Abstract
A new composite film was developed by combining Gelidium corneum (GC) with chitosan to enhance the physicochemical characteristics of GC film. In addition, to confer new functional property on the GC-chitosan composite film, various amounts (0.5%, 1.0%, and 1.5%) of java citronella essential oil (JCEO) were incorporated into the film. As the concentration of JCEO increased, the extensibility of the GC-chitosan film improved. In addition, the film became more opaque due to decreased light transmission. Especially, ultraviolet light was completely blocked in the composite films containing JCEO. Radical scavenging activities of the films also increased with increasing JCEO content, indicating that the films have antioxidant activity. Therefore, GC-chitosan composite film containing JCEO is applicable in food packaging to preserve food quality by retarding lipid oxidation.
Keywords: Antioxidant activity, Chitosan, Composite film, Gelidium corneum, Java citronella essential oil
Introduction
Eco-friendly packaging materials have been extensively studied as a replacement for synthetic plastic packaging materials to prevent environmental pollution (Andretta et al., 2019). To develop environment friendly packaging films, polysaccharides as a biodegradable polymer have been used as a film base material (Ortega et al., 2014).
Gelidium corneum (GC) is a red alga that consists of a large amount of agar, and there have been a few studies conducted on the properties of its film (Hong et al., 2009; Jang et al., 2011; Ku et al., 2008; Lim et al., 2010a; 2010b). GC films have been known to have poor mechanical properties (Lim et al., 2010a; 2010b). Ku et al. (2008) reported that the tensile strength (TS) value of GC extract film prepared from GC bleached with chlorine dioxide and treated with ozone gas was 2.32 MPa. It was also reported that films prepared with whole GC including pulp fraction had better mechanical properties than GC extract films (Jang et al., 2011; Lim et al., 2010a). In addition, GC films blended with other proteins have improved rigidity, and the TS value was increased to 6.23 MPa after blending with gelatin (Hong et al., 2009) Thus, the mechanical properties of GC film can be enhanced by combining them with other biopolymers to develop composite films.
Chitosan, a deacetylated chitin, is an abundant and biodegradable material with a good film-forming ability. In particular, chitosan films have good mechanical properties (Martínez Camacho et al., 2010). Chitosan can also hold aromatic compounds because it forms hydrogen bonds with the constituents of essential oils (Xu et al., 2018). However, there is currently no study on the use of chitosan in the development of GC composite films for improved physicochemical characteristics.
To develop a novel packaging with functional properties, incorporation of active materials into biodegradable films have been studied (Andretta et al., 2019). In particular, essential oils incorporated into biodegradable films can retard lipid oxidation in foods because essential oils can confer antioxidant effects due to their high amount of phenolic compounds (Benavides et al., 2012). Among them, java citronella essential oil (JCEO) is known to have antioxidant properties due to the presence of citronellal, citronellol, and geraniol (Teixeira et al., 2013).
This study was conducted to develop a GC-chitosan composite film as a novel biodegradable film, which has not been studied yet, in order to improve the physicochemical properties of GC film. In addition, to confer an antioxidant activity to the composite film, JCEO was added as an active material to develop a packaging film with functional properties to inhibit the deterioration of food quality caused by lipid oxidation.
Materials and methods
Materials
As a film-base material, GC was harvested in Jeju, Korea. JCEO was obtained from Gooworl Co. (Daegu, Korea). Chitosan (deacetylation 75–85%) was purchased from Showa Chemical (Tokyo, Japan). Fructose, ABTS, and DPPH were purchased from Sigma-Aldrich (St. Louis, MO, USA). Tryptic soy broth (TSB), brain heart infusion (BHI), and nutrient broth (NB) were purchased from Becton–Dickinson Co. (Sparks, NV, USA).
GC powder preparation
GC (100 g) was washed to remove contaminants, mixed with distilled water (1:10, w/v), heated at 120 °C for 2 h, and dried for 24 h (Lim et al., 2010a). After drying, the dried GC was ground and sieved through an 80-mesh sieve.
Preparation of composite films
The ratio of GC and chitosan as well as the concentrations of GC and fructose were determined based on preliminary experiments. GC powder (1.5%, w/v) and fructose (1.5%, w/v) as a plasticizer were dispersed in distilled water and heated while stirring at 90 °C for 30 min. To make chitosan solution, chitosan (1 g) was dissolved in acetic acid solution (100 mL) and stirred for 6 h. After the filtration of each solution, GC solution and chitosan solution were mixed at a ratio of 7:3 (v/v). JCEO (0.5%, 1.0%, and 1.5%) and Tween 80 (0.2 g/g essential oil) were then added to the solution. The solution was sonicated and homogenized at 8,000 rpm for 3 min using a homogenizer (IKA, Staufan, Germany). The solution was filtered using a four-layer gauze, and the filtrate (35 mL) was cast onto the glass plate (11 cm × 12.5 cm). After drying at 25 °C for 16 h, the obtained films were conditioned for a day (25 °C, 50% relative humidity (RH)).
Thickness, TS, and elongation at break (EB)
The films were cut into pieces (2.5 cm × 10.5 cm) and a thickness gauge (Mitutoyo, Tokyo, Japan) was used to determine the thickness of the films. An Instron universal testing machine (Testometric Co., Lancashire, UK) was used for the measurement of TS and EB.
Moisture content (MC) and water solubility (WS)
The film (2 cm × 2 cm) was weighed, dried at 105 °C for a day, and re-weighed. The difference in the weight represents the MC of the film. To obtain WS, the film was dried and immersed in distilled water for a day with shaking, and then re-dried at 105 °C. The WS was determined as the difference between the weights of the film.
Water vapor permeability (WVP)
The film (2 cm × 2 cm) was attached to the top of a polymethylacrylate cup filled with distilled water (15 mL). The cup weight was measured every 1 h, and WVP value was obtained after 8 h at 25 °C and 50% RH.
Optical properties
To evaluate the color, each film was analyzed using a colorimeter (CR-400 M, Minolta, Tokyo, Japan). Opacity was obtained by measuring the absorbance at 600 nm using a spectrophotometer (UV-2450, Shimadzu Co., Kyoto, Japan). To evaluate the light transmittance, the absorbance was measured at a wavelength range of 200 nm to 800 nm.
Antioxidant activity
The ABTS radical scavenging activity of the film was determined based on an established method (Bonilla et al., 2013). The film extract (0.1 g film in 10 mL distilled water) was prepared. ABTS solution was diluted until the absorbance was 0.7. The film extract (60 µL) and diluted ABTS solution (2940 µL) were mixed and left to react in the dark. After 10 min, the absorbance was measured at 734 nm. And DPPH radical scavenging activity was also evaluated (Hashemi and Khaneghah, 2017). The film extract (0.1 mL) and 1 mM DPPH solution (3.9 mL) were mixed and reacted for 1 h, and the absorbance was measured at 517 nm.
Fourier transform infrared (FTIR) analysis
The FTIR analysis was conducted using a vacuum infrared spectrometer (Billerica, MA, USA). The spectra were obtained at wavenumber range of 400–4000/cm.
Thermal stability
Thermogravimetric analysis (TGA) and derivative thermogravimetric (DTGA) analysis were performed using a TGA analyzer (Mettler Toledo, Columbus, OH, USA) to examine the thermal stability of the films. The films were heated from 25 to 700 °C. The weight loss (%) and the residues of the film (%) were measured.
Statistical analysis
SAS software (SAS Institute Inc., Cary, NC, USA) was used for analysis of experimental data and all values are expressed as mean ± standard deviation. All experiments were carried out at least five times.
Results and discussion
Mechanical properties
As a preliminary experiment, a neat GC film was prepared and its mechanical properties were determined (data not shown). The TS of the prepared GC film was 2.91 MPa, which is similar to the value reported in a previous study (Ku et al., 2008), indicating that the rigidity of the GC film needs to be improved for application as a food packaging material. Meanwhile, the TS of a chitosan film was 38.18 MPa, which suggests that chitosan films have better mechanical properties than neat GC films. Therefore, chitosan was combined with GC to make a composite film with enhanced rigidity. The TS of the GC-chitosan composite film was 20.21 MPa, which is approximately seven-fold of that of the neat GC film. The TS and EB of the GC-chitosan composite films were also affected by the addition of JCEO (Table 1). With increasing concentration of JCEO, the TS and EB of the composite film decreased and increased, respectively. However, there was no significant (p < 0.05) difference between the composite films containing 1.0% and those containing 1.5% JCEO. The TS and EB values were 20.21 MPa and 21.34%, respectively, for the GC-chitosan composite film without JCEO, whereas the composite film containing 1.5% JCEO had decreased TS (10.62 MPa) and increased EB (25.02%). Because JCEO can function as a plasticizer, the composite film network could be weakened, resulting in enhanced flexibility of the films. In addition, JCEO exists in the form of droplets inside the film, which can easily be deformed and increase the extensibility of the films, similar to alginate films containing oregano essential oil (Benavides et al., 2012).
Table 1.
Physical properties of the GC-chitosan composite films containing JCEO
| JCEO (%) | Thickness (mm) | Tensile strength (MPa) | Elongation at break (%) | Water solubility (%) | Moisture content (%) | Water vapor permeability (10−9 g/m s Pa) |
|---|---|---|---|---|---|---|
| 0 | 0.076 ± 0.005b | 20.21 ± 0.68a | 21.34 ± 0.65b | 19.44 ± 0.53c | 20.97 ± 1.09c | 4.01 ± 0.08b |
| 0.5 | 0.085 ± 0.001a | 17.06 ± 0.86b | 23.54 ± 0.77ab | 19.64 ± 0.52c | 21.14 ± 0.44c | 4.32 ± 0.26b |
| 1 | 0.087 ± 0.003a | 13.32 ± 1.15c | 24.01 ± 0.95a | 25.68 ± 1.40b | 25.46 ± 1.38b | 4.82 ± 0.78ab |
| 1.5 | 0.090 ± 0.003a | 10.62 ± 2.44c | 25.02 ± 2.00a | 28.16 ± 0.72a | 28.56 ± 0.65a | 5.31 ± 0.18a |
Mean ± S.D., n = 5
a–cAny means in the same column followed by different letters are significantly (p <0.05) different by Duncan’s multiple range test
WS and MC
The WS of the films is an important characteristic because it is associated with the biodegradability of the films (Maizura et al., 2007). The WS and MC of GC-chitosan composite films are represented in Table 1. There was no significant (p < 0.05) difference between the WS and MC of the films without JCEO and those of the films with 0.5% JCEO. In contrast, the addition of 1.0% JCEO and 1.5% JCEO increased the WS and MC of the films. The WS and MC were 25.68% and 25.46% for the composite film containing 1.0% JCEO, and they were 28.16% and 28.56% for the film containing 1.5% JCEO, respectively. Thus, the addition of JCEO above 1% affected both WS and MC. The JCEO droplets could loosen the film network, causing water molecules to diffuse into the film and increasing the WS (Hosseini et al., 2009). Jouki et al. (2014) also explained that the weakened film network resulted in an increase in the soluble matter of quince seed mucilage films in water. Furthermore, the addition of JCEO loosened the film matrix, resulting in increased MC.
WVP
Water barrier property is a parameter to decide the shelf life of foods. Addition of JCEO to the composite film in this study affected the WVP of the film. As the concentration of JCEO increased, the WVP increased. There was no significant difference between the composite film and the composite film containing 0.5% of JCEO, whereas the WVP of the composite film containing 1.5% JCEO increased from 4.01 × 10−9 g/m s Pa to 5.31 × 10−9 g/m s Pa. This increase can be explained by a loose film matrix and increased water evaporation by the addition of JCEO, thereby enhancing the passage of water through the films (Atef et al., 2015). In addition, loose film network could be formed because water molecules act as a plasticizer (Maizura et al., 2007), resulting in higher WVP with the addition of JCEO.
Optical properties
The optical property of the films influences the appearance and the application of the films. With increasing concentration of JCEO, L* and a* value of the composite films decreased, whereas b* value, ΔE, and opacity increased (Table 2). The L* value was the lowest (90.53) and b* value was the highest (22.24) for the GC-chitosan composite film containing 1.5% JCEO owing to the yellow color of JCEO. In addition, as the concentration of JCEO increased, the film became more opaque, which means that there was a reduction in light transmittance (Fig. 1). With increasing JCEO concentration, the transmittance decreased in the range between 360 nm and 800 nm. Furthermore, in the UV light region, the films containing JCEO completely blocked the light, whereas the film without JCEO could not block the light. Light transmittance was affected by the interactions between the polymer chains and JCEO as well as the distribution of JCEO among the polymer chains because light scattering could occur at the interface of essential oil droplets (Atef et al., 2015). Thus, the GC-chitosan composite films containing JCEO could block UV and visible light effectively, and these characteristics could delay lipid oxidation in foods.
Table 2.
Optical properties of the GC-chitosan composite films containing JCEO
| JCEO (%) | L* | a* | b* | ΔE | Opacity (A/mm) |
|---|---|---|---|---|---|
| 0 | 93.75 ± 0.40a | − 1.99 ± 0.08a | 13.14 ± 0.60a | – | 7.71 ± 0.10c |
| 0.5 | 92.60 ± 0.09b | − 2.25 ± 0.07b | 16.45 ± 0.28b | 3.39 ± 0.27c | 8.14 ± 0.40c |
| 1.0 | 91.65 ± 0.16c | − 2.66 ± 0.04c | 20.18 ± 0.39c | 7.24 ± 0.42b | 10.34 ± 0.31b |
| 1.5 | 90.53 ± 0.27d | − 2.86 ± 0.21c | 22.24 ± 0.41d | 9.57 ± 0.44a | 13.39 ± 0.96a |
Mean ± S.D., n = 5
a–dAny means in the same column followed by different letters are significantly (p <0.05) different by Duncan’s multiple range test
Fig. 1.
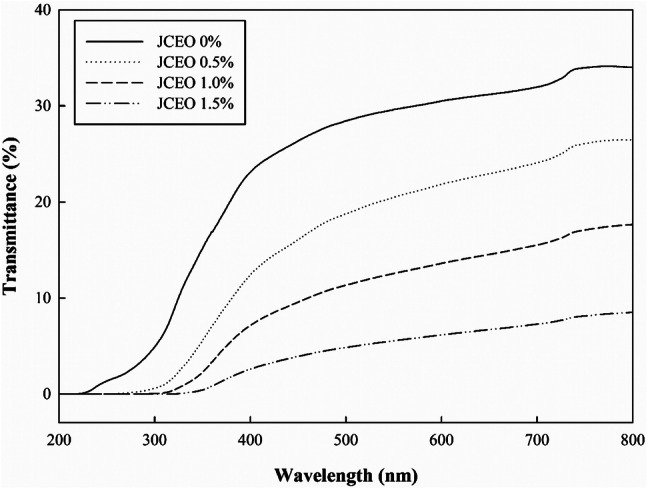
UV-visible light transmittance of the GC-chitosan composite films containing JCEO
Antioxidant activities
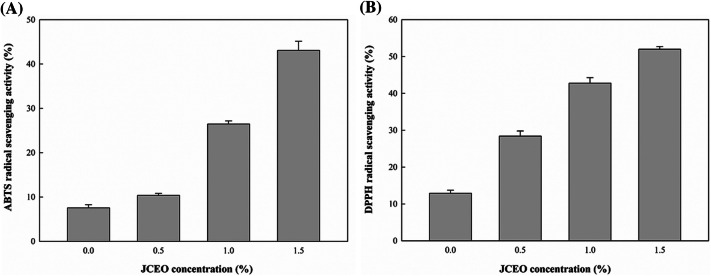
The radical scavenging activities of the GC-chitosan composite films were evaluated (Fig. 2). The composite film without JCEO showed some antioxidant activities (7.5% and 12.91% for ABTS and DPPH, respectively) because free radicals could interact with residual amino groups of chitosan (Siripatrawan and Harte, 2010). Similarly, Moradi et al. (2012) observed 12% DPPH radical scavenging activity in neat chitosan films. As the amount of JCEO in the composite film increased, antioxidant activities also increased. ABTS and DPPH radical scavenging activities of the composite film containing 1.5% JCEO were 43.08% and 51.98%, respectively. Increase in the antioxidant activities of the film containing JCEO is mainly due to the components of JCEO, such as citronellol and geraniol, which have antioxidant activity (Teixeira et al., 2013). Baek et al. (2018) also reported that the antioxidant activities of the films containing essential oils such as cinnamon essential oils are influenced by the components of the essential oils. According to a previous study (Pires et al., 2013), the DPPH radical scavenging activity of hake protein films with citronella essential oil was reported to be approximately 70%. Thus, these results indicate that GC-chitosan composite films containing JCEO can be effective in delaying lipid oxidation in foods owing to the radical scavenging activities.
Fig. 2.
Antioxidant activities of the GC-chitosan composite films containing JCEO. (A) ABTS radical scavenging activity, (B) DPPH radical scavenging activity
FTIR analysis
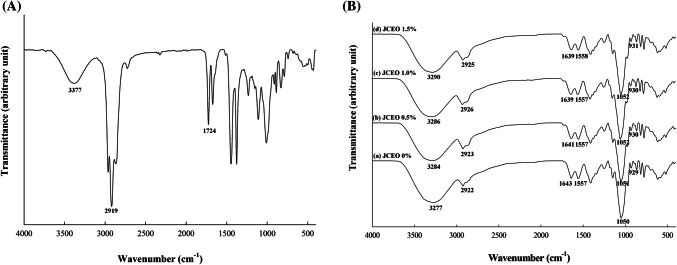
To examine the molecular interactions between the GC-chitosan composite film matrix and JCEO, FTIR analysis was performed. The spectrum of JCEO is shown in Fig. 3a. The band at 3377/cm corresponds to hydroxyl group stretching, and the band at 2919/cm is associated with methylene C–H stretching (Wany et al., 2014). And the band at 1724/cm is owing to aldehyde or ketone groups from phenolic compounds in JCEO (Wany et al., 2014). In contrast, in the spectra of the GC-chitosan composite film (Fig. 3b), the band at 3277/cm corresponds to hydroxyl group stretching vibration and N–H stretching (Shen and Kamdem, 2015) and the band at 2922/cm is related to C=O and C–H stretching vibration (Cao et al., 2018). The band at 1639/cm indicates the stretching of conjugated peptide bonds (Jumaidin et al., 2016), and the band at 1557/cm corresponds to N–H bending that is from amine groups of chitosan (Shen and Kamdem, 2015). And the bands observed at 1050/cm and 929/cm are associated with C–O stretching of 3,6-anhydro-galactose. Jumaidin et al. (2016) and Freile Pelegrín et al. (2007) also explained that these bands are characteristics of agar molecules. The addition of JCEO to the GC-chitosan composite film affected the interactions and chemical bonds of film components. The band at 3277/cm was shifted to 3284, 3286, and 3290/cm for the composite films containing 0.5%, 1.0%, and 1.5% JCEO, respectively. The changes in the band were attributed to the interactions between JCEO and GC/chitosan by the addition of JCEO, indicating that the amount of hydrogen bonds increased between hydroxyl groups of JCEO and hydroxyl groups or amine groups of GC and chitosan (Cao et al., 2018). The intensity of the band at 2922/cm also increased proportionally with increasing JCEO concentration, which was associated with C–H stretching of the JCEO. In addition, in the spectra of the composite films, the band of JCEO at 1724/cm disappeared and new bands appeared at 1643/cm and 1557/cm. These changes were mainly due to the intermolecular interactions between aldehyde or ketone groups of JCEO and amine groups of chitosan molecules, and subsequently the amount of added JCEO affected the intensity and wavenumber of these bands (Jumaidin et al., 2016).
Fig. 3.
FTIR spectra of JCEO and the GC-chitosan composite films containing JCEO. (A) JCEO, (B) GC-chitosan composite films containing JCEO
TGA analysis
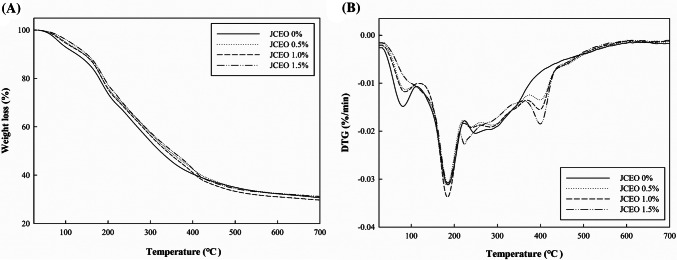
To investigate the thermal stability of the GC-chitosan composite films containing JCEO, TGA analysis was performed (Fig. 4). Thermal decomposition of the films took place in four stages. The first stage occurred at about 80–90 °C as water and acetic acid molecules were evaporated. The second stage occurred because of the degradation of low molecular weight molecules and plasticizers. In the third stage, the dehydration of saccharides rings and the decomposition of polymers occurred at 200–300 °C (Pires et al., 2013; Shen and Kamdem, 2015). In the range between 80 °C and 400 °C, the composite films containing JCEO showed less weight loss than the film without JCEO, indicating that the films containing JCEO had slightly higher thermal stability because the interactions between the polymer chains and JCEO could retard the degradation of the films (Xu et al., 2019). The fourth decomposition of the films did not appear in the control film, but in the films containing JCEO. The decomposition of the components of JCEO and surfactants, which are stable at high temperature, occurred at this stage. This stage was similarly observed by Shen and Kamdem (2015), who reported that the decomposition of the components of citronella essential oil and Tween 80 that are stable at high temperature occurred, leaving 34% residue after decomposition. In this study, after the decomposition of the GC-chitosan composite films at above 400 °C, the amount of residue was approximately 30% for the composite films.
Fig. 4.
TGA (A) and DTGA (B) of the GC-chitosan composite films containing JCEO
In conclusion, the GC-chitosan composite film containing JCEO was developed as a new packaging material in this study. Compared with a neat GC film, the developed GC-chitosan composite film in this study had an improved TS. Additionally, the extensibility of the composite film was increased with the addition of JCEO and light-blocking ability was improved. The composite films containing JCEO also exhibited radical scavenging activities, which could retard the lipid oxidation of foods. Therefore, the developed GC-chitosan composite films with JCEO is applicable as an active biodegradable packaging material.
Acknowledgements
This study was supported by a Grant from the National Research Foundation of Korea.
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Eun-Jeong Go, Email: ko8546882@naver.com.
Kyung Bin Song, Email: kbsong@cnu.ac.kr.
References
- Andretta R, Luchese CL, Tessaro IC, Spada JC. Development and characterization of pH–indicator films based on cassava starch and blueberry residue by thermocompression. Food Hydrocoll. 2019;93:317–324. doi: 10.1016/j.foodhyd.2019.02.019. [DOI] [Google Scholar]
- Atef M, Rezaei M, Behrooz R. Characterization of physical, mechanical, and antibacterial properties of agar–cellulose bionanocomposite films incorporated with savory essential oil. Food Hydrocoll. 2015;45:150–157. doi: 10.1016/j.foodhyd.2014.09.037. [DOI] [Google Scholar]
- Baek SK, Kim S, Song K. Characterization of Ecklonia cava alginate films containing cinnamon essential oils. Int. J. Mol. Sci. 2018;19:3545. doi: 10.3390/ijms19113545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benavides S, Villalobos Carvajal R, Reyes JE. Physical, mechanical and antibacterial properties of alginate film: effect of the crosslinking degree and oregano essential oil concentration. J. Food Eng. 2012;110:232–239. doi: 10.1016/j.jfoodeng.2011.05.023. [DOI] [Google Scholar]
- Bonilla J, Talón E, Atarés L, Vargas M, Chiralt A. Effect of the incorporation of antioxidants on physicochemical and antioxidant properties of wheat starch–chitosan films. J. Food Eng. 2013;118:271–278. doi: 10.1016/j.jfoodeng.2013.04.008. [DOI] [Google Scholar]
- Cao T, Yang SY, Song K. Development of burdock root inulin/chitosan blend films containing oregano and thyme essential oils. Int. J. Mol. Sci. 2018;19:131. doi: 10.3390/ijms19010131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freile Pelegrín Y, Madera Santana T, Robledo D, Veleva L, Quintana P, Azamar JA. Degradation of agar films in a humid tropical climate: thermal, mechanical, morphological and structural changes. Polym. Degrad. Stabil. 2007;92:244–252. doi: 10.1016/j.polymdegradstab.2006.11.005. [DOI] [Google Scholar]
- Hashemi SMB, Khaneghah AM. Characterization of novel basil-seed gum active edible films and coatings containing oregano essential oil. Prog. Org. Coat. 2017;110:35–41. doi: 10.1016/j.porgcoat.2017.04.041. [DOI] [Google Scholar]
- Hong YH, Lim GO, Song KB. Physical properties of Gelidium corneum–gelatin blend films containing grapefruit seed extract or green tea extract and its application in the packaging of pork loins. J. Food Sci. 2009;74:C6–C10. doi: 10.1111/j.1750-3841.2008.00987.x. [DOI] [PubMed] [Google Scholar]
- Hosseini MH, Razavi SH, Mousavi MA. Antimicrobial, physical and mechanical properties of chitosan–based films incorporated with thyme, clove and cinnamon essential oils. J. Food Process Preserv. 2009;33:727–743. doi: 10.1111/j.1745-4549.2008.00307.x. [DOI] [Google Scholar]
- Jang SA, Shin YJ, Seo YB, Song KB. Effects of various plasticizers and nanoclays on the mechanical properties of red algae film. J. Food Sci. 2011;76:N30–N34. doi: 10.1111/j.1750-3841.2011.02089.x. [DOI] [PubMed] [Google Scholar]
- Jouki M, Yazdi FT, Mortazavi SA, Koocheki A. Quince seed mucilage films incorporated with oregano essential oil: physical, thermal, barrier, antioxidant and antibacterial properties. Food Hydrocoll. 2014;36:9–19. doi: 10.1016/j.foodhyd.2013.08.030. [DOI] [Google Scholar]
- Jumaidin R, Sapuan SM, Jawaid M, Ishak MR, Sahari J. Characteristics of thermoplastic sugar palm starch/agar blend: thermal, tensile, and physical properties. Int. J. Biol. Macromol. 2016;89:575–581. doi: 10.1016/j.ijbiomac.2016.05.028. [DOI] [PubMed] [Google Scholar]
- Ku KJ, Hong YH, Song KB. Mechanical properties of a Gelidium corneum edible film containing catechin and its application in sausages. J. Food Sci. 2008;73:C217–C221. doi: 10.1111/j.1750-3841.2008.00700.x. [DOI] [PubMed] [Google Scholar]
- Lim GO, Hong YH, Song KB. Application of Gelidium corneum edible films containing carvacrol for ham packages. J. Food Sci. 2010;75:C90–C93. doi: 10.1111/j.1750-3841.2009.01431.x. [DOI] [PubMed] [Google Scholar]
- Lim GO, Jung SA, Song KB. Physical and antimicrobial properties of Gelidium corneum/nano-clay composite film containing grapefruit seed extract or thymol. J. Food Eng. 2010;98:415–420. doi: 10.1016/j.jfoodeng.2010.01.021. [DOI] [Google Scholar]
- Maizura M, Fazilah A, Norziah MH, Karim AA. Antibacterial activity and mechanical properties of partially hydrolyzed sago starch–alginate edible film containing lemongrass oil. J. Food Sci. 2007;72:C324–C330. doi: 10.1111/j.1750-3841.2007.00427.x. [DOI] [PubMed] [Google Scholar]
- Martínez Camacho AP, Cortez Rocha MO, Ezquerra Brauer JM, Graciano Verdugo AZ, Rodriguez Félix F, Castillo Ortega MM, Plascencia Jatomea M. Chitosan composite films: thermal, structural, mechanical and antifungal properties. Carbohydr. Polym. 2010;82:305–315. doi: 10.1016/j.carbpol.2010.04.069. [DOI] [Google Scholar]
- Moradi M, Tajik H, Rohani SMR, Oromiehie AR, Malekinejad H, Aliakbarlu J, Hadian M. Characterization of antioxidant chitosan film incorporated with Zataria multiflora Boiss essential oil and grape seed extract. LWT-Food Sci. Technol. 2012;46:477–484. doi: 10.1016/j.lwt.2011.11.020. [DOI] [Google Scholar]
- Ortega Toro R, Jiménez A, Talens P, Chiralt A. Properties of starch–hydroxypropyl methylcellulose based films obtained by compression molding. Carbohydr. Polym. 2014;109:155–165. doi: 10.1016/j.carbpol.2014.03.059. [DOI] [PubMed] [Google Scholar]
- Pires C, Ramos C, Teixeira B, Batista I, Nunes ML, Marques A. Hake proteins edible films incorporated with essential oils: physical, mechanical, antioxidant and antibacterial properties. Food Hydrocoll. 2013;30:224–231. doi: 10.1016/j.foodhyd.2012.05.019. [DOI] [Google Scholar]
- Shen Z, Kamdem DP. Development and characterization of biodegradable chitosan films containing two essential oils. Int. J. Biol. Macromol. 2015;74:289–296. doi: 10.1016/j.ijbiomac.2014.11.046. [DOI] [PubMed] [Google Scholar]
- Siripatrawan U, Harte BR. Physical properties and antioxidant activity of an active film from chitosan incorporated with green tea extract. Food Hydrocoll. 2010;24:770–775. doi: 10.1016/j.foodhyd.2010.04.003. [DOI] [Google Scholar]
- Teixeira B, Marques A, Ramos C, Neng NR, Nogueira JM, Saraiva JA, Nunes ML. Chemical composition and antibacterial and antioxidant properties of commercial essential oils. Ind. Crop. Prod. 2013;43:587–595. doi: 10.1016/j.indcrop.2012.07.069. [DOI] [Google Scholar]
- Wany A, Kumar A, Nallapeta S, Jha S, Nigam VK, Pandey DM. Extraction and characterization of essential oil components based on geraniol and citronellol from java citronella (Cymbopogon winterianus Jowitt) Plant Growth Regul. 2014;73:133–145. doi: 10.1007/s10725-013-9875-7. [DOI] [Google Scholar]
- Xu T, Gao C, Feng X, Huang M, Yang Y, Shen X, Tang X. Cinnamon and clove essential oils to improve physical, thermal and antimicrobial properties of chitosan–gum arabic polyelectrolyte complexed films. Carbohydr. Polym. 2019;217:116–125. doi: 10.1016/j.carbpol.2019.03.084. [DOI] [PubMed] [Google Scholar]
- Xu T, Gao C, Yang Y, Shen X, Huang M, Liu S, Tang X. Retention and release properties of cinnamon essential oil in antimicrobial films based on chitosan and gum arabic. Food Hydrocoll. 2018;84:84–92. doi: 10.1016/j.foodhyd.2018.06.003. [DOI] [Google Scholar]