Abstract
In December 2019, many pneumonia cases with unidentified sources appeared in Wuhan, Hubei, China, with clinical symptoms like viral pneumonia. Deep sequencing analysis of samples from lower respiratory tract revealed a novel coronavirus, called 2019 novel coronavirus (2019-nCoV). Currently there is a rapid global spread. World Health Organization declare the disease a pandemic condition. The pathologic source of this disease was a new RNA virus from Coronaviridae family, which was named COVID-19. SARS-CoV-2 entry starts with the binding of the spike glycoprotein expressed on the viral envelope to ACE2 on the alveolar surface followed by clathrin-dependent endocytosis of the SARS-CoV-2 and ACE2 complex. SARS-CoV-2 enters the cells through endocytosis process, which is possibly facilitated, via a pH dependent endosomal cysteine protease cathepsins. Once inside the cells, SARS-CoV-2 exploits the endogenous transcriptional machinery of alveolar cells to replicate and spread through the entire lung. Endosomal acidic pH for SARS-CoV-2 processing and internalization is critical. After entering the cells, it possibly activates or hijack many intracellular pathways in favor of its replication. In the current opinion article, we will explain the possible involvement of unfolded protein response as a cellular stress response to the SARS-CoV-2 infection.
Keywords: Endoplasmic reticulum, Unfolded protein response, PERK, IRE1, Spliced XBP1
Opinion
Currently, there is no specific and effective antiviral therapy for covid-19 and patients are offered only supportive therapy (Chen et al., 2020). To curb the spread of the disease, measures aimed at controlling the potential sources of infection and early diagnosis are followed, while proper personal hygiene, supportive treatment and the clear publication of epidemic information are also recommended. From a public health perspective, there is an urgent need to find antiviral therapies and to develop an effective vaccine to stop or at least limit the pandemic caused by Covid-19. In this sense, working on the development of antivirals that target essential elements of viral replication cycle requires an intensive research.
The main function of the endoplasmic reticulum (ER) in eukaryotic cells is to synthesize and fold the transmembrane proteins (Ghavami et al., 2018; Iranpour et al., 2016; Yeganeh et al., 2013) and those that are going to be secreted and regulate secretome of the cells (Logue et al., 2018; Talty et al., 2019). However, entry of excess amount of proteins to the ER protein folding system disrupts the balance between protein synthesis demand and ER folding capacity, resulting in the accumulation of the unfolded proteins in the lumen of the organelle. Continued accumulation of the unfolded proteins in the ER lumen triggers ER stress response, which is initiated to help the organelle return to homeostasis (Almanza et al., 2019). The changes in the ER initiate the activation of signalling pathways known collectively as the unfolded protein response (UPR) (Almanza et al., 2019; Hombach-Klonisch et al., 2018; Yeganeh et al., 2015).
The UPR response is mediated by three ER transmembrane sensors: the protein kinase R (PKR)-like endoplasmic reticulum kinase (PERK), the activating transcriptional factor 6 (ATF6), and inositol-requiring enzyme 1 alpha (IRE1α) (Lee et al., 2003; Mehrbod et al., 2019; Shi et al., 1998; Szegezdi et al., 2006; Wang et al., 2000). Specifically, these sensors are transmembrane proteins with an intraluminal domain that recognizes unfolded proteins inside the ER, and a cytosolic domain that will transmit the signal and activate the subsequent response. UPR resolves ER stress by diverse mechanisms, to improve ER folding capacity by inducing the expression of molecular chaperones, a reduction in the global protein synthesis to reduce their influx into the ER and ER-associated degradation (ERAD) (REF). However, if the ER stress persists and is not resolved, UPR may lead to activation of signalling pathways that induce apoptosis (Ghavami et al., 2014; Szegezdi et al., 2006; Tabas and Ron, 2011).
The replication of coronaviruses takes place in the cytoplasm and is directly related to the ER. Diverse studies have shown that the replication of coronavirus induces ER stress and, consequently, UPR in infected cells. UPR modulates a wide range of signalling pathways that are essential to the cell function such as the mitogen-activated protein (MAP) kinase pathways, inflammatory responses, apoptosis, autophagy and innate immunity. Thus, it is not surprising that the existence of ER stress/UPR can significantly affect the patient's antiviral response and is a key factor in virus-host interaction (Fung and Liu, 2019; Ma et al., 2018; Shi et al., 2019; Zou et al., 2019).
ER stress has been evident in cells infected with various viruses, including: severe acute respiratory syndrome coronavirus (SARS-CoV), Murine hepatitis virus (MHV), avian infectious bronchitis virus (IBV) (Chan et al., 2006; Fung and Liu, 2019; Liao et al., 2013; Shi et al., 2019; Versteeg et al., 2007; Yeung et al., 2008). The likely mechanisms responsible for ER stress and induction of UPR response upon coronavirus infection is excessive synthesis, modification and folding of viral proteins; the severe restructuring of the ER membrane for the formation of double membrane vesicles (DMVs) for viral genome replication; and the exhaustion of the ER membrane due to continued formation of new virions and autophagy (Fung et al., 2014; Fung and Liu, 2014). Different coronaviruses have developed the ability to subvert or utilize certain aspects of UPR and overcome protein translation shutdown to benefit their own replication and pathogenesis by ensuring the production of viral proteins (Fung et al., 2016).
In one study, it was analysed in SARS-CoV how the early secretory pathway interacts with the induction of a reticulovesicular cytoplasmic network and the viral replication/transcription complex that is anchored to this network (Knoops et al., 2010). Treatment with brefeldin A, a drug that prevents the assembly of proteins of the Coatomer Protein I (COP–I) complex, hence disrupting the transport from the ER to the Golgi complex, partly inhibited reticulovesicular network formation and viral RNA synthesis, but did not completely block viral RNA synthesis. The authors conclude that a reduced level of reticulovesicular cytoplasmic network formation can be maintained in the presence of brefeldin A, suggesting that the early secretory pathway is unlikely to be intimately involved in coronavirus replication.
Another work reported that delayed brain tumour cell line infected by MHV responded with the activation of both IRE1 and ATF6 pathways evidenced by an IRE1-mediated splicing of XBP-1 (X-box binding protein 1) mRNA and the cleavage of ATF6 (Bechill et al., 2008). However, a reduced induction of downstream UPR target genes was observed. Ultimately, the virus alters the UPR, preventing the induction of UPR-responsive genes, which induces the blockage of protein synthesis in the host cell and favours the translation of viral proteins. This modified response would allow MHV to escape the innate defence cell signalling pathways during coronavirus replication. In yet another project, researchers investigated the capability of porcine epidemic diarrhoea virus (PEDV) infection to induce UPR in Vero cells (Wang et al., 2014). They have shown that, the presence of the virus in the cell induced a UPR, while the silencing of PERK by shRNA considerably increased virus loads in the cells. The treatment with an ER stress inducer, 2-deoxy-D-glucose (2-DG), reduced the degree of PEDV infection via altering viral protein translation during the early stage of virus infection and reducing the virus assembly. The antiviral effects of an small compound inhibitor, named K22 ((Z)-N-(3-(4-(4-bromophenyl)-4-hydroxypiperidin-1-yl)-3-oxo-1-phenylprop-1-en-2 yl)benzamide), that specifically targets this membrane-bound RNA replication step by blocking the formation of DMVs was assayed in primary human epithelia cultures (Lundin et al., 2014). K22 exerted strong anti-coronavirus activities, including SARS-CoV and MERS–CoV, during the early stages of infection through severe loss of DMV formation resulting in almost complete inhibition of RNA synthesis.
It has been reported that in severe cases of COVID-19, hypoxaemia is induced by the pneumonic process and might have several adverse effects in patients (Rello et al., 2020). There are several lines of defence mechanisms in response to hypoxia, which is a consequence of hypoxaemia, including responses triggers from mitochondria and ER (Bartoszewska and Collawn, 2020). The main goal of these responses to hypoxia is restoring oxygen level and promoting cell survival in these conditions (Bartoszewska and Collawn, 2020). Therefore, in prolonged hypoxia induction via COVID-19, the UPR is triggered to help the cells to survive. However, prolonged hypoxia would possibly drive UPR role from survival to death and apoptotic mode, which possibly is one of the causes of cellular and organ damage in COVID-19 cases.
Recent investigation has showed that IRE1 axis of UPR is involved in regulation of the secretome of the cells via production of spliced XBP (XBPs) (Logue et al., 2018). The investigators have developed a specific inhibitor, which competitively inhibits RNAase activity of IRE1 (Logue et al., 2018; Sanches et al., 2014) and showed that it inhibits secretome of the breast cancer cells (Logue et al., 2018). On the other hand, recent reports indicate that SARS-Coronavirus induces UPR through its Open Reading Frame-8b (ORF-8b). It also activates NLR Family Pyrin Domain Containing 3 (NLRP3) inflammasomes in macrophages which is involved in regulation of IL-1β and IL-18. ORF-8b also activates autophagy flux in the infected cells, which might be indirectly involved in regulation of cytokine processing in the infected cells (Shi et al., 2019). Therefore, targeting the RNAase activity of IRE1, could potentially be an ideal approach to modulate Covid-19 infection and pathogenesis via modulation of the secretome of macrophages.
Yet another study has showed that SARS-CoV activated PERK arm of UPR with subsequent increased phosphorylation of eukaryotic initiation factor 2 alpha (eIF2α). Activation of the PERK regulates innate immunity by suppression of type 1 interferon (IFN) signaling (Minakshi et al., 2009). Therefore, it suggests a potential role for UPR in attenuating IFN responses and innate immunity in Coronavirus infected cells. Hence, recently developed potent PERK inhibitors (GSK-PERK inhibitor) could also serve as potential therapeutics for controlling Covid 19 infection.
Several compounds can act as metal ionophores which diffuse through lipid membranes as they transport metal ions between extracellular and intracellular spaces and between different cellular compartments. Ionophore-metal complexes passes across lipid vesicle membranes, becoming protonated upon entry which triggers the release of the bound metal inside the cellular organelle. This interferes with the acidification of the lysosomal compartment and affects autophagy, leading to the release of degradative enzymes into the cytosol upon disruption of lysosomes, triggering stress and subsequent apoptosis. In Rous sarcoma virus (RSV), metal ionophores (8-Hydroxyquinoline and several of its derivatives) can inhibit proteasome with subsequent disruption of viral replication processes through the inhibition of Ribonucleic Acid-Dependent Deoxyribonucleic Acid Polymerase (Rohde et al., 1976). Zinc (Zn) ionophores can exert their effects on both autophagy (Rohde et al., 1976) and proteasome (Te Velthuis et al., 2010) with their effects being extensively evaluated in cancer (Ding and Lind, 2009) and HIV infection (Lee et al., 2019). The observed effects were due to the inhibition of the RNA-dependent RNA polymerase elongation and template binding. Usage of Zinc and Zinc ionophores may enhance the therapeutic effects of targeting autophagy and replication in COVID-19 by several compounds which along pyrithione include dithiocarbamates, disulfiram and quinoline derivatives such as clioquinol. Since Zn and Zn ionophores interact with both the autophagy machinery and the replicative apparatus in SARS-CoV, such interactions likely involve ER stress and UPR induction.
In summary SARS-CoV-2 can possibly activate UPR and hijack this pathway for the benefit of its own infection process. In Scheme 1 a brief summary of the possible role of autophagy and UPR in SARS-CoV-2 infection is summarized. Considering that the replication of coronaviruses causes ER stress and induces UPR in the infected cells, additional research on coronavirus-induced UPR could help identify new targets for antiviral agents and facilitate the development of effective vaccines against covid-19. Interfering with or manipulating the coronavirus-induced UPR may provide new therapeutic targets that contribute to infection control and the pathogenesis of this emergent coronavirus.
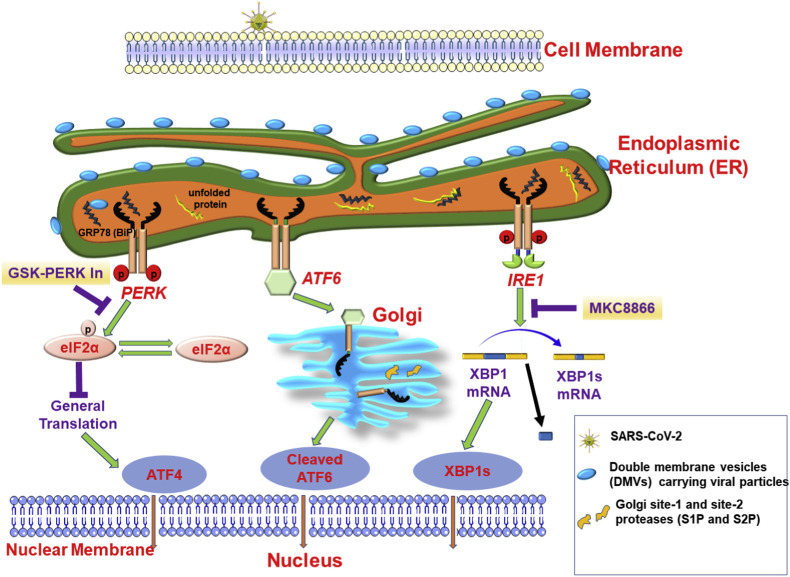
Scheme 1.
SARS-CoV-2 spike protein interacts with the lung epithelial cells through Angiotensin-Converting Enzyme 2 (ACE2) and being internalized to the cells via endocytosis. SARS-CoV-2 uses intracellular trafficking and double membrane vesicles (DMVs) for replication. During the process of viral replication possibly DMVs carrying viral particles interacts with ER chaperons (ATF6 = activating transcription factor 6; IRE1 = inositol requiring enzyme-1; PERK = PKR-like endoplasmic reticulum kinase; GRP78(BiP) = binding immunoglobulin protein). and initiates unfolded protein response (UPR). In addition, the protein load to the ER protein synthesis machinery during viral protein synthesis can possibly induce production of unfolded protein and initiate UPR during SARS-CoV2 infection. Inhibitors of the PERK (GSK-PERK inhibitor) and IRE1 RNAase activity (MKC8866) can regulate the UPR response in infected cells through modulating the innate immunity and the cellular secretome, respectively. Therefore, these inhibitors hold promise as potential therapeutics for controlling Covid-19 infection. [eIF2α = eukaryotic translation initiation factor 2 alpha; XBP1s = spliced X-box binding protein 1].
Author agreement
Hereby, all authors agree the publication of the article by European Journal of Pharmacology.
CRediT authorship contribution statement
Antoni Sureda: Writing - original draft. Javad Alizadeh: Writing - original draft, participation in revision Seyed Fazel Nabavi: Writing - original draft. Ioana Berindan Neagoe: Writing - original draft. Cosmin Andrei Cismaru: Writing - original draft. Philippe Jeandet: Writing - original draft. Marek J. Los: Writing - original draft, Writing - original draft. Emilio Clementi: Writing - original draft. Seyed Mohammad Nabavi: Writing - original draft. Saeid Ghavami: Writing - original draft, revision.
Acknowledgements
A. Sureda was supported by the Institute of Health Carlos III (CIBEROBN CB12/03/30038).
Contributor Information
Marek J. Łos, Email: mjelos@gmail.com.
Seyed Mohammad Nabavi, Email: Nabavi208@gmail.com.
Saeid Ghavami, Email: saeid.ghavami@umanitoba.ca.
References
- Almanza A., Carlesso A., Chintha C., Creedican S., Doultsinos D., Leuzzi B., Luís A., McCarthy N., Montibeller L., More S. Endoplasmic reticulum stress signalling–from basic mechanisms to clinical applications. FEBS J. 2019;286:241–278. doi: 10.1111/febs.14608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartoszewska S., Collawn J.F. Unfolded protein response (UPR) integrated signaling networks determine cell fate during hypoxia. Cell. Mol. Biol. Lett. 2020;25:18. doi: 10.1186/s11658-020-00212-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechill J., Chen Z., Brewer J.W., Baker S.C. Coronavirus infection modulates the unfolded protein response and mediates sustained translational repression. J. Virol. 2008;82:4492–4501. doi: 10.1128/JVI.00017-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan C.-P., Siu K.-L., Chin K.-T., Yuen K.-Y., Zheng B., Jin D.-Y. Modulation of the unfolded protein response by the severe acute respiratory syndrome coronavirus spike protein. J. Virol. 2006;80:9279–9287. doi: 10.1128/JVI.00659-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Liu Q., Guo D. Emerging coronaviruses: genome structure, replication, and pathogenesis. J. Med. Virol. 2020;92(4):418–423. doi: 10.1002/jmv.25681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding W.Q., Lind S.E. Metal ionophores–an emerging class of anticancer drugs. IUBMB Life. 2009;61:1013–1018. doi: 10.1002/iub.253. [DOI] [PubMed] [Google Scholar]
- Fung T.S., Huang M., Liu D.X. Coronavirus-induced ER stress response and its involvement in regulation of coronavirus–host interactions. Virus Res. 2014;194:110–123. doi: 10.1016/j.virusres.2014.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung T.S., Liao Y., Liu D.X. Regulation of stress responses and translational control by coronavirus. Viruses. 2016;8(7):184. doi: 10.3390/v8070184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung T.S., Liu D.X. Coronavirus infection, ER stress, apoptosis and innate immunity. Front. Microbiol. 2014;5:296. doi: 10.3389/fmicb.2014.00296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung T.S., Liu D.X. The ER stress sensor IRE1 and MAP kinase ERK modulate autophagy induction in cells infected with coronavirus infectious bronchitis virus. Virology. 2019;533:34–44. doi: 10.1016/j.virol.2019.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghavami S., Sharma P., Yeganeh B., Ojo O.O., Jha A., Mutawe M.M., Kashani H.H., Los M.J., Klonisch T., Unruh H., Halayko A.J. Airway mesenchymal cell death by mevalonate cascade inhibition: integration of autophagy, unfolded protein response and apoptosis focusing on Bcl2 family proteins. Biochim. Biophys. Acta. 2014;1843:1259–1271. doi: 10.1016/j.bbamcr.2014.03.006. [DOI] [PubMed] [Google Scholar]
- Ghavami S., Yeganeh B., Zeki A.A., Shojaei S., Kenyon N.J., Ott S., Samali A., Patterson J., Alizadeh J., Moghadam A.R. Autophagy and the unfolded protein response promote profibrotic effects of TGF-β1 in human lung fibroblasts. Am. J. Physiol. Lung Cell Mol. Physiol. 2018;314:L493–L504. doi: 10.1152/ajplung.00372.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hombach-Klonisch S., Mehrpour M., Shojaei S., Harlos C., Pitz M., Hamai A., Siemianowicz K., Likus W., Wiechec E., Toyota B.D. Glioblastoma and chemoresistance to alkylating agents: involvement of apoptosis, autophagy, and unfolded protein response. Pharmacol. Therapeut. 2018;184:13–41. doi: 10.1016/j.pharmthera.2017.10.017. [DOI] [PubMed] [Google Scholar]
- Iranpour M., Moghadam A.R., Yazdi M., Ande S.R., Alizadeh J., Wiechec E., Lindsay R., Drebot M., Coombs K.M., Ghavami S. Apoptosis, autophagy and unfolded protein response pathways in Arbovirus replication and pathogenesis. Expet Rev. Mol. Med. 2016;18 doi: 10.1017/erm.2015.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoops K., Swett-Tapia C., van den Worm S.H., Te Velthuis A.J., Koster A.J., Mommaas A.M., Snijder E.J., Kikkert M. Integrity of the early secretory pathway promotes, but is not required for, severe acute respiratory syndrome coronavirus RNA synthesis and virus-induced remodeling of endoplasmic reticulum membranes. J. Virol. 2010;84:833–846. doi: 10.1128/JVI.01826-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee A.-H., Iwakoshi N.N., Glimcher L.H. XBP-1 regulates a subset of endoplasmic reticulum resident chaperone genes in the unfolded protein response. Mol. Cell. Biol. 2003;23:7448–7459. doi: 10.1128/MCB.23.21.7448-7459.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.A., Elliott J.H., McMahon J., Hartogenesis W., Bumpus N.N., Lifson J.D., Gorelick R.J., Bacchetti P., Deeks S.G., Lewin S.R. Population pharmacokinetics and pharmacodynamics of disulfiram on inducing latent HIV‐1 transcription in a phase IIb trial. Clin. Pharmacol. Therapeut. 2019;105:692–702. doi: 10.1002/cpt.1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao Y., Fung T.S., Huang M., Fang S.G., Zhong Y., Liu D.X. Upregulation of CHOP/GADD153 during coronavirus infectious bronchitis virus infection modulates apoptosis by restricting activation of the extracellular signal-regulated kinase pathway. J. Virol. 2013;87:8124–8134. doi: 10.1128/JVI.00626-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logue S.E., McGrath E.P., Cleary P., Greene S., Mnich K., Almanza A., Chevet E., Dwyer R.M., Oommen A., Legembre P. Inhibition of IRE1 RNase activity modulates the tumor cell secretome and enhances response to chemotherapy. Nat. Commun. 2018;9:1–14. doi: 10.1038/s41467-018-05763-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundin A., Dijkman R., Bergström T., Kann N., Adamiak B., Hannoun C., Kindler E., Jonsdottir H.R., Muth D., Kint J. Targeting membrane-bound viral RNA synthesis reveals potent inhibition of diverse coronaviruses including the middle East respiratory syndrome virus. PLoS Pathog. 2014;10 doi: 10.1371/journal.ppat.1004166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y., Wang C., Xue M., Fu F., Zhang X., Li L., Yin L., Xu W., Feng L., Liu P. The coronavirus transmissible gastroenteritis virus evades the type I interferon response through IRE1α-mediated manipulation of the microRNA miR-30a-5p/SOCS1/3 axis. J. Virol. 2018;92 doi: 10.1128/JVI.00728-18. e00728-00718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehrbod P., Ande S.R., Alizadeh J., Rahimizadeh S., Shariati A., Malek H., Hashemi M., Glover K.K.M., Sher A.A., Coombs K.M., Ghavami S. The roles of apoptosis, autophagy and unfolded protein response in arbovirus, influenza virus, and HIV infections. Virulence. 2019;10:376–413. doi: 10.1080/21505594.2019.1605803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minakshi R., Padhan K., Rani M., Khan N., Ahmad F., Jameel S. The SARS Coronavirus 3a protein causes endoplasmic reticulum stress and induces ligand-independent downregulation of the type 1 interferon receptor. PloS One. 2009;4 doi: 10.1371/journal.pone.0008342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rello J., Storti E., Belliato M., Serrano R. Clinical phenotypes of SARS-CoV-2: implications for clinicians and researchers. Eur. Respir. J. 2020;55(5):1–4. doi: 10.1183/13993003.01028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohde W., Mikelens P., Jackson J., Blackman J., Whitcher J., Levinson W. Hydroxyquinolines inhibit ribonucleic acid-dependent deoxyribonucleic acid polymerase and inactivate Rous sarcoma virus and herpes simplex virus. Antimicrob. Agents Chemother. 1976;10:234–240. doi: 10.1128/aac.10.2.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanches M., Duffy N.M., Talukdar M., Thevakumaran N., Chiovitti D., Canny M.D., Lee K., Kurinov I., Uehling D., Al-Awar R. Structure and mechanism of action of the hydroxy–aryl–aldehyde class of IRE1 endoribonuclease inhibitors. Nat. Commun. 2014;5:1–16. doi: 10.1038/ncomms5202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi C.-S., Nabar N.R., Huang N.-N., Kehrl J.H. SARS-Coronavirus Open Reading Frame-8b triggers intracellular stress pathways and activates NLRP3 inflammasomes. Cell Death Discov. 2019;5:1–12. doi: 10.1038/s41420-019-0181-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y., Vattem K.M., Sood R., An J., Liang J., Stramm L., Wek R.C. Identification and characterization of pancreatic eukaryotic initiation factor 2 α-subunit kinase, PEK, involved in translational control. Mol. Cell. Biol. 1998;18:7499–7509. doi: 10.1128/mcb.18.12.7499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szegezdi E., Logue S.E., Gorman A.M., Samali A. Mediators of endoplasmic reticulum stress‐induced apoptosis. EMBO Rep. 2006;7:880–885. doi: 10.1038/sj.embor.7400779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabas I., Ron D. Integrating the mechanisms of apoptosis induced by endoplasmic reticulum stress. Nat. Cell Biol. 2011;13:184–190. doi: 10.1038/ncb0311-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talty A., Deegan S., Ljujic M., Mnich K., Naicker S.D., Quandt D., Zeng Q., Patterson J.B., Gorman A.M., Griffin M.D. Inhibition of IRE1α RNase activity reduces NLRP3 inflammasome assembly and processing of pro-IL1β. Cell Death Dis. 2019;10:1–11. doi: 10.1038/s41419-019-1847-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Te Velthuis A.J., van den Worm S.H., Sims A.C., Baric R.S., Snijder E.J., van Hemert M.J. Zn2+ inhibits coronavirus and arterivirus RNA polymerase activity in vitro and zinc ionophores block the replication of these viruses in cell culture. PLoS Pathog. 2010;6 doi: 10.1371/journal.ppat.1001176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Versteeg G.A., van de Nes P.S., Bredenbeek P.J., Spaan W.J. The coronavirus spike protein induces endoplasmic reticulum stress and upregulation of intracellular chemokine mRNA concentrations. J. Virol. 2007;81:10981–10990. doi: 10.1128/JVI.01033-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Li J.-r., Sun M.-x., Ni B., Huan C., Huang L., Li C., Fan H.-j., Ren X.-f., Mao X. Triggering unfolded protein response by 2-deoxy-d-glucose inhibits porcine epidemic diarrhea virus propagation. Antivir. Res. 2014;106:33–41. doi: 10.1016/j.antiviral.2014.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Shen J., Arenzana N., Tirasophon W., Kaufman R.J., Prywes R. Activation of ATF6 and an ATF6 DNA binding site by the endoplasmic reticulum stress response. J. Biol. Chem. 2000;275:27013–27020. doi: 10.1074/jbc.M003322200. [DOI] [PubMed] [Google Scholar]
- Yeganeh B., Moghadam A.R., Alizadeh J., Wiechec E., Alavian S.M., Hashemi M., Geramizadeh B., Samali A., Lankarani K.B., Post M. Hepatitis B and C virus-induced hepatitis: apoptosis, autophagy, and unfolded protein response. World J. Gastroenterol. 2015;21:13225. doi: 10.3748/wjg.v21.i47.13225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeganeh B., Moghadam A.R., Tran A.T., Rahim M.N., Ande S.R., Hashemi M., Coombs K.M., Ghavami S. Asthma and influenza virus infection: focusing on cell death and stress pathways in influenza virus replication. Iran. J. Allergy, Asthma Immunol. 2013:1–17. [PubMed] [Google Scholar]
- Yeung Y.-S., Yip C.-W., Hon C.-C., Chow K.Y., Ma I.C., Zeng F., Leung F.C. Transcriptional profiling of Vero E6 cells over-expressing SARS-CoV S2 subunit: insights on viral regulation of apoptosis and proliferation. Virology. 2008;371:32–43. doi: 10.1016/j.virol.2007.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou D., Xu J., Duan X., Xu X., Li P., Cheng L., Zheng L., Li X., Zhang Y., Wang X. Porcine epidemic diarrhea virus ORF3 protein causes endoplasmic reticulum stress to facilitate autophagy. Vet. Microbiol. 2019;235:209–219. doi: 10.1016/j.vetmic.2019.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]