Abstract
Gamma radiation changes the molecular structure and activity of proteins, which in turn changes their physiological effects. Sericin, one of the silk peptides, has beneficial effects to humans such as inducing apoptosis, acting as an anti-oxidant. The effects of gamma irradiation on the physiological activity of fibroin have been studied, but its effects on sericin alone have not yet been established. In this study, we assessed the effects of gamma irradiation on sericin (I-sericin) in regard to its inflammatory effects in vitro and in vivo. Our results showed that I-sericin (5 kGy) significantly increased nitric oxide production, proliferation of immune cells, and effectively attenuated lipopolysaccharide (LPS)-induced inflammation. The mice were fed I-sericin for 4 weeks and treated with LPS; they exhibited significantly increased proliferation of lymphocytes, activation of NK cells and decreased secretion of inflammatory cytokines These results suggest gamma-irradiated I-sericin as a valuable functional food supplement by immune-enhancing and anti-inflammation effects.
Keywords: Sericin, Gamma irradiation, Immune enhancement, Anti-inflammation
Introduction
Food irradiation kills microbes and insects and is a highly effective conservation technique that reduces dependence on chemical fumigants and preservatives. It reduces food losses due to microbial spoilage and insect damage (Roberts, 2014; Verde et al., 2013). Specifically, irradiation with gamma rays is known to be safe and effective in food storage by reducing microorganisms and viruses and preventing addition of biological hazards and toxic substances such as N-nitrosamine and biologically active amines (Lafortune et al., 2005). In addition, gamma irradiation has been reported to change physiological properties of proteins by altering their structure (Valdes-Diaz et al., 2007). Gamma irradiation results in changes in physiological properties through fragmentation, cross-linking, aggregation, and oxidation by oxygen radicals (Davies, 1987; Dogbevi et al., 1999). Therefore, there are efforts to exploit these irradiation-induced changes in physiological properties of organic compounds to develop various therapeutic agents (Brandstetter et al., 2009; Lee et al., 2012; Pereira et al., 2017).
The immune system is a biological defense from various external factors such as pathogenic bacteria, toxins, and viruses. The immune system is mainly divided into innate immunity and adaptive immunity. The innate immunity consists of inflammatory cells such as neutrophils, mast cells, and macrophages. Unlike adaptive immunity, it is a nonspecific immune response to antigens (Medzhitov, 2007). Macrophages play a key role in immune function as phagocytic cells, which digest and eliminate external factors in the immune response. Macrophages are activated by external factors and produce an inflammatory immune response, including secretion of inflammatory cytokines such as tumor necrosis factor-alpha (TNF-α), interleukin (IL)-2, and IL-10 (Minami et al., 2008). Excessive cytokine secretion causes tissue damage, increases risk of cancer, and can serve as a biomarker of control of the inflammatory response (Lin and Karin, 2007).
Silk peptide refers to amino acids and a peptide-linked component of amino acids and comprises silk. It is commonly known as a continuous protein fiber produced by lepidopteran larvae such as silkworms, spiders, and mites (Altman et al., 2003). Generally, a silk fiber consists of two types of protein: fibroin (75%) and sericin (25%). Sericin has anti-oxidant properties, can induce apoptosis (Dash et al., 2008), and can improve diabetes (Ryu, 2014). However, most studies on silk proteins are focused on fibroin, the main protein. Specifically, research has described the effect of gamma radiation on silk fibroin structure (Kojthung et al., 2008) but not yet sericin.
Thus, we investigated the changes in the physiological properties of sericin in response to gamma radiation, specifically its ability to activate the immune response. These data give insight into whether gamma-irradiated sericin could be useful as a functional food supplement.
Materials and methods
Sericin irradiation and solubility measurement
Sericin was purchased from Worldway Co., LTD. (Sejong, Korea). Sericin was separated and freeze-dried to obtain a sericin powder and was dissolved to a concentration of 2.5, 5, 7.5, 10, or 20 mg/ml in 0.01 M phosphate buffer. Sericin solution was irradiated at 0, 2.5, 5.0, 7.5, or 10.0 kGy in a cobalt-60 irradiator (IR-79, Nordion International Ltd., Ontario, Canada). At this time, the source strength was approximately 1.2 MCi, and the dose rate was 10 kGy per hr. The absorbed dose was assessed using a 5-mm-diameter alanine dosimeter (Bruker Instruments, Rheinstetten, Germany). The dosimetry system was used after standardization in accordance with the International Atomic Energy Agency (IAEA) standard. The irradiated sericin solution was lyophilized and stored at 4 °C.
Gamma-irradiated sericin powders were dissolved in distilled water at a concentration of 1.0 mg/mL. After filtration using a 0.20-μL filtration filter, the solubility of sericin was determined using a BCA assay. The amount of protein was calculated by substituting the absorbance of the samples into the protein quantification curve obtained using bovine serum albumin as a standard protein. The sericin not treated with gamma rays was dissolved in distilled water and prepared as a control. The controls were used to prepare a calibration curve.
Cell culture
RAW 264.7 cells (ATCC, Rockville, MD, USA) were grown in Dulbecco’s modified Eagle medium (DMEM, containing 10% FBS and 1% antibiotics) in a 37 °C humidified atmosphere with 5% CO2. The media was changed every 2–3 days. Cells were seeded in 100-mm cell culture dishes (Nunc, Rochester, NY, USA) 24 h before treatment. In the in vitro assays, spleen cells were isolated from 6-week-old mice.
NO production, immune cells proliferation, and viability assay
RAW 264.7 and spleen cells were plated at a density of 2 × 104 cells/well in 48-well plates (Nunc). After 24 h incubation, cells were treated with various concentrations of I-sericin (10, 50, 100 μg/ml) or sericin (100 μg/ml) for 24 h. Cell proliferation and viability were determined using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) (Sigma, Sydney, Australia) assay. The index of NO production was assessed using a commercial NO assay kit (Griess reagent, Promega, Madison, WI, USA). Absorbance was determined using a multiplate reader spectrophotometer (PowerWave2, BioTek Instruments, Winooski, VT, USA) at 540 nm.
Animal and drug administration
Six-week-old male BALB/c mice (n = 10) were purchased from Damul Science (Daejeon, Korea) with weight ranging from 20 to 22 g. All mice were cared for in accordance with institutional ethical guidelines for the care and use of experimental animals at Chonbuk National University (CBNU 2018-066). All mice were housed on a 12-h light/dark cycle and provided a standard laboratory pellet diet and water ad libitum at a constant temperature (25 ± 2 °C) and humidity (approximately 60%). At the beginning of the experiment, the mice were acclimatized for 1 week. Subsequently, mice were randomly assigned to one of the following six groups: normal (non-treated mice), LPS only (LPS 20 mg/kg, i.p., once), I-sericin groups (LPS + each I-sericin 100, 200, and 400 mg/kg), and sericin (LPS + 400 mg/kg). The body weight and food intakes were determined every day during the experimental period. After 4 weeks of I-sericin supplementation, macrophages were isolated from mice (Seo et al., 2014), and blood samples were collected from the abdominal artery of anesthetized mice. Serum was immediately separated from the blood sample by centrifuge. Subsequently, the spleen of each mouse was perfused, collected, placed in liquid nitrogen, and stored at − 72 °C until analyses.
Werstern blot
After the cells were treated in the same manner as the method for measuring cell viability, total protein from RAW 264.7 cell lysates were subjected to sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis using 8-12% gels. Protein bands were then transferred to PVDF membranes (BioRad, Hercules, CA, USA), which were blocked with 5% skim milk in PBS then incubated with a 1:1000 or 1:500 v/v dilution of primary antibodies against TNF-α, IL-2, IL-10 (Abcam, Cambridge, MA, UK) and β-actin (Cell Signaling, Danvers, MA, USA) in PBS with 1% skim milk overnight at 4 °C. The blots were then incubated with peroxidase-conjugated goat anti-rabbit IgG and anti-mouse IgG (1:10,000 v/v, Millipore, CA, USA) for 1 h. The immunoreactions were visualized with SuperSignal West Dura Extended Duration Substrate (Thermo Scientific, CA, USA) on a ChemiImager analyzer system (Alpha Innotech, San Leandro, CA, USA).
Spleen cell proliferation
The proliferation of spleen cells was determined using MTT assay using spleen cells isolated from mice fed I-sericin (100, 200, 400 mg/kg) or sericin (400 mg/kg) for 4 weeks (Ryu et al., 2006). Spleen cells were plated at 2 × 104 cells/well on 48-well plates (Nunc). The cells were treated with 5 μg/ml of concanavalin A (ConA) or 15 ng/mL of lipopolysaccharide (LPS). After 24 h of incubation, cell proliferation was measured. Absorbance was determined using a multiplate reader spectrophotometer (PowerWave2, BioTek Instruments, Winooski, VT, USA) at 540 nm.
Immune cytokine analysis
The concentrations of TNF-α (Abcam, Cambridge, MA, UK), interferon gamma (IFN-γ), IL-2 and L-10, (Thermo Scientific, MA, USA) in serum and macrophages were determined with ELISA kits. Absorbance was measured at 450 nm using a microplate reader.
NK cell cytotoxicity analysis
NK cell cytotoxicity was determined using cytotoxicity detection kit of lactate dehydrogenase (LDH) (Takara Bio, Tokyo, Japan) (Kang et al., 2015). Spleen cells isolated from normal mice were plated at 2 × 104 cells/well on a 48-well plate (Nunc) with YAC cells at a 200:1 ratio. After a 24-h incubation, cells were treated with I-sericin (10, 50, 100 μg/ml) or sericin (100 μg/ml) for 24 h, and the LDH assay was performed. NK cell cytotoxicity was assessed by isolating the spleen cells of mice fed I-sericin (100, 200 or 400 mg/kg) or sericin (400 mg/kg) for 4 weeks. The experiment proceeded in the same way as the in vitro experiment.
Statistical analysis
Results are expressed as mean ± standard error (SE). Data were analyzed using Student’s t test for two groups. p values less than 0.05 were considered statistically significant (Andrikopoulos et al., 2008).
Results and discussion
I-sericin regulates NO production
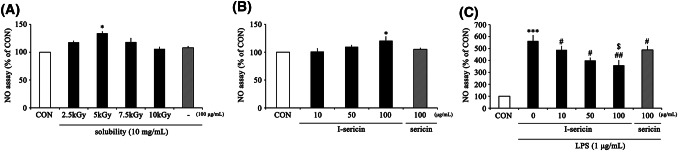
Macrophages detect pathogens, apoptotic cells, and antigen-presenting cells (Birk et al., 2001) and are activated by intrusion of nonspecific antigens (Chou et al., 2013). Activated macrophages secrete NO to inhibit the antigens that cause harmful infections (Ortiz-Andrellucchi et al., 2008). However, excessive NO production by activated macrophages can generate inflammatory damage by DNA mutations (Mu et al., 2001). Thus, regulation of NO production plays a key role in the body’s immune system. We first established the effects of NO product activity in RAW 264.7 cells after treatment with various doses of gamma-irradiation and concentrations of I-sericin. NO production was significantly increased after gamma irradiation with 5 kGy (Fig. 1A). The solubility of sericin significantly decreased with increasing irradiation dose. Also, the solubility of sericin at 5 kGy was highest at 10 mg/mL: 91.1% (Table 1). Based on these data, we used 5 kGy gamma-irradiated sericin in all subsequent experiments. NO production was significantly increased after treatment with 100 μg/ml I-sericin (Fig. 1B). NO production in cells treated with LPS alone was significantly increased, whereas co-treatment with I-sericin produced a significant decrease in a dose-dependent manner (Fig. 1C). Compared to using 100 μg/ml sericin, cells treated with I-sericin showed lower NO production. These results suggest that I-sericin can activate immunity against the initial infections. In addition, I-sericin has been shown to significantly attenuate NO production by external toxins.
Fig. 1.
Effect of I-sericin on NO production in RAW 264.7 cells. (A) NO production of RAW 264.7 cells after treatment with sericin irradiated with increasing doses of gamma rays (2.5–10 kGy). (B) NO production of RAW 264.7 cells after treatment with increasing concentrations of I-sericin (10, 50, 100 μg/ml) or sericin (100 μg/ml) for 24 h. (C) NO production of RAW 264.7 cells after treatment with 1 μg/ml LPS for 24 h after pretreatment with I-sericin (10, 50, 100 μg/ml) or sericin (100 μg/ml) for 1 h. I-sericin; gamma-irradiated sericin. Data are expressed as mean ± SE (n = 3). *p < 0.05; ***p < 0.001, compared with CON. #p < 0.05; ##p < 0.01 compared with LPS only. $p < 0.05, compared with sericin 100
Table 1.
Change of solubilities of sericin solutions gamma-irradiated under the different concentration
| Concentration (mg/ml) | Irradiation dose (kGy) | ||||
|---|---|---|---|---|---|
| 0 | 2.5 | 5 | 7.5 | 10 | |
| 2.5 | 100 | 93.8* | 90.5* | 88.4* | 87.2* |
| 5 | 100 | 94.2* | 90.2* | 88.4* | 86.9* |
| 7.5 | 100 | 93.7* | 90.5* | 89.2* | 87.2* |
| 10 | 99.8 | 93.2* | 91.1* | 88.4* | 87* |
| 20 | 99.2 | 92.5* | 86.7* | 88.6* | 76.8** |
*p < 0.05; **p < 0.01, compared with non-irradiation (n = 20)
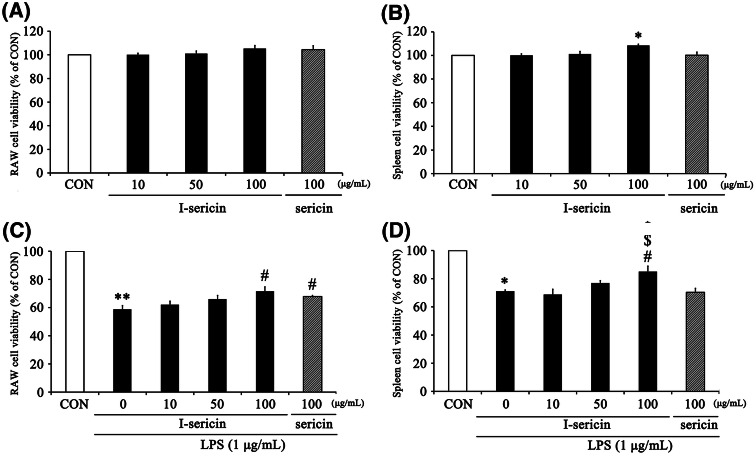
I-sericin increased cell proliferation in immune cells
The spleen is the most important lymphatic organ in our body, accounting for approximately 25% of the total lymphatic organ weight. The spleen has an immune response to blood-derived antigens and acts to remove aged and damaged cells. It also forms secondary lymphoid organs containing T lymphocytes and B lymphocytes. Therefore, proliferation of spleen cells is highly influential in the immune system (Zalys et al., 2000). In the present study, we assessed the effects of I-sericin and sericin on the proliferation of RAW 264.7 cells and spleen cells.
To assess the cell proliferation induced by both I-sericin and sericin in RAW 264.7 and spleen cells, cells were treated with I-sericin or sericin for 24 h. Cell proliferation by I-sericin at 100 μg/ml was showed increasing tendency (Fig. 2A), but no significance difference was observed. I-sericin at 100 μg/ml significantly increased cell proliferation in spleen cells (Fig. 2B). RAW 264.7 and spleen cells were pretreated with I-sericin and sericin for 1 h prior to incubation with 1 μg/ml LPS for 24 h. The cell viability of I-sericin-treated cells was significantly increased compared with that of LPS alone (Fig. 2C, D).
Fig. 2.
Effects of I-sericin on cell proliferation and viability in RAW 264.7 and ex vivo murine spleen cells. Proliferation of RAW 264.7 (A) and spleen (B) cells treated with I-sericin (10, 50, 100 μg/ml) or sericin (100 μg/ml) for 24 h. Viability of RAW 264.7 (C) and spleen cells (D) after treatment with 1 μg/ml LPS for 24 h after pretreatment with I-sericin (10, 50, 100 μg/ml) or sericin (100 μg/ml) for 1 h. I-sericin; gamma-irradiated sericin. Data are expressed as mean ± SE (n = 3). *p < 0.05; **p < 0.01, compared with CON. #p < 0.05, compared with LPS only. $p < 0.05, compared with sericin 100
In the in vivo experiment, we isolated the spleens from mice fed I-sericin and sericin and assessed cell proliferation. There was no significant difference in body weight or food intake between the groups (Not suggested). I-sericin significantly increased amount of spleen cells compared to the controls in a dose-dependent manner. Specifically, compared to using the same concentration (400 mg/kg) of sericin, cells treated with I-sericin showed a significantly higher cell proliferation (Fig. 3). This result shows that I-sericin helps to increase immunity by inducing the proliferation of macrophages and spleen cells.
Fig. 3.
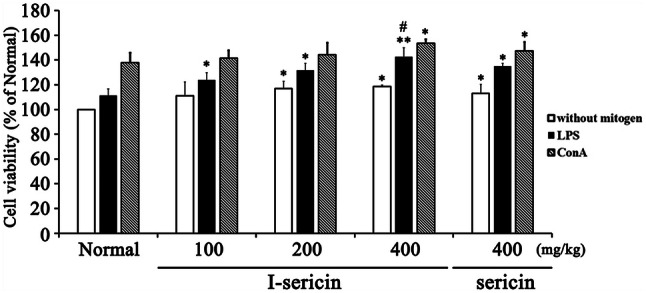
Cell proliferation of spleen cells treated with I-sericin and mitogen. Spleen cells isolated from mice fed I-sericin in the absence or presence of the LPS or ConA for 24 h. I-sericin; gamma-irradiated sericin. Data are expressed as mean ± SE (n = 10). *p < 0.05; **p < 0.01, compared with normal. #p < 0.05, compared with sericin at 400 mg/kg
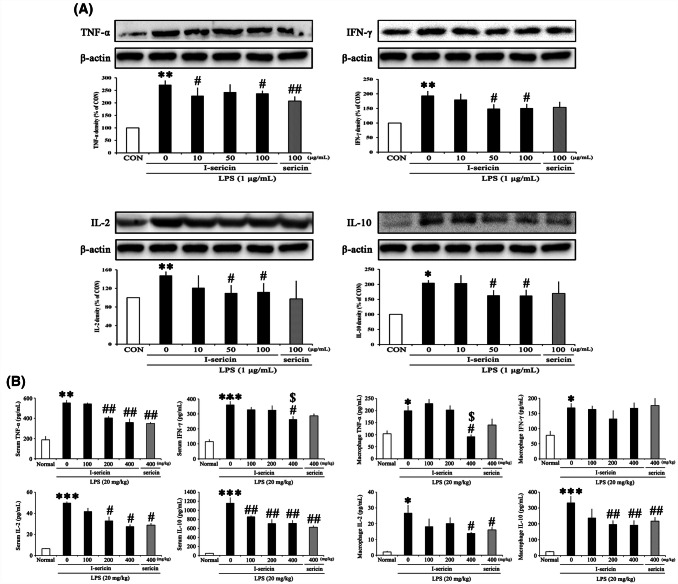
I-sericin decreased pro-inflammatory protein expression and cytokine level
Activated macrophages secrete pro-inflammatory cytokines such as TNF-α, IFN-γ, IL-2, and IL-10. These inflammatory cytokines promote the mass production of NO by further activating surrounding activated macrophages and inducing iNOS expression (Han et al., 2016; MacMicking et al., 1997). Also, constant cytokine overexpression is associated with chronic diseases such as atherosclerosis, cancer, and Alzheimer’s disease (Moller and Villiger, 2006; Ungefroren et al., 2011).
TNF-α secreted from macrophages plays a central role by activating eosinophils and promoting the production of granular leukocytes (Andrews et al., 1990). IFN-γ and IL-2, pro-inflammatory cytokines, are secreted by Th1 helper cells (Th1). IFN-γ increases activated NK cells by stimulating the innate immune response to monocytes and dendritic cells (Vivier et al., 2008). IL-2 regulates the proliferation of NK cells and macrophages by acting directly on recurrent NK cells (Wang et al., 2000). IL-10, a pro-inflammatory cytokine, is secreted by Th2 helper cells (Th2). IL-10 is secreted as an inhibitor when an inflammatory reaction occurs and regulates the proliferation and differentiation of T cells, B cells, natural killer cells, antigen-presenting cells, and other immune cells (Taylor et al., 2006).
To evaluate the protein expression of I-sericin and sericin in RAW 264.7 were pretreated with I-sericin and sericin for 1 h prior to incubation with 1 μg/ml LPS for 24 h. Protein expressions in LPS alone were significantly increased, whereas pretreatment with I-sericin significantly decreased the protein expression in a dose-dependent manner. Specifically, compared to using the same concentration of non-irradiated sericin (100 μg/ml), cells treated with I-sericin showed a lower level of cytokines (Fig. 4A).
Fig. 4.
Effect of I-sericin on inflammatory protein expression and cytokines secretion. (A) Expression of inflammatory protein in RAW 264.7. I-sericin; gamma-irradiated sericin. Data are expressed as mean ± SE (n = 3). *p < 0.05; **p < 0.01, compared with CON. #p < 0.05; ##p < 0.01 compared with LPS only. (B) Secretion of inflammatory cytokines in serum and macrophages. I-sericin; gamma-irradiated sericin. Data are expressed as mean ± SE (n = 10). *p < 0.05; **p < 0.01; ***p < 0.001, compared with CON. #p < 0.05; ##p < 0.01, compared with LPS only
To assess cytokine levels of I-sericin and sericin in serum and macrophages were collected from mice fed I-sericin (100, 200 or 400 mg/kg) or sericin (400 mg/kg) and isolated. Cytokines in LPS alone were significantly increased, whereas co-treatment with I-sericin significantly decreased the cytokine level in a dose-dependent manner. Specifically, compared to using the same concentration of non-irradiated sericin (100 μg/ml), cells treated with I-sericin showed a lower level of cytokines (Fig. 4B). Therefore, I-sericin might have an inhibitory effect on inflammation by regulating cytokines.
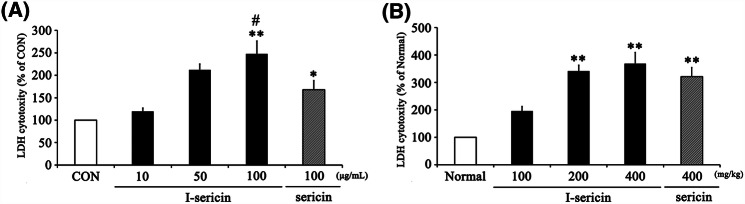
I-sericin increased NK cell activity
NK cells, which are activated early in the immune responses, recognize abnormal cells, tumor cells, and virus-infected cells and bind to the cells to pass toxic granules to them and induce apoptosis (Biron et al., 1999; Frese-Schaper et al., 2014). NK cells express a variety of inhibitory and activating receptors on the surface. Determining the activation of NK cells is a signal balance between inhibition and activation receptors; normal cells expressing ligands of normal receptors cannot activate NK cells (Fernandez et al., 2005). Therefore, we next assessed NK cell activation effects of I-sericin using LDH assays. As shown in Fig. 5, I-sericin and sericin significantly increased LDH cytotoxity compared to the controls both in vitro and in vivo in a dose-dependent manner. Specifically, using the same concentration (100 μg/ml) of I-sericin and sericin, cells treated with I-sericin showed higher NK cell activity. Therefore, these results showed that I-sericin has a beneficial effect on NK cell activity and the immune response.
Fig. 5.
Effect of I-sericin on NK cell activity in spleen cells. (A) NK cell activity in in vitro experiment. I-sericin; gamma-irradiated sericin. Data are expressed as mean ± SE (n = 3). *p < 0.05; **p < 0.01, compared with control (CON). #p < 0.05, compared with sericin at 100 μg/ml. (B) NK cell activity in in vivo experiment. I-sericin; gamma-irradiated sericin. Data are expressed as mean ± SE (n = 10). **p < 0.01, compared with Normal
In conclusion, our studies suggest that gamma irradiation of sericin at 5 kGy can increase its ability to activate innate immunity by enhancing NO production. Moreover, the present results show that I-sericin has immune-enhancing effects and protects against LPS-induced inflammation. These findings suggest that I-sericin has promise as a nutritional supplement for immune enhancement and inhibition of inflammatory disease. However, further studies should be required to determine the safety and the structural changes of sericin by gamma irradiation.
Acknowledgements
This work was supported by a Grant from the National Research Foundation of Korea, funded by the Korean government (NRF- 2016M2A2A6A04913728).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
In-Bong Song, Email: sib17@naver.com.
Hye-Ju Han, Email: hanhyeju0929@gmail.com.
Jungkee Kwon, Email: jkwon@jbnu.ac.kr.
References
- Altman GH, Diaz F, Jakuba C, Calabro T, Horan RL, Chen J, Lu H, Richmond J, Kaplan DL. Silk-based biomaterials. Biomaterials. 2003;24:401–416. doi: 10.1016/S0142-9612(02)00353-8. [DOI] [PubMed] [Google Scholar]
- Andrews JS, Berger AE, Ware CF. Characterization of the receptor for tumor necrosis factor (TNF) and lymphotoxin (LT) on human T lymphocytes. TNF and LT differ in their receptor binding properties and the induction of MHC class I proteins on a human CD4 + T cell hybridoma. J. Immunol. 1990;144:2582–2591. [PubMed] [Google Scholar]
- Andrikopoulos S, Blair AR, Deluca N, Fam BC, Proietto J. Evaluating the glucose tolerance test in mice. Am. J. Physiol. Endocrinol. Metab. 2008;295:E1323–E1332. doi: 10.1152/ajpendo.90617.2008. [DOI] [PubMed] [Google Scholar]
- Birk RW, Gratchev A, Hakiy N, Plitz O, Schiefzewski K, Guillot P, Orfanos CE, Goerdt S. Alternative activation of antigen-presenting cells: concepts and clinical relevance. Hautarzt. 2001;52:193–200. doi: 10.1007/s001050051289. [DOI] [PubMed] [Google Scholar]
- Biron CA, Nguyen KB, Pien GC, Cousens LP, Salazar-Mather TP. Natural killer cells in antiviral defense: function and regulation by innate cytokines. Annu. Rev. Immunol. 1999;17:189–220. doi: 10.1146/annurev.immunol.17.1.189. [DOI] [PubMed] [Google Scholar]
- Brandstetter S, Berthold C, Isnardy B, Solar S, Elmadfa I. Impact of gamma-irradiation on the antioxidative properties of sage, thyme, and oregano. Food Chem. Toxicol. 2009;47:2230–2235. doi: 10.1016/j.fct.2009.06.009. [DOI] [PubMed] [Google Scholar]
- Chou ST, Peng HY, Hsu JC, Lin CC, Shih Y. Achillea millefolium L. essential oil inhibits LPS-induced oxidative stress and nitric oxide production in RAW 264.7 macrophages. Int. J. Mol. Sci. 2013;14:12978–12993. doi: 10.3390/ijms140712978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dash R, Mandal M, Ghosh SK, Kundu SC. Silk sericin protein of tropical tasar silkworm inhibits UVB-induced apoptosis in human skin keratinocytes. Mol. Cell Biochem. 2008;311:111–119. doi: 10.1007/s11010-008-9702-z. [DOI] [PubMed] [Google Scholar]
- Davies KJ. Protein damage and degradation by oxygen radicals. I. general aspects. J. Biol. Chem. 1987;262:9895–9901. [PubMed] [Google Scholar]
- Dogbevi MK, Vachon C, Lacroix M. Physicochemical and microbiological changes in irradiated fresh pork loins. Meat. Sci. 1999;51:349–354. doi: 10.1016/S0309-1740(98)00133-8. [DOI] [PubMed] [Google Scholar]
- Fernandez NC, Treiner E, Vance RE, Jamieson AM, Lemieux S, Raulet DH. A subset of natural killer cells achieves self-tolerance without expressing inhibitory receptors specific for self-MHC molecules. Blood. 2005;105:4416–4423. doi: 10.1182/blood-2004-08-3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frese-Schaper M, Keil A, Yagita H, Steiner SK, Falk W, Schmid RA, Frese S. Influence of natural killer cells and perforinmediated cytolysis on the development of chemically induced lung cancer in A/J mice. Cancer Immunol. Immun. 2014;63:571–580. doi: 10.1007/s00262-014-1535-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Y, Wu J, Liu T, Hu Y, Zheng Q, Wang B, Lin H, Li X. Separation, characterization and anticancer activities of a sulfated polysaccharide from Undaria pinnatifida. Int. J. Biol. Macromol. 2016;83:42–49. doi: 10.1016/j.ijbiomac.2015.11.049. [DOI] [PubMed] [Google Scholar]
- Kang IS, Kim RI, Kim GS, Kim NR, Shin JY, Kim C. Effects of Agaricus blazei Murill water extract on immune response in BALB/c mice. J. Korean Soc. Food Sci. Nutr. 2015;44:1629–1636. doi: 10.3746/jkfn.2015.44.11.1629. [DOI] [Google Scholar]
- Kojthung A, Meesilpa P, Sudatis B, Treeratanapiboon L, Udomsangpetch R, Oonkhanond B. Effects of gamma radiation on biodegradation of Bombyx mori silk fibroin. Int. Biodeter. Biodegr. 2008;62:487–490. doi: 10.1016/j.ibiod.2007.12.012. [DOI] [Google Scholar]
- Lafortune R, Caillet S, Lacroix M. Combined effects of coating, modified atmosphere packaging, and gamma irradiation on quality maintenance of ready-to-use carrots (Daucus carota) J. Food Protect. 2005;68:353–359. doi: 10.4315/0362-028X-68.2.353. [DOI] [PubMed] [Google Scholar]
- Lee SS, Lee EM, Hong SH, Yoo SH, Cho JY, Lee IC, Chung BY. Enhanced formation of quercetin by combined use of gamma ray and H2O2 from cyanidin-3-O-xylosylrutinoside. Radiat. Phys. Chem. 2012;81:1132–1135. doi: 10.1016/j.radphyschem.2011.11.028. [DOI] [Google Scholar]
- Lin WW, Karin M. A cytokine-mediated link between innate immunity, inflammation, and cancer. J. Clin. Invest. 2007;117:1175–1183. doi: 10.1172/JCI31537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacMicking J, Xie QW, Nathan C. Nitric oxide and macrophage function. Annu. Rev. Immunol. 1997;15:323–350. doi: 10.1146/annurev.immunol.15.1.323. [DOI] [PubMed] [Google Scholar]
- Medzhitov R. Recognition of microorganisms and activation of the immune response. Nature. 2007;449:819–826. doi: 10.1038/nature06246. [DOI] [PubMed] [Google Scholar]
- Minami M, Shimizu K, Okamoto Y, Folco E, Ilasaca ML, Feinberg MW, Aikawa M, Libby P. Prostaglandin E receptor type 4-associated protein interacts directly with NF-kappaB1 and attenuates macrophage activation. J. Biol. Chem. 2008;283:9692–9703. doi: 10.1074/jbc.M709663200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moller B, Villiger PM. Inhibition of IL-1, IL-6, and TNF-alpha in immune-mediated inflammatory diseases. Springer Semin. Immunopathol. 2006;27:391–408. doi: 10.1007/s00281-006-0012-9. [DOI] [PubMed] [Google Scholar]
- Mu MM, Chakravortty D, Sugiyama T, Koide N, Takahashi K, Mori I, Yoshida TY. The inhibitory action of quercetin on lipopolysaccharide-induced nitric oxide production in RAW 264.7 macrophage cells. J. Endotoxin Res. 2001;7:431–438. doi: 10.1179/096805101101533034. [DOI] [PubMed] [Google Scholar]
- Ortiz-Andrellucchi A, Sanchez-Villegas A, Rodriguez-Gallego C, Lemes A, Molero T, Soria A, Pena-Quintana L, Santana M, Ramirez O, Garcia J, Cabrera F, Cobo J, Serra-Majem L. Immunomodulatory effects of the intake of fermented milk with Lactobacillus casei DN114001 in lactating mothers and their children. Br. J. Nutr. 2008;100:834–845. doi: 10.1017/S0007114508959183. [DOI] [PubMed] [Google Scholar]
- Pereira E, Antonio AL, Rafalski A, Barreira JCM, Barros L, Oliveira MBPP, Ferreira ICFR. Electron-beam irradiation as an alternative to preserve nutritional, chemical and antioxidant properties of dried plants during extended storage periods. LWT-Food Sci. Technol. 2017;82:386–395. doi: 10.1016/j.lwt.2017.04.069. [DOI] [Google Scholar]
- Roberts PB. Food irradiation is safe: Half a century of studies. Radiation Phys. Chem. 2014;105:78–82. doi: 10.1016/j.radphyschem.2014.05.016. [DOI] [Google Scholar]
- Ryu SP. Silkworm pupae powder ingestion increases fat metabolism in swim-trained rats. J. Exerc. Nutrition Biochem. 2014;18:141–149. doi: 10.5717/jenb.2014.18.2.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu HS, Kim J, Kim HS. Enhancing effect of Sorghum bicolor L. Moench (Sorghum, su-su) extracts on mouse spleen and macrophage cell activation. J. Korean Soc. Food Sci. Nutr. 2006;19:176–182. [Google Scholar]
- Seo MJ, Kang BW, Kim MJ, Lee HH, Seo KI, Kim KH, Jeong YK. The Effect of Cordycepin on the Production of Pro-inflammatory Cytokines in Mouse Peritoneal Macrophages. J. Korean Soc. Food Sci. Nutr. 2014;46:68–72. [Google Scholar]
- Taylor A, Verhagen J, Blaser K, Akdis M, Akdis CA. Mechanisms of immune suppression by interleukin-10 and transforming growth factor-β: the role of T regulatory cells. Immunology. 2006;117:433–442. doi: 10.1111/j.1365-2567.2006.02321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungefroren H, Sebens S, Seidl D, Lehnert H, Hass R. Interaction of tumor cells with the microenvironment. Cell Commun. Signal. 2011;9:18. doi: 10.1186/1478-811X-9-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdes-Diaz G, Rodriguez-Calvo S, Perez-Gramatges A, Rapado-Paneque M, Fernandez-Lima FA, Ponciano CR, da Silveira EF. Effects of gamma radiation on phase behaviour and critical micelle concentration of Triton X-100 aqueous solutions. J. Colloid Interf. Sci. 2007;311:253–261. doi: 10.1016/j.jcis.2007.02.081. [DOI] [PubMed] [Google Scholar]
- Verde SC, Trigo MJ, Sousa MB, Ferreira A, Ramos AC, Nunes I, Junqueira C, Melo R, Santos PM, Botelho ML. Effects of gamma radiation on raspberries: safety and quality issues. J. Toxicol. Env. Health A. 2013;76:291–303. doi: 10.1080/15287394.2013.757256. [DOI] [PubMed] [Google Scholar]
- Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S. Functions of natural killer cells. Nat. Immunology. 2008;9:503–510. doi: 10.1038/ni1582. [DOI] [PubMed] [Google Scholar]
- Wang KS, Frank DA, Ritz J. Interleukin-2 enhances the response of natural killer cells to interleukin-12 through up-regulation of the interleukin-12 receptor and STAT4. Blood. 2000;95:3183–3190. doi: 10.1182/blood.V95.10.3183. [DOI] [PubMed] [Google Scholar]
- Zalys RS, Zagon I, H.Bonneau R, Lang CM, JMcLaughlin P. In vivo effects of chronic treatment with [Met5]-enkephalin on hematological values and natural killer cell activity in athymic mice. Life Sci. 66: 829-834 (2000) [DOI] [PubMed]