Abstract
Thirty polymorphic SSRs, derived from RNA sequencing of Tinospora cordifolia (willd.), were utilized for genetic diversity and population structure evaluation among 96 accessions collected from ten different geographical regions of India. A total of 7611 SSRs were identified from 268149 transcripts. Of all SSR loci, 69.07% of them were tri-nucleotide repeat motifs, followed by di-nucleotide repeat motifs (12.82%). A total of 230 alleles were generated by 30 SSRs with an average of 7.67 alleles per locus with comparatively higher polymorphic information content (average 0.68). The expected (He) and observed (Ho) heterozygosity means were 0.71 and 0.12, respectively. All the loci showed significant deviation from Hardy–Weinberg Equilibrium (HWE). The neighbor joining clustering based on jaccard’s coefficient grouped all the 96 accessions into three major cluster which was also in congruence with model-based structure plot. The result of molecular variance (AMOVA) revealed higher genetic variance within populations than among populations. The result reflects an existence of high level of genetic diversity in the collected accessions of T. cordifolia. The accessions Tc131, Tc31, Tc129, Tc38, Tc16, Tc59, Tc60, Tc17, Tc106 and Tc130 was found to be potential and diverse in nature and the SSRs TCSSR-18, TCSSR-37, TCTSSR-59, TCTSSR-92, TCTSSR-123 and TCTSSR-126 as potential markers. These accessions and newly developed SSR markers provide valuable resource and could be strategically utilized for further genetic improvement of T. cordifolia.
Electronic supplementary material
The online version of this article (10.1007/s13205-020-02300-7) contains supplementary material, which is available to authorized users.
Keywords: Tinospora cordifolia, Simple sequence repeat (SSR), Genetic diversity, AMOVA
Introduction
Tinospora cordifolia (Willd.) Hook.f. & Thomason (Menispermaceae, 2n = 22) is one of the important traditional medicinal plants described in the Ayurveda system of medicine. It is commonly known as Giloy (Hindi) and Guduchi (Sanskrit), and is distributed throughout the tropical parts of Indian subcontinent (Sri Lanka and Bangladesh) and also in China (Kumawat et al. 2019; Spandana et al. 2013). It is a large, glabrous, deciduous and succulent climber with greenish-yellow flower. The leaves are simple, alternate, heart-shaped and half way round with long petiole. Fruits are yellowish-red in color, fleshy, in clusters of 1-3 and rounded on dense stalk with sub-terminal scars. The major active compounds of T. cordifolia includes alkaloids, diterpenoids, lactones, glycosides, steroids, sesquiterpenoids, phenolics, aliphatic compounds and polysaccharides (Sharma et al. 2019; Kumawat et al. 2019; Kumar et al. 2018; Mittal et al. 2014; Saha and Ghosh 2012; Gupta and Sharma 2011; Patel and Mishra 2011). T. cordifolia is one of the most frequently used ayurvedic medicines to treat a diverse range of health disorders (Dhama et al. 2016; Thomas et al. 2016; Srivastava 2011; Ghosh et al. 2011) and is designated as miracle herb of the 21st century according by the Ministry of Ayurvedic, Yoga, Naturopathy, Unani, Siddha and Homeopathy (AYUSH), Government of India. In spite of the immense medicinal properties and application of T. cordifolia, little information is available about its breeding system, genetic variability and population genetic structure. The knowledge of genetic variability of any plant is considered as prerequisite for its efficient exploitation and utilization, to develop suitable conservation strategies and deploy suitable breeding technique for its genetic improvement (Fu 2015). Various molecular markers have been utilized for understanding the domestication, evolution, distinctiveness, population structure and extent of genetic variation within and between species (Bakatoushi and Ahmed 2018). Molecular markers such as RAPD and ISSR (Malik et al. 2019; Nazneen et al. 2019, Lade et al. 2018; Rana et al. 2012; Shinde and Dhalwal 2010) ITS and cpDNA markers (Ahmed et al. 2006), isozyme (Kalpesh and Mohan 2009), and also gene targeted SCoT markers (Paliwal et al. 2013) have been utilized to unravel the genetic diversity and distinctiveness among limited accessions of T. cordifolia considering only small geographical area. Albeit, there is information available, where SSR markers have been used to estimate the genetic diversity in small number of accessions collected mostly from local or small geographical areas, which do not reflect a comprehensive account of the actual genetic diversity (Gargi et al. 2017; Paliwal et al. 2016; Singh et al. 2014, 2016). Therefore, it is important to estimate the genetic potential in diverse and large number of accessions from a wider range of phyto-climatic zones. Among various marker systems, SSRs is considered as highly potential to estimate the genetic variability due to wide coverage of the genome, reproducibility, high level of polymorphism and co-dominant inheritance (Kalia et al. 2010). Considering the medicinal significance and restricted marker-based studies in T. cordifolia, more SSR markers needs to be developed, which could substantially contribute towards estimation of genetic diversity more comprehensively and identify the diverse populations for future prospection. The present study was, therefore, undertaken to enrich the SSR resources of T. cordifolia by extracting novel SSRs from transcriptome sequences developed at CSIR-National Botanical Research Institute (CSIR-NBRI), Lucknow (India), and utilize a subset of newly developed SSRs to analyse the genetic diversity and population genetic structure in 96 accessions of T. cordifolia.
Materials and methods
Plant materials
The plant materials used in the present investigation were collected from various phyto-geographical zones of India. The stem cuttings of almost equal length and width were planted in the experimental field at CSIR-NBRI, Lucknow and support was provided with the help of iron pipes (Fig. S1). A total of 96 accessions were selected for the present study collected from ten different states namely Uttar Pradesh, Madhya Pradesh, Jammu & Kashmir, Maharashtra, Kerala, Gujarat, Andhra Pradesh, Karnataka, Chhattisgarh and Haryana (Table S1). The total genomic DNA was isolated from the young leaves using CTAB (Cetyl trimethylammonium bromide) method following Porebski et al. (1997). The DNA quality was checked by agarose gel electrophoresis and quantified by Nanodrop (Thermo Nano drop ND 1000).
RNA isolation and sequencing
Approximately 100 mg of fresh young leaves was grounded into a fine powder with liquid nitrogen. Total RNA was extracted and cleaned using Spectrum™ Plant Total RNA Kit (SIGMA-ALDRICH) and RNase –free DNase I, respectively, for transcriptome sequencing. The RNA concentration and purity were determined using Nanodrop (Thermo Nano drop ND 1000) and agarose gel electrophoresis (1.2%), respectively. High-quality RNA samples with concentration of ≥ 600 ng/µL; RIN value ≥ 6.8; OD A260/280 ≥ 2.10 was used to construct complementary DNA (cDNA) libraries, which were then sequenced using the Illumina HiSeq™ 2000 platform (SciGenom Labs Pvt Ltd).
SSR identification and primer designing
The assembled transcriptome sequence data was subjected to SSR identification using MIcroSAtellite tool (MISA; https://webblast.ipk-gatersleben.de/misa/) (Thiel et al. 2003). The major criteria for SSR identification was set as: minimum number of repeat units: ten for di-nucleotides, five for tri- and tetra-nucleotides, and four for penta- and hexa-nucleotides. The primer pairs were designed using Primer 3 v2.23 (http://primer3.sourceforge.net) with the following parameters: primer length of 18–27 bp, GC content of 40–60%, annealing temperature of 55–60 °C, and target PCR product size of 100–300 bp.
PCR amplification and data acquisition
A random set of 135 SSR primers was commercially synthesized (Eurofins Genomics India Pvt. Ltd.) for genotyping. First, all the 135 SSR primers were PCR amplified with 4 accessions of T. cordifolia to identify polymorphic SSRs. Furthermore, the polymorphic SSRs were used to amplify the 96 accessions. The PCR reactions was carried out in 10 µL reaction volume containing 20 ng of template DNA, 1X PCR buffer, 2.0 mM MgCl2, 0.25 mM each dNTPs, 1U Taq DNA polymerase (Qiagen), 1.0 pmol forward and reverse primers. The PCR amplification was performed using C1000 Touch™ Thermal Cycler (BIO-RAD, USA) with an initial denaturation of 5.0 min at 95 °C followed by 36 cycles of denaturation for 30 s at 95 °C, annealing for 45 s at 52–55 °C (primer specific), extension for 1.0 min at 72 °C and final extension for 10.0 min at 72 °C. The PCR products were first confirmed on 2.5% agarose gel and subsequently, separated on 6% polyacrylamide gel electrophoresis and visualized by silver staining. The size of PCR products were measured against the 100 bp DNA ladder (Promega, USA) and amplicon size was calculated using online tool fragment size calculator (biotools.nubic.northwestern.edu/SizeCalc.html).
SSR data analysis
All the SSR fragments were manually scored as allele size and also transformed into binary matrix data, i.e., 0 for the absence and 1 for the presence of the band. The statistical analysis of SSR allelic data was performed using Power Marker (Liu and Muse 2005) to measure observed heterozygosity (Ho), allele frequency, gene diversity or expected heterozygosity (He) and polymorphic information content (PIC) value. To identify the genetic relationship among the accessions, pairwise genetic dissimilarities across all the 96 accessions was calculated using Jaccard’s coefficient with using DARwin 5.0.128 software (Perrier et al. 2003). The calculated dissimilarity matrix was then used to construct a neighbor joining tree with a bootstrap value of 1000 replicate. Furthermore, the Bayesian clustering was also performed using STRUCTURE v 2.3.3 software to determine the number of subpopulations. The presumed population (K) was set to 2 to 15 with five separate runs per K and 30,000 burn-in period and 100,000 iterations for each run. The optimal K value was calculated using structure harvester (Earl and Von Holdt 2012) and analysing by ΔK statistic and L(K) according to Evanno et al. (2005). The molecular variance (AMOVA) and confirmation of the Hardy–Weinberg equilibrium (HWE) was performed using GenAlEx 6.502 (Excoffier et al. 2009). In addition, the relative discriminatory power of genetic loci/combinations of loci for better population assignment was determined using WHICHLOCI software (Banks et al. 2003). The analysis was done with criteria of Use Critical Population Method at LOD score 3.0 and the threshold of minimum percentage of assigned to population was set to 95.0.
Results
Frequency and distribution of SSRs
The Illumina Hiseq 2500 sequencing generated > 246.0 million reads which were assembled into 268,149 transcripts. In total, 155,821 unique contigs were obtained and a total of 7611 SSR loci identified were distributed in 7457 sequences. Among the identified SSR, 154 were found to be in compound form, while others were of ideal one-repeat type (Table 1). Out of 7611 SSRs, the most abundant were tri-nucleotide repeats (69.1%), followed by di-nucleotide (12.8%), tetra-nucleotide repeats (8.2%), penta-nucleotide (6.6%) and hexa-nucleotide repeats (1.4%) (Fig. S2). The most common motifs were AAG/CTT repeats (10.7%) followed by AAT/ATT (8.2%) for tri-nucleotides and AG/CT (3.7%) repeats for di-nucleotides (Fig. S3).
Table 1.
Summary of SSRs mined from transcriptome data of Tinospora cordifolia
| Parameters used in screening | Numbers |
|---|---|
| Total number of sequences examined | 268149 |
| Total size of examined sequences (bp) | 225518219 |
| Total number of identified SSRs | 7611 |
| Number of SSR containing sequences | 7457 |
| Number of sequences containing more than 1 SSR | 521 |
| Number of SSRs present in compound formation | 154 |
Validation of SSRs and genotyping
Out of 7611, a total of 2981 primer pairs were successfully designed flanking the SSRs motifs. However, the flanking sequences of rest of SSRs were not found suitable for primer designing. A random set of 135 primers were selected and commercially synthesized (Eurofins Genomics India Pvt. Ltd.) for validation (Supplementary File 1). The initial screening of SSR primers with 4 DNA was done to optimize the PCR conditions. After several round of PCR optimization, finally a set of 56 (41.48%) primer pairs were found to be good which generated amplification products with expected size, 46 (34.07%) showed non-specific products and 33 (24.44%) did not amplified any fragment (Supplementary File 1). The 56 SSR primers were then amplified with 96 accessions and amplicon resolved on 6% polyacrylamide gel electrophoresis (PAGE) and visualized through silver staining (Fig. S4). Out of 56 SSRs, 30 were found to be polymorphic among 96 accessions, while the rest 26 were monomorphic. These 30 polymorphic SSRs generated a total of 230 alleles ranged from 2 to 12 alleles with an average of 7.67 ± 2.88 alleles per locus (Table 2). The maximum number of alleles (12) was exhibited by TCTSSR-37 and TCTSSR-59 followed by TCTSSR-123, TCTSSR-104, TCTSSR-60, TCTSSR-92, TCTSSR-18 (11 alleles) and TCTSSR-06 (10 alleles), respectively. Out of 30, 22 SSRs (73%) had alleles range between 6.00 and 12.00, while 8 SSRs (27%) between 2.0 and 5.0. The PIC value was found to be comparatively higher as 80% SSRs showed PIC value in range of 0.61 to 0.85 (Table 2, Fig. S5). The observed heterozygosity value ranges from 0.00 to 0.78 with an average of 0.12 ± 0.22. The gene diversity was in between 0.40 and 0.85 with an average of 0.71 ± 0.13. Out of 30, 18 SSRs (60%) revealed highly informative gene diversity value range between 0.71 and 0.85, while 8 SSRs (27%) revealed moderate values ranging between 0.61 and 0.70 and 4 SSRs (13%) had lower gene diversity values < 0.60. The major allele frequency ranged between 0.21 (TCTSSR-92) to 0.76 (TCTSSR-124) with an average of 0.41 ± 0.15. The SSRs 40% (12) showed major allele frequency between 0.41 and 0.80, while 30% (9 SSRs) ranged between 0.31 and 0.40. All the SSRs showed significant Chi square test for Hardy–Weinberg equilibrium test at P < 0.001.
Table 2.
Amplification pattern of polymorphism of 30 polymorphic SSR used for diversity analysis in T. cordifolia
| SSR_ID | Sequence (5′–3′) Forward Primer | Sequence (5′–3′) Reverse Primer | SSR motifs | MAF | Na | He | Ho | PIC | HWE | |
|---|---|---|---|---|---|---|---|---|---|---|
| χ2 | p value | |||||||||
| TCTSSR-37 | GAATGGTTCACGGTGGATTC | TGACCTGTTAAAGGGGCAGT | (AC)10 | 0.30 | 12.00 | 0.79 | 0.09 | 0.77 | 787.31 | 0.000*** |
| TCTSSR-59 | TGCTGTTGTTGAGAACTCGTG | GGGATGGGAATGGGAGTAGT | (ACT)5 | 0.26 | 12.00 | 0.85 | 0.00 | 0.84 | 1034.00 | 0.000*** |
| TCTSSR-70 | AAGCTTCCAGCAGCAACAAT | TCAGCACGTGTTAAGGACCC | (AGT)5 | 0.26 | 7.00 | 0.81 | 0.00 | 0.80 | 576.00 | 0.000*** |
| TCTSSR-75 | ATGAAGGAGCCTTGCATTTG | ATGGATTTGCTGTTGCTTCC | (AGC)5 | 0.26 | 8.00 | 0.81 | 0.00 | 0.79 | 672.00 | 0.000*** |
| TCTSSR-78 | TGCATAAACCATTCTGTGTGTG | CCAGTCAATCCCGTAACTTCA | (AAT)5 | 0.42 | 5.00 | 0.70 | 0.00 | 0.66 | 384.00 | 0.000*** |
| TCTSSR-79 | CTCCAGTTAACCGATCCAGC | ATCTCCGATCCTCGATTCCT | (AGA)5 | 0.63 | 4.00 | 0.54 | 0.00 | 0.49 | 288.00 | 0.000*** |
| TCTSSR-93 | GGGCAAGTTCTGATTGAATGT | CCTTCGATTCAAGGGATTCA | (TCT)5 | 0.39 | 5.00 | 0.74 | 0.00 | 0.71 | 376.00 | 0.000*** |
| TCTSSR-104 | GCCGATTGCTTCGTAGATGT | CTCGAAACCCCCAATTCTC | (AG)10 | 0.36 | 11.00 | 0.80 | 0.11 | 0.79 | 604.10 | 0.000*** |
| TCTSSR-124 | AATTCAGGGAATAGTGGGGG | GTAAGACCCCGAACCGTACA | (TGG)5 | 0.76 | 4.00 | 0.40 | 0.00 | 0.39 | 282.00 | 0.000*** |
| TCTSSR-131 | TCTTGTTCCTACTCCACCGC | AACTCAGCTTGAAGGGCAAA | (ATC)6 | 0.38 | 9.00 | 0.72 | 0.14 | 0.69 | 578.50 | 0.000*** |
| TCTSSR-123 | CCATTTTTGCTTTGATTGGAA | AAGTTGAACCAAATCCTGCC | (AAT)7 | 0.49 | 11.00 | 0.67 | 0.22 | 0.64 | 400.65 | 0.000*** |
| TCTSSR-60 | ACGCGAATCTGCTTCTTGTT | TTGCTGTTGCGATTTAGGTG | (AAC)5 | 0.51 | 11.00 | 0.69 | 0.14 | 0.68 | 503.31 | 0.000*** |
| TCTSSR-6 | GAACGAAGCGAACAGCAAAT | GGGGTGAATGAAGAGAAGATTG | (AG)10 | 0.46 | 10.00 | 0.70 | 0.04 | 0.67 | 663.45 | 0.000*** |
| TCTSSR-84 | ATGATGCATGCAAATGGAGA | GTATGGGCATGTCGTGTGAG | (AGT)5 | 0.39 | 8.00 | 0.71 | 0.07 | 0.67 | 374.01 | 0.000*** |
| TCTSSR-94 | GTAGTCCGCACCCATCTCAT | ATGCCACGATTAACTACGCC | (TGG)5 | 0.38 | 7.00 | 0.71 | 0.00 | 0.67 | 576.00 | 0.000*** |
| TCTSSR-40 | CCATCAGGCCATTTTCAATC | GCCTTCCTAGAAGCCCAACT | (AC)10 | 0.35 | 9.00 | 0.80 | 0.45 | 0.78 | 302.86 | 0.000*** |
| TCTSSR-46 | CTTCGATCGTCTCTCTCGCT | TGAAGGGCTCAAGGTTATGG | (CT)10 | 0.31 | 5.00 | 0.73 | 0.00 | 0.70 | 360.00 | 0.000*** |
| TCTSSR-135 | AGATCATTGGTGGGAAACCA | TCATCATGCCAATTTTCCAA | (TTC)5 | 0.59 | 4.00 | 0.54 | 0.00 | 0.47 | 279.00 | 0.000*** |
| TCTSSR-133 | GCCCAAGTCCAGAATCAGTC | GAGGAGGCCCAACTGTGTAA | (ATA)5 | 0.48 | 6.00 | 0.63 | 0.08 | 0.58 | 280.78 | 0.000*** |
| TCTSSR-126 | CCTTGAAAATGGAGCTGGAA | GAGGTTTTGGGTTTTGGGTT | (CTT)5 | 0.30 | 8.00 | 0.82 | 0.00 | 0.82 | 651.00 | 0.000*** |
| TCTSSR-82 | GGTGCACGATTGCATTGTAG | CAATGACCACATTCTTCTGCTT | (ATT)5 | 0.55 | 5.00 | 0.64 | 0.00 | 0.61 | 380.00 | 0.000*** |
| TCTSSR-96 | TTTCACTCCCAATCACACCA | GCTCTTCGAAGGTGAAAACG | (AGA)5 | 0.30 | 7.00 | 0.80 | 0.01 | 0.78 | 470.17 | 0.000*** |
| TCTSSR-49 | TATCACGAAGCGTAGGGACC | GACCACTATTGCATTTTCCGA | (TGC)5 | 0.46 | 6.00 | 0.69 | 0.25 | 0.65 | 211.50 | 0.000*** |
| TCTSSR-67 | TCCCTACCCCTTTCCTTCAG | CCACTTGGTCACCTGGTTTT | (AAG)5 | 0.72 | 2.00 | 0.40 | 0.00 | 0.32 | 96.00 | 0.000*** |
| TCTSSR-61 | TGAAATCATCAAGGTGGCAA | TTCTTTACGGCTCCAACGAC | (AGA)5 | 0.33 | 9.00 | 0.79 | 0.78 | 0.77 | 240.55 | 0.000*** |
| TCTSSR-85 | GCTTCCCACAATAACTTCGC | CTTGCCACCTACTCGTGTGA | (AAC)5 | 0.27 | 9.00 | 0.78 | 0.05 | 0.76 | 475.72 | 0.000*** |
| TCTSSR-69 | ATTGCAGATGGCAATGACAA | AACTGACCCTGACCCATTTG | (AAT)5 | 0.38 | 7.00 | 0.75 | 0.17 | 0.73 | 363.79 | 0.000*** |
| TCTSSR-92 | TCAGTGAAGGGGGAAGAACA | TGCTTACCCCACCAAAATTC | (AAG)5 | 0.21 | 11.00 | 0.85 | 0.65 | 0.84 | 264.70 | 0.000*** |
| TCTSSR-18 | CAAAGACCAGCTCATTTTCTTG | GCGATTTAAGGTGACAAATTACC | (AG)10 | 0.26 | 11.00 | 0.82 | 0.13 | 0.80 | 742.41 | 0.000*** |
| TCTSSR-90 | CTTGGGCAGCCTAATTCAAG | TTTGTTGCTCAAAAGCTTGC | (ACA)5 | 0.49 | 7.00 | 0.64 | 0.11 | 0.59 | 268.88 | 0.000*** |
| Mean ± SD | 0.41 ± 0.15 | 7.67 ± 2.88 | 0.71 ± 0.13 | 0.12 ± 0.22 | 0.68 ± 0.14 | |||||
MAF major allele frequency, Na number of alleles, He expected heterozygosity or gene diversity, Ho observed heterozygosity, PIC polymorphic information content; HWE: deviation from Hardy–Weinberg equilibrium with significant p < 0.001; ***p < 0.001
Genetic diversity and population structure
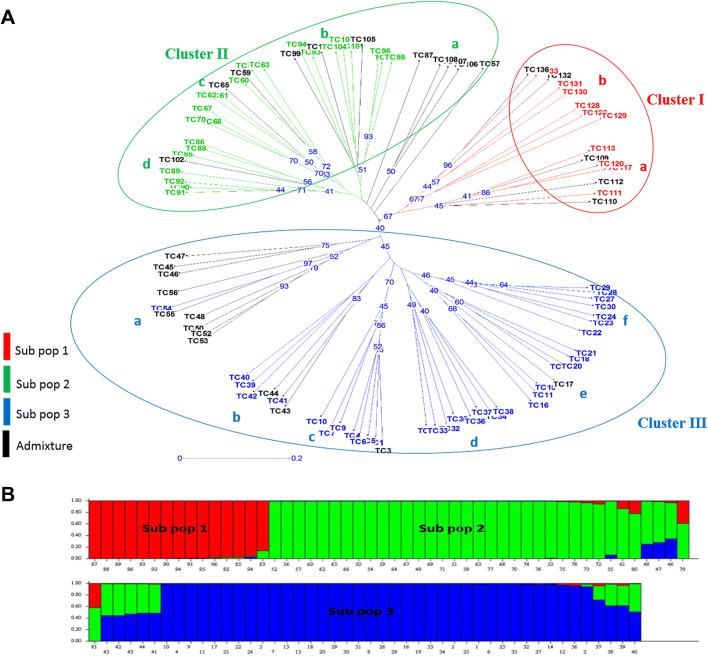
The allelic data was used to calculate a pairwise genetic distance based on Jaccard’s coefficient to understand the genetic relationship among the 96 accessions. The pairwise genetic dissimilarity varied from 0.26 to 1.0 with an average of 0.89 ± 0.05. The maximum genetic dissimilarity of 0.99 was observed among Tc131-Tc31, Tc129-Tc38, Tc16-Tc59, Tc16-Tc60, Tc17-Tc106, Tc4-Tc130 and 0.98 among Tc23-TC55, Tc16-Tc63, Tc16-Tc90, Tc10-Tc128, Tc15-Tc128, Tc9-Tc112, Tc9-131, Tc7-Tc129 and Tc5-Tc129. The minimum genetic dissimilarity (0.26) was observed between Tc95 and Tc96 followed by Tc53 and Tc52 (0.27), Tc28 and Tc29 (0.28). The neighbor joining (NJ) dendrogram grouped all the 96 accessions into three main clusters: cluster I, cluster II and cluster III (Fig. 1a). The cluster III comprised of 47 accessions (49%), followed by cluster II (34 accessions; 35%) and cluster I (15 accessions; 16%). The cluster I could be subdivided into two sub-clusters namely Ia with 7 accessions and Ib with 8 accessions. Similarly, cluster II was separated into four sub-clusters as IIa, IIb, IIc, IId with 5, 11, 10 and 8 accessions, respectively. Likewise, the cluster III was separated into six sub-clusters IIIa, IIIb, IIIc, IIId, IIIe and IIIf, each consisting of 10, 6, 8, 8, 8 and 7 accessions, respectively. Furthermore, the model-based simulation was performed to investigate the subpopulations among the 96 accessions. The population structure analysis revealed 3 subpopulations (K = 3) based on maximum ΔK value obtained through the structure harvester (Fig. S6). The bar plot of model-based clustering revealed that the subpopulation 1 confined 15 accessions (15.62%), among which 10 accessions (66.66%) were found to be pure lines and 5 admixture type (Fig. 1b). The subpopulation 2 comprised 41 accessions (42.70%) with 24 (58.53%) accessions as pure lines, 17 admixture and subpopulation 3 had 40 accessions (41.66%) in which 33 (82.5%) accessions as pure lines, 7 admixtures. The NJ dendrogram and STRUCTURE analysis revealed almost similar patterns of accession compositions. The NJ tree as well as the STRUCTURE plot showed that the accessions composition of subpopulation 1 was coincided by both the methods. The accessions of subpopulation 2 also corresponded with cluster II, with the exception of seven accessions that grouped in cluster III.
Fig. 1.
Distance- and Bayesian Model-based genetic clustering of 96 accessions of Tinospora cordifolia using 30 SSR markers. a Dendrogram was constructed in DARwin v 5.0.128 based on Jaccard’s coefficient using NJ method. Robustness of the node of the phylogenetic tree was assessed from 1000 bootstrap replicates and bootstrap values of > 40% are shown (each color is indicative of a separate group and color coding is according to STRUCTURE analysis data). b Population structure analysis of Tinospora cordifolia accessions for K = 3 subpopulations (pop1–pop3) using STRUCTURE v 2.3.3 application
Furthermore, to understand the population differentiation, AMOVA analysis was carried out which revealed that 78% genetic variation was observed within the population and 22% was among the population (Table 3). The average pairwise ФPT (Similar To fst) was 0.22 which eventually supported the structuring in the populations. The maximum genetic diversity (He) was recorded for population 3 (0.12 ± 0.009), followed by population 2 (0.10 ± 0.010) and population 1(0.11 ± 0.010) with an average of 0.11 ± 0.005 (Table 4). Similarly, population 3 revealed maximum effective number of alleles per locus (Ne), number of alleles per locus (Na) and Shannon’s information index (I). The mean value for effective number of alleles, Nei genetic diversity and Shannon’s information index were 0.11 ± 0.005, 1.17 ± 0.010 and 0.19 ± 0.008, respectively. The locus score derived from WHICHLOCI analysis showed that the TCTSSR-126, had highest assignment score (2.631), whereas TCTSSR-79 displayed the lowest score (0.701) (Fig. S7, a; Table 5). The ‘‘overall’’ panel without defining the critical population included four SSR markers: TCTSSR-131, TCTSSR-126, TCTSSR-1230 and TCTSSR-85. In this panel, the locus scores varied from 2.27 (TCTSSR-131) to 2.63 for TCTSSR-126, with an average value of 2.44 (Table 5). About 96.87% of the simulated accessions were accurately assigned to their original population using the most effective markers (Table 5). Similarly, in critical population method for population 1, TCTSSR-126 had the highest Locus score (1.92) and TCTSSR-79 the lowest (0.394) (Fig. S7, b). A panel of three marker loci, TCTSSR-126, TCTSSR-06 and TCTSSR-131 had 97.91% correct assignment of accessions to population 1. For Population 2, the Locus score varied from 0.55 for TCTSSR-79 to 1.67 for TCTSSR-126 (Fig. S7, c). The population 2 panel had 96.87% assignment accuracy of accessions and included loci TCTSSR-126 (1.67), TCTSSR-123 (1.60), TCTSSR-85 (1.53) and TCTSSR-46 (1.47). For population 3, the locus score varied from 0.45 for TCTSSR-79 to 2.0 for TCTSSR-85 (Fig. S7, d). The four markers, TCTSSR-85 (2.0), TCTSSR-84 (1.79), TCTSSR-49 (1.79) and TCTSSR-96 (1.67), accounted for 97.91% assignment accuracy of accessions.
Table 3.
Analysis of molecular variance for 96 accessions from three populations
| Source | Degree of freedom | Sum of squares | Variance | % variance |
|---|---|---|---|---|
| Among pops | 2 | 456.292 | 6.944 | 22 |
| Within pops | 93 | 2293.833 | 24.665 | 78 |
| Total | 95 | 2750.125 | 31.609 | 100 |
FST = 0.220, P < 0.010
Table 4.
Different genetic diversity estimates for three populations of T. cordifolia based on SSR data
| Population | Na | Ne | I | He |
|---|---|---|---|---|
| pop 1 (15) | 1.05 ± 0.065 | 1.17 ± 0.017 | 0.18 ± 0.014 | 0.11 ± 0.010 |
| pop2 (34) | 1.12 ± 0.065 | 1.16 ± 0.018 | 0.17 ± 0.014 | 0.10 ± 0.010 |
| pop3 (47) | 1.49 ± 0.057 | 1.17 ± 0.016 | 0.21 ± 0.013 | 0.12 ± 0.009 |
| Mean | 1.22 ± 0.037 | 1.17 ± 0.010 | 0.19 ± 0.008 | 0.11 ± 0.005 |
Na number of alleles, Ne effective number of alleles, I Shannon’s information index, He expected heterozygosity
Table 5.
Estimation of correct assignments and incorrect assignments of loci to one population at a time using critical population method by WHICHLOCI ver 1.0 Software for three populations of Tinospora cordifolia
| Marker | Panels (including markers) with the corresponding locus score | |||
|---|---|---|---|---|
| Overall (whole population) | Population 1 | Population 2 | Population 3 | |
| TCTSSR-06 | 1.68 | |||
| TCTSST-46 | 1.47 | |||
| TCTSSR-49 | 1.79 | |||
| TCTSSR-84 | 1.79 | |||
| TCTSSR-85 | 2.53 | 1.53 | 2.00 | |
| TCTSSR-96 | 1.67 | |||
| TCTSSR123 | 2.36 | 1.60 | ||
| TCTSSR-126 | 2.63 | 1.92 | 1.67 | |
| TCTSSR-131 | 2.27 | 1.63 | ||
| Mean of Locus score | 2.44 | 1.74 | 1.56 | 1.81 |
| Correct assignment (%) | 96.87 | 97.91 | 96.87 | 97.91 |
| Not correct assignment (%) | 3.12 | 2.08 | 3.12 | 2.08 |
No critical population method used for “Overall” panel
Discussion
The knowledge about the level of genetic diversity provides basic parameters to any plant for its sustainable exploitation and utilization. The genetic diversity could be evaluated based on morphological traits and DNA markers. The DNA markers provide more precise informations on as they are rarely affected by environmental variables (Sharma et al. 2008). Various RAPD based studies in T. cordifolia have been reported; however, the results of such studies could not be so reliable due to low reproducibility and low level of polymorphism of RAPD marker (Li et al. 2011; Hamza et al. 2013). Contrary, the SSR markers revealed higher heterozygosity and thus can be attributed to detection of high polymorphism which makes them the most preferred DNA marker (Rana et al. 2015; Sharma et al. 2015). There are, however, few reports available in the public domain on the development and characterization of SSR markers in T. cordifolia, wherein limited number of accessions were analyzed (Gargi et al. 2017; Paliwal et al. 2016; Singh et al. 2016). Gargi et al. (2017) extracted SSRs from enriched libraries and utilized 23 SSRs to assess the genetic diversity among 30 accessions collected from very narrow geographical region of India. While, Singh et al. (2016) identified transcriptome derived SSRs and deployed over 24 accessions to understand the level of genetic diversity. All these studies carried out with limited number of accessions mostly collected from narrow geographical region and thus majority of the genetic variation remains unexplored. Therefore, in the present investigation large set of accessions from wider geographical regions was estimated for genetic variability assessment. In addition, SSR marker resource in T. cordifolia also enriched with new SSR identified from RNA-seq data. A total of 246 million reads and 268149 assembled transcripts were generated which were greater than the results of previous studies (Singh et al. 2016). This indicates that the transcriptome data was efficiently assembled and appropriate for marker development. The present study revealed that the tri-nucleotide repeats were found to be the most abundant (69.1%) to di-nucleotide (12.8%) and tetra-nucleotide repeats (8.2%). This was also in agreement with the earlier reports, wherein tri-nucleotide found to be most abundant (57.88%) than the di-nucleotide (36.87%) (Singh et al. 2016). The dominancy of tri-nucleotide SSR repeats was also observed in other plants such as Curcuma alismatifolia (Taheri et al. 2019), Elymus L. (Zhang et al. 2019), Panicum miliaceum L. (Hou et al. 2017), Capsicum annuum (Lu et al. 2012); however, di-nucleotide SSR was found to be dominant in Notopterygium incisum (Jia et al. 2019), Brassica rapa L. spp. pekinensis (Ding et al. 2015). This discrepancy might be due to search criteria, algorithm used and selection constraints of plant species (Shi et al. 2017). The preponderance of tri-nucleotide repeat motifs considered to be usual for SSRs, as insertions or deletions inside translated regions do not interact with open reading frames, whereas frame-shift mutations restrict the expansion of other motif forms (Wang et al. 2018). The AAG/CTT repeats was the most frequent tri-nucleotide repeats (10.7%) in the present data set which was also in corroboration with previous findings, where the most prevalent SSR repeats in T. cordifolia was AAG/CTT, (25.60%) (Singh et al. 2016). Among the di-nucleotide, AG/CT was the most frequently observed (3.7%) repeats, which was also in congruence with the earlier studies (Singh et al. 2016; Zheng et al. 2013).
The allele range and average number of alleles in the present investigation was found to be comparatively higher than the previous reports in T. cordifolia (Gargi et al. 2017; Paliwal et al. 2016; Singh et al. 2016) which indicated that the accessions used have higher level of genetic variability. The present study also revealed significantly higher average PIC value (0.68) and five SSRs out of 30 showed > 0.8 PIC value indicated high polymorphism. Furthermore, the HWE analysis indicated that the SSRs was randomly distributed in the genome and found suitable to be used for genetic diversity assessment. Based on allele and PIC value the SSRs, TCTSSR-59, TCTSSR-92, TCTSSR-126, TCSSR-18 and TCSSR-37 was identified as potential marker which could be utilized for genetic studies on other set of accessions of T. cordifolia. Likewise, the accessions Tc131, Tc31, Tc129, Tc38, Tc16, Tc59, Tc16, Tc60, Tc17, Tc106 and Tc130 was identified as diverse and potential accessions. These diverse accessions could be further utilized in bio-prospection programme for new biomolecules. The clustering pattern of accessions revealed that the accessions are independent of their geographical affiliations; however, majority of the accessions from Jammu & Kashmir clustered together in cluster II. The Bayesian Model-based structuring and NJ dendrogram revealed similar patterns of population structuring amongst the T. cordifolia populations which suggests that any one of these two methods are efficient to differentiate the accessions based on their genetic variations. Furthermore, the importance of genetic structure, represented by the AMOVA analysis indicated that 78% of the genetic variation resided within the populations and 22% among the three populations. The FST value (0.22) derived by AMOVA analysis supported the strong population stratification among the accessions. The discriminatory power of markers is determined by their efficiency in correct population assignments and inversely correlated with their propensity for causing false assignments (Agrama et al. 2012; Banks et al. 2003). The locus score of a marker is positively associated with the corresponding genetic diversity, PIC, and number of alleles. In the ‘‘overall’’ panel analysis, four markers with high locus scores presented more proximity towards high PICs, genetic diversity and high allele numbers. The SSR markers TCTSSR-79 and TCTSSR-124 had the lowest locus scores, because their polymorphism and diversity were the more towards lowest (PIC = 0.49 and 0.39, diversity = 0.54 and 0.40, allele number = 4, respectively) among the 30 markers. Among the nine SSR markers selected in the four panels, four highly diversified markers (TCTSSR-131, TCTSSR-126, TCTSSR-123 and TCTSSR-85) were common to more than one panel.
Conclusions
The present investigation revealed that the considerable level of genetic variability exists across the T. cordifolia accessions. The study has resulted into the identification of several genetically diverse accessions (Tc131, Tc31, Tc129, Tc38, Tc16, Tc59, Tc16, Tc60, Tc17, Tc106 and Tc130), which could be further utilized in bio-prospecting of T. cordifolia for its valuable traits. The highly informative SSRs such as TCTSSR-59, TCTSSR-92, TCTSSR-126, TCSSR-18, TCTSSR-131, TCTSSR-123 and TCSSR-37 could be utilized for sorting out the large set of germplasm.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Authors thank Director, CSIR-NBRI, Lucknow facilities and encouragement. This work was financially supported by the CSIR through HCP 010 and BSC 107 projects.
Author contributions
SL performed the experiments and prepared the manuscript, HKY and SL analyzed the data. TSR, HKY and VP designed, coordinated the experiments and improved the manuscript.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest in the publication.
Contributor Information
Suchita Lade, Email: suchitalade.sl@gmail.com.
Veena Pande, Email: veena_kumaun@yahoo.co.in.
Tikam Singh Rana, Email: ranats@nbri.res.in.
Hemant Kumar Yadav, Email: h.yadav@nbri.res.in.
References
- Agrama HA, McClung AM, Yan W. Using minimum DNA marker loci for accurate population classification in rice (Oryza sativa L.) Mol Breed. 2012;29:413–425. [Google Scholar]
- Ahmed SM, Verma V, Qazi PH, Ganaie MM, Bakshi SK, Qazi GN. Molecular phylogeny in Indian Tinospora species by DNA based molecular markers. Plant Syst Evol. 2006;256:75–87. [Google Scholar]
- Bakatoushi REL, Ahmed DGA. Evaluation of genetic diversity in wild populations of Peganum harmala L., a medicinal plant. J Genet Eng Biotechol. 2018;16:143–151. doi: 10.1016/j.jgeb.2017.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks MA, Eichert W, Olsen JB. Which genetic loci have greater population assignment power? Bioinformatics. 2003;19:1436–1438. doi: 10.1093/bioinformatics/btg172. [DOI] [PubMed] [Google Scholar]
- Dhama K, Sachan S, Khandia R, Munjal A, Iqbal Hafiz MN, Latheef SK, Karthik K, Samad HA, Tiwari R, Dadar M. Medicinal and beneficial health applications of Tinospora cordifolia (Guduchi): a miraculous herb countering various diseases/disorders and its immunomodulatory effects. Bentham Sci. 2016;10(2):96–111. doi: 10.2174/1872214811666170301105101. [DOI] [PubMed] [Google Scholar]
- Ding Q, Li J, Wang F, Zhang Y, Li H, Zhang J, Gao J. Characterization and development of EST-SSRs by deep transcriptome sequencing in Chinese Cabbage (Brassica rapa L. ssp. Pekinensis) J Genomics. 2015;20:473028. doi: 10.1155/2015/473028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earl DA, Von Holdt BM. STRUCTURE HARVESTER: a website and program for visualizing STRUCTURE output and implementing the Evano method. Conserv Genet Resour. 2012;4:359–366. [Google Scholar]
- Evanno G, Regnaut S, Goudet J. Detecting the number of clusters of individuals using the software STRUCTURE: a simulation study. Mol Ecol. 2005;14:2611–2620. doi: 10.1111/j.1365-294X.2005.02553.x. [DOI] [PubMed] [Google Scholar]
- Excoffier L, Hofer T, Foll M. Detecting loci under selection in a hierarchically structured population. Heredity. 2009;103:285–298. doi: 10.1038/hdy.2009.74. [DOI] [PubMed] [Google Scholar]
- Fu YB. Understanding crop genetic diversity under modern plant breeding. Theor Appl Genet. 2015;128:2131–2142. doi: 10.1007/s00122-015-2585-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gargi M, Thakur S, Anand SS, Choudhary S, Bhardwaj P. Development and characterization of genomic microsatellite markers in Tinospora cordifolia. J Genet. 2017;8(96):e25–e30. doi: 10.1007/s12041-017-0777-8. [DOI] [PubMed] [Google Scholar]
- Ghosh AK, Martyr CD, Steffey M, Wang YF, Agniswamy J, Amano M, et al. Design of substituted bis-tetrahydrofuran (bis-THF)-derived potent HIV-1 protease inhibitors, protein-ligand X-ray structure, and convenient syntheses of bis-THF and substituted bis-THF ligands. ACS Med Chem Lett. 2011;2:298–302. doi: 10.1021/ml100289m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta R, Sharma V. Ameliorative effects of Tinospora cordifolia root extract on histopathological and biochemical changes induced by aflatoxin-b (1) in mice kidney. Toxicol Int. 2011;18:94–98. doi: 10.4103/0971-6580.84259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamza H, Abederrahim MAB, Elbekkay M, Ferchichi A. Comparison of the effectiveness of ISSR and SSR markers in determination of date palm (Phoenix dactylifera L.) agronomic traits. Aust J Crop Sci. 2013;7(6):763–769. [Google Scholar]
- Hou S, Sun Z, Li Y, Wang Y, Ling H, Xing G, Han Y, Li H. Transcriptomic analysis, genic SSR development and genetic diversity of Proso Millet (Panicum miliaceum; Poaceae) Appl Plant Sci. 2017;5(7):1600137. doi: 10.3732/apps.1600137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia Y, Bai JQ, Liu ML, Jiang ZF, Wu Y, Fang MF, Li ZH. Transcriptome analysis of the endangered Notopterygium incisum: cold-tolerance gene discovery and identification of EST-SSR and SNP markers. Plant Divers. 2019;41(1):1–6. doi: 10.1016/j.pld.2019.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalia RK, Rai MK, Kalia S, Singh R, Dhawan AK. Microsatellite markers: an overview of the recent progress in plants. Euphytica. 2010;177:309–334. [Google Scholar]
- Kalpesh I, Mohan JSS. Assessment of genetic diversity in the medicinal climber Tinospora cordifolia (Willd.) Miers (Menispermaceae) from Gujarat India. Afr J Biotechnol. 2009;8:6499–6505. [Google Scholar]
- Kumar V, Singh S, Singh A, Dixit AK, Srivastava B, Sidhu GK, Singh R, Meena AK, Sigh RP, Subhose V, Prakash O. Phytochemical, antioxidant, antimicrobial, and protein binding qualities of hydro-ethanolic extract of Tinospora cordifolia. JBAP. 2018;8(3):192–200. [Google Scholar]
- Kumawat NK, Dushyant K, Kumar VJ, Padmapriya B, Manoj NT. Tinospora cordifolia: a wonderful miracle herb of 21st century of India. Medicinal plants. Int J Phytomed Relat Ind. 2019;11(2):117–122. [Google Scholar]
- Lade S, Sikarwar PS, Ansari A, Khatoon S, Kumar N, Yadav HK, Ranade SA. Diversity in a widely distributed dioecious medicinal plant, Tinospora cordifolia (Willd.) Miers. ex. Hook F. and Thomas. Current Sci. 2018;114(7):1520–1526. [Google Scholar]
- Li L, Wanapu C, Huang X, Huang T, Li Q, Peng Y, Huang G. Comparison of AFLP and SSR for genetic diversity analysis of Brassica napus hybrids. J Agric Sci. 2011;3(3):101–110. [Google Scholar]
- Liu K, Muse SV. Power Marker: an integrated analysis environment for genetic marker analysis. Bioinformatics. 2005;21:2128–2129. doi: 10.1093/bioinformatics/bti282. [DOI] [PubMed] [Google Scholar]
- Lu FH, Cho MC, Park YJ. Transcriptome profiling and molecular marker discovery in red pepper, Capsicum annuum L. TF68. Mol Biol Rep. 2012;39:3327–3335. doi: 10.1007/s11033-011-1102-x. [DOI] [PubMed] [Google Scholar]
- Malik A, Arya A, Kaushik V, Sindhu A. Studies on genetic variability of Tinospora cordifolia collected from different agroclimatic zones of Haryana using RAPD markers. Res Crop. 2019;20(2):407–412. [Google Scholar]
- Mittal J, Sharma MM, Batra A. Tinospora cordifolia: a multipurpose medicinal plant—a review. J Med Plants. 2014;2(2):32–47. [Google Scholar]
- Nazneen HF, Naik BA, Ramesh P, Sekhar AC, Shankar PC. Genetic diversity using random amplified polymorphic DNA (RAPD) and inter-simple sequence repeats (ISSR) markers in Tinospora cordifolia from the Rayalseema region in Andhra Pradesh. Afr J Biotechnol. 2019;18(10):231–241. [Google Scholar]
- Paliwal R, Singh R, Singh AK, Kumar S, Kumar A, Majumdar RS. Molecular characterization of Giloe (Tinospora cordifolia (Willd.) Miers ex Hook F & Thoms.) accessions using Start Codon Targeted (SCoT) markers. Int J Med Arom Plants. 2013;3(4):413–422. [Google Scholar]
- Paliwal R, Singh R, Choudhary DR, Singh AK, Kumar S, Kumar A, Bhatt KC, Singh R, Mahato AK, Singh NK, Singh R. Development of genomic simple sequence repeats (g-SSR) markers in Tinospora cordifolia and their application in diversity analysis. Plant Gene. 2016;5:118–125. [Google Scholar]
- Patel MB, Mishra S. Hypoglycemic activity of alkaloidal fraction of Tinospora cordifolia. Phyto Med. 2011;18:045–052. doi: 10.1016/j.phymed.2011.05.006. [DOI] [PubMed] [Google Scholar]
- Perrier X, Flori A, Bonnot F. Data analysis methods. In: Hamon P, Seguin M, Perrier X, Glaszmann JC, editors. Genetic diversity of cultivated tropical plants. Enfield: Enfield Science Publishers Montpellier; 2003. pp. 43–76. [Google Scholar]
- Porebski S, Bailey GL, Baum BR. Modification of a CTAB DNA extraction protocol for plants containing high polysaccharide and polyphenols components. Plant Mol Biol Rep. 1997;15:8–15. [Google Scholar]
- Rana V, Thakur K, Sood R, Sharma V, Sharma TR. Genetic diversity analysis of Tinospora cordifolia germplasm collected from north western Himalayan region of India. J Genet. 2012;91:99–103. doi: 10.1007/s12041-012-0137-7. [DOI] [PubMed] [Google Scholar]
- Rana JC, Chahota RK, Sharma V, Rana M, Verma N, Verma B, Sharma TR. Genetic diversity and structure of Pyrus accessions of Indian Himalayan region based on morphological and SSR markers. Tree Genet Genomes. 2015;11:821. [Google Scholar]
- Saha S, Ghosh S. Tinospora cordifolia: one plant, many roles. Anc Sci Life. 2012;31(4):151. doi: 10.4103/0257-7941.107344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma RK, Gupta P, Sharma V, Sood A, Mohapatra T, Ahuja PS. Evaluation of rice and sugarcane SSR markers for phylogenetic and genetic diversity analyses in bamboo. Genome. 2008;51:91–103. doi: 10.1139/g07-101. [DOI] [PubMed] [Google Scholar]
- Sharma V, Rana M, Katoch M, Sharma PK, Ghani M, Rana JC, Sharma TR, Chahota RK. Development of SSR and ILP markers in horse gram (Macrotyloma uniflorum), their characterization, cross-transferability and relevance for mapping. Mol Breed. 2015;35:102. [Google Scholar]
- Sharma P, Dwivedee BP, Bisht D, Dash AK, Kumar D. The chemical constituents and diverse pharmacological importance of Tinospora cordifolia. Heliyon. 2019;5:e02437. doi: 10.1016/j.heliyon.2019.e02437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi T, Li R, Chen Q, Li Y, Pan F, Chen Q. De novo sequencing of seed transcriptome and development of genic-SSR markers in common buckwheat (Fagopyrum esculentum) Mol Breed. 2017;37:147. [Google Scholar]
- Shinde VM, Dhalwal K. DNA fingerprinting of Tinospora cordifolia using RAPD analysis. J Global Pharma Techol. 2010;2:38–42. [Google Scholar]
- Singh K, Kadyan S, Panghal M, Yadav JP. Assessment of genetic diversity in Tinospora cordifolia by inter simple sequence repeats (ISSR) and expressed sequence tagged- simple sequence repeats (EST-SSR) Int J Pharm Pharm Sci. 2014;6(10):520–524. [Google Scholar]
- Singh R, Kumar R, Mahato AK, Paliwal R, Singh AK, Kumar S, Marla SS, Kumar A, Singh NK. De novo transcriptome sequencing facilitates genomic resource generation in Tinospora cordifolia. Funct Integr Genomics. 2016;16(5):581–591. doi: 10.1007/s10142-016-0508-x. [DOI] [PubMed] [Google Scholar]
- Spandana U, Ali SL, Nirmala T, Santhi M, SipaiBabu SD. A reviewon Tinospora cordifolia. Int J Curr Pharm Rev Res. 2013;4(2):61–68. [Google Scholar]
- Srivastava P. Tinospor cordifolia (Amrita)-A miracle herb and lifeline to many diseases. Int J med Arom Plants. 2011;1(2):57–61. [Google Scholar]
- Taheri S, Abdullah TL, Rafii MY, Harikrishna JA, Werbrouck SPO, Teo CH, Sahebi M, Azizi P. De novo assembly of transcriptomes, mining, and development of novel EST-SSR markers in Curcuma alismatifolia (Zingiberaceae family) through Illumina sequencing. Sci Rep. 2019;9:3047. doi: 10.1038/s41598-019-39944-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiel T, Michalek W, Varshney RK, Graner A. Exploiting EST databases for the development and characterization of gene-derived SSR-markers in barley (Hordeum vulgare L.) Theor Appl Genet. 2003;106:411–422. doi: 10.1007/s00122-002-1031-0. [DOI] [PubMed] [Google Scholar]
- Thomas A, Rajesh EK, Suresh Kuma D. The significance of Tinospora crispa in treatment of diabetes mellitus. Phyto Ther Res. 2016;30:357–366. doi: 10.1002/ptr.5559. [DOI] [PubMed] [Google Scholar]
- Wang H, Lei Y, Yan L, Wan L, Cai Y, Yang Z, Lv J, Zhang X, Xu C, Liao B. Development and validation of simple sequence repeat markers from Arachis hypogaea transcript sequences. Crop J. 2018;6:172–180. [Google Scholar]
- Zhang Z, Xie W, Zhao Y, Zhang J, Wang N, Ntakirutimana F, Yan J, Wang Y. EST-SSR marker development based on RNA-sequencing of E. sibiricus and its application for phylogenetic relationships analysis of seventeen Elymus species. BMC Plant Biol. 2019;19:235. doi: 10.1186/s12870-019-1825-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng X, Pan C, Diao Y, You Y, Yang C, Hua Z. Development of microsatellite markers by transcriptome sequencing in two species of Amorphophallus (Araceae) BMC Genomics. 2013;14(1):490. doi: 10.1186/1471-2164-14-490. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.