Abstract
Urushiols are important active compounds found in the sap of the lacquer tree (Rhus verniciflua Stokes). Recently, various biological effects of urushiols, such as antioxidant, antimicrobial, and anticancer activities, have been reported. However, urushiols can also induce skin allergies. Nevertheless, the lacquer tree has traditionally been used in Korea as a folk medicine. In this study, we evaluated the absorption and metabolism of 3-pentadecylcatechol (PDC), a natural urushiol. PDC (48.0 mg/kg body wt.) in 1 mL propylene glycol was orally administered to rats (Sprague-Dawley, male, 6 weeks old). Blood plasma, urine, and feces were collected, separately. PDC was not detected in the extracts from rat blood plasma and urine. However, 89.4 ± 5.2% of the orally administered PDC was detected in the feces extracts, indicating that PDC was predominantly excreted and not absorbed.
Keywords: Urushiol, 3-pentadecylcatechol, Rhus verniciflua Stokes, Absorption metabolism
Introduction
Cardiovascular diseases (CVDs) are a leading cause of death worldwide. The World Health Organization has reported that over half (50.3%) of the top 10 causes of death worldwide in 2016 were CVDs, ischemic heart disease, and stroke (World Health Organization, 2018). In particular, atherosclerosis is a major cause of CVD (Falk, 2006). Previous studies revealed that the most important chemoattractant of atherogenesis is oxidized low-density lipoprotein (LDL), which is abundant in atherosclerotic plaques (Willerson and Kereiakes, 2003). Therefore, it is recognized that the consumption of antioxidants or antioxidant-rich foods can prevent and improve arteriosclerosis through the inhibition of LDL oxidation (Esterbauer et al., 1992; Kamada et al., 2005; Moon et al., 2001; Vaya et al., 2003).
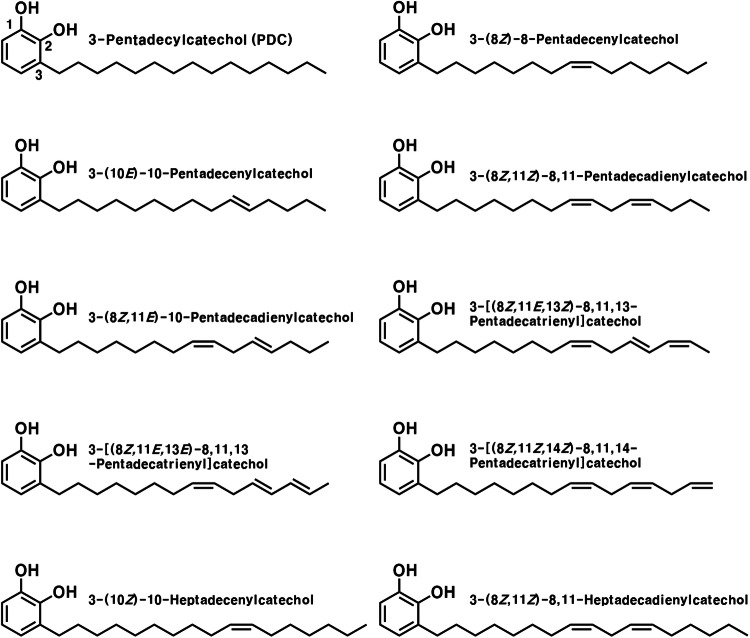
Urushiols are the principal compounds in the sap of the lacquer tree (Rhus verniciflua Stokes), comprising 60 to 70% of its content (Hatada et al., 1994). The basic structure of urushiols comprises 15 or 17 saturated or unsaturated carbon chains connected to the C-3 of catechol; ten different derivatives have already been isolated and reported (Fig. 1) (Hatada et al., 1994). Urushiols are compounds with unique properties that cause severe contact dermatitis (Ma et al., 2012; Wakabayashi et al., 2005; Zepter et al., 1997). Nevertheless, the lacquer tree has been used for thousands of years in China, Japan, and Korea as a protective coating material for surfaces (Jeong et al., 2015; Lee, 2012) and as a traditional folk medicine for gastric disorders and improvement of blood circulation (Park et al., 2016; Suk et al., 2011). Additionally, there are many reports on the various beneficial effects of urushiols, such as their antioxidant, antimicrobial, and anticancer activities (Choi et al., 2001; Hong et al., 2013; Kim et al., 1997; Kim et al., 2009; Kim et al., 2013; Suk et al., 2011).
Fig. 1.
Structure of urushiol derivatives isolated from Rhus verniciflua Stokes
In our previous study, 3-pentadecylcatechol (PDC, Fig. 1), a derivative of urushiol, exhibited marginally higher radical-scavenging activity than α-tocopherol, and exerted an inhibitory effect against the lipid peroxidation of liposome membranes and rat blood plasma (Kim et al., 2014). Furthermore, PDC exhibited strong lipophilicity and affinity to liposome membranes (Kim et al., 2014); therefore, the use of urushiols as a therapeutic agent for arteriosclerosis is to be expected. However, the absorption and metabolism of urushiol have not been previously reported; therefore, in the present study, we evaluated the absorption, metabolism, and excretion of PDC in vivo.
Materials and methods
Chemicals
3-Pentadecylcatechol (PDC) and 3-pentadecylveratrole (1,2-dimethoxy-3-pentadecylbenzene, PDV) were chemically synthesized, as previously described (Kim et al., 2014). β-Glucuronidase type H-1 (from Helix pomatia, EC 3.2.1.31) and heparin sodium salt (from porcine intestinal mucosa) were obtained from Sigma-Aldrich Chemical Co. (St. Louis, MO, USA). All other chemicals and solvents were of analytical grade.
Animals
All experimental procedures were approved by the Institutional Animal Care and Use Committee of Chonnam National University (No. CNU IACUC-YB-2012-26, Gwangju, Republic of Korea). Six-week-old male Sprague-Dawley rats, weighing 180–200 g, were supplied by Samtako Bio Korea (Osan, Korea). The animals were maintained in an environmentally controlled facility at 20 ± 1 °C and 55 ± 5% humidity, under a 12 h dark/light cycle, and were provided water and normal feed (Harlan Rodent diet, 2018S, by Samtako Bio Korea) ad libitum. The rats were acclimatized to the environment for 1 d before the experiments were commenced (Lee et al., 2017).
Oral administration of PDC and sample collection
All rats were fasted for 15 h and were deprived of water for 3 h before PDC administration. PDC was dissolved in 1 mL propylene glycol, and was orally administered at 150 μmol (48.0 mg/kg body weight) to three rats of each group. For the bioavailability study, blood samples were collected under light anesthesia (diethyl ether) from the abdominal aorta of the rats at 0, 1, 2, 4, 8, and 12 h, into heparinized tubes, after PDC administration. The plasma samples were obtained by centrifuging blood at 2767 × g, 4 °C for 15 min, and were stored at – 80 °C until use (Griffiths and Barrow, 1972; Lee et al., 2017; Shimoi et al., 2003).
For the excretion study, three rats were kept in a metabolic cage after the oral administration of PDC, and the urine and feces were collected for up to 24 h post-administration. The volume of the urine and the weight of the feces samples were measured; subsequently, all samples were stored at – 80 °C until use (Griffiths and Barrow, 1972; Lee et al., 2017; Shimoi et al., 2003).
Determination of the presence or absence of PDC in rat plasma
Rat plasma (100 μL) was mixed with 200 μL β-glucuronidase type H-1 (500 units, from H. pomatia) solution in 0.1 M sodium acetate buffer (pH 5.0) (Lee et al., 2017). The mixture was incubated at 37 °C for 2 h, and the reaction mixture was extracted in 1 mL MeCN. Following this, the mixture was vortexed for 30 s, sonicated for 30 s, and centrifuged for 15 min at 4 °C and 2767 × g. The supernatant was concentrated in vacuo and dissolved in 100 μL MeOH. Twenty microliters of the resulting solution was injected onto an ODS HPLC column (UG 120, 5 μm, 4.6 × 250 mm, Shiseido, Tokyo, Japan). HPLC analysis was performed on a Shimadzu LC-6AD instrument with an SPD-M20A detector. An isocratic mobile phase of 92% MeOH was used. The column temperature was maintained at 40 °C, and the flow rate was 1 mL/min. The elution pattern was monitored through the measurement of the absorbance at 275 nm.
Determination of the presence or absence of PDC in rat urine
Rat urine (1 mL) was mixed with β-glucuronidase type H-1 (5000 units, from H. pomatia) solution in 0.1 M sodium acetate buffer (pH 5.0, 2 mL) (Shimoi et al., 2003). The mixture was incubated at 37 °C for 2 h. The reaction mixture was extracted thrice with EtOAc (3 mL), and the EtOAc layers were collected and concentrated. The concentrate was dissolved in MeOH (100 μL). Twenty microliters of the resulting solution was analyzed using HPLC, as described above, but with different solvent conditions. The mobile phase was a gradient of 5% MeOH containing 2% AcOH (Solvent A) and 100% MeOH (Solvent B). The gradient program for HPLC analysis was 100% A/0% B to 0% A/100% B for 0− 50 min and isocratic 0% A/100% B for 15 min.
Determination of the presence or absence of PDC in rat feces
Lyophilized rat feces (1 g/dry weight) were mixed with MeOH (containing 2% AcOH, 10 mL) and homogenized (T-25 Ultra Turrax, IKA, Germany) (Griffiths and Barrow, 1972). The mixture was sonicated (30 s) and centrifuged (2767 × g, 15 min, 4 °C). The supernatant was collected, and the residue extraction was repeated with the same conditions. After centrifugation, the supernatants were collected and concentrated in vacuo. The concentrate was pretreated (enzyme treatment and extraction) following the same procedure described above for urine. Twenty microliters of the resulting solution was analyzed under the HPLC conditions described above, but with different solvent conditions. The mobile phase comprised gradient elution with 15% MeOH containing 2% AcOH (Solvent A) and 100% MeOH (Solvent B), with the following program used for HPLC: 100% A/0% B to 20% A/80% B for 0− 20 min, 0% A/100% B for 20− 45 min, and isocratic 0% A/100% B for 15 min.
Results and discussion
Urushiols are significant constituents of the sap of the lacquer tree (Rhus verniciflua Stokes) (Hatada et al., 1994). Even though urushiols are known to induce contact dermatitis (Ma et al., 2012; Wakabayashi et al., 2005; Zepter et al., 1997), the lacquer tree extract, in which urushiols are a major component (Hatada et al., 1994), is used for its various beneficial effects, such as anti-inflammatory and anticancer activities (Choi et al., 2014; Kim et al., 2006). Urushiols are amphipathic substances with an antioxidative active site of catechol, and their inhibitory effects on biomembrane peroxidation have been reported (Kim et al., 2014). However, metabolic studies are needed to confirm their usefulness after absorption into the blood in order to utilize urushiols in the blood and circulatory system. Therefore, in this study, the absorption and metabolism of PDC, a natural urushiol derivative, were evaluated to assess its potential as a therapeutic agent for arteriosclerosis.
The presence or absence of PDC in rat plasma
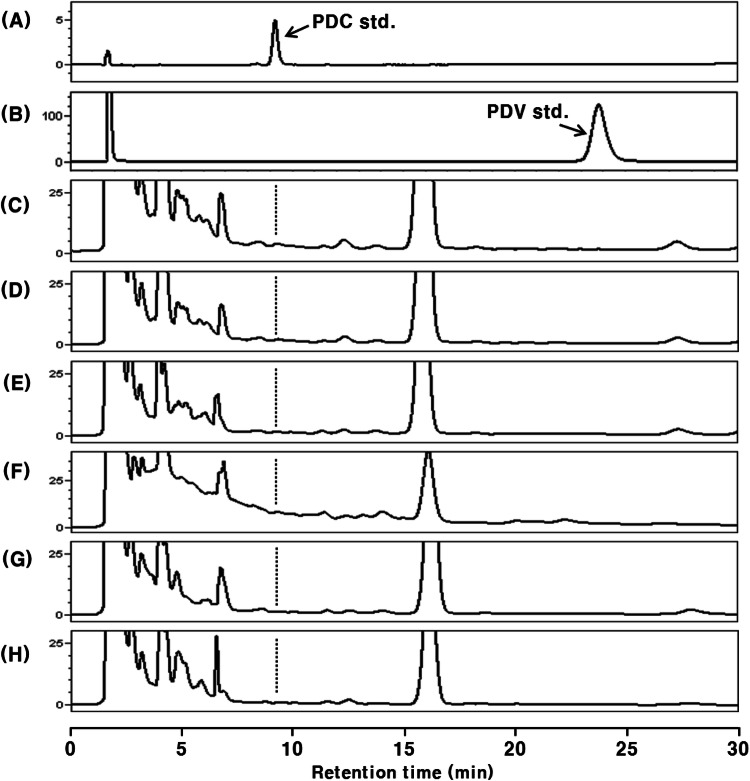
Rat blood plasma after oral administration of PDC was analyzed by HPLC–PDA (Fig. 2). To confirm the presence or absence of PDC in the intact form, all plasma samples were analyzed without β-glucuronidase type H-1 treatment (Fig. 2). None of the plasma samples before the treatment with β-glucuronidase type H-1 showed a peak at the same retention time (tR 9.2 min) as the PDC standard in the HPLC chromatograms (Fig. 2).
Fig. 2.
HPLC chromatograms of PDC-administered (48.0 mg/kg body weight) rat plasma extracts without β-glucuronidase H-1 treatment. The data are representative of three experiments. (A) PDC standard; (B) PDV standard; (C) control plasma; (D) 1 h after administration; (E) 2 h after administration, (F) 4 h after administration; (G) 8 h after administration; (H) 12 h after administration
Generally, some phenolic compounds are absorbed in their intact form into the blood (McGhie et al., 2003; Tsuda et al., 1999), whereas most are absorbed as conjugated metabolites; where the hydroxyl group of the phenolic compounds binds with glucuronic acid and/or sulfuric acid (Crespy et al., 1999; Lee et al., 2017; Moon et al., 2000; Moon et al., 2001 ; Murota and Terao, 2003). This mechanism increases the polarity of these compounds and promotes the excretion of foreign matter, such as phenolics and drugs (Omiecinski et al., 2011). We expected PDC to exist in a similar metabolic form in the blood. Accordingly, the blood plasma was treated with β-glucuronidase type H-1, which can hydrolyze the links of O-glucuronic acid and O-sulfuric acid (Lee et al., 2017), but PDC (tR 9.2 min) was still not detected in the plasma sample (Fig. 3).
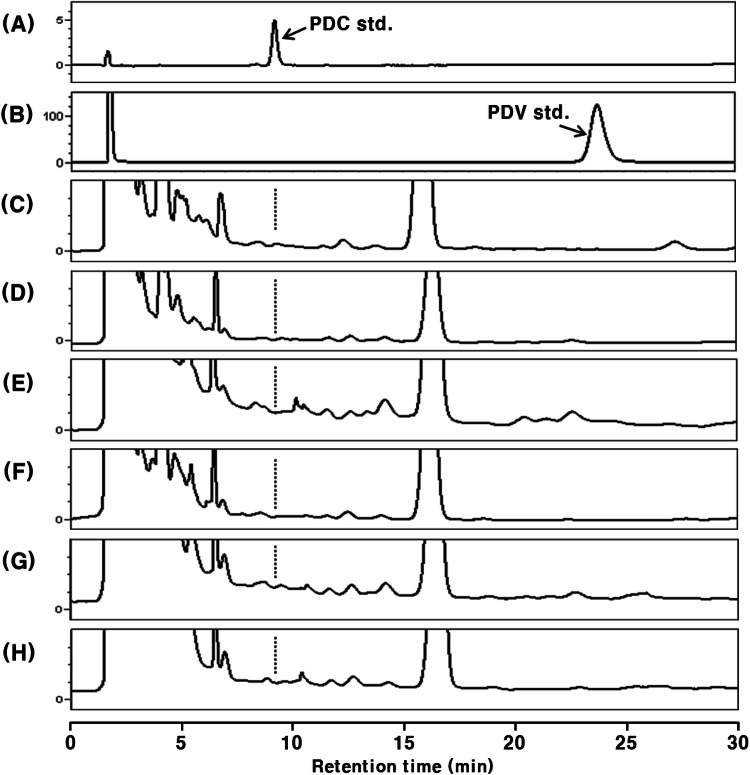
Fig. 3.
HPLC chromatograms of PDC-administered (48.0 mg/kg body weight) rat plasma extracts with β-glucuronidase H-1 treatment. The data are representative of three experiments. (A) PDC standard; (B) PDV standard; (C) control plasma; (D) 1 h after administration; (E) 2 h after administration, (F) 4 h after administration; (G) 8 h after administration; (H) 12 h after administration
It is well known that a part of the phenolic compounds absorbed into the blood undergoes methylation in the liver (Piskula and Terao, 1998). Therefore, the presence or absence of PDC in the methylated form, which may be one of the possible metabolites, was determined (Figs. 2 and 3). The pentadecylveratrole (1,2-dimethoxy-3-pentadecylbenzene, PDV), an intermediate in PDC synthesis, was detected at tR 23.9 min (Figs. 2B and 3B). However, no significant peaks corresponding to monomethylated PDC and PDV were detected between peaks of free PDC and PDV on the HPLC chromatograms of plasma samples. Considering the relative polarity of these compounds and the characteristics of the ODS column, it was suggested that methylated PDC and its conjugated metabolite(s) are not present in the rat blood plasma after oral administration of PDC. Under these analytical conditions, the detection limit for both PDC and PDV was 15.0 pmol.
The presence or absence of PDC in rat urine
To confirm whether PDC was absorbed, we analyzed the urine of rats after PDC administration, before and after β-glucuronidase type H-1 treatment. The representative HPLC chromatograms are shown in Fig. 4. Similar to the results obtained with the plasma, PDC (tR 55.4 min) was not detected (Fig. 4D, E) in urine. In addition, PDV as well as the peaks presumed to be monomethylated PDC were not found (data not shown) as in plasma. Therefore, it was suggested that orally administered PDC was not absorbed, or that only a minimal amount might have been absorbed.
Fig. 4.
HPLC chromatograms of rat urine extracts after oral administration of PDC (48.0 mg/kg body weight). (A) PDC standard; (B) control urine (without β-glucuronidase H-1 treatment); (C) control urine (with β-glucuronidase H-1 treatment); (D) urine after PDC administeration (without β-glucuronidase H-1 treatment); (E) urine after PDC administeration (with β-glucuronidase H-1 treatment)
The presence or absence of PDC in rat feces
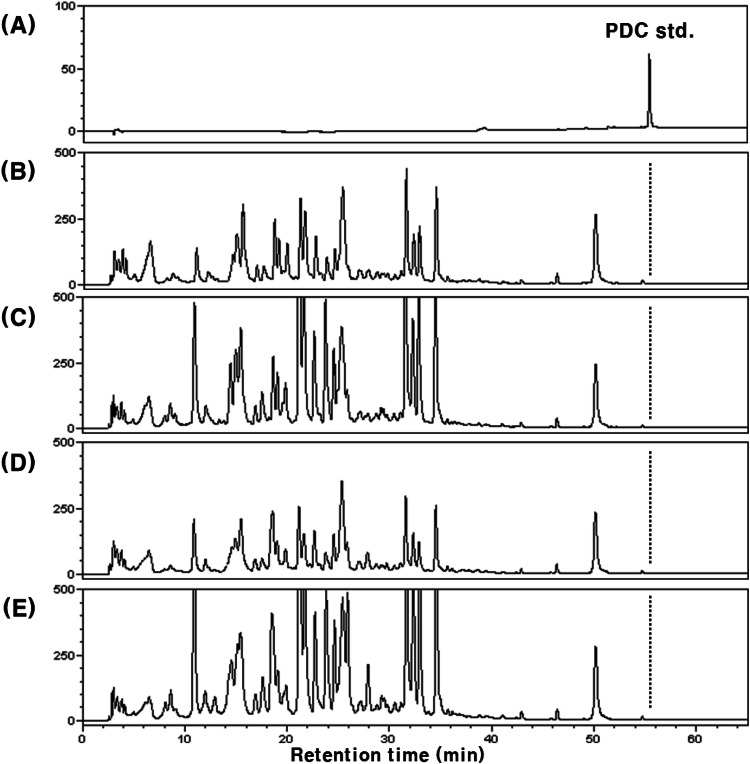
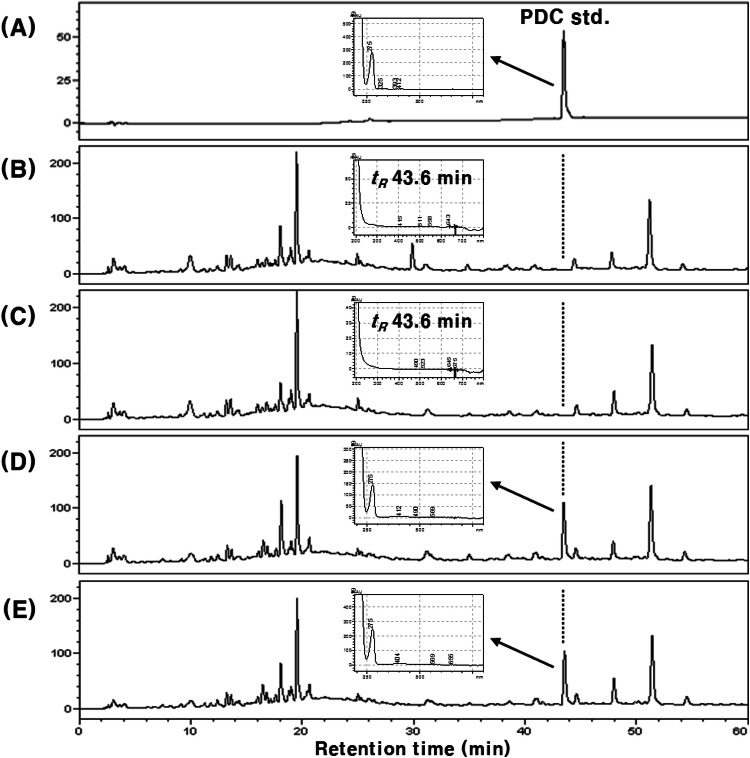
Unlike our expectations, PDC was not detected both in plasma and urine, even after 12 h and 24 h of oral PDC administration, respectively. As our results showed that PDC was not absorbed, the feces of PDC-treated rats were analyzed to confirm whether PDC was excreted. The representative HPLC chromatograms are shown in Fig. 5. The peak (tR 43.6 min) consistent with the retention time of standard PDC was detected on HPLC chromatograms before and after the treatment with β-glucuronidase type H-1 (Fig. 5D, E). The UV spectra of the three peaks (Fig. 5A, D and E) detected at tR 43.6 min were also consistent. The content of total PDC in feces was 89.4 ± 5.2% of that of the orally administered PDC. Additionally, the peak area of PDC did not increase after treatment with β-glucuronidase type H-1 (Fig. 5E). Studies on quercetin, a representative vegetable flavonoid, showed that a portion of the absorbed metabolites (glucuronide and/or sulfate) was excreted back into the small intestine, and then reabsorbed after hydrolysis by the intestinal microorganisms, or excreted through feces (Crespy et al., 1999; Murota and Terao, 2003). Therefore, the peak area of PDC in feces did not change after the treatment with β-glucuronidase type H-1 because intestinal absorption or re-secretion did not occur. On the other hand, for the remaining about 10% of orally administered PDC, which was not detected in feces, we assumed that it might be the affection by microorganisms in large intestine. It has been reported that rutin (quercetin 3-O-β-rutinoside) is rarely absorbed in the small intestine after oral administration as it is difficult to hydrolyze, and mainly absorbed in the large intestine with reaction of microorganisms after it reached the large intestine (Crespy et al., 1999; Shimoi et al., 2003). In addition, the conversion or degradation of dietary phenols by the microorganisms in the large intestine is also well known (Kawabata et al. 2019; Wang et al. 2020). In our results, PDC was not detected from the rat blood plasma after 8 and 12 h of PDC administration, meaning that PDC was also not absorbed in large intestine. Therefore, it was considered that PDC might be also converted to other compounds by microorganisms in the large intestine like rutin and other dietary phenols (Crespy et al., 1999; Shimoi et al., 2003, Kawabata et al., 2019; Wang et al., 2020), even though their structural characteristics are different. Our results confirmed that orally administered PDC was not absorbed and that the majority was excreted in feces. Even though further studies on the absorption of other urushiol derivatives are needed, our results imply that the various in vivo beneficial effects of the lacquer-tree extract might be due to the constituent flavonoids, such as fustin, fisetin, and sulfuretin, rather than urushiols (Lee et al., 2002; Lee et al., 2012; Lee et al., 2015).
Fig. 5.
HPLC chromatograms of rat feces extracts after oral administration of PDC (48.0 mg/kg body weight) and correlated UV/VIS spectra (tR 43.6 min). (A) PDC standard; (B) control feces (without β-glucuronidase H-1 treatment); (C) control feces (with β-glucuronidase H-1 treatment); (D) feces after PDC administeration (without β-glucuronidase H-1 treatment); (E) feces after PDC administeration (with β-glucuronidase H-1 treatment)
In our recent study (Jeong, 2018), we synthesized an ionized PDC (C21H34O2Na2), a compound more polar than PDC, while maintaining the basic structure of PDC (C21H36O2), and eliminating its allergenicity. Even though we expected an increase in absorption with the change in molecular polarity, similar to PDC, the ionized PDC was also not absorbed into the blood after oral administration. Though further studies are required, we presume the structural characteristics of PDC and ionized PDC—with their long chain and the benzene ring—might affect their effective absorption into the blood.
As described above, rutin, which mainly reaches the large intestine with little absorption in small intestine, exerts antioxidant and anti-inflammatory effects in the colonic mucosa (Gautam et al., 2016; Hussein et al., 2014). In addition, a small number of green tea catechins were reported to be absorbed into the small intestine, but a considerable amount were transferred to the colon (Spencer, 2003). Yamamoto et al. reported that iron-induced lipid peroxidation of large intestinal mucosa was suppressed by intake of green tea catechins in rat (Yamamoto et al., 2006). Furthermore, it has been reported that chlorogenic acid, a compound that has a low absorption ratio reaches the large intestine in large amounts after oral administration (Olthof et al., 2003) and inhibits colon cancer metastasis (Kang et al., 2011). Therefore, it is expected that PDC may also contribute to the improved antioxidative activity of the colon.
In summary, we analyzed the absorption and metabolism of PDC, a natural urushiol derivative; PDC was not detected in blood plasma and urine after oral administration, but was detected in the feces. Though further studies are required, our results indicate that there are difficulties in using PDC as an orally administered anti-arteriosclerosis factor, owing to its low absorbability; however, its antioxidant activity might prove effective for the prevention of colon-related diseases. In addition, studies on the biological activities of R. verniciflua Stokes, mainly focused on urushiols, should also be reconsidered, using different approaches.
Acknowledgements
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (No. NRF-2017R1D1A1B03034520).
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Hang Yeon Jeong, Email: wjdgkddus@naver.com.
Hyoung Jae Lee, Email: ableleehj@gmail.com.
Jeong-Yong Cho, Email: jyongcho17@jnu.ac.kr.
Jae-Hak Moon, Email: nutrmoon@jnu.ac.kr.
References
- Choi HS, Seo HS, Kim SR, Choi YK, Jang BH, Shin YC, Ko SG. Antiinflammatory and antiproliferative effects of Rhus verniciflua Stokes in RAW264.7 cells. Mol. Med. Rep. 2014;9:311–315. doi: 10.3892/mmr.2013.1775. [DOI] [PubMed] [Google Scholar]
- Choi JY, Park CS, Choi JO, Rhim HS, Chun HJ. Cytotoxic effect of urushiol on human ovarian cancer cells. J. Microbiol. Biotechnol. 2001;11:399–405. [Google Scholar]
- Crespy V, Morand C, Manach C, Besson C, Demigne C, Remesy C. Part of quercetin absorbed in the small intestine is conjugated and further secreted in the intestinal lumen. Am. J. Physiol. 1999;277:G120–G126. doi: 10.1152/ajpgi.1999.277.1.G120. [DOI] [PubMed] [Google Scholar]
- Esterbauer H, Waeg G, Puhl H, Dieber-Rotheneder M, Tatzber F. Inhibition of LDL oxidation by antioxidants. EXS. 1992;62:145–157. doi: 10.1007/978-3-0348-7460-1_15. [DOI] [PubMed] [Google Scholar]
- Falk E. Pathogenesis of atherosclerosis. J. Am. Coll. Cardiol. 2006;47:C7–C12. doi: 10.1016/j.jacc.2005.09.068. [DOI] [PubMed] [Google Scholar]
- Gautam R, Singh M, Gautam S, Rawat JK, Saraf SA, Kaithwas G. Rutin attenuates intestinal toxicity induced by Methotrexate linked with anti-oxidative and anti-inflammatory effects. BMC Complement. Altern. Med. 2016;16:99. doi: 10.1186/s12906-016-1069-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths LA, Barrow A. Metabolism of flavonoid compounds in germ-free rats. Biochem. J. 1972;130:1161–1162. doi: 10.1042/bj1301161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatada K, Kitayama T, Nishiura T, Nishimoto A, Simonsick WJ, Vogl O. Structural analysis of the components of Chinese lacquer “Kuro-urushi”. Macromol. Chem. Phys. 1994;195:1865–1870. [Google Scholar]
- Hong SH, Suk KT, Choi SH, Lee JW, Sung HT, Kim CH, Kim EJ, Kim MJ, Han SH, Kim MY, Baik SK, Kim DJ, Lee GJ, Lee S, Park SH, Ryu OH. Anti-oxidant and natural killer cell activity of Korean red ginseng (Panax ginseng) and urushiol (Rhus vernicifera Stokes) on non-alcoholic fatty liver disease of rat. Food Chem. Toxicol. 2013;55:586–591. doi: 10.1016/j.fct.2013.01.022. [DOI] [PubMed] [Google Scholar]
- Hussein SA, AbouZaid OAR, Abdel-Maksoud HA, Khadija AAA. Anti-inflammatory and antioxidant effect of rutin on 2,4,6-trinitrobenzene sulfonic acid induced ulcerative colitis in rats. Benha Vet. Med. J. 2014;27:208–220. [Google Scholar]
- Jeong H, Heo J, Son B, Choi D, Park TH, Chang M, Hong J. Intrinsic hydrophobic cairnlike multilayer films for antibacterial effect with enhanced durability. ACS Appl. Mater. Interfaces. 2015;7:26117–26123. doi: 10.1021/acsami.5b07613. [DOI] [PubMed] [Google Scholar]
- Jeong HY. Bioavailability and anti-Helicobacter pylori activity of urushiol derivatives. PhD thesis, Chonnam National University, Gwangju, Republic of Korea (2018)
- Kamada C, Da Silva EL, Ohnishi-Kameyama M, Moon JH, Terao J. Attenuation of lipid peroxidation and hyperlipidemia by quercetin glucoside in the aorta of high cholesterol-fed rabbit. Free Radic. Res. 2005;39:185–194. doi: 10.1080/10715760400019638. [DOI] [PubMed] [Google Scholar]
- Kang NJ, Lee KW, Kim BH, Bode AM, Lee HJ, Heo YS, Boardman L, Limburg P, Lee HJ, Dong Z. Coffee phenolic phytochemicals suppress colon cancer metastasis by targeting MEK and TOPK. Carcinogenesis. 2011;32:921–928. doi: 10.1093/carcin/bgr022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawabata K, Yoshioka Y, Terao J. Role of intestinal microbiota in the bioavailability and physiological functions of dietary polyphenols. Molecules. 2019;24:370. doi: 10.3390/molecules24020370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D, Jeon S, Seo J. The preparation and characterization of urushiol powders (YPUOH) based on urushiol. Prog. Org. Coat. 2013;76:1465–1470. [Google Scholar]
- Kim HS, Yeum JH, Choi SW, Lee JY, Cheong IW. Urushiol/polyurethane-urea dispersions and their film properties. Prog. Org. Coat. 2009;65:341–347. [Google Scholar]
- Kim JH, Kim HP, Jung CH, Hong MH, Hong MC, Bae HS, Lee SD, Park SY, Park JH, Ko SG. Inhibition of cell cycle progression via p27Kip1 upregulation and apoptosis induction by an ethanol extract of Rhus verniciflua Stokes in AGS gastric cancer cells. Int. J. Mol. Med. 2006;18:201–208. [PubMed] [Google Scholar]
- Kim JY, Cho JY, Ma YK, Lee YG, Moon JH. Nonallergenic urushiol derivatives inhibit the oxidation of unilamellar vesicles and of rat plasma induced by various radical generators. Free Radic. Biol. Med. 2014;71:379–389. doi: 10.1016/j.freeradbiomed.2014.03.041. [DOI] [PubMed] [Google Scholar]
- Kim MJ, Choi YH, Kim WG, Kwak SS. Antioxidative activity of urushiol derivatives from the sap of lacquer tree (Rhus verniciflua Stokes) Korean J. Plant Res. 1997;10:227–230. [Google Scholar]
- Lee HJ, Jeong HY, Jin MR, Lee HJ, Cho JY, Moon JH. Metabolism and antioxidant effect of malaxinic acid and its corresponding aglycone in rat blood plasma. Free Radic. Biol. Med. 2017;110:399–407. doi: 10.1016/j.freeradbiomed.2017.06.020. [DOI] [PubMed] [Google Scholar]
- Lee JC, Lim KT, Jang YS. Identification of Rhus verniciflua Stokes compounds that exhibit free radical scavenging and anti-apoptotic properties. Biochim. Biophys. Acta. 2002;1570:181–191. doi: 10.1016/s0304-4165(02)00196-4. [DOI] [PubMed] [Google Scholar]
- Lee NH. Korean lacquer art technique - Korean lacquer craft of Goryeo to Joseon Dynasties. J. Chinese Lacq. 2012;31:29–39. [Google Scholar]
- Lee WJ, Kang JE, Choi JH, Jeong ST, Kim MK, Choi HS. Comparison of the flavonoid and urushiol content in different parts of Rhus verniciflua Stokes grown in Wonju and Okcheon. Korean J. Food Sci. Technol. 2015;47:158–163. [Google Scholar]
- Lee YR, Hwang JK, Koh HW, Jang KY, Lee JH, Park JW, Park BH. Sulfuretin, a major flavonoid isolated from Rhus verniciflua, ameliorates experimental arthritis in mice. Life Sci. 2012;90:799–807. doi: 10.1016/j.lfs.2012.04.015. [DOI] [PubMed] [Google Scholar]
- Ma X, Lu R, Miyakoshi T. Recent advances in research on lacquer allergy. Allergol. Int. 2012;61:45–50. doi: 10.2332/allergolint.11-RA-0324. [DOI] [PubMed] [Google Scholar]
- McGhie TK, Ainge GD, Barnett LE, Cooney JM, Jensen DJ. Anthocyanin glycosides from berry fruit are absorbed and excreted unmetabolized by both humans and rats. J. Agric. Food Chem. 2003;51:4539–4548. doi: 10.1021/jf026206w. [DOI] [PubMed] [Google Scholar]
- Moon JH, Nakata R, Oshima S, Inakuma T, Terao J. Accumulation of quercetin conjugates in blood plasma after the short-term ingestion of onion by women. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2000;279:R461–R467. doi: 10.1152/ajpregu.2000.279.2.R461. [DOI] [PubMed] [Google Scholar]
- Moon JH, Tsushida T, Nakahara K, Terao J. Identification of quercetin 3-O-beta-D-glucuronide as an antioxidative metabolite in rat plasma after oral administration of quercetin. Free Radic. Biol. Med. 2001;30:1274–1285. doi: 10.1016/s0891-5849(01)00522-6. [DOI] [PubMed] [Google Scholar]
- Murota K, Terao J. Antioxidative flavonoid quercetin: implication of its intestinal absorption and metabolism. Arch. Biochem. Biophys. 2003;417:12–17. doi: 10.1016/s0003-9861(03)00284-4. [DOI] [PubMed] [Google Scholar]
- Olthof MR, Hollman PCH, Buijsman MNCP, van Amelsvoort JMM, Katan MB. Chlorogenic acid, quercetin-3-rutinoside and black tea phenols are extensively metabolized in humans. J. Nutr. 2003;133:1806–1814. doi: 10.1093/jn/133.6.1806. [DOI] [PubMed] [Google Scholar]
- Omiecinski CJ, Vanden Heuvel JP, Perdew GH, Peters JM. Xenobiotic metabolism, disposition, and regulation by receptors: from biochemical phenomenon to predictors of major toxicities. Toxicol. Sci. 2011;120(Suppl):S49–S75. doi: 10.1093/toxsci/kfq338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JM, Lee JH, Na CS, Lee D, Lee JY, Satoh M, Lee MY. Heartwood extract of Rhus verniciflua Stokes and its active constituent fisetin attenuate vasoconstriction through calcium-dependent mechanism in rat aorta. Biosci. Biotechnol. Biochem. 2016;80:493–500. doi: 10.1080/09168451.2015.1107464. [DOI] [PubMed] [Google Scholar]
- Piskula MK, Terao J. Accumulation of (–)-epicatechin metabolites in rat plasma after oral administration and distribution of conjugation enzymes in rat tissues. J. Nutr. 1998;128:1172–1178. doi: 10.1093/jn/128.7.1172. [DOI] [PubMed] [Google Scholar]
- Shimoi K, Yoshizumi K, Kido T, Usui Y, Yumoto T. Absorption and urinary excretion of quercetin, rutin, and alphaG-rutin, a water soluble flavonoid, in rats. J. Agric. Food Chem. 2003;51:2785–2789. doi: 10.1021/jf026108a. [DOI] [PubMed] [Google Scholar]
- Spencer JPE. Metabolism of tea flavonoids in the gastrointestinal tract. J. Nutr. 2003;133:3255S–3261S. doi: 10.1093/jn/133.10.3255S. [DOI] [PubMed] [Google Scholar]
- Suk KT, Baik SK, Kim HS, Park SM, Paeng KJ, Uh Y, Jang IH, Cho MY, Choi EH, Kim MJ, Ham YL. Antibacterial effects of the urushiol component in the sap of the lacquer tree (Rhus verniciflua Stokes) on Helicobacter pylori. Helicobacter. 2011;16:434–443. doi: 10.1111/j.1523-5378.2011.00864.x. [DOI] [PubMed] [Google Scholar]
- Tsuda T, Horio F, Osawa T. Absorption and metabolism of cyanidin 3-O-beta-D-glucoside in rats. FEBS Lett. 1999;449:179–182. doi: 10.1016/s0014-5793(99)00407-x. [DOI] [PubMed] [Google Scholar]
- Vaya J, Mahmood S, Goldblum A, Aviram M, Volkova N, Shaalan A, Musa R, Tamir S. Inhibition of LDL oxidation by flavonoids in relation to their structure and calculated enthalpy. Phytochemistry. 2003;62:89–99. doi: 10.1016/s0031-9422(02)00445-4. [DOI] [PubMed] [Google Scholar]
- Wakabayashi T, Hu DL, Tagawa YI, Sekikawa K, Iwakura Y, Hanada K, Nakane A. IFN-γ and TNF-α are involved in urushiol-induced contact hypersensitivity in mice. Immunol. Cell Biol. 2005;83:18–24. doi: 10.1111/j.1440-1711.2005.01310.x. [DOI] [PubMed] [Google Scholar]
- Wang Y, Berhow MA, Black M, Jeffery EH. A comparison of the absorption and metabolism of the major quercetin in brassica, quercetin-3-O-sophoroside, to that of quercetin aglycone, in rats. Food Chem. 2020;311:125880. doi: 10.1016/j.foodchem.2019.125880. [DOI] [PubMed] [Google Scholar]
- Willerson JT, Kereiakes DJ. Endothelial dysfunction. Circulation. 2003;108:2060–2061. doi: 10.1161/01.CIR.0000099580.72044.83. [DOI] [PubMed] [Google Scholar]
- World Health Organization: The top 10 causes of death. https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death. Accessed Jun. 18, 2019.
- Yamamoto M, Miyamoto S, Moon JH, Murota K, Hara Y, Terao J. Effect of dietary green tea catechin preparation on oxidative stress parameters in large intestinal mucosa of rats. Biosci. Biotechnol. Biochem. 2006;70:286–289. doi: 10.1271/bbb.70.286. [DOI] [PubMed] [Google Scholar]
- Zepter K, Häffner A, Soohoo LF, De Luca D, Tang HP, Fisher P, Chavinson J, Elmets CA. Induction of biologically active IL-1 beta-converting enzyme and mature IL-1 beta in human keratinocytes by inflammatory and immunologic stimuli. J. Immunol. 1997;159:6203–6208. [PubMed] [Google Scholar]