Abstract
Cytokine storm, multiorgan failure, and particularly acute respiratory distress syndrome (ARDS) is the leading cause of mortality and morbidity in patients with COVID-19. A fulminant ARDS kills the majority of COVID-19 victims.
Pirfenidone (5-methyl-1-phenyl-2-[1H]-pyridone), is a novel anti-fibrotic agent with trivial adverse effects. Pirfenidone is approved for the treatment of Idiopathic Pulmonary Fibrosis (IPF) for patients with mild to moderate disease. Pirfenidone could inhibit apoptosis, downregulate ACE receptors expression, decrease inflammation by several mechanisms and ameliorate oxidative stress and hence protect pneumocytes and other cells from COVID-19 invasion and cytokine storm simultaneously. Based on the pirfenidone mechanism of action and the known pathophysiology of COVID-19, I believe that pirfenidone has the potential for the treatment of COVID-19 patients.
Abbreviations: COVID-19, Coronavirus Disease 2019; COVID-19-SARS, Coronavirus Disease 2019 – Severe Acute Respiratory Syndrome; ARDS, Acute respiratory distress syndrome; IPF, Idiopathic Pulmonary Fibrosis; TGF-β1, Transforming growth factor β1; TGF, Connective tissue growth factor; PDGF, Platelet-derived growth factors; TNF-α, Tumor necrosis factor-α; ADP, Adenosin Diphosphate; ACE, Angiotensin Converting Enzyme; NADPH, Nicotinamide adenine dinucleotide phosphate; ECM, Extracellular matrix; FDA, US Food and Drug Administration
Keywords: COVID-19, Pirfenidone, Treatment, Cytokine storm, Oxidative stress, Inflammation, Angiotensin converting enzyme receptor, Acute respiratory distress syndrome
Introduction
COVID-19
Every minute, an American dies of COVID-19. Cytokine storm, multiorgan failure and particularly acute respiratory distress syndrome (ARDS) are the leading causes of mortality and morbidity in patients with COVID-19. A fulminant ARDS kills the majority of COVID-19 victims [1]. Also, there are whispers that some of the survivors might develop pulmonary sequels. Further investigations and follow-ups are warranted in this case [2], [3]. A large number of suggested treatments such as ivermectin, hydroxychloroquine, and azithromycin are currently under investigation. Among them, hydroxychloroquine, azithromycin, and recently remdesivir showed acceptable results in clinical trials, as of June 2020 [4], [5], [6], [7], [8], [9], [10]. Nevertheless, the results of these interventions are not completely satisfactory and studies for other medications are still warranted .
Pirfenidone
Pirfenidone (5-methyl-1-phenyl-2-[1H]-pyridone), is a novel anti-fibrotic agent with trivial adverse effects [11], [12], [13]. Pirfenidone is approved for the treatment of Idiopathic Pulmonary Fibrosis (IPF) in humans for patients with mild to moderate disease [14], [15].
Diverse action mechanisms have been suggested for pirfenidone, among them are downregulating effects on a series of cytokines, including transforming growth factor (TGF)- β1, connective tissue growth factor (CTGF), platelet-derived growth factors (PDGF), and tumor necrosis factor (TNF)- α [16], [17], [18], [19], [20]. Additionally, pirfenidone is a reactive oxygen species (ROS) scavenger, and last but not the list, pirfenidone downregulates the expression of ACE receptor, the major cellular receptor for COVID-19 [21], [22], [23]. Additioally, some other characteristics of pirfenidone makes it an appropriate treatment for COVID-19, among them are anti-apoptotic and anti-fibrotic effects of pirfenidone. The details of the hypothesis have been discussed below. Based on known pirfenidone mechanism of action and the pathophysiology of COVID-19, I believe that pirfenidone has the potential for the treatment of COVID-19 patients.
The Hypothesis/theory
Cytokine storm, severe inflammation, oxidative stress, and reactive oxygen species damage and increased permeability of vascular bed are responsible for the development of ARDS and multi-organ damage in patients with COVID-19 [24], [25]. Elderly patients, particularly those with comorbidities such as DM, cardiovascular disorder, and cancer are at increased risk of severe manifestation of COVID-19 [26], [27]. Pathologic manifestation of COVID-19 under microscope includes the presence of exudate, vascular congestion, inflammatory clusters with fibrinoid material and multinucleated giant cell. Reactive alveolar hyperplasia and fibroblastic proliferation have been shown in alive patients with COVID-19 who underwent a lung biopsy due to cancer before the diagnosis of COVID-19 [28], [29].
Almost all of the current promising treatments have anti-inflammatory characteristics, beyond their antibiotic effects. For example, both azithromycin and hydroxychloroquine possess anti-inflammatory effects [4].
Pirfenidone, a novel anti-fibrotic agent is known to have several anti-fibrotic, anti-inflammatory, oxygen radical scavenger/antioxidant effects [11], [22], [23], [30], [31].
Anti-inflammatory effects of pirfenidone
The anti-inflammatory effects of pirfenidone have been shown in several experimental studies. It has been shown that pirfenidone inhibits TNF-α secretion and decrease a large number of other inflammatory cytokines as well [20], [32], [33], [34]. Additionally, Li et al. in a recent study shown that pirfenidone ameliorates lipopolysaccharide-induced pulmonary inflammation and fibrosis by blocking NLRP3 inflammasome activation [35], [36].
Anti-fibrotic effects of pirfenidone
It has been shown in several studies that pirfenidone significantly inhibits TGF- β 1-induced fibronectin synthesis [17], [18]. Down-regulating of profibrotic gene expression and collagen secretion has been shown in humans and animal models treated with pirfenidone [37], [38], [39]. Reduction of overexpression of TGF- β in inflammatory conditions plays a key role in the antifibrotic activity of pirfenidone [38], [40].
Pirfenidone inhibits collagen I fibril formation and causes a reduction in collagen fibril bundles [39], [41], [42]. It has been shown that pirfenidone has pleiotropic actions on both the immune system and extracellular matrix (ECM), such as hyaluronan, a major component of the ECM that regulates tissue injury and repair [43]. Recently, the upregulation of RGS2 has been suggested as a novel mechanism of amelioration of pulmonary fibrosis with pirfenidone treatment [44].
Protection against oxidative stress and lipid peroxidation
The followings are probable endpoints of an overactive inflammatory response and WBC free radical formation in Microsome (via microsomal NADPH cytochrome c reductase) and Mitochondria (NADH-quinone oxidoreductase of the inner/outer membranes): excitotoxicity, damage to lipids and proteins, apoptosis, ADP-ribosylation, injury to mitochondrial DNA, and impaired NO activity [11], [22], [45]. Cytoskeletal damage and lipid peroxidation are the other destructive effects of inflammation and severe oxidative stress due to cytokine storm [23], [45], [46]. Hence, the antioxidant character of pirfenidone makes it potent for the treatment of hyperimmune response [11], [22], [23], [30], [31].
Lipid peroxidation, which is initiated by generated superoxide in the cyclic reduction–oxidation is one of the mechanisms of cytokine storm-inflammation-oxidative stress end-organ-damage and pulmonary toxicity [11]. It has been shown that pirfenidone could inhibit NADPH dependent lipid peroxidation [22], [45].
Anti-apoptotic effects of pirfenidone
It has been shown that Fas-dependent alveolar apoptosis that results in inflammatory reaction and finally interstitial fibrosis is responsible for the battle against viruses and also responsible for sequels of infections such as Poxvirus, bacterial LPS, etc [35], [47]. On the other hand, it has been shown that pirfenidone could decrease apoptosis [19], [48], [49], [50], [51].
Down regulation of ACE receptor expression
ACE receptors are the major COVID-19-SARS virus receptor in humans. Trials that targeted the inhibition of these receptors with antibodies are under investigation [52]. Surprisingly, it has been shown that pirfenidone inhibits the AT1R/p38 MAPK pathway, decreased angiotensin-converting enzyme (ACE), angiotensin II, and angiotensin II type 1 receptor expression, and strongly enhanced liver X receptor-α expression [21]. This will not only protect cells from developing fibrosis (LXR-α) also by decreasing the ACE receptor expression decrease entrance of the COVID-19-SARS virus into cells.
With respect to the known characteristics of pirfenidone (anti-inflammatory, anti-fibrotic, antioxidant) and our current understanding of severe COVID-19 pathophysiology (cytokine storm, inflammation, probable fibrosis, hyper-immunity and as a result oxidative stress, it is rational to suggest pirfenidone application in the treatment of patients with moderate to severe COVID-19-SARS.
Evaluation of the hypothesis
Uncontrolled overreaction of the immune system to the virus leads to the release of numerous inflammatory cytokines, further superoxide production, ARDS development and subsequently matrix remodeling and overproduction of collagen and other matrix components that may cause fibrosis in survivors [25], [53], [54]. Cytokine storm, an uncontrolled immune reaction is responsible for the development of multi-organ damage and ARDS in patients with COVID-19-SARS [53].
Anti-inflammatory effects of pirfenidone have been shown in several animal studies and clinical trials. The antioxidant activity of pirfenidone has been verified in several experimental studies [20], [24], [25], [54], [32], [33], [34]. Furthermore, the anti-fibrotic effects of pirfenidone have been shown in several clinical trials and tend to FDA approval of this drug for the treatment of patients with IPF [14], [22], [55], [56], [57], [58].
Based on pirfenidone characteristics and therapeutic effects, I have previously suggested the treatment of paraquat poisoning with pirfenidone which is gradually opened its space in the treatment protocols of patients with paraquat poisoning [11], [59], [60], [61], [62]. Previously, Saha et al. successfully treated the patients with post H1N1 ARDS pulmonary fibrosis with combined pirfenidone, azithromycin, and prednisolone [63]. To the best of my knowledge, the mechanisms of post H1N1 ARDS fibrosis and paraquat poisoning and COVID-19 share similarities. Additionally, pirfenidone successfully improved treatment of post-H1N1 ARDS fibrosis, hence it seems equitable to evaluate the potential of pirfenidone in the treatment of COVID-19 [63]. Also, pirfenidone has been suggested and tried successfully in the treatment of ARDS due to white smoke-induced ARDS [11]. As another example, Zinc Chloride smoke (white smoke) inhalation induced severe ARDS has been successfully treated with a combination of pirfenidone and corticosteroids [35], [64].
Verification of the hypothesis
Pirfenidone has been approved by the FDA for the treatment of patients with IPF. It has been tolerated very well with trivial side effects [15], [65], [66].
The current situation enforced clinicians and agencies to relax strict preclinical approval and extensive experimentation before starting human experimental treatment and clinical trials. The fact that our hands are empty in the battle against COVID-19, and an urgent need for treatment, enforced us to try any possible probably safe treatment, and those approved medications with low side effects are among the suggested and tried medications. Actually, our current standard of care is based on these experiments.
Nevertheless, a limited number of labs have access to animal models of COVID-19-SARS and can conduct experimental studies parallel or before human trials. We have no time to wait for animal modeling, and animal models do not necessarily provide valid shreds of evidence in this case in terms of toxicity or efficacy of treatments, because mortality of this virus is almost always due to interaction of the virus with human immune system and animals are not appropriate surrogate models here [67].
At the end of the day, only a well-designed double-blind randomized controlled clinical trial is the accepted method to appropriately analyze this hypothesis.
Consequences of the hypothesis and conclusion
In a limited number of patients, COVID-19 present as a fulminant cytokine storm, ARDS, and end-organ damage. But the death toll of this limited number of patients surpassed a one and a half million recently. This is a human tragedy that calls for immediate intervention.
New therapeutic strategies are considered in the treatment of COVID-19. However, to the best of my knowledge, pirfenidone has not been tried yet. As discussed above, I believe that pirfenidone could be a safe add on to the current protocols of COVID-19 treatment, with trivial side effects and plenty of potential benefits.
During the reviewing process of this article, some other studies proposed similar point of view [68], [69], [70]. For example, parallel to what I discussed here, George et all, also pointed to the shared risk factors of COVID-19 and IPF, and mentioned that the burden of lung fibrosis following COVID-19 is likely to be high; they concluded that given the scale of the pandemic, the global burden of fibrotic lung disease will probably increase considerably.
They also suggested a therapeutic rationale for application of approved antifibrotic therapy in acute exacerbations of IPF. Pirfenidone and nintedanib are among them.
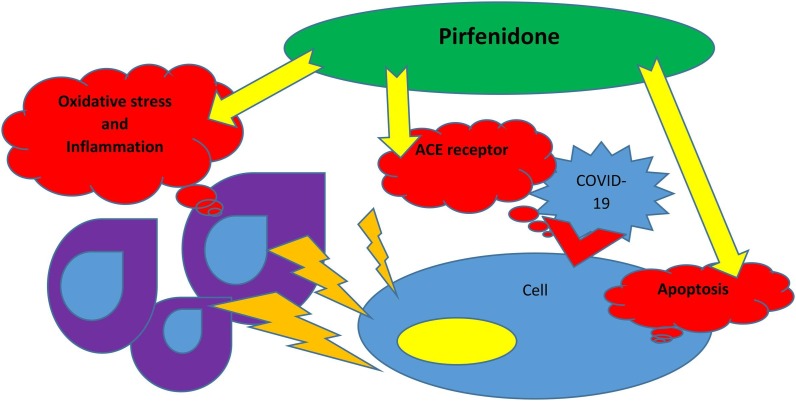
In conclusion, pirfenidone could inhibit apoptosis, downregulate ACE receptors expression, decrease inflammation by several mechanisms and ameliorate oxidative stress and hence protect pneumocytes and other cells from COVID-19 invasion and cytokine storm simultaneously (Fig. 1 ).
Fig. 1.
Pirfenidone could inhibit apoptosis, downregulate ACE receptors expression, decrease inflammation by several mechanisms and ameliorate oxidative stress and hence protect pneumocytes and other cells from the COVID-19 invasion and cytokine storm simultaneously.
Declaration of Competing Interest
The author declares that he have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
I would like to cordially thank all of my colleagues in the front line of battle against COVID-19, all brave health care works, nurses, clinicians, scientists and lab workers all around the world, particularly my colleagues in Beth Israel Deaconess Medical Center, Harvard Medical School, and Hackensack Meridian Health Mountainside Medical Center, namely Dr. Bijal S. Mehta, M.D., FACP.
Financial support
None.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.mehy.2020.110005.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Cascella M, Rajnik M, Cuomo A, Dulebohn SC, Di Napoli R. Features, Evaluation and Treatment Coronavirus (COVID-19). StatPearls. Treasure Island (FL)2020. [PubMed]
- 2.Lupia Tommaso, Scabini Silvia, Mornese Pinna Simone, Di Perri Giovanni, De Rosa Francesco Giuseppe, Corcione Silvia. 2019 novel coronavirus (2019-nCoV) outbreak: a new challenge. J Global Antimicrob Resist. 2020;21:22–27. doi: 10.1016/j.jgar.2020.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang G, Gong T, Wang G, Wang J, Guo X, Cai E, et al. Timely Diagnosis and Treatment Shortens the Time to Resolution of Coronavirus Disease (COVID-19) Pneumonia and Lowers the Highest and Last CT Scores From Sequential Chest CT. AJR American journal of roentgenology. 2020:1-7. [DOI] [PubMed]
- 4.Gautret P, Lagier JC, Parola P, Hoang VT, Meddeb L, Mailhe M, et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. International journal of antimicrobial agents. 2020:105949. [DOI] [PMC free article] [PubMed]
- 5.Schlagenhauf P, Grobusch MP, Maier JD, Gautret P. Repurposing antimalarials and other drugs for COVID-19. Travel medicine and infectious disease. 2020:101658. [DOI] [PMC free article] [PubMed]
- 6.Rosa SGV, Santos WC. Clinical trials on drug repositioning for COVID-19 treatment. Revista panamericana de salud publica = Pan American journal of public health. 2020;44:e40. [DOI] [PMC free article] [PubMed]
- 7.Bonovas S., Piovani D. Compassionate Use of Remdesivir in Covid-19. N England J Med. 2020;382 doi: 10.1056/NEJMc2015312. [DOI] [PubMed] [Google Scholar]
- 8.Al-Tawfiq JA, Al-Homoud AH, Memish ZA. Remdesivir as a possible therapeutic option for the COVID-19. Travel medicine and infectious disease. 2020;34:101615. [DOI] [PMC free article] [PubMed]
- 9.Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC, et al. Remdesivir for the Treatment of Covid-19 - Preliminary Report. The New England journal of medicine. 2020. [DOI] [PubMed]
- 10.Davies M, Osborne V, Lane S, Roy D, Dhanda S, Evans A, et al. Remdesivir in Treatment of COVID-19: A Systematic Benefit-Risk Assessment. Drug safety. 2020. [DOI] [PMC free article] [PubMed]
- 11.Seifirad Soroush, Keshavarz Amirhossein, Taslimi Shervin, Aran Shima, Abbasi Hamidreza, Ghaffari Alireza. Effect of pirfenidone on pulmonary fibrosis due to paraquat poisoning in rats. Clin Toxicol. 2012;50(8):754–758. doi: 10.3109/15563650.2012.718783. [DOI] [PubMed] [Google Scholar]
- 12.Tzouvelekis A, Karampitsakos T, Ntolios P, Tzilas V, Bouros E, Markozannes E, et al. Longitudinal “Real-World” Outcomes of Pirfenidone in Idiopathic Pulmonary Fibrosis in Greece. Frontiers in medicine. 2017;4:213. [DOI] [PMC free article] [PubMed]
- 13.Giri Shri N., Wang Qingjian, Xie Yan, Lango Jozsef, Morin Dexter, Margolin Solomon B., Buckpitt Alan R. Pharmacokinetics and metabolism of a novel antifibrotic drug pirfenidone, in mice following intravenous administration. Biopharm. Drug Dispos. 2002;23(5):203–211. doi: 10.1002/bdd.311. [DOI] [PubMed] [Google Scholar]
- 14.Taniguchi H., Ebina M., Kondoh Y., Ogura T., Azuma A., Suga M., Taguchi Y., Takahashi H., Nakata K., Sato A., Takeuchi M., Raghu G., Kudoh S., Nukiwa T. Pirfenidone in idiopathic pulmonary fibrosis. Eur Respir J. 2010;35(4):821–829. doi: 10.1183/09031936.00005209. [DOI] [PubMed] [Google Scholar]
- 15.Azuma Arata, Nukiwa Toshihiro, Tsuboi Eiyasu, Suga Moritaka, Abe Shosaku, Nakata Koichiro, Taguchi Yoshio, Nagai Sonoko, Itoh Harumi, Ohi Motoharu, Sato Atsuhiko, Kudoh Shoji. Double-blind, placebo-controlled trial of pirfenidone in patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2005;171(9):1040–1047. doi: 10.1164/rccm.200404-571OC. [DOI] [PubMed] [Google Scholar]
- 16.Lin X, Yu M, Wu K, Yuan H, Zhong H. Effects of pirfenidone on proliferation, migration, and collagen contraction of human Tenon's fibroblasts in vitro. Investigative ophthalmology & visual science. 2009;50(8):3763-70. [DOI] [PubMed]
- 17.Shihab Fuad S., Bennett William M., Yi Hong, Andoh Takeshi F. Pirfenidone treatment decreases transforming growth factor-beta1 and matrix proteins and ameliorates fibrosis in chronic cyclosporine nephrotoxicity. Am J Transplant. 2002;2(2):111–119. doi: 10.1034/j.1600-6143.2002.020201.x. [DOI] [PubMed] [Google Scholar]
- 18.Card J.W., Racz W.J., Brien J.F., Margolin S.B., Massey T.E. Differential effects of pirfenidone on acute pulmonary injury and ensuing fibrosis in the hamster model of amiodarone-induced pulmonary toxicity. Toxicol Sci. 2003;75(1):169–180. doi: 10.1093/toxsci/kfg167. [DOI] [PubMed] [Google Scholar]
- 19.Grattendick K.J., Nakashima J.M., Feng L., Giri S.N., Margolin S.B. Effects of three anti-TNF-alpha drugs: etanercept, infliximab and pirfenidone on release of TNF-alpha in medium and TNF-alpha associated with the cell in vitro. Int Immunopharmacol. 2008;8(5):679–687. doi: 10.1016/j.intimp.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 20.Cain W.C., Stuart R.W., Lefkowitz D.L., Starnes J.D., Margolin S., Lefkowitz S.S. Inhibition of tumor necrosis factor and subsequent endotoxin shock by pirfenidone. Int J Immunopharmacol. 1998;20(12):685–695. doi: 10.1016/s0192-0561(98)00042-3. [DOI] [PubMed] [Google Scholar]
- 21.Li C., Han R., Kang L., Wang J., Gao Y., Li Y. Pirfenidone controls the feedback loop of the AT1R/p38 MAPK/renin-angiotensin system axis by regulating liver X receptor-alpha in myocardial infarction-induced cardiac fibrosis. Sci Rep. 2017;7 doi: 10.1038/srep40523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fois AG, Posadino AM, Giordo R, Cossu A, Agouni A, Rizk NM, et al. Antioxidant Activity Mediates Pirfenidone Antifibrotic Effects in Human Pulmonary Vascular Smooth Muscle Cells Exposed to Sera of Idiopathic Pulmonary Fibrosis Patients. Oxidative medicine and cellular longevity. 2018;2018:2639081. [DOI] [PMC free article] [PubMed]
- 23.Giri S.N., Leonard S., Shi X., Margolin S.B., Vallyathan V. Effects of pirfenidone on the generation of reactive oxygen species in vitro. J Environ Pathol Toxicol Oncology. 1999;18(3):169–177. [PubMed] [Google Scholar]
- 24.Zhang C., Wu Z., Li J.W., Zhao H., Wang G.Q. The cytokine release syndrome (CRS) of severe COVID-19 and Interleukin-6 receptor (IL-6R) antagonist Tocilizumab may be the key to reduce the mortality. Int J Antimicrob Agents. 2020;105954 doi: 10.1016/j.ijantimicag.2020.105954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu D, Yang XO. TH17 responses in cytokine storm of COVID-19: An emerging target of JAK2 inhibitor Fedratinib. Journal of microbiology, immunology, and infection = Wei mian yu gan ran za zhi. 2020. [DOI] [PMC free article] [PubMed]
- 26.Gou FX, Zhang XS, Yao JX, Yu DS, Wei KF, Zhang H, et al. [Epidemiological characteristics of COVID-19 in Gansu province]. Zhonghua liu xing bing xue za zhi = Zhonghua liuxingbingxue zazhi. 2020;41(0):E032. [DOI] [PubMed]
- 27.Adhikari SP, Meng S, Wu YJ, Mao YP, Ye RX, Wang QZ, et al. Epidemiology, causes, clinical manifestation and diagnosis, prevention and control of coronavirus disease (COVID-19) during the early outbreak period: a scoping review. Infectious diseases of poverty. 2020;9(1):29. [DOI] [PMC free article] [PubMed]
- 28.Cui Y., Tian M., Huang D., Wang X., Huang Y., Fan L. A 55-Day-Old Female Infant infected with COVID 19: presenting with pneumonia, liver injury, and heart damage. J Infect Dis. 2020 doi: 10.1093/infdis/jiaa113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang Tianbing, Du Zhe, Zhu Fengxue, Cao Zhaolong, An Youzhong, Gao Yan, Jiang Baoguo. Comorbidities and multi-organ injuries in the treatment of COVID-19. Lancet. 2020;395(10228):e52. doi: 10.1016/S0140-6736(20)30558-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tanaka Ken-Ichiro, Azuma Arata, Miyazaki Yuri, Sato Keizo, Mizushima Tohru. Effects of lecithinized superoxide dismutase and/or pirfenidone against bleomycin-induced pulmonary fibrosis. Chest. 2012;142(4):1011–1019. doi: 10.1378/chest.11-2879. [DOI] [PubMed] [Google Scholar]
- 31.Ji Xu, Naito Yukiko, Weng Huachun, Ma Xiao, Endo Kosuke, Kito Naoko, Yanagawa Nariaki, Yu Yang, Li Jie, Iwai Naoharu. Renoprotective mechanisms of pirfenidone in hypertension-induced renal injury: through anti-fibrotic and anti-oxidative stress pathways. Biomed. Res. 2013;34(6):309–319. doi: 10.2220/biomedres.34.309. [DOI] [PubMed] [Google Scholar]
- 32.Poulin Braim Amy E., MacDonald Melinda H., Bruss Michael L., Grattendick Ken J., Giri Shri N., Margolin Solomon B. Effects of intravenous administration of pirfenidone on horses with experimentally induced endotoxemia. Am J Vet Res. 2009;70(8):1031–1042. doi: 10.2460/ajvr.70.8.1031. [DOI] [PubMed] [Google Scholar]
- 33.Shi Q, Liu X, Bai Y, Cui C, Li J, Li Y, et al. In vitro effects of pirfenidone on cardiac fibroblasts: proliferation, myofibroblast differentiation, migration and cytokine secretion. PloS one. 2011;6(11):e28134. [DOI] [PMC free article] [PubMed]
- 34.Hale Martha L., Margolin Solomon B., Krakauer Teresa, Roy Chad J., Stiles Bradley G. Pirfenidone blocks the in vitro and in vivo effects of staphylococcal enterotoxin B. IAI. 2002;70(6):2989–2994. doi: 10.1128/IAI.70.6.2989-2994.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li Yi, Li Haitao, Liu Shuai, Pan Pinhua, Su Xiaoli, Tan Hongyi, Wu Dongdong, Zhang Lemeng, Song Chao, Dai Minhui, Li Qian, Mao Zhi, Long Yuan, Hu Yongbin, Hu Chengping. Pirfenidone ameliorates lipopolysaccharide-induced pulmonary inflammation and fibrosis by blocking NLRP3 inflammasome activation. Mol Immunol. 2018;99:134–144. doi: 10.1016/j.molimm.2018.05.003. [DOI] [PubMed] [Google Scholar]
- 36.Wang Yongliang, Wu Yongquan, Chen Jiawei, Zhao Shumei, Li Hongwei. Pirfenidone attenuates cardiac fibrosis in a mouse model of TAC-induced left ventricular remodeling by suppressing nlrp3 inflammasome formation. Cardiology. 2013;126(1):1–11. doi: 10.1159/000351179. [DOI] [PubMed] [Google Scholar]
- 37.Conte E., Gili E., Fagone E., Fruciano M., Iemmolo M., Vancheri C. Effect of pirfenidone on proliferation, TGF-beta-induced myofibroblast differentiation and fibrogenic activity of primary human lung fibroblasts. Eur J Pharm Sci. 2014;58:13–19. doi: 10.1016/j.ejps.2014.02.014. [DOI] [PubMed] [Google Scholar]
- 38.Yang Y, Ye Y, Lin X, Wu K, Yu M. Inhibition of pirfenidone on TGF-beta2 induced proliferation, migration and epithlial-mesenchymal transition of human lens epithelial cells line SRA01/04. PloS one. 2013;8(2):e56837. [DOI] [PMC free article] [PubMed]
- 39.Li G., Ren J., Hu Q., Deng Y., Chen G., Guo K. Oral pirfenidone protects against fibrosis by inhibiting fibroblast proliferation and TGF-beta signaling in a murine colitis model. Biochem Pharmacol. 2016;117:57–67. doi: 10.1016/j.bcp.2016.08.002. [DOI] [PubMed] [Google Scholar]
- 40.Iyer S.N., Gurujeyalakshmi G., Giri S.N. Effects of pirfenidone on transforming growth factor-beta gene expression at the transcriptional level in bleomycin hamster model of lung fibrosis. J Pharmacol Exp Therap. 1999;291(1):367–373. [PubMed] [Google Scholar]
- 41.Knüppel Larissa, Ishikawa Yoshihiro, Aichler Michaela, Heinzelmann Katharina, Hatz Rudolf, Behr Jürgen, Walch Axel, Bächinger Hans Peter, Eickelberg Oliver, Staab-Weijnitz Claudia A. A novel antifibrotic mechanism of nintedanib and pirfenidone. inhibition of collagen fibril assembly. Am J Respir Cell Mol Biol. 2017;57(1):77–90. doi: 10.1165/rcmb.2016-0217OC. [DOI] [PubMed] [Google Scholar]
- 42.Iyer S.N., Gurujeyalakshmi G., Giri S.N. Effects of pirfenidone on procollagen gene expression at the transcriptional level in bleomycin hamster model of lung fibrosis. J Pharmacol Exp Therap. 1999;289(1):211–218. [PubMed] [Google Scholar]
- 43.Tzouvelekis A, Wolters PJ. Pirfenidone in the kaleidoscope: reflecting mechanisms through different angles. The European respiratory journal. 2018;52(5). [DOI] [PubMed]
- 44.Xie Y, Jiang H, Zhang Q, Mehrotra S, Abel PW, Toews ML, et al. Upregulation of RGS2: a new mechanism for pirfenidone amelioration of pulmonary fibrosis. Respiratory research. 2016;17(1):103. [DOI] [PMC free article] [PubMed]
- 45.Misra H.P., Rabideau C. Pirfenidone inhibits NADPH-dependent microsomal lipid peroxidation and scavenges hydroxyl radicals. Mol Cell Biochem. 2000;204(1–2):119–126. doi: 10.1023/a:1007023532508. [DOI] [PubMed] [Google Scholar]
- 46.Du Xiaogu, Schelegle Edward, Mohr F. Charles, Margolin Solomon B., Giri Shri N., Al-Bayati Mohammed Ali. Amelioration of doxorubicin-induced cardiac and renal toxicity by pirfenidone in rats. Cancer Chemother Pharmacol. 2004;53(2):141–150. doi: 10.1007/s00280-003-0703-z. [DOI] [PubMed] [Google Scholar]
- 47.Bień Karolina, Sokołowska Justyna, Bąska Piotr, Nowak Zuzanna, Stankiewicz Wanda, Krzyzowska Malgorzata. Fas/FasL Pathway Participates in regulation of antiviral and inflammatory response during mousepox infection of lungs. Mediators Inflamm. 2015;2015:1–13. doi: 10.1155/2015/281613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shihab Fuad S., Bennett William M., Yi Hong, Andoh Takeshi F. Effect of pirfenidone on apoptosis-regulatory genes in chronic cyclosporine nephrotoxicity. Transplantation. 2005;79(4):419–426. doi: 10.1097/01.tp.0000151721.99418.48. [DOI] [PubMed] [Google Scholar]
- 49.Chen K., Chen L., Ouyang Y., Zhang L., Li X., Li L. Pirfenidone attenuates homocysteineinduced apoptosis by regulating the connexin 43 pathway in H9C2 cells. Int J Mol Med. 2020;45(4):1081–1090. doi: 10.3892/ijmm.2020.4497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang J., Yang Y., Xu J., Lin X., Wu K., Yu M. Pirfenidone inhibits migration, differentiation, and proliferation of human retinal pigment epithelial cells in vitro. Mol Vision. 2013;19:2626–2635. [PMC free article] [PubMed] [Google Scholar]
- 51.Xiong Yong, Liu Yuan, Cao Liu, Wang Dehe, Guo Ming, Jiang Ao, Guo Dong, Hu Wenjia, Yang Jiayi, Tang Zhidong, Wu Honglong, Lin Yongquan, Zhang Meiyuan, Zhang Qi, Shi Mang, Liu Yingle, Zhou Yu, Lan Ke, Chen Yu. Transcriptomic characteristics of bronchoalveolar lavage fluid and peripheral blood mononuclear cells in COVID-19 patients. Emerg Microbes Infect. 2020;9(1):761–770. doi: 10.1080/22221751.2020.1747363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sun P., Lu X., Xu C., Wang Y., Sun W., Xi J. CD-sACE2 Inclusion Compounds: an effective treatment for corona virus disease 2019 (COVID-19) J Med Virol. 2020 doi: 10.1002/jmv.25804. [DOI] [PubMed] [Google Scholar]
- 53.Zhou Fei, Yu Ting, Du Ronghui, Fan Guohui, Liu Ying, Liu Zhibo, Xiang Jie, Wang Yeming, Song Bin, Gu Xiaoying, Guan Lulu, Wei Yuan, Li Hui, Wu Xudong, Xu Jiuyang, Tu Shengjin, Zhang Yi, Chen Hua, Cao Bin. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Luo Pan, Liu Yi, Qiu Lin, Liu Xiulan, Liu Dong, Li Juan. Tocilizumab treatment in COVID‐19: a single center experience. J Med Virol. 2020;92(7):814–818. doi: 10.1002/jmv.25801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ogura Takashi, Azuma Arata, Inoue Yoshikazu, Taniguchi Hiroyuki, Chida Kingo, Bando Masashi, Niimi Yuka, Kakutani Shinichi, Suga Moritaka, Sugiyama Yukihiko, Kudoh Shoji, Nukiwa Toshihiro. All-case post-marketing surveillance of 1371 patients treated with pirfenidone for idiopathic pulmonary fibrosis. Respir Investig. 2015;53(5):232–241. doi: 10.1016/j.resinv.2015.06.001. [DOI] [PubMed] [Google Scholar]
- 56.Bando Masashi, Yamauchi Hiroyoshi, Ogura Takashi, Taniguchi Hiroyuki, Watanabe Kentaro, Azuma Arata, Homma Sakae, Sugiyama Yukihiko, The Japan Pirfenidone Clinical Study Group Clinical experience of the long-term use of pirfenidone for idiopathic pulmonary fibrosis. Intern. Med. 2016;55(5):443–448. doi: 10.2169/internalmedicine.55.5272. [DOI] [PubMed] [Google Scholar]
- 57.Taguchi Yoshio, Ebina Masahito, Hashimoto Seishu, Ogura Takashi, Azuma Arata, Taniguchi Hiroyuki, Kondoh Yasuhiro, Suga Moritaka, Takahashi Hiroki, Nakata Koichiro, Sugiyama Yukihiko, Kudoh Shoji, Nukiwa Toshihiro. Efficacy of pirfenidone and disease severity of idiopathic pulmonary fibrosis: Extended analysis of phase III trial in Japan. Respir Investig. 2015;53(6):279–287. doi: 10.1016/j.resinv.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 58.Tzouvelekis Argyrios, Ntolios Paschalis, Karampitsakos Theodoros, Tzilas Vasilios, Anevlavis Stavros, Bouros Evangelos, Steiropoulos Paschalis, Koulouris Nikolaos, Stratakos Grigoris, Froudarakis Marios, Bouros Demosthenes. Safety and efficacy of pirfenidone in severe idiopathic pulmonary fibrosis: a real-world observational study. Pulm Pharmacol Ther. 2017;46:48–53. doi: 10.1016/j.pupt.2017.08.011. [DOI] [PubMed] [Google Scholar]
- 59.Rasooli Rokhsana, Pourgholamhosein Fatemeh, Kamali Younes, Nabipour Fatemeh, Mandegary Ali. Combination therapy with pirfenidone plus prednisolone ameliorates paraquat-induced pulmonary fibrosis. Inflammation. 2018;41(1):134–142. doi: 10.1007/s10753-017-0671-9. [DOI] [PubMed] [Google Scholar]
- 60.Pourgholamhossein Fateme, Rasooli Rokhsana, Pournamdari Mostafa, Pourgholi Leyla, Samareh-Fekri Mitra, Ghazi-Khansari Mahmoud, Iranpour Maryam, Poursalehi Hamid-Reza, Heidari Mahmoud-Reza, Mandegary Ali. Pirfenidone protects against paraquat-induced lung injury and fibrosis in mice by modulation of inflammation, oxidative stress, and gene expression. Food Chem Toxicol. 2018;112:39–46. doi: 10.1016/j.fct.2017.12.034. [DOI] [PubMed] [Google Scholar]
- 61.Blanco-Ayala T, Anderica-Romero AC, Pedraza-Chaverri J. New insights into antioxidant strategies against paraquat toxicity. Free radical research. 2014;48(6):623-40. [DOI] [PubMed]
- 62.Seifirad S. Pirfenidone could decrease paraquat-induced pulmonary fibrosis in rats. Tzu Chi Medical Journal. 2013;25(2):130.
- 63.Saha A., Vaidya P.J., Chavhan V.B., Achlerkar A., Leuppi J.D., Chhajed P.N. Combined pirfenidone, azithromycin and prednisolone in post-H1N1 ARDS pulmonary fibrosis. Sarcoidosis Vasculitis and Diffuse Lung Disease. 2018;35(1):85–90. doi: 10.36141/svdld.v35i1.6393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mahboob H, Richeson Iii R, McCain R. Zinc Chloride Smoke Inhalation Induced Severe Acute Respiratory Distress Syndrome: First Survival in the United States with Extended Duration (Five Weeks) Therapy with High Dose Corticosteroids in Combination with Lung Protective Ventilation. Case reports in critical care. 2017;2017:7952782. [DOI] [PMC free article] [PubMed]
- 65.Tzouvelekis A, Karampitsakos T, Ntolios P, Tzilas V, Bouros E, Markozannes E, et al. Corrigendum: Longitudinal “Real-World” Outcomes of Pirfenidone in Idiopathic Pulmonary Fibrosis in Greece. Frontiers in medicine. 2017;4:257. [DOI] [PMC free article] [PubMed]
- 66.Azuma A, Taguchi Y, Ogura T, Ebina M, Taniguchi H, Kondoh Y, et al. Exploratory analysis of a phase III trial of pirfenidone identifies a subpopulation of patients with idiopathic pulmonary fibrosis as benefiting from treatment. Respiratory research. 2011;12:143. [DOI] [PMC free article] [PubMed]
- 67.Seifirad S, Haghpanah V. Inappropriate modeling of chronic and complex disorders: How to reconsider the approach in the context of predictive, preventive and personalized medicine, and translational medicine. The EPMA journal. 2019;10(3):195-209. [DOI] [PMC free article] [PubMed]
- 68.George PM, Wells AU, Jenkins RG. Pulmonary fibrosis and COVID-19: the potential role for antifibrotic therapy. The Lancet Respiratory medicine. 2020. [DOI] [PMC free article] [PubMed]
- 69.Bian Y.Q., Ma J., Ren Y., Zhang Y.L., Qiao Y.J. [Discovery of intervention effect of Chinese herbal formulas on COVID-19 pulmonary fibrosis treated by VEGFR and FGFR inhibitors]. Zhongguo Zhong yao za zhi = Zhongguo zhongyao zazhi = China J Chin Mater Med. 2020;45(7):1481–1487. doi: 10.19540/j.cnki.cjcmm.20200315.401. [DOI] [PubMed] [Google Scholar]
- 70.Spagnolo P, Balestro E, Aliberti S, Cocconcelli E, Biondini D, Casa GD, et al. Pulmonary fibrosis secondary to COVID-19: a call to arms? The Lancet Respiratory medicine. 2020. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.