Abstract
Background
Eribulin, a nontaxane synthetic inhibitor of microtubule dynamics, is widely used to manage locally advanced or metastatic breast cancer (MBC). Eribulin has demonstrated immunomodulatory activity on the tumour microenvironment. Baseline neutrophil-to-lymphocyte ratio (NLR), a marker of immune status, may predict progression-free survival in eribulin treatment. This post hoc analysis assessed predictors for overall survival (OS).
Methods
The phase 3 open-label study (EMBRACE) of eribulin versus treatment of physician’s choice (TPC) in patients with MBC provided source data. Baseline absolute lymphocyte counts (ALCs) and NLR were evaluable in 751 and 713 patients, respectively.
Results
Eribulin prolonged OS versus TPC in patients with baseline ALC ≥ 1500/µl (hazard ratio [HR] 0.586; 95% confidence interval [CI] 0.437–0.784; P < 0.001). There was no significant difference by treatment for ALC < 1500/µl (HR 1.002; 95% CI 0.800–1.253; P = 0.989). Univariate and multivariate analyses were performed and identified baseline ALC as a potential predictor of OS in eribulin-treated patients. Interaction analysis of OS supported 1500/µl as a potentially differential cutoff value. NLR at a cutoff value of 3 was associated with prolonged OS (eribulin group). However, similar results were also observed in the TPC group, without apparent interaction effect, suggesting that NLR may be a general prognostic marker rather than a specific predictor of OS for eribulin.
Discussion
This hypothesis-generating study speculates that baseline ALC may be an independent predictor for longer OS in eribulin-treated MBC patients and could be clinically impactful because it can be evaluated without the need for additional invasive procedures.
Trial Registration
Electronic supplementary material
The online version of this article (10.1007/s12282-020-01067-2) contains supplementary material, which is available to authorized users.
Keywords: Eribulin, Absolute lymphocyte count, Overall survival, Metastatic breast cancer, Treatment of physician’s choice
Introduction
Eribulin, a synthetic inhibitor of microtubule dynamics, is widely used for locally advanced or metastatic breast cancer (MBC) after one or two previous lines of chemotherapy in Europe and the United States, respectively [1, 2]. In Japan, eribulin has been approved for inoperable or recurrent breast cancer, following treatment with an anthracycline and a taxane [3]. Eribulin monotherapy versus treatment of physician’s choice (TPC) in patients with MBC (EMBRACE) was a phase 3, open-label, randomised study of 762 patients (eribulin, n = 508; TPC, n = 254) [4]. Overall survival (OS) was significantly longer in the eribulin group versus the TPC group (median OS: 13.1 vs 10.6 months, respectively; hazard ratio [HR] 0.81; 95% confidence interval [CI], 0.66–0.99; P = 0.041). However, there was no statistically significant difference in progression-free survival (PFS). Although the impact of eribulin on OS distinguishes it from other neoplastic agents (including taxanes) in pretreated MBC, the exact mechanism behind this difference in efficacy outcomes is not well understood.
In addition to its antimitotic effect, eribulin facilitates antineoplastic activity in MBC by reversing epithelial-to-mesenchymal transition (EMT) and inducing vascular remodelling [5, 6]. Kashiwagi et al. [7] reported that the number of tumour-infiltrating lymphocytes (TILs) in the tumour microenvironment may be a predictor of eribulin’s therapeutic effect in patients with triple-negative MBC; patients with higher numbers of TILs had significantly longer PFS than patients with lower numbers of TILs. In a single-institute retrospective study, Miyagawa et al. [8] demonstrated that neutrophil-to-lymphocyte ratio (NLR), a marker of systemic immunity, was significantly associated with PFS in eribulin-treated patients but not in those treated with nab-paclitaxel for MBC. Median PFS of patients with a baseline NLR < 3 was significantly longer than patients with a baseline NLR ≥ 3 (242 days vs 98 days; HR 0.37; 95% CI 0.18–0.71) [8].
NLR and TILs are markers of immunological status associated with predicting outcomes in patients with cancer [9–13]. Therefore, we speculate that eribulin’s ability to mediate immunological regulation in patients with MBC may clarify why there is a greater impact on OS compared with PFS. This post hoc analysis used data from EMBRACE to evaluate absolute lymphocyte count (ALC) and NLR as predictors of OS with eribulin.
Patients and methods
Clinical study design
The study design and primary efficacy and safety results of EMBRACE have been reported previously [4]. Briefly, patients with ≥ 2 previous chemotherapy regimens (including an anthracycline and taxane, for locally recurrent breast cancer or MBC) were randomised 2:1 to receive eribulin or TPC. Patients with baseline ALC and NLR assessments (i.e., the last nonmissing result prior to the first administration of study drug) were included in the analysis for association between baseline ALC/NLR and OS/PFS outcomes. Over 90% of baseline blood samples were collected within 3 days prior to initial administration of eribulin or TPC. If these blood samples were unavailable, blood samples obtained during screening were utilised.
Approval was obtained from independent ethics committees and regulatory authorities in participating countries, and all patients provided written informed consent. EMBRACE was conducted in accordance with the World Medical Association Declaration of Helsinki (WMA General Assembly, Tokyo, 2004) guidelines of the Committee for Proprietary Medicinal Products/International Conference for Harmonisation/Good Clinical Practice (CPMP/ICH/135/95) and local ethical/legal requirements.
Treatments
Eribulin was administered intravenously at a dose of 1.4 mg/m2 during a 2–5-min infusion on days 1 and 8 of a 21-day cycle. TPC was administered according to local practice; both eribulin and TPC continued until disease progression, unacceptable toxic effects, patient or physician request to discontinue, or serious protocol noncompliance [4].
Post hoc analysis of patient outcomes
OS was measured from the date of randomisation to the date of death from any cause. OS was censored at the last date that patients were known to be alive. PFS was defined as the time from the date of randomisation to the date of radiological disease progression based on independent review or death from any cause, whichever occurred first. PFS was censored at the date of last radiological assessment.
Statistical analyses
The Kaplan–Meier method was used to estimate OS/PFS distribution. The cutoff value for baseline ALC and NLR was set at 1500/µl [14] and 3 [8], respectively. HRs of the high-ALC or low-NLR group versus the low-ALC or high-NLR group were estimated from the stratified Cox proportional hazard model with ALC or NLR group as an independent variable and stratified by the randomisation stratification factors: HER2/neu status, prior capecitabine treatment, and geographical region. Subgroup analyses were conducted in a similar manner. Differences between groups were evaluated using the stratified log-rank test with randomisation stratification factors. Interactions between treatment and baseline ALC/NLR were explored using a stratified Cox proportional hazard model with treatment, ALC/NLR category, and their interaction, as covariates. For baseline ALC, the interactions were also explored broadly with different cutoff values. Univariate/multivariate analyses for baseline factors were performed using the Cox proportional hazard model to identify predictors for OS. Backward selection was used with a significance level of 10% for retaining the factors in the multivariate model. Multiplicity adjustments were not used for any analyses. Analyses were performed using SAS version 9.3 (SAS Institute Inc., Cary, NC, USA).
Results
Patient characteristics
762 Patients were enrolled in the intent-to-treat population and were randomised to receive eribulin (n = 508) or TPC (n = 254). There were evaluable baseline ALC results for 500 (eribulin) and 251 (TPC) patients (751 total; Online resource Fig. 1). Baseline characteristics were similar between groups with ≥ 1500/µl (high ALC) and < 1500/µl (low ALC) in both treatment groups with the exception of the number of patients with > 2 organs involved (high ALC; 19–24% vs low ALC; 30–39%) and the number of patients from North America/Western Europe/Australia (high ALC; 53–55% vs low ALC; 69–70%) (Table 1). There were evaluable baseline NLR results for 475 (eribulin) and 238 (TPC) patients (713 total). Baseline characteristics were similar between ≥ 3 (high NLR) and < 3 (low NLR) patients in both the eribulin and TPC groups except for the number of patients with > 2 organs involved (high NLR; 32–41% vs low NLR; 20–26%), > 3 prior chemotherapy regimens (high NLR; 60–62% vs low NLR; 47–50%) and the number of patients from North America/Western Europe/Australia (high NLR; 74–75% vs low NLR; 57–60%) (Online Resource Table 1). Patients were heavily pretreated (median, 4 previous chemotherapy regimens). Median (interquartile range) baseline ALCs for eribulin, TPC, and overall groups were 1308 (1000, 1814), 1307 (991, 1697), and 1307 (1000, 1776), respectively. Median (interquartile range) baseline NLRs for eribulin, TPC, and overall groups were 3.05 (2.13, 4.44), 3.06 (2.14, 4.19), and 3.05 (2.14, 4.41), respectively.
Table 1.
Demographic and baseline characteristics by ALC group
| Characteristic, n (%) | Eribulin (n = 500) | TPCa (n = 251) | ||
|---|---|---|---|---|
| ALC ≥ 1500/μl (n = 199) | ALC < 1500/μl (n = 301) | ALC ≥ 1500/μl (n = 92) | ALC < 1500/μl (n = 159) | |
| Geographical region | ||||
| North America/Western Europe/Australia | 106 (53) | 212 (70) | 51 (55) | 110 (69) |
| Eastern Europe | 66 (33) | 62 (21) | 29 (32) | 34 (21) |
| Latin America/South Africa | 27 (14) | 27 (9) | 12 (13) | 15 (9) |
| HER2 status | ||||
| Positive | 34 (17) | 48 (16) | 16 (17) | 23 (14) |
| Negative | 146 (73) | 221 (73) | 69 (75) | 121 (76) |
| Unknown | 19 (10) | 32 (11) | 7 (8) | 15 (9) |
| Prior capecitabine treatment | ||||
| Yes | 136 (68) | 228 (76) | 65 (71) | 122 (77) |
| No | 63 (32) | 73 (24) | 27 (29) | 37 (23) |
| ECOG performance status | ||||
| 0 | 87 (44) | 127 (42) | 40 (43) | 63 (40) |
| ≥ 1 | 110 (55) | 168 (56) | 51 (55) | 94 (59) |
| Age group | ||||
| < 65 years | 166 (83) | 242 (80) | 76 (83) | 120 (75) |
| ≥ 65 years | 33 (17) | 59 (20) | 16 (17) | 39 (25) |
| ER status | ||||
| Positive | 136 (68) | 195 (65) | 55 (60) | 114 (72) |
| Negative | 47 (24) | 95 (32) | 33 (36) | 39 (25) |
| Unknown | 16 (8) | 11 (4) | 4 (4) | 6 (4) |
| PgR status | ||||
| Positive | 103 (52) | 148 (49) | 43 (47) | 78 (49) |
| Negative | 68 (34) | 126 (42) | 37 (40) | 65 (41) |
| Unknown | 28 (14) | 27 (9) | 12 (13) | 16 (10) |
| HR status | ||||
| Positive | 141 (71) | 203 (67) | 61 (66) | 116 (73) |
| Negative | 38 (19) | 85 (28) | 26 (28) | 37 (23) |
| Unknown | 20 (10) | 13 (4) | 5 (5) | 6 (4) |
| Triple negative | ||||
| Triple negative | 27 (14) | 65 (22) | 21 (23) | 30 (19) |
| Non-triple negative | 172 (86) | 236 (78) | 71 (77) | 129 (81) |
| Site of disease | ||||
| Visceral disease | 160 (80) | 247 (82) | 74 (80) | 135 (85) |
| Nonvisceral disease | 36 (18) | 52 (17) | 17 (18) | 22 (14) |
| Number of organs involved | ||||
| ≤ 2 | 159 (80) | 208 (69) | 69 (75) | 95 (60) |
| > 2 | 37 (19) | 91 (30) | 22 (24) | 62 (39) |
| Number of prior chemotherapy regimens | ||||
| ≤ 3 | 111 (56) | 127 (42) | 44 (48) | 69 (43) |
| > 3 | 88 (44) | 172 (57) | 48 (52) | 89 (56) |
| Number of prior chemotherapy regimens for locally advanced or metastatic disease | ||||
| ≤ 3 | 165 (83) | 221 (73) | 66 (72) | 112 (70) |
| > 3 | 34 (17) | 80 (27) | 26 (28) | 46 (29) |
| Refractory to taxanesb | ||||
| Yes | 154 (77) | 249 (83) | 76 (83) | 127 (80) |
| No | 45 (23) | 52 (17) | 16 (17) | 32 (20) |
ALC absolute lymphocyte count, ECOG Eastern Cooperative Oncology Group, ER oestrogen receptor, HER2 human epidermal growth factor receptor-2, HR hormone receptor, PgR progesterone receptor, TPC treatment of physician’s choice
aTPC was defined as any single-agent chemotherapy, or hormonal or biological therapy, approved for the treatment of cancer
bDisease progression on or within 6 months of taxane treatment
OS and PFS outcomes
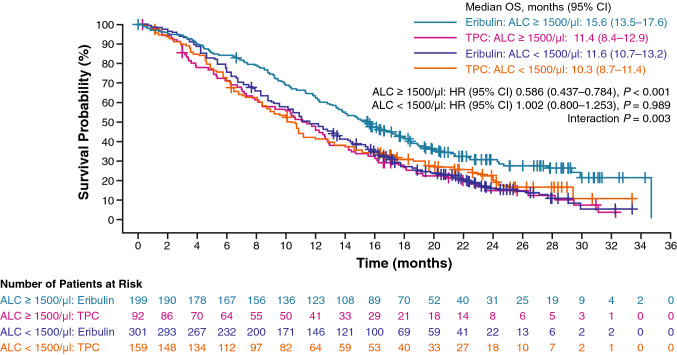
Using the cutoff value of ALC (“high” ≥ 1500/μl and “low” < 1500/μl), OS was compared in patients treated with eribulin (high ALC, n = 199 [40%]; low ALC, n = 301 [60%]) versus TPC (high ALC, n = 92 [37%]; low ALC, n = 159 [63%]). OS was prolonged in the eribulin versus the TPC group in patients with high ALC (median 15.6 months vs 11.4 months; HR 0.586; 95% CI 0.437–0.784; P < 0.001); in patients with low ALC, the difference was not significant (median, 11.6 months vs 10.3 months; HR 1.002; 95% CI 0.800–1.253; P = 0.989) (Fig. 1). Moreover, statistical signals of an interaction effect between treatment and baseline ALC were found; OS was longer in the high-ALC group versus the low-ALC group (HR 0.631, 95% CI 0.505–0.789) with eribulin but not TPC. Although this association was found in PFS within each treatment, there were no significant differences in PFS between the eribulin and TPC arms in either the high- or low-ALC groups (Online Resource Fig. 2).
Fig. 1.
Interaction between treatment and baseline ALC (< 1500/µL vs ≥ 1500/µL) on OS. In patients with high ALC, the median OS was 15.6 and 11.4 months in the eribulin and TPC arms, respectively (HR 0.586; 95% CI 0.437–0.784; P < 0.001). In patients with low ALC, the median OS was 11.6 and 10.3 months in the eribulin and TPC arms, respectively (HR 1.002; 95% CI 0.800–1.253; P = 0.989). ALC absolute lymphocyte count, CI confidence interval, HR hormone receptor, OS overall survival
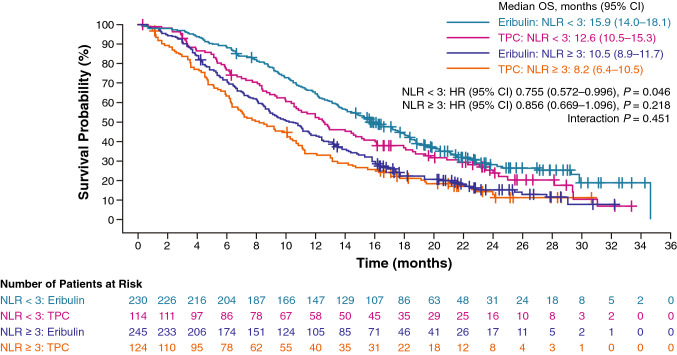
OS was also compared using an NLR cutoff value of 3 (“high” ≥ 3 and “low” < 3) in patients treated with eribulin (high NLR, n = 245 [52%]; low NLR, n = 230 [48%]) vs TPC (high NLR, n = 124 [52%]; low NLR, n = 114 [48%]). OS was prolonged with eribulin versus the TPC group in patients with low NLR (median, 15.9 months vs 12.6 months; HR 0.755; 95% CI: 0.572–0.996; P = 0.046); in patients with high NLR, the difference was not significant (median, 10.5 months vs 8.2 months; HR 0.856; 95% CI 0.669–1.096; P = 0.218). There were no statistical signals of an apparent interaction effect between treatment and baseline NLR (Fig. 2). OS was longer in the low-NLR group versus the high-NLR group within each treatment; a similar association was also found for PFS (Online Resource Fig. 3).
Fig. 2.
Interaction between treatment and baseline NLR (< 3 vs ≥ 3) on OS. In patients with high NLR, the median OS was 10.5 and 8.2 months in the eribulin and TPC arms, respectively (HR 0.856; 95% CI 0.669–1.096; P = 0.218). In patients with low NLR, the median OS was 15.9 and 12.6 months in the eribulin and TPC arms, respectively (HR 0.755; 95% CI 0.572–0.996; P = 0.046). CI confidence interval, OS overall survival
Univariate/multivariate analyses of baseline factors for OS
Univariate/multivariate analyses were conducted to explore baseline predictors of OS and confirm baseline ALC as a predictor of OS, adjusting for other potential confounding factors (Table 2). Six parameters were identified as predictors of OS in the eribulin group: (1) prior capecitabine use, (2) ECOG performance status, (3) HR status, (4) number of organs involved, (5) refractory to taxanes, and (6) baseline ALC. In the TPC group, four of these factors (with the exception of prior capecitabine use and baseline ALC) affected OS.
Table 2.
Univariate/ multivariate analyses of the baseline factors for OS in EMBRACE
| Parameter | Eribulin | TPC | ||||||
|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | |||||
| Hazard Ratio (95% CI) | P-value | Hazard Ratio (95% CI) | P-value | Hazard Ratio (95%CI) | P-value | Hazard Ratio (95% CI) | P-value | |
| North America/Western Europe/Australia vs Latin America/South Africa |
0.783 (0.568–1.079) |
0.1349 | – | – |
0.845 (0.531–1.343) |
0.4757 | – | – |
| Eastern Europe (vs Latin America/South Africa) |
0.736 (0.513–1.055) |
0.0951 | – | – |
0.703 (0.420–1.175) |
0.1786 | – | – |
| HER2 status (positive vs negative) |
1.106 (0.845–1.446) |
0.4630 | – | – |
1.387 (0.964–1.995) |
0.0783 | – | – |
| Prior capecitabine (yes vs no) |
1.262 (1.000–1.593) |
0.0497 |
1.292 (1.006–1.660) |
0.0447 |
1.316 (0.954–1.816) |
0.0942 | – | – |
| ECOG performance status (0 vs ≥ 1) |
0.593 (0.482–0.729) |
< 0.0001 |
0.598 (0.481–0.745) |
< 0.0001 |
0.526 (0.394–0.704) |
< 0.0001 |
0.563 (0.418–0.758) |
0.0002 |
| Age group (< 65 years vs ≥ 65 years) |
0.904 (0.705–1.160) |
0.4278 | – | – |
1.307 (0.924–1.848) |
0.1301 | – | – |
| ER status (positive vs negative) |
0.726 (0.582–0.906) |
0.0047 | – | – |
0.815 (0.599–1.110) |
0.1946 | – | – |
| PgR status (positive vs negative) |
0.795 (0.644–0.982) |
0.0331 | – | – |
1.079 (0.804–1.449) |
0.6114 | – | – |
| HR status (positive vs negative) |
0.664 (0.528–0.834) |
0.0004 |
0.649 (0.512–0.822) |
0.0003 |
0.770 (0.557–1.066) |
0.1151 | – | – |
| Triple negative (vs non-triple negative) |
1.475 (1.148–1.895) |
0.0023 | – | – |
1.305 (0.924–1.843) |
0.1309 | – | – |
| Site of disease (visceral vs nonvisceral) |
1.265 (0.960–1.667) |
0.0952 | – | – |
1.479 (0.992–2.207) |
0.0550 | – | – |
| Number of organs involved (≤ 2 vs > 2) |
0.615 (0.493–0.766) |
< 0.0001 |
0.559 (0.442–0.707) |
< 0.0001 |
0.615 (0.462–0.818) |
0.0009 |
0.689 (0.514–0.925) |
0.0132 |
| Number of prior chemotherapy regimens (≤ 3 vs > 3) |
0.870 (0.711–1.063) |
0.1734 | – | – |
0.865 (0.654–1.144) |
0.3102 | – | – |
| Number of prior chemotherapy regimens for locally advanced or metastatic disease (≤ 3 vs > 3) |
0.752 (0.599–0.944) |
0.0142 | – | – |
0.817 (0.606–1.103) |
0.1868 | – | – |
| Refractory to taxanesa (yes vs no) |
1.682 (1.283–2.206) |
0.0002 |
1.694 (1.267–2.264) |
0.0004 |
0.912 (0.649–1.283) |
0.5980 | – | – |
| Baseline ALC (≥ 1500/μL vs < 1500/μL) |
0.670 (0.542–0.827) |
0.0002 |
0.761 (0.607–0.955) |
0.0183 |
1.098 (0.826–1.459) |
0.5208 | – | – |
ALC absolute lymphocyte count, CI confidence interval, ECOG Eastern Cooperative Oncology Group, ER oestrogen receptor, HER2 human epidermal growth factor receptor-2, HR hormone receptor, PgR progesterone receptor
aDisease progression on or within 6 months of taxane treatment
Subgroup analyses for baseline ALC effects on OS
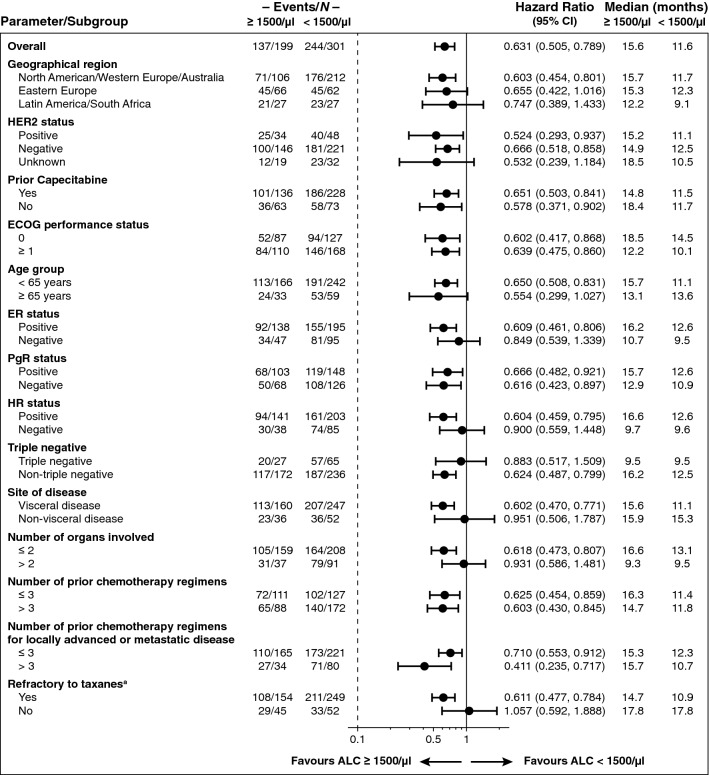
Subgroup analysis on OS for baseline ALC (≥ 1500/μl vs < 1500/μl) in patients treated with eribulin indicated that high ALC consistently favoured OS in all parameters analysed, with the exception of patients not refractory to taxanes (Fig. 3). In patients treated with TPC, the subgroup analysis did not demonstrate a consistent correlation between high ALC and OS (Online Resource Fig. 4).
Fig. 3.
The forest plot for baseline ALC (< 1500/µl vs ≥ 1500/µl) effects on OS for the eribulin arm indicates that high ALC consistently favoured OS in all parameters analysed, except for patients who were not refractory to taxanes. ALC absolute lymphocyte count, CI confidence interval, ECOG Eastern Cooperative Oncology Group, ER oestrogen receptor, HER2 human epidermal growth factor receptor-2, HR hormone receptor, OS overall survival, PgR progesterone receptor. aDisease progression on or within 6 months of taxane treatment
Cutoff value of ALC
An interaction analysis of OS was performed between treatment and baseline ALC in EMBRACE to evaluate baseline ALC as a predictive factor of eribulin’s effect and to confirm the cutoff values of ALC (Online Resource Table 2). Numerically longer median OS was observed in the eribulin group compared with the TPC group regardless of baseline ALC. However, HRs of eribulin versus TPC in the high-ALC groups were consistently lower than those in the low-ALC groups. Moreover, the benefits of eribulin in high-ALC groups were greater than in the low-ALC groups across the cutoff values of 1400–1700/μl (interaction P < 0.05). Notable differential effects were observed around cutoff values of 1500/μl (interaction P = 0.003). The HR of eribulin versus TPC was 0.586 (95% CI 0.437–0.784) in patients with baseline ALC ≥ 1500/μl.
Discussion
In this post hoc analysis conducted with data from EMBRACE, a high ALC (≥ 1500/µl) was found to be a significant and independent predictor for longer OS in patients treated with eribulin, but not in those treated with TPC.
Previously, Miyagawa et al. [8] found that NLR was significantly associated with PFS in eribulin-treated patients. Recently, Araki et al. [14] reported that baseline ALC at a cutoff value of 1500/µl is a predictor for PFS in HER2-positive advanced breast cancer treated with pertuzumab and trastuzumab, irrespective of combination chemotherapy regimens including eribulin. Collectively, these results suggest that the status of the tumour microenvironment affects the efficacy of eribulin. Therefore, this analysis focused on baseline ALC and NLR as peripheral immunological biomarkers associated with the efficacy of eribulin. In EMBRACE [4], at a cutoff value of 3, NLR was associated with prolonged PFS and OS in the eribulin group. However, similar results were also observed in the TPC group, without apparent interaction effect. This suggests that NLR may be a general prognostic marker rather than a specific predictor of OS for eribulin, although NLR cannot be excluded as a potential predictor for other agents. Therefore, it seems that ALC might be a superior predictive marker of improved OS with eribulin than NLR. Based on this analysis, ALC appears to be a specific predictor of OS for eribulin, but not a general prognostic factor. To the best of our knowledge, this is the first report demonstrating ALC as a predictive marker for OS benefit in MBC. Considering these outcomes, baseline ALC may be used as a specific biomarker to predict the survival benefit conferred by eribulin treatment in patients with MBC.
Eribulin induced the remodelling of the tumour vasculature in a preclinical xenograft model and in patients with breast cancer in several studies [6, 15–17]. Additionally, eribulin induced reoxygenation by vascular remodelling in patients with advanced breast cancer and decreased transforming growth factor-beta (TGF-β), which is typically associated with hypoxic conditions [15]. In a retrospective analysis of eribulin responders (patients with MBC), programmed cell death-1 (PD-1), programmed cell death ligand-1 (PD-L1), and forkhead box P3 levels reportedly decreased, while infiltrated CD8+ T-cell levels increased, but these findings were not observed in the nonresponder group [18]. As PD-1/PD-L1 pathways and TGF-β have potent immunosuppressive effects [19], these results suggested that eribulin may have had an immunomodulatory effect mediated through vascular remodelling, especially in the responder group.
Other research has demonstrated that baseline ALC was associated with longer OS in melanoma patients treated with ipilimumab, an anti-CTLA-4 antibody [20]. Ku et al. [21] reported a trend towards improved OS in a high-ALC group in patients with melanoma treated with ipilimumab (median OS, 13.3 vs 5.1 months; P = 0.06). Ku et al. concluded that baseline ALC has the potential to be an immunological predictive index for OS when treated with immune checkpoint inhibitors (ICIs).
Considering that ALC may be predictive of the efficacy of ICIs, ALC may potentially reflect the immune microenvironment within the tumour. Reportedly, hypoxia induces an increase in the expression of PD-L1 via the hypoxia-inducible factor-1 (HIF-1) transcription factor in myeloid-derived suppressor cells [22]. HIF-1 also regulated TGF-β, which has potent immunosuppressive effects and promotes the growth of breast cancer. TGF-β also plays a role in EMT [23]. As eribulin may potentially possess immunoregulatory effects, via vascular remodelling, we speculate eribulin may induce reversal of EMT and this effect may contribute to prolonging OS. Moreover, we speculate that tumours with high-ALC levels in peripheral blood may reflect a favourable immune microenvironment that potentiates benefit from eribulin treatment.
We identified that high ALC (≥ 1500/µl) was a significant and independent predictor for longer OS in patients treated with eribulin through our evaluation of data from EMBRACE. However, we cannot exclude the possibility that treatment with prior chemotherapy may have influenced baseline ALC, despite exclusion of patients who had received previous treatment within 3 weeks of EMBRACE. Therefore, baseline ALC and NLR should be further evaluated in patients receiving first-line treatment with eribulin. Additionally, as this was a post hoc analysis, these results should be considered hypothesis generating. Considering this limitation, prospective studies or additional investigations are required to further validate the results of this report.
Conclusions
In this hypothesis-generating analysis of EMBRACE, patients with high baseline ALC (≥ 1500/µl) showed longer OS with eribulin treatment, but not with TPC. Generally, ALC demonstrated potential as an immunological predictive index, hence, we suggest that longer OS in eribulin-treated patients is associated with modulation of the tumour microenvironment, such as the immune-regulation system. These results may be useful in selecting patients who may have greater OS benefits with eribulin. As ALC is a simple biomarker that can be evaluated without the need for additional invasive procedures, clinical utility of ALC may be important not only for predicting eribulin efficacy but also in considering biomarkers for use in combination therapy with ICIs.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors thank Hiroyuki Shiiba for coordinating and Yukinori Sakata for his critical reading of the manuscript.
Author contributions
YM: participated in the study design, data analysis/interpretation, manuscript preparation, manuscript editing, and manuscript review. YY: participated in the study design, data analysis/interpretation, manuscript preparation, manuscript editing, and manuscript review. KS: participated in the study design, data analysis/interpretation, statistical analysis, manuscript preparation, manuscript editing, and manuscript review. KM: participated in the data analysis/interpretation, manuscript preparation, manuscript editing, and manuscript review. MS: participated in the data analysis/interpretation, manuscript preparation, manuscript editing, and manuscript review. KA: participated in the data analysis/interpretation, manuscript preparation, manuscript editing, and manuscript review. KN: participated in the data analysis/interpretation, manuscript preparation, manuscript editing, and manuscript review. SN: participated in the data analysis/interpretation, manuscript preparation, manuscript editing, and manuscript review. TS: participated in the data analysis/interpretation, manuscript preparation, manuscript editing, and manuscript review. JW: participated in the data analysis/interpretation, manuscript preparation, manuscript editing, and manuscript review. JMP-G: participated in the data analysis/interpretation, manuscript preparation, manuscript editing, and manuscript review. JC: participated in the data analysis/interpretation, manuscript preparation, manuscript editing, and manuscript review.
Funding
This work was supported by Eisai Inc., Woodcliff Lake, NJ, USA. The sponsor (Eisai Inc.) participated in the design of this analysis, data analysis, data interpretation, manuscript review, manuscript approval, and decision to submit for publication. Medical writing assistance was provided by Jessica Pannu, PharmD, of Oxford PharmaGenesis Inc., Newtown, PA, USA, with funding provided by Eisai Inc.
Compliance with ethical standards
Conflict of interest
Y. Miyoshi: reports grants from Eisai, Chugai, MSD, Kyowa-Kirin, Eli Lilly, and Taiho; personal fees from Eisai, Chugai, AstraZeneca, Eli Lilly, and Pfizer. Y. Yoshimura: is an employee of Eisai Co., Ltd. K. Saito: is an employee of Eisai Inc. K. Muramoto: is an employee of Eisai Co., Ltd. M. Sugawara: is an employee of Eisai Co., Ltd. K. Alexis: is a former employee of Eisai Inc. K. Nomoto: is an employee of Eisai Inc. S. Nakamura: reports grants from Chugai, Daiichi Sankyo, Eisai, Kyowa-Kirin, Novartis, Pfizer, Taiho, and Takeda; personal fees from AstraZeneca, Bayer, Chugai, Daiichi Sankyo, Eisai, Eli Lilly, Kyowa-Kirin, Nippon Kayaku, Novartis, Pfizer, Taiho, and Takeda. T. Saeki: has received grants from AstraZeneca, Eisai, MSD, Ono Pharmaceutical, Kyowa-Kirin, Daiichi Sankyo, Sawai, Taiho, Chugai, Nihon Medi-Physics, Nippon Kayaku, Novartis, Hamamatsu Photonics, and Fuji Pharma; personal fees from AstraZeneca, Ono Pharmaceutical, Kyowa-Kirin, Taiho, Chugai, Novartis, and Fuji Pharma. J. Watanabe: reports personal fees from Chugai, Daiichi Sankyo, Eisai, Eli Lilly, Novartis, and Pfizer. J.M. Perez-Garcia: reports an advisory role with Roche and Lilly. J. Cortes: reports consulting for Roche, Celgene, Cellestia, AstraZeneca, Biothera Pharmaceutical, Merus, Seattle Genetics, Daiichi Sankyo, Erytech, Athenex, Polyphor, Lilly, Servier, Merck Sharp & Dohme, and GSK; honoraria for Roche, Novartis, Celgene, Eisai, Pfizer, Samsung Bioepis, Lilly, and Merck Sharp & Dohme; research funding to the institution for Roche, Ariad pharmaceuticals, AstraZeneca, Baxalta GMBH/Servier Affaires, Bayer healthcare, Eisai, F. Hoffman-La Roche, Guardant health, Merck Sharp & Dohme, Pfizer, Piqur Therapeutics, Puma C, Queen Mary University of London, and Seagen; stock/patents/intellectual property of MedSIR.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Approval was obtained from independent ethics committees (Oxfordshire Research Ethics Committee; reference number 07/Q1606/22) and regulatory authorities in participating countries. EMBRACE was conducted in accordance with the World Medical Association Declaration of Helsinki (WMA General Assembly, Tokyo, 2004) guidelines of the Committee for Proprietary Medicinal Products/International Conference for Harmonisation/Good Clinical Practice (CPMP/ICH/135/95) and local ethical/legal requirements.
Informed consent
Informed consent was obtained from all individual participants included in the study. This article does not contain any studies with animals performed by any of the authors.
Footnotes
Some of the data were previously presented in an abstract at the San Antonio Breast Cancer Symposium; December 10–14, 2019, San Antonio, TX, USA.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Halaven (eribulin mesylate) [prescribing information]. Woodcliff Lake, NJ: Eisai Inc.; 2017.
- 2.Halaven 0.44 mg/ml solution for injection [summary of product characteristics]. Hertfordshire, UK: Eisai Europe Limited; 2019.
- 3.Halaven [Japanese prescribing information]. Tokyo, Japan: Eisai Co., Ltd; 2016.
- 4.Cortes J, O'Shaughnessy J, Loesch D, Blum JL, Vahdat LT, Petrakova K, et al. Eribulin monotherapy versus treatment of physician's choice in patients with metastatic breast cancer (EMBRACE): a phase 3 open-label randomised study. Lancet. 2011;377:914–923. doi: 10.1016/S0140-6736(11)60070-6. [DOI] [PubMed] [Google Scholar]
- 5.Yoshida T, Ozawa Y, Kimura T, Sato Y, Kuznetsov G, Xu S, et al. Eribulin mesilate suppresses experimental metastasis of breast cancer cells by reversing phenotype from epithelial-mesenchymal transition (EMT) to mesenchymal-epithelial transition (MET) states. Br J Cancer. 2014;110:1497–1505. doi: 10.1038/bjc.2014.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Funahashi Y, Okamoto K, Adachi Y, Semba T, Uesugi M, Ozawa Y, et al. Eribulin mesylate reduces tumor microenvironment abnormality by vascular remodeling in preclinical human breast cancer models. Cancer Sci. 2014;105:1334–1342. doi: 10.1111/cas.12488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kashiwagi S, Asano Y, Goto W, Takada K, Takahashi K, Noda S, et al. Use of tumor-infiltrating lymphocytes (TILs) to predict the treatment response to eribulin chemotherapy in breast cancer. PLoS ONE. 2017;12:e0170634. doi: 10.1371/journal.pone.0170634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miyagawa Y, Araki K, Bun A, Ozawa H, Fujimoto Y, Higuchi T, et al. Significant association between low baseline neutrophil-to-lymphocyte ratio and improved progression-free survival of patients with locally advanced or metastatic breast cancer treated with eribulin but not with nab-paclitaxel. Clin Breast Cancer. 2018;18:400–409. doi: 10.1016/j.clbc.2018.03.002. [DOI] [PubMed] [Google Scholar]
- 9.Noh H, Eomm M, Han A. Usefulness of pretreatment neutrophil to lymphocyte ratio in predicting disease-specific survival in breast cancer patients. J Breast Cancer. 2013;16:55–59. doi: 10.4048/jbc.2013.16.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ethier JL, Desautels D, Templeton A, Shah PS, Amir E. Prognostic role of neutrophil-to-lymphocyte ratio in breast cancer: a systematic review and meta-analysis. Breast Cancer Res. 2017;19:2. doi: 10.1186/s13058-016-0794-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu H, Zhang T, Ye J, Li H, Huang J, Li X, et al. Tumor-infiltrating lymphocytes predict response to chemotherapy in patients with advance non-small cell lung cancer. Cancer Immunol Immunother. 2012;61:1849–1856. doi: 10.1007/s00262-012-1231-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kocián P, Šedivcová M, Drgáč J, Cerná K, Hoch J, Kodet R, et al. Tumor-infiltrating lymphocytes and dendritic cells in human colorectal cancer: their relationship to KRAS mutational status and disease recurrence. Hum Immunol. 2011;72:1022–1028. doi: 10.1016/j.humimm.2011.07.312. [DOI] [PubMed] [Google Scholar]
- 13.Lee WS, Kang M, Baek JH, Lee JI, Ha SY. Clinical impact of tumor-infiltrating lymphocytes for survival in curatively resected stage IV colon cancer with isolated liver or lung metastasis. Ann Surg Oncol. 2013;20:697–702. doi: 10.1245/s10434-012-2752-1. [DOI] [PubMed] [Google Scholar]
- 14.Araki K, Ito Y, Fukada I, Kobayashi K, Miyagawa Y, Imamura M, et al. Predictive impact of absolute lymphocyte counts for progression-free survival in human epidermal growth factor receptor 2-positive advanced breast cancer treated with pertuzumab and trastuzumab plus eribulin or nab-paclitaxel. BMC Cancer. 2018;18:982. doi: 10.1186/s12885-018-4888-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ueda S, Saeki T, Takeuchi H, Shigekawa T, Yamane T, Kuji I, et al. In vivo imaging of eribulin-induced reoxygenation in advanced breast cancer patients: a comparison to bevacizumab. Br J Cancer. 2016;114:1212–1218. doi: 10.1038/bjc.2016.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ito K, Hamamichi S, Abe T, Akagi T, Shirota H, Kawano S, et al. Antitumor effects of eribulin depend on modulation of the tumor microenvironment by vascular remodeling in mouse models. Cancer Sci. 2017;108:2273–2280. doi: 10.1111/cas.13392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao S, Yu W, Ukon N, Tan C, Nishijima KI, Shimizu Y, et al. Elimination of tumor hypoxia by eribulin demonstrated by 18F-FMISO hypoxia imaging in human tumor xenograft models. EJNMMI Res. 2019;9:51. doi: 10.1186/s13550-019-0521-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goto W, Kashiwagi S, Asano Y, Takada K, Morisaki T, Fujita H, et al. Eribulin promotes antitumor immune responses in patients with locally advanced or metastatic breast cancer. Anticancer Res. 2018;38:2929–2938. doi: 10.21873/anticanres.12541. [DOI] [PubMed] [Google Scholar]
- 19.Li MO, Wan YY, Sanjabi S, Robertson AK, Flavell RA. Transforming growth factor-beta regulation of immune responses. Annu Rev Immunol. 2006;24:99–146. doi: 10.1146/annurev.immunol.24.021605.090737. [DOI] [PubMed] [Google Scholar]
- 20.Muto Y, Kitano S, Tsutsumida A, Namikawa K, Takahashi A, Nakamura Y, et al. Investigation of clinical factors associated with longer overall survival in advanced melanoma patients treated with sequential ipilimumab. J Dermatol. 2019;46:498–506. doi: 10.1111/1346-8138.14865. [DOI] [PubMed] [Google Scholar]
- 21.Ku GY, Yuan J, Page DB, Schroeder SE, Panageas KS, Carvajal RD, et al. Single-institution experience with ipilimumab in advanced melanoma patients in the compassionate use setting: lymphocyte count after 2 doses correlates with survival. Cancer. 2010;116:1767–1775. doi: 10.1002/cncr.24951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Noman MZ, Desantis G, Janji B, Hasmim M, Karray S, Dessen P, et al. PD-L1 is a novel direct target of HIF-1α, and its blockade under hypoxia enhanced MDSC-mediated T cell activation. J Exp Med. 2014;211:781–790. doi: 10.1084/jem.20131916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Taylor MA, Lee YH, Schiemann WP. Role of TGF-β and the tumor microenvironment during mammary tumorigenesis. Gene Expr. 2011;15:117–132. doi: 10.3727/105221611X13176664479322. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.