Abstract
We report a rare case of renal metastasis from primary hepatocellular carcinoma (HCC). A mass in the right kidney of a 71-year-old man was detected by follow-up computed tomography (CT) for HCC. He was diagnosed as having primary HCC 18 years ago and had undergone partial hepatectomy, transarterial chemoembolization, and pulmonary segmentectomy for primary HCC and its metastasis over 10 years. Eight years after this, follow-up CT revealed a right kidney mass, and laboratory testing showed an elevated level of protein induced by vitamin K absence II (PIVKA-II). We performed laparoscopic radical nephrectomy for the right kidney mass. Histopathology revealed renal metastasis from primary HCC. To date, only a small number of cases of renal metastasis from HCC have been reported.
Keywords: Renal metastasis, Hepatocellular carcinoma, Neoplasmas, Acarboxyprothrombin
Introduction
Renal metastasis is rare from primary tumors. Lung, breast, stomach and contralateral kidney are reported as primary tumor sites [1]. Although metastasis from hepatocellular carcinoma (HCC) occurs mainly in the lung, lymph nodes, peritoneum and bone [2], there are very few reports of renal metastasis from HCC [1]. In this case report, we present the clinical course of renal metastasis from HCC after 8 years of metastasis-free survival and review the previously reported cases. To our best knowledge, this is the 10th case of renal metastasis from HCC to be published in the literature.
Case report
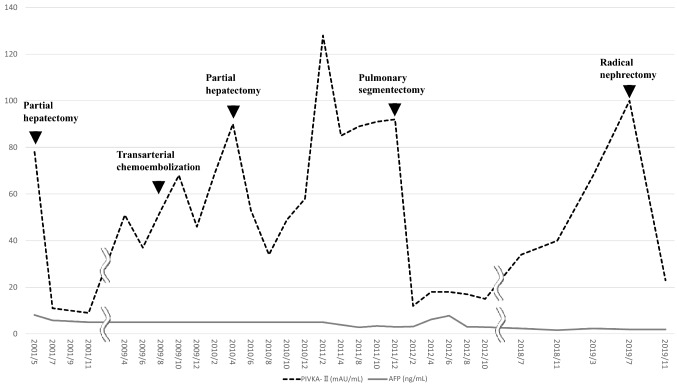
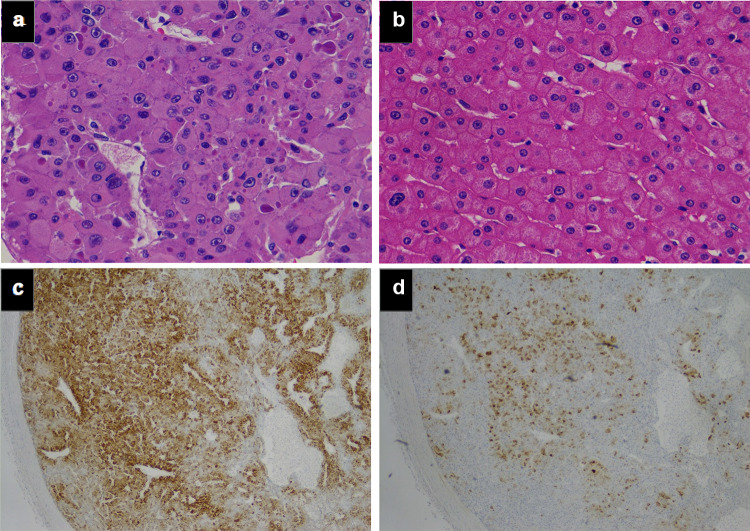
A 71-year-old man presented with an incidental right renal mass revealed by follow-up computed tomography (CT) for HCC in July 2019. The patient had suffered from hepatitis B and developed HCC in 2001. He undergone partial hepatectomy in July 2001, transarterial chemoembolization in August 2009, partial hepatectomy again in July 2010 for intrahepatic metastasis, and pulmonary segmentectomy for lung metastasis in January 2011. Follow-up CT examinations showed no recurrence or metastasis for almost 8 years. In July 2019, however, a CT scan showed a 21 × 14-mm mass in the right kidney with enhancement in the early phase and washout in the late portal phase (Fig. 1a–c). Protein induced by vitamin K absence II (PIVKA-II), one of the markers of HCC, was elevated from 34 mAU/mL (normal range, < 40 mAU/mL) in July 2018 to 100 mAU/mL in July 2019 (Fig. 2). The level of alpha-fetoprotein (AFP) was maintained under the reference value. His liver function showed Child–Pugh class A. Three months later, in October 2019, the tumor had increased to 25 × 25-mm size (Fig. 1d–f). We decided to perform radical nephrectomy, considering that the right kidney mass might be derived from HCC, because the tumor size had rapidly increased and the level of PIVKA-II was elevated. The patient thus underwent retroperitoneal laparoscopic radical nephrectomy. Surgical findings revealed no evidence of invasion to the peri-renal fat and diaphragm. The procedure resulted in 15 mL of blood loss and no perioperative complications. Hematoxylin and eosin staining revealed that the tumor had a trabecular cell pattern and large nuclei, which were similar to the findings from the primary HCC (Fig. 3a–b). Immunohistochemical staining showed the tumor to be positive for hepatocyte-paraffin 1 (Hep-par1) and glypican 3 (GPC3) (Fig. 3c–d). Hep-par1 is normally positive for hepatocytes, and GPC3 is specific for fetal liver [3, 4]. According to these findings, the tumor was diagnosed as renal metastasis from HCC. The patient was discharged 8 days after the operation. Abdominal magnetic resonance imaging (MRI) performed 1 month after surgery showed no recurrence. The patient’s level of PIVKA-II decreased to 23 mAU/mL, within the normal limit, 25 days after the radical nephrectomy (Fig. 2).
Fig. 1.
Triple-phase abdominal computed tomography (CT) for a right kidney tumor. a Plain phase in July 2019. A 21 × 14-mm mass was located in the right kidney. b Arterial phase in July 2019. Kidney mass shows partly enhancement. c Portal phase in July 2019. Mass enhancement shows slight washout. d–f Plain, arterial, portal phase in October 2019 right before the nephrectomy. Tumor size was 25 × 25 mm
Fig. 2.
Time course of the tumor marker values during the treatment period. Protein induced by vitamin K absence II (PIVKA-II) and alpha-fetoprotein (AFP) were measured every 2–4 months
Fig. 3.
Histopathological findings of the right kidney tumor and original HCC. Tumor cells obtained by radical nephrectomy. A trabecular cell pattern and large nuclei are seen (hematoxylin and eosin [H&E] staining) (a). Tumor cells from the partial hepatectomy performed in 2011 (H&E staining) (b). Positive staining for hepatocyte-paraffin 1 (c) and glypican 3 (d) of the tumor obtained by the present radical nephrectomy. Pictures a and b were showed in original magnification × 400. Pictures c and d were × 100
Discussion
Renal metastasis is very rare, and its rate of occurrence is reported to be 7.2% from autopsies of patients with any malignant disease [5]. The primary tumor is reported to be the lung (19.8%), breast (12.3%), stomach (11.1%) and contralateral kidney (8.6%) [1]. Renal metastasis from HCC is also uncommon. In search of the literature using PubMed, we could only find nine reported cases of renal metastasis from HCC [6–14]. Among these nine cases, the precise clinical course was reported in five cases (Table 1) [10–14], four of men and one of a woman. The kidney tumor size in three reports was over 40 mm (meeting the National Comprehensive Cancer Network criteria of T1b), and the kidney tumor had ruptured in two cases. Only one case reported the clinical course of tumor markers, including PIVKA-II and AFP [14]. In that case, the elevation of PIVKA-II was related to the emergence of intrahepatic and lung metastases. In our case, the level of PIVKA-II also changed depending on the status of the metastasis (Fig. 2), but the AFP remained below the standard value. The outcome in three cases was death within 3 months after initial treatment: two patients were treated with transarterial embolization and the other chose palliative care. The outcome in the other two cases was survival of more than 3 months after initial treatment: One patient was treated with open radical nephrectomy, and the other underwent transarterial embolization. Sorafenib was administered as further treatment in these two patients. Drugs target vascular endothelial growth factor receptor2 (VEGFR2) are reported as treatments for unresectable hepatocellular carcinoma. Recent clinical trials has shown that sorafenib and lenvatinib significantly improves overall survival in unresectable HCC [15] [16]. In our case, we will administer sorafenib or lenvatinib if other metastasis occur in the future.
Table 1.
Reports of renal metastasis from hepatocellular carcinoma
| References | Age | Sex | Size (mm) | Initial presentation | Other metastasis | Initial treatment | Further treatment | Diagnosis | Outcome following initial treatment |
|---|---|---|---|---|---|---|---|---|---|
| Fukushima et al. [10] | 52 | M | 78 × 86 | Gross hematuria | Intrahepatic | Transarterial embolization | Interferon-α | Needle biopsy | Died of disease at 2 months |
| Mezawa et al. [11] | 67 | M | None | Back pain | Intrahepatic, lung, brain | Transarterial embolization | Palliative | Autopsy | Died of disease at 3 months |
| Aron et al. [12] | 74 | M | None | Gross hematuria and back pain | None | Palliative | None | Needle biopsy | Died of disease at 6 weeks |
| Ong et al. [13] | 70 | M | 55 × 45 | None (routine MRI) | Intrahepatic, lung | Open radical nephrectomy | Sorafenib | Nephrectomy | Alive with disease for over 3 months |
| Kinoshita et al. [14] | 76 | F | 80 × 60 | Back pain | None | Transarterial embolization | Sorafenib | CT and tumor marker | Alive with disease for over 12 months |
| Present case | 71 | M | 21 × 14 | None (routine CT) | None | Laparoscopic radical nephrectomy | None | Nephrectomy | Alive with disease for over 3 months |
CT computed tomography, MRI magnetic resonance imaging
In summary, we reported the case of a 71-year-old man with renal metastasis from HCC and elevation of PIVKA-II after 8 years of metastasis-free survival. We performed radical nephrectomy, because the tumor had rapidly increased in size and the level of PIVKA-II was elevated. Sorafenib or lenvatinib remain choices for further treatment if metastasis recurs. Clinicians need to be aware of the potential occurrence of a renal tumor in patients with a past medical history of HCC. Further accumulation of evidence from additional reports is required to reveal a proper strategy for treating renal metastasis from HCC.
Abbreviations
- HCC
Hepatocellular carcinoma
- CT
Computed tomography
- PIVKA-II
Protein induced by vitamin K absence II
- AFP
Alpha-fetoprotein
- MRI
Magnetic resonance imaging
- Hep-par1
Hepatocyte-paraffin 1
- GPC3
Glypican 3
Author contributions
YR, SY, GK, IS and TJ performed the operation and managed the patient postoperatively. BT, SK and YW performed the pathological analysis. YR and SY drafted the manuscript. All authors read and approved the final manuscript.
Funding
No funding was received for this case report.
Data availability
Data and material will be available on request to the corresponding author.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflicts of interest.
Informed consent
Informed consent was obtained from the patient for the publication of this case report.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Wagle DG, Moore RH, Murphy GP. Secondary carcinomas of the kidney. J Urol. 1975;114(1):30–32. doi: 10.1016/S0022-5347(17)66935-0. [DOI] [PubMed] [Google Scholar]
- 2.Sawabe M, Nakamura T, Kanno J, et al. Analysis of morphological factors of hepatocellular carcinoma in 98 autopsy cases with respect to pulmonary metastasis. Acta Pathol Jpn. 1987;37(9):1389–1404. doi: 10.1111/j.1440-1827.1987.tb02261.x. [DOI] [PubMed] [Google Scholar]
- 3.Fan Z, van de Rijn M, Montgomery K, et al. Hep par 1 antibody stain for the differential diagnosis of hepatocellular carcinoma: 676 tumors tested using tissue microarrays and conventional tissue sections. Mod Pathol. 2003;16(2):137–144. doi: 10.1097/01.MP.0000052103.13730.20. [DOI] [PubMed] [Google Scholar]
- 4.Shirakawa H, Suzuki H, Shimomura M, et al. Glypican-3 expression is correlated with poor prognosis in hepatocellular carcinoma. Cancer Sci. 2009;100(8):1403–1407. doi: 10.1111/j.1349-7006.2009.01206.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bracken RB, Chica G, Johnson DE, et al. Secondary renal neoplasms: an autopsy study. South Med J. 1979;72(7):806–807. doi: 10.1097/00007611-197907000-00013. [DOI] [PubMed] [Google Scholar]
- 6.Hsu YB, Lee PH, Sheu JC, et al. Hepatocellular carcinoma with metastasis to the kidney: report of a case. J Formos Med Assoc. 1994;93(1):71–74. [PubMed] [Google Scholar]
- 7.Sanz Mayayo E, Mayayo Dehesa T, Gómez García I, et al. Renal metastasis of hepatocellular carcinoma. Actas Urol Esp. 2003;27(5):387–390. doi: 10.1016/S0210-4806(03)72941-6. [DOI] [PubMed] [Google Scholar]
- 8.D'Antonio A, Caleo A, Caleo O, et al. Hepatocellular carcinoma metastatic to the kidney mimicking renal oncocytoma. Hepatobiliary Pancreat Dis Int. 2010;9(5):550–552. [PubMed] [Google Scholar]
- 9.Miyamoto M, Sudo T, Kuyama T. Spontaneous rupture of hepatocellular carcinoma: a review of 172 Japanese cases. Am J Gastroenterol. 1991;86(1):67–71. [PubMed] [Google Scholar]
- 10.Fukushima M, Isoyama E, Sakaridani N, et al. Renal metastasis originating from liver cancer. Nihon Hinyokika Gakkai Zasshi. 1996;87(3):710–713. doi: 10.5980/jpnjurol1989.87.710. [DOI] [PubMed] [Google Scholar]
- 11.Mezawa S, Homma H, Doi T, et al. Re: spontaneous rupture of renal metastasis of hepatocellular carcinoma: management by emergency transcatheter arterial embolization. Cardiovasc Intervent Radiol. 2001;24(2):143–144. doi: 10.1007/s002700000381. [DOI] [PubMed] [Google Scholar]
- 12.Aron M, Nair M, Hemal AK. Renal metastasis from primary hepatocellular carcinoma. A case report and review of the literature. Urol Int. 2004;73(1):89–91. doi: 10.1159/000078812. [DOI] [PubMed] [Google Scholar]
- 13.Ong KW, Joseph B, Gyomber DV, et al. Nephrectomy for a renal metastasis of undiagnosed hepatocellular carcinoma arising from an orthotopic liver transplant undertaken for cryptogenic cirrhosis. Korean J Urol. 2013;54(10):715–717. doi: 10.4111/kju.2013.54.10.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kinoshita O, Ichijo Y, Yoneda M, et al. Spontaneous rupture of renal metastasis from hepatocellular carcinoma. Case Rep Surg. 2017;2017:8607061. doi: 10.1155/2017/8607061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shen A, Tang Y, et al. A systematic review of sorafenib in Child-Pugh A patients with unresectable hepatocellular carcinoma. J Clin Gastroenterol. 2013;47:871–880. doi: 10.1097/MCG.0b013e3182a87cfd. [DOI] [PubMed] [Google Scholar]
- 16.Cheng AL, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomized phase 3 non-inferiority trial. Lancet. 2018;391(10126):1163–1173. doi: 10.1016/S0140-6736(18)30207-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data and material will be available on request to the corresponding author.