Abstract
The novel coronavirus 2019 (SARS-CoV-2) was first identified in January 2020 and has since evolved into a pandemic affecting >200 countries. The severity of presentation is variable and carries a mortality between 1% and 3%. We continue to learn about the virus and the resulting acute respiratory illness and hypercoagulability; however, much remains unknown. In our early experience in a high-volume center, we report a series of four cases of acute peripheral artery ischemia in patients with COVID-19 in the setting of elevated D-dimer levels.
Keywords: COVID-19, Arterial thromboembolism, D-dimer
As the novel coronavirus 2019 (SARS-CoV-2) pandemic spreads across the world, early recognition of the spectrum of symptoms and patterns of clinical presentation is critical for optimal management. Many patients with coronavirus disease (COVID-19), the illness caused by SARS-CoV-2, present with minimal respiratory symptoms; however, 15% to 20% may present with severe acute respiratory disease.1 New data and expeditious reporting in the literature2 continue to provide new information about best practices and treatment strategies, leading to evolving guidelines, sometimes on a daily basis.
Case reports
Respiratory support remains the primary concern. Many patients present with a rapidly progressive acute respiratory distress syndrome secondary to cytokine storm in the setting of elevated levels of multiple inflammatory markers.3 This hyperinflammatory state has been linked previously to disseminated intravascular coagulation and a hypercoagulable state. Other viral infections, like dengue and influenza due to H1N1 infection, have shown a similar hypercoagulable state, with patients presenting with venous and arterial thromboembolic events.4 New York City has become the epicenter with >100,000 diagnosed cases of COVID-19 to date. Our institution has seen a large volume: >6000 emergency department visits, >2500 admissions, and management of >250 critically ill patients. We present a series of four cases of patients with COVID-19-associated arterial thromboembolism. Permission was obtained from the patient or next of kin for all these cases.
Case 1
A 58-year-old man with hypertension, hyperlipidemia, and diabetes presented with fever, cough, and hypoxia and was admitted with a diagnosis of COVID-19. Treatment was initiated with hydroxychloroquine and azithromycin. Tocilizumab was also initiated because of progression of hypoxemia and elevated interleukin 6 levels. On hospital day (HD) 5, he developed word finding difficulty and new-onset right foot pain with numbness. A neurologist diagnosed the patient with small-volume infarct within the left middle cerebral artery territory. A lower extremity arterial duplex ultrasound scan was obtained, demonstrating a popliteal embolus. A therapeutic heparin infusion was started, and urgent operative revascularization was planned because of progressive limb ischemia. However, hypoxemia rapidly progressed, requiring urgent intubation and maximum ventilatory support. Given this instability, operative intervention was deferred. D-dimer level showed a rapid increase during the subsequent 2 days: HD 1, 369 ng/mL; HD 5 (thromboembolic event), 6715 ng/mL; and HD 6, >10,000 ng/mL. Progressive hemodynamic and respiratory instability developed despite vasopressors and prone positioning. After discussion with his family, an order of do not resuscitate was made with no further escalation of care, and he died on HD 6.
Case 2
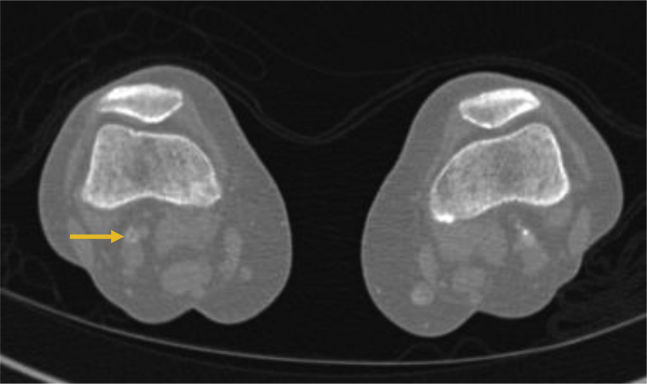
A 78-year-old woman with atrial fibrillation receiving apixaban presented with sudden onset of right leg and foot pain with numbness. Her vital signs were unremarkable, except for an oxygen saturation of 95% on breathing of room air. She denied cough or shortness of breath. She complained of malaise for 1 week. She was motor and sensory intact but had nonpalpable pedal pulses, and workup for acute limb ischemia was initiated. She was systemically heparinized, and a computed tomography (CT) scan demonstrated an embolus of the right popliteal artery (Fig 1). Laboratory values were remarkable for a white blood cell count of 21,000/μL. Imaging of the lungs demonstrated ground-glass opacities. A presumptive diagnosis of COVID-19 was made and later confirmed. The D-dimer level on admission was noted to be elevated at 1912 ng/mL. Her symptoms of rest pain resolved with therapeutic heparinization. She was admitted for management of COVID-19. During the ensuing days, she remained pain free; D-dimer level dropped to 495 ng/mL on HD 2, and she was discharged home.
Fig 1.
Computed tomography (CT) angiography demonstrating right popliteal embolus (arrow).
Case 3
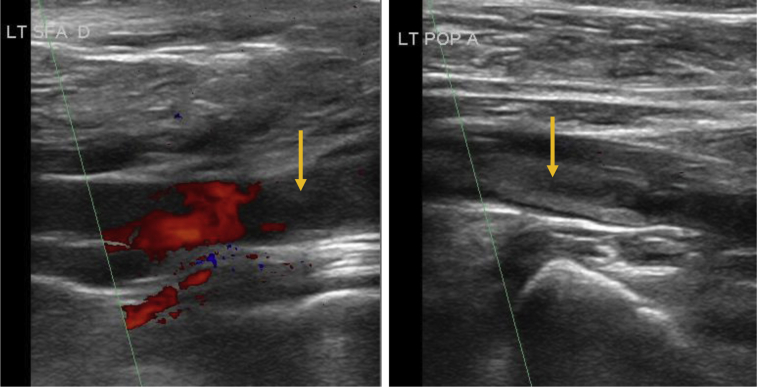
A 54-year-old man, otherwise in good health, presented with acute left leg pain for the past 3 days and acutely worsening chronic cough. He was found to be hypoxic on admission (Spo2 90%) and placed on oxygen support with confirmed SARS-CoV-19 infection. The D-dimer level at admission was 6802 ng/mL. He was also diagnosed with pulmonary embolism (PE), and anticoagulation was administered. Arterial duplex ultrasound imaging showed isolated popliteal artery occlusion with reconstitution of tibial arteries (Fig 2). In addition, a venous duplex ultrasound examination identified a soleal vein deep venous thrombosis. His foot pain improved, but he remained with a severely abnormal chest radiograph with multiple areas of infiltrates. Given his severely compromised and tenuous pulmonary status and improvement of ischemic symptoms, expectant management was pursued. Some residual pain remained, with a D-dimer level that decreased to 3601 ng/mL on HD 3. He ultimately underwent diagnostic angiography, which identified a mural thrombus in the left common iliac artery. He underwent a successful iliac and popliteal embolectomy with removal of a platelet-rich embolus. His postoperative course was otherwise unremarkable, and he was discharged home on HD 8.
Fig 2.
Ultrasound image demonstrating left popliteal embolus (arrow).
Case 4
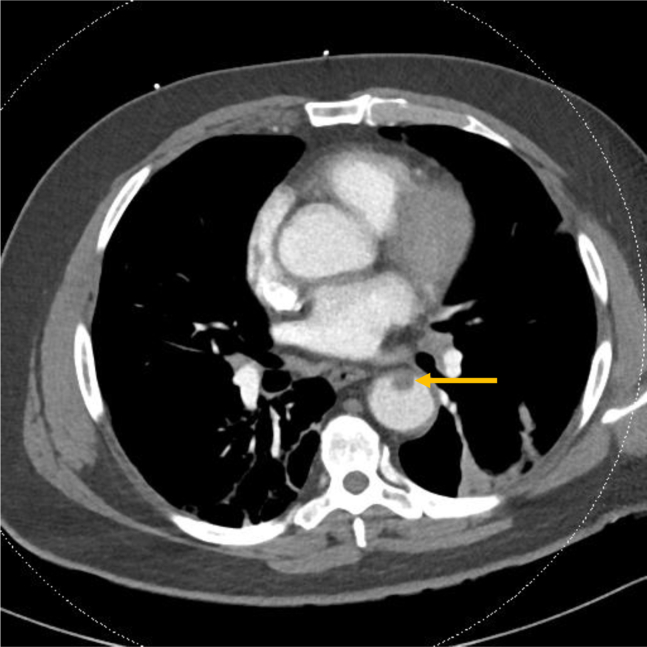
A 63-year-old man with diabetes presented with left pleuritic and flank pain, fevers, and malaise for 2 weeks. He was tachycardic with an oxygen saturation of 95% on breathing of room air. Laboratory values were remarkable for hyperglycemia and lymphopenia, and he was diagnosed with diabetic ketoacidosis in the setting of COVID-19. D-dimer level at admission was 541 ng/mL and increased to 2584 ng/mL on HD 3. CT scan of the chest to rule out PE was performed and identified an aortic thrombus in the descending aorta (Fig 3). Intravenous therapeutic heparin was initiated, and he is being managed for viral pneumonia. He was discharged home on HD 3, and his pain improved.
Fig 3.
Computed tomography (CT) angiography demonstrating mural thrombus (arrow) in the descending thoracic aorta.
Discussion
Herein we present a series of four cases of large-vessel thromboembolism that we have identified in our very early experience in treating patients with COVID-19. These cases occurred in the setting of elevated D-dimer levels and a diagnosis of COVID-19, with no prior history of peripheral artery disease. Of note, in two of these cases, the presentation to the emergency service was initially for leg ischemia, with subsequent expeditious recognition of signs of COVID-19, which was then confirmed with rapid testing. Surgical and endovascular intervention was not undertaken in two of the three cases of peripheral ischemia because of severe respiratory decompensation. One patient died of the pulmonary complications, and urgent revascularization was deferred in the other two patients because of a tenuous pulmonary status, with subsequent stabilization or improvement of arterial ischemic symptoms on systemic anticoagulation with intravenous unfractionated heparin. One patient underwent delayed revascularization. In the fourth case, the arterial thrombus was an incidental finding in the setting of an elevated D-dimer level and concern for PE.
Hemostatic balance can be disrupted by numerous mechanisms and can vary from a minor disruption to severe disseminated intravascular coagulation. Viral illnesses have previously been associated with a hypercoagulable state. Viral hepatitis has been shown to be associated with the development of venous thromboembolic events, particularly portal vein thrombosis.5 Patients with human immunodeficiency virus infection have shown an incidence of venous thromboembolism of up to 7%.6 Similar thrombotic events have been shown to occur in the setting of influenza H1N1 and varicella.4,7 The mechanism and degree of thrombotic dysfunction are variable. Viral infection has been associated with reduced platelet function secondary to decreased production, platelet consumption, and production of autoantibodies. Endothelial cells play a significant role in regulation of the coagulation cascade. Viral infection can result in dysregulation of this pathway secondary to activation of the procoagulant factors. Finally, antiphospholipid antibodies may develop during viral infections.8
The majority of described virus-associated thrombotic events affect the venous bed. Among previously reported thromboembolic events within arterial beds, the coronary vessels have been predominantly involved.4,9 The altered coagulation mechanisms and affected vascular beds in COVID-19 infection are unclear. It is known that these patients experience a severe inflammatory state with elevated D-dimer levels and a hypercoagulable state. Interestingly, in a study of COVID-19 patients, elevated D-dimer levels were shown to be associated with an elevated risk of in-hospital death.10
The primary cause and the incidence are unknown, but the embolus formation is occurring in the setting of a hypercoagulable state and is most likely of a cardiogenic source or large-vessel mural thrombus formation. A paradoxical embolus (venous thrombosis with a patent foramen ovale) is another possibility. Given the need for prudent resource use, few echocardiographic examinations, ultrasound scans, and CT scans have been performed at present. It is unclear whether other vascular territories are similarly afflicted; our findings suggest that is a possibility. Some of these events may be acute arterial ischemia in the peripheral and possibly other arterial beds. Consequently, it is critical to be aware of the risk of thromboembolic events in the setting of elevated D-dimer levels in patients with COVID-19. Because this is early in our experience, it is unclear to us what will be the resultant clinical course. Furthermore, it is unclear whether there is a role for prophylactic systemic anticoagulation. Tang et al11 reported a better prognosis in patients with COVID-19 and severe infections and elevated (>6000 ng/mL) D-dimer levels treated with anticoagulants. In our very limited series, D-dimer levels decreased with systemic heparinization in two patients, along with stabilization in their clinical course.
Conclusions
Appropriate surgical management of COVID-19 patients with arterial thromboembolic events is still being delineated on a case-by-case basis. It is largely being guided by the degree of stability of the patient's respiratory status, which may deteriorate rapidly in the acute, severely ill COVID-19 patient. Patients who are critically ill may be at too high risk to undergo any surgical or endovascular attempts at limb salvage. Anticoagulation is essential while it is being determined whether the patient should undergo immediate or deferred surgical therapy. If the patient recovers, revascularization can be considered if the limb is still salvageable, but amputation may be the only option if the limb progresses to nonviability. Timing of intervention after pulmonary recovery will be the most complex aspect of decision-making. For patients with acute-onset lower extremity ischemia and new diagnosis of symptomatic COVID-19, initial management with anticoagulation alone may be prudent. It is now known that some of these patients (such as the patient in case 1) will die of acute severe respiratory decompensation. Clinical predictors of such decompensation are still being identified, one of which may indeed be severely elevated D-dimer levels.
Footnotes
Author conflict of interest: none.
The editors and reviewers of this article have no relevant financial relationships to disclose per the Journal policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
References
- 1.Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rubin E.J., Baden L.R., Morrissey S., Campion E.W. Medical journals and the 2019-nCoV outbreak. N Engl J Med. 2020;382:866. doi: 10.1056/NEJMe2001329. [DOI] [PubMed] [Google Scholar]
- 3.Yang X., Yu Y., Xu J., Shu H., Xia J., Liu H. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475–478. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bunce P.E., High S.M., Nadjafi M., Stanley K., Liles W.C., Christian M.D. Pandemic H1N1 influenza infection and vascular thrombosis. Clin Infect Dis. 2011;52:e14–e17. doi: 10.1093/cid/ciq125. [DOI] [PubMed] [Google Scholar]
- 5.Galli L., Gerdes V.E., Guasti L., Squizzato A. Thrombosis associated with viral hepatitis. J Clin Transl Hepatol. 2014;2:234–239. doi: 10.14218/JCTH.2014.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jackson B.S., Pretorius E. Pathological clotting and deep vein thrombosis in patients with HIV. Semin Thromb Hemost. 2019;45:132–140. doi: 10.1055/s-0038-1676374. [DOI] [PubMed] [Google Scholar]
- 7.Zhang Y., Luo G., Huang Y., Yu Q., Wang L., Li K. Risk of stroke/transient ischemic attack or myocardial infarction with herpes zoster: a systematic review and meta-analysis. J Stroke Cerebrovasc Dis. 2017;26:1807–1816. doi: 10.1016/j.jstrokecerebrovasdis.2017.04.013. [DOI] [PubMed] [Google Scholar]
- 8.Goeijenbier M., van Wissen M., van de Weg C., Jong E., Gerdes V.E., Meijers J.C. Review: viral infections and mechanisms of thrombosis and bleeding. J Med Virol. 2012;84:1680–1696. doi: 10.1002/jmv.23354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nadel J., O'Dwyer E., Emmanuel S., Huang J., Cheruvu S., Sammel N. High-risk coronary plaque, invasive coronary procedures, and cardiac events among HIV-positive individuals and matched controls. J Cardiovasc Comput Tomogr. 2016;10:391–397. doi: 10.1016/j.jcct.2016.07.018. [DOI] [PubMed] [Google Scholar]
- 10.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tang N., Bai H., Chen X., Gong J., Li D., Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18:1094–1099. doi: 10.1111/jth.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]