Abstract
To investigate the effect of fermentation on texturized vegetable protein (TVP), TVPs extruded at 40 and 50% feed moisture contents (MC) were fermented using Bacillus subtilis at 37 °C. Physicochemical, structural and microbial properties of TVPs were determined at 0, 12, 24, 36, 48, and 60 h after fermentation. Integrity index of fermented TVPs did not change significantly until 24–36 h after fermentation. Springiness and cohesiveness remained stable after fermentation in all samples. Significant total color change was observed during fermentation. The pH values dropped initially and rose again with a coincident increase in nitrogen solubility index and viable cell count of B. subtilis. During fermentation, TVP extruded at 50% MC maintained higher chewiness, hardness, integrity index, and layered structure than that extruded at 40% MC. The study demonstrated that fermenting the TVP extruded at 50% MC has potential to produce a new type of TVP based B. subtilis fermented food.
Keywords: Extrusion, Fermentation, Texturized vegetable protein, Physicochemical properties, Structural properties
Introduction
Worldwide improvements in peoples’ nutrition, agricultural production, and markets recently have drawn an increased attention to texturized vegetable protein (TVP). TVP is a plant protein processed food produced by extrusion technique and is noticeably similar with meat in fibrous structure, appearance, color, and taste. Purified soy protein isolates with 90% or more protein content is a widely used raw material for processing TVP. TPV is popular as low-cost and healthy food not only due to similarity with meat but also due to high protein, low fat, and no cholesterol contents (Asgar et al., 2010; Singh et al., 2008). TVP is widely consumed as meat extender or directly as meat analogs of hamburger patties, sausages, steak, sliced meats, and many other products. However, it has never been tried to consume as fermented product which has many advantages for nutrition and health.
Bacillus subtilis is an important microorganism for fermented soybean products in many countries including Korea (doenjang, cheonggukjang, kochujang, etc.), Japan (natto), Thailand (thua-nao), India (kinema), and West Africa (dawadawa) (Kiers et al., 2000; Kim et al., 2010). B. subtilis has high activity of proteolysis and fermentation of soybean by B. subtilis can be completed within a short time. B. subtilis fermented soybean products contain increased amount of free amino acids, vitamins such as thiamine (Vitamin B1), riboflavin (Vitamin B2), niacin, and folate (Ginting and Arcot, 2004; Shresthal et al., 2010). In contrast, the indigestible disaccharides, polysaccharides, and anti-nutrients in raw soybeans are reduced in fermented products (Esteves et al., 2010; Sarkar and Tamang, 1995). Moreover, soybeans fermented by B. subtilis contain higher isoflavone which can reduce the risks of cardiovascular diseases, prostate, breast, and colon cancers. Likewise, the antioxidant activity of DPPH (2, 2-dipheny-1 picrylhydrazyl) is also enhanced (Shrestha et al., 2010).
On the other hand, regardless of those properties, the slimy and rotten appearance of B. subtilis fermented foods along with strong beany smell lead to less consumption by large number of population (Park et al., 2012; Shrestha et al., 2010). Attempts have been made to increase the consumption of those products (Shrestha et al., 2010). It was noticeable that B. subtilis fermented soybean products with good texture such as high chewiness and hardness were preferred by taste panelists during sensory evaluation (Omafuvbe et al., 2002, 2007; Tamang and Nikkuni, 1996). Therefore, improving the textural properties of B. subtilis fermented products may be an alternative way to draw more consumers’ interest on them. Since TVP made from soy protein have good textural properties as described early, it is worthy to try using TPV as a raw material for B. subtilis fermented products. Moreover, unlike soybean, prolong soaking process can be excluded during preparation prior to fermentation of TVP.
It has been reported that B. subtilis fermentation of TVP showed high protease activity and produce higher amount of soybean peptides within short period of time when compared with other substrates. Angiotensin converting enzyme inhibitory activity, which plays important role to lower blood pressure in patients with hypertension, was also higher in fermented TVP than that of other fermented soybean products such as miso paste and natto (Kim et al., 2010). With such a good potential as a functional food after bioconversion with B. subtilis, it is also necessary to maintain the textural properties of TVP. TVP is originally intended to mimic the real meat, and nevertheless, the texture of TVP should remain even after fermentation.
Until now, there is no information on how textural properties of TVP change after fermentation. Although Kim et al. (2010), Kim and Lee (2010) and Ojokoh and Yimin (2011) studied the effect of fermentation on TVP, these studies focused only on chemical composition, nutritional quality and production of biologically active compounds. The scientific study on physical properties of B. subtilis fermented TVP is needed.
In the study of Kim et al. (2010), the enzyme activities and production of biologically active compounds were dependent upon the fermentation time during fermentation of TVP with B. subtilis (KCCM 10775P). It can be assumed that textural properties of fermented TVP can also be varied with the fermentation time. In addition, the texture of TVP originally depends on the extrusion process parameters among which feed moisture content is the most important one (Akdogan, 1996; Gu and Ryu, 2017). Since the current study tends to focus on physical properties especially textural characteristics of fermented TVP, both feed moisture content during extrusion and fermentation time should be taken as important factors.
Earlier work by Samard et al. (2019) have done extrusion of isolated soy protein (ISP), wheat gluten (WG) and corn starch (CS) (50:40:10 w/w) at 30% feed moisture content. However, the resulting TVP were found to have more spongy structure than fibrous and meat-like structure. Theoretically, mixture of ISP, WG, and CS should have texturized properly due to ability of ISP and WG to replicate meat texture and ability of CS to enhance cohesion between protein molecules. Meanwhile, Park et al. (2017) and Samard and Ryu (2019) reported that extrusion of ISP and WG conducted at > 40% feed moisture content could form fibrous structure. According to these, it was assumed that feed moisture content during the blend of ISP, WG and CS should also be increased to more than 30% for production of TVP with desirable textural properties. Therefore, two feed moisture contents, 40 and 50%, were selected for extrusion of TVP products in this experiment.
Considering all of the above, the present study aimed to examine the effect of B. subtilis fermentation on physical, structural, chemical and microbial properties of TVPs, extruded at two different moisture contents and fermented for different times.
Materials and methods
Materials
ISP, WG, and CS were obtained from Wachen Industry Co. (Quingdao, China), Roquette Freres (Lestrem, France) and Qone Co. Ltd. (Incheon, Korea), respectively. The protein content of ISP was 90% (dry basis) while that of WG was 83% (dry basis). The moisture content (MC) of the mixture of ISP, WG, and CS was 6.04% prior to extrusion.
Extrusion process
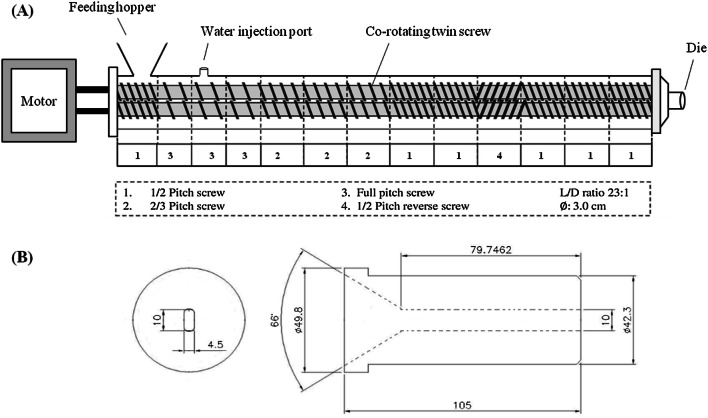
TVPs were prepared by extruding the mixture of ISP (500 g/kg), WG (400 g/kg), and CS (100 g/kg) with the use of co-rotating intermeshing twin-screw extruder (THK 31T, Incheon Machinery Co., Incheon, Korea). The extrusion process conditions were set at two different feed MC of 40 and 50%, 100 g/min feed rate, 200 rpm screw speed and 130 °C die temperature. During extrusion, desired MC was controlled by injecting water directly into the feeding zone with a pump (Grundfos DDA 30-4, Pfinztal, Germany). The screw and die configurations used in experiment are shown in Fig. 1. Immediately after extrusion, The TVPs were cut into 2 cm pieces and dried at 50 °C for 12 h until the MC became less than 2%.
Fig. 1.
A Screw (THK 31T) and B die configurations used for extrusion
Preparation of starter culture
Bacillus subtilis (KACC 15935) was obtained from Korean Agricultural Culture Collection (Wanju, Jeollabuk-do, Korea). B. subtilis was grown on nutrient agar at 35 °C for 24 h. The single colony was then transferred into 4 mL of nutrient broth and grown as a shaking culture at 35 °C. After 24 h, the bacterial culture was transferred into nutrient broth (0.5% v/v) and cultivated as a shake culture at 35 °C for 24 h. Finally, the bacterial culture was diluted with sterilized distilled water to give an absorbance of 1.5 at 660 nm in a spectrophotometer (Biochrom Lightwave II, Cambridge, England).
Fermentation of TVP samples
One and half kilogram of each TVP (extruded previously at two different MC) were taken and equally distributed 300 g (dry weight) into 2000 mL conical flasks plugged with cotton wool. The flasks were then autoclaved for 15 min at 121 °C for sterilization. After autoclaving, TVPs were cooled at room temperature for 2 h and then mixed with sterilized distilled water to achieve 50% MC. Then the sterilized TVPs were inoculated with starter culture (5% w/w). The TVPs were then fermented at 37˚C up to 60 h in low temperature incubator (HB-103MP, Hanbaek Scientific Co., Gyeonggi-do, Korea). The flasks were removed after 0, 12, 24, 36, 48, and 60 h of fermentation. After every removal, the fermented TVPs were kept at 4 °C to stop fermentation, and then texture profile analysis, integrity index, pH and viable cell number were measured as soon as possible. A part of the fermented samples were freeze-dried and microstructure was studied. The rest freeze-dried samples were ground into powders of particle size less than 0.5 mm and stored at − 20 °C until the completion of color measurement and chemical analysis. Physicochemical and microbial properties of raw TVPs (i.e. extruded, unautoclaved, and unfermented samples) were also analyzed to compare with those of fermented TVPs.
Texture profile analysis
Texture profile analysis of raw (extruded, unautoclaved, and unfermented) and fermented TVPs was carried out with Sun Rheometer (Compac-100 II, Sun Sci. Co., Tokyo, Japan). Firstly, MC of fermented TVPs was measured in accordance with the standard methods of AOAC (1990). Then, MC of raw TVPs was adjusted to be the same as that of fermented TVPs by means of hydration. To obtain the same MC (50%) as fermented ones, raw TVPs were hydrated at 80 °C for 30 min and drained on 20-mesh sieves for 15 min before measurement for texture profile analysis. The fermented TVPs were not hydrated, and measured directly. As measurement conditions, the cross head speed was set as 100 mm/min with the distance between two supports of 11 mm and the maximum peak stress of 10 kg. The hardness, cohesiveness, springiness, and chewiness were measured by compressing the sample (approximately 1.5 × 1.0 × 1.5 cm3) with a 25 mm diameter cylinder probe for 2 times. Each sample was measured for 10 times.
Integrity index
The integrity index indicates whether the texturized products had the structural integrity after being retorted under various processing conditions during post-extrusion. Integrity index was determined as previously described by Ma et al. (2018). The raw (extruded, unautoclaved, and unfermented) TVPs were hydrated at 80 °C for 30 min before analysis. The fermented TVPs were not hydrated prior to analysis. Each sample was measured for triplicates. Integrity index was calculated by using the following formula (1):
| 1 |
Color
A colorimeter (Minolta JP/CR-300, Konica Minolta, Tokyo, Japan) was used to determine the color of ground raw (extruded, unautoclaved, and unfermented) and fermented TVPs. The lightness was denoted by the L value (from black 0 to white 100). The redness and yellowness were denoted by a values (from green − 60 to red + 60) and the b values (from blue − 60 to yellow + 60), respectively. The color was calibrated against the standard white tile (L = + 97.26, a = − 0.02, and b = + 1.77). Each sample was measured in triplicates. The total color change (ΔE) of TVP samples during fermentation was calculated as Eq. (2) using the color values of raw TVP as standard.
| 2 |
where the subscript “0” indicates initial color values of raw TVPs.
Microstructure
The microstructures of fermented TVPs extruded at different MC were obtained by high resolution field emission scanning electron microscopy (MIRA III LMH, Tescan Co., Cranberry Township, PA). Freeze-dried samples were fixed on the stabs and sputter-coated with silver-palladium alloy before observation. All samples were observed at 1500× magnification with an accelerating voltage of 10 kV.
pH
The raw (extruded, unautoclaved, and unfermented) and fermented TVPs (4 g wet weight) were blended with 16 mL of distilled water in a homogenizer (IKA® T10 basic Ultra-Turrax®, Staufen, Germany) at 1453×g for 2 min. The pH of the slurry was measured with a pH meter (inoLab pH 720, Weilheim, Germany). Each sample was measured in triplicates.
Nitrogen solubility index
Soluble nitrogen of raw (extruded, unautoclaved, and unfermented) and fermented TVPs was extracted by following the method described by Daun and Kisilowsky (1999). Total nitrogen of raw and fermented samples was extracted using a method of Starcher (2001). To measure the soluble and total nitrogen contents, the supernatant (0.05 mL) was mixed with 1.67 mL of Ninhydrin reagent in microfuge tube and boiled in a water bath at 100 °C for 10 min. The tubes were removed from water bath, cooled and the absorbance was measured at 575 nm. The blank contained water instead of sample. The standard curve was prepared by using albumin. Each sample was measured in triplicates. The nitrogen solubility index was calculated using the following formula (3):
| 3 |
Viable cell count of Bacillus subtilis
The fermented TVPs (1 g wet weight) were homogenized with 10 mL of sterilized distilled water and further dilutions were made. Appropriate dilutions (1 mL) were plated in triplicate in nutrient agar using the spread plate method. Inoculated plates were incubated at 35 °C for 24 h after which the colonies were counted and expressed as colony forming unit cfu/g wet weight of sample.
Statistical analysis
The data were analyzed for statistical significance using two-way analysis of variance (ANOVA) in IBM SPSS statistical software (version 23, SPSS, Chicago, IL, USA). Mean comparison was done by using Duncan’s multiple range tests at 5% level of significance.
Results and discussion
Texture profile analysis
The effect of fermentation on the texture profile (springiness, cohesiveness, chewiness, and hardness) of TVPs extruded at two different feed moisture contents (MC) is shown in Table 1. Springiness (p < 0.001), cohesiveness (p < 0.05), chewiness (p < 0.05), and hardness (p < 0.05) were significantly affected only by fermentation time. The effect of MC and the interaction effect between MC and fermentation time were not statistically significant.
Table 1.
Texture profile analysis of TVPs extruded at two different moisture contents and fermented by Bacillus subtilis for different times
| MC (%) during extrusion | Fermentation time (h) | Springiness (%) | Cohesiveness (%) | Chewiness (N) | Hardness (N) |
|---|---|---|---|---|---|
| 40 | Raw | 97.41 ± 4.15a | 88.85 ± 8.11a | 19.50 ± 0.69a | 21.99 ± 7.39a |
| 0 | 97.30 ± 2.58a | 89.79 ± 5.49a | 17.15 ± 0.36ab | 19.16 ± 4.23abc | |
| 12 | 93.09 ± 3.01bcd | 83.99 ± 4.57ab | 16.66 ± 0.60abc | 19.27 ± 6.67abc | |
| 24 | 94.15 ± 6.24abcd | 82.27 ± 8.56ab | 14.99 ± 0.46abc | 17.10 ± 4.96abcd | |
| 36 | 92.10 ± 3.62cd | 80.23 ± 6.83b | 14.50 ± 0.52abcd | 16.50 ± 5.40abcd | |
| 48 | 92.96 ± 3.27bcd | 82.35 ± 6.46ab | 12.64 ± 0.39bcd | 15.67 ± 5.54abcd | |
| 60 | 93.69 ± 3.54abcd | 84.87 ± 3.51ab | 9.90 ± 0.53d | 11.48 ± 5.84d | |
| 50 | Raw | 96.69 ± 3.04ab | 86.27 ± 9.36ab | 17.54 ± 0.65ab | 20.36 ± 7.28ab |
| 0 | 95.99 ± 3.26abc | 83.93 ± 5.81ab | 15.97 ± 0.34abc | 16.58 ± 6.04abcd | |
| 12 | 96.83 ± 3.69ab | 85.89 ± 6.54ab | 14.90 ± 0.29abc | 16.78 ± 4.58abcd | |
| 24 | 95.91 ± 2.45abc | 83.68 ± 6.39ab | 16.27 ± 0.16abc | 15.10 ± 3.27bcd | |
| 36 | 93.47 ± 2.93abcd | 80.84 ± 9.11b | 16.46 ± 0.58abc | 19.45 ± 7.54abc | |
| 48 | 90.69 ± 2.07d | 82.62 ± 4.22ab | 16.46 ± 0.51abc | 19.48 ± 5.95abc | |
| 60 | 90.36 ± 5.76d | 81.12 ± 7.59b | 11.86 ± 0.62cd | 13.15 ± 6.25cd |
Each value represents mean ± standard deviation of ten replications. Means followed by the same letter in the same column are not significantly different based on Duncan’s multiple range test at 5% level of significance
TVP: texturized vegetable protein; MC: moisture content; Raw: extruded, unautoclaved, and unfermented TVP; Fermentation time 0 h: extruded, autoclaved, and just before the start of fermentation
Springiness expresses the extent to which a sample returns to its original size after compression and removal of the load (Zhao and Zheng, 2009). In TVP extruded at 40% MC, the lowest springiness value was obtained at 36 h fermentation. However, it was statistically similar with that of 12, 24, 48, and 60 h fermented samples. On the other hand, springiness of TVP extruded at 50% MC was lowest at 60 h after fermentation and it was not statistically significant from that of 36 and 48 h fermented samples.
Cohesiveness describes the force required to excite the strength of the internal bonds which structure the body of the food product (Zhao and Zheng, 2009). Cohesiveness of fermented TVP (extruded at 40% MC) ranged from 80.23 to 89.79% and the lowest value was observed at 36 h after fermentation. It was statistically significant only from that of 0 h fermented sample (i.e. extruded and autoclaved TVP just before the start of fermentation). Likewise, cohesiveness of fermented TVP (extruded at 50% MC) ranged from 80.84 to 85.89% and the lowest was observed in 36 h fermented sample. However, it was also statistically similar that of all other fermentation time.
Hardness and chewiness values exhibited the decreasing trend with increase in fermentation time. In TVP extruded at 40% MC, The lowest hardness was observed in 60 h fermented sample (11.48 N) and it was significantly different from that of 0 and 12 h fermented samples. For TVP (extruded at 50% MC), the lowest value was found at 60 h after fermentation (13.15 N). With the decrease values in hardness, chewiness of fermented TVPs also decreased with gradual increase in fermentation time from 0 to 60 h.
Similar results were reported by several other studies that fermentation reduces the hardness of cooked soybeans (Hu et al., 2010; Shrestha et al., 2010; Wei et al., 2001). B. subtilis during Thua Nao fermentation secretes proteinases that play major role in proteolysis of soybean proteins (Vissessanguan et al., 2005). Hu et al. (2010) described that three extra cellular enzymes namely neutral proteases, alkaline protease, and esterase are mainly responsible for proteolytic activity of B. subtilis which alter the protein structure. This can explain the lower level of hardness and chewiness after fermentation of TVPs in this experiment.
Although the effect of MC during extrusion was not statistically significant for texture profile analysis, raw TVP extruded at 40% MC showed slightly higher springiness, cohesiveness, hardness, and chewiness than that of TVP extruded at 50% MC. On the other hand, other textural properties (integrity index and microstructures) were significantly affected by MC during extrusion (Table 2 and Fig. 2). It is possible that effect of extrusion MC is more contributed to structural integrity and microstructure modification than to texture profile properties. Likewise, interaction effect between feed MC during extrusion and fermentation time were not statistically significant. However, TVP extruded at 50% MC maintained numerically higher chewiness and hardness than those extruded at 40% MC at the end of the fermentation. This difference might be attributable to microstructure difference between TVPs extruded under two different MC as presented in Fig. 2.
Table 2.
Integrity index of TVPs extruded at two different moisture contents and fermented by Bacillus subtilis for different times
| MC (%) during extrusion | Fermentation time (h) | Integrity index (%) |
|---|---|---|
| 40 | Raw | 74.92 ± 3.24a |
| 0 | 52.37 ± 5.52de | |
| 12 | 54.25 ± 6.20de | |
| 24 | 59.90 ± 4.96bcd | |
| 36 | 39.37 ± 2.58f | |
| 48 | 36.01 ± 2.82f | |
| 60 | 34.43 ± 3.48f | |
| 50 | Raw | 79.83 ± 3.60a |
| 0 | 73.38 ± 4.23a | |
| 12 | 76.29 ± 3.43a | |
| 24 | 64.04 ± 7.20b | |
| 36 | 62.71 ± 1.93bc | |
| 48 | 55.87 ± 1.10cde | |
| 60 | 52.00 ± 4.50e |
Each value represents mean ± standard deviation of three replications. Means followed by the same letter in the same column are not significantly different based on Duncan’s multiple range test at 5% level of significance
TVP: texturized vegetable protein; MC: moisture content; Raw: extruded, unautoclaved, and unfermented TVP; Fermentation time 0 h: extruded, autoclaved, and just before the start of fermentation
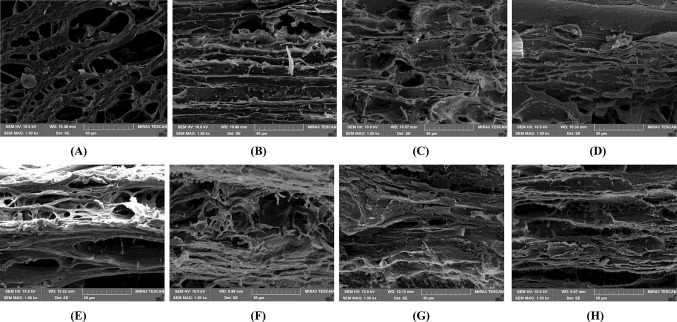
Fig. 2.
Scanning electron micrographs of texturized vegetable protein. A–D TVPs extruded at 40% moisture content and fermented with Bacillus subtilis for 0, 36, 48, and 60 h respectively; E–H TVPs extruded at 50% moisture content and fermented with Bacillus subtilis for 0, 36, 48, and 60 h respectively
Integrity index
The effect of fermentation on the integrity index of TVPs extruded at two different MC is summarized in Table 2. Integrity index was significantly affected by moisture content during extrusion and fermentation time (p < 0.001), with a significant interaction (p < 0.001) among the factors. The integrity index of TVP extruded at 50% MC showed higher integrity index (79.83%) than that of TVP extruded at 40% MC (74.92%). Also, TVP extruded at 50% MC maintained significantly higher integrity index (73.38%) than that of TVP extruded at 40% MC (52.37%) even after autoclaving just before the start of fermentation (i.e. 0 h fermentation time).
In TVP sample extruded at 40% MC, the integrity index did not change significantly up to 24 h after fermentation. In samples extruded at 50% MC, the decrease in integrity index started from 36 h. At the end of the fermentation, TVP extruded at 50% MC retained significantly higher integrity index (52.00%) when compared with that of TVP extruded at 40% MC (34.43%). This indicated that TVP extruded at higher MC (50%) had better texturization and more suitable for further processing conditions (fermentation in this study). Similar results were also reported by Gu and Ryu (2017).
After texturization, the protein molecules of vegetable protein (commonly soy protein) are rearranged into a layered and cross linked structure which can withstand the disruption due to further heating or other processing (Alam et al., 2016). The structural integrity and characteristic chewy texture of TVP can still remain after hydration, cooking, retorting, or other preparation ways for consumption (Asgar et al., 2010). These advantages of TVP might be the reasons for the stable and unchanged integrity index of fermented TVPs until 24 h (in TVP extruded at 40% MC) and 36 h after fermentation (in TVP extruded at 50% MC) in this study.
Color
Color values (lightness, redness, and yellowness) and total color change between raw (extruded, unautoclaved, and unfermented) and fermented TVPs are presented in Table 3. Extrusion MC as well as fermentation time significantly affected the lightness, redness, yellowness, and color difference (p < 0.001) with a highly significant interaction (p < 0.001) between the factors. As the MC was increased from 40 to 50% during extrusion, lightness (L) of raw TVP decreased, but redness (a) and yellowness (b) increased. In all samples, lightness (L value) and yellowness (b value) decreased with increased fermentation time. On the other hand, redness (a value) and total color change (ΔE) were found to be increased significantly as the fermentation time was increased from 0 (extruded, autoclaved, and just before the start of fermentation) to 60 h. Ryang et al. (2016) found out the similar results that the L value of doenjang sample (fermented soybean paste) decreased while the a value increased. Shrestha et al. (2010) also reported that the kinema (Bacillus fermented soybean) flours were considerably darker when compared with soyflours.
Table 3.
Color properties of TVPs extruded at two different moisture contents and fermented by Bacillus subtilis for different times
| MC (%) during extrusion | Fermentation time (h) | Lightness (L) | Redness (a) | Yellowness (b) | Total color change (ΔE)1 |
|---|---|---|---|---|---|
| 40 | Raw | 70.63 ± 0.17a | 3.09 ± 0.05i | 24.93 ± 0.32b | – |
| 0 | 68.63 ± 0.32b | 8.49 ± 0.23c | 24.22 ± 0.08bcd | 5.81 ± 0.13i | |
| 12 | 55.11 ± 0.32h | 11.12 ± 0.29a | 23.31 ± 0.56de | 17.56 ± 0.24c | |
| 24 | 56.15 ± 0.49g | 10.23 ± 0.38b | 21.90 ± 0.79f | 16.45 ± 0.41d | |
| 36 | 55.43 ± 0.38gh | 10.28 ± 0.36b | 21.76 ± 0.99f | 17.13 ± 0.35cd | |
| 48 | 53.08 ± 0.54i | 10.93 ± 0.42a | 21.33 ± 1.03f | 19.58 ± 0.49b | |
| 60 | 51.85 ± 0.68j | 11.28 ± 0.28a | 19.95 ± 0.26g | 21.09 ± 0.73a | |
| 50 | Raw | 67.83 ± 0.53b | 3.43 ± 0.14hi | 26.04 ± 0.29a | – |
| 0 | 65.99 ± 0.49c | 3.62 ± 0.07h | 26.02 ± 0.10a | 1.85 ± 0.49j | |
| 12 | 61.49 ± 0.57d | 6.90 ± 0.24f | 26.13 ± 0.60a | 7.25 ± 0.42h | |
| 24 | 61.30 ± 0.19d | 6.45 ± 0.07g | 23.21 ± 0.07e | 7.73 ± 0.16h | |
| 36 | 60.01 ± 0.65e | 7.60 ± 0.13de | 24.56 ± 0.34bc | 9.00 ± 0.49g | |
| 48 | 57.81 ± 0.19f | 7.35 ± 0.23e | 24.12 ± 0.44bcde | 10.94 ± 0.17f | |
| 60 | 53.62 ± 1.27i | 7.98 ± 0.04d | 23.92 ± 0.32cde | 15.07 ± 1.25e |
Each value represents mean ± standard deviation of three replications. Means followed by the same letter in the same column are not significantly different based on Duncan’s multiple range test at 5% level of significance
TVP: texturized vegetable protein; MC: moisture content; Raw: extruded, unautoclaved, and unfermented TVP; Fermentation time 0 h: extruded, autoclaved, and just before the start of fermentation
1The total color change (ΔE) of TVP samples during fermentation was calculated using the color values of raw TVP as a standard
Hydrolysates of protein and sugar hydrolyzed from carbohydrates are important sources for Millard Reaction (MR) which occurs between reducing sugars and amino compounds (Langer and Rzeski, 2014; Wang et al., 2013). Therefore, as described by Zhang et al. (2015), the degree of protein hydrolysis during fermentation might be associated with the significant color change of the fermented TVP products in our experiment. TVP extruded at lower MC (40%) exhibited significantly higher total color change (ΔE) and redness (a) than those of TVP extruded at higher MC (50%) during fermentation.
Microstructure modification of fermented TVPs
The microstructures of TVPs extruded under different MC and fermented were examined at different fermentation times (0, 36, 48, and 60 h) by scanning electron microscopy. Electron micrographs obtained are presented in Fig. 2 which shows how microstructure of TVPs changed during fermentation.
At 0 h fermentation time (i.e. before the start of fermentation), both of TVP extruded at 40% MC (Fig. 2A) and at 50% MC (Fig. 2E) exhibited layer and fibrous protein network structure with air cells. However, the difference in microstructures of two samples was also observed. TVP extruded at 50% MC had smaller air cells, and more compact and rigid fibrous layers when compared with that extruded at 40% MC. This may be attributed by the increased formation of disulfide cross-links, hydrogen, ionic, and hydrophobic bonds during extrusion under 50% MC. The results are in accordance with findings of Samard et al. (2019) who reported that TVP extruded from blend of SPI, WG and CS (50:40:10 w/w) extruded at low MC had more spongy and less fibrous structure.
During fermentation, air cells disappeared and the microstructure of all fermented TVPs was modified as the protein network was diminished gradually in both of sample extruded at 40% (Fig. 2B–D) and that extruded at 50% MC (Fig. 2F–H). Similar results can also be seen in the study of Zhao and Zheng (2009) in which the open spaces of mao-tofu gradually disappeared during fermentation with Mucor sp. This microstructure modification of fermented TVPs was seemed to be related with growth of B. subtilis (Fig. 3C) and its enzymatic activity during fermentation. After fermented for 60 h, TVP extruded at 50% MC retained more layer structure than that extruded at 40% MC, which might explain higher integrity index of sample extruded at 50% MC and fermented for 60 h when compared with that of sample extruded at 40% MC and fermented for 60 h as presented in Table 2.
Fig. 3.
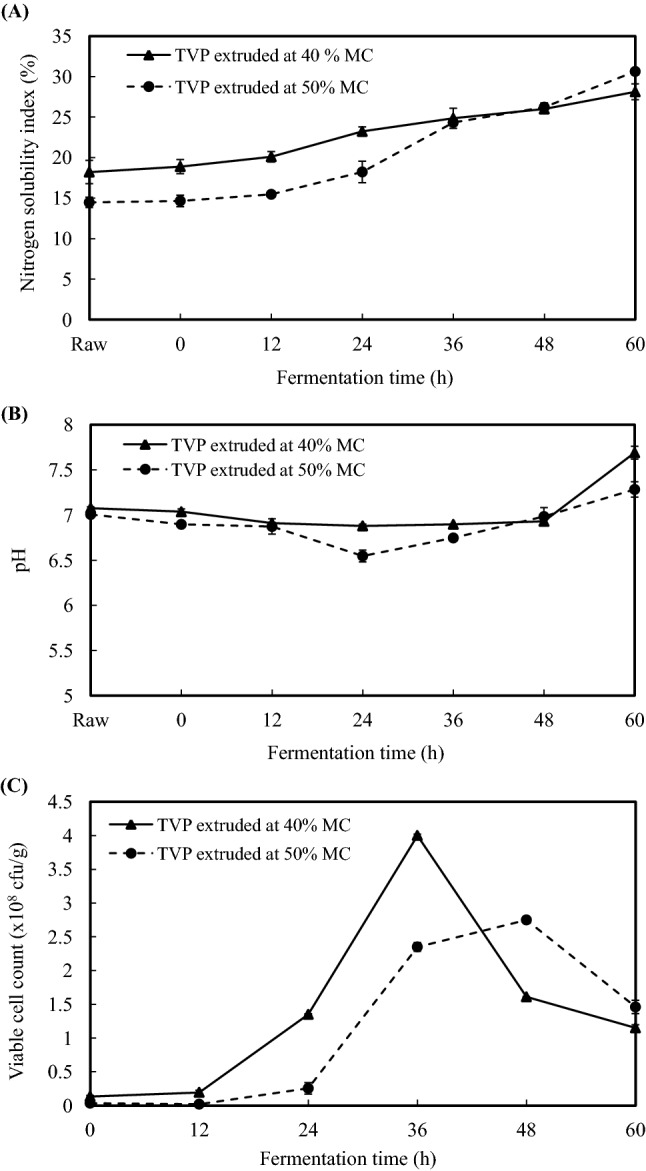
Changes in A nitrogen solubility index, B pH, and C viable cell count of fermented texturized vegetable proteins (extruded at two different moisture contents) in relation to fermentation time. Each value represents the mean ± standard deviation of three replications. TVP: texturized vegetable protein; MC: moisture content; Raw: extruded, unautoclaved, and unfermented TVP; Fermentation time 0 h: extruded, autoclaved, and just before the start of fermentation
Nitrogen solubility index
The degree of hydrolyzed and denatured proteins can be measured as nitrogen solubility index (NSI) which evaluates the solubility of proteins in water (Ojokoh and Yimin, 2011). Among the various functional properties of proteins, solubility is the most important one since many other functional performances of proteins vary according to their ability to dissolve into solution initially (Kim et al., 1990).
The changes in NSI of TVPs extruded at different feed MC and fermented for different times are shown in Fig. 3A. NSI was significantly affected by extrusion moisture content (p < 0.01) and fermentation time (p < 0.001), but the interaction effect among the factors was not significant statistically. NSI value of raw (i.e. extruded, unautoclaved, and unfermented samples) TVP extruded at 40% MC was higher than that of raw TVP extruded at 50% MC. This finding is in agreement with the findings of Gu and Ryu (2017) who reported that soy protein isolates extruded at higher MC had lower NSI due to better texturization after extrusion.
NSI of the all the fermented samples gradually increased along with increased fermentation time. In TVP sample extruded at 40% MC, significant increase in NSI was observed from 12 h after fermentation (20.11%) and the increased continued up to 28.12% after 60 h fermentation. In TVP sample extruded at 50% MC, NSI started to significantly increase from 24 h after fermentation and continuously increased up to 30.64% at 60 h after fermentation.
The findings of this study were in agreement with several other studies which also reported that NSI could increase after fermentation (Amadou et al., 2010; Kim et al., 2006; Shrestha et al., 2010). Ojokoh and Yimin (2011) reported the similar trend that fermentation of extruded soy samples increased the NSI when compared with extruded unfermented samples. Hydrolysis of proteins into smaller peptides and finally ammonia is responsible for the increase amount of nitrogen soluble in water and also the flavor of fermented products (Shrestha et al., 2010).
pH and viable cell count
Figure 3B illustrates the pH values of the raw (extruded, unautoclaved, and unfermented) and fermented TVPs extruded at different moisture contents. The pH values were significantly affected by fermentation time (p < 0.001), however, effects of MC and interaction among factors were not statistically significant. During fermentation, the initial pH of raw TVP (extruded at 40% MC) was 7.08 and it decreased significantly up to 6.88 at 24 h after fermentation. Then, the pH increased gradually from 36 h after fermentation and reached the maximum of 7.69 after 60 h after fermentation. The same trend was observed in TVP (extruded at 50% MC) in which the initial pH was 7.01 and then decreased up to 6.55 at 24 h after fermentation. After that, the pH increased again and reached the maximum of 7.28 at 60 h after fermentation. The initial decrease in pH value in this study has been observed also in the traditional Bacillus fermentation of soybeans by Sarkar et al. (1993). It was assumed that sugars instead of proteins were utilized as growth substrates in the beginning and acids released due to hydrolysis of carbohydrates by Bacillus enzymes which lead to decrease in pH. At the later phase of fermentation, proteolysis occurred and the use of amino acids as carbon and energy sources enhanced the release of ammonia and subsequent increase of pH (Sarkar et al., 1993; Sarkar and Tamang, 1995).
Figure 3C shows the changes in viable cell count of B. subtilis in the fermented TVPs extruded at different feed MC throughout the fermentation period. Extrusion MC (p < 0.01) as well as fermentation time (p < 0.001) significantly affected the viable cell no. of B. subtilis, with a highly significant interaction (p < 0.001) among the factors. In fermented TVP (extruded at 40% MC), the viable cell count of B. subtilis increased from an initial 1.4 × 107 to 4.2 × 108 cfu/g at 36 h after fermentation. Then the bacterial no. started to decline after 48 h fermentation. The same trend of bacterial cell count was observed in another fermented TVP (extruded at 50% MC). The initial viable cell no. of 0.4 × 107 cfu/g increased to 2.8 × 108 cfu/g at 48 h after fermentation and then the bacterial cell no. started to decrease.
The similar pattern in viable cell count of Bacillus was also observed in other studies (Omafuvbe, 2006; Sarkar et al., 1993; Vijayalakshmi et al., 2013). Although B. subtilis growth showed similar trend in both TVP during fermentation, the viable cell count during exponential phase was significantly higher in fermented TVP extruded at 40% MC than that of fermented TVP extruded at 50% MC. This might be related with higher rate of proteolysis which leads to lower hardness, chewiness, integrity index and higher NSI in at the end of fermentation in TVP extruded at 40% MC.
In conclusion, B. subtilis fermentation could impair the textural properties of TVP. However, TVP extruded at higher MC (50%) maintained higher chewiness, hardness, and structural integrity than that extruded at lower MC (40%) at the end of fermentation. Therefore, 50% feed MC during extrusion is more suitable for producing B. subtilis fermented TVP with desirable textural properties. This finding is also supported by SEM results. In addition, the trend of pH increase and bacterial growth were coincided with the increase in NSI and total color change indicating the good proteolysis activity during fermentation of TVP. Considering all the findings of this study, it can be concluded that there is a potential to develop a new type of fermented product with good textural properties especially chewiness, hardness, structural integrity, and layered structure by combining extrusion and fermentation technologies. Further studies should be conducted to analyze sensory properties regarding the consumers’ acceptance of fermented TVP.
Acknowledgements
This work was supported by the research grant of the Kongju National University in 2019.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
The Thiri Maung, Email: thiriyau08@gmail.com.
Bon Yeob Gu, Email: gby9rn@naver.com.
Mi Hwan Kim, Email: mhkim@kongju.ac.kr.
Gi Hyung Ryu, Email: ghryu@kongju.ac.kr.
References
- Akdogan H. Pressure, torque and energy responses of a twin screw extruder at high moisture contents. Food Res. Int. 1996;29:423–429. doi: 10.1016/S0963-9969(96)00036-1. [DOI] [Google Scholar]
- Alam MS, Kaur J, Khaira H, Gupta K. Extrusion and extruded products: changes in quality attributes as affected by extrusion process parameters: a review. Crit. Rev. Food Sci. Nutr. 2016;56:445–473. doi: 10.1080/10408398.2013.779568. [DOI] [PubMed] [Google Scholar]
- Amadou I, Amza T, Foh MBK, Kamara MT, Le GW. Influence of Lactobacillus plantarum Lp6 fermentation on the functional properties of soybean protein meal. Emir. J. Food Agric. 2010;22:456–465. doi: 10.9755/ejfa.v22i6.4663. [DOI] [Google Scholar]
- AOAC. Official Method of Analysis of AOAC International 15th ed. Association of Official Analytical Chemists, Washington, DC, USA (1990)
- Asgar MA, Fazilah A, Huda N, Bhat R, Kaim AA. Nonmeat protein alternatives as meat extenders and meat analogs. Compr. Rev. Food Sci. Food Saf. 2010;9:513–529. doi: 10.1111/j.1541-4337.2010.00124.x. [DOI] [PubMed] [Google Scholar]
- Daun J and Kisilowsky M. Nitrogen solubility index (NSI) of canola seed and meal produced at Canadian and Japanese crushing plants. Canadian Grain Commission, Winnipeg, Canada (1999). Available from: http://www.regional.org.au/au/gcirc/5/222.htm. Accessed Jun 04, 2018.
- Esteves EA, Martino HSD, Olivieira FCE, Bressan J, Costa NMB. Chemical composition of soybeans cultivar lacking lipoxygenases. Food Chem. 2010;122:238–242. doi: 10.1016/j.foodchem.2010.02.069. [DOI] [Google Scholar]
- Ginting E, Arcot J. High-performance liquid chromatographic determination of naturally occurring folates during tempeh preparation. J. Agric. Food Chem. 2004;52:7752–7758. doi: 10.1021/jf040198x. [DOI] [PubMed] [Google Scholar]
- Gu BY, Ryu GH. Effects of moisture content and screw speed on physical properties of extruded soy protein isolate. J. Korean Soc. Food Sci. Nutr. 2017;46:751–758. [Google Scholar]
- Hu Y, Ge C, Yuan W, Zhu R, Zhang W, Du L, Xue J. Characterization of fermented black soybean natto inoculated with Bacillus natto during fermentation. J. Sci. Food Agric. 2010;90:1194–1202. doi: 10.1002/jsfa.3947. [DOI] [PubMed] [Google Scholar]
- Kiers JL, Van laeken AEA, Rombouts FM, Nout MJR. In vitro digestibility of Bacillus fermented soya bean. Int. J. Food Microbiol. 2000;60:163–169. doi: 10.1016/S0168-1605(00)00308-1. [DOI] [PubMed] [Google Scholar]
- Kim JE, Hwang K, Lee SP. ACE inhibitory and hydrolytic enzyme activities in textured vegetable protein in relation to the solid state fermentation period using Bacillus subtilis HA. Food Sci. Biotechnol. 2010;19:487–495. doi: 10.1007/s10068-010-0068-0. [DOI] [Google Scholar]
- Kim JE, Lee SP. Evaluation of radical scavenging activity and physical properties of textured vegetable protein fermented by solid culture with Bacillus subtilis HA according to fermentation time. J. Korean Soc. Food Sci. Nutr. 2010;39:872–879. doi: 10.3746/jkfn.2010.39.6.872. [DOI] [Google Scholar]
- Kim KM, Kim HR, Park HJ. The quality characteristics of chunggugjang prepared by Bacillus subtilis NRLSI IV on the different inoculum levels and fermentation times. Korean J. Community Living Sci. 2006;17:123–131. [Google Scholar]
- Kim SY, Park PSW, Rhee KC. Functional properties of proteolytic enzyme modified soy protein isolate. J. Agric. Food Chem. 1990;38:851–856. [Google Scholar]
- Langer E, Rzeski W. Biological properties of melanoidins: a review. Int. J. Food Prop. 2014;17:344–353. doi: 10.1080/10942912.2011.631253. [DOI] [Google Scholar]
- Ma X, Gu BY, Ryu GH. Optimization of extrusion variables for improving the qualities of textured vegetable protein with green tea using response surface methodology. Food Eng. Prog. 2018;22(1):1–8. doi: 10.13050/foodengprog.2018.22.1.1. [DOI] [Google Scholar]
- Ojokoh AO, Yimin W. Effect of fermentation on chemical composition and nutritional quality of extruded and fermented soya products. Int. J. Food Eng. 2011;7:1–16. doi: 10.2202/1556-3758.1857. [DOI] [Google Scholar]
- Omafuvbe BO, Abiose SHA, Shonukan OO. Fermentation of soybean (Glycine max) for soy-daddawa production by starter cultures of Bacillus. Food Microbiol. 2002;19:561–566. doi: 10.1006/fmic.2002.0513. [DOI] [Google Scholar]
- Omafuvbe BO, Esosuakpo EO, Oladejo TS, Toye AA. Effect of soaking and roasting dehulling methods of soybean on Bacillus fermentation of soy-daddawa. Am. J. Food Technol. 2007;2:257–264. doi: 10.3923/ajft.2007.257.264. [DOI] [Google Scholar]
- Omafuvbe BO. Effect of salt on the fermentation of soybean (Glycine max) into daddawa using Bacillus subtilis as starter culture. Afr. J. Biotechnol. 2006;5:1001–1005. [Google Scholar]
- Park JH, Chatpaisarn A, Ryu GH. Effects of gluten and moisture contents on texturization of extruded soy protein isolate. J. Korean Soc. Food Sci. Nutr. 2017;46:473–480. doi: 10.3746/jkfn.2017.46.4.473. [DOI] [Google Scholar]
- Park MJ, General T, Lee SP. Physicochemical properties of roasted soybean flour bioconverted by solid-state fermentation using Bacillus subtilis and Lactobacillus plantarum. Prev. Nutr. Food Sci. 2012;17:36–45. doi: 10.3746/pnf.2012.17.1.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryang JH, Kim NH, Lee BS, Kim CT, Rhee MS. Destruction of Bacillus cereus spores in a thick soy bean paste (doenjang) by continuous ohmic heating with five sequential electrodes. Lett. Appl. Microbiol. 2016;63:66–73. doi: 10.1111/lam.12588. [DOI] [PubMed] [Google Scholar]
- Samard S, Gu BY, Ryu GH. Effects of extrusion types, screw speed and addition of wheat gluten on physicochemical characteristics and cooking stability of meat analogues. J. Sci. Food Agric. 2019;99:4922–4931. doi: 10.1002/jsfa.9722. [DOI] [PubMed] [Google Scholar]
- Samard S, Ryu GH. A comparison of physicochemical characteristics, texture, and structure of meat analogue and meats. J. Sci. Food Agric. 2019;99:2708–2715. doi: 10.1002/jsfa.9438. [DOI] [PubMed] [Google Scholar]
- Sarkar PK, Cook PE, Owens JD. Bacillus fermentation of soybeans. World J. Microbiol. Biotechnol. 1993;9:295–299. doi: 10.1007/BF00383066. [DOI] [PubMed] [Google Scholar]
- Sarker PK, Tamang JP. Changes in the microbial profile and proximate composition during natural and controlled fermentations of soybeans to produce Kinema. Food Microbiol. 1995;12:317–325. doi: 10.1016/S0740-0020(95)80112-X. [DOI] [Google Scholar]
- Shrestha AK, Dhal NR, Ndungutse V. Bacillus fermentation of soybean: a review. J. Food Sci. Technol. Nepal. 2010;6:1–9. doi: 10.3126/jfstn.v6i0.8252. [DOI] [Google Scholar]
- Singh P, Kumar R, Sabapathy SN, Bawa AS. Functional and edible uses of soy protein products. Compr. Rev. Food Sci. Food Saf. 2008;7:14–28. doi: 10.1111/j.1541-4337.2007.00025.x. [DOI] [Google Scholar]
- Starcher B. A ninhydrin-based assay to quantitate the total protein content of tissue samples. Anal. Biochem. 2001;292:125–129. doi: 10.1006/abio.2001.5050. [DOI] [PubMed] [Google Scholar]
- Tamang JP, Nikkuni S. Selection of starter cultures for the production of kinema, a fermented soybean food the Himalaya. World J. Microbiol. Biotechnol. 1996;12:629–635. doi: 10.1007/BF00327727. [DOI] [PubMed] [Google Scholar]
- Vijayalakshmi S, Ranjitha J, Devi Rajeswari V. Enzyme production ability by Bacillus subtilis and Bacillus licheniformis—a comparative study. Asian J. Pharm. Clin. Res. 2013;6:235–238. [Google Scholar]
- Vissessanguan W, Benjakul S, Potachareon W, Panya A, Riebroy S. Accelerated proteolysis of soy proteins during fermentation of Thua-Nao inoculated with Bacillus subtilis. J. Food Biochem. 2005;29:349–366. doi: 10.1111/j.1745-4514.2005.00012.x. [DOI] [Google Scholar]
- Wang H, Li YY, Cheng YQ, Yin LJ, Li LT. Effect of the maillard reaction on angiotensin I-converting enzyme (ACE)-inhibitory activity of douchi during fermentation. Food Bioprocess Tech. 2013;6:297–301. doi: 10.1007/s11947-011-0596-5. [DOI] [Google Scholar]
- Wei Q, Wolf-Hall C, Chang KC. Natto characteristics as affected by steaming time, Bacillus strain, and fermentation time. J. Food Sci. 2001;66:167–173. doi: 10.1111/j.1365-2621.2001.tb15601.x. [DOI] [Google Scholar]
- Zhang Y, Ma L, Wang X. Correlation between protein hydrolysates and color during fermentation of mucor-type douche. Int. J. Food Prop. 2015;18:2800–2812. doi: 10.1080/10942912.2015.1013632. [DOI] [Google Scholar]
- Zhao X, Zheng X. A primary study on texture modification and proteolysis of mao-tofu during fermentation. Afr. J. Biotechnol. 2009;8:2294–2300. [Google Scholar]