early in life at a pre-symptomatic stage, i.e. after initial identification by newborn screening, in order to prevent or at least decelerate irreversible lung damage (14). This is precisely where new imaging techniques can be of value; ideally, this means that imaging procedures would also start early in life when measures of lung function are normal and chest x-ray is too insensitive to sufficiently detect disease-related changes. Although it has been stressed recently that novel CT scanner generations will deliver acceptable image quality at high scanning speeds even in infants at very low radiation dose (15), the remaining radiation dose is a concern for both providers and families, particularly in young children who are more sensitive to radiation-induced malignancy (16, 17). Further, some centers may generate CT images in both inspiration and expiration in young children (to gain some functional information), and general anesthesia and mechanical ventilation may be required, which also pose risks (18). Also, other procedures apart from cross-sectional imaging for assessment of CF lung disease severity may be required during the chronic disease process, such as emergency imaging in case of pulmonary embolism or hemorrhage, with subsequent fluoroscopy-guided interventions, or ICU treatments, that increase the cumulative radiation burden (19, 20). Thus, the biggest single advantage of MRI for life-long imaging surveillance of CF lung disease severity remains its lack of ionizing radiation. This also means it may be repeated multiple times as an end-point in clinical studies monitoring early and long-term treatment response, and preliminary results of the first randomized multicenter trial employing MRI as an endpoint are now available (21–23). The University of Heidelberg, for example, now performs annual contrast-enhanced surveillance MRI in all patients with CF starting with diagnosis, together with chest x-ray and clinical work-up (20). In their experience, imaging results greatly assist in selecting adequate therapy, and especially in directing courses of antibiotic therapy for pulmonary exacerbations (personal communication). Apart from being free of radiation, morphological MRI may harvest not only structural details for diagnosis, but also employ the combination of T1 and T2 contrasts and Gadolinium-enhancement for specification of imaging findings. The most obvious is to differentiate airway wall thickening from mucus filling by T1 and T2, and to detect active airway wall inflammation by increased Gd-uptake. More recently it was shown that Aspergillus superinfection may be detected by a locally reversed mucus signal on T1 and T2 (24). There is vast potential to take advantage of the range of structural and functional techniques with longitudinal MRI, which outclass CT techniques in terms of scope, finesse and temporal resolution, even though spatial resolution is generally lower.
While there is considerable excitement about functional imaging techniques involving hyperpolarized gases or dynamic changes in proton density (discussed in subsequent sections), most clinical, translational, and research MRI performed to date in CF lung disease has involved traditional proton MRI. Thus, the bulk of this review focuses on these techniques, which range from very straightforward Cartesian sequences that have existed for decades to cutting-edge techniques that involve 3D spiral trajectories or non-contrast perfusion techniques. Each section below details the broad categories of sequences that are currently employed, with discussion of technical details, advantages/disadvantages, and feasibility for certain patient populations.
Traditional Cartesian sequences for structure
Structural changes of airways and lung parenchyma in cystic fibrosis are often characterized by T2 -weighted sequences as well as T1 -weighted imaging pre- and post-contrast administration. A published, semi-quantitative MR reader score can evaluate specific morphological changes : (1) bronchiectasis/bronchial wall thickening, (2) mucus plugging, (3) abscesses/sacculations (circular, > 1.5 cm structures which are air filled or show an air fluid level, (4) consolidations > 2 cm, and (5) special findings, i.e. pleural changes (25). Findings are evaluated for each lobe, and a semi-quantitative score is assigned as follows: 0 = no abnormality; 1 = <50% of the lobe involved; 2 =≥50% of the lobe involved (26). A pulse-sequence strategy for three-dimensional structural imaging has to address all three major challenges associated with proton MRI in the lung, namely short T2*, low proton density, and respiratory motion (27). While newer radial and spiral k-space trajectories (often called UTE, or ultra-short echo-time sequences) offer strong advantages and are discussed below, Cartesian sequences remain ubiquitous and will serve an important role in the near future.
A Cartesian T2 -weighted fast spin sequence will visualize pulmonary infiltrates, inflammatory bronchial wall thickening, and mucus and fluid accumulations, as demonstrated in Figure 1 (28). As the robust workhorse for T2 imaging, a fast spin echo half-Fourier acquisition sequence with T2 weighting is recommended with the following parameters at 1.5T: TR 560 ms, TE 30 ms, FA 150°, spatial resolution 1.3 × 1.3 × 5 mm, (29). This sequence is chosen for four reasons: (1) it is a single shot sequence causing few breathing artifacts; (2) it allows ECG triggering to reduce cardiac motion artifacts; (3) it allows coverage of the whole thorax in one breath hold (approximately 18 s, each for coronal and transverse slice orientation; (4) by using a TE of 28–30 ms, the resulting images provide good fluid visualization (28).
Figure 1:
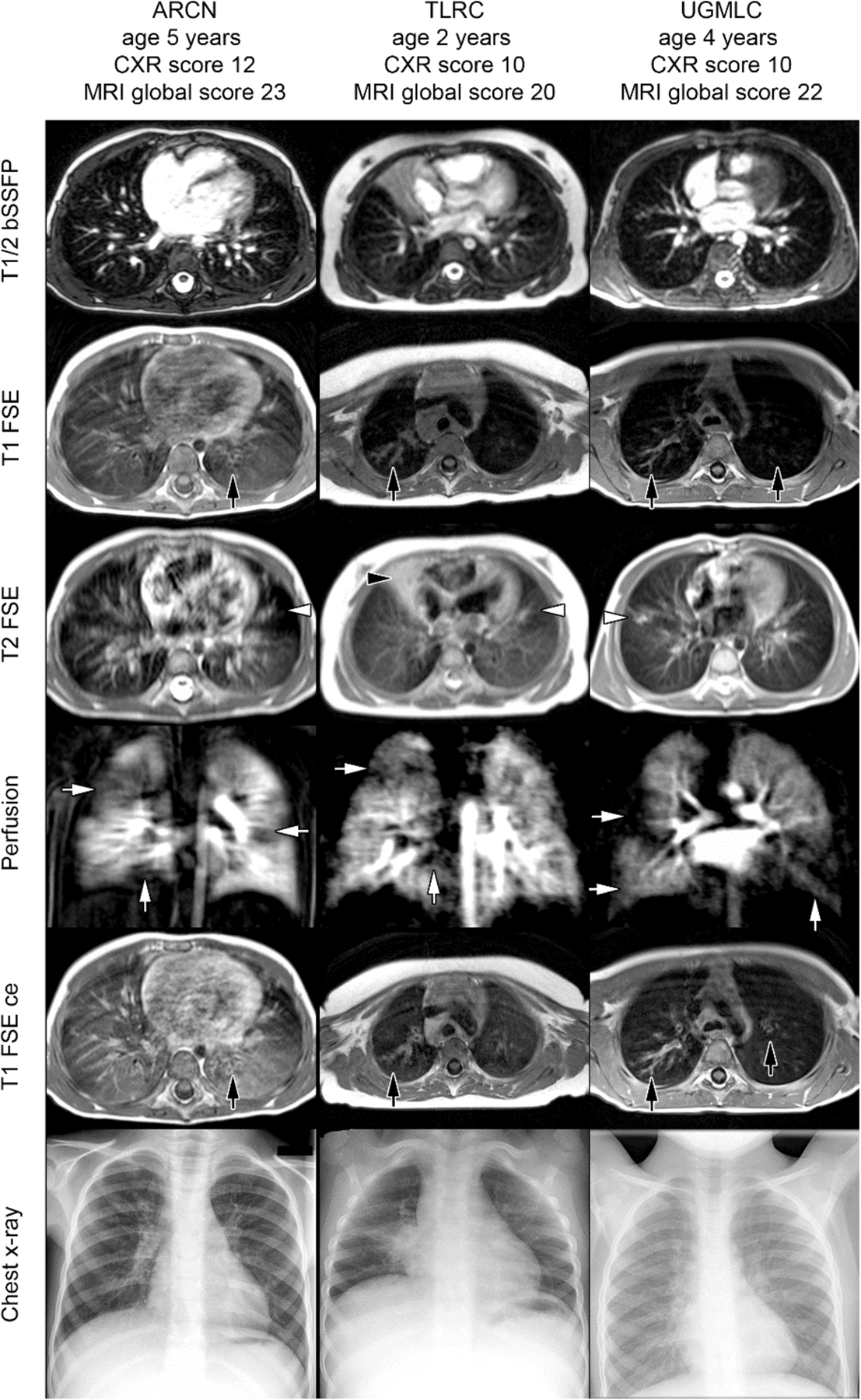
Examples of T1, T2, and perfusion-weighted images in infants and children with cystic fibrosis. Here, bronchiectasis and wall thickening were identified well on T1-weighted images, with and without contrast (black arrows, T1 FSE and T1 FSE ce, respectively), while mucus plugging and consolidations were identified with T2 weightings (white arrows, T2 FSE). A balanced steady-state free precession sequence (T1/2 bSSFP), contrast perfusion, and chest x-ray are shown for comparison. Reprinted with permission from J Cyst Fibros 2018; 17: 518.
For Cartesian T1 -weighted imaging a three-dimensional (3D) gradient echo sequence with short echo time can be employed for the assessment of the mediastinum, pulmonary nodules, masses, and consolidations. The same sequence may be repeated with fat saturation after the administration of contrast material to assess structural changes for enhancement indicating active inflammation (28). A volume interpolated gradient echo sequence with the following parameters is a reasonable choice, for either 1.5T or 3T: parallel acquisition factor two, TR = 3.7 ms, TE = minimum, ideally below 1 ms; FA = 8°, spatial resolution 1.0 × 1.0 × 5 mm, patient-adapted FOV, transverse orientation, with breath hold (approximately 19 s). This sequence was chosen for three reasons: coverage of the whole thorax in one breath hold, short echo time, and high spatial resolution (29).
Radial and spiral methods
Free-induction-decay imaging via radial and spiral trajectories originating at the center of k-space are not a new invention, but the advent of modern computing has now made the techniques feasible in translational and some clinical settings. These MRI sequences are often dubbed “ultrashort echo”, or UTE, because of the short time (≤ 0.2 ms) between initial rf excitation and data acquisition, even though there is no actual gradient-induced or spin echo. The advantages of UTE techniques for CF lung disease are multiple. The very short TE allows data acquisition in lung parenchyma before it can decay via T2*; for lung imaging at 3T (T2* = 0.8 ms), 88% of the lung parenchyma signal remains at an echo time of 0.1 ms, providing a solution to two problems discussed in the Cartesian section above: short T2* and low proton density. Radial and spiral UTE techniques are also inherently robust to motion, because of oversampling at the center of k-space. The combination of short echo times and robustness to motion allow translational and clinical implementations that begin to rival CT imaging in terms of resolution, even in very young patients (Figure 2). Recent studies have demonstrated high UTE MRI sensitivity to early CF lung disease in 1–2 year olds, with comparison to CT (Figure 2) (26), and high agreement with CT in assessing segmental bronchiectasis in adults (30), in addition to quantitative differentiation of disease with ventilation techniques (discussed below) (31). Importantly, longitudinal follow up can reveal the long-term dynamics of disease in a way impractical with CT because of radiation concerns (Figure 3). These traditional UTE techniques that have been previously employed use a radial acquisition that requires many trajectories to adequately sample the edges of k-space, leading to longer scan times than most Cartesian sequences. A radial sequence with the following parameters is a reasonable choice: TR ~4ms (minimum); TE = 0.2 ms or less; FA = Ernst angle; spatial resolution of 1.5 × 1.5 × 1.5 mm; patient-adapted FOV; readout duration equivalent to T2* or less; and golden angle sampling. One alternative that can save time uses adaptive or density-compensated techniques that slow the trajectory at higher k-space values (8, 32). Other implementations use spiral trajectories that adapt to the short T2* by decreasing the rate of spiral radius increase with increasing k-values, providing more efficient k-space sampling while retaining robustness to motion (Figure 4) (33, 34). One additional advantage of center-out radial and spiral trajectories is the sensitivity to diaphragmatic motion, via the first point of the free-induction decay (35). If trajectories are acquired carefully such that subsets adequately fill k-space, image reconstructions can be made from binned data at different points of the respiratory cycle (Figure 5) (32).
Figure 2:
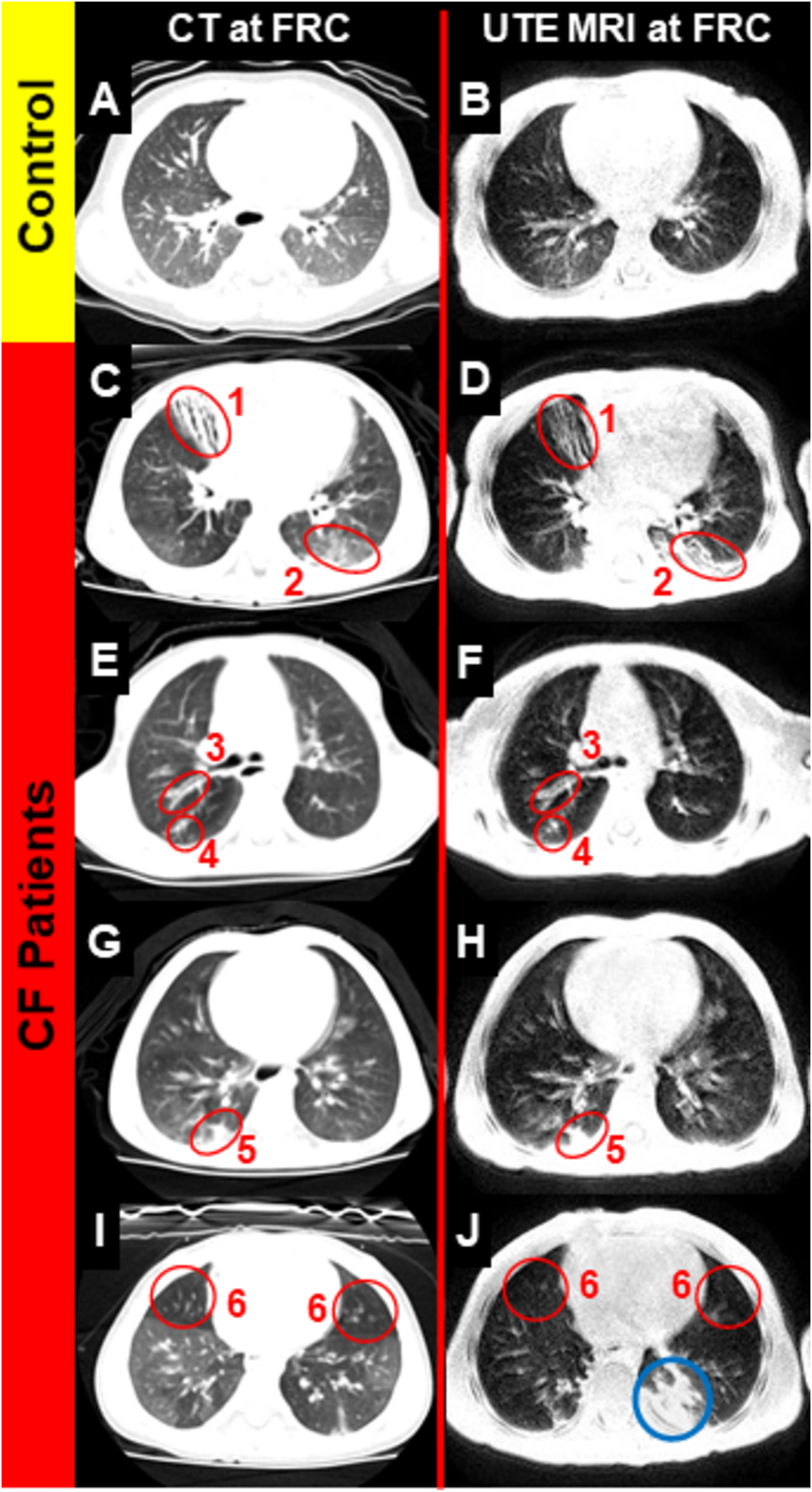
Comparison of radial UTE (stack of stars) and CT in a healthy patient and four CF patients, all 2–3 years old. Each of 6 categories of CT-identified abnormalities is identified near-equally via both modalities: (1) bronchiectasis, (2) ground-glass opacities, (3) bronchial-wall thickening, (4) mucus plugging, (5) consolidations, and (6) air trapping. Reprinted with permission from the American Thoracic Society (26).
Figure 3:

Female cystic fibrosis patient 2 years old at baseline with relatively severe disease. Annual chest x-ray and surveillance MRI in stable clinical situation are shown, demonstrating persistent middle lobe atelectasis (black arrow), adjacent pleural reaction (black arrowhead), as well as bronchiectasis with mucus plugging of the right superior lobe (white arrow). Note the progressive bronchiectasis inside the atelectatic middle lobe and otherwise relatively stable morphological findings in contrast to changing perfusion abnormalities (same image planes are shown). Perfusion abnormalities tend to re-occur in similar segments (white arrowhead).
Figure 4:
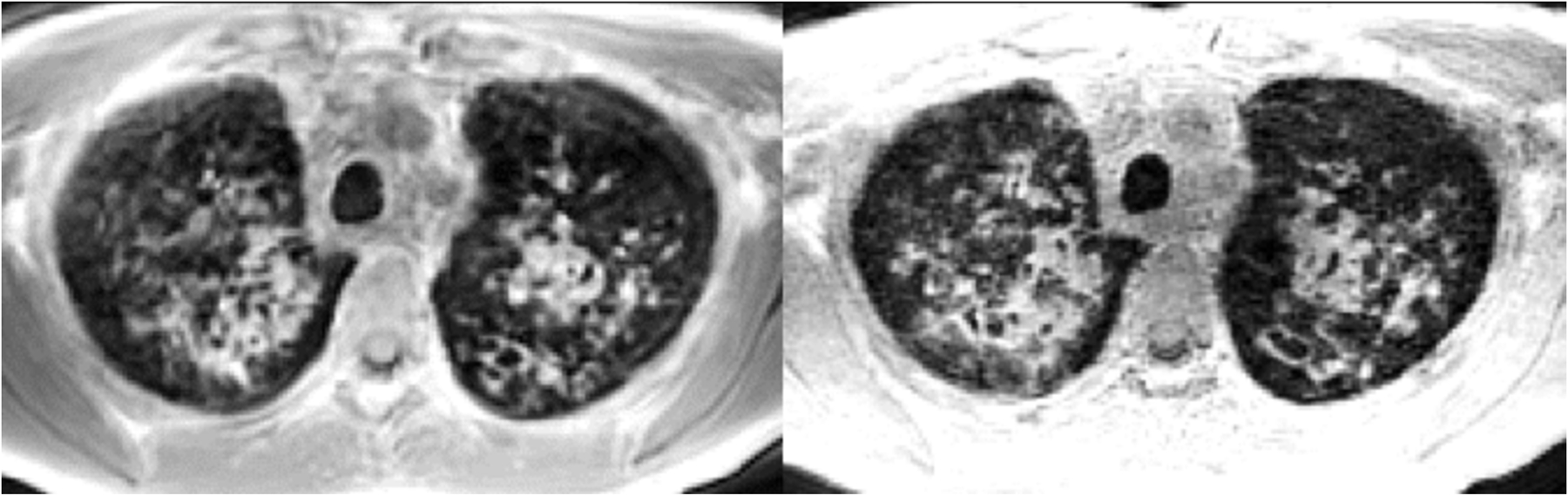
An example of an apical axial slice from center-out trajectories in k-space with phase encoding in the z-direction (left, stack of stars, 1.5 × 1.5 × 5 mm3) and with spiral trajectories with isotropic resolution (1.5 mm, FLORET sequence). Bronchiectasis and mucus plugs are easily identifiable in this 12-year-old CF patient.
Figure 5:

UTE reconstructions at tidal inspiration (right) and tidal expiration (left) in a healthy adult, taken during one free-breathing MRI sequence in approximately 8 minutes. Red and blue diaphragmatic windows are in the same physical location in space, demonstrating diaphragmatic movement. Reprinted with permission from Magn Reson Med 2016; 77: 1284.
Reader Scoring Systems for Structural Abnormalities
Structural abnormalities in CF lung disease, acquired by one or more of the methods above, may be separated into two groups according to their reversibility as a function of treatment. Mucus plugs and pathophysiologically linked airway wall inflammation are variable over time, and thus may also be reversible by therapy (21, 22, 36) . Similarly, air-trapping, which is linked to airway obstruction, seems to be highly variable over time (37)). On the other hand, bronchiectasis, cystic sacculations, and emphysema are usually considered irreversible lung destruction (38–40) Persistent consolidations may result in “destroyed lobes” which often contain visible bronchiectasis but no aerated lung anymore. Additionally, dilated bronchial arteries may be considered a sign of advanced irreversible destruction as well. Although bronchiectasis has been clearly defined(41) , large overlap with so-called airway dilatation is likely, and thus reversibility of so-called bronchiectasis has been reported as well (42). For example, Mott et al. described that bronchiectasis in young children with CF (mean age 2.0 years) was reversed in 26% of paired scans over one year (43). It is conceivable that in early CF lung disease, some reversibility of inflammation-induced airway dilatation may occur, but ultimately leads to progressive bronchiectasis and cystic sacculations, which have characterized the disease since its identification and are likely permanent after formation.
In an attempt to transform the manifold morphological changes of the CF lung into numerical values in which the scale reflects relative severity, and which can be subjected to statistical evaluation, scoring systems have been introduced. As a first modality, the presence and extent of visual impressions such as parallel lines, mottled shadows or consolidations were assessed on chest radiographs, sometimes complemented by diaphragmatic signs of hyperinflation (44–48). With increased use of cross-sectional CT, additional scoring systems have been introduced specifically for CT, which directly assessed CT-defined morphological changes such as airway wall thickening, bronchiectasis, mucus plugging or consolidations. Further, mosaic attenuation on inspiratory CT or air-trapping on expiratory CT – if present – were assessed as functional impairment (11, 49–51).. Apart from visual scoring, computer supported assessment (52) as well as fully automatic segmentation of the airway tree and lung parenchyma quantification including air-trapping on three-dimensional multidetector CT datasets is possible (37, 53–57).
By analogy, an MRI scoring system was introduced by Eichinger et al. in 2012, which assesses airway wall thickening and bronchiectasis as a combined item as a concession to the limited resolution of MRI and the link between inflammatory wall thickening and airway dilatation mentioned above (25). Further, mucus plugging, sacculations or abscesses, consolidations and pleural reactions are assessed. Recently, a score for mosaic signal inhomogeneity has been added for infants and preschool children (58). For a systematic assessment of subtracted 4D perfusion datasets, a perfusion score is used. All of these subscores are assessed by lobe on a rating scale of 0 to 2, depending on the extent of the disease manifestation with 0 = no manifestation, 1 = extent < 50% of a lobe, and 2 = extent ≥ 50% of the lobe. Further, the MRI score was cross-validated against chest x-ray (28) and CT (26, 59), as well as against LCI as a measure of lung function (22). The proposed scoring system is specifically adapted to a standardized protocol available on state-of-the-art scanners across vendors and applicable to all age-groups (21) and was validated in a multi-center setting (60).
With the introduction of UTE MRI to CF, CT-like resolution is available, but the typical contrasts of MRI are often lost. This may allow for a modification of the MRI scoring system with a more granular assessment down to a segmental level (26). However, the differentiation of airway wall inflammation detectable by contrast enhancement, high T2-values of mucus plugs, reversed signal on T1 vs. T2 in case of allergic bronchopulmonary aspergillosis (61) may be lost by transition to UTE-only imaging, which is why novel combined protocols for morphological assessment are advisable. However, UTE MRI may facilitate a software-based quantification due to enhanced resolution and signal-to-background ratios, with approaches adapted from CT.
Angiography and perfusion MRI in CF
The pulmonary blood supply can be measured using MR perfusion methods, both with and without contrast agents (Figure 3). The lungs have a dual arterial blood supply through the pulmonary arterial circulation, which drives the gas exchange mechanism and oxygenation of blood, and the bronchial arterial circulation, which is derived from the aorta and delivers oxygen-rich blood to the vital lung structures. These circulations have abundant anastomoses, which may be more dominant depending on pathological processes. The bronchial circulation cannot overcome hypoxia, but it can keep structures intact by providing oxygen-rich blood supply where the pulmonary arterial system is compromised. The pulmonary arterial circulation, however, will be affected by changes in ventilation in the lungs, via the hypoxic vasoconstriction response of the pulmonary arterial capillary bed. This allows for optimal matching of perfusion and ventilation, which in turn allows higher efficiency of gas exchange. This process may become strained with more widespread pathological processes, such as pneumonia, where increased amounts of blood are shunted away from non-ventilated lung, ultimately leading to hypoxia. Similarly, with obstruction of the pulmonary arteries, such as seen in acute and chronic thromboembolism, the regional perfusion is decreased, leading to an increase in pulmonary artery pressure centrally and shunting of blood to those areas where perfusion and gas exchange are achievable.
In CF lung disease there is an increasing competition between the hypoxic vasoconstrictive response, the need for gas exchange and the need for oxygen by airways and lung parenchyma related tissues. Mucus plugging will lead to peripheral airflow obstruction and the vasoconstrictive response will shunt blood to more well-ventilated lung areas (Figure 1). In addition, post-obstructive inflammatory changes will lead to an increased demand of oxygen-rich blood to allow the immune system to do its work (hyperperfusion and delivery of white cells). These two demands will increase the blood flow through the bronchial circulation, and hypertrophy of these critical vessels often arises. This may cause complications, such as bronchial arterial hemorrhage, which may be fatal. The assessment of pulmonary perfusion is therefore not just essential to understand the gas exchange mechanism and abnormalities in patients with CF but will also need to focus on the systemic blood supply by applying delayed imaging.
Contrast methods
Magnetic resonance angiography (MRA) allows for the contrast study of either the pulmonary arterial circulation or the systemic circulation. The difference is only related to a difference in timing of imaging, such that the trigger for image acquisition is set in the right ventricle for the pulmonary arterial visualization and in the descending aorta for visualization of the bronchial arterial circulation. Although this method may be useful to allow for morphological assessment of these systems, it is not feasible at present to allow for a more functional assessment, such as the evaluation of shunts or the precise depiction of perfusion.
Dynamic contrast-enhanced perfusion MRI allows for the visualization of a T1-contrast bolus as it circulates, first through the pulmonary arterial system, and then to the left heart, aorta, and the bronchial circulation. Therefore, this method provides much greater insight into the overall pulmonary and bronchial circulation and its interactions. As a physiological response to airway obstruction and local hypoxia, delivery of blood to the affected lung region is downregulated (62); thus, perfusion imaging is an excellent tool to monitor functional impairment in CF, which may be easier to apply in broad practice than hyperpolarized gas MRI (also in Figure 1 and discussed below). Perfusion deficits as part of the overall CF MRI score have been demonstrated to correlate well (r = 0.57 – 0.84, p < 0.01–0.001) with the lung clearance index (LCI) in 97 infants to adult CF patients (22). However, a number of patients with significant lung disease and perfusion abnormalities on MRI showed a normal LCI in this study. As a concern, LCI may be unable to detect completely obstructed segments where tracer gas is neither washed-in nor -out, but which may be depicted by perfusion MRI. This may in part explain why correlation is imperfect especially in young patients with mild CF lung disease (22). Contrast methods are used routinely in adult clinical scans and have become a part of both adult and pediatric clinical CF practice at many European academic sites. In pulmonary exacerbation, MRI scores are increased with more severe perfusion defects compared to patients in stable clinical condition that are partially reversible after antibiotic therapy in all age-groups, indicating that perfusion MRI may be a sensitive tool for detecting therapy response in general (Figure 6) (21, 22, 63).
Figure 6:

MRI during and 1-month after acute exacerbations in a 6-year-old patient with cystic fibrosis. The airway-wall thickening and mucus plugging (white arrows) largely resolved after treatment with intravenous antibiotics, as did consolidations (black arrows) and perfusion deficits (black arrowheads). Reprinted with permission from the American Thoracic Society (21)
US pediatric sites have recently reduced their utilization of contrast out of an abundance of caution and as a result of an FDA statement(64). Alternative contrast agents, such as ultra-small particles of iron oxide (USPIO) are increasingly employed, but largely unused in standard clinical care at present(65).
Non-contrast perfusion
With uncertainty of long-term effect of Gd in the brain currently discussed world-wide, it is increasingly desirable to transfer the established information obtained from contrast-enhanced MR techniques to non-contrast MR methods. However, this transition to MRI without contrast-enhancement is still underway with intensive efforts of investigators by utilizing innovative approaches including T1 mapping, self-gated non-contrast MRI, diffusion-weighted imaging, and phase-resolved functional techniques (66–69). One such promising methodology using normalized T1 mapping and arterial spin labeling (ASL) has been well validated against multiple inert-gas elimination techniques and has been employed in CF research studies but has yet to see full translation to more widespread use in the lung (70–72). Another promising approach that may be used in future multi-site studies is the Fourier decomposition technique, which capitalizes on an ability to separate MRI signal changes at the frequency of breathing from the frequency of cardiac motion (Figure 8) (69). In a recent study it was shown that ventilation and perfusion defects detected by Fourier decomposition MRI also correlate similarly well with the LCI (r = 0.76 and r = 0.85, p < 0.0001) in 40 children aged 6–18 years (73).
Figure 8:

Non-contrast ventilation (c) and perfusion (d) imaging via Fourier decomposition MRI in a school-age female with CF, compared to contrast perfusion (b) and Tw-weighted imaging (a). Reprinted with permission from Rofo 2016; 188: 834.
Hyperpolarized-Gas MRI
Hyperpolarized gas MRI provides an exquisite means of imaging lung ventilation heterogeneity in obstructive airways diseases. The bulk of work performed in CF in the past has focused on the use of 3He gas, as historically it has been easier to polarize and provides intrinsically higher image SNR than 129Xe, though recent engineering advances and gas availability are making 129Xe much more attractive for current and future studies. Figure 7 demonstrates near-identical 129Xe and 3He MRI in a series of CF patients, with a 3D volume rendering. Imaging of lung ventilation is typically performed at breath-hold (typically after a lung inflation of 1L or less, beginning at FRC) using multi-slice or 3D gradient echo or steady state free precession sequences and dedicated chest transmit-receive coils. The first studies employed static breath-hold 3He ventilation imaging and were performed in adults with manifest CF lung disease (74) as diagnosed by spirometry and demonstrated clear sensitivity to ventilation heterogeneity. Follow-up studies in adults with breath-hold 3He ventilation imaging have shown concordance with some of the structural changes seen on 1H MRI and CT (75). Ventilation imaging has been assessed by qualitative reader analysis and quantitatively via the percentage of non-ventilated volume (often called ventilation defect percentage, or VDP, derived with co-registered 1H images of the lung cavity). These images have been shown to be reliable and robust measurements with good scan-to-scan repeatability (76, 77) in studies performed in CF patients at both 1.5T and 3T, with impressively-high sensitivity compared to pulmonary function testing (Figure 9) (78). These methods have also has proven sensitivity to detect and quantify regional changes in lung ventilation in response to; bronchodilator in adult CF patients (79), new gene specific mucolytic therapies (80) and also to percussive chest physiotherapy used for mucus clearance in children (81, 82). With a demonstrated higher diagnostic sensitivity to early stage lung disease than spirometry, CT and LCI (83, 84), and the ability to track sub-clinical early lung disease progression with high sensitivity (78, 85), the potential of hyperpolarized gas ventilation MRI in the clinical assessment of pediatric CF is great. Earlier findings with 3He are now being well corroborated with parallel studies using 129Xe (78, 84, 86), which bodes well for future clinical translation of this cheaper and more readily available isotope (natural abundance xenon ~ £25 /L). Altes et al. demonstrated that 3He-MRI can be used in a single-center setting for an interventional trial with ivacaftor, by showing that after 4 weeks of treatment in a small group of 8 adolescent to adult CF patients with a G551D-CFTR mutation total ventilation defects decreased by 8.2% from baseline (80). The same group also showed that hyperpolarized gas MRI with 3He is feasible in non-sedated infants ≤ 4 years, which was initially thought problematic because 3He MRI is usually acquired in static single or multiple breath-hold (87). Smith et al. provided the first long-term data on 3He-MRI in 14 school-age children with CF by measuring ventilation defect percentage in two examinations 1.3 – 2.0 years apart (Figure 10). Compared to spirometry and multiple breath wash-out, ventilation defect percentage showed the strongest change over time in this relatively small cohort (85).
Figure 7:

3He and 129Xe ventilation images from a series of CF patients with differing degrees of obstructive lung disease. The images convey equivalent qualitative and quantitative information with some subtle differences related to defect size. Both sets of images are acquired in 3D with a bSSFP sequence and can be visualized as a 3D volume rendering.
Figure 9:

Axial 129Xe MRI in age-matched control and CF patients, both with normal FEV1. 129Xe ventilation MRI is able to detect obstructive defects that are invisible to traditional pulmonary function testing. Group comparisons demonstrate clear separation via ventilation defect percentage but not via FEV1. Graph reprinted with permission from J Cyst Fibros 2017; 16: 275.
Figure 10:

3He ventilation MRI, demonstrating increasing severity of defects over 1.5 – 2 years, in 10–12-year-old CF patients. Reprinted with permission from Am J Respir Crit Care Med 2018: 197: 397.
As a result of these and similar studies, hyperpolarized-gas MRI has now been adopted as part of diagnostic clinical imaging work up in some centers as part of the clinical review, and a Xe MRI multi-site consortium has been formed for the use of such imaging in clinical trials.
Hyperpolarized gas MRI also has dynamic sensitivity to air flow in CF lungs using time resolved imaging (88), and multi-breath washout imaging has been used to regionally measure washout rates (89) in CF lungs to provide some regional insight in to the complementary MBW method of measuring ventilation heterogeneity.
In addition to straightforward ventilation imaging with hyperpolarized gas, there is high sensitivity to airspace enlargement via diffusion weighted imaging (90–92) and, for 129Xe MRI only, a direct method for measuring and quantifying regional gas exchange(93). 129Xe is a large atom and dissolves very slightly in liquids and tissue, with a large (~200 ppm) chemical shift between the gas phase and two visible, dissolved peaks. These two dissolved peaks correspond to 129Xe in red blood cells (217 ppm) and 129Xe in tissue or blood plasma (198 ppm), with near-constant exchange between compartments (94, 95). By using variants of typical fat-water separation (e.g., Dixon techniques), separate quantitative maps of the three phases can be generated, providing a direct measure of gas exchange. While gas-exchange imaging has yet to be applied to cystic fibrosis, there is strong potential for its use to understand the complex changes and interaction that can occur as a result of regional ventilation, perfusion, airway, and parenchymal abnormalities in CF.
Structure-Function Relationships
An important marker of CF lung disease severity is airway wall thickening and dilatation that can be quantified by dedicated post-processing software on CT datasets only. Several groups demonstrated that severity of airway changes, assessed by scoring or by software, is associated with limitations in airflow obstruction (96–98), and, moreover, that imaging is more sensitive to disease progression than spirometry (78, 83, 99). Importantly, small peripheral airways are not visible on CT or MRI but their obstruction may be detected indirectly by mosaicism on inspiratory and air-trapping on expiratory CT or by ventilation MRI (37, 78, 83). Here, MRI in particular can excel with its potential for functional imaging. For more than two decades, hyperpolarized MRI has been studied extensively as a tool to directly display and quantify ventilation abnormalities in obstructive airway diseases. In general, hyperpolarized gas MRI can and should be combined with morphologic CT or MRI to fully harvest the potential of combined functional and morphological cross-sectional imaging. Also, combined imaging allows for a more precise lung segmentation for the calculation of ventilation defect percentage (100).
MRI techniques not yet applied to CF lung disease
A relatively straightforward method for ventilation imaging, albeit with low overall contrast, is the combination of T1 mapping with oxygen inhalation. Here, the paramagnetic properties of pure O2 are used to induce a decrease of the T1 relaxation time in ventilated lung regions (101–103). Some recent work suggests that T1 mapping alone may be reflective of lung perfusion (104–106), and the added value of MRI with O2 inhalation is a matter of ongoing debate. Not yet established in CF are perfluorinated gases for ventilation imaging, which do not require the process of hyperpolarization and may potentially be combined with LCI measurements because SF6, for example, is an LCI tracer gas as well (107). Experience with this technique in general is limited, and there are disadvantages that include the requirement of multi-breath imaging of a high concentration of a dense gas that increases the work of breathing.
Overview and Summary
Pulmonary MRI has made enormous strides within the past 20 years and now can provide significant functional, specificity, and safety advantages over CT, albeit at lower spatial resolution. Structural and functional MRI can be performed at any age, longitudinally, to understand changes in individual patients or genotypes over time, in addition to quantification of treatment response. Traditional proton techniques allow proton density and T1/T2 contrast to be obtained, each within a single breath hold, providing depiction of structural abnormalities and active inflammation, which can be quantified using reader scoring or computer-aided techniques. While CT remains the clinical gold standard for lung imaging in CF, modern UTE techniques rival CT in resolution for depiction and quantification of functional and structural changes, which are an early indicator of reversible and irreversible airway and parenchymal abnormalities. Angiographic and perfusion MRI techniques can also be employed longitudinally, to visualize changes in pulmonary and bronchial circulation that routinely occur in CF lung disease. While contrast-enhanced perfusion is used routinely, non-contrast techniques promise to improve safety and provide diagnostic-quality images in the future. Functional information (ventilation and perfusion) can be obtained from proton images alone, using techniques such as Fourier decomposition. Hyperpolarized-gas MRI, increasingly using 129Xe over 3He, are now becoming more widespread and have been demonstrated to have exquisite sensitivity to early airway obstruction in CF via ventilation MRI and to gas-exchange abnormalities in other obstructive diseases via images of dissolved Xe in red blood cells and tissue. The sensitivity of 129Xe MRI helps ensure it will have a bright future in personalized medicine, management of early CF lung disease, and in future clinical trials. By combining structural and functional techniques, regional structure-function relationships can be illuminated, giving insight into pathophysiology of disease and providing potential for improved clinical management. While we will continue to see progress within the field of pulmonary MRI for CF lung disease, present capabilities available at most CF centers allow MRI to be adopted for routine clinical management of individual patients.
ACKNOWLEDGMENTS:
GRANT SUPPORT: NIH R01 HL131012 (JCW, JPC), R44 HL123299 (JCW, JPC), Scottish Imaging Network Platform of Scientific Excellence (SINAPSE)
LITERATURE
- 1.Spielberg DR, Clancy JP. Cystic Fibrosis and Its Management Through Established and Emerging Therapies. Annu Rev Genom Hum G 2016; 17: 155–175. [DOI] [PubMed] [Google Scholar]
- 2.Ratjen F, Hug C, Marigowda G, Tian S, Huang X, Stanojevic S, Milla CE, Robinson PD, Waltz D, Davies JC, group VXi. Efficacy and safety of lumacaftor and ivacaftor in patients aged 6–11 years with cystic fibrosis homozygous for F508del-CFTR: a randomised, placebo-controlled phase 3 trial. Lancet Respir Med 2017; 5: 557–567. [DOI] [PubMed] [Google Scholar]
- 3.Stanojevic S, Ratjen F. Physiologic endpoints for clinical studies for cystic fibrosis. J Cyst Fibros 2016; 15: 416–423. [DOI] [PubMed] [Google Scholar]
- 4.Milla CE, Ratjen F, Marigowda G, Liu F, Waltz D, Rosenfeld M, * VXPBIG. Lumacaftor/Ivacaftor in Patients Aged 6–11 Years with Cystic Fibrosis and Homozygous for F508del-CFTR. Am J Respir Crit Care Med 2017; 195: 912–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davies J, Sheridan H, Bell N, Cunningham S, Davis SD, Elborn JS, Milla CE, Starner TD, Weiner DJ, Lee PS, Ratjen F. Assessment of clinical response to ivacaftor with lung clearance index in cystic fibrosis patients with a G551D-CFTR mutation and preserved spirometry: a randomised controlled trial. Lancet Respir Med 2013; 1: 630–638. [DOI] [PubMed] [Google Scholar]
- 6.Amin R, Subbarao P, Lou W, Jabar A, Balkovec S, Jensen R, Kerrigan S, Gustafsson P, Ratjen F. The effect of dornase alfa on ventilation inhomogeneity in patients with cystic fibrosis. The European respiratory journal 2011; 37: 806–812. [DOI] [PubMed] [Google Scholar]
- 7.Amin R, Subbarao P, Jabar A, Balkovec S, Jensen R, Kerrigan S, Gustafsson P, Ratjen F. Hypertonic saline improves the LCI in paediatric patients with CF with normal lung function. Thorax 2010; 65: 379–383. [DOI] [PubMed] [Google Scholar]
- 8.Rosenfeld M, Farrell PM, Kloster M, Swanson JO, Vu T, Brumback L, Acton JD, Castile RG, Colin AA, Conrad CK, Hart MA, Kerby GS, Hiatt PW, Mogayzel PJ, Johnson RC, Davis SD. Association of lung function, chest radiographs and clinical features in infants with cystic fibrosis. Eur Respir J 2013; 42: 1545–1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davis SD, Brody AS, Emond MJ, Brumback LC, Rosenfeld M. Endpoints for clinical trials in young children with cystic fibrosis. Proc Am Thorac Soc 2007; 4: 418–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Szczesniak R, Turkovic L, Andrinopoulou ER, Tiddens H. Chest imaging in cystic fibrosis studies: What counts, and can be counted? J Cyst Fibros 2017; 16: 175–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brody AS, Klein JS, Molina PL, Quan J, Bean JA, Wilmott RW. High-resolution computed tomography in young patients with cystic fibrosis: distribution of abnormalities and correlation with pulmonary function tests. J Pediatr 2004; 145: 32–38. [DOI] [PubMed] [Google Scholar]
- 12.Perez-Rovira A, Kuo W, Petersen J, Tiddens HA, de Bruijne M. Automatic airway-artery analysis on lung CT to quantify airway wall thickening and bronchiectasis. Med Phys 2016; 43: 5736. [DOI] [PubMed] [Google Scholar]
- 13.Kuo W, Kemner-van de Corput MP, Perez-Rovira A, de Bruijne M, Fajac I, Tiddens HA, van Straten M, group E-CSCs. Multicentre chest computed tomography standardisation in children and adolescents with cystic fibrosis: the way forward. The European respiratory journal 2016; 47: 1706–1717. [DOI] [PubMed] [Google Scholar]
- 14.Ramsey BW, Banks-Schlegel S, Accurso FJ, Boucher RC, Cutting GR, Engelhardt JF, Guggino WB, Karp CL, Knowles MR, Kolls JK, LiPuma JJ, Lynch S, McCray PB Jr., Rubenstein RC, Singh PK, Sorscher E, Welsh M. Future directions in early cystic fibrosis lung disease research: an NHLBI workshop report. Am J Respir Crit Care Med 2012; 185: 887–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuo W, Ciet P, Tiddens HA, Zhang W, Guillerman RP, van Straten M. Monitoring cystic fibrosis lung disease by computed tomography. Radiation risk in perspective. Am J Respir Crit Care Med 2014; 189: 1328–1336. [DOI] [PubMed] [Google Scholar]
- 16.O’Connell OJ, McWilliams S, McGarrigle A, O’Connor OJ, Shanahan F, Mullane D, Eustace J, Maher MM, Plant BJ. Radiologic imaging in cystic fibrosis: cumulative effective dose and changing trends over 2 decades. Chest 2012; 141: 1575–1583. [DOI] [PubMed] [Google Scholar]
- 17.Dournes G, Marthan R, Berger P, Laurent F. Excess Risk of Cancer from Computed Tomography Scan Is Small but Not So Low as to Be Incalculable. Am J Respir Crit Care Med 2015; 192: 1396–1397. [DOI] [PubMed] [Google Scholar]
- 18.Sly PD, Gangell CL, Chen L, Ware RS, Ranganathan S, Mott LS, Murray CP, Stick SM. Risk factors for bronchiectasis in children with cystic fibrosis. N Engl J Med 2013; 368: 1963–1970. [DOI] [PubMed] [Google Scholar]
- 19.Mall MA, Stahl M, Graeber SY, Sommerburg O, Kauczor HU, Wielputz MO. Early detection and sensitive monitoring of CF lung disease: Prospects of improved and safer imaging. Pediatric pulmonology 2016; 51: S49–S60. [DOI] [PubMed] [Google Scholar]
- 20.Wielpütz MO, Eichinger M, Biederer J, Wege S, Stahl M, Sommerburg O, Mall MA, Kauczor HU, Puderbach M. Imaging of Cystic Fibrosis Lung Disease and Clinical Interpretation. RoFo : Fortschritte auf dem Gebiete der Rontgenstrahlen und der Nuklearmedizin 2016; 188: 834–845. [DOI] [PubMed] [Google Scholar]
- 21.Wielpütz MO, Puderbach M, Kopp-Schneider A, Stahl M, Fritzsching E, Sommerburg O, Ley S, Sumkauskaite M, Biederer J, Kauczor H-U, Eichinger M, Mall MA. Magnetic Resonance Imaging Detects Changes in Structure and Perfusion, and Response to Therapy in Early Cystic Fibrosis Lung Disease. Am J Respir Crit Care Med 2014; 189: 956–965. [DOI] [PubMed] [Google Scholar]
- 22.Stahl M, Wielputz MO, Graeber SY, Joachim C, Sommerburg O, Kauczor HU, Puderbach M, Eichinger M, Mall MA. Comparison of Lung Clearance Index and Magnetic Resonance Imaging for Assessment of Lung Disease in Children with Cystic Fibrosis. Am J Respir Crit Care Med 2017; 195: 349–359. [DOI] [PubMed] [Google Scholar]
- 23.Stahl M, Graeber S, Sommerburg O, Ricklefs I, Diekmann G, Dopfer C, Barth S, Schlegtendal A, Wielpütz M, Kauczor H. Randomized, double-blind, controlled pilot study on safety and efficacy of hypertonic saline as preventive inhalation therapy in infants with CF (PRESIS) Pediatric pulmonology: WILEY 111 RIVER ST, HOBOKEN 07030–5774, NJ USA; 2017. p. S302–S302. [Google Scholar]
- 24.Dournes G, Berger P, Refait J, Macey J, Bui S, Delhaes L, Montaudon M, Corneloup O, Chateil J-F, Marthan R. Allergic bronchopulmonary aspergillosis in cystic fibrosis: MR imaging of airway mucus contrasts as a tool for diagnosis. Radiology 2017; 285: 261–269. [DOI] [PubMed] [Google Scholar]
- 25.Eichinger M, Optazaite D-E, Kopp-Schneider A, Hintze C, Biederer J, Niemann A, Mall MA, Wielpütz MO, Kauczor H-U, Puderbach M. Morphologic and functional scoring of cystic fibrosis lung disease using MRI. European Journal of Radiology 2012; 81: 1321–1329. [DOI] [PubMed] [Google Scholar]
- 26.Roach DJ, Cremillieux Y, Fleck RJ, Brody AS, Serai SD, Szczesniak RD, Kerlakian S, Clancy JP, Woods JC. Ultrashort Echo-Time Magnetic Resonance Imaging Is a Sensitive Method for the Evaluation of Early Cystic Fibrosis Lung Disease. Annals of the American Thoracic Society 2016; 13: 1923–1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miller GW, Mugler JP, Sá RC, Altes TA, Prisk GK, Hopkins SR. Advances in functional and structural imaging of the human lung using proton MRI. NMR in Biomedicine 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Puderbach M, Eichinger M, Gahr J, Ley S, Tuengerthal S, Schmahl A, Fink C, Plathow C, Wiebel M, Muller FM, Kauczor HU. Proton MRI appearance of cystic fibrosis: comparison to CT. Eur Radiol 2007; 17: 716–724. [DOI] [PubMed] [Google Scholar]
- 29.Scholz O, Denecke T, Böttcher J, Schwarz C, Mentzel H-J, Streitparth F, Maurer M, Pfeil A, Huppertz A, Mehl A. MRI of cystic fibrosis lung manifestations: sequence evaluation and clinical outcome analysis. Clinical radiology 2017; 72: 754–763. [DOI] [PubMed] [Google Scholar]
- 30.Dournes G, Menut F, Macey J, Fayon M, Chateil JF, Salel M, Corneloup O, Montaudon M, Berger P, Laurent F. Lung morphology assessment of cystic fibrosis using MRI with ultra-short echo time at submillimeter spatial resolution. Eur Radiol 2016; 26: 3811–3820. [DOI] [PubMed] [Google Scholar]
- 31.Zha W, Kruger SJ, Johnson KM, Cadman RV, Bell LC, Liu F, Hahn AD, Evans MD, Nagle SK, Fain SB. Pulmonary ventilation imaging in asthma and cystic fibrosis using oxygen-enhanced 3D radial ultrashort echo time MRI. J Magn Reson Imaging 2018; 47: 1287–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Higano NS, Hahn AD, Tkach JA, Cao X, Walkup LL, Thomen RP, Merhar SL, Kingma PS, Fain SB, Woods JC. Retrospective respiratory self-gating and removal of bulk motion in pulmonary UTE MRI of neonates and adults. Magn Reson Med 2017; 77: 1284–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Robison RK, Anderson AG 3rd, Pipe JG. Three-dimensional ultrashort echo-time imaging using a FLORET trajectory. Magn Reson Med 2017; 78: 1038–1049. [DOI] [PubMed] [Google Scholar]
- 34.Willmering MM, Robison RK, Wang H, Pipe JG, Woods JC. Implementation of the FLORET UTE sequence for lung imaging. Magn Reson Med 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zucker EJ, Cheng JY, Haldipur A, Carl M, Vasanawala SS. Free-breathing pediatric chest MRI: Performance of self-navigated golden-angle ordered conical ultrashort echo time acquisition. J Magn Reson Imaging 2018; 47: 200–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shah RM, Sexauer W, Ostrum BJ, Fiel SB, Friedman AC. High-resolution CT in the acute exacerbation of cystic fibrosis: evaluation of acute findings, reversibility of those findings, and clinical correlation. AJR Am J Roentgenol 1997; 169: 375–380. [DOI] [PubMed] [Google Scholar]
- 37.Loeve M, Rosenow T, Gorbunova V, Hop WC, Tiddens HA, de Bruijne M. Reversibility of trapped air on chest computed tomography in cystic fibrosis patients. Eur J Radiol 2015; 84: 1184–1190. [DOI] [PubMed] [Google Scholar]
- 38.Sobonya RE, Taussig LM. Quantitative aspects of lung pathology in cystic fibrosis. Am Rev Respir Dis 1986; 134: 290–295. [DOI] [PubMed] [Google Scholar]
- 39.Cantin AM, Hartl D, Konstan MW, Chmiel JF. Inflammation in cystic fibrosis lung disease: Pathogenesis and therapy. J Cyst Fibros 2015; 14: 419–430. [DOI] [PubMed] [Google Scholar]
- 40.Esterly JR, Oppenheimer EH. Cystic fibrosis of the pancreas: structural changes in peripheral airways. Thorax 1968; 23: 670–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ooi GC, Khong PL, Chan-Yeung M, Ho JC, Chan PK, Lee JC, Lam WK, Tsang KW. High-resolution CT quantification of bronchiectasis: clinical and functional correlation. Radiology 2002; 225: 663–672. [DOI] [PubMed] [Google Scholar]
- 42.Javidan-Nejad C, Bhalla S. Bronchiectasis. Radiol Clin North Am 2009; 47: 289–306. [DOI] [PubMed] [Google Scholar]
- 43.Mott LS, Park J, Murray CP, Gangell CL, de Klerk NH, Robinson PJ, Robertson CF, Ranganathan SC, Sly PD, Stick SM, Arest CF. Progression of early structural lung disease in young children with cystic fibrosis assessed using CT. Thorax 2012; 67: 509–516. [DOI] [PubMed] [Google Scholar]
- 44.Chrispin AR, Norman AP. The systematic evaluation of the chest radiograph in cystic fibrosis. Pediatr Radiol 1974; 2: 101–105. [DOI] [PubMed] [Google Scholar]
- 45.Terheggen-Lagro S, Truijens N, van Poppel N, Gulmans V, van der Laag J, van der Ent C. Correlation of six different cystic fibrosis chest radiograph scoring systems with clinical parameters. Pediatr Pulmonol 2003; 35: 441–445. [DOI] [PubMed] [Google Scholar]
- 46.Benden C, Wallis C, Owens CM, Ridout DA, Dinwiddie R. The Chrispin-Norman score in cystic fibrosis: doing away with the lateral view. The European respiratory journal 2005; 26: 894–897. [DOI] [PubMed] [Google Scholar]
- 47.Weatherly MR, Palmer CG, Peters ME, Green CG, Fryback D, Langhough R, Farrell PM. Wisconsin cystic fibrosis chest radiograph scoring system. Pediatrics 1993; 91: 488–495. [PubMed] [Google Scholar]
- 48.Cleveland RH, Stamoulis C, Sawicki G, Kelliher E, Zucker EJ, Wood C, Zurakowski D, Lee E. Brasfield and Wisconsin scoring systems have equal value as outcome assessment tools of cystic fibrosis lung disease. Pediatr Radiol 2014; 44: 529–534. [DOI] [PubMed] [Google Scholar]
- 49.Bhalla M, Turcios N, Aponte V, Jenkins M, Leitman BS, McCauley DI, Naidich DP. Cystic fibrosis: scoring system with thin-section CT. Radiology 1991; 179: 783–788. [DOI] [PubMed] [Google Scholar]
- 50.Helbich TH, Heinz-Peer G, Eichler I, Wunderbaldinger P, Gotz M, Wojnarowski C, Brasch RC, Herold CJ. Cystic fibrosis: CT assessment of lung involvement in children and adults. Radiology 1999; 213: 537–544. [DOI] [PubMed] [Google Scholar]
- 51.Robinson TE, Leung AN, Northway WH, Blankenberg FG, Chan FP, Bloch DA, Holmes TH, Moss RB. Composite spirometric-computed tomography outcome measure in early cystic fibrosis lung disease. Am J Respir Crit Care Med 2003; 168: 588–593. [DOI] [PubMed] [Google Scholar]
- 52.Rosenow T, Oudraad MC, Murray CP, Turkovic L, Kuo W, de Bruijne M, Ranganathan SC, Tiddens HA, Stick SM, Australian Respiratory Early Surveillance Team for Cystic F. PRAGMA-CF. A Quantitative Structural Lung Disease Computed Tomography Outcome in Young Children with Cystic Fibrosis. Am J Respir Crit Care Med 2015; 191: 1158–1165. [DOI] [PubMed] [Google Scholar]
- 53.Goris ML, Zhu HJ, Blankenberg F, Chan F, Robinson TE. An automated approach to quantitative air trapping measurements in mild cystic fibrosis. Chest 2003; 123: 1655–1663. [DOI] [PubMed] [Google Scholar]
- 54.Montaudon M, Berger P, de Dietrich G, Braquelaire A, Marthan R, Tunon-de-Lara JM, Laurent F. Assessment of airways with three-dimensional quantitative thin-section CT: in vitro and in vivo validation. Radiology 2007; 242: 563–572. [DOI] [PubMed] [Google Scholar]
- 55.Wielputz MO, Weinheimer O, Eichinger M, Wiebel M, Biederer J, Kauczor HU, Heussel CP, Mall MA, Puderbach M. Pulmonary emphysema in cystic fibrosis detected by densitometry on chest multidetector computed tomography. PloS one 2013; 8: e73142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mets OM, Roothaan SM, Bronsveld I, Luijk B, van de Graaf EA, Vink A, de Jong PA. Emphysema Is Common in Lungs of Cystic Fibrosis Lung Transplantation Patients: A Histopathological and Computed Tomography Study. PloS one 2015; 10: e0128062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Konietzke P, Weinheimer O, Wielputz MO, Savage D, Ziyeh T, Tu C, Newman B, Galban CJ, Mall MA, Kauczor HU, Robinson TE. Validation of automated lobe segmentation on paired inspiratory-expiratory chest CT in 8–14 year-old children with cystic fibrosis. PloS one 2018; 13: e0194557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Leutz-Schmidt P, Stahl M, Sommerburg O, Eichinger M, Puderbach MU, Schenk JP, Alrajab A, Triphan SMF, Kauczor HU, Mall MA, Wielputz MO. Non-contrast enhanced magnetic resonance imaging detects mosaic signal intensity in early cystic fibrosis lung disease. Eur J Radiol 2018; 101: 178–183. [DOI] [PubMed] [Google Scholar]
- 59.Sileo C, Corvol H, Boelle PY, Blondiaux E, Clement A, Ducou Le Pointe H. HRCT and MRI of the lung in children with cystic fibrosis: comparison of different scoring systems. J Cyst Fibros 2014; 13: 198–204. [DOI] [PubMed] [Google Scholar]
- 60.Wielputz MO, von Stackelberg O, Stahl M, Jobst BJ, Eichinger M, Puderbach MU, Nahrlich L, Barth S, Schneider C, Kopp MV, Ricklefs I, Buchholz M, Tummler B, Dopfer C, Vogel-Claussen J, Kauczor HU, Mall MA. Multicentre standardisation of chest MRI as radiation-free outcome measure of lung disease in young children with cystic fibrosis. J Cyst Fibros 2018; 17: 518–527. [DOI] [PubMed] [Google Scholar]
- 61.Dournes G, Berger P, Refait J, Macey J, Bui S, Delhaes L, Montaudon M, Corneloup O, Chateil JF, Marthan R, Fayon M, Laurent F. Allergic Bronchopulmonary Aspergillosis in Cystic Fibrosis: MR Imaging of Airway Mucus Contrasts as a Tool for Diagnosis. Radiology 2017; 285: 261–269. [DOI] [PubMed] [Google Scholar]
- 62.Hopkins SR, Wielpütz MO, Kauczor H-U. Imaging lung perfusion. J Appl Physiol 2012; 113: 328–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Grasemann H, Ciet P, Amin R, McDonald N, Klingel M, Tiddens H, Ratjen F, Grosse-Wortmann L. Changes in magnetic resonance imaging scores and ventilation inhomogeneity in children with cystic fibrosis pulmonary exacerbations. The European respiratory journal 2017; 50. [DOI] [PubMed] [Google Scholar]
- 64.Levine D, McDonald RJ, Kressel HY. Gadolinium Retention After Contrast-Enhanced MRI. JAMA 2018; 320: 1853–1854. [DOI] [PubMed] [Google Scholar]
- 65.Zhou XY, Jeffris KE, Yu EY, Zheng B, Goodwill PW, Nahid P, Conolly SM. First in vivo magnetic particle imaging of lung perfusion in rats. Phys Med Biol 2017; 62: 3510–3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Triphan SM, Jobst BJ, Breuer FA, Wielputz MO, Kauczor HU, Biederer J, Jakob PM. Echo time dependence of observed T1 in the human lung. J Magn Reson Imaging 2015; 42: 610–616. [DOI] [PubMed] [Google Scholar]
- 67.Veldhoen S, Weng AM, Knapp J, Kunz AS, Stab D, Wirth C, Segerer F, Hebestreit H, Malzahn U, Kostler H, Bley TA. Self-gated Non-Contrast-enhanced Functional Lung MR Imaging for Quantitative Ventilation Assessment in Patients with Cystic Fibrosis. Radiology 2017; 283: 242–251. [DOI] [PubMed] [Google Scholar]
- 68.Ciet P, Bertolo S, Ros M, Andrinopoulou ER, Tavano V, Lucca F, Feiweier T, Krestin GP, Tiddens H, Morana G. Detection and monitoring of lung inflammation in cystic fibrosis during respiratory tract exacerbation using diffusion-weighted magnetic resonance imaging. The European respiratory journal 2017; 50. [DOI] [PubMed] [Google Scholar]
- 69.Voskrebenzev A, Gutberlet M, Klimes F, Kaireit TF, Schonfeld C, Rotarmel A, Wacker F, Vogel-Claussen J. Feasibility of quantitative regional ventilation and perfusion mapping with phase-resolved functional lung (PREFUL) MRI in healthy volunteers and COPD, CTEPH, and CF patients. Magn Reson Med 2018; 79: 2306–2314. [DOI] [PubMed] [Google Scholar]
- 70.Hopkins SR, Wielputz MO, Kauczor HU. Imaging lung perfusion. J Appl Physiol (1985) 2012; 113: 328–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sa RC, Henderson AC, Simonson T, Arai TJ, Wagner H, Theilmann RJ, Wagner PD, Prisk GK, Hopkins SR. Measurement of the distribution of ventilation-perfusion ratios in the human lung with proton MRI: comparison with the multiple inert-gas elimination technique. J Appl Physiol (1985) 2017; 123: 136–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Donnola SB, Dasenbrook EC, Weaver D, Lu L, Gupta K, Prabhakaran A, Yu X, Chmiel JF, McBennett K, Konstan MW, Drumm ML, Flask CA. Preliminary comparison of normalized T1 and non-contrast perfusion MRI assessments of regional lung disease in cystic fibrosis patients. J Cyst Fibros 2017; 16: 283–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nyilas S, Bauman G, Sommer G, Stranzinger E, Pusterla O, Frey U, Korten I, Singer F, Casaulta C, Bieri O, Latzin P. Novel magnetic resonance technique for functional imaging of cystic fibrosis lung disease. The European respiratory journal 2017; 50. [DOI] [PubMed] [Google Scholar]
- 74.Donnelly LF, MacFall JR, McAdams HP, Majure JM, Smith J, Frush DP, Bogonad P, Charles HC, Ravin CE. Cystic fibrosis: combined hyperpolarized 3He-enhanced and conventional proton MR imaging in the lung--preliminary observations. Radiology 1999; 212: 885–889. [DOI] [PubMed] [Google Scholar]
- 75.McMahon CJ, Dodd JD, Hill C, Woodhouse N, Wild JM, Fichele S, Gallagher CG, Skehan SJ, van Beek EJ, Masterson JB. Hyperpolarized 3helium magnetic resonance ventilation imaging of the lung in cystic fibrosis: comparison with high resolution CT and spirometry. Eur Radiol 2006; 16: 2483–2490. [DOI] [PubMed] [Google Scholar]
- 76.Kirby M, Svenningsen S, Ahmed H, Wheatley A, Etemad-Rezai R, Paterson NA, Parraga G. Quantitative evaluation of hyperpolarized helium-3 magnetic resonance imaging of lung function variability in cystic fibrosis. Academic radiology 2011; 18: 1006–1013. [DOI] [PubMed] [Google Scholar]
- 77.O’Sullivan B, Couch M, Roche JP, Walvick R, Zheng S, Baker D, Johnson M, Botfield M, Albert MS. Assessment of repeatability of hyperpolarized gas MR ventilation functional imaging in cystic fibrosis. Academic radiology 2014; 21: 1524–1529. [DOI] [PubMed] [Google Scholar]
- 78.Thomen RP, Walkup LL, Roach DJ, Cleveland ZI, Clancy JP, Woods JC. Hyperpolarized (129)Xe for investigation of mild cystic fibrosis lung disease in pediatric patients. J Cyst Fibros 2017; 16: 275–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mentore K, Froh DK, de Lange EE, Brookeman JR, Paget-Brown AO, Altes TA. Hyperpolarized HHe 3 MRI of the lung in cystic fibrosis: assessment at baseline and after bronchodilator and airway clearance treatment. Academic radiology 2005; 12: 1423–1429. [DOI] [PubMed] [Google Scholar]
- 80.Altes TA, Johnson M, Fidler M, Botfield M, Tustison NJ, Leiva-Salinas C, de Lange EE, Froh D, Mugler JP 3rd. Use of hyperpolarized helium-3 MRI to assess response to ivacaftor treatment in patients with cystic fibrosis. J Cyst Fibros 2017; 16: 267–274. [DOI] [PubMed] [Google Scholar]
- 81.Woodhouse N, Wild JM, van Beek EJ, Hoggard N, Barker N, Taylor CJ. Assessment of hyperpolarized 3He lung MRI for regional evaluation of interventional therapy: a pilot study in pediatric cystic fibrosis. J Magn Reson Imaging 2009; 30: 981–988. [DOI] [PubMed] [Google Scholar]
- 82.Bannier E, Cieslar K, Mosbah K, Aubert F, Duboeuf F, Salhi Z, Gaillard S, Berthezene Y, Cremillieux Y, Reix P. Hyperpolarized 3He MR for sensitive imaging of ventilation function and treatment efficiency in young cystic fibrosis patients with normal lung function. Radiology 2010; 255: 225–232. [DOI] [PubMed] [Google Scholar]
- 83.Marshall H, Horsley A, Taylor CJ, Smith L, Hughes D, Horn FC, Swift AJ, Parra-Robles J, Hughes PJ, Norquay G, Stewart NJ, Collier GJ, Teare D, Cunningham S, Aldag I, Wild JM. Detection of early subclinical lung disease in children with cystic fibrosis by lung ventilation imaging with hyperpolarised gas MRI. Thorax 2017; 72: 760–762. [DOI] [PubMed] [Google Scholar]
- 84.Kanhere N, Couch MJ, Kowalik K, Zanette B, Rayment JH, Manson D, Subbarao P, Ratjen F, Santyr G. Correlation of Lung Clearance Index with Hyperpolarized (129)Xe Magnetic Resonance Imaging in Pediatric Subjects with Cystic Fibrosis. Am J Respir Crit Care Med 2017; 196: 1073–1075. [DOI] [PubMed] [Google Scholar]
- 85.Smith L, Marshall H, Aldag I, Horn F, Collier G, Hughes D, West N, Horsley A, Taylor CJ, Wild J. Longitudinal Assessment of Children with Mild Cystic Fibrosis Using Hyperpolarized Gas Lung Magnetic Resonance Imaging and Lung Clearance Index. Am J Respir Crit Care Med 2018; 197: 397–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Walkup LL, Thomen RP, Akinyi TG, Watters E, Ruppert K, Clancy JP, Woods JC, Cleveland ZI. Feasibility, tolerability and safety of pediatric hyperpolarized (129)Xe magnetic resonance imaging in healthy volunteers and children with cystic fibrosis. Pediatr Radiol 2016; 46: 1651–1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Altes TA, Meyer CH, Mata JF, Froh DK, Paget-Brown A, Gerald Teague W, Fain SB, de Lange EE, Ruppert K, Botfield MC, Johnson MA, Mugler JP 3rd. Hyperpolarized helium-3 magnetic resonance lung imaging of non-sedated infants and young children: a proof-of-concept study. Clinical imaging 2017; 45: 105–110. [DOI] [PubMed] [Google Scholar]
- 88.Koumellis P, van Beek EJ, Woodhouse N, Fichele S, Swift AJ, Paley MN, Hill C, Taylor CJ, Wild JM. Quantitative analysis of regional airways obstruction using dynamic hyperpolarized 3He MRI-preliminary results in children with cystic fibrosis. J Magn Reson Imaging 2005; 22: 420–426. [DOI] [PubMed] [Google Scholar]
- 89.Horn FC, Deppe MH, Marshall H, Parra-Robles J, Wild JM. Quantification of regional fractional ventilation in human subjects by measurement of hyperpolarized 3He washout with 2D and 3D MRI. Journal of applied physiology (Bethesda, Md : 1985) 2014; 116: 129–139. [DOI] [PubMed] [Google Scholar]
- 90.Woods JC, Choong CK, Yablonskiy DA, Bentley J, Wong J, Pierce JA, Cooper JD, Macklem PT, Conradi MS, Hogg JC. Hyperpolarized 3He diffusion MRI and histology in pulmonary emphysema. Magn Reson Med 2006; 56: 1293–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Stewart NJ, Chan HF, Hughes PJC, Horn FC, Norquay G, Rao M, Yates DP, Ireland RH, Hatton MQ, Tahir BA, Ford P, Swift AJ, Lawson R, Marshall H, Collier GJ, Wild JM. Comparison of (3) He and (129) Xe MRI for evaluation of lung microstructure and ventilation at 1.5T. J Magn Reson Imaging 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yablonskiy DA, Sukstanskii AL, Woods JC, Gierada DS, Quirk JD, Hogg JC, Cooper JD, Conradi MS. Quantification of lung microstructure with hyperpolarized 3He diffusion MRI. J Appl Physiol (1985) 2009; 107: 1258–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wang Z, Robertson SH, Wang J, He M, Virgincar RS, Schrank GM, Bier EA, Rajagopal S, Huang YC, O’Riordan TG, Rackley CR, McAdams HP, Driehuys B. Quantitative analysis of hyperpolarized (129) Xe gas transfer MRI. Med Phys 2017; 44: 2415–2428. [DOI] [PubMed] [Google Scholar]
- 94.Wang JM, Robertson SH, Wang Z, He M, Virgincar RS, Schrank GM, Smigla RM, O’Riordan TG, Sundy J, Ebner L, Rackley CR, McAdams P, Driehuys B. Using hyperpolarized (129)Xe MRI to quantify regional gas transfer in idiopathic pulmonary fibrosis. Thorax 2018; 73: 21–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Qing K, Ruppert K, Jiang Y, Mata JF, Miller GW, Shim YM, Wang C, Ruset IC, Hersman FW, Altes TA, Mugler JP 3rd. Regional mapping of gas uptake by blood and tissue in the human lung using hyperpolarized xenon-129 MRI. J Magn Reson Imaging 2014; 39: 346–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Montaudon M, Berger P, Cangini-Sacher A, de Dietrich G, Tunon-de-Lara JM, Marthan R, Laurent F. Bronchial measurement with three-dimensional quantitative thin-section CT in patients with cystic fibrosis. Radiology 2007; 242: 573–581. [DOI] [PubMed] [Google Scholar]
- 97.Wielputz MO, Eichinger M, Weinheimer O, Ley S, Mall MA, Wiebel M, Bischoff A, Kauczor HU, Heussel CP, Puderbach M. Automatic airway analysis on multidetector computed tomography in cystic fibrosis: correlation with pulmonary function testing. J Thorac Imaging 2013; 28: 104–113. [DOI] [PubMed] [Google Scholar]
- 98.de Jong PA, Lindblad A, Rubin L, Hop WC, de Jongste JC, Brink M, Tiddens HA. Progression of lung disease on computed tomography and pulmonary function tests in children and adults with cystic fibrosis. Thorax 2006; 61: 80–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.De Jong PA, Nakano Y, Lequin MH, Mayo JR, Woods R, Pare PD, Tiddens H. Progressive damage on high resolution computed tomography despite stable lung function in cystic fibrosis. European Respiratory Journal 2004; 23: 93–97. [DOI] [PubMed] [Google Scholar]
- 100.Horn FC, Tahir BA, Stewart NJ, Collier GJ, Norquay G, Leung G, Ireland RH, Parra-Robles J, Marshall H, Wild JM. Lung ventilation volumetry with same-breath acquisition of hyperpolarized gas and proton MRI. NMR Biomed 2014; 27: 1461–1467. [DOI] [PubMed] [Google Scholar]
- 101.Stadler A, Jakob PM, Griswold M, Stiebellehner L, Barth M, Bankier AA. T1 mapping of the entire lung parenchyma: Influence of respiratory phase and correlation to lung function test results in patients with diffuse lung disease. Magn Reson Med 2008; 59: 96–101. [DOI] [PubMed] [Google Scholar]
- 102.Chen Q, Jakob P, Griswold M, Levin D, Hatabu H, Edelman R. Oxygen enhanced MR ventilation imaging of the lung. Magnetic Resonance Materials in Physics, Biology and Medicine 1998; 7: 153–161. [DOI] [PubMed] [Google Scholar]
- 103.Jakob PM, Wang T, Schultz G, Hebestreit H, Hebestreit A, Hahn D. Assessment of human pulmonary function using oxygen-enhanced T(1) imaging in patients with cystic fibrosis. Magn Reson Med 2004; 51: 1009–1016. [DOI] [PubMed] [Google Scholar]
- 104.Jobst BJ, Triphan SM, Sedlaczek O, Anjorin A, Kauczor HU, Biederer J, Ley-Zaporozhan J, Ley S, Wielputz MO. Functional Lung MRI in Chronic Obstructive Pulmonary Disease: Comparison of T1 Mapping, Oxygen-Enhanced T1 Mapping and Dynamic Contrast Enhanced Perfusion. PloS one 2015; 10: e0121520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Triphan SMF, Breuer FA, Gensler D, Kauczor H-U, Jakob PM. Oxygen enhanced lung MRI by simultaneous measurement of T1 and T2* during free breathing using ultrashort TE. Journal of Magnetic Resonance Imaging 2015; 41: 1708–1714. [DOI] [PubMed] [Google Scholar]
- 106.Triphan SMF, Jobst BJ, Breuer FA, Wielpütz MO, Kauczor H-U, Biederer J, Jakob PM. Echo time dependence of observed T1 in the human lung. Journal of Magnetic Resonance Imaging 2015; 42: 610–616. [DOI] [PubMed] [Google Scholar]
- 107.Gutberlet M, Kaireit TF, Voskrebenzev A, Lasch F, Freise J, Welte T, Wacker F, Hohlfeld JM, Vogel-Claussen J. Free-breathing Dynamic (19)F Gas MR Imaging for Mapping of Regional Lung Ventilation in Patients with COPD. Radiology 2018; 286: 1040–1051. [DOI] [PubMed] [Google Scholar]


