Abstract
The recent recovery of mutations in vesicular trafficking genes causing congenital heart disease (CHD) revealed an unexpected role for the endocytic pathway. We now show that mice with a C4232R missense mutation in Low density lipoprotein receptor related protein 1 (LRP1) exhibit atrioventricular septal defects with double outlet right ventricle. Lrp1m/m mice exhibit shortened outflow tracts (OFT) and dysmorphic hypocellular cushions with reduced proliferation and increased apoptosis. Lrp1m/m embryonic fibroblasts show decreased cell motility and focal adhesion turnover associated with retention of mutant LRP1 in endoplasmic reticulum and reduced LRP1 expression. Conditional deletion of Lrp1 in cardiac neural crest cells (CNC) replicates the full CHD phenotype. Cushion explants showed defective cell migration, with gene expression analysis indicating perturbation of Wnt and other signaling pathways. Thus, LRP1 function in CNCs is required for normal OFT development with other cell lineages along the CNC migratory path playing a supporting role.
Subject terms: Embryology, Disease model
Lin et al. find that mutation in endocytic trafficking protein Lrp1 causes congenital heart defects in mice due to a requirement for Lrp1 in the neural crest lineage, where it regulates outflow tract lengthening. This study provides insights into how Lrp1 and the neural crest contribute to heart development.
Introduction
Congenital heart disease affects nearly 1% of neonates per year in the United States1 and is the leading cause of neonatal death2. With improved survival made possible by advances in critical care and surgical palliation, there are now over 2 million children and adults with CHD in the United States3. However, surviving CHD adults continue to face medical challenges, including rehospitalization, surgical re-intervention, or even need for heart transplantation. The development of better clinical management strategies will require a better understanding of the developmental etiology and pathomechanisms of CHD.
An unexpected role for the endocytic pathway in CHD4 pathogenesis was revealed by a large-scale mouse forward genetic screen for mutations causing CHD4. Among these was a missense mutation in Lrp1, a member of the multifunctional low-density lipoprotein receptor-related protein (LRP) family5,6. The LRP1 precursor is a 600 kD protein that is cleaved in the trans-Golgi network, generating a 515 kD α-chain (amino acid 20–3944) and a membrane bound 85 kD β-chain (amino acid 3945–4545). The α-chain of LRP1 contains various repeated motifs and the β-chain contains the C4232R mutation and two cytoplasmic NPxY sequences5 that serve as signals for endocytosis5,6. LRP1 was originally described to mediate endocytosis of lipoproteins, but it is now also appreciated that LRP1 can bind dozens of other ligands. This includes many different transmembrane receptors—LRP1 modulates their activity and downstream intracellular signaling, including their internalization, sorting to different endosomal compartments, recycling, and their degradation5,6.
Mice homozygous for a missense mutation in the cytoplasmic domain of the LRP1 β-chain (Lrp1m/m) survive to mid-gestation, dying prenatally from structural heart defects4. This stands in marked contrast to the peri-implantation lethality of Lrp1 knockout (KO) mice7. This Lrp1C4232R (Lrp1m/m) mouse model reveals a role for Lrp1 in CHD pathogenesis. Using this unique Lrp1m/m missense mutant and conditional KO mouse models with a floxed allele of Lrp1 (Lrp1f/f) crossed with different Cre drivers, we now investigate the pathomechanism for CHD arising from Lrp1 perturbation. Our studies indicate an essential role for Lrp1 in the cardiac neural crest (CNC) lineage, and a non-cell autonomous role in other cell lineages along the NCC migratory path. Further experiments with fibroblasts derived from Lrp1m/m mice suggest that this likely involves LRP1 regulation of cell motility and cell signaling required for proper NCC targeting to the heart.
Results
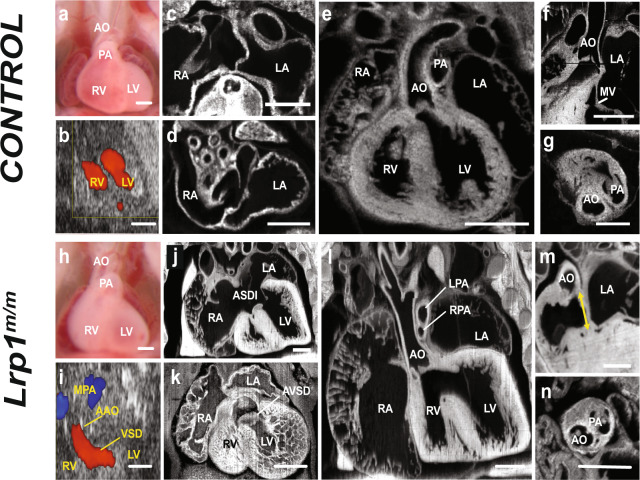
Mice harboring the Lrp1C4232R mutation exhibit homozygous lethality at E15.5, with rare stillborn pups. In addition to CHD, Lrp1m/m embryos also exhibited extracardiac defects such as micrognathia and cleft palate, with some mutants exhibiting gastroschisis with liver protruding outside the abdominal cavity (Supplementary Fig. 1). Cardiovascular assessments conducted using fetal echocardiography8 and episcopic confocal microscopy (ECM)9 showed outflow tract (OFT) malalignment defects in the Lrp1m/m mutants. Side-by-side positioning of the OFT was typically observed with blood flow across the ventricular septum, indicating possible double outlet right ventricle (DORV) (Fig. 1i, l, n). Also, commonly seen was atrioventricular septal defect (AVSD) with blood flow mixing between all four cardiac chambers (Fig. 1i–k).
Fig. 1. Homozygous Lrp1m/m mutants exhibit a spectrum of cardiac defects.
Compared with E14.5 normal control (a–g), Lrp1m/m mutant embryo showed side-by-side great arteries (h, i, l, n). Echocardiogram using Vevo2100 of control embryo indicated intact ventricular septum with no evidence of interventricular shunt (b), which was confirmed by histopathology with ECM (e). Representative fetal ultrasound of the Lrp1m/m mutant (i) showed a VSD and side-by-side great vessels with aorta positioned to the right of the pulmonary artery. This was also confirmed by ECM (l), which showed double outflow right ventricle with both the aorta (AO) and pulmonary artery (LPA and RPA) arise from RV and a large VSD. Compared to the wild-type control (c, d), the homozygous Lrp1m/m mutant exhibited ASD primum (ASDI) (j), a part of AVSD, and ventricular part defect of AVSD (k). While there is aorta to mitral valve continuity in controls (f), the Lrp1m/m mutant showed loss of the continuity between aortic valve and mitral valve, with a gap between the mitral and aortic valves (m, yellow arrowhead). Instead of aorta being to the right and posterior to the pulmonary artery (g), the Lrp1m/m mutant exhibited a side-by-side and rightward aorta (AO) to pulmonary artery (PA) relationship (n). Scale bars: 0.5 mm.
During heart development, a single outflow forms that subsequently divides to generate the aortic and pulmonary arteries. Initially, both arteries are connected to the RV, but with development, the aortic root shifts, becoming wedged between the AV valves, causing the aorta to lie over the left ventricle10. During this same developmental window, endocardial/epithelial–mesenchymal transition (EMT) occurs11, generating cardiac cushions that later remodel to form the semilunar and AV valves12. ECM imaging showed all the mutants have CHD, the majority (90.63%) exhibiting DORV with AVSD (Supplementary Fig 2), 6.25% having isolated DORV, and 3.12% with isolated AVSD (Fig. 1, Supplementary Fig. 2).
Lineage-specific roles of Lrp1 in cardiac development
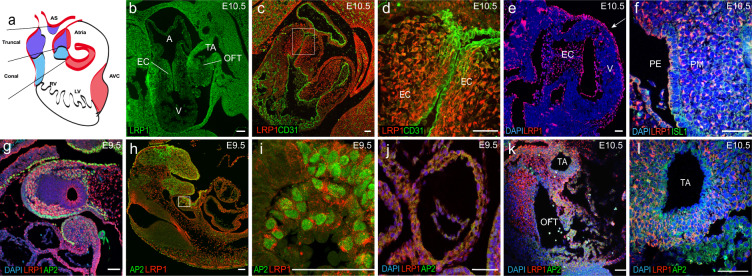
To determine whether Lrp1 could play a role that is autonomous to cell populations essential for development of the OFT and endocardial cushions, we examined LRP1 expression in the E10.5 embryo using immunohistochemistry with a LRP1 antibody. LRP1 expression was observed in many cell types in the heart, including the OFT (Fig. 2b, j–l), the mesenchymal cells in the atrioventricular (AV) canal (Fig. 2b–e), the OFT cushion (Fig. 2b, k) and in the epicardium (Fig. 2e). Antibody staining with ISL1, a second heart field (SHF) marker13, showed colocalization of LRP1 and ISL1 in pharyngeal mesoderm (PM) and endoderm (PE) (Fig. 2f). AP2α, a neural crest marker14, also colocalized with LRP1 in the OFT (Fig. 2j–l) and CNC/mesenchymal cells (Fig. 2g–i). Surprisingly, LRP1 expression was not observed in the endocardium/endothelial cells (Fig. 2b, c). The endocardium normally undergoes EMT to generate mesenchymal cells in the cardiac cushions11. These AV cushions function as primitive valves that later will remodel into mature cardiac valves.
Fig. 2. LRP1 expression in different cell linages that are important for cardiac development in the developing heart.
Panel a is schematic representation of embryonic heart showing AVC and OFT (conal part and truncal part). Immunostaining in E9.5 and E10.5 embryos demonstrated LRP1 is expressed in mesenchymal cells in the endocardial cushion, with no expression seen in the CD31 positive endocardium (b–d) at E10.5; epicardium (e white arrow) at E10.5 pharyngeal endoderm and pharyngeal arch (b, f) at E10.5, neural crest/mesenchymal cells (g–i) at E9.5; outflow tract (i) at E9.5, outflow tract (j) at E10.5 and truncal artery (k) at E10.5. LRP1 and Islet-1 (ISL1) are expressed in (f) pharyngeal endoderm and pharyngeal mesoderm. LRP1 and AP2α both expressed in the neural crest/mesenchymal cells (g–i) and outflow tract (j–l). i The magnified view from the white box of figure (h). Scale bars: 50 µm.
Since Lrp1 expresses in different cell lineages in the critical period in the developing heart, we used a floxed Lrp1 (Lrp1f/f) allele15 with different Cre drivers to orchestrate conditional Lrp1 deletion in cell lineages associated with cardiac development. The Cre drivers used included Wnt116, Mef2c-anterior heart field-Cre targeting cells17, Nkx2–518, Tie219, Twist220,21, and Nfat1c22, in the CNC (Wnt1-Cre16), anterior heart field/PM (Mef2c-Cre17), first heart field (Nkx2–5-Cre18), PE/PM (NKx2–5-Cre18), endothelial/endocardial cells and mesenchymal cells of AV cushion (Tie2-Cre19), mesenchymal cells of AV cushion (Twist2-Cre20,21), and endothelial/endocardial cells (Nfat1c-Cre22), respectively.
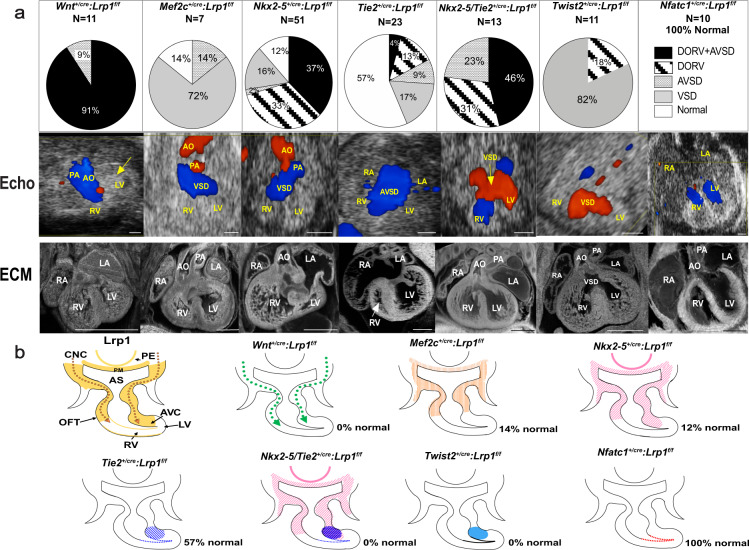
Wnt1-Cre16: To examine Lrp1 requirement in the CNC lineage, we conditionally ablated Lrp1 using the neural crest-specific Cre recombinase Wnt1-Cre16. Wnt1+/cre: Lrp1f/f mutant embryos did not survive past E14.5. Significantly, 100% of the Wnt1+/cre: Lrp1f/f embryos exhibited CHD: 91% with DORV and AVSD, and 9% with AVSD. This replicates the CHD phenotype and with same penetrance as seen in the Lrp1m/m mutant (Fig. 3). These findings suggest CHD in the Lrp1m/m mutant may arise from a cell autonomous requirement for Lrp1 in the CNC cells.
Fig. 3. Conditional Cre deletion of floxed Lrp1 allele.
Lrp1 conditional knockout in different cell lineages during cardiac development. a Pie charts illustrate the percentage of each cardiac phenotype. Representative echocardiograms and ECM pictures in each conditional knockout are demonstrated in (a). Targeting Lrp1 deletion in cardiac neural crest cells using Wnt1-Cre recapitulated the cardiac phenotype of Lrp1m/m mutants with AVSD and DORV in 91% of Wnt1+/Cre: Lrp1f/f mutants, yellow arrow indicated pericardial hemorrhage. Deletion of Lrp1 in the AHF derivatives using the Mef2c-AHF-Cre yielded septation defects. Nkx2–5-Cre mediated deletion of Lrp1 yielded mostly DORV with or without AVSD. Totally, 43% of mutants generated with the Tie2-Cre drivers had septation defects. Combined deletion of Lrp1 mediated by both Tie2-Cre and Nkx2–5-Cre recapitulated the high penetrance of the DORV/AVSD phenotype observed in the Lrp1m/m mutant and increase the penetrance of AVSD. Deletion of Lrp1 in the mesenchymal cells of AV cushion with Twist2-Cre drivers showed 82% of mutants with membranous VSD and 18% mutants with DORV. There were no cardiac abnormalities with the Nfatc1-Cre drivers, consistent with absence of Lrp1 expression in the endocardium of atrioventricular cushion. b Schematic representation of LRP1 expression (yellow) in developing heart around E10.5–E11.5. Lrp1 expresses in pharyngeal endoderm (PE) and pharyngeal mesoderm (PM), mesenchymal cells of outflow tract cushion, atrioventricular cushion, neural crest cell migration pathway as well as epicardial cells. Ablation of Lrp1 in neural crest cells using Wnt1-Cre (Wnt1+/Cre:Lrp1f/f) is illustrated in dotted green arrows. Ablation of Lrp1 using Mef2c-AHF-Cre (Mef2c-AHF+/cre: Lrp1f/f) is illustrated in orange. Ablation of LRP1 expression using Nkx2–5-Cre (Nkx2–5+/Cre:Lrp1f/f) is illustrated in pink, specifically in PM, PE, AHF, OFT cushion and AV canal cushion. Ablation of Lrp1 expression using Tie2-Cre (Tie2+/Cre: Lrp1f/f) is illustrated in navy-blue hatch (mesenchymal cells of atrioventricular cushion) and navy-blue dots (endothelium of atrioventricular canal and endocardium of ventricle). The ablation of Lrp1 in double knockout of Nkx2–5-Cre and Tie2-Cre (Nkx2–5+/Cre Tie2+/Cre: Lrp1f/f) is illustrated in purple and navy blue. Ablation of Lrp1 in Twist2-Cre is expressed in azure (royal blue). Ablation of Lrp1 expression in Nfatc1-Cre is expressed in red. Scale bars: 0.5 mm.
We next used Mef2c-AHF-Cre17 to test whether Lrp1 in the anterior heart field is essential for formation of the OFT17. With Mef2c-AHF-Cre17 deletion, 14% of the Mef2c-AHF+/Cre: Lrp1f/f embryos exhibited no CHD, while 72% had a membranous VSD, with one mutant (14%) exhibiting a partial AVSD (Fig. 3). None of Mef2c-AHF +/Cre:Lrp1f/f exhibited either DORV or DORV/AVSD as seen in the original Lrp1m/m mutant.
Nkx2–5 driven Cre18: Using the Nkx2–5-Cre driver18, we investigated the requirement for Lrp1 in myocardial cells from the first and SHF18. Analysis of 51 Nkx2–5+/cre:Lrp1f/f embryos showed 88% have CHD. This included 37% (N = 19) with AVSD and DORV, 33% (N = 17) with isolated DORV, 16% (N = 6) with membranous VSD, and 2% (N = 1) with unbalanced AVSD with dominant left ventricle (Fig. 3).
Tie219, Twist220,21, and Nfatc122 driven Cre: Using Tie2-Cre19, we targeted Lrp1 deletion in the endothelial/endocardial lineage, and also cells in the cushion mesenchyme19. With Tie2-Cre19 deletion, only 43% of the 23 Cre deleted embryos (Tie2+/cre: Lrp1f/f) have cardiac defects. This included 17% with membranous VSD (N = 4), 13% (N = 3) with DORV/subaortic VSD, 9% (N = 2) with isolated AVSD, and one (4%) with DORV/AVSD (Fig. 3). As Lrp1 is not expressed in endothelial/endocardial cells but is highly expressed in the cushion mesenchyme, this suggested that the CHD resulting from Tie2-Cre19 deletion may reflect a requirement for Lrp1 function in endocardium derived cushion mesenchyme. Hence, we further assessed Lrp1 deletion using the mesenchymal specific Twist2-Cre20,21 and endocardial specific Nfatc1-Cre22. All the mutant embryos with Twist2-Cre20,21 deletion (Twist2+/cre: Lrp1f/f) had septal defects with membranous VSD in 82% (N = 9) and isolated DORV in 18 (N = 2). In contrast, all ten embryos generated with Nfatc1-Cre22 deletion (Nfatc1+/cre: Lrp1f/f) had normal cardiovascular anatomy, suggesting the cushion mesenchymal cells targeted by Tie2-Cre are not of endocardial origin (Fig. 3).
To test whether Lrp1 in myocardial cells and in non-endocardium derived cushion mesenchyme contribute synergistically to heart development, we knocked out Lrp1 using the Nkx2–5-Cre18 and Tie2-Cre919 drivers simultaneously. This resulted in synergistic effects, with 100% of the embryos exhibiting CHD, as compared to 43% with Tie2+/Cre:Lrp1f/f and 88% with Nkx2–5 +/Cre:Lrp1f/f (Fig. 3). There was a tenfold increase in the incidence of DORV with AVSD—46% with Tie2+/Cre/Nkx2–5+/Cre:Lrp1f/f double Cre deletion vs. 4% with Tie2+/Cre:Lrp1f/f. Overall, there was a marked increase in AVSD—69% with the Tie2+/Cre/Nkx2–5+/Cre:Lrp1f/f double Cre deletion vs. 13% with Tie2 +/Cre: Lrp1f/f and 39% with Nkx2–5+/Cre:Lrp1f/f (Fig. 3). These results suggest both myocardial cells and non-endocardium derived cushion mesenchyme are essential for supporting Lrp1-mediated heart development.
Together, these data show Lrp1 function is primarily required in the CNC lineage, with a secondary requirement in the myocardial cells of the first and SHF and in cushion mesenchyme of non-endocardial origin.
OFT and defects in development of the endocardial cushion
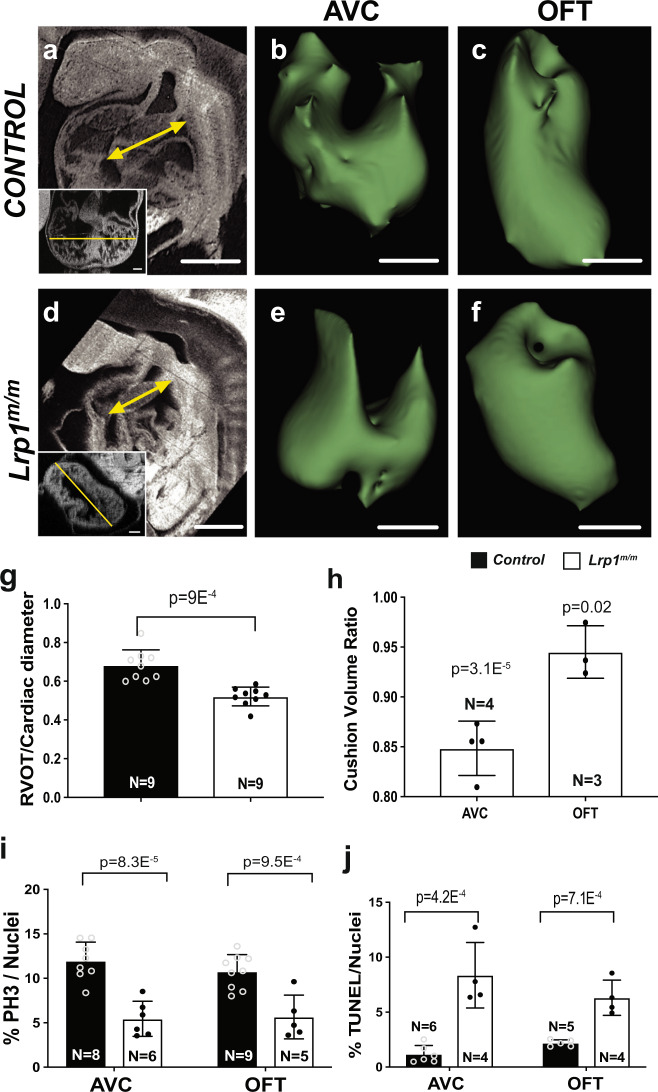
To determine the developmental processes contributing to the OFT malalignment and endocardial cushion defects in the Lrp1m/m mutants, we conducted ECM imaging and 3D reconstructions of the E10.5 heart from Lrp1m/m mutant and littermate control embryos. Measurements of the OFT length with cardiac diameter normalization showed a significant reduction in OFT length of the Lrp1 mutant embryos compared to littermate controls (Fig. 4a, d, g). Volumetric analysis of the atrioventricular (AV) and outflow (OFT) cardiac cushions with cardiac diameter normalization showed a significant reduction in the volume of both cardiac cushions in the Lrp1m/m mutant embryos (Fig. 4b, c, e–h). Analysis of cell proliferation with pH3 staining23 revealed a decrease in mitotic cells in the AVC/OFT cushion of Lrp1m/m embryos (Fig. 4i, Supplementary Fig 3a). Transferase-mediated dUTP-biotin nick-end labeling (TUNEL) staining24 showed increased apoptosis in the endocardial cushions of Lrp1m/m mutants (Fig. 4j, Supplementary Fig. 3b).
Fig. 4. Lrp1m/m mutant hearts exhibit decreased OFT length, decreased AVC and OFT cushion volume, decreased proliferation and increased apoptosis at E10.5.
ECM in the sagittal plane of an E10.5 wildtype (a) and Lrp1m/m mutant (d) embryos are shown, indicating reduction in length of mutant OFT versus control (yellow arrow in (a, d)). Insert panels in a and d demonstrated the cardiac diameter (yellow line) in the corresponding embryo. g Quantitative measurement using histopathology images showed a significant decrease in the length of the OFT with cardiac diameter normalization in the homozygous Lrp1m/m mutant hearts as compared to the controls (combined wildtype and Lrp1+/m hearts). Three-dimensional (3D) reconstructions of AVC (b, e) and OFT (c, f) were generated from ECM of Lrp1m/m mutant hearts and controls at E10.5. h Quantitative measurement using histopathology images and 3D slicer to process the images showed a significant decrease in the volume (with normalization of cardiac diameter) of the AVC and OFT in the homozygous Lrp1m/m mutant hearts as compared with the controls (combined wildtype and Lrp1+/m hearts). i Quantitative results of cell proliferation using the ratio of positive pH3 cells/total cushion cells in AVC and OFT cushion. k Quantitative results of the ratio of positive TUNEL cells/total cushion cells. Statistical comparison was performed using unpaired two-way Student′s t test. Error bars show standard deviation. Scale bars: 0.5 mm in (a, d); 0.1 mm in (b, c, e, f).
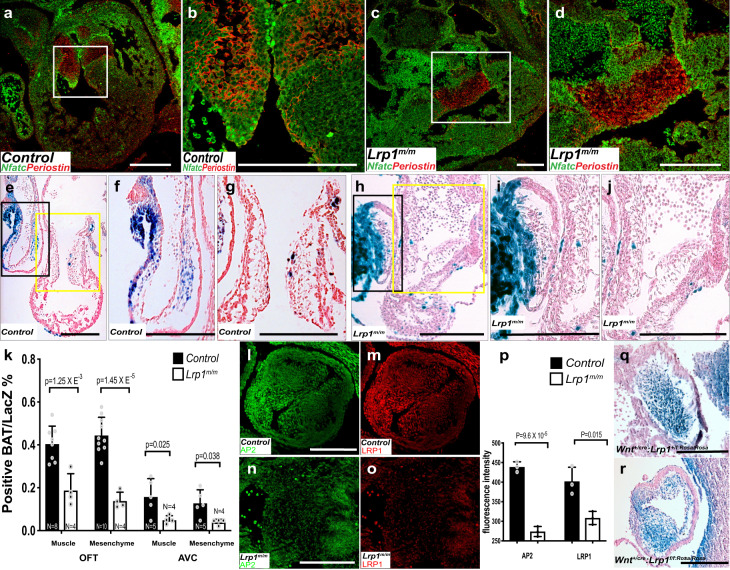
Further analysis of the E12.5 Lrp1m/m mutant heart revealed a single primitive, undivided AV cushion as compared to two distinct, well separated AV cushions (the mitral and tricuspid valve anlage) in wild-type embryos (Fig. 5a–d). Immunostaining of Periostin, an extracellular matrix protein expressed in the cardiac cushions, revealed deformed and immature endocardial cushions in the Lrp1m/m mutants (Fig. 5a–d).
Fig. 5. Abnormal atrioventricular and outflow tract cushion development was observed in Lrp1m/m mutants.
Immunostaining of NFATC-1 (green) and Periostin (red) in wildtype (a, b) and Lrp1m/m mutant (c, d) embryos at E12.5. b, d Magnified views of the white box from (a, c). Compared with the well-formed two atrioventricular valves in the wildtype control, Lrp1m/m mutant demonstrated primitive undivided endocardial cushion morphology. Representative images from X-gal and Eosin stained tissue sections from E10.5 control (Lrp1m/+) (e–g) and Lrp1m/m mutant (h–j). f, i The high magnified view of the black box e and h (OFT). g, j The high magnified view of the yellow box from (e, h) (AV cushion). X-gal staining e–j demonstrated diminished expression of LacZ expression in the Lrp1m/m AVC (e, f, h, i) and OFT cushion (e, g, h, j). k Quantitative analysis of the percentage of positive BAT/LacZ cells in the muscle and mesenchyme portion of OFT and AVC. Lrp1m/m mutant had hypocellular AVC and OFT cushions (h–j). l–o Immunostaining of AP2α (green) and LRP1 (red) in Lrp1m/m mutant (n, o) and control (l, m). Lrp1m/m mutant had decreased expression of AP2α and LRP1 in the outflow tract at E10.5–E11.5 (l–o). p Quantitative analysis of the fluorescence intensity of AP2α and LRP1 demonstrated decreased expression of both AP2α and LRP1 in Lrp1m/m mutant OFT. (q, r) LacZ staining of OFT in Wnt1+/cre: Lrp1f/f/rosa/rosa and control (Wnt1+/cre: Lrp1f/+/rosa/rosa), which demonstrated decreased LacZ expression in the Wnt1+/cre: Lrp1f/f/rosa/rosa mutant. Scale bars: 200 μm. Statistical comparison was performed using unpaired two-way Student's t test. Error bars show standard deviation.
Given the known importance of Wnt signaling in cardiac valvular morphogenesis, we crossed a Wnt BAT-LacZ reporter25,26 into the Lrp1m/m mutant line to assess in situ Wnt signaling in the developing cardiac cushions. X-gal staining revealed a marked decrease in LacZ expressing cells in the AV and OFT cushions of the E10.5 Lrp1m/m mutant embryos as compared to control (Fig. 5e–k). This was associated with hypocellularity characterized by decreased abundance of mesenchymal cells in the endocardial and OFT cushions (Fig. 5e–j), these results consistent with the reduced cell proliferation and increased apoptosis observed earlier in the E10.5 embryo AVC and OFT cushions (Fig. 4i, j).
To further delineate the role of LRP1 in OFT development, immunostaining of AP2-α14, a marker for neural crest cell and LRP1 in E10.5 embryos was used. Compared with controls (Fig. 5l, m), Lrp1m/m mutants demonstrated significant decreased expression of AP2-α and LRP1 (Fig. 5n–p) in the developing OFT. Consistent with the conditional deletion of LRP1 in the neural crest lineage16 results in OFT defects (DORV with AVSD, DORV), a Cre lineage tracing of the neural crest cells, Wnt1-Cre16 with Rosa/LacZ reporter27, demonstrated decreased expression of the Wnt1/LacZ cells in the OFT at E10.5 embryos (Fig. 5q, r), which is consistent with the decrease in Wnt BAT-LacZ expression in the AV and OFT cushions of Lrp1m/m mutants (Fig. 5e–k). These data indicate neural crest cells require LRP1 to migrate to the cardiac OFT properly.
Gene expression analysis indicates EMT and cell migration defects
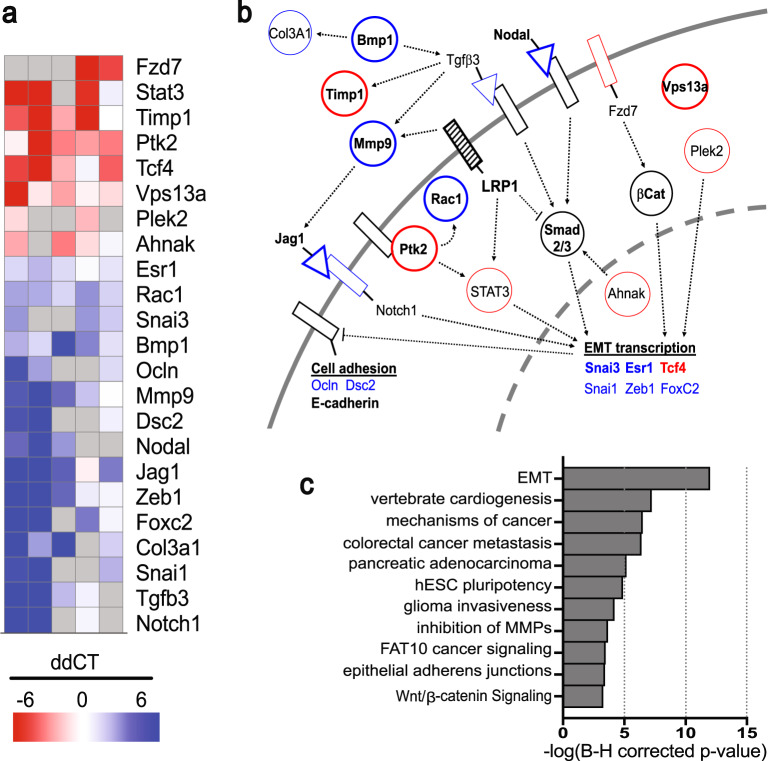
Since the hypocellularity associated with both the Lrp1m/m endocardial and OFT cushions suggest a hindrance in the ability of Lrp1m/m cells to undergo proper EMT, leading to cardiac cushion defects, we next sought to investigate the molecular perturbation underlying the cardiac cushion defects in the Lrp1m/m mutants. We used a polymerase chain reaction (PCR) array focused on EMT to examine gene expression alterations in the E10.5 AV cushions (Fig. 6a, b). We conducted these real-time PCR experiments on five independent mutant and littermate control embryo pairs. When we analyzed altered genes using Integrated Pathway Analysis, several impacted pathways related to EMT stood out (Fig. 6c). These included cell–cell adhesion and cell invasiveness, vertebrate cardiogenesis, cancer metastasis, glioma invasiveness, inhibition of matrix metalloproteinase, and epithelial adherens junctions (Fig. 6b, c). Also observed were changes in Wnt/catenin signaling26,28 and gene expression changes related to bone morphogenetic protein (Bmp)/transforming growth factor-β (Tgf-β)12, and Notch signaling12—all cell signaling pathways with important roles in valvular morphogenesis12 (Fig. 6b).
Fig. 6. Quantitative differential expression of EMT-related genes by real-time PCR.
Quantitative comparison of EMT-related gene expression in Lrp1m/m vs. WT in the endocardial cushion at E10.5, prepared using Morpheus (Broad Institute) (a). a Genes which were repeatably over- (red) or under-expressed (blue) are shown. After discarding measurements with unacceptably low copy number (gray; raw Ct > 40), replicate ddCt values were filtered with an outlier test (ROUT, Q = 10%) and samples with three replicates were tested against a H0 that ddCt = 0 using a one-sample two-tailed t test. Genes that passed (p < 0.05) are shown, along with other strongly responsive genes (p ≥ 0.05, but mean > variance). Red indicates genes upregulated in Lrp1m/m and blue indicates downregulated. The functional relationships between these genes is shown in (b); bolded names or outlines indicate genes with significant overexpression or underexpression and lighter names or outlines indicates strongly responsive but not significant. Color coding is the same as in (a); black outlines or names represent key genes that are not overexpressed or underexpressed in this analysis. The major cellular pathways represented by this gene set are shown in (c), as determined by Ingenuity Pathway Analysis.
Cell migration defects due to reduced FA turnover
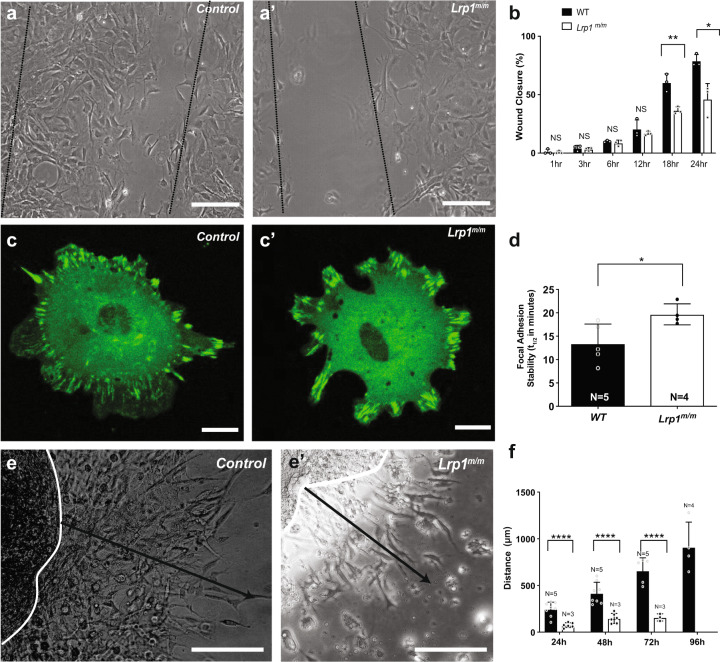
Given the importance of directional cell migration in the targeting of NCC to the heart, we tested whether the Lrp1C4232R mutation inhibits the migration of fibroblasts from Lrp1m/m embryos. This entailed growing the MEFs to confluency, then making a scratch in the monolayer to create a wound gap to stimulate cell migration. Lrp1m/m mutant MEFs showed a significant reduction in the rate of wound closure (Fig. 7a, a′, b). This was due to alterations in the speed of cell locomotion, but no change in the directionality of cell migration was observed (Fig. 7a, a′). Consistent with this, Golgi apparatus oriented normally with the direction of cell migration, indicating the ability of cells to polarize was unaffected (Supplementary Fig. 4a, d).
Fig. 7. Lrp1 mutation causes migration defects.
a, a′ Representative image of wound scratch assay from (a) a wild-type control and (a′) a mutant MEF, 18 h after scratch. b Quantitation of wound gap closure showed Lrp1m/m mutant MEFs have decreased cell motility compared to control with decreased filling of the scratch “gap” compared with controls. % indicates the percentage of closing the gap, 100% means close the gap completely. c Wild-type and (c′) Lrp1m/m mutant fibroblast cells were transfected with vinculin-GFP and vinculin turnover was measured using live-cell imaging. d Quantitative measurement revealed slower focal adhesion turn over in MEF from Lrp1m/m mutant, as measured by the half-life of the overlap between segmented focal adhesions at time 0 and time n. e, e′ Representative imagine of AVC explant migration assay from a wild-type control (e) and Lrp1m/m mutant (e′). Black line indicated the migration distance at 48 h. f Quantitative analysis of the distance of migration of endocardial cushion explants from E10.5 control and Lrp1m/m embryos showed near complete loss of cell migration from the Lrp1m/m mutant explants Statistical comparison was performed using unpaired two-way Student′s t test. Error bars show standard deviation.
Notably, LRP1 regulates the trafficking of integrins, which regulate focal adhesions (FA) and link the extracellular matrix to the actin cytoskeleton for cell migration and matrix adhesion29. To determine whether our Lrp1m/m mutation alters the structure or behavior of FA, we used quantitative confocal imaging analysis with an antibody to vinculin to measure the number, area, and length of FA in Lrp1m/m MEFs. This analysis showed no net change in focal contacts in mutant vs. wild-type MEFs (Supplementary Fig. 4b, c, e–g). Since FA are dynamic structures regulated by turnover, we conducted live cell imaging with transient transfection of a vinculin-GFP reporter to measure the FA turnover rate. Our analysis showed turnover in the FA “footprint” (Fig. 7c, c′, d, Supplementary Video 1) is significantly reduced in Lrp1m/m MEFs, consistent with the reduced cell migration rate seen for the mutant MEFs in the wound closure assay (Fig. 7a, a′, b). As formation of the endocardial cushion involves EMT, a process associated with cell invasion and migration11, we tested migration of cushion cells by plating AV cushion explants on a collagen gel matrix. This analysis showed a marked reduction in migration (Fig. 7e, e′, f) of Lrp1m/m mutant explants.
LRP1 C4232R mutation alters endocytic trafficking
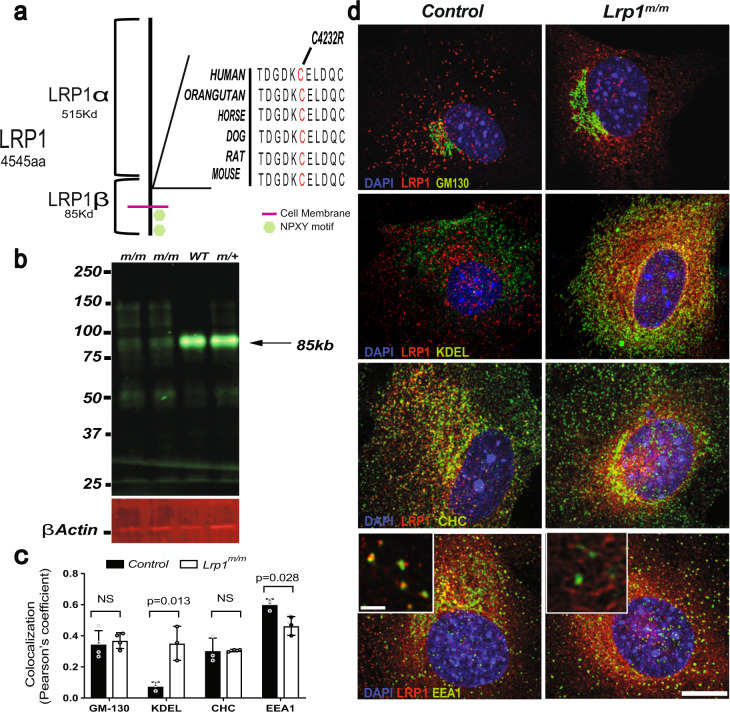
Since the Lrp1 missense mutation C4232R is situated in a highly conserved cysteine residue within one of the extracellular EGF repeat domains of the protein that bind membrane proteins/ligands5,6, we next sought to determine how Lrp1C4232R is altered at the protein level (Fig. 8a). Western blot of protein extraction from the liver of Lrp1m/m showed the LRP1 protein is expressed, though at reduced levels, suggesting that the protein could be degraded during or after synthesis (Fig. 8b).
Fig. 8. LRP1 C4232R mutant protein affects the expression of the 85 kD (LRP1-β) domain and has increased retention in the endoplasmic reticulum.
Schematic diagram of LRP1 protein. a LRP1 consists of 4545 amino acids. The mutant line 1554 (MGI 96828) harbors a missense (C4232R) mutation in the extracellular region encoding the EGF repeat domain of LRP1. The cysteine residue mutated at position 4232 is conserved among species. b Immunoblotting analysis showed strongly reduced expression of the 85 kD LRP1 protein in liver lysates from the Lrp1m/m homozygous mutant, as compared with Lrp1+/m and wild-type embryos. c Quantitative measurement of colocalization between LRP1 and the markers of cellular compartments using Pearson′s coefficient demonstrates (c, d) Lrp1m/m has increased localization to the ER (KDEL) and decreased localization in early endosomes (EEA1). Error bars showed mean standard deviation. d Immunostaining for LRP1 and markers for the Golgi apparatus (GM130), endoplasmic reticulum (ER) marker (KDEL), clathrin marker (CHC), and the early endosome marker (EEA1). Scale bars: 10 µm in (d); 2 µm in magnified box in (d).
To test whether trafficking of LRP1C4232R is normal, we used confocal microscopy to measure its colocalization with various cell compartments, including an endoplasmic reticulum (ER) resident protein (KDEL), a Golgi marker (GM130), clathrin heavy chain (CHC) (Fig. 8c, d), and the early endosome marker (EEA1)30 (Fig. 8c, d). In wild-type MEFs, LRP1 showed the expected strong colocalization with early endosome marker EEA1, but less so with KDEL, GM130, and CHC (Fig. 8d). In contrast, in Lrp1m/m MEFs, LRP1 had reduced co-localization with early endosomes, but strong co-localization with KDEL (Fig. 8c, d), indicating ER retention of the mutant LRP1C4232R protein. These results suggest the substantial reduction in the 85 kD LRP1 β-chain seen in the Western blot is likely due to retention and ER-associated degradation of misfolded LRP1 protein or due to problems cleaving the 600 kD protein to 515 and 85 kD subunits.
Discussion
We showed that a missense mutation in Lrp1, Lrp1C4232R, causes prenatal lethality with DORV and AVSD. This was embryologically associated with shortening of the OFT and hypoplastic/under-development AV cushions. The Lrp1C4232R mutation is hypomoprhic, with retention in the ER and marked reduction of the 85 kD LRP1 β-chain, likely a reflection of degradation of misfolded protein or problems during cleavage of the of 600 kD LRP1 protein. Using Cre drivers we found the CHD phenotypes and disease penetrance of the Lrp1 mutation can be fully replicated with Wnt1-cre, suggesting that LRP1 function during OFT development is required cell autonomously in the CNC cells.
While Lrp1 deletion mediated by other Cre drivers also generated cardiac defects, unlike Wnt-1+/Cre:Lrp1f/f in which 100% of the mutant embryos exhibited CHD, fewer cardiac defects were seen in Nkx2–5+/Cre:Lrp1f/f, Mef2c-AHF+/Cre:Lrp1f/f and Tie2+/Cre:Lrp1f/f deletion embryos (12%, 14%, and 57% had normal cardiac anatomy, respectively). These Cre drivers have broad expression domains encompassing embryonic tissues with Lrp1 expression found along the CNC migratory path, including myocardium of the first/SHF, PE and PM, and cushion mesenchyme. Hence, Lrp1 expression in these additional cell lineages may provide signaling function that can ensure quantitative recruitment of neural crest cells to the heart. Consistent with this model, Nkx2–5+/Cre/Tie2+/Cre: Lrp1f/f double Cre deletion exert additive effects, yielding more severe phenotypes with higher heart defect penetrance than either Nkx2–5+/Cre or Tie2+/Cre Cre driver alone.
The requirement for CNC cells in heart development is well described31–34, but what function they provide remains largely unknown. Neural crest cells are required for OFT alignment and septation28,31–34, and in the development of the endocardial cushions26. Proper great artery connections with their respective ventricles involve rotation of the developing great arteries and wedging of the aorta between the AV valves to achieve mitral-aortic fibrous continuity10. Our observations in the Lrp1m/m mutants with decreased expression of AP2-α expression and decreased Wnt BAT-LacZ expression in the developing OFT would suggest that CNC cells may impact this process through regulating OFT lengthening by providing migratory cues for recruiting SHF cells and endothelial-derived cells to the distal OFT. Conditional deletion of CNC using Wnt1-Cre driver had decreased expression of neural crest cells in the OFT cushion at E10.5 indicating the necessity of LRP1 expression in the developing heart for proper CNC cell migration. Consistent with this, CNC ablation can lead to shortening of the OFT with fewer myocardial cells added to the distal OFT31.
Our analysis of the cardiac cushion explants and MEFs from the Lrp1m/m mutant embryos revealed a cell migration defect. This was associated with reduction in the rate of FA turnover. LRP1 is known to regulate cell surface recycling of integrins that link the actin cytoskeleton with the extracellular matrix in FA29. Our Lrp1C4232R mutation is situated in the β-chain harboring the endocytic NPxY1/NpXy2 and YXXL motifs. Previous studies of MEFs with a Lrp1 knock-in replacement of the NPxY2 motif showed elevated cell surface β1-integrin expression29. This was associated with increased cell adhesion and decreased cell migration29, results similar to our findings. Based on these findings, we propose a primary CNC migration defect may drive the OFT malalignment phenotype in the Lrp1m/m mutant. We note a previous study of mice with Nkx2–5-Cre mediated KO of Fak encoding FA kinase yielded CHD comprising DORV or overriding aorta35. As Nkx2–5-Cre deletion of Lrp1 also generated DORV, but with reduced penetrance compared to the Wnt1+/Cre: Lrp1f/f, this would suggest there is a secondary requirement for Lrp1 in cells of the SHF to orchestrate normal OFT lengthening.
Gene expression analysis of the cardiac cushions showed disruption of Wnt/catenin signaling, as well as perturbation of Bmp/Tgfβ, and Notch signaling. KO mice with disruption of these three cells signaling pathways all show OFT defects, including DORV36. In contrast, OFT malalignment defects are not observed with disruption of NFAT, a signaling pathway also known to play important roles in valvular morphogenesis37,38. The disruption of these cell signaling pathways may secondarily contribute to the cushion mesenchyme hypoplasia. This cushion defect may also cause AVSD and perturb wedging of the aorta to disrupt OFT alignment.
Unexpectedly, we found evidence for altered EMT in Lrp1m/m mice. Cardiac cushions in the e10.5 OFT, which depend strongly on EMT for development38, are dysmorphic and hypocellular in Lrp1m/m mice. Fibroblasts derived from Lrp1m/m embryos had reduced FA turnover and reduced directional migration both in 2D assays and in 3D explant cultures. Importantly, this defect was not caused by loss of directional migration, as the Golgi apparatus oriented normally towards a gap ‘scratched′ in a confluent monolayer. These data suggest that the EMT pathway could be a key effector of Lrp1 during heart development, a question that we intend to pursue more detail in subsequent studies.
In summary, we show, to our knowledge, for the first time, deficiency in an endocytic trafficking protein in the cardiac NCC can cause CHD from disruption of OFT lengthening and the expansion of AV cushion mesenchyme. Further investigations into how disruption of endocytic trafficking may affect neural crest function may help elucidate the developmental etiology for DORV and give insights into the broader role of endocytic trafficking in CHD pathogenesis.
Methods
Recovery of the Lrp1 mutation and mouse breeding
Mouse studies were conducted under an approved University of Pittsburgh Institutional Animal Care and Use of Committee protocol. Breeding for the mutagenesis screen was described previously4, which used both male and female mice between 6 weeks and 6 months of age in a C57BL6/J background. Genomic DNA for line 1554 (MGI: 5437079) was harvested from a mutant embryo displaying AVSD, DORV, and pulmonary stenosis, then sequence captured using Agilent SureSelect Mouse All Exon Kit V1 and sequenced using Illumina HiSeq 2000 with a goal of 50× as the minimum target-sequence coverage (BGI America). Sequences were aligned to the C57BL/6J mouse reference genome (mm9) then analyzed using CLCBio Genomic Workbench and the Genome Analysis Toolkit (GATK) software. Annotation of sequence variants was performed using ANNOVAR (http://www.openbioinformatics.org/annovar/) then filtered against custom in-house scripts as well as dbSNP128 (https://www.ncbi.nlm.nih.gov/projects/SNP/snp_summary.cgi?build_id=128). Ten homozygous coding mutations were identified (Supplementary Table 1) More than 10 generations of breeding line 1554 has further validated the Lrp1 mutation as responsible for the displayed phenotypes.
Mouse lines
Line 1554 (MGI:5437079) harboring a Lrp1C4232R missense mutation (Lrp1m/m) previously recovered from a large scale mouse mutagenesis screen4 is maintained in a C57BL6/J strain background, having been backcrossed for more than ten generations with C57BL6/J mice. A Lrp1flox/flox (Lrp1f/f) allele was obtained from the Jackson Laboratory [Lrp1tm2Her/J #012604]15 which was generated by inserting a floxed Neo cassette and a single loxP site into the downstream of exon 2 of Lrp1 gene. This floxed allele (Lrp1f/f) was crossed with mice carrying different Cre drivers for targeting Lrp1 deletion in different cell lineages: this includes intercrossing with Wnt1-Cre [Wnt1-Cre [Tg(Wnt1-GAL4)11Rth/J Jackson Labs Stock #003829]. This line uses an enhancer of the Wnt1 gene to express Cre in early NCCs16. Nkx2–5IRESCRE [NKx2–5KItm2(cre)Rph/J, Jackson Labs Stock #024637] allele is a knock in of the Cre Recombinase into the Nkx2 homeobox 5 locus, leaving the protein coding region unaltered17.Tie2-Cre [Tg(Tek-cre)1Ywa/J Jackson Labs #008863]18 expresses Cre under the control of the tyrosine kinase Tek18. Nfatc1-Cre was developed with an insertion of Cre downstream of the mouse Nfatc1 stop codon19. Cre expression was seen in endocardial and in the mesenchymal cells (from the daughter cells of endocardial cells) of the developing heart19, this line was kindly provided by Bin Zhou, Einstein College of Medicine, NY19. Mef2c-AHF-Cre, a transgenic line using Mef2c promoter elements, was kindly provided by B. Black (UCSF, CA)20.
Genotyping
Genotyping was performed using GoTaqTM Hot Start Polymerase: Green Master Mix, 2× (Promega, PRM5122) and Dimethyl Sulfoxide (DMSO) (Sigma, D2650). PCRs were performed on SimpliAmpTM Thermal Cycler (Fisher Scientific, A24811). Primer sequences are listed in Supplementary Table 2.
Ultrasound assessment of cardiac structure
In utero fetal echocardiography was performed with the Visualsonics Vevo 2100 ultrasound system8. Our ultrasound phenotyping pipeline utilized a standard combination of two-dimentional, color flow and spectral Doppler imaging. This was conducted from E13.5 to E15.5, spanning the time when OFT septation and cardiac chamber formation are completed8.
ECM histopathology and volumetric analysis
Fetuses identified with CHD by ultrasound screening were harvested and embedded in paraffin for serial histological imaging using ECM9. The serial image stacks were digitally sectioned in different imaging planes and reconstructed in three-dimensions (3D) for CHD diagnosis. DORV is defined as both great arteries arising from the RV with aorta >50% over the RV and/or with aortic-mitral valve discontinuity. 3D Slicer was used to segment and quantify the endocardial and OFT cushions from reconstructions of the serial image stacks from E10.5 samples.
Sodium dodecyl sulfate (SDS) polyacrylamide gel electrophoresis and immunoblotting
Mouse tissues/cells were lysed in lysis buffer (Lysis Buffer 25 mM Tris-HCl (pH 7.4), 150 mM NaCl 5 mM EDTA, 0.5% w/v TritonX-100 and 50 µM phenylmethylsulfonyl fluoride and complete mini EDTA free protease inhibitor (Roche-Sigma 11836170001). Proteins were quantified using bicinchoninic acid (Pierce) or Bradford (BioRad). Samples with 30 μg of total protein were boiled in SDS sample buffer. SDS denatured samples were resolved on gels prepared from a 30:.04 acrylamide-bis-acrylamide. Gels were transferred on PVDF membrane. Membranes were blocked in Odyssey blocking buffer (PBS) (LI-COR) for 1 h at room temperature. Blocked polyvinylidene fluoride (PVDF) membranes were incubated with antibodies in odyssey blocking buffer in PBST at room temp for 2 h. After washing in PBST, secondary antibody combinations were IRdye 800CW donkey anti-rabbit IgG (1:10,000) and IRDye 680RD donkey anti-mouse IgG (1:10,000) (LI-COR) in PBST and 0.01% SDS for 1 h in dark. Immunoblots were washed four times in PBST with a final wash in PBS. Dried immunoblots were scanned in IR Odyssey imager-classic (LI-COR).
Whole mount β-galactosidase staining
Embryos were dissected in phosphate-buffered saline (PBS) and fixed with 4% paraformaldehyde in PBS. For detection of β-galactosidase activity, embryos were stained overnight at 37 °C in standard staining solution containing X-gal.
Analysis of cell proliferation and apoptosis
Embryos embedded in paraffin were sectioned and stained using antibodies to anti-phosphorylated histone H3 antibody (pH3) for assessing cell proliferation21 and deoxynucleotidyl TUNEL with a Roche TUNEL assay kit to measure apoptosis22.
Immunohistochemistry and confocal microscopy
Cells or embryos were fixed in either 4% paraformaldehyde or −20 °C methanol. Antibody incubation was carried at room temperature for an hour with cells and overnight at 4 °C for tissue sections using different primary antibodies (Supplementary Table 3). Incubation of secondary antibodies (Supplementary Table 4) were carried out at room temperature for one hour. All immunohistochemistry results are from at least three mutants and three controls. Imaging was conducted using Leica TCS-SP8 or Olympus fluoview confocal microscope. Images were quantitatively analyzed using ImageJ and/or CellProfiler.
Wound closure assay
Mouse embryonic fibroblasts (MEFs) were isolated from E12.5 to E13.5 mouse embryos. MEFs were grown to confluency, and then a scratch in the monolayer was made using a 20 µL pipette tip attached to a vacuum line, and time lapse images were obtained at 1, 3, 6, 18, and 24 h using a 20× objective to measure the extent of wound closure. After wound closure, a second scratch was made, and 18 h later, cells were fixed and stained with anti-Giantin antibody to measure the angle between the center of mass of the Golgi apparatus and the center of mass of the nucleus, relative to the edge of the wound. Only cells within 100 microns of the wound edge were chosen for angle measurements, while cells >200 microns from the wound edge were used as non-polarized controls.
Cushion explant cell migration assay
The endocardial cushion from E10.5 embryos was excised and explanted endocardial side down onto rat tail collagen gel (Enzo Life Sciences) and cultured at 37 °C. Cushions were imaged after attachment to the gel, then at 24 and 48 h post-explant to measure gel invasion by cushion cells using ImageJ.
Vinculin FA and stability assays
MEF cells were stained for Vinculin (Sigma, 1:400, V9131). Images were processed to increase contrast and quantify size and number of FA. Particles less than 0.45 µm2 were excluded from quantification.
Cells were transfected with mEmerald-vinculin (Addgene, 54302) using TransIt-X2 (Mirus Biosciences) following manufacturer′s directions. Totally, 48–72 h after transfection, the cells were imaged on a Leica SP8 confocal microscope in resonant scanning mode. A 40×/1.3NA oil immersion objective was used for imaging and adaptive focus control was used to maintain the focal plane during acquisition. To separate FA from cellular background, a narrow pinhole (1 AU) was used and the focal plane set as low as possible. Images were collected every 45 s for 70 min using low laser power (<1%) to prevent photodamage.
To measure the stability of FA, cells were pre-processed using Fiji software (Gaussian blurring to reduce noise, followed by background subtraction). FA were then segmented by intensity thresholding, and a single intensity threshold was used to segment FA at all subsequent time points. The % of segmented pixels overlapping with segmented FA at time 0 was measured for each time point, then the overlap % was graphed vs. time and a mono-exponential decay curve was fit to the plots using Prism 8 (GraphPad, Inc.). A greater mono-exponential decay kinetic (min−1) indicates faster turnover of FA.
Quantitative PCR array
Changes in expression of EMT-related genes were analyzed using a RT2 Profiler Mouse EMT PCR Array (PAMM-090Z; QIAGEN). Total RNA of E10.5 endocardial cushion tissue was isolated using RNeasy Plus kit (QIAGEN), then cDNA was synthesized and amplified using Ovation RNA-Esq system V2 kit (TECAN, CA). Amplification and real-time analysis were performed with ABI 7900HT. After normalizing transcript levels to Gapdh, relative mRNA levels in control vs. mutant cushions were calculated according to the comparative Ct (ddCt) method. Cardiac cushions from five pairs of mutants and controls at E10.5 were run on five independent microarrays. Due to the small size of endocardial cushion at E10.5, high variability was expected in transcript measurements. Thus, this assay was run several times to improve the experiment′s signal to noise ratio. In each run, samples with unacceptably low copy number (raw Ct > 40) were excluded for quality control purposes. Then the ddCt values for each transcript were filtered with an outlier test (ROUT, Q = 10%) in Prism 8 (Graphpad Inc.), and column statistics were performed on remaining ddCt values using a one-sample t test against a hypothetical value of 0. Two types of transcript were identified: strongly responsive genes (p < 0.05), and weakly responsive genes (p ≥ 0.05, but the mean > variance). These genes were then entered into IPA software (Qiagen, Inc.) for pathway analysis.
Statistics and reproducibility
Experiments were performed using multiple wildtype and mutant embryos from the same litter. The results generated from mutants from the same litter were averaged, with the same done for wildtype embryos. Then, experiments were replicated with more litters, with each litter representing an n of 1. Multiple litters were used for all statistics. For experiments comparing wild-type and mutants, statistical comparison was performed using unpaired two-way Student's t test for most and Morpheus (Broad Institute) and one-sample two-tailed t test for EMT-related gene expression comparisons.
Reporting summary
Further information on experimental design is available in the Nature Research Reporting Summary linked to this paper.
Supplementary information
Description of Additional Supplementary Items
Acknowledgements
The authors would like to thank Bin Zhou and Brian Black for Nfatc1-Cre and Mef2c-AHF-Cre, respectively. This work is supported by the Department of Critical Care, University of Pittsburgh (start-up fund) and American Heart Association Scientist Development Award (#17SDG33670982) to J.H.L. and NIH Grants HL098180, HL132024, and HL142788 to C.W.L.
Author contributions
These authors contributed equally to this work: J.H.L., T.F., A.J., and J.M. Conceived and designed the experiments: J.H.L., A.J., T.F., and C.W.L. Performed the experiments: J.H.L., A.J., T.F., J.M., J.X., A.A., G.G., L.M., and T.R. Data analysis: J.H.L., T.F., J.M., and X.X. Wrote the paper: J.H.L., T.F., J.M., and C.W.L.
Data availability
The authors declare that all data supporting the findings of this study are available within the paper
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Jiuann-Huey I. Lin, Timothy N. Feinstein, Anupma Jha, Jacob T. McCleary.
Supplementary information
Supplementary information is available for this paper at 10.1038/s42003-020-1035-9.
References
- 1.Reller MD, Strickland MJ, Riehle-Colarusso T, Mahle WT, Correa A. Prevalence of congenital heart defects in Atlanta, 1998-2005. J. Pediatr. 2008;153:807–813. doi: 10.1016/j.jpeds.2008.05.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang Q, et al. Racial differences in infant mortality attributable to birth defects in the United States, 1989-2002. Birth Defects Res. A Clin. Mol. Teratol. 2006;76:706–713. doi: 10.1002/bdra.20308. [DOI] [PubMed] [Google Scholar]
- 3.Marelli A, et al. Estimating the congenital heart disease population in the united states in 2010—what are the numbers? J. Am. Coll. Cardiol. 2012;59:E787–E787. doi: 10.1016/S0735-1097(12)60788-8. [DOI] [Google Scholar]
- 4.Li Y, et al. Global genetic analysis in mice unveils central role for cilia in congenital heart disease. Nature. 2015;521:520–524. doi: 10.1038/nature14269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Strickland DK, Au DT, Cunfer P, Muratoglu SC. Low-density lipoprotein receptor-related protein-1: role in the regulation of vascular integrity. Arterioscler. Thromb. Vasc. Biol. 2014;34:487–498. doi: 10.1161/ATVBAHA.113.301924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.May P, Herz J, Bock HH. Molecular mechanisms of lipoprotein receptor signalling. Cell. Mol. Life Sci. 2005;62:2325–2338. doi: 10.1007/s00018-005-5231-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Herz J, Clouthier DE, Hammer RE. LDL receptor-related protein internalizes and degrades uPA-PAI-1 complexes and is essential for embryo implantation. Cell. 1992;71:411–421. doi: 10.1016/0092-8674(92)90511-A. [DOI] [PubMed] [Google Scholar]
- 8.Liu X, et al. Interrogating congenital heart defects with noninvasive fetal echocardiography in a mouse forward genetic screen. Circ. Cardiovasc. Imaging. 2014;7:31–42. doi: 10.1161/CIRCIMAGING.113.000451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu X, Tobita K, Francis RJ, Lo CW. Imaging techniques for visualizing and phenotyping congenital heart defects in murine models. Birth Defects Res. C. Embryo Today. 2013;99:93–105. doi: 10.1002/bdrc.21037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ebadi A, Spicer DE, Backer CL, Fricker FJ, Anderson RH. Double-outlet right ventricle revisited. J. Thorac. Cardiovasc. Surg. 2017;154:598–604. doi: 10.1016/j.jtcvs.2017.03.049. [DOI] [PubMed] [Google Scholar]
- 11.Li Y, Lui KO, Zhou B. Reassessing endothelial-to-mesenchymal transition in cardiovascular diseases. Nat. Rev. Cardiol. 2018;15:445–456. doi: 10.1038/s41569-018-0023-y. [DOI] [PubMed] [Google Scholar]
- 12.Combs MD, Yutzey KE. Heart valve development: regulatory networks in development and disease. Circ. Res. 2009;105:408–421. doi: 10.1161/CIRCRESAHA.109.201566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cai CL, et al. Isl1 identifies a cardiac progenitor population that proliferates prior to differentiation and contributes a majority of cells to the heart. Dev. Cell. 2003;5:877–889. doi: 10.1016/S1534-5807(03)00363-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brewer S, Feng W, Huang J, Sullivan S, Williams T. Wnt1-Cre-mediated deletion of AP-2alpha causes multiple neural crest-related defects. Dev. Biol. 2004;267:135–152. doi: 10.1016/j.ydbio.2003.10.039. [DOI] [PubMed] [Google Scholar]
- 15.Rohlmann A, Gotthardt M, Willnow TE, Hammer RE, Herz J. Sustained somatic gene inactivation by viral transfer of Cre recombinase. Nat. Biotechnol. 1996;14:1562–1565. doi: 10.1038/nbt1196-1562. [DOI] [PubMed] [Google Scholar]
- 16.Danielian PS, Muccino D, Rowitch DH, Michael SK, McMahon AP. Modification of gene activity in mouse embryos in utero by a tamoxifen-inducible form of Cre recombinase. Curr. Biol. 1998;8:1323–1326. doi: 10.1016/S0960-9822(07)00562-3. [DOI] [PubMed] [Google Scholar]
- 17.Verzi MP, McCulley DJ, De Val S, Dodou E, Black BL. The right ventricle, outflow tract, and ventricular septum comprise a restricted expression domain within the secondary/anterior heart field. Dev. Biol. 2005;287:134–145. doi: 10.1016/j.ydbio.2005.08.041. [DOI] [PubMed] [Google Scholar]
- 18.Stanley EG, et al. Efficient Cre-mediated deletion in cardiac progenitor cells conferred by a 3′UTR-ires-Cre allele of the homeobox gene Nkx2-5. Int. J. Dev. Biol. 2002;46:431–439. [PubMed] [Google Scholar]
- 19.Kisanuki YY, et al. Tie2-Cre transgenic mice: a new model for endothelial cell-lineage analysis in vivo. Dev. Biol. 2001;230:230–242. doi: 10.1006/dbio.2000.0106. [DOI] [PubMed] [Google Scholar]
- 20.Sosic D, Richardson JA, Yu K, Ornitz DM, Olson EN. Twist regulates cytokine gene expression through a negative feedback loop that represses NF-kappaB activity. Cell. 2003;112:169–180. doi: 10.1016/S0092-8674(03)00002-3. [DOI] [PubMed] [Google Scholar]
- 21.Goddard LM, et al. hemodynamic forces sculpt developing heart valves through a KLF2-WNT9B paracrine signaling axis. Dev. Cell. 2017;43:274–289. doi: 10.1016/j.devcel.2017.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu B, et al. Endocardial cells form the coronary arteries by angiogenesis through myocardial-endocardial VEGF signaling. Cell. 2012;151:1083–1096. doi: 10.1016/j.cell.2012.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Veras E, Malpica A, Deavers MT, Silva EG. Mitosis-specific marker phospho-histone H3 in the assessment of mitotic index in uterine smooth muscle tumors: a pilot study. Int. J. Gynecol. Pathol. 2009;28:316–321. doi: 10.1097/PGP.0b013e318193df97. [DOI] [PubMed] [Google Scholar]
- 24.Negoescu A, Guillermet C, Lorimier P, Brambilla E, Labat-Moleur F. Importance of DNA fragmentation in apoptosis with regard to TUNEL specificity. Biomed. Pharmacother. 1998;52:252–258. doi: 10.1016/S0753-3322(98)80010-3. [DOI] [PubMed] [Google Scholar]
- 25.Maretto S, et al. Mapping Wnt/beta-catenin signaling during mouse development and in colorectal tumors. Proc. Natl Acad. Sci. USA. 2003;100:3299–3304. doi: 10.1073/pnas.0434590100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liebner S, et al. Beta-catenin is required for endothelial-mesenchymal transformation during heart cushion development in the mouse. J. Cell Biol. 2004;166:359–367. doi: 10.1083/jcb.200403050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. [letter] Nat. Genet. 1999;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- 28.Hurlstone AF, et al. The Wnt/b-catenin pathway regulates cardiac valve formation. Nature. 2003;425:633–637. doi: 10.1038/nature02028. [DOI] [PubMed] [Google Scholar]
- 29.Rabiej VK, et al. Low density lipoprotein receptor-related protein 1 mediated endocytosis of β1-integrin influences cell adhesion and cell migration. Exp. Cell Res. 2016;340:102–115. doi: 10.1016/j.yexcr.2015.11.020. [DOI] [PubMed] [Google Scholar]
- 30.Laatsch A, et al. Low density lipoprotein receptor-related protein 1 dependent endosomal trapping and recycling of apolipoprotein E. PLoS ONE. 2012;7:e29385. doi: 10.1371/journal.pone.0029385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yelbuz TM, et al. Shortened outflow tract leads to altered cardiac looping after neural crest ablation. Circulation. 2002;106:504–510. doi: 10.1161/01.CIR.0000023044.44974.8A. [DOI] [PubMed] [Google Scholar]
- 32.Phillips HM, et al. Neural crest cells are required for correct positioning of the developing outflow cushions and pattern the arterial valve leaflets. Cardiovasc. Res. 2013;99:452–460. doi: 10.1093/cvr/cvt132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ma P, et al. Cardiac neural crest ablation results in early endocardial cushion and hemodynamic flow abnormalities. Am. J. Physiol. Heart Circ. Physiol. 2016;311:H1150–H1159. doi: 10.1152/ajpheart.00188.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jain R, et al. Cardiac neural crest orchestrates remodeling and functional maturation of mouse semilunar valves. J. Clin. Invest. 2011;121:422–430. doi: 10.1172/JCI44244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hakim ZS, et al. Conditional deletion of focal adhesion kinase leads to defects in ventricular septation and outflow tract alignment. Mol. Cell Biol. 2007;15:5352–5364. doi: 10.1128/MCB.00068-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Obler D, Juraszek AL, Smoot LB, Natowicz MR. Double outlet right ventricle: aetiologies and associations. J. Med. Genet. 2008;45:481–497. doi: 10.1136/jmg.2008.057984. [DOI] [PubMed] [Google Scholar]
- 37.Lin Chien-Jung, Lin Chieh-Yu, Chen Chen-Hao, Zhou Bin, Chang Ching-Pin. Partitioning the heart: mechanisms of cardiac septation and valve development. Development. 2012;139:3277–3299. doi: 10.1242/dev.063495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang H, et al. Yap1 is required for endothelial to mesenchymal transition of the atrioventricular cushion. J. Biol. Chem. 2014;289:18681–18692. doi: 10.1074/jbc.M114.554584. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Items
Data Availability Statement
The authors declare that all data supporting the findings of this study are available within the paper