Abstract
Background
Patients with lung cancers may have disproportionately severe coronavirus disease 2019 (COVID-19) outcomes. Understanding the patient-specific and cancer-specific features that impact the severity of COVID-19 may inform optimal cancer care during this pandemic.
Patients and methods
We examined consecutive patients with lung cancer and confirmed diagnosis of COVID-19 (n = 102) at a single center from 12 March 2020 to 6 May 2020. Thresholds of severity were defined a priori as hospitalization, intensive care unit/intubation/do not intubate ([ICU/intubation/DNI] a composite metric of severe disease), or death. Recovery was defined as >14 days from COVID-19 test and >3 days since symptom resolution. Human leukocyte antigen (HLA) alleles were inferred from MSK-IMPACT (n = 46) and compared with controls with lung cancer and no known non-COVID-19 (n = 5166).
Results
COVID-19 was severe in patients with lung cancer (62% hospitalized, 25% died). Although severe, COVID-19 accounted for a minority of overall lung cancer deaths during the pandemic (11% overall). Determinants of COVID-19 severity were largely patient-specific features, including smoking status and chronic obstructive pulmonary disease [odds ratio for severe COVID-19 2.9, 95% confidence interval 1.07–9.44 comparing the median (23.5 pack-years) to never-smoker and 3.87, 95% confidence interval 1.35–9.68, respectively]. Cancer-specific features, including prior thoracic surgery/radiation and recent systemic therapies did not impact severity. Human leukocyte antigen supertypes were generally similar in mild or severe cases of COVID-19 compared with non-COVID-19 controls. Most patients recovered from COVID-19, including 25% patients initially requiring intubation. Among hospitalized patients, hydroxychloroquine did not improve COVID-19 outcomes.
Conclusion
COVID-19 is associated with high burden of severity in patients with lung cancer. Patient-specific features, rather than cancer-specific features or treatments, are the greatest determinants of severity.
Key words: chemotherapy, COVID-19, immunotherapy/checkpoint blockade, lung cancer, small molecule agents
Introduction
Patients with cancers, particularly those with lung cancers, have been reported by multiple series to have disproportionally increased severity outcomes from coronavirus disease 2019 (COVID-19), including higher rates of hospitalization and death.1, 2, 3, 4 It is unknown whether lung cancer itself or other pre-existing factors such as age, genetic variation in immunity, smoking history, underlying cardiopulmonary disease, and/or cancer-directed treatments predisposes an individual to significant symptoms of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. We previously explored the impact of programmed cell death protein 1 (PD-1) blockade therapy on COVID-19 severity and did not find a clinically meaningful signal.5 No population cohort to date has had sufficient detail and follow-up to address these issues or to characterize recovery from COVID-19. We hypothesized that a deeply annotated analysis of the experience of patients with lung cancers and COVID-19 from a single center in New York City, one of the epicenters of COVID-19 worldwide, would help address these ongoing issues to provide guidance and insight regarding both COVID-19 and cancer care in real-time during this pandemic.
Methods
Ethics approval
This retrospective study was approved by the Institutional Review Board at Memorial Sloan Kettering Cancer Center (MSK) (protocol 20-142), which granted a waiver of informed consent.
Patients
Our study population included all patients with a diagnosis of lung cancer being treated at MSK who had a positive SARS-CoV-2 RT-PCR test between the first case identified on 12 March 2020 through 6 May 2020. Patients were followed through 11 May 2020. We used several data sources to identify patients including International Classification of Diseases diagnosis codes, pathology reports, institutional databases, and survey of physicians in the Thoracic Oncology Service at MSK. Patients with suspected but unconfirmed COVID-19, or patients already receiving hospice care alone at the time of diagnosis of COVID-19, or who did not have any information detailing their history, disposition, or vital status after the positive test were excluded. Overall, we identified 102 patients for this analysis. Patients with known COVID-19 diagnosis were included irrespective of whether COVID-19 was diagnosed at MSK (n = 61) or other health care facilities (n = 41).
Patient records were manually reviewed to identify demographics, prior smoking history, baseline clinical characteristics, comorbid conditions, pathology characteristics, treatments, symptoms, laboratory values, disease course, and vital status. Smoking history was collected based on a detailed self-reporting survey. Additional details were manually reviewed in the medical history. Molecular testing results were obtained through institutional databases. Medications were obtained through pharmacy records. Baseline laboratory values included complete blood count, serum creatinine, liver function tests, and inflammatory/injury markers obtained on the day of the positive test. If no blood tests were obtained on the date of the SARS-CoV-2 test, we used the value the day before or the day after. Patients who were readmitted for COVID-19 symptoms after their initial COVID-19 hospitalization were coded as a single hospitalization course. Extubation was defined as independence from invasive mechanical ventilator support. Extracted results were entered into a clinical data form for subsequent analysis.
Data elements extracted from the medical records were predefined by clinicians and researchers JL, HR, and MDH after reviewing existing literature on COVID-19. Data was manually abstracted by JL, HR, JVE, IP, and MDH, each trained clinicians or researchers skilled at clinical data abstraction. Abstraction results were spot checked independently by a second individual in the research team for reliability.
To examine the proportion of deaths among patients with lung cancer at our institution which were attributable to COVID-19, we used institutional databases to identify all patients with a diagnosis of lung cancer who died over the same time period of our primary analysis cohort. Abstraction results were examined by HR and MDH for reliability.
To examine the association of human leukocyte antigen (HLA) genotype with COVID-19 severity, we identified a control group using institutional databases to identify patients with lung cancers who were not known to have COVID-19 and who had MSK-IMPACT next generation sequencing in order to infer imputed class I HLA allele supertypes.
To examine the impact of cancer therapy on the severity of COVID-19, we defined exposure to prior programmed cell death protein 1/programmed cell deathligand 1 (PD-(L)1) blockade as most recent dose within 6 weeks of positive SARS-CoV-2 RT-PCR test, exposure to prior chemotherapy plus PD-(L)1 blockade as most recent dose within 3 weeks of positive SARS-CoV-2 RT-PCR test, exposure to prior chemotherapy alone as most recent dose within 3 weeks of positive SARS-CoV-2 RT-PCR test, and exposure to prior tyrosine kinase inhibitor as most recent dose within 1 week of positive SARS-CoV-2 RT-PCR test.
Study outcomes
Outcomes of interest included dates of hospitalization, admission to intensive care unit (ICU), intubation and invasive mechanical ventilation, transition to do not intubate (DNI) (specifically in place of otherwise urgent intensification of care and/or intervention such as intubation for treatment of hypoxic respiratory failure), death (at home or inpatient), and recovery. Disposition included discharge date. Status of the patient on the date of the database lock (recovered, improving, pending, or died) was determined by clinicians reviewing all available hospital records leading up to the date of the database lock (11 May 2020). For patients being treated at MSK who were admitted to outside hospitals for care of COVID-19 (n = 30), details of hospital course and severity outcomes were obtained when possible by review of existing outside hospital records and from personal communication with primary oncologists [n = 1 unknown highest level of care (ICU versus non-ICU hospital floor), status known for all other patients]. Patients who were in a health care facility at the time of diagnosis of COVID-19 and thereafter transitioned to hospice (n = 2) were included in the ‘hospitalization’ analysis. Patients with unknown status for a given severity outcome (n = 1 for ICU/intubation/DNI) were coded as unknown and removed from analysis for that outcome.
Recovered was defined as a combination of (i) 14 days post negative swab per the institution standard and (ii) at least 3 days since resolution of COVID-19 symptoms per Centers for Disease Control and Prevention (CDC) guidelines. Improving was defined as existence of a preponderance of evidence in the medical record (dates of disease course, notes, vital signs, and laboratory values) the patient was steadily improving with a trajectory toward recovery from COVID-19. The status of all other patients who had not died was categorized as pending.
HLA analysis
Of the 102 patients, 46 had MSK-IMPACT next generation sequencing data to infer imputed HLA-A and HLA-B alleles. Each cohort's patients' HLA-A and HLA-B type were categorized into 12 supertypes, as previously defined using anchor residue specificity similarities6; other HLA-A and HLA-B types that were not categorizable into one of these supertypes were included in a separate miscellaneous group called ‘Other A,B’. Patients were classified as having severe COVID-19 if they met criteria for ICU/intubation/DNI and/or died. All others were classified as having mild COVID-19.
HLA-A and HLA-B types were available for 5166 control patients with lung cancers and no known diagnosis of COVID-19; HLA-A and B types were categorized into supertypes as above. The control group was used to establish a null distribution. We examined if there were differences in the mild and severe cohorts as compared with the control group. All comparisons were two-sided and Bonferroni corrected for multiple hypothesis testing.
Statistical analysis
Briefly, our pre-planned primary analysis was to evaluate the impact of baseline characteristics of interest on the severity and recovery of COVID-19 in patients with lung cancer. We used literature review of COVID-19 publications and directed acyclic graphs to identify relationships between baseline features of interest and examined features that may directly impact COVID-19 infection severity and recovery.
COVID-19 severity outcomes of interest were defined a priori as: hospitalization, death, and a composite metric of severe disease (ICU stay, intubation and invasive mechanical ventilation, and/or transition to DNI). We calculated odds ratios (ORs) using a univariate logistic regression model to examine baseline characteristics and treatments that could affect COVID-19 severity. Logistic regression was chosen as the method of analysis because (i) our outcomes of interest were binary (e.g. death or not) rather than time to event and (ii) we had prolonged follow-up on patients (median 25 days), with the final status of COVID-19 in our patients known for 89%. Age, pack-years smoked, and body mass index were treated as continuous variables. Given the plateauing effects of pack-years smoked, this variable was a priori log10 transformed.
We carried out descriptive statistics characterizing the disease course of COVID-19 in this population of patients with lung cancer. Survival and recovery analyses were estimated using the Kaplan–Meier method. For the survival analysis, patients were censored at the date of last contact before the end of the follow-up period. The recovery analysis was described by a cumulative incidence function with competing events being death and recovery from COVID-19. Fisher's exact test and the Mann–Whitney U test were used to calculate P values. All 95% confidence interval (CI) estimates reflect an alpha level of 0.025 in each tail. The 95% CI for ORs were calculated using the Baptista–Pike method. Statistical analyses were carried out using Python 3.7.3 (Python Software Foundation, https://www.python.org/) using matplotlib, Prism 8.4.2 (GraphPad Software, La Jolla, CA), and R 3.6.2 (R foundation for Statistical Computing, Vienna, Austria) using GLM.
Results
We identified 102 consecutive patients with lung cancers and a SARS-CoV-2-positive swab between 12 March 2020 and 6 May 2020 (Table 1 ). Patients were followed until 11 May 2020, inclusive of updated follow-up on patients previously reported.5 Median follow-up was 25 days (interquartile range 10–36 days). The median age was 68 years old (range 31–91 years old). Most patients had metastatic or active lung cancer (72%, n = 73/102), and the median pack-year smoking history was 23.5 (range 0–120 pack-years). Common chronic conditions included hypertension (56%, n = 57/102) and chronic obstructive pulmonary disease (COPD, 24%, n = 24/102).
Table 1.
Baseline characteristics and clinical course of patients with lung cancers and + severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) test
| Patients characteristics | Patients (n = 102) no./total no.a (%) |
|---|---|
| Age | |
| Median (IQR), years | 68 (61–75) |
| Sex | |
| Female | 53/102 (52) |
| Male | 49/102 (48) |
| Race, patient reported | |
| White | 64/102 (63) |
| Black | 16/102 (16) |
| Asian | 13/102 (13) |
| Other | 3/102 (3) |
| Unknown | 6/102 (6) |
| Ethnicity, patient reported | |
| Hispanic or Latino | 9/102 (9) |
| Non-Hispanic or Latino | 85/102 (83) |
| Unknown | 8/102 (8) |
| Prior smoking history | |
| Median (range) – pack-year | 24 (0–120) |
| ≥5 pack-years | 27/102 (27) |
| Lung cancer specific features | |
| Non-small-cell lung cancer | 94/102 (92) |
| Small-cell lung cancer | 7/102 (7) |
| Metastatic or active lung cancerb | 73/102 (72) |
| Prior thoracic surgery or radiation therapy | 54/102 (53) |
| Comorbid conditions | |
| COPDc | 24/102 (24) |
| Non-COPD lung diseased | 28/102 (28) |
| Obesity (BMI ≥ 30) | 30/102 (29) |
| Hypertension | 57/102 (56) |
| Congestive heart failuree | 7/102 (7) |
| Diabetes mellitus | 27/102 (27) |
| Clinical course | |
| Hospitalization | 63/102 (62) |
| Admission of ICU/receipt of intubation/transition to DNI | 34/101a (34) |
| Admission to ICU | 21/101a (21) |
| Receipt of intubation and mechanical ventilation | 18/100a (18) |
| Transition to DNR/DNIf | 18/97a (19) |
| Death | 25/102 (25) |
BMI, body mass index; COPD, chronic obstructive pulmonary disease; DNI, do not intubate; DNR, do not resuscitate; ICU, intensive care unit; IQR, interquartile range.
Denominators reflect available data; unless specified, unknowns are not included.
Metastatic or active lung cancer was defined as patients with metastatic lung cancer or patients undergoing active treatment of lung cancer (e.g. neoadjuvant or adjuvant therapy).
COPD was defined as anyone with this diagnosis listed as a part of the past medical history plus either an abnormal pulmonary function test interpreted as consistent with COPD or had inhalers for COPD listed in the outpatient medication record. Patients with only radiologic evidence of COPD or a note in the medical record the diagnosis was in question were not included.
Non-COPD lung disease was defined as underlying lung disease other than COPD (e.g. reactive airways disease, pneumonitis, abnormal pulmonary function test interpreted as underlying lung disease, etc.).
Congestive heart failure was defined as anyone with NYHA functional class I–IV disease. As such, anyone with this diagnosis listed as a part of the past medical history or an abnormal cardiac echocardiogram demonstrating evidence of structural heart disease consistent with this diagnosis was included.
An additional four patients received intubation and invasive mechanical ventilation and subsequently elected not to receive further necessary intensification of care or interventions.
The patients who died from confirmed COVID-19 during the follow-up period represented 11% of all deaths among patients with lung cancers at our institution. The peak percent of deaths related to COVID-19 was approximately 20% in April 2020 (Figure 1 A). Most deaths occurred within a week of COVID-19 diagnosis; an estimated 85% (95% CI 78% to 92%), 81% (95% CI 73% to 89%), and 78% (95% CI 70% to 87%) of patients were alive at 1, 2, and 4 weeks, respectively (Figure 1B). Although symptoms such as cough (70%) and fever (59%) were common, the constellation of symptoms within a given patient at presentation were variable (Figure 1C). Of 102 patients, 62% (63/102) required hospital admission and 25% died (25/102) (Figure 1D). Of the patients who required ICU level of care (21%, n = 21), 14% recovered and 72% died.
Figure 1.
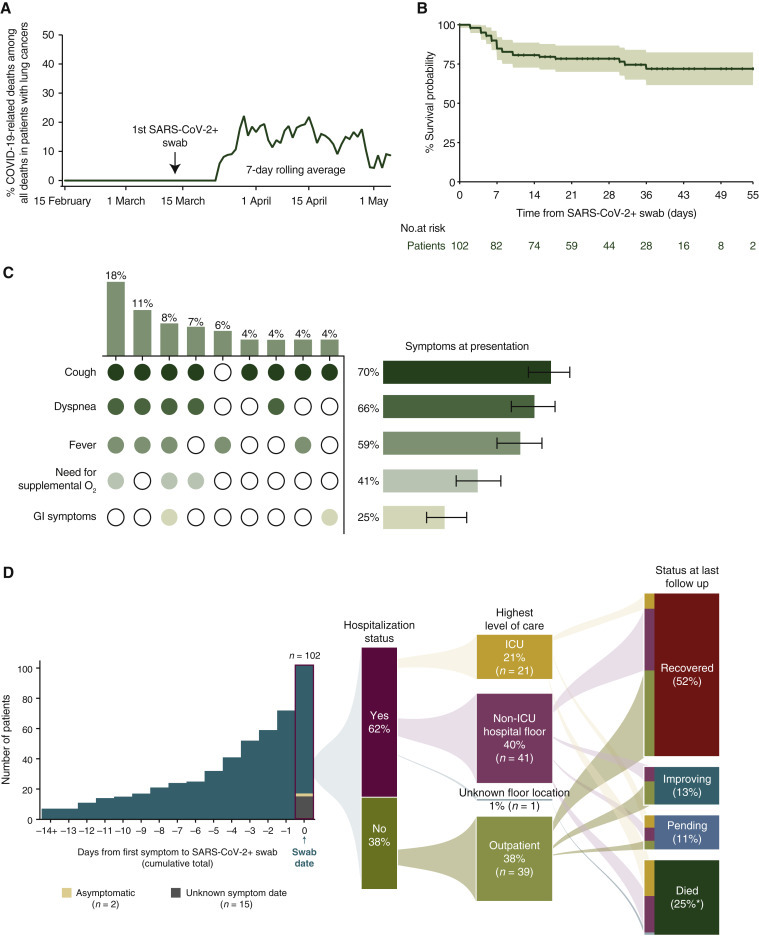
COVID-19 in patients with lung cancers.
(A) Estimated percent of COVID-19-related deaths that occurred during the study period among all deaths of patients with lung cancers shown as a 7-day rolling average (green line). The first positive SARS-CoV-2 test in a patient with lung cancer occurred on 12 March 2020. (B) Kaplan–Meier estimated survival probability starting from date of positive SARS-CoV-2-positive swab. Confidence band represents the 95% confidence interval (CI). (C) Presenting signs and symptoms of COVID-19 infection in patients with known information for these symptoms (n = 89). Figure shows both single and clusters of signs and symptoms. Vertical bars and corresponding heatmap represents frequency of sign and symptom clusters that occurred >4%. (D) Patients were identified starting from the first case on 12 March 2020 through 6 May 2020, and followed until 11 May 2020. Median follow up was 25 days (IQR 10–36 days). Symptom presentation, hospitalization status, highest level of care, and patient COVID-19 status at time of last follow up. Vertical bar graph represents the cumulative number of symptomatic patients (98%, n = 85/87; 87 had a known symptom start date) leading up to the date of the positive SARS-CoV-2 swab. ∗percentages may add up to greater than 100% due to rounding.
COVID-19, coronavirus disease 2019; GI, gastrointestinal; ICU, intensive care unit; IQR, interquartile range; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
a Percentages may add up to >100% due to rounding.
Among patients with lung cancers, several baseline clinical features consistently associated with increased risk of COVID-19 severity, including age, smoking history, COPD, and hypertension (Figure 2A and B). Congestive heart failure (CHF) associated with increased risk of COVID-19 severity, although baseline CHF was present in a small number of patients in this analysis (n = 7). Cancer-specific features, such as presence of active/metastatic lung cancer or history of prior thoracic radiation or thoracic surgery, did not appear to impact severity of COVID-19. Histology, presence of targetable oncogenes, or PD-L1 immunohistochemistry expression also did not impact severity (supplementary Figure S1A, available at Annals of Oncology online).
Figure 2.
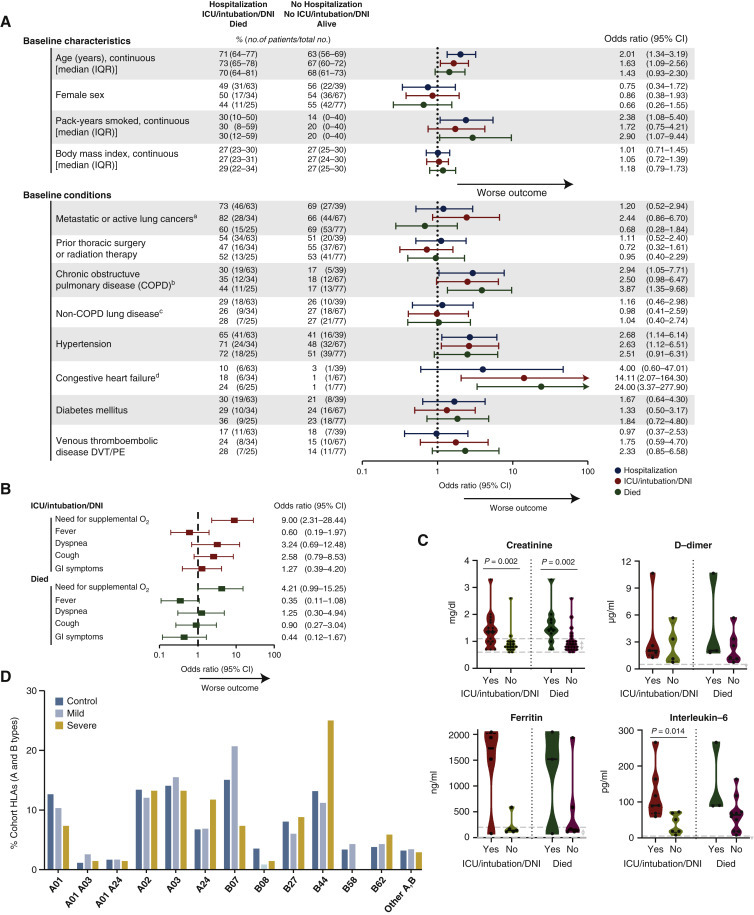
Impact of cancer-specific and additional laboratory features on severity of COVID-19 in lung cancer.
(A) Odds ratios (ORs) for the impact of baseline characteristics of patients on severity outcomes associated with COVID-19 [rate of hospitalization; ICU admission, intubation, and/or change to DNI status to avoid intensification of care (e.g. intubation) (ICU/intubation/DNI); or death]. ORs were calculated using univariate logistic regression. Error bars represent 95% CIs. The x-axis is on a log10 scale. For the continuous variables: ORs for age compare an older person and younger person with an age difference of 10 years; ORs for pack-years smoked compare median smoking (23.5 pack-years) to never smoking (0 pack-years); ORs for body mass index (BMI) compare a person with higher BMI to lower BMI with a difference of 5 kg/m2. (B) Among hospitalized patients, showing ORs for the impact of symptoms at presentation with lung cancers and COVID-19 on severity outcomes (ICU/intubation/DNI and death). ORs were calculated using univariate logistic regression. Error bars represent 95% CIs. The x-axis is on a log10 scale. (C) Among hospitalized patients, baseline creatinine and inflammatory markers (D-dimer, ferritin, interleukin-6) of patients who experienced severe COVID-19 outcomes (ICU/intubation/DNI and death). Dots represent individual values. Dashed lines within violins represent median, 25% percentile, and 75% percentile. Violins show min-max ranges and kernel density estimate distributions of each group. Gray dashed lines represent the normal range for that laboratory value. The Mann–Whitney U test was used for calculating P values. (D) The frequency of imputed HLA-A and HLA-B alleles among control, mild, and severe patients with lung cancers and COVID-19.
CI, confidence interval; COVID-19, coronavirus disease 2019; DNI, do not intubate; DVT, deep venous thrombosis; GI, gastrointestinal; HLA, human leukocyte antigen; ICU, intensive care unit; IQR, interquartile range; PE, pulmonary embolism.
ametastatic or active lung cancer was defined as patients with metastatic lung cancer or patients undergoing active treatment for lung cancer (e.g. neoadjuvant or adjuvant therapy); bCOPD was defined as anyone with this diagnosis listed as a part of the past medical history plus either an abnormal pulmonary function test interpreted as consistent with COPD or had inhalers for COPD listed in the outpatient medication record. Patients with only radiologic evidence of COPD or a note in the medical record the diagnosis was in question were not included; cNon-COPD lung disease was defined as underlying lung disease other than COPD (eg reactive airways disease, pneumonitis, abnormal pulmonary function test interpreted as underlying lung disease, etc.); dCongestive heart failure was defined as anyone with NYHA functional class I-IV disease. As such, anyone with this diagnosis listed as a part of the past medical history or an abnormal cardiac echocardiogram demonstrating evidence of structural heart disease consistent with this diagnosis was included.
Among hospitalized patients, the need for supplemental oxygen at presentation associated with increased odds of severe COVID-19 illness [OR 9.0, 95% CI 2.31–28.44 for ICU admission, intubation, and/or change to DNI status to avoid intensification of care (ICU/intubation/DNI) and OR 4.21, 95% CI 0.99–15.25 for death] (Figure 2B). Elevated creatinine at initial presentation associated with increased severity (P = 0.002 for ICU/intubation/DNI; P = 0.002 for death) (Figure 2C). Levels of inflammatory markers at presentation were generally elevated in patients who developed more severe COVID-19 (Figure 2C). Other laboratory results at presentation, such as white blood cell count, absolute lymphocyte count, and liver function tests, did not differentiate severity of COVID-19 (supplementary Figure S1B, available at Annals of Oncology online).
As the adaptive immune response to SARS-CoV-2 antigens may be relevant for determining severity of COVID-19,7 we examined the association between HLA-A and HLA-B alleles on outcomes. Imputation of HLA-A and HLA-B alleles were available in 46 of 102 (45%) patients with lung cancers and known COVID-19 and were organized into 12 HLA supertypes based on similarity of antigen presentation.6 Patients with mild (n = 29, no ICU/intubation/DNI or death) or severe (n = 17, ICU/intubation/DNI or death) disease were compared with the control cohort of 5166 patients with lung cancers and no known COVID-19. Generally, the frequencies of HLA-A and HLA-B alleles were similar in mild and severe COVID-19 cases compared with controls (Figure 2D). Although HLA-B44 supertype was numerically more common among patients with severe COVID-19, this was not significant after adjustment for multiple hypothesis testing.
We examined the impact of cancer therapy on the severity of COVID-19, a particularly critical question to guide real-time cancer care during this pandemic. Similar to our previous results,5 recent PD-(L)1 blockade with or without chemotherapy did not associate with increased severity of COVID-19 (Figure 3A and B). We did not observe a consistent impact of severity in patients who recently received chemotherapy or tyrosine kinase inhibitors (Figure 3C and D).
Figure 3.
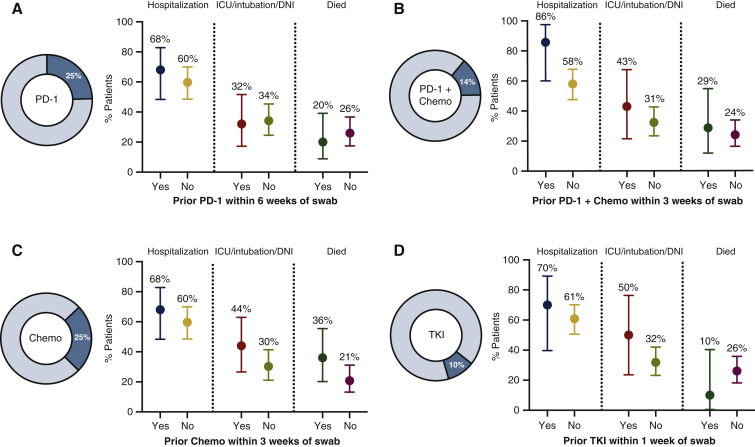
Impact of cancer therapy on COVID-19 severity in patients with lung cancers.
For each figure in this series, the % of all patients (n = 102) who received various cancer therapy regimens during the time period defined before the swab date. Dots show (left) the rate of hospitalization (middle) ICU admission, intubation, and/or change to DNI status to avoid intensification of care (e.g. intubation) (right) or death among patients with COVID-19 with known status of the outcome displayed. The four treatment categories of interest included (A) prior PD-1 blockade within 6 weeks of swab, (B) prior PD-1 blockade and chemotherapy within 3 weeks of swab, (C) prior chemotherapy within 3 weeks of swab, and (D) prior tyrosine kinase inhibitor (TKI) within 1 week of swab. There was no significant difference in severity outcomes in any of the comparisons. Error bars represent 95% confidence intervals.
chemo, chemotherapy; COVID-19, coronavirus disease 2019; DNI, do not intubate; ICU, intensive care unit; PD-1, programmed cell death protein 1.
Although the rates of severe COVID-19 appear to be increased in patients with lung cancers, recovery occurs in the majority of patients (65% patients recovered or were improving as of this writing). As expected, the rates and timing of recovery associated with the highest level of care during the disease course (3-week recovery rate in outpatients, 60%, 95% CI 43% to 77%; in patients requiring non-ICU hospitalization, 20%, 95% CI 8% to 33%; in patients requiring ICU care, 5%, 95% CI 0% to 15%) (Figure 4 A). The median time to recovery (by definition, a minimum of 14 days) for outpatients, patients requiring non-ICU hospitalization, and patients requiring ICU care was 18 days, 34 days, and not reached, respectively. Among patients requiring ICU care, 5 of 18 (28%) patients who required intubation have since been extubated and 3 (17%) have been discharged to home or a rehabilitation facility.
Figure 4.
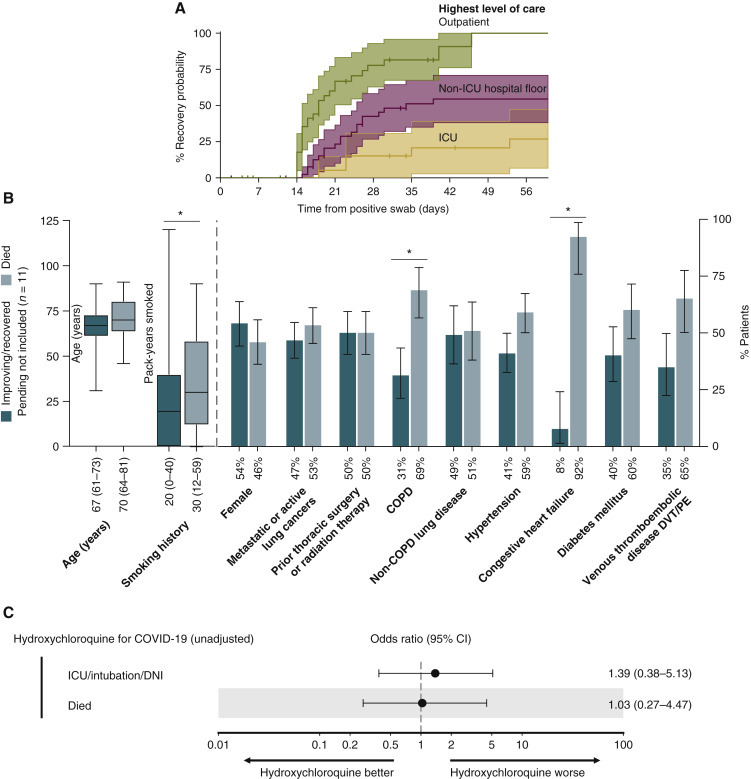
Recovery from COVID-19 in patients with lung cancers.
(A) Cumulative probability of recovery by highest level of care: outpatient (n = 39), non-ICU hospital floor (n = 41), and ICU (n = 21). Recovered was defined as a combination of at least 14 days from positive swab date and asymptomatic for 72 h. Two patients among patients categorized as ‘non-ICU hospital floor’ were residing in an inpatient facility at the time of diagnosis of COVID-19. (B) Rate of baseline characteristics among patients who are either improving or recovered from COVID-19 (n = 66) compared with died (n = 25). Patients with pending clinical status were not included (n = 11). Significant odds ratios are denoted by asterisks. Fewer pack-years smoked, continuous (OR 0.45, 95% CI 0.18–0.93); absence of COPD (OR 0.31, 95% CI 0.90–0.12); absence of congestive heart failure (OR 0.05, 95% CI 0.004–0.35). Percent of cases or median (interquartile range) are below each bar. Error bars represent 95% CIs. (C) ORs for the impact of inpatient hydroxychloroquine use for COVID-19 on severity outcomes associated with COVID-19 (ICU/intubation/DNI and death). ORs were calculated using univariate logistic regression. Error bars represent 95% CIs. The x-axis is on a log10 scale.
CI, confidence interval; COPD, chronic obstructive pulmonary disease; COVID-19, coronavirus disease 2019; DNI, do not intubate; DVT, deep venous thrombosis; ICU, intensive care unit; OR, odds ratio; PE, pulmonary embolism.
Diminished smoking history (OR 0.45, 95% CI 0.18–0.93), absence of COPD (OR 0.31, 95% CI 0.90–0.12), and absence of CHF (OR 0.05, 95% CI 0.004–0.35) were associated with increased odds of recovery (Figure 4B).
Treatment with inpatient hydroxychloroquine [73%, n = 35/48, median time from hospitalization to start was 0 days, interquartile range (0–0.75 days)] was not associated with improved outcomes (OR for ICU/intubation/DNI 1.39, 95% CI 0.37–4.56, P = 0.7, and OR death 1.03, 95% CI 0.26–3.55, P = 0.99) (Figure 4C).
Discussion
In our study of 102 patients with lung cancers and COVID-19, we examined the disease course, impact of cancer-directed treatments, and determinants of severity/recovery and identified several important implications.
Similar to what has been described in the literature,1 , 8 the disease course of COVID-19 is more severe in patients with lung cancers. Although COVID-19 led to death in approximately one-quarter of patients with lung cancer, COVID-19 represented a minority of the deaths among patients with lung cancers that occurred during this period. This finding amplifies the importance of maintaining urgent focus on the needs of patients with cancer and optimizing cancer care within the context of the local prevalence of SARS-CoV-2 infection.9
The course of SARS-CoV-2 infection in lung cancer was longer and more severe than that reported in the general US population.10, 11, 12 About one-third of patients experienced a milder outpatient course, two thirds needed hospitalization, and one-quarter of patients died. Although more frequently severe, these observations mirror common phenotypes of COVID-19 illness reported in the literature for the general population13 , 14—a mild and/or asymptomatic outpatient course, a rapid and fatal presentation, and a subacute lingering course that generally leads to recovery.
The diversity of these COVID-19 phenotypes is undoubtedly in part related to underlying clinical features, such as smoking history, age, hypertension, and COPD as seen in our report. Recent reports, including a cohort of patients with thoracic cancers mostly from epicenters in Europe with somewhat differing health care resources (33% death rate, but 8.3% ICU admission, 5% rate of intubation), were ambiguous as to whether active systemic cancer therapy increases severity of COVID-19.2 , 3 , 15, 16, 17 Importantly, we did not identify cancer-specific features that impacted severity; active or metastatic cancer at the time of the SARS-CoV-2-positive swab, prior thoracic surgery, thoracic radiation, near-term systemic therapies, and pathologic features of lung cancer were similar across ranges of COVID-19 severity. Our report a priori focused on the impact of systemic anticancer therapy in time periods proximal to the SARS-CoV-2-positive swab (1–6 weeks), with the goal of approximating the prospective decision making that will be relevant for active cancer therapy in the context of a new or suspected COVID-19 diagnosis. Our findings suggest that risk factors leading to lung cancer and its related chronic medical conditions, rather than cancer itself or cancer-directed treatments, are the primary drivers of severity of COVID-19 in patients with lung cancers.
The severity of COVID-19 may also relate to the biological underpinnings of SARS-CoV-2 host-virus immune response.18 , 19 We examined the impact of HLA alleles on the severity of COVID-19. Class I HLA alleles contribute to determining the quantity and quality of COVID-19 antigens presented for adaptive immune response, which may impact the effectiveness of acute and memory T-cell response. The generally similar distribution of HLA supertypes among controls and patients with COVID-19 may align with a recent report describing CD8+ T-cell responses to SARS-CoV-2 epitopes in most patients who had recovered from COVID-19 or were immunized with vaccine.7 , 20 Future studies with larger samples sizes will be needed to precisely define the impact of HLA alleles on the severity of COVID-19.
Despite the burden of COVID-19 severity in patients with lung cancers, recovery was achieved in more than half of the patients, including a small (but important) fraction of patients initially requiring intubation and invasive mechanical ventilation. Absence of COPD, absence of CHF, and fewer pack-years smoked were predictors of achieving recovery. These results are consistent with reports describing cardiopulmonary disease and/or its risk factors disproportionately leading to worse COVID-19 outcomes in unselected patient populations,21, 22, 23 likely driven by multiple factors including age, underlying organ injury, direct SARS-CoV-2 invasion, and hyperinflammation.22 , 24
Consistent with a recent retrospective study out of New York City25 and a randomized controlled study in China,26 our preliminary investigation of hydroxychloroquine did not find an improvement in COVID-19 severity outcomes among patients with lung cancer.
A limitation of our study was the sample size, which impacts the ability to perform adjustments for multiple potential confounders (such as effect modifiers and confounders by indication) with smaller effect sizes. Larger sample sizes and cohorts are needed to confirm the generalizability of our results.
Our results characterize COVID-19 in patients with lung cancers, highlighting both the urgent vulnerability of patients with lung cancer during this pandemic as well as the persistently critical need to continue, and drive improvements in, optimal cancer care.
Acknowledgements
The authors thank Pranay Sinha and Jean W. Liew for helpful discussions related to this analysis.
Funding
This work was supported by Memorial Sloan Kettering Cancer Center Support Grant/Core [grant number P30-CA008748] and the Druckenmiller Center for Lung Cancer Research at Memorial Sloan Kettering Cancer Center; the National Institutes of Health [grant numbers T32-CA009207 to JL, K30-UL1TR00457 to JL, HHSN272201400008C to MŁ, 7R01AI081848-04 to BDG, 1R01CA240924-01 to BDG]; Damon Runyon Cancer Research Foundation [grant number CI-98-18 to MDH]. BDG and DH are supported under a collaboration by Stand Up To Cancer, a program of the Entertainment Industry Foundation, the Society for Immunotherapy of Cancer, and the Lustgarten Foundation. BDG is a The Pershing Square Sohn Prize—Mark Foundation Fellow supported by funding from The Mark Foundation for Cancer Research. MDH is a member of the Parker Institute for Cancer Immunotherapy.
Disclosure
JL has received honoraria from Targeted Oncology. IRP has been a compensated consultant for Pfizer and AstraZeneca. KCA reports compensated consulting for AstraZeneca. She has received non-monetary research support from Novartis and Takeda (to her institution). JEC reports compensated consulting for AstraZeneca, Bristol-Myers Squibb, Merck, and Genentech; and research funding to institution from AstraZeneca, Bristol-Myers Squibb, Merck, and Genentech. RMD reports personal equity ownership in CVS Caremark and Roche and immediate family member with equity ownership in Pfizer, Eli Lilly, Cigna Corporation, and Baxter Bioscience. AED reports honoraria/advisory boards for Ignyta/Genentech/Roche, Loxo/Bayer/Lilly, Takeda/Ariad/Millenium, TP Therapeutics, AstraZeneca, Pfizer, Blueprint Medicines, Helsinn, Beigene, BergenBio, Hengrui Therapeutics, Exelixis, Tyra Biosciences, Verastem, MORE Health, Abbvie, 14ner/Elevation Oncology, Remedica Ltd., ArcherDX, Monopteros; associated research paid to institution from Pfizer, Exelixis, GlaxoSmithKlein, Teva, Taiho, PharmaMar; research funding from Foundation Medicine; royalties from Wolters Kluwer; other support from Merck (food/beverage), Puma (food/beverage), Merus, Boehringer Ingelheim; and CME honoraria from Medscape, OncLive, PeerVoice, Physicians Education Resources, Targeted Oncology, Research to Practice, Axis, Peerview Institute, Paradigm Medical Communications, WebMD. WVL receives institutional research funding from Daiichi Sankyo, Amgen, and Abbvie; has been a compensated consultant for PharmaMar, G1 Therapeutics, AstraZeneca, Jazz Pharmaceuticals. BTL receives institutional research funding from Genentech, Lilly, Amgen, Daiichi Sankyo, AstraZeneca, Hengrui Therapeutics, BioMedValley Discoveries, Illumina, GRAIL, Guardant Health and MORE Health; has two institutional patents at Memorial Sloan Kettering Cancer Center (US62/685,057, US62/514,661); has been a compensated consultant/advisor for Roche/Genentech, Thermo Fisher Scientific, Guardant Health, Hengrui Therapeutics, Mersana Therapeutics, and Lilly; received travel support from Resolution Bioscience and MORE Health. AN has been a compensated consultant for Bayer. MDO has been a compensated consultant for PharmMar, Novartis, and Targeted Oncology; received travel support from Bristol-Myers Squibb and Merck; received honoraria from OncLive. PKP reports honoraria/advisory boards for Boehringer Ingelheim, Celgene, EMD Serono, Celithera, AstraZeneca, Abbvie, and Lilly Oncology; was compensated for participation in an independent data safety monitoring committee for Takeda. GJR receives institutional research funding from Mirati, Merck, Pfizer, Novartis, Roche, and Takeda. CMR has been a compensated consultant regarding oncology drug development with AbbVie, Amgen, Ascentage, Astra Zeneca, Bicycle, Celgene, Daiichi-Sankyo, Genentech/Roche, Ipsen, Jazz, Lilly, Pfizer, Pharmamar, Syros, and Vavotek; serves on the scientific advisory boards of Bridge Medicines and Harpoon Therapeutics. HAY receives institutional research funding from AstraZeneca, Novartis, Pfizer, Lilly, Cullinan, and Daiichi-Sankyo; has been a compensated consultant for AstraZeneca and Daiichi-Sankyo. MGZ has received consulting fees from GlaxoSmithKline (2020), Epizyme (2017), Aldeyra Therapeutics (2019), Novocure (2019), and Atara (2018) and honoraria from Medical Learning Institute (2019) and OncLive (2019). Memorial Sloan Kettering receives research funding from the Department of Defense, the National Institutes of Health, GlaxoSmithKline, Epizyme, Polaris, Sellas Life Sciences, Bristol Myers Squibb, Millenium/Takeda, Curis, and Roche for research conducted by MGZ. MGZ serves as Chair of the Board of Directors of the Mesothelioma Applied Research Foundation. MGZ reports grants from National Institutes of Health/National Cancer Institute. Memorial Sloan Kettering has an institutional agreement with IBM for Watson for Oncology and receives royalties from IBM. MGZ is an employee of Memorial Sloan Kettering. BDG has received honoraria for speaking engagements from Merck, Bristol-Myers Squibb, and Chugai Pharmaceuticals; has been a compensated consultant for PMV Pharma and Rome Therapeutics of which he is a cofounder. MGK receives personal fees from AstraZeneca, Pfizer, Regeneron, and Daiichi-Sankyo; received honoraria for participation in educational programs from WebMD, OncLive, Physicians Education Resources, Prime Oncology, Intellisphere, Creative Educational Concepts, Peerview, i3 Health, Paradigm Medical Communications, AXIS, Carvive Systems, AstraZeneca, and Research to Practice; received travel support from AstraZeneca, Pfizer, Regeneron, and Genentech. MGK is an employee of Memorial Sloan Kettering. Memorial Sloan Kettering has received research funding from The National Cancer Institute (USA), The Lung Cancer Research Foundation, Genentech Roche, and PUMA Biotechnology for research conducted by MGK. MSK has licensed testing for EGFR T790M to MolecularMD. MDH receives institutional research funding from Bristol-Myers Squibb; has been a compensated consultant for Merck, Bristol-Myers Squibb, AstraZeneca, Genentech/Roche, Nektar, Syndax, Mirati, Shattuck Labs, Immunai, Blueprint Medicines, Achilles, and Arcus; received travel support/honoraria from AstraZeneca, Eli Lilly, and Bristol-Myers Squibb; has options from Shattuck Labs, Immunai, and Arcus; has a patent filed by his institution related to the use of tumor mutation burden to predict response to immunotherapy (PCT/US2015/062208), which has received licensing fees from PGDx. The remaining authors have declared no conflicts of interest.
Supplementary data
References
- 1.Dai M., Liu D., Liu M., et al. Patients with cancer appear more vulnerable to SARS-COV-2: a multi-center study during the COVID-19 outbreak. Cancer Discov. 2020;10:783–791. doi: 10.1158/2159-8290.CD-20-0422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Horn L., Whisenant J.G., Torri V., et al. Thoracic Cancers International COVID-19 Collaboration (TERAVOLT): impact of type of cancer therapy and COVID therapy on survival. J Clin Oncol. 2020;38 3818_suppl: LBA111. [Google Scholar]
- 3.Zhang L., Zhu F., Xie L., et al. Clinical characteristics of COVID-19-infected cancer patients: a retrospective case study in three hospitals within Wuhan, China. Ann Oncol. 2020;31:894–901. doi: 10.1016/j.annonc.2020.03.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liang W., Guan W., Chen R., et al. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21:335–337. doi: 10.1016/S1470-2045(20)30096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Luo J., Rizvi H., Egger J.V., et al. Impact of PD-1 blockade on severity of COVID-19 in patients with lung cancers. Cancer Discov. 2020 doi: 10.1158/2159-8290.CD-20-0596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sidney J., Peters B., Frahm N., et al. HLA class I supertypes: a revised and updated classification. BMC Immunol. 2008;9:1. doi: 10.1186/1471-2172-9-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grifoni A., Weiskopf D., Ramirez S.I., et al. Targets of T cell responses to SARS-CoV-2 coronavirus in humans with COVID-19 disease and unexposed individuals. Cell. 2020;181(7) doi: 10.1016/j.cell.2020.05.015. 1489-1501.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mehta V., Goel S., Kabarriti R., et al. Case fatality rate of cancer patients with COVID-19 in a New York Hospital System. Cancer Discov. 2020 doi: 10.1158/2159-8290.CD-20-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hanna T.P., Evans G.A., Booth C.M. Cancer, COVID-19 and the precautionary principle: prioritizing treatment during a global pandemic. Nat Rev Clin Oncol. 2020;17:268–270. doi: 10.1038/s41571-020-0362-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Richardson S., Hirsch J.S., Narasimhan M., et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323:2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cummings M.J., Baldwin M.R., Abrams D., et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet. 2020;395:1763–1770. doi: 10.1016/S0140-6736(20)31189-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lewnard J.A., Liu V.X., Jackson M.L., et al. Incidence, clinical outcomes, and transmission dynamics of severe coronavirus disease 2019 in California and Washington: prospective cohort study. BMJ. 2020;369:m1923. doi: 10.1136/bmj.m1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bhatraju P.K., Ghassemieh B.J., Nichols M., et al. Covid-19 in critically ill patients in the Seattle Region - case series. N Engl J Med. 2020;382:2012–2022. doi: 10.1056/NEJMoa2004500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sutton D., Fuchs K., D'Alton M., Goffman D. Universal screening for SARS-CoV-2 in women admitted for delivery. N Engl J Med. 2020;382:2163–2164. doi: 10.1056/NEJMc2009316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barlesi F., Foulon S., Bayle A., et al. Outcome of cancer patients infected with COVID-19, including toxicity of cancer treatments. Presented at the AACR Virtual Annual Meeting. 2020 [Google Scholar]
- 16.Robilotti E.V., Babady N.E., Mead P.A., et al. Determinants of COVID-19 disease severity in patients with cancer. Nat Med. 2020 doi: 10.1038/s41591-020-0979-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuderer N.M., Choueiri T.K., Shah D.P., et al. Clinical impact of COVID-19 on patients with cancer (CCC19): a cohort study. Lancet. 2020;395(10241):1907–1918. doi: 10.1016/S0140-6736(20)31187-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Siddiqi H.K., Mehra M.R. COVID-19 illness in native and immunosuppressed states: a clinical-therapeutic staging proposal. J Heart Lung Transplant. 2020;39:405–407. doi: 10.1016/j.healun.2020.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vardhana S.A., Wolchok J.D. The many faces of the anti-COVID immune response. J Exp Med. 2020;217:e20200678. doi: 10.1084/jem.20200678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhu F.-C., Li Y.-H., Guan X.-H., et al. Safety, tolerability, and immunogenicity of a recombinant adenovirus type-5 vectored COVID-19 vaccine: a dose-escalation, open-label, non-randomised, first-in-human trial. Lancet. 2020;395:1845–1854. doi: 10.1016/S0140-6736(20)31208-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang J., Zheng Y., Gou X., et al. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: a systematic review and meta-analysis. Int J Infect Dis. 2020;94:91–95. doi: 10.1016/j.ijid.2020.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clerkin K.J., Fried J.A., Raikhelkar J., et al. COVID-19 and cardiovascular disease. Circulation. 2020;141:1648–1655. doi: 10.1161/CIRCULATIONAHA.120.046941. [DOI] [PubMed] [Google Scholar]
- 23.Docherty A.B., Harrison E.M., Green C.A., et al. Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ. 2020;369:m1985. doi: 10.1136/bmj.m1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoffmann M., Kleine-Weber H., Schroeder S., et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280.e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Geleris J., Sun Y., Platt J., et al. Observational study of hydroxychloroquine in hospitalized patients with Covid-19. N Engl J Med. 2020;382(25):2411–2418. doi: 10.1056/NEJMoa2012410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tang W., Cao Z., Han M., et al. Hydroxychloroquine in patients with mainly mild to moderate coronavirus disease 2019: open label, randomised controlled trial. BMJ. 2020;369:m1849. doi: 10.1136/bmj.m1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


