Abstract
Purpose
Obesity and insulin resistance have been associated with poor prognosis in breast cancer (BC). The present prospective study aimed to investigate the impact of metabolic syndrome (MetS) and its components on early BC (eBC) patients’ outcome.
Methods
MetS was defined by the presence of 3 to 5 of the following components: waist circumference > 88 cm, blood pressure ≥ 130/≥ 85 mmHg, serum levels of triglycerides ≥ 150 mg/dL, high density lipoprotein < 50 mg/dL and fasting glucose ≥ 110 mg/dL. Seven hundred and seventeen patients with data on ≥ 4 MetS components at BC diagnosis were enrolled. Study population was divided into two groups: patients with < 3 (non-MetS) vs. ≥ 3 components (MetS). Categorical variables were analyzed by Chi-square test and survival data by log-rank test and Cox proportional hazards regression model.
Results
Overall, 544 (75.9%) and 173 (24.1%) women were categorized as non-MetS and MetS, respectively. MetS patients were more likely to be older, postmenopausal, and insulin-resistant compared to non-MetS patients (p < 0.05). In multivariate analysis, MetS patients had a numerically higher risk of relapse [disease-free survival (DFS), hazard ratio (HR) 1.51, p = 0.07] and a significantly higher risk of death compared to non-MetS patients [overall survival (OS), HR 3.01, p < 0.0001; breast cancer-specific survival (BCSS), HR 3.16, p = 0.001]. Additionally, patients with 1 to 2 components of MetS had an increased risk of dying compared to patients with 0 components (OS, HR 4.90, p = 0.01; BCSS, HR 6.07, p = 0.02).
Conclusions
MetS correlated with poor outcome in eBC patients. Among patients without full criteria for MetS diagnosis, the presence of 1 or 2 components of the syndrome may predict for worse survival.
Keywords: Breast cancer, Metabolic syndrome, Metabolic syndrome components, Breast cancer outcome
Introduction
Breast cancer (BC) represents the most common cancer among women, with about 2 million of new cancer cases estimated in 2018 worldwide. Incidence rate varies across world regions, ranging from 26 to 28 per 100,000 in developing countries (i.e., South-Central Asia and Middle Africa) to 92–94 per 100,000 in the more developed ones (i.e., Western Europe, Australia, and New Zealand) [1]. This difference in BC incidence can be explained by different dietary and nutritional habits with a higher consumption of fatty, low-fiber, and processed food in westernized countries [2, 3]. This unhealthy diet, often correlated with physical inactivity, is considered one of the most important causes of the so-called “obesity epidemic” [4], associated with cardiovascular events and deaths [5].
Obesity has been associated with postmenopausal BC incidence [6, 7], BC subtypes [8] and poor survival [9] through different mechanisms including an increased estrogen production from circulating androgens [10] and the promotion of a low-grade chronic inflammation state [11, 12]. Similarly, diabetes has been correlated with increased BC risk [13] and poor survival [14, 15], in part due to the activation of the oncogenic Ras-MAPK and PI3K/Akt pathways in breast cells [16, 17].
Abdominal obesity and high fasting glycaemia combined with dyslipidemia and hypertension are diagnostic criteria of a more complex metabolic disorder known as “Metabolic Syndrome” (MetS) [18]. Initially linked to cardiovascular diseases, MetS has been recently associated with increased cancer risk [19], particularly colon-rectal [20] and BC [21, 22] in previous studies.
We have previously demonstrated that the concomitant presence of obesity and diabetes worsened early breast cancer (eBC) patients’ outcome compared to presence of only one or none of these comorbidities [23], suggesting not only a close link between these medical conditions, but also an outcome worsening as the number of the metabolic alterations increased.
We therefore hypothesized that MetS could be associated with a worse outcome in eBC patients. Large studies [24–26] retrospectively correlated MetS with worse prognosis in eBC patients. However, prospective studies evaluating the association between MetS and eBC patients’ long-term outcome are still missing. In this study, we comprehensively investigated the association between MetS or its individual components and BC outcome in a large prospective cohort of eBC patients.
Materials and methods
Study population
Overall, 955 eBC patients were prospectively enrolled in this study between January 2009 and December 2013 at University Hospital Federico II and National Cancer Institute G. Pascale, Naples, Italy. Clinical data [age, height, weight, waist circumference, blood pressure, fasting glucose, triglycerides, total cholesterol, high density lipoprotein (HDL), low density lipoprotein (LDL)] and tumor characteristics [tumor size (T), nodal status (N), tumor stage, estrogen receptor (ER) and progesterone receptor (PgR) expression, grading (G), ki67, HER2 status] were collected before starting systemic (neo)adjuvant therapy. The homeostatic model assessment for insulin resistance (HOMA-IR) score was calculated as fasting glucose (mmol/l) multiplied by fasting insulin (µUI/l) divided by 22.5 [26]. Immunohistochemical (IHC) BC subtypes were identified and categorized according to the 13th St. Gallen International Breast Cancer Conference (2013) Expert Panel [27].
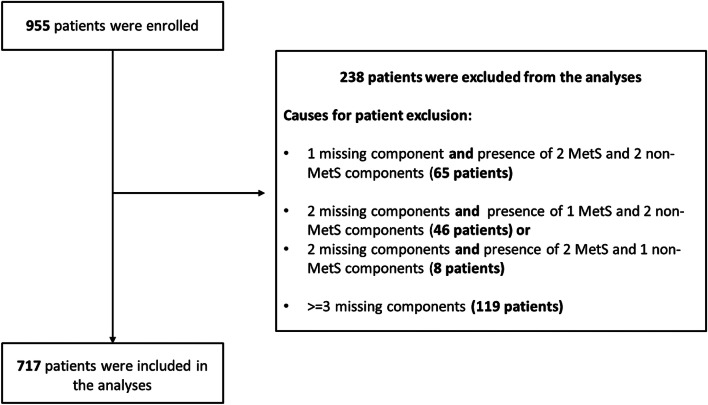
MetS was defined by the presence of 3 to 5 of the following variables: waist circumference > 88 cm, blood pressure ≥ 130/ ≥ 85 mmHg, triglycerides ≥ 150 mg/dL, HDL < 50 mg/dL and fasting glucose ≥ 110 mg/dL, according to the National Cholesterol Education Program Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults—NCEP-ATPIII criteria [28]. A total of 717 patients (75.1%) had complete data to define or not the presence of MetS, thus were included in the current analysis (Fig. 1). Study population was divided into 2 main groups: (1) patients with less than 3 components (non-MetS); (2) patients with 3 or more components (MetS). The study was approved by the Institutional Review Board of the University of Naples Federico II (IRB approval number 75/15) and participants provided written informed consent to participate. The records and data of patients were anonymized and de-identified prior to analysis.
Fig. 1.
Study flow chart. Metabolic Syndrome (MetS) was defined by the presence of 3 of the following variables: waist circumference > 88 cm, blood pressure ≥ 130/ ≥ 85 mmHg, triglycerides ≥ 150 mg/dL, HDL < 50 mg/dL and fasting glucose ≥ 110 mg/dL. Patients were excluded from the study if they had missing components that precluded investigators from assessing accurate MetS status as detailed in the flow chart
Statistical analyses
Descriptive statistics for the categorical data were reported. The Chi-square test was used to assess the association between MetS and non-MetS groups and clinico-pathological variables. Patients’ outcomes were analyzed in terms of disease-free survival (DFS; with local, contralateral, and distant disease recurrence as well as secondary primary tumors and death from any cause defined as the event), overall survival (OS; with death from any cause defined as the event) and breast cancer-specific survival (BCSS; death from the disease). Univariate analyses were performed using the Kaplan–Meier method. Numbers of events and survival percentage were reported, and Log-rank test was conducted to determine the statistically significant difference. Cox multivariate analysis for DFS, OS and BCSS was done either for singular components of MetS or for the combination of them into “number of MetS components”. For each variable, the best category was used as the reference group for the calculations of hazard ratios (HR) and 95% confidence intervals (CI). Statistical analyses were performed using IBM® SPSS® Statistics, version 25 (IBM Corp., Armonk, NY, USA). All statistical analyses were of an exploratory nature, with p values of less than 0.05 considered significant, without any adjustments for multiplicity applied.
Results
Patient demographics, clinical and pathological characteristics
Overall, 173 (24.1%) and 544 (75.9%) women were categorized as MetS and non-MetS, respectively. Clinical data and tumor characteristics, according to the presence or absence of MetS, are reported in Table 1. MetS group had more elderly [age > 55 years; 130 (75.1%) vs. 198 (36.4%), p < 0.0001] and postmenopausal [152 (87.9%) vs. 271 (49.8%), p < 0.0001] women than non-MetS groups. Patients with MetS were also more likely to be insulin-resistant, as HOMA-IR score higher than 5 was found in 43 out of 173 (29.3%) MetS vs. 25 out of 544 (5.7%) non-MetS groups (p < 0.0001).
Table 1.
Distribution of patients’ clinico-pathological and metabolic characteristics
| Characteristics | non-MetS 544 (75.9%) |
MetS 173 (24.1%) |
p-value |
|---|---|---|---|
| Age | < 0.0001 | ||
| ≤ 55 years | 346 (63.6) | 43 (24.9) | |
| > 55 years | 198 (36.4) | 130 (75.1) | |
| Menopause | < 0.0001 | ||
| Post-menopause | 271 (49.8) | 152 (87.9) | |
| Pre-menopause | 273 (50.2) | 21 (12.1) | |
| Stage | 0.2 | ||
| I–II | 433 (82.6) | 127 (78.4) | |
| III | 91 (17.4) | 35 (21.6) | |
| Therapy | 0.4 | ||
| Chemo only | 80 (15.4) | 25 (15.9) | |
| Hormone only | 188 (36.2) | 65 (41.4) | |
| Chemo + hormone | 251 (48.4) | 67 (42.7) | |
| IHC-subtypesa | 0.7 | ||
| Luminal A | 207 (39.7) | 71 (42.0) | |
| Luminal B | 149 (28.5) | 48 (28.4) | |
| Her2 positive | 91 (17.4) | 31 (18.3) | |
| Triple negative | 75 (14.4) | 19 (11.2) | |
| HOMA-IR score | < 0.0001 | ||
| Normal (< 2.6) | 312 (71.7) | 57 (38.8) | |
| Medium (2.6–5) | 98 (22.5) | 47 (32.0) | |
| High (> 5) | 25 (5.7) | 43 (29.3) | |
| Patient status | < 0.0001 | ||
| Alive without disease relapse | 385 (70.8) | 97 (56.1) | |
| Alive with disease relapse | 68 (12.5) | 15 (8.7) | |
| Death for the disease | 41 (7.5) | 34 (19.7) | |
| Death for other causes | 7 (1.3) | 7 (4.0) | |
| Lost to follow-up | 43 (7.9) | 20 (11.6) |
IHC immunohistochemical
aSt Gallen categorization 2013: Luminal A: ER+, PgR > = 20%, ki67 < 20%, Her2−; Luminal B: ER+; PgR < 20% or ki67 > = 20%; Her2−; Her2positive: any ER and PgR, any ki67, Her2+; Triple negative: ER−; PgR−; Her2−; ki67 any
bNCEP—ATP III criteria
Death rates for BC and other causes were higher in patients with MetS vs non-MetS. Incidence for death for BC and death for other causes were 34 (19.7%) vs. 41 (7.5%) and 7 (4.0%) vs. 7 (1.3%) in patients with MetS vs non-MetS, respectively (p < 0.0001).
No statistically significant differences in tumor stage or IHC-subtypes were identified between the two groups and the presence of MetS did not influence the choice of (neo)adjuvant systemic therapy.
Survival analysis
In univariate analysis, patients with MetS were more likely to recur and die from BC. After a median follow-up time of 7.1 years from diagnosis, rates for DFS, OS and BCSS were 71.2% vs. 79.8% (p = 0.008), 75.9% vs. 91.1% (p < 0.0001) and 80.0% vs. 92.4% (p < 0.0001), in patients with MetS vs. non-MetS, respectively (Fig. 2a–c; Table 2). Interestingly, among patients with non-MetS, patients with 1 or 2 components of MetS had an increased risk of recurrence and death for BC compared to patients with no component at all. However, these risks were lower when compared with those of patients with MetS. Specifically, rates for DFS, OS and BCSS were 80.2% vs. 79.2% vs. 71.2% (p = 0.02), 98.9% vs. 88.1% vs. 75.9% (p < 0.0001) and 96.7% vs. 89.9% vs. 80.0% (p < 0.0001), in patients with ≥ 3 vs. 1–2 vs. 0 components of MetS (Fig. 3a–c; Table 2). Other factors associated to both DFS and OS rates in univariate analysis were tumor stage, IHC-subtypes, type of (neo)adjuvant therapy, triglycerides, and fasting glucose levels. Age, waist circumference, blood pressure and HDL levels correlated to OS only (Table 2).
Fig. 2.
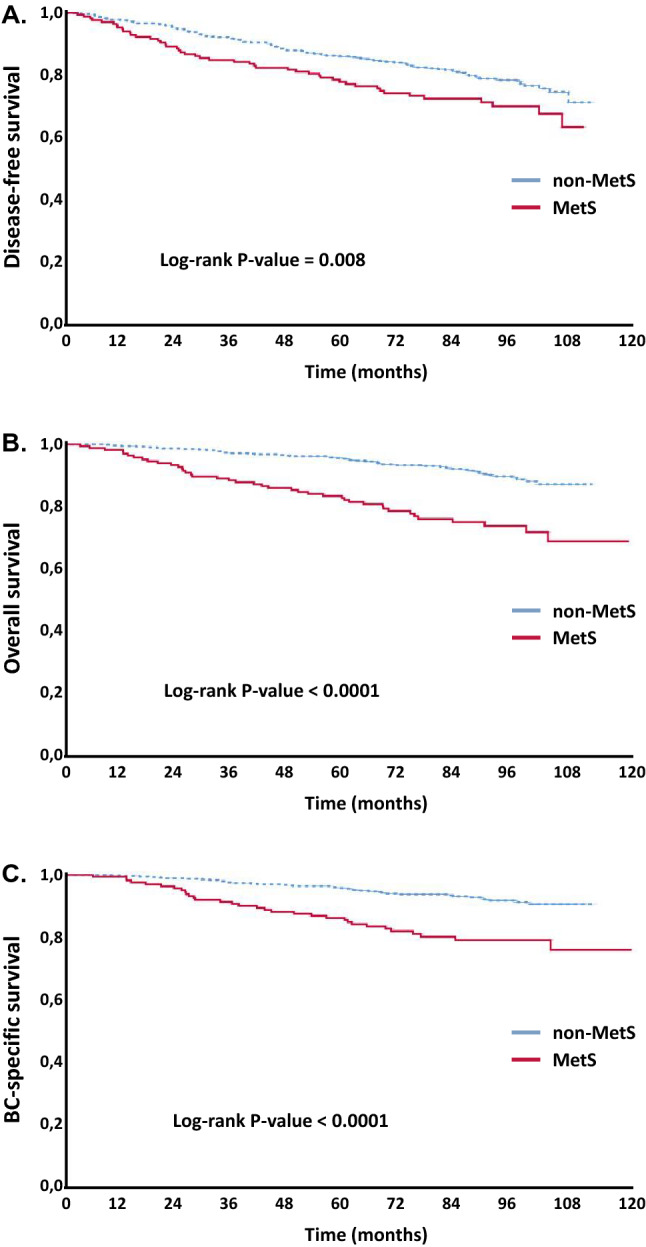
Disease-free Survival (a), Overall Survival (b) and Breast Cancer-Specific Survival (c) according to metabolic syndrome (MetS)
Table 2.
Disease-free survival and overall survival results: Univariate analysis
| Variable | DFS rates | OS rates | ||||
|---|---|---|---|---|---|---|
| No. of events | % | Log-rankap-value | No. of events | % | Log-rankap-value | |
| MetS | 0.008 | < 0.0001 | ||||
| No | 109 | 79.8 | 48 | 91.1 | ||
| Yes | 49 | 71.2 | 41 | 75.9 | ||
| No. of MetS comp.b | 0.02 | < 0.0001 | ||||
| 0 components | 24 | 80.2 | 5 | 98.9 | ||
| 1–2 components | 68 | 79.2 | 39 | 88.1 | ||
| 3–5 components | 49 | 71.2 | 41 | 75.9 | ||
| Age | 0.1 | < 0.0001 | ||||
| ≤ 55 years | 78 | 79.8 | 26 | 93.3 | ||
| > 55 years | 80 | 75.2 | 63 | 80.5 | ||
| Stage | < 0.0001 | < 0.0001 | ||||
| I–II | 100 | 82.1 | 456 | 90.0 | ||
| III | 46 | 62.0 | 30 | 75.2 | ||
| IHC-subtypes | 0.001 | 0.04 | ||||
| Luminal A | 52 | 81.2 | 32 | 88.4 | ||
| Luminal B | 41 | 79 | 24 | 87.7 | ||
| HER2 positive | 36 | 70 | 16 | 86.7 | ||
| Triple negative | 25 | 72.8 | 16 | 82.6 | ||
| Therapy | < 0.0001 | < 0.0001 | ||||
| Chemotherapy only | 32 | 69.5 | 22 | 79.0 | ||
| Hormone only | 34 | 86.6 | 22 | 91.3 | ||
| Chemo + hormone | 70 | 78.0 | 29 | 90.9 | ||
| HOMA-IR score | 0.9 | 0.1 | ||||
| Normal (< 2.6) | 83 | 77.3 | 51 | 86.1 | ||
| Medium (2.6–5) | 26 | 81.9 | 10 | 93.1 | ||
| High (> 5) | 19 | 71.2 | 8 | 87.9 | ||
| Waist circumference | 0.1 | < 0.0001 | ||||
| ≤ 88 cm | 72 | 79.8 | 30 | 91.6 | ||
| > 88 cm | 84 | 75.5 | 58 | 83.1 | ||
| Blood pressure | 0.8 | 0.03 | ||||
| < 130; < 85 mmHg | 80 | 78.1 | 40 | 89.0 | ||
| ≥ 130; ≥ 85 mmHg | 64 | 77.5 | 47 | 83.5 | ||
| HDL | 0.1 | 0.04 | ||||
| ≥ 50 mg/dL | 93 | 77.6 | 52 | 87.5 | ||
| < 50 mg/dL | 37 | 72.6 | 26 | 80.7 | ||
| Triglycerides | < 0.0001 | < 0.0001 | ||||
| < 150 mg/dL | 110 | 80.8 | 44 | 92.3 | ||
| ≥ 150 mg/dL | 45 | 62.8 | 43 | 64.2 | ||
| Fasting glucose | 0.003 | 0.001 | ||||
| < 110 mg/dL | 123 | 79.2 | 64 | 89.2 | ||
| ≥ 110 mg/dL | 34 | 70.2 | 25 | 78.1 | ||
aKaplan–Meier univariate analysis
bNCEP—ATP III criteria
Fig. 3.
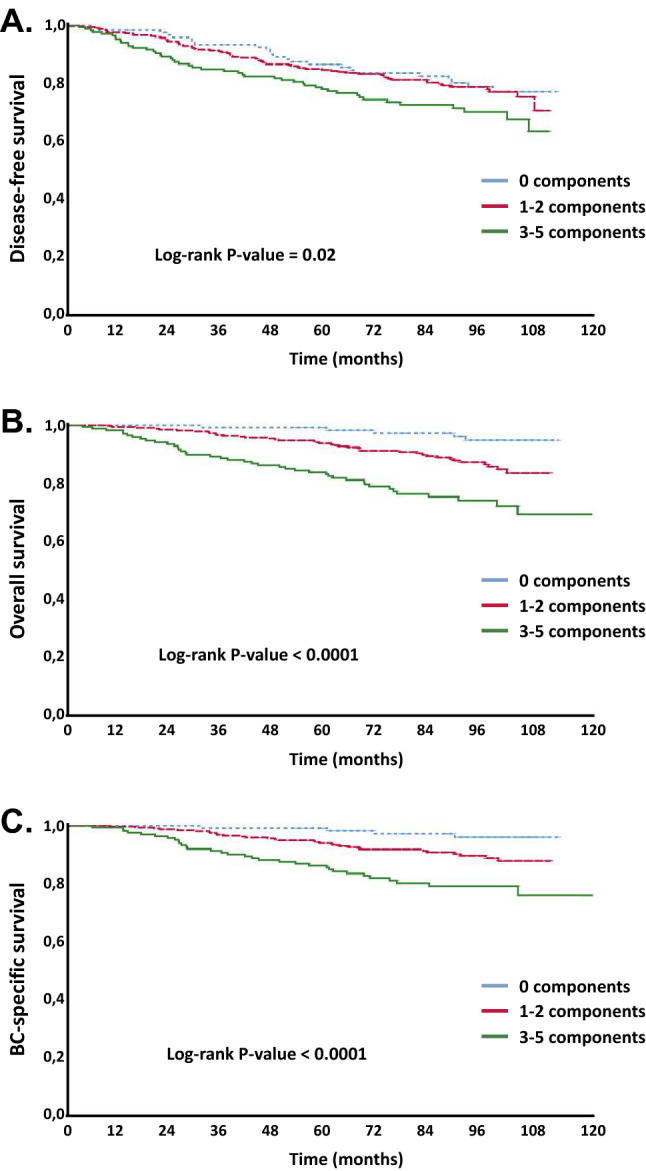
Disease-free Survival (a), Overall Survival (b) and Breast Cancer-Specific Survival (c) according to number of MetS components
In Cox regression models (Table 3), adjusted for age, tumor stage, IHC-subtypes and therapy, MetS was associated with a threefold increased risk of BC mortality (HR for BCSS = 3.16, 95% confidence interval (CI) 1.64–6.07, p = 0.001) and of death (HR for OS = 3.01, 95% CI 1.72–5.28, p < 0.0001) compared to patients with non-MetS. A numerical, but not statistically significant, difference in DFS was observed. High waist circumference (> 88 cm), high blood pressure (≥ 130; ≥ 85 mmHg), high triglycerides (≥ 150 mg/dL), and high fasting glucose (≥ 110 mg/dL) were individually associated with increased risk of death (HR for OS = 2.34, 95% CI 1.32–4.14, p = 0.003; HR 1.99, 95% CI 1.15–3.64, p = 0.01; HR 3.58, 95% CI 2.08–6.17, p < 0.0001; and HR 2.26, 95% CI 1.26–4.05, p = 0.006, respectively) and death for BC (HR for BCSS = 3.24, 95% CI 1.64–6.41, p = 0.001; HR 2.02, 95% CI 1.07–3.81, p = 0.03; HR 3.10, 95% CI 1.59–6.05, p = 0.001; and HR 2.49, 95% CI 1.25–4.96, p = 0.009, respectively) in multivariable adjusted models (Table 3). For DFS, patients with triglycerides ≥ 150 mg/dL and fasting glucose ≥ 110 mg/dL had an increased risk of relapse (HR for DFS = 1.66, 95% CI 1.01–2.74, p = 0.04 and HR 1.70, 95% CI 1.04–2.76, p = 0.03, respectively).
Table 3.
Cox multivariate analysis of BC risk for DFS, OS and BC-specific survival
| Variable | Disease-free survival | Overall-survival | BC-specific survival | ||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | (95% CI) | p-value | HR | (95% CI) | p-value | HR | (95% CI) | p-value | |
| MetS | |||||||||
| No | 1† | 1† | 1† | ||||||
| Yes | 1.51 | 0.96–2.38 | 0.07 | 3.01 | 1.72–5.28 | < 0.0001 | 3.16 | 1.64–6.07 | 0.001 |
| Waist circumference | |||||||||
| ≤ 88 cm | 1† | 1† | 1† | ||||||
| > 88 cm | 1.36 | 0.91–2.02 | 0.1 | 2.34 | 1.32–4.14 | 0.003 | 3.24 | 1.64–6.41 | 0.001 |
| Blood pressure | |||||||||
| < 130; < 85 mmHg | 1† | 1† | 1† | ||||||
| ≥ 130; ≥ 85 mmHg | 1.26 | 0.83–1.92 | 0.3 | 1.99 | 1.15–3.64 | 0.01 | 2.02 | 1.07–3.81 | 0.03 |
| HDL | |||||||||
| ≥ 50 mg/dL | 1† | 1† | 1† | ||||||
| < 50 mg/dL | 0.86 | 0.53–1.38 | 0.5 | 0.65 | 0.35–1.17 | 0.1 | 0.54 | 0.27–1.05 | 0.07 |
| Triglycerides | |||||||||
| < 150 mg/dL | 1† | 1† | 1† | ||||||
| ≥ 150 mg/dL | 1.66 | 1.01–2.74 | 0.04 | 3.58 | 2.08–6.17 | < 0.0001 | 3.10 | 1.59–6.05 | 0.001 |
| Fasting glucose | |||||||||
| < 110 mg/dL | 1† | 1† | 1† | ||||||
| ≥ 110 mg/dL | 1.70 | 1.04–2.76 | 0.03 | 2.26 | 1.26–4.05 | 0.006 | 2.49 | 1.25–4.96 | 0.009 |
| Number of MetS comp.a | 0.04 | 0.001 | 0.003 | ||||||
| 0 components | 1† | 1† | 1† | ||||||
| 1–2 components | 1.48 | 0.86–2.57 | 0.1 | 4.90 | 1.47–16.35 | 0.01 | 6.07 | 1.41–26.21 | 0.02 |
| 3–5 components | 2.26 | 1.18–4.33 | 0.01 | 12.2 | 3.49–43.01 | < 0.0001 | 15.97 | 3.49–73.16 | < 0.0001 |
Adjusted for terms of Age (< 40,40–45,46–55,56–65,66–75,75 +); Stage (I–II, III); IHC- subtypes (Luminal A: ER+, PgR > = 20%, ki67 < 20%, Her2−; Luminal B: E+;PgR < 20% or ki67 > = 20%; Her2−; Her2positive: any ER and PgR, any ki67, Her2+; Triple negative: ER−; PgR−; Her2−; ki67 any) and Therapy (Chemotherapy only, Hormone only, Chemo + hormone)
aWald test
Compared to individuals without any component of MetS present, the risk of death and death for BC increased steeply as the number of MetS components increased (Table 3). Patients with 3–5 components had over twofold higher risk of relapse (HR for DFS = 2.26, 95% CI 1.18–4.33, p = 0.01), 12-fold higher risk of death (HR for OS = 12.2, 95% CI 3.49–43.01, p < 0.0001) and nearly 16-fold higher risk of death for BC (HR for BCSS = 15.97, 95% CI 3.49–73.16, p < 0.0001) than patients with 0 components (Table 3). Interestingly, patients with 1–2 MetS components presented about fivefold higher risk of death (HR for OS = 4.90, 95% CI 1.47–16.35, p = 0.01), sixfold risk of death for BC (HR for BCSS = 6.07, 95% CI 1.41–26.21, p = 0.02) but no significant increased risk of relapse compared to patients with no components at all (Table 3).
Discussion
In this large prospective study, we have found that MetS was significantly associated with increased risk of dying in general and of dying from breast cancer in eBC patients receiving (neo)adjuvant therapy at a median follow-up time of 7.1 years. To our knowledge, this is one of the first prospective studies correlating MetS with poor long-term outcome in a large cohort of eBC patients in this setting.
Our findings are consistent with those from previous studies [24, 25, 29, 30]. In a cohort of 4,216 eBC patients, the presence of MetS at diagnosis was associated with a 1.5-fold increased risk of recurrence or second primary BC and 1.65-fold increased risk of BC-specific mortality compared with patients with no MetS [24]. Similarly, a study in 10,014 patients reported twofold increase in BC mortality with MetS [30], while another study (N = 288,834) reported a 23% higher risk of BC mortality in only older (> 60 years) women with MetS without any impact in younger patients [25]. Interestingly, MetS also correlated with an enhanced risk of new BC events (defined as loco-regional recurrences, distant metastasis or new primary BC) in a prospective study using 2,092 eBC patients; however, an impact on survival was not evaluated [29]. As other reports [29, 30], our study found that patients with MetS were more likely to be older and postmenopausal compared to those with no MetS. However, differently from previous reports [31, 32] MetS was not associated with adverse pathological features in our study, as no correlation between IHC-defined BC subtypes, tumor stage at diagnoses and presence of MetS was found. The presence of MetS at diagnoses also did not influence the choice of (neo)adjuvant treatment administered.
In our study, even the presence of a single component of MetS such as high waist circumference, blood pressure, fasting glucose or triglycerides, was strongly associated with increased risk of BC mortality, regardless other well-known prognostic factors such as age, tumor stage, IHC-subtypes and therapy. Other studies have also investigated the impact of individual MetS components on BC outcome with differing results. Higher risk of BC mortality was reported in women in the highest tertile of total cholesterol (29% higher risk) and blood pressure (41% higher risk) [33] and in patients with high waist circumference (HR 1.32), high cholesterol (HR 1.24), and hypertension (HR 1.24) [34]. In addition, BC outcomes correlated with hyperglycemia [35] and higher waist-to-hip ratio [36]. Increased insulin levels, due to insulin resistance (IR) also directly correlated with BC relapse and mortality [37]. However, we could not detect any significant correlation between HOMA-IR score and increased risk of BC relapse or mortality, which is consistently with previous findings from our group as well as others [23, 29]. These data suggest that a complex interaction between metabolic alterations caused by altered glucose metabolism, rather than the presence of IR alone, may be more relevant for BC outcome.
Interestingly, in this study, we also demonstrate that, among patients without MetS, the risk of BC mortality increased significantly as the number of MetS components increased. We observed a sixfold and 16-fold increase in risk of BC mortality among women with 1–2 components and 3–5 of MetS, respectively, compared with women with no components. These results indicate that higher the extent of metabolic health impairment, worse the outcomes in eBC patients.
The mechanisms by which MetS can increase BC risk and worsen patients’ prognosis are partially understood. Each of the metabolic alterations included in MetS may play a critical role in BC biology. Increased glycaemia and IR have shown to promote malignant cell growth [38]. Insulin mediates insulin like growth factor (IGF-1) production, resulting in a hyper-activation of Ras-MAPK and PI3K/Akt pathways in malignant cells [16] and increases serum free estrogen levels by reducing the concentration of the sex hormone binding globulin [39]. Obesity, not only promotes estrogen production, as the aromatase enzyme synthesizes estrogens in adipose tissue from circulating androgens [10], but is also associated with a low-grade chronic inflammation. This is characterized by reduced levels of anti-inflammatory cytokines (such as adiponectin) [11] and high levels of pro-inflammatory cytokines [12] [as tumor necrosis factor alfa (TNFα), interleukin (IL) 1β, IL-6 and IL-8] that can exert mitogenic, anti-apoptotic, and angiogenic effects, thus promoting disease progression. Importantly, in mammary gland, the interaction with BC cells may promote transformation of mammary adipocytes into the so-called “cancer associated adipocytes” (CAA), which may enhance tumor growth and progression [40] through lipolytic activity and the secretion of adipokines [41]. Moreover, the adipocyte/tumor cell crosstalk may negatively affect response to systemic treatment [42] and mediate endocrine resistance in BC cells, particularly in the presence of high glucose levels [43]. Pre-clinical studies have shown that dyslipidemia, in an Apolipoprotein (Apo) E knockout (ApoE−/−) mice model, promote tumor growth and metastasis development through activation of PI3K/Akt signal pathway [44] due to increased cholesterol levels. Finally, low serum HDL, as markers of increased androgen levels, are also associated with BC risk [45]. Taken together, multiple molecular mechanisms related to various metabolic alterations within MetS may be responsible for an increased risk of BC development and progression. These mechanisms may function independently in presence of only one metabolic alteration to impact patient outcome or cooperatively when multiple MetS components are present to further worsen the recurrence risk and survival.
Our study and findings have several strengths and limitations. First, our large prospective cohort of patients is fully characterized with regard to clinical and tumor features, objective baseline measures of MetS, and subsequent treatment. This information has allowed us to comprehensively evaluate the effect of MetS components on patients’ outcome by a multivariate model adjusted for known prognostic variables. Second, exposure and covariate data were obtained at baseline before treatment could interfere with the metabolic parameters included in the study. Third, data on BC mortality were obtained from patient’s charts, thereby minimizing the risk of death misclassification. On the other hand, we did not have information about the medical treatments for diabetes, hypertension, and dyslipidemia, which may have led to un underestimation of the number of patients with MetS. However, our analysis focused on the real-time laboratory results of patients may be more appropriate to assess functional/uncontrolled MetS. Future studies determining the effects of lifestyle and/or therapeutic interventions to treat MetS on BC progression, risk of recurrence, and survival may help improve clinical management of BC patients with MetS. In addition, small number of patients included in some of our sub-group analysis may have limited the power to detect significant associations between clinical variables and should be confirmed in future studies.
In summary, we demonstrate here that the presence of MetS at diagnosis correlates with poor outcome in eBC patients. Compared to patients without any criterion for MetS at diagnosis, patients with only 1 or 2 components of MetS have worse survival. In addition, the prognosis worsens with the presence of even more components of MetS. These findings strongly support testing for components of MetS in all eBC patients at diagnoses and during (neo)adjuvant treatment to improve survival.
Acknowledgements
We would like to express our special thanks to all patients who participated in the study and to Dr. Maurizio Montella († May 2, 2019).
Funding
There was no specific funding supporting this research.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Giuseppe Buono, Email: giuseppe.buono88@gmail.com.
Carmine De Angelis, Email: carmine.deangelis1@unina.it.
References
- 1.https://gco.iarc.fr/today/data/factsheets/cancers/20-Breast-fact-sheet.pdf
- 2.Monteiro CA, Moubarac JC, Cannon G, et al. Ultra-processed products are becoming dominant in the global food system. Obes Rev. 2013;14:2–28. doi: 10.1111/obr.12107. [DOI] [PubMed] [Google Scholar]
- 3.Monteiro CA, Moubarac JC, Levy RB, et al. Household availability of ultra-processed foods and obesity in nineteen European countries. Public Health Nutr. 2018 doi: 10.1017/S1368980017001379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zobel EH, Hansen TW, Rossing P, von Scholten BJ. Global changes in food supply and the obesity epidemic. Curr Obes Rep. 2016 doi: 10.1007/s13679-016-0233-8. [DOI] [PubMed] [Google Scholar]
- 5.Hubert HB, Feinleib M, McNamara PM, Castelli WP. Obesity as an independent risk factor for cardiovascular disease: a 26-year follow-up of participants in the Framingham Heart Study. Circulation. 1983 doi: 10.1161/01.CIR.67.5.968. [DOI] [PubMed] [Google Scholar]
- 6.White AJ, Nichols HB, Bradshaw PT, Sandler DP. Overall and central adiposity and breast cancer risk in the sister study. Cancer. 2015;121:3700–3708. doi: 10.1002/cncr.29552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Neuhouser ML, Aragaki AK, Prentice RL, et al. Overweight, obesity, and postmenopausal invasive breast cancer risk: A secondary analysis of the women’s health initiative randomized clinical trials. JAMA Oncol. 2015 doi: 10.1001/jamaoncol.2015.1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crispo A, Montella M, Buono G, et al. Body weight and risk of molecular breast cancer subtypes among postmenopausal Mediterranean women. Curr Res Transl Med. 2016 doi: 10.1016/j.retram.2016.01.004. [DOI] [PubMed] [Google Scholar]
- 9.Jiralerspong S, Goodwin PJ. Obesity and breast cancer prognosis: Evidence, challenges, and opportunities. J Clin Oncol. 2016;34:4203–4216. doi: 10.1200/JCO.2016.68.4480. [DOI] [PubMed] [Google Scholar]
- 10.Chen Y, Wen YY, Li ZR, et al. The molecular mechanisms between metabolic syndrome and breast cancer. Biochem Biophys Res Commun. 2016;471:391–395. doi: 10.1016/j.bbrc.2016.02.034. [DOI] [PubMed] [Google Scholar]
- 11.Hauner D, Hauner H. Metabolic syndrome and breast cancer: is there a link? Breast Care. 2014;9:277–281. doi: 10.1159/000365951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Skurk T, Alberti-Huber C, Herder C, Hauner H. Relationship between adipocyte size and adipokine expression and secretion in human adipose tissue. J Clin Endocrinol Metab. 2007;92:1023–1033. doi: 10.1210/jc.2006-1055. [DOI] [PubMed] [Google Scholar]
- 13.De Bruijn KMJ, Arends LR, Hansen BE, et al. Systematic review and meta-analysis of the association between diabetes mellitus and incidence and mortality in breast and colorectal cancer. Br J Surg. 2013;100:1421–1429. doi: 10.1002/bjs.9229. [DOI] [PubMed] [Google Scholar]
- 14.Zhao XB, Ren GS. Diabetes mellitus and prognosis in women with breast cancer: a systematic review and meta-analysis. Med (United States) 2016 doi: 10.1097/MD.0000000000005602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peairs KS, Barone BB, Snyder CF, et al. Diabetes mellitus and breast cancer outcomes: a systematic review and meta-analysis. J Clin Oncol. 2011 doi: 10.1200/JCO.2009.27.3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khandekar MJ, Cohen P, Spiegelman BM. Molecular mechanisms of cancer development in obesity. Nat Rev Cancer. 2011;11:896–898. doi: 10.1038/nrc3174. [DOI] [PubMed] [Google Scholar]
- 17.García-Jiménez C, Gutiérrez-Salmerón M, Chocarro-Calvo A, et al. From obesity to diabetes and cancer: epidemiological links and role of therapies. Br J Cancer. 2016;114:716–722. doi: 10.1038/bjc.2016.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alberti K, Eckel RH, Grundy SM, et al. Harmonizing the metabolic syndrome a joint interim statement of the international diabetes federation task force on epidemiology and prevention for the study of obesity. Circulation. 2009 doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 19.Esposito K, Chiodini P, Colao A, et al. Metabolic syndrome and risk of cancer: a systematic review and meta-analysis. Diabetes Care. 2012 doi: 10.2337/dc12-0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jinjuvadia R, Lohia P, Jinjuvadia C, et al. The association between metabolic syndrome and colorectal neoplasm: systemic review and meta-analysis. J. Clin. Gastroenterol. 2013;47:33–44. doi: 10.1097/MCG.0b013e3182688c15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Agnoli C, Berrino F, Abagnato CA, et al. Metabolic syndrome and postmenopausal breast cancer in the ORDET cohort: a nested case-control study. Nutr Metab Cardiovasc Dis. 2010 doi: 10.1016/j.numecd.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Esposito K, Chiodini P, Capuano A, et al. Metabolic syndrome and postmenopausal breast cancer: systematic review and meta-analysis. Menopause. 2013 doi: 10.1097/GME.0b013e31828ce95d. [DOI] [PubMed] [Google Scholar]
- 23.Buono G, Crispo A, Giuliano M, et al. Combined effect of obesity and diabetes on early breast cancer outcome: a prospective observational study. Oncotarget. 2017 doi: 10.18632/oncotarget.22977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Calip GS, Malone KE, Gralow JR, et al. Metabolic syndrome and outcomes following early-stage breast cancer. Breast Cancer Res Treat. 2014 doi: 10.1007/s10549-014-3157-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bjørge T, Lukanova A, Jonsson H, et al. Metabolic syndrome and breast cancer in the Me-Can (metabolic syndrome and cancer) project. Cancer Epidemiol Biomark Prev. 2010 doi: 10.1158/1055-9965.EPI-10-0230. [DOI] [PubMed] [Google Scholar]
- 26.Matthews DR, Hosker JP, Rudenski AS, et al. Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985 doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 27.Goldhirsch A, Winer EP, Coates AS, et al. Personalizing the treatment of women with early breast cancer: highlights of the st gallen international expert consensus on the primary therapy of early breast Cancer 2013. Ann Oncol. 2013 doi: 10.1093/annonc/mdt303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Expert Panel on Detection and Treatment of High Blood Cholesterol in Adults, E (2001) Executive summary of the third report of the national cholesterol (NCEP) expert panel on detection, evaluation and treatment of high blood cholesterol in adults (adult treatment panel III). J Am Med Assoc [DOI] [PubMed]
- 29.Berrino F, Villarini A, Traina A, et al. Metabolic syndrome and breast cancer prognosis. Breast Cancer Res Treat. 2014 doi: 10.1007/s10549-014-3076-6. [DOI] [PubMed] [Google Scholar]
- 30.Gathirua-Mwangi WG, Song Y, Monahan PO, et al. Associations of metabolic syndrome and C-reactive protein with mortality from total cancer, obesity-linked cancers and breast cancer among women in NHANES III. Int J Cancer. 2018 doi: 10.1002/ijc.31344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Healy LA, Ryan AM, Carroll P, et al. Metabolic syndrome, central obesity and insulin resistance are associated with adverse pathological features in postmenopausal breast cancer. Clin Oncol. 2010 doi: 10.1016/j.clon.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 32.Maiti B, Kundranda MN, Spiro TP, Daw HA. The association of metabolic syndrome with triple-negative breast cancer. Breast Cancer Res Treat. 2010 doi: 10.1007/s10549-009-0591-y. [DOI] [PubMed] [Google Scholar]
- 33.Emaus A, Veierød MB, Tretli S, et al. Metabolic profile, physical activity, and mortality in breast cancer patients. Breast Cancer Res Treat. 2010 doi: 10.1007/s10549-009-0603-y. [DOI] [PubMed] [Google Scholar]
- 34.Dibaba DT, Ogunsina K, Braithwaite D, Akinyemiju T. Metabolic syndrome and risk of breast cancer mortality by menopause, obesity, and subtype. Breast Cancer Res Treat. 2019 doi: 10.1007/s10549-018-5056-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Monzavi-Karbassi B, Gentry R, Kaur V, et al. Pre-diagnosis blood glucose and prognosis in women with breast cancer. Cancer Metab. 2016 doi: 10.1186/s40170-016-0147-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Borugian MJ, Sheps SB, Kim-Sing C, et al. Waist-to-hip ratio and breast cancer mortality. Am J Epidemiol. 2003 doi: 10.1093/aje/kwg236. [DOI] [PubMed] [Google Scholar]
- 37.Goodwin PJ, Ennis M, Pritchard KI, et al. Fasting insulin and outcome in early-stage breast cancer: results of a prospective cohort study. J Clin Oncol. 2002 doi: 10.1200/JCO.20.1.42. [DOI] [PubMed] [Google Scholar]
- 38.Warburg O. On the origin of cancer cells. Science. 1956 doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 39.Lipworth L, Adami HO, Trichopoulos D, et al. Serum steroid hormone levels, sex hormone-binding globulin, and body mass index in the etiology of postmenopausal breast cancer. Epidemiology. 1996 doi: 10.1097/00001648-199601000-00017. [DOI] [PubMed] [Google Scholar]
- 40.Wiseman BSWZ. Stromal effects on mammary gland development and breast cancer. Science. 2002;296:1046–1049. doi: 10.1126/science.1067431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nieman KM, Romero IL, Van Houten B, Lengyel E. Adipose tissue and adipocytes support tumorigenesis and metastasis. Biochim Biophys Acta. 2013;1831:1533–1541. doi: 10.1016/j.bbalip.2013.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lehuédé C, Li X, Dauvillier S, et al. Adipocytes promote breast cancer resistance to chemotherapy, a process amplified by obesity: Role of the major vault protein (MVP) Breast Cancer Res. 2019 doi: 10.1186/s13058-018-1088-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ambrosio MR, D’Esposito V, Costa V, et al. Glucose impairs tamoxifen responsiveness modulating connective tissue growth factor in breast cancer cells. Oncotarget. 2017;2:3. doi: 10.18632/oncotarget.22552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Alikhani N, Ferguson RD, Novosyadlyy R, et al. Mammary tumor growth and pulmonary metastasis are enhanced in a hyperlipidemic mouse model. Oncogene. 2013 doi: 10.1038/onc.2012.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gillmer MD. Mechanism of action/effects of androgens on lipid metabolism. Int J Fertil. 1992;37:83–92. [PubMed] [Google Scholar]