Abstract
Cardiorespiratory fitness is an independent risk factor for cardiovascular disease and shortened life expectancy in breast cancer survivors. This randomised controlled trial (n = 153) was designed for patients with a physically inactive lifestyle prediagnosis and concurrently referred to adjuvant chemotherapy. We compared two 12-week exercise interventions aimed at physiological and patient-reported outcomes (cardiorespiratory fitness, muscle strength, metabolic markers, physical activity, pain, fatigue), including a 39-week follow-up. A supervised hospital-based moderate to high intensity group exercise intervention was compared to an instructed home-based individual pedometer intervention. The two 12-week interventions included oncologists’ recommendations and systematic health counselling. Outcomes were measured at baseline and week 6, 12 and 39. Primary outcome cardiorespiratory fitness declined significantly during chemotherapy and was restored in both interventions at follow-up. The interventions effectively engaged breast cancer patients in sustaining physical activities during and following adjuvant treatment. A composite metabolic score improved significantly. Positive cardiorespiratory fitness responders had improved clinical effects on fatigue, pain and dyspnoea versus negative responders. We conclude that a loss of cardiorespiratory fitness among physically inactive breast cancer patients may be restored by early initiated interventions and by adapting to physical activity recommendations, leading to a decreased cardiovascular risk profile in breast cancer survivors.
Subject terms: Quality of life, Translational research, Breast cancer
Introduction
Physical inactivity and sedentary behaviour represent a challenge for global health due to a significant risk of pre-mature death from chronic diseases such as cardiovascular diseases, diabetes and cancer1,2. In breast cancer (BC), physical inactivity and obesity has consistently been associated with an increased risk of recurrence3,4, though physiological, biological and molecular pathways are partly known5–8. Low cardiorespiratory fitness (CRF) increases risk for coronary heart disease and affects survival in BC survivors9, similar to asymptomatic women10, low-risk adults11 and high-risk sedentary populations12,13. Compared with healthy sedentary Americans, a relative 25% decline in CRF has been shown to be a primary recurring effect in the BC trajectory14, a finding confirmed in a German cross-sectional study15. The causal mechanisms involved in this decline in CRF in patients with BC may be related to a cascade of factors in the oxygen delivery system15–17. These factors may include direct chemotherapy toxic impairment on the left ventricular function and endothelial damage, in combination lifestyle factors with a negative effect, such as physical inactivity and weight gain17,18.
Various studies point out the importance of having built-in, regular physical activity (PA) in multimodal cancer supportive care and rehabilitation19–21. There appears to be a potential health gain related to moderate and high intensity PA, with the highest effect on CRF and mortality observed in individuals performing high intensity exercise compared to controls or sedentary populations22,23. A recent systematic review on cancer survivors24, e.g. sedentary BC survivors25, found that walking was the strongest PA modality preference. Nonetheless, less attention has been paid to recruiting physically inactive cancer patients in exercise programmes26. It remains unclear what the optimal setting, timing during cancer trajectory, dosage and combination of exercise and health-promoting components that best facilitate patient adherence and symptom management to support physiological improvements and sustainable lifestyle changes in a physically inactive BC population27. Prevention of a decline in CRF may have a salutogenic medical effect that necessitates the integration of lifestyle modifications in BC oncology28–30 that specifically support behavioural aspects when recommended PA guidelines are not met.
However, recent randomised controlled trials (RCTs) among patients with BC have raised concerns about the potential interventional effect on CRF during adjuvant chemotherapy and at follow-up23,31,32. This concern appears to derive from a shift in chemotherapy regimens incorporating taxane-based chemotherapy, implicating an altered patient symptom profile33,34. In the randomised feasibility study preceding the present RCT, we used Danish national PA guidelines as the initial screening inclusion criteria35. This simplified two-item tool was able to detect physically inactive BC patients at onset of adjuvant chemotherapy with a high correlation of low VO2peak at baseline compared with the Scandinavian background population27. Qualitative in-depth studies indicate that a recommendation from clinicians to exercise at the specific timepoint around chemotherapy onset is ideal for recruiting subjects and initiating PA36. Moreover, we proposed an overall framework for transforming behavioural change towards re-thinking PA into post-cancer life priorities in an at-risk, physically inactive BC population36.
The present RCT aimed to compare the effects of two 12-week exercise interventions on physiological outcomes (e.g. CRF, muscle strength, body composition, blood cholesterol and insulin) and patient-reported outcomes (e.g. PA, pain, fatigue, dyspnoea and anxiety), including a 39-week follow-up. The population comprised screened, physically inactive BC patients at onset of adjuvant chemotherapy that included sequential anthracycline and cyclophosphamide with docetaxel or paclitaxel-based regimens.
Patients and methods
Research design and study population
This study was designed as an assessor single-blinded two-centre RCT comparing a structured 12-week supervised hospital-based group exercise intervention (Group 1 (Gr. 1)) versus a 12-week instructed home-based individual pedometer intervention (Group 2 (Gr. 2)) during adjuvant chemotherapy among confirmed physically inactive patients with BC. Both interventions were combined with health and symptom guidance given by a clinical nurse specialist. The primary outcome of interest was CRF (CRF/VO2peak). The RCT was analysed and published according to Consolidated Standards of Reporting Trials (CONSORT) guidelines37.
Patients who had symptomatic heart disease, bone metastasis, received neo-adjuvant chemotherapy, suffered from psychotic conditions or did not understand Danish were excluded. Patients who met the national criteria for leisure time PA were not eligible for the study27,35,38.
Setting
This study was conducted at the Departments of Oncology, Copenhagen University Hospital, Rigshospitalet and Herlev Hospital at the Centre for Integrated Rehabilitation of Cancer Patients (CIRE), Copenhagen, Denmark and was established and supported by the Danish Cancer Society and the Novo Nordic Foundation. CIRE adheres to three key intervention principles: (1) early initiation of an intervention during cancer treatment; (2) exercise/physical activity and (3) patient activation (EEX-ACT)39.
Approvals
Ethics approval and consent to participate
All patients provided informed written consent and all methods were performed in accordance with the relevant guidelines and regulations. This study was approved by the Scientific Committee of the Capital Region of Denmark (file no. H3-2013-155), and the Danish Data Protection Agency (file no. 2011-41-6349). Trial registration: Current Controlled Trials 13/03/2014 ISRCTN13816000.
Procedure
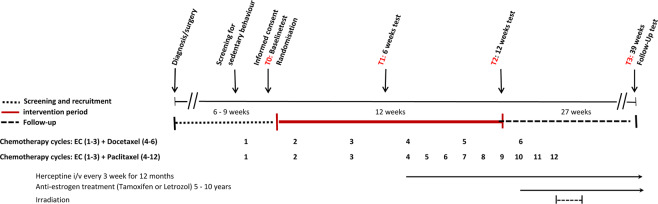
Eligible BC patients were identified with a pre-screening instrument based on PA guidelines from the Danish Health and Medicines Authority (>150 minutes of regular and moderate recreational PA and at least 2 × 20 min of strenuous exercise per week)35. The oncologists or primary nurse present during the patients’ adjuvant chemotherapy provided the initial screening assessment of these two PA parameters. A clinical nurse specialist on the exercise team subsequently gave in-depth information to physically inactive BC patients about the study’s rationale, tests and intervention. Baseline testing was planned between the first and second cycle of epirubicin and cyclophosphamide (see Fig. 1). Patients were stratified by age (<48>) and hospital setting and then randomly assigned 1:1, either to Gr. 1 or Gr. 2, by the Copenhagen Trial Unit. Informed consent was obtained from every participant included in the study.
Figure 1.
Global study overview during chemotherapy.
Interventions
The protocol for this study described and compared the two pragmatic interventions in more detail38, while the feasibility study investigated the validity of the PA screening tool related to the primary outcome (CRF), patient acceptance, interventional safety, adherence and programme feasibility27,36.
Supervised exercise intervention
12-week supervised hospital-based group exercise intervention and health counselling and symptom guidance.
Patients were offered a 12-week supervised exercise programme (PART1: six weeks, 9 h/week and PART2: six weeks, 6 h/week) in groups of 10–14 patients supervised by an exercise physiologist/physical therapist and a clinical nurse specialist. PART1 included three training sessions per week and one restorative session comprising high-low-intensity components (cardiorespiratory training on stationary bikes, resistance training, relaxation training and massage). PART2 comprised the sports floorball games, dance and circuit training. The total training volume in PART1 and PART2 corresponded to approximately a 40-metabolic equivalent of task hours per week19. Pre-exercise safety screening took place before each session and involved moderate-to-high-intensity physical training components19,38.
Instructed pedometer intervention
Individual 12-week instructed home-based pedometer intervention and health counselling and symptom guidance.
The instructed pedometer intervention was a 12-week individually organised programme designed to progressively support increased PA and was predominantly provided by a clinical nurse specialist in cancer and exercise. Patients were encouraged to enhance their PA levels and to avoid physical inactivity by integrating exercise into activities of daily living. The initial explicit goal was to achieve a low to moderate recreational PA level of 30 min/day (aerobic walking) and, stepwise, to achieve 7,500 steps/day five times per week, with the ultimate goal of incorporating 150 minutes of moderate-to-vigorous PA per week40,41. The Omron Walking Style Pro 2.0 Pedometer made it possible to visually portray the patients’ exercise achievements on a daily, weekly and monthly basis at a scheduled instruction and evaluation meeting baseline at weeks 2, 4, 6, 9 and 12. Pedometer data were transferred electronically to investigators.
Health counselling and symptom guidance
Patients in both interventions received individual test feedback and health counselling and symptom guidance at baseline and at weeks 6, 12 and 39 to help counteract barriers and to support individual motivational aspects to initiate and prioritise leisure time PA27,38. The theoretical framework for initiating and adopting behavioural change at onset and during adjuvant chemotherapy focused on recognising pre-exercise history, individual goal planning, family resources and the immediate pitfalls during anticancer treatment as crucial determinants for the individual to rethink regular exercise as pivotal for their health and post-cancer life priorities36.
Assessments, outcomes and data protection
Patient assessment of primary outcome, CRF/VO2peak, and secondary outcomes was conducted at time of inclusion, baseline (TO); week 6, midway (T1); week 12, completed (T2); and week 39, follow-up (T3) (six months after intervention and chemotherapy and irradiation completed) (Table 1). Assessments were blinded to test personnel (affiliated health professionals trained by an exercise physiologist). Fasting, whole-body dual-energy X-ray absorptiometry was performed by medical technicians who were not involved in group allocation or interventional activities at Copenhagen University Hospital, Rigshospitalet, Department of Clinical Physiology, Nuclear Medicine and PET. Figure 1 provides a global study overview.
Table 1.
Outcome assessments, instruments and timepoints.
| ASSESSMENT | INSTRUMENT/METHOD | TIMEPOINT |
|---|---|---|
|
Primary outcome Cardiorespiratory fitness/oxygen uptake |
Maximal oxygen uptake (VO2peak); incremental test on cycle ergometer (Monark Ergomedic 839E) with direct measures of respiratory gases27,38 Assessment of physical exertion on physiological tests (1.10 and >1.15) and the Borg Scale42 |
T0, T1, T2, T3 T0, T1, T2, T3 |
| Secondary physiological outcomes | ||
| Body composition (fat mass, lean mass, bone density) | Fasting whole-body DXA scan (fat percent, visceral fat, lean mass, android/gynoid ratio, bone mass density43,44 | T0, T2, T3 |
| Muscle strength |
Isokinetic maximum knee extension strength (600/sec.) (Contrex MJ Isokinetic Dynamometer CMV AG, Switzerland) Maximum load (one repetition maximum = 1RM) measured at knee extension, leg press and lateral pull (Technogym (Gambettola, Italy) |
T0, T1, T2, T3 T0, T1, T2, T3 |
| Biomarkers | Lipids, cholesterols, blood glucose, insulin14 | T0, T2, T3 |
| Metabolic syndrome profile | Composite score: cholesterol, triglyceride, blood glucose, blood pressure, android/gynoid (AG) ratio45 | |
| Blood pressure | Test, A&D Medical UA-852 Digital Blood Pressure Monitor | T0, T1, T2, T3 |
| Haemoglobin | Test | T0, T1, T2, T3 |
| Physical activity (objective) | Pedometer in home-based exercise programme, Omron Walking Style Pro 2.027 |
T0 to T2 continuous (solely pedometer group) |
| Pulse | Pulse sensor during supervised exercise intervention, Polar Team System 2, Polar, Finland | T0 to T2 continuous (supervised exercise group only) |
| Psychometric measures | ||
| Health-related quality of life | European Organization for Research and Treatment of Cancer Quality of Life Questionnaire46 | T0, T1, T2, T3 |
| Anxiety and depression | Hospital Anxiety and Depression Scale47 | T0, T1, T2, T3 |
| Physical activity (subjective), labour market, lifestyle factors (smoking, alcohol, diet) | Self-developed questionnaire with guideline-based physical activity scale35 | T0, T1, T2, T3 |
| Clinical characteristics and treatment | Medical records | Continuous |
| Diagnosis, stage, anti-cancer treatment | ||
Abbreviations: TO = baseline; T1 = week 6, midway; T2 = week 12, completed; T3 = week 39, follow-up; DXA = dual-energy X-ray absorptiometry; 1RM = one repetition maximum.
All outcomes were entered into a secure database with tracking hosted by the Copenhagen Trial Unit at Copenhagen University Hospital. Data access for study researchers was provided when the last sequentially numbered patient had completed the T2 assessment (Table 1).
Power calculation and statistics
Based on the a priori assumption that the supervised exercise intervention will be more effective in maintaining or increasing aerobic capacity (VO2peak), sample size was based on an expected mean difference between groups of VO2peak 180 ml/min and with a standard deviation (SD) of 2419,23,27. With a power of 0.9, a dropout rate of 25% and assuming that the population would comprise 70% true non-training, physically inactive patients (VO2peak measure compared against the background population)48, the final sample included n = 77 in each study arm, yielding 154 patients.
The principal analyses of primary and secondary outcomes employed the intention-to-treat approach by including all available data. Data were reported as means and SDs. Change scores and differences between change scores were derived from the linear mixed model along with corresponding 95% confidence intervals using the delta method. No attempts at imputation beyond those implicit in linear mixed models were implemented for subjects with missing follow-up data, but pattern mixture models were performed for the primary outcome dropout analyses49. Baseline demographic characteristics were provided with 95% confidence intervals.
Results
Patients
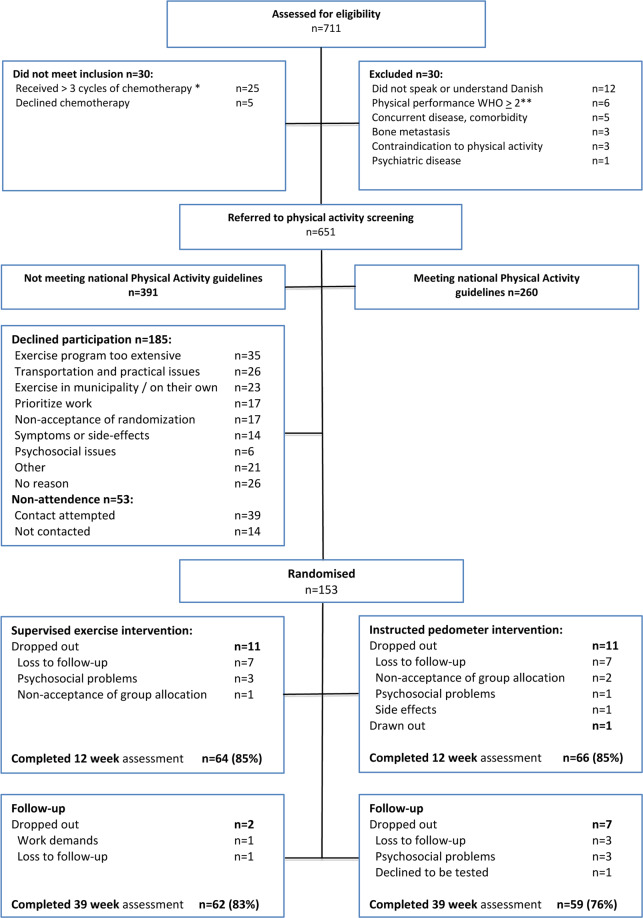
A total of 711 patients were screened for eligibility, 60% of whom were considered physically inactive prior to their diagnosis, while 40% met national PA recommendations. Study recruiters failed to establish contact with 53 patients (13.5%). With 185 patients declining to participate, the study acceptance rate was 45%. The study attrition rate was 15% for each study group at T2. At T3 it was 17% for Gr. 1 and 24% for Gr. 2 (Fig. 2). Test adherence at week 12 was 85% in both the supervised exercise and the instructed pedometer interventions. Programme adherence measured for 12-week completers was 1617/2304 or 70.2% for supervised exercise participation and 378/396 or 95.5% for PA pedometer instruction meetings.
Figure 2.
Consort flow chart: recruitment and completion.
No adverse events prompting medical attention occurred during the supervised exercise sessions. Three minor training-related injuries occurred (pulled muscle, sprained foot, back problem). Two patients experienced a lower dyspnoea threshold during moderate intensity training with no further action taken. Another two minor adverse events (temporary swelling and foot pain) related to the instructed pedometer intervention were reported.
The stratified randomisation procedure balanced baseline characteristics across groups (Table 2). Five patients (3%) reported doing some moderate to strenuous PA, while approximately one-third (36%) reported doing low to moderately active PA > 150 minutes per week. At baseline, 90% had a VO2peak lower than the age-matched background population of Scandinavian women. Participants received four courses of chemotherapy during the intervention period consisting of one or two courses of epirubicin and cyclophosphamide followed by two or three courses of docetaxel-based chemotherapy. For nineteen participants, one series of docetaxel was replaced with three series of paclitaxel as standard treatment, equally distributed between groups.
Table 2.
Baseline characteristics.
| Total (N = 153) | Supervised hospital- based exercise intervention (n=75) | Instructed pedometer intervention (n=78) | diff(95% confidence interval) | |
|---|---|---|---|---|
| Age, mean ± SD (y) | 51.7 ± 9.4 | 51.5 ± 9.6 | 52.0 ± 9.3 | −0.5 (−3.5 to 2.5) |
| BMI, mean ± SD (kg/m2) | 26.1 ± 5.1 | 26.2 ± 5.3 | 26.0 ± 4.9 | 0.2 (−1.4 to 1.9) |
| Marital status n (%) | ||||
| Single/divorced/widowed | 50 (33) | 26 (35) | 24 (31) | 3 (−12 to 18.1) |
| Married/living together | 103 (67) | 49 (65) | 54 (69) | −4 (−18.1 to 12) |
| Education n (%) | ||||
| Lower | 12 (7.9) | 7 (9.3) | 6 (7.7) | 1.6 (−8.1 to 9.2) |
| Secondary | 48 (31.8) | 25 (33.3) | 24 (30.8) | 2.5 (−12.8 to 17) |
| Advanced | 91 (60.3) | 43 (58.9) | 48 (61.5) | −2.6 (−18.3 to 13) |
| Smoking n (%) | ||||
| Never/ex-smoker* | 138 (90.8) | 68 (90.7) | 70 (89.7) | 1.0 (−9.4 to 9) |
| Current | 15 (9.8) | 7 (9.3) | 8 (10.3) | −1.0 (−9 to 9.4) |
| Alcohol | ||||
| Intake per week, median | 2 (0 to 5.5) | 2 (1 to 6.5) | 2 (0 to 5) | 0 (0 to 1) |
| Physical activity. Prediagnosis No. (%) | ||||
| Light-moderate PA <150 min/week | 99 (65) | 44 (59) | 55 (71) | −12 (−27 to 3.2) |
| Light-moderate PA >150 min/week | 54 (35) | 31 (41) | 23 (29) | 11.8 (−3.2 to 27) |
| Strenuous activity <2 × 20 min/week | 148 (97) | 73 (97) | 75 (96) | 1.2 (−4.4 to 6.8) |
| Strenuous activity >2 × 20 min/week | 5 (3) | 2 (3) | 3 (4) | −1.2 (−6.8 to 4.4) |
| Cancer stage, n (%) | ||||
| Stage 1 | 56 (36.6) | 31 (41.3) | 25 (31.1) | 9.3 (−5.9 to 24.5) |
| Stage 2 | 81 (52.9) | 36 (48.0) | 45 (57.7) | −9.7 (−25.4 to 6.1) |
| Stage 3 | 16 (10.5) | 8 (10.7) | 8 (10.3) | 0.4 (−9.3 to 10.1) |
| Breast surgery, n (%) | ||||
| Lumpectomy | 90 (58.8) | 47 (62.7) | 43 (55.1) | 7.5 (−8 to 23.1) |
| Mastectomy | 56 (36.6) | 26 (34.7) | 30 (38.5) | −3.8 (−19 to 11.5) |
| Mastectomy plus expander | 7 (4.6) | 2 (2.7) | 5 (6.4) | −3.7 (−10.3 to 2.8) |
| Chemotherapy, n (%) | ||||
| Q3Wy CE × 3 -> Q3W docetaxel × 3 | 130 (85.0) | 66 (88.0) | 64 (82.1) | 5.9 (−5.3 to 17.2) |
| Q3W CE × 3 -> weekly paclitaxel × 9 | 19 (12.4) | 8 (10.7) | 11 (14.1) | −3.4 (−13.9 to 7) |
| Other | 4 (2.6) | 1 (1.3) | 3 (3.9) | −2.5 (−7.5 to 2.5) |
| Pegfilgrastim n (%) | 114 (75) | 56 (75) | 58 (74) | |
| Radiotherapy, n (%) | 121 (79.1) | 58 (77.3) | 63 (80.8) | −3.4 (−16.3 to 9.5) |
| Herceptin, n (%) | 44 (28.8) | 22 (29.3) | 22 (28.2) | 1.1 (−13.2 to 15.5) |
| Endocrine treatment, n (%) | 118 (77.1) | 59 (78.7) | 59 (75.6) | 3.0 (−10.3 to 16.3) |
| Tamoxifen | 62 (41) | 33 (44) | 29 (37) | — |
| Letrozole | 44 (29) | 21 (28) | 23 (29) | — |
| Other | 12 (8) | 5 (7) | 7 (9) | — |
Abbreviations: diff=difference; BMI = body mass index; *cessation>1 year; Q3W = every three weeks; CE = cyclophosphamide and epirubicin.
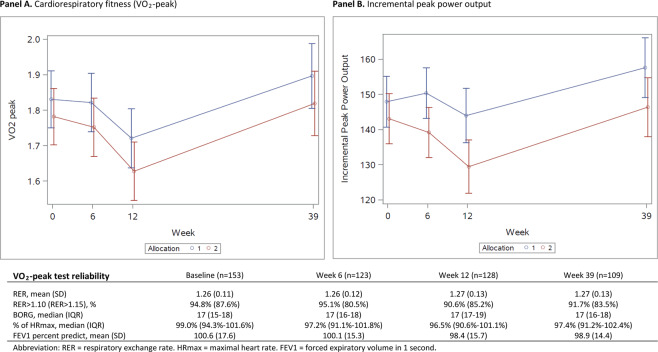
Primary outcome
The incremental test on the cycle ergometer with direct measures of respiratory gases was performed with a high reliability (Fig. 3). The primary outcome, VO2peak, decreased significantly in both study groups (Gr. 1: 6.2%, Gr. 2: 9.1%) from baseline to week 12 (T2) without any between-group difference. Both study groups restored VO2peak from week 12 to week 39 (p < 0.0001) with no significant between-group differences observed (Fig. 3, Panel A). There was a higher attrition rate in Gr. 2 among 12 patients with lower mean VO2peak baseline values, which is in accordance with the power calculation and exceeded the minimal clinical relevance of 180 ml/min at week 12. However, a dropout analysis at week 12 showed no differences in socio-demographics or treatment characteristics among completers and non-completers. At week 39 the difference in baseline VO2peak between completers and non-completers did not exceed the minimal clinically important threshold.
Figure 3.
Primary outcome.
The incremental peak power output (IPPO) differed significantly between study groups, favouring the supervised exercise intervention but did not remain significant at T3 (Fig. 3, Panel B). Similar to VO2peak measures, the mean baseline IPPO was lower among patients with no follow-up assessments.
Secondary outcomes
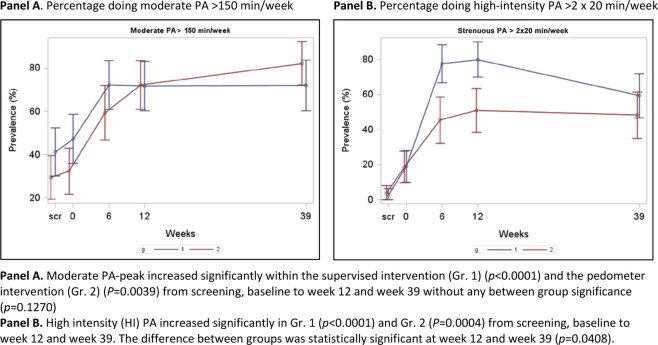
Self-reported physical activity level
Figure 4 Panels A and B presents the self-reported prevalence in PA in accordance with Danish national PA guidelines. Both study groups showed a highly significant increase in moderate PA from baseline (T0) to intervention completion (T2) that remained at the week-39 follow-up (T3). The incorporation of high-intensity PA components was increased significantly in both study groups; however, there was a significant difference in favour of the supervised exercise intervention compared to the instructed pedometer intervention during the 12-week period and at T3 (p = 0.0408). At T3, more than 50% met the national PA guidelines for adults.
Figure 4.
Percentage doing moderate and high intensity physical activity.
Muscle strength and isokinetic measures
The supervised exercise intervention showed within-group increases in strength using the one repetition maximum test (knee extension, lateral pull and leg press), with significant between-group differences visible at intervention completion (T2) and sustained at follow-up (T3). The isokinetic measures significantly favoured the supervised exercise intervention when it concluded (T2), but there was no between-group significance at week 39 (T3), where baseline isokinetic measures were restored (Table 3).
Table 3.
Strength (1RM) and isokinetic measures (Contrex).
| Variable | Allocation | T0 (n = 153) | T1 (n = 124) | T2 (n = 127) | T3 (n = 112) | (95% CI) | p | Between group | p |
|---|---|---|---|---|---|---|---|---|---|
| group | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | diff kg (95% CI) | ||||
| 1RM knee extension (kg) | 1 | 39 (10) | 47 (10) | 8 (6 to 10) | <0.0001 | 8 (5 to 10) | *<0.0001 | ||
| 2 | 41 (12) | 41 (12) | 0 (−2 to 2) | 0.8138 | — | ||||
| 1 | 39 (10) | 49 (10) | 10 (7 to 12) | <0.0001 | 8 (4 to 11) | *<0.0001 | |||
| 2 | 42 (13) | 43 (13) | 2 (−1 to 4) | 0.1493 | — | ||||
| 1 | 39 (9) | 46 (10) | 7 (4 to 9) | <0.0001 | 6 (2 to 9) | *0.0022 | |||
| 2 | 41 (12) | 43 (12) | 1 (−1 to 4) | 0.3197 | — | ||||
| 1RM lateral pull (kg) | 1 | 29 (7) | 33 (7) | 4 (2 to 6) | <0.0001 | 3 (1 to 5) | *0.0173 | ||
| 2 | 30 (8) | 32 (7) | 1 (−1 to 3) | 0.168 | — | ||||
| 1 | 30 (7) | 36 (7) | 6 (4 to 8) | <0.0001 | 4 (1 to 7) | *0.0050 | |||
| 2 | 30 (8) | 32 (7) | 2 (0 to 4) | 0.0525 | — | ||||
| 1 | 29 (7) | 33 (6) | 4 (2 to 6) | 0.0001 | 3 (0 to 6) | *0.0287 | |||
| 2 | 30 (7) | 31 (7) | 1 (−1 to 3) | 0.4116 | — | ||||
| 1RM leg press (kg) | 1 | 84 (27) | 106 (33) | 21 (16 to 26) | <0.0001 | 20 (13 to 27) | *<0.0001 | ||
| 2 | 85 (36) | 86 (38) | 1 (−4 to 6) | 0.6769 | — | ||||
| 1 | 84 (27) | 108 (36) | 24 (18 to 29) | <0.0001 | 22 (14 to 29) | *<0.0001 | |||
| 2 | 86 (36) | 87 (38) | 2 (−3 to 7) | 0.4468 | — | ||||
| 1 | 82 (26) | 103 (37) | 21 (16 to 27) | <0.0001 | 18 (10 to 26) | *<0.0001 | |||
| 2 | 84 (35) | 87 (36) | 3 (−3 to 8) | 0.2951 | — | ||||
| Contrex Peak right | 1 | 117 (23) | 118 (23) | 1 (−2 to 4) | 0.4679 | 4 (0 to 8) | 0.0518 | ||
| 2 | 119 (29) | 116 (29) | −3 (−6 to 0) | 0.0447 | |||||
| 1 | 118 (23) | 118 (22) | 1 (−3 to 4) | 0.6659 | 6 (1 to 11) | *0.0126 | |||
| 2 | 120 (29) | 114 (27) | −5 (−9 to −2) | 0.0019 | |||||
| 1 | 116 (23) | 115 (23) | −1 (−5 to 3) | 0.5158 | 2 (−3 to 8) | 0.422 | |||
| 2 | 120 (30) | 116 (29) | −4 (−8 to 0) | 0.0752 | |||||
| Contrex Peak left | 1 | 116 (23) | 120 (21) | 4 (1 to 6) | 0.0191 | 5 (1 to 9) | *0.0246 | ||
| 2 | 119 (29) | 117 (28) | −1 (−4 to 2) | 0.3916 | |||||
| 1 | 116 (24) | 118 (24) | 2 (−2 to 5) | 0.2956 | 6 (1 to 11) | *0.0269 | |||
| 2 | 119 (30) | 115 (30) | −4 (−7 to 0) | 0.0354 | — | ||||
| 1 | 116 (22) | 116 (22) | 1 (−4 to 5) | 0.7467 | 4 (−2 to 11) | 0.1879 | |||
| 2 | 121 (30) | 116 (28) | −4 (−8 to 1) | 0.1245 | — |
Abbreviations: diff=difference; TO = baseline; T1 = week 6, midway; T2 = week 12, completed; T3 = week 39, follow-up; n = maximum n for patients at baseline test and associated follow-up test; *=between-group significance p < 0.05; SD = standard deviation; CI = confidence interval; diff = difference; 1RM = one repetition maximum test; allocation group 1 = supervised exercise intervention; allocation group 2 = instructed pedometer intervention.
Body composition
Body weight remained stable during the intervention period, with a significant decrease at T3 in the supervised exercise intervention, though there were no between-group differences. Lean body mass increased significantly from T0-T2 and dropped to baseline levels at T3. Fat mass decreased in the supervised exercise intervention at follow-up (T3) with no difference observed between groups. In both groups there was a significant loss of bone mass content from T0 to T3, while bone mass density had increased significantly in both study groups at T3. T-scores decreased significantly from T0 to T3 without reaching levels for osteopenia50. (Table 4).
Table 4.
DXA scan body mass measures.
| Variable | Group | T0: Mean (SD) (n = 153) | T2: Mean (SD) (n = 128) | T3: Mean (SD) (n = 115) | Delta (95% CI) | p | diff (95% CI) | p |
|---|---|---|---|---|---|---|---|---|
| Weight (kg) | 1 | 74.7 (14.3) | 74.6 (13.9) | −0.1 (−1 to 0.8) | 0.8320 | −0.4 | 0.5866 | |
| 2 | 73 (13.7) | 73.3 (14) | 0.3 (−0.7 to 1.2) | 0.576 | (−1.7 to 1.0) | |||
| 1 | 75.1 (14.7) | 74 (13.6) | −1.1 (−2.1 to −0.2) | 0.0215 | −0.6 | 0.4068 | ||
| 2 | 72.7 (13) | 72.1 (13.2) | −0.6 (−1.5 to 0.4) | 0.2594 | (−2.0 to 0.8) | |||
| Fat mass (g) | 1 | 29342 (10039) | 28169 (9815) | −1152 (−2307 to 3) | 0.0507 | −834 (−2457 to 788) | 0.3120 | |
| 2 | 28060 (9358) | 27767 (9601) | −318 (−1457 to 822) | 0.5834 | ||||
| 1 | 29859 (10179) | 30269 (11690) | 456 (−736 to 1648) | 0.4522 | 795 (−894 to 2484) | 0.3548 | ||
| 2 | 27678 (8754) | 27353 (8707) | −339 (−1536 to 858) | 0.5770 | ||||
| Bone mass content (g) | 1 | 2497.2 (346.2) | 2492.5 (350.8) | −4.2 (−22.8 to 14.5) | 0.6608 | −13.3 | 0.3191 | |
| 2 | 2407.9 (337.9) | 2417.2 (333.2) | 9.1 (−9.3 to 27.5) | 0.3299 | (−39.4 to 12.9) | |||
| 1 | 2482.7 (346.4) | 2433.1 (335.5) | −48.4 (−67.6 to −29.1) | <0.0001 | −12.9 | 0.3513 | ||
| 2 | 2412.3 (348.6) | 2376.5 (344.6) | −35.4 (−54.8 to −16.1) | 0.0004 | (−40.2 to 14.3) | |||
| Lean body mass (g) | 1 | 42842 (5218) | 43916 (5304) | 1048 (592 to 1505) | <0.0001 | 486 (−156 to 1127) | 0.1372 | |
| 2 | 42545 (5465) | 43102 (5710) | 563 (112 to 1013) | 0.0146 | ||||
| 1 | 42733 (5380) | 42722 (5057) | −78 (−549 to 394) | 0.7456 | 82 (−586 to 750) | 0.8086 | ||
| 2 | 42556 (5402) | 42442 (5398) | −160 (−633 to 314) | 0.5064 | ||||
| Bone mineral density | 1 | 1.20 (0.12) | 1.23 (0.23) | 0.03 (0.00 to 0.07) | 0.0696 | 0.02 (−0.03 to 0.07) | 0.459 | |
| 2 | 1.16 (0.19) | 1.18 (0.12) | 0.01 (−0.02 to 0.05) | 0.4283 | ||||
| 1 | 1.19 (0.11) | 1.18 (0.11) | −0.01 (−0.04 to 0.03) | 0.6288 | 0.00 (−0.05 to 0.05) | 0.9815 | ||
| 2 | 1.16 (0.19) | 1.15 (0.12) | −0.01 (−0.04 to 0.03) | 0.6066 | ||||
| T-score (SD) | 1 | 1.21 (1.19) | 1.19 (1.18) | −0.02 (−0.08 to 0.05) | 0.6585 | −0.04 (−0.13 to 0.06) | 0.4343 | |
| 2 | 0.93 (1.24) | 0.95 (1.18) | 0.02 (−0.04 to 0.09) | 0.5056 | ||||
| 1 | 1.14 (1.15) | 0.99 (1.10) | −0.15 (−0.22 to −0.08) | <0.0001 | 0.03 (−0.07 to 0.13) | 0.5395 | ||
| 2 | 0.86 (1.24) | 0.69 (1.21) | −0.18 (−0.25 to −0.11) | <0.0001 |
Abbreviations: TO = baseline; T1 = week 6, midway; T2 = week 12, completed; T3 = week 39, follow-up; DXA = dual-energy X-ray absorptiometry; n = maximum n for patients at baseline test and associated follow-up test; 1 = group 1 supervised exercise intervention; 2 = group 2 pedometer exercise intervention; SD = standard deviation; CI = confidence interval.
Blood markers
Blood markers showed no between-group difference (P-glucose, P-insulin, P-cholesterol, high-density lipoprotein cholesterol, high-density lipoprotein cholesterol), except for P-triglyceride, which was significantly lowered in the pedometer group at week 39 (T3). Total cholesterol generally remained elevated (5.3–5.4 SD 1.1) and unchanged. Blood glucose and cholesterol remained relatively stable, though a slightly significant increase was visible for high-density lipoprotein in Gr. 2 at follow-up (T3). For a full analysis see online Supplemental Material.
A composite metabolic syndrome score of five biological and physiological variables was calculated for each group (Fig. 5). Three (or more) out of five risk variables were classified as a case. The findings revealed a significant improvement in the metabolic risk profile from baseline to week 39 for both groups (Gr. 1, p = 0.0493; Gr. 2, p = 0.0156).
Figure 5.
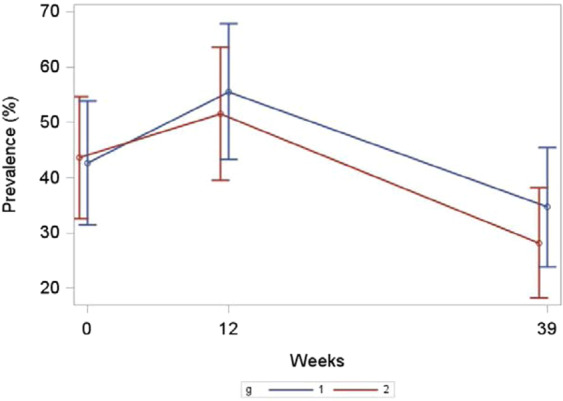
Metabolic syndrome.
Selected patient-reported outcomes
There were no significant between-group changes on the European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire (EORTC QLQ) or the Hospital Anxiety Depression Scale. Fatigue, pain, dyspnoea and insomnia worsened significantly during chemotherapy without between-group differences and was restored significantly to baseline levels at follow-up. Anxiety remained relatively stable during the study period, whereas depression increased from baseline to T2 and was significantly below the baseline values at T3 (Table 5).
Table 5.
Selected patient-reported outcomes.
| Panel A: | Group | T0 (n = 153) | T1 (n = 125) | T2 (n = 126) | T3 (n = 117) | (95% CI) | p Value | Diff. | p |
|---|---|---|---|---|---|---|---|---|---|
| HADS | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | (95% CI) | Value | |||
| HADS Anxiety | 1 | 4.4 (4.0) | 4.4 (3.9) | 1.0 (0.4 to 1.7) | 0.002 | 0.9 (0.0 to 1.8) | 0.0591 | ||
| (Scale 0–21) | 2 | 5.2 (3.8) | 4.8 (3.9) | 0.2 (−0.5 to 0.8) | 0.6483 | ||||
| 1 | 4.4 (4) | 4.0 (3.7) | 1.3 (0.5 to 2.1) | 0.0017 | −0.3 (−1.4 to 0.8) | 0.6251 | |||
| 2 | 5.2 (3.9) | 5.3 (3.9) | 1.5 (0.8 to 2.3) | 0.0001 | |||||
| 1 | 4.3 (4.0) | 3.9 (3.7) | −0.8 (−1.5 to −0.1) | 0.0304 | −0.3 (−1.4 to 0.7) | 0.5193 | |||
| 2 | 5.0 (3.8) | 4.8 (4.0) | −0.5 (−1.2 to 0.3) | 0.2108 | |||||
| HADS Depression | 1 | 3.3 (2.8) | 4.3 (3.3) | 1.0 (0.4 to 1.7) | 0.002 | 0.9 (0 to 1.8) | 0.0591 | ||
| (Scale 0–21) | 2 | 3.7 (3.5) | 4.0 (3.7) | 0.2 (−0.5 to 0.8) | 0.6483 | ||||
| 1 | 3.3 (2.8) | 4.6 (3.4) | 1.3 (0.5 to 2.1) | 0.0017 | −0.3 (−1.4 to 0.8) | 0.6251 | |||
| 2 | 3.7 (3.4) | 5.4 (4.0) | 1.5 (0.8 to 2.3) | 0.0001 | |||||
| 1 | 3.2 (2.8) | 2.5 (2.7) | −0.8 (−1.5 to −0.1) | 0.0304 | −0.3 (−1.4 to 0.7) | 0.5193 | |||
| 2 | 3.4 (3.2) | 3.1 (3.1) | −0.5 (−1.2 to 0.3) | 0.2108 | |||||
| Panel B: Selected EORTC scales | |||||||||
| EORTC Global Health Status | 1 | 60 (21) | 52 (24) | −8 (−14 to −3) | 0.0021 | −6 (−14 to 1) | 0.1125 | ||
| (Scale 0–100) | 2 | 60 (21) | 58 (23) | −2 (−8 to 3) | 0.3780 | ||||
| 1 | 61 (21) | 56 (22) | −4 (−10 to 2) | 0.1905 | 6 (−2 to 15) | 0.1288 | |||
| 2 | 62 (22) | 51 (20) | −11 (−16 to −5) | 0.0005 | |||||
| 1 | 62 (21) | 76 (19) | 15 (9 to 21) | <0.0001 | 8 (0 to 16) | 0.0517 | |||
| 2 | 63 (21) | 69 (23) | 7 (1 to 13) | 0.0208 | |||||
| EORTC Fatigue | 1 | 42 (25) | 52 (26) | 10 (3 to 17) | 0.0036 | 8 (−2 to 18) | 0.0977 | ||
| (Scale 0–100) | 2 | 49 (25) | 50 (26) | 2 (−5 to 9) | 0.5333 | ||||
| 1 | 42 (25) | 58 (27) | 17 (9 to 25) | <0.0001 | 5 (−6 to 16) | 0.3491 | |||
| 2 | 48 (25) | 59 (25) | 12 (4 to 19) | 0.0033 | |||||
| 1 | 40 (25) | 29 (24) | −12 (−18 to −5) | 0.0005 | −4 (−13 to 6) | 0.4300 | |||
| 2 | 46 (24) | 38 (26) | −8 (−15 to −1) | 0.0168 | |||||
| 1 | 17 (21) | 31 (32) | 15 (7 to 24) | 0.0004 | 9 (−3 to 21) | 0.1357 | |||
| EORTC Pain | 2 | 22 (26) | 29 (31) | 6 (−2 to 15) | 0.1236 | ||||
| (Scale 0–100) | 1 | 18 (21) | 32 (30) | 16 (8 to 24) | <0.0001 | −3 (−14 to 8) | 0.6209 | ||
| 2 | 22 (26) | 42 (29) | 19 (11 to 27) | <0.0001 | |||||
| 1 | 17 (20) | 22 (22) | 6 (−1 to 12) | 0.0778 | 6 (−3 to 15) | 0.1973 | |||
| 2 | 20 (24) | 21 (23) | 0 (−7 to 6) | 0.9464 | |||||
| 1 | 10 (22) | 20 (23) | 11 (4 to 17) | 0.0026 | 4 (−5 to 14) | 0.3656 | |||
| EORTC Dyspnoea | 2 | 13 (22) | 19 (27) | 6 (−1 to 13) | 0.0707 | ||||
| (Scale 0–100) | 1 | 10 (22) | 28 (31) | 18 (9 to 27) | 0.0001 | 2 (−11 to 14) | 0.7901 | ||
| 2 | 12 (22) | 30 (31) | 16 (8 to 25) | 0.0003 | |||||
| 1 | 10 (22) | 10 (19) | 0 (−7 to 7) | 0.9566 | 0 (−10 to 9) | 0.9885 | |||
| 2 | 8 (17) | 12 (22) | 0 (−6 to 7) | 0.9407 | |||||
Abbreviations: *=between-group difference; TO = baseline; T1 = week 6, midway; T2 = week 12, completed; T3 = week 39, follow-up; 1 = group 1 supervised exercise intervention; 2 = group 2 pedometer exercise intervention; SD = standard deviation; CI = confidence interval; EORTC = European Organisation for Research and Treatment of Cancer − Quality of Life Questionnaire; HADS = Hospital Anxiety Depression Scale.
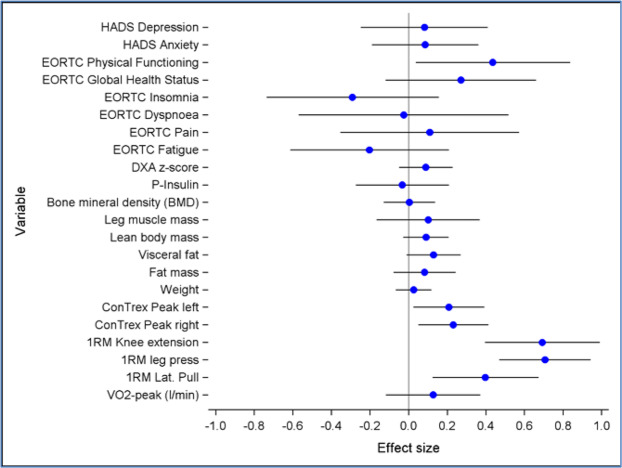
Figure 6 provides an overview and comparison of effect sizes (cohens d) between study groups at T2 assessment for the primary outcome, VO2peak, and secondary measures. In general, effect sizes reflect benefits favouring the supervised exercise intervention in physiological outcomes and physical functioning, whereas effects on patient-reported measures were heterogenous between groups.
Figure 6.
Global overview of effect sizes.
Explorative analysis of VO2 peak
By merging VO2peak data across study groups, we dichotomised VO2peak into positive responders/unchanged (n = 41, 32%) and negative responders >2% (n = 87, 68%) from baseline to week 12. Positive/unchanged responders had improved clinical effects on EORTC QLQ scores versus negative responders: fatigue (51.3 SD 28 vs. 60.0 SD 24.6); pain (26.9 SD 25.8 vs. 41.7 SD 30.7); and dyspnoea (17.9 SD 26.3 vs. 30.7 SD 28.9). There were no significant differences in baseline scores or at follow-up (T3) between responders and decliners.
Correlation analyses between changes in (delta), physiological outcomes (VO2peak, muscle strength) and (delta) selected patient-reported outcome scales (fatigue, pain, dyspnoea) at week 12 revealed a very weak correlation between strength and delta fatigue, pain and dyspnoea, whereas VO2peak demonstrated a significant weak correlation on EORTC QLQ fatigue, pain and dyspnoea. Parametric testing indicated that an improvement on VO2peak with 100 ml/min would decrease fatigue by 4 points, whereas, e.g. a gain in lean body mass by 0.5 kg would result in a decrease in fatigue by 1.4 points. Delta VO2peak (100 ml) or delta leg press (10 kg) improvements were significantly correlated with reduced pain scores (average 3 EORTC QLQ points). (Table 6).
Table 6.
Correlations and parametric values between delta physiological outcomes and delta selected patient-reported outcome scales.
| Panel A: Correlation | Δ Fatigue | Δ Pain | Δ Dyspnoea |
|---|---|---|---|
| Δ Contrex right | −0.12 (−0.29, 0.06) | −0.01 (−0.19, 0.16) | 0.04 (−0.14, 0.22) |
| Δ Contrex left | −0.04 (−0.22, 0.13) | −0.03 (−0.21, 0.14) | −0.04 (−0.22, 0.13) |
| Δ Lean mass | −0.18 (−0.34, 0.00) | −0.11 (−0.28, 0.07) | −0.04 (−0.21, 0.14) |
| Δ Leg press | −0.07 (−0.25, 0.11) | −0.22 (−0.38, −0.04) | −0.12 (−0.29, 0.07) |
| Δ VO2peak | −0.31 (−0.46, −0.14) | −0.25 (−0.41, −0.08) | −0.27 (−0.42, −0.09) |
| Panel B: Parametric | |||
| Δ Contrex right (5 N m) | −1.3 (−3.3, 0.7) | −0.1 (−2.1, 1.8) | 0.5 (−1.7, 2.7) |
| Δ Contrex left (5 N m) | −0.5 (−2.4, 1.4) | −0.4 (−2.3, 1.5) | −0.5 (−2.6, 1.6) |
| Δ Lean Mass (0.5 kg) | −1.4 (−2.8, 0.0) | −0.8 (−2.3, 0.6) | −0.3 (−2.0, 1.3) |
| Δ Leg press (10 kg) | −0.8 (−3.2, 1.7) | −2.7 (−5.2, −0.3) | −1.8 (−4.5, 1.0) |
| Δ VO2peak (100 ml) | −4.0 (−6.1, −1.8) | −3.2 (−5.4, −1.1) | −3.7 (−6.1, −1.3) |
Discussion
Contrary to our a priori hypothesis, we observed no in-between group difference on the primary outcome, VO2peak (CRF), in supervised hospital-based moderate to high intensity group exercise intervention versus an instructed home-based individual pedometer programme. A decline in VO2peak was observed in both groups after onset of chemotherapy, and VO2peak was fully restored at week 39. Our results disprove a lasting CRF loss due to chemotherapy, as previously suggested14–16. Other intervention RCTs have found an even further reduction in CRF during adjuvant taxane-based chemotherapy, though some natural improvement in CRF may occur in RCT controls23,31,32. In observational studies CRF has been inversely associated with mortality in clinical and population cohorts11,51–54. Furthermore, physically inactive survivors of BC are at increased risk of post-treatment cardiovascular disease and cancer recurrence29,55,56, and only few powered intervention RCTs have explored the adaptationally positive impact of regular PA on disease-free survival and all-cause mortality57. There is also a lack of studies explicitly recruiting cancer patients to PA interventions during treatment who were physically inactive/sedentary prediagnosis26,58. Consequently, we cannot clarify whether results from the vast majority of studies and meta-analyses are derived from selective populations of cancer patients with a predominant preference for PA21,59–61, or whether recruitment, intervention activities and outcome expectations can be transferred to an at-risk subgroup with less PA experience and beyond the context of a trial environment. Patient-reported PA levels, a secondary outcome, supported our physiological findings, and we suggest that a loss in CRF can be restored by sustainable adaption to national PA guidelines across intervention approaches and among physically inactive survivors with BC.
The two-item screening tool based on national PA guidelines identified 60% as being physically inactive against 40% meeting PA guidelines at chemotherapy onset. The study acceptance rate of 45% is comparable to exercise RCTs that do not exclusively recruit screened, physically inactive patients with BC referred to adjuvant chemotherapy32,62. The randomly considered study attrition rate was acceptable for both groups. In addition to the level of physical inactivity identified, we observed several physiological and biological indicators of metabolic syndrome that were raised among 42% of the recruited population, constituting an elevated risk profile. At baseline, 23 patients (15%) were medically treated for a cardiovascular risk component and were moderately overweight, with an average body mass index >26 (SD 5.15]. Several studies have observed weight gain following BC treatment associated with an increased metabolic long-term risk63–65. Metabolic syndrome may increase BC recurrence three-fold and BC-specific mortality approximately two-fold66. CRF and muscle strength have been shown to have an inversely independent association on metabolic cardiovascular risk factors in adults67. Likewise, moderate to vigorous leisure time PA is inversely associated with metabolic syndrome68. Thus, the improved metabolic risk profile and stable weight development achieved in the present study validate the cohesion of physiological and patient-reported PA outcomes, suggesting the treatment complementary effectiveness of the interventions to improve important clinical risk outcomes. We believe this transverse cohesion, along with the systematic recruitment of physically inactive BC patients, constitutes the primary clinical importance in the approach and findings of this trial.
In line with several RCTs and metanalysis evaluating combined cardio and resistance exercise interventions31,32,69,70, our findings indicate a significant positive effect on physical functioning in the supervised exercise group, with an effect size considered subjectively meaningful71. Similarly, in agreement with previous studies evaluating the effect of resistance exercise19,23,31,32,72, we found that participation in the supervised exercise intervention maintained or improved muscle strength in the lower and upper extremities, with one repetition maximum strength increases corresponding to 20% and 22% (knee extension and leg press, respectively) and 17% in the lateral pull exercise post-intervention. Muscle strength after chemotherapy for BC survivors without intervention has been found to be 12–16% lower in the upper extremities, and 25% lower in the lower extremities, compared to healthy women72. This is of potential clinical importance as declines in muscle strength have been associated with loss of function in BC survivors, affecting the ability to perform recreational and daily living activities73. Considering the relationship between muscle strength and sarcopenia, our findings have potential clinical implications as BC sarcopenia, even early stage, has been associated with poorer survival and shorter time to tumour progression74. Our results indicate that exercise during adjuvant chemotherapy, with supervised heavy-load resistance training, provides the strongest effects and may ameliorate the detrimental effects of chemotherapy on skeletal muscles72,74,75, irrespective of cardiorespiratory deterioration.
EORTC patient-reported outcomes showed an increasing symptom profile on, e.g. fatigue, pain, dyspnoea and insomnia during the intervention period and without any in-between group differences. However, the explorative analyses showed that, across groups, the individuals who were able to maintain or improve CRF (33%) showed significant improvement on several EORTC QLQ scales. The correlation analyses between physiological outcomes and patient-reported outcomes suggested that CRF is inversely correlated with reduced fatigue, pain and dyspnoea, while muscle indicators had almost no correlation. This finding is in line with the study’s rationale and overall assumptions38. These results support the notion that pathways and interplay between physiological measures and patient perceived benefits on symptomatology and side effects are complex and far from being understood31.
The two present interventions were designed to appeal to and motivate BC patients who did not meet the Danish national PA guidelines and were concurrently referred to adjuvant chemotherapy. The findings showed that interventions were equally effective with respect to supporting behavioural changes towards adopting an adequate transition to PA in everyday life activities for several participants, during and post chemotherapy. It has been suggested that health professional counselling rooted in cognitive behavioural therapy and possessing a central basis in PA interventions has a positive effect on maintaining PA adherence rate for patients with cancer76. Two studies preceding the present RCT indicated that recommendations from the oncologist, the screening procedure, the objective physiological tests and the counselling sessions with the clinical nurse specialist were crucial to patient recruitment, adherence and patients feeling safe when initiating PA during their treatment cycles27,36. The recurring counselling sessions were carried out face to face between the clinical nurse specialist and patients. Besides serving as motivation, these sessions, by incorporating social cognitive theory77 and behavioural change strategies36,78, targeted exercise intolerance by taking into account the individual patient’s symptom profile, reducing side effect-related dropout and by addressing the habits and structural barriers in daily living. Accordingly, we suggest that interventions should be integrated into a treatment context that aligns with patient needs and preferences in terms of becoming physically active during adjuvant chemotherapy36. Consequently, exercise oncology ideology based primarily on physiological mechanisms and exercise prescriptions79 may potentially underestimate the role the human dynamic plays, as well as the environmental impact on motivation and PA sustainability36.
One of the strengths in the present study is the focus on a longitudinal clinical profile in PA screened survivors with breast cancer by using gold standard measures on various physiological outcomes with a low risk of bias. The clinicians who pre-screened for physical inactivity present at adjuvant chemotherapy secured recruitment of the targeted population at risk, underpinning the validity and reliability of the two-item screening tool. A potential selection bias relates to the fact that patients were likely to be more highly educated. This is consistent, on the one hand, with the inverse social gradient in BC populations80,81, but could indicate, on the other, selection of the most highly motivated among the less active. It is a weakness that we did not incorporate a non-intervention control group, but this was not possible for ethical reasons associated with standard hospital exercise programmes19. The 15% dropout rate at T2 may have caused a selection bias, though we did not identify explanatory variables significantly associated with the attrition.
Conclusions
In conclusion, our study shows that beneficial effects can be obtained from supervised intensive, hospital-based exercise programmes but also from a less demanding pedometer exercise intervention under guidance and counselling from committed health professionals. Both interventions were effective in supporting formerly inactive BC patients in sustaining PA activities during and following adjuvant treatment. Restored CRF at follow-up and metabolic indicators showed an improved health profile and subsequently a decline in cardiovascular risk. Clinicians should address patient-specific modifiable risks within a medical and preventative scope, especially to support motivational progress and to counteract cumbersome barriers, such as treatment-related toxicity, factors that constantly challenge motivation and exercise intolerance in physically inactive BC survivors.
Supplementary information
Acknowledgements
This research was supported by grants from the Center for Integrated Rehabilitation of Cancer patients (CIRE), which was established in 2011 and is supported by the Danish Cancer Society and the Novo Nordisk Foundation. The project also received grants from TrygFonden Denmark and Toyota-Fonden Denmark. The Copenhagen Trial Unit CTU hosted and protected data until study completion.
Author contributions
All authors have contributed substantially to the creation and revision of the manuscript. T.M. was the first author and primary investigator and, jointly with L.A., C.A., C.L., K.B., B.E. and M.R. had a leading role during the writing process. K.B.C., T.M. and C.L. were responsible for study analyses. K.B., C.A., C.L. and T.M. had a leading role in the study coordination of tests and intervention activities. C.A., C.L., T.M. and K.B. were primarily responsible for delivering the interventions. P.O. performed, analysed and coordinated the DXA scan. C.K., P.T., T.B., U.B., M.T. and B.E. screened and identified patients in the oncology departments. T.M., C.A., C.K., P.T., T.B. and U.B. informed patients and obtained written informed consent.
Data availability
Anonymous data are available upon from the corresponding author (TM) upon reasonable request. The datasets generated and/or analysed during the current study are available from the corresponding author upon reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-66513-9.
References
- 1.Global status report on noncommunicable diseases 2010. (WHO, 2011).
- 2.Colditz GA, Wolin KY, Gehlert S. Applying what we know to accelerate cancer prevention. Sci. Transl. Med. 2012;4:127rv124. doi: 10.1126/scitranslmed.3003218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Friedenreich CM, Gregory J, Kopciuk KA, Mackey JR, Courneya KS. Prospective cohort study of lifetime physical activity and breast cancer survival. Int. J. Cancer. 2009;124:1954–1962. doi: 10.1002/ijc.24155. [DOI] [PubMed] [Google Scholar]
- 4.Holmes MD, Chen WY, Feskanich D, Kroenke CH, Colditz GA. Physical activity and survival after breast cancer diagnosis. JAMA. 2005;293:2479–2486. doi: 10.1001/jama.293.20.2479. [DOI] [PubMed] [Google Scholar]
- 5.Dethlefsen C, et al. Exercise-Induced Catecholamines Activate the Hippo Tumor Suppressor Pathway to Reduce Risks of Breast Cancer Development. Cancer Res. 2017;77:4894–4904. doi: 10.1158/0008-5472.CAN-16-3125. [DOI] [PubMed] [Google Scholar]
- 6.Hojman P, Gehl J, Christensen JF, Pedersen BK. Molecular Mechanisms Linking Exercise to Cancer Prevention and Treatment. Cell Metab. 2018;27:10–21. doi: 10.1016/j.cmet.2017.09.015. [DOI] [PubMed] [Google Scholar]
- 7.Margolis KL, et al. Physical activity in different periods of life and the risk of breast cancer: the Norwegian-Swedish Women’s Lifestyle and Health cohort study. Cancer Epidemiol. Biomarkers Prev. 2005;14:27–32. [PubMed] [Google Scholar]
- 8.Hursting SD, Dunlap SM. Obesity, metabolic dysregulation, and cancer: a growing concern and an inflammatory (and microenvironmental) issue. Ann. N. Y. Acad. Sci. 2012;1271:82–87. doi: 10.1111/j.1749-6632.2012.06737.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peel JB, et al. A prospective study of cardiorespiratory fitness and breast cancer mortality. Med. Sci. Sports Exerc. 2009;41:742–748. doi: 10.1249/MSS.0b013e31818edac7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mora S, et al. Ability of exercise testing to predict cardiovascular and all-cause death in asymptomatic women: a 20-year follow-up of the lipid research clinics prevalence study. JAMA. 2003;290:1600–1607. doi: 10.1001/jama.290.12.1600. [DOI] [PubMed] [Google Scholar]
- 11.Barlow CE, et al. Cardiorespiratory fitness and long-term survival in “low-risk” adults. J. Am. Heart Assoc. 2012;1:e001354. doi: 10.1161/JAHA.112.001354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barlow CE, Shuval K, Balasubramanian BA, Kendzor DE, Pettee Gabriel K. Sitting Time, Physical Activity, and Cardiorespiratory Fitness: Cooper Center Longitudinal Study Cohort. J. Phys. Act. Health. 2016;13:17–23. doi: 10.1123/jpah.2014-0430. [DOI] [PubMed] [Google Scholar]
- 13.Gulati M, et al. The prognostic value of a nomogram for exercise capacity in women. N. Engl. J. Med. 2005;353:468–475. doi: 10.1056/NEJMoa044154. [DOI] [PubMed] [Google Scholar]
- 14.Jones LW, et al. Cardiopulmonary function and age-related decline across the breast cancer survivorship continuum. J. Clin. Oncol. 2012;30:2530–2537. doi: 10.1200/JCO.2011.39.9014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klassen O, et al. Cardiorespiratory fitness in breast cancer patients undergoing adjuvant therapy. Acta Oncol. 2014;53:1356–1365. doi: 10.3109/0284186X.2014.899435. [DOI] [PubMed] [Google Scholar]
- 16.Kirkham AA, Davis MK. Exercise Prevention of Cardiovascular Disease in Breast Cancer Survivors. J. Oncol. 2015;2015:917606. doi: 10.1155/2015/917606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jones LW, Haykowsky MJ, Swartz JJ, Douglas PS, Mackey JR. Early breast cancer therapy and cardiovascular injury. J. Am. Coll. Cardiol. 2007;50:1435–1441. doi: 10.1016/j.jacc.2007.06.037. [DOI] [PubMed] [Google Scholar]
- 18.Herman DR, Ganz PA, Petersen L, Greendale GA. Obesity and cardiovascular risk factors in younger breast cancer survivors: The Cancer and Menopause Study (CAMS) Breast Cancer Res. Treat. 2005;93:13–23. doi: 10.1007/s10549-005-2418-9. [DOI] [PubMed] [Google Scholar]
- 19.Adamsen L, et al. Effect of a multimodal high intensity exercise intervention in cancer patients undergoing chemotherapy: randomised controlled trial. BMJ. 2009;339:b3410. doi: 10.1136/bmj.b3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fong DY, et al. Physical activity for cancer survivors: meta-analysis of randomised controlled trials. BMJ. 2012;344:e70. doi: 10.1136/bmj.e70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Furmaniak AC, Menig M, Markes MH. Exercise for women receiving adjuvant therapy for breast cancer. Cochrane Database Syst. Rev. 2016;9:CD005001. doi: 10.1002/14651858.CD005001.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leitzmann MF, et al. Physical activity recommendations and decreased risk of mortality. Arch. Intern. Med. 2007;167:2453–2460. doi: 10.1001/archinte.167.22.2453. [DOI] [PubMed] [Google Scholar]
- 23.Courneya KS, et al. Effects of exercise dose and type during breast cancer chemotherapy: multicenter randomized trial. J. Natl Cancer Inst. 2013;105:1821–1832. doi: 10.1093/jnci/djt297. [DOI] [PubMed] [Google Scholar]
- 24.Wong JN, McAuley E, Trinh L. Physical activity programming and counseling preferences among cancer survivors: a systematic review. Int. J. Behav. Nutr. Phys. Act. 2018;15:48. doi: 10.1186/s12966-018-0680-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rogers LQ, Courneya KS, Verhulst S, Markwell SJ, McAuley E. Factors associated with exercise counseling and program preferences among breast cancer survivors. J. Phys. Act. Health. 2008;5:688–705. doi: 10.1123/jpah.5.5.688. [DOI] [PubMed] [Google Scholar]
- 26.Bourke, L. et al. Interventions for promoting habitual exercise in people living with and beyond cancer. Cochrane Database Syst Rev, CD010192, 10.1002/14651858.CD010192.pub2 (2013). [DOI] [PubMed]
- 27.Moller T, et al. The challenge of preserving cardiorespiratory fitness in physically inactive patients with colon or breast cancer during adjuvant chemotherapy: a randomised feasibility study. BMJ open. sport. Exerc. Med. 2015;1:e000021. doi: 10.1136/bmjsem-2015-000021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lenihan DJ, Cardinale D, Cipolla CM. The compelling need for a cardiology and oncology partnership and the birth of the International CardiOncology Society. Prog. Cardiovasc. Dis. 2010;53:88–93. doi: 10.1016/j.pcad.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 29.Schmitz KH, Prosnitz RG, Schwartz AL, Carver JR. Prospective surveillance and management of cardiac toxicity and health in breast cancer survivors. Cancer. 2012;118:2270–2276. doi: 10.1002/cncr.27462. [DOI] [PubMed] [Google Scholar]
- 30.Jones LW, et al. Exercise and Risk of Cardiovascular Events in Women With Nonmetastatic Breast Cancer. J. Clin. Oncol. 2016;34:2743–2749. doi: 10.1200/JCO.2015.65.6603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mijwel S, et al. Highly favorable physiological responses to concurrent resistance and high-intensity interval training during chemotherapy: the OptiTrain breast cancer trial. Breast Cancer Res. Treat. 2018;169:93–103. doi: 10.1007/s10549-018-4663-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van Waart H, et al. Effect of Low-Intensity Physical Activity and Moderate- to High-Intensity Physical Exercise During Adjuvant Chemotherapy on Physical Fitness, Fatigue, and Chemotherapy Completion Rates: Results of the PACES Randomized Clinical Trial. J. Clin. Oncol. 2015;33:1918–1927. doi: 10.1200/JCO.2014.59.1081. [DOI] [PubMed] [Google Scholar]
- 33.Ejlertsen B, et al. Adjuvant Cyclophosphamide and Docetaxel With or Without Epirubicin for Early TOP2A-Normal Breast Cancer: DBCG 07-READ, an Open-Label, Phase III, Randomized Trial. J. Clin. Oncol. 2017;35:2639–2646. doi: 10.1200/JCO.2017.72.3494. [DOI] [PubMed] [Google Scholar]
- 34.Ejlertsen, B. Adjuvant chemotherapy in early breast cancer. Dan Med J63 (2016). [PubMed]
- 35.Physical activity: recommendations for adults (18–64 years old), https://www.sst.dk/en/health-and-lifestyle/physical-activity# (2010).
- 36.Adamsen L, Andersen C, Lillelund C, Bloomquist K, Moller T. Rethinking exercise identity: a qualitative study of physically inactive cancer patients’ transforming process while undergoing chemotherapy. BMJ open. 2017;7:e016689. doi: 10.1136/bmjopen-2017-016689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moher D, et al. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. Int. J. Surg. 2012;10:28–55. doi: 10.1016/j.ijsu.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 38.Moller T, et al. At cancer diagnosis: a ‘window of opportunity’ for behavioural change towards physical activity. A randomised feasibility study in patients with colon and breast cancer. BMJ open. 2013;3:e003556. doi: 10.1136/bmjopen-2013-003556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Adamsen, L. et al. Exercise in cancer survivors: The Center for Integreted Rehabilitation for Cancer Patients (CIRE) discusses the role that exercise can play for cancer survivors. Horizon 2020 Projects - Portal, 100–103 (2015).
- 40.Bravata DM, et al. Using pedometers to increase physical activity and improve health: a systematic review. Jama. 2007;298:2296–2304. doi: 10.1001/jama.298.19.2296. [DOI] [PubMed] [Google Scholar]
- 41.Pal S, Cheng C, Ho S. The effect of two different health messages on physical activity levels and health in sedentary overweight, middle-aged women. BMC Public. Health. 2011;11:204. doi: 10.1186/1471-2458-11-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Borg G. Perceived exertion as an indicator of somatic stress. Scand. J. rehabilitation Med. 1970;2:92–98. [PubMed] [Google Scholar]
- 43.Going SB, et al. Detection of small changes in body composition by dual-energy x-ray absorptiometry. Am. J. Clin. Nutr. 1993;57:845–850. doi: 10.1093/ajcn/57.6.845. [DOI] [PubMed] [Google Scholar]
- 44.Irwin ML, et al. Exercise improves body fat, lean mass, and bone mass in breast cancer survivors. Obesity. 2009;17:1534–1541. doi: 10.1038/oby.2009.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Prasad H, Ryan DA, Celzo MF, Stapleton D. Metabolic syndrome: definition and therapeutic implications. Postgrad. Med. 2012;124:21–30. doi: 10.3810/pgm.2012.01.2514. [DOI] [PubMed] [Google Scholar]
- 46.Aaronson NK, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J. Natl Cancer Inst. 1993;85:365–376. doi: 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- 47.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta psychiatrica Scandinavica. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 48.Astrand I. Aerobic work capacity in men and women with special reference to age. Acta Physiol. Scand. Suppl. 1960;49:1–92. [PubMed] [Google Scholar]
- 49.Ratitch B, O’Kelly M, Tosiello R. Missing data in clinical trials: from clinical assumptions to statistical analysis using pattern mixture models. Pharm. Stat. 2013;12:337–347. doi: 10.1002/pst.1549. [DOI] [PubMed] [Google Scholar]
- 50.Lorentzon M, Cummings SR. Osteoporosis: the evolution of a diagnosis. J. Intern. Med. 2015;277:650–661. doi: 10.1111/joim.12369. [DOI] [PubMed] [Google Scholar]
- 51.Balady GJ. Survival of the fittest–more evidence. N. Engl. J. Med. 2002;346:852–854. doi: 10.1056/NEJM200203143461111. [DOI] [PubMed] [Google Scholar]
- 52.Jones LW, et al. Rationale and design of the Exercise Intensity Trial (EXCITE): A randomized trial comparing the effects of moderate versus moderate to high-intensity aerobic training in women with operable breast cancer. BMC Cancer. 2010;10:531. doi: 10.1186/1471-2407-10-531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kraus WE, Douglas PS. Where does fitness fit in? N. Engl. J. Med. 2005;353:517–519. doi: 10.1056/NEJMe058132. [DOI] [PubMed] [Google Scholar]
- 54.Lahart IM, Metsios GS, Nevill AM, Carmichael AR. Physical activity, risk of death and recurrence in breast cancer survivors: A systematic review and meta-analysis of epidemiological studies. Acta Oncol. 2015;54:635–654. doi: 10.3109/0284186X.2014.998275. [DOI] [PubMed] [Google Scholar]
- 55.Brown JC, Winters-Stone K, Lee A, Schmitz KH. Cancer, physical activity, and exercise. Compr. Physiol. 2012;2:2775–2809. doi: 10.1002/cphy.c120005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Speck RM, Courneya KS, Masse LC, Duval S, Schmitz KH. An update of controlled physical activity trials in cancer survivors: a systematic review and meta-analysis. J. Cancer Surviv. 2010;4:87–100. doi: 10.1007/s11764-009-0110-5. [DOI] [PubMed] [Google Scholar]
- 57.Courneya KS, et al. Effects of exercise during adjuvant chemotherapy on breast cancer outcomes. Med. Sci. Sports Exerc. 2014;46:1744–1751. doi: 10.1249/MSS.0000000000000297. [DOI] [PubMed] [Google Scholar]
- 58.Courneya KS, Rogers LQ, Campbell KL, Vallance JK, Friedenreich CM. Top 10 research questions related to physical activity and cancer survivorship. Res. Q. Exerc. sport. 2015;86:107–116. doi: 10.1080/02701367.2015.991265. [DOI] [PubMed] [Google Scholar]
- 59.Markes, M., Brockow, T. & Resch, K. L. Exercise for women receiving adjuvant therapy for breast cancer. Cochrane Database Syst Rev, Cd005001, 10.1002/14651858.CD005001.pub2 (2006). [DOI] [PubMed]
- 60.McNeely ML, et al. Effects of exercise on breast cancer patients and survivors: a systematic review and meta-analysis. Cmaj. 2006;175:34–41. doi: 10.1503/cmaj.051073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kim CJ, Kang DH, Park JW. A meta-analysis of aerobic exercise interventions for women with breast cancer. West. J. Nurs. Res. 2009;31:437–461. doi: 10.1177/0193945908328473. [DOI] [PubMed] [Google Scholar]
- 62.Mijwel S, et al. Adding high-intensity interval training to conventional training modalities: optimizing health-related outcomes during chemotherapy for breast cancer: the OptiTrain randomized controlled trial. Breast Cancer Res. Treat. 2018;168:79–93. doi: 10.1007/s10549-017-4571-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gadea E, Thivat E, Planchat E, Morio B, Durando X. Importance of metabolic changes induced by chemotherapy on prognosis of early-stage breast cancer patients: a review of potential mechanisms. Obes. Rev. 2012;13:368–380. doi: 10.1111/j.1467-789X.2011.00957.x. [DOI] [PubMed] [Google Scholar]
- 64.Heideman WH, Russell NS, Gundy C, Rookus MA, Voskuil DW. The frequency, magnitude and timing of post-diagnosis body weight gain in Dutch breast cancer survivors. Eur. J. Cancer. 2009;45:119–126. doi: 10.1016/j.ejca.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 65.Saquib N, et al. Weight gain and recovery of pre-cancer weight after breast cancer treatments: evidence from the women’s healthy eating and living (WHEL) study. Breast Cancer Res. Treat. 2007;105:177–186. doi: 10.1007/s10549-006-9442-2. [DOI] [PubMed] [Google Scholar]
- 66.Dieli-Conwright CM, et al. Effects of Aerobic and Resistance Exercise on Metabolic Syndrome, Sarcopenic Obesity, and Circulating Biomarkers in Overweight or Obese Survivors of Breast Cancer: A Randomized Controlled Trial. J. Clin. Oncol. 2018;36:875–883. doi: 10.1200/jco.2017.75.7526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wijndaele K, et al. Muscular strength, aerobic fitness, and metabolic syndrome risk in Flemish adults. Med. Sci. Sports Exerc. 2007;39:233–240. doi: 10.1249/01.mss.0000247003.32589.a6. [DOI] [PubMed] [Google Scholar]
- 68.Wijndaele K, et al. Sedentary behaviour, physical activity and a continuous metabolic syndrome risk score in adults. Eur. J. Clin. Nutr. 2009;63:421–429. doi: 10.1038/sj.ejcn.1602944. [DOI] [PubMed] [Google Scholar]
- 69.Carayol M, et al. Short- and long-term impact of adapted physical activity and diet counseling during adjuvant breast cancer therapy: the “APAD1” randomized controlled trial. BMC Cancer. 2019;19:737. doi: 10.1186/s12885-019-5896-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Juvet LK, et al. The effect of exercise on fatigue and physical functioning in breast cancer patients during and after treatment and at 6 months follow-up: A meta-analysis. Breast. 2017;33:166–177. doi: 10.1016/j.breast.2017.04.003. [DOI] [PubMed] [Google Scholar]
- 71.Osoba D, Rodrigues G, Myles J, Zee B, Pater J. Interpreting the significance of changes in health-related quality-of-life scores. J. Clin. Oncol. 1998;16:139–144. doi: 10.1200/jco.1998.16.1.139. [DOI] [PubMed] [Google Scholar]
- 72.Klassen O, et al. Muscle strength in breast cancer patients receiving different treatment regimes. J. cachexia, sarcopenia muscle. 2017;8:305–316. doi: 10.1002/jcsm.12165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Harrington S, Padua D, Battaglini C, Michener LA. Upper extremity strength and range of motion and their relationship to function in breast cancer survivors. Physiother. Theory Pract. 2013;29:513–520. doi: 10.3109/09593985.2012.757683. [DOI] [PubMed] [Google Scholar]
- 74.Christensen JF, et al. Muscle dysfunction in cancer patients. Ann. Oncol. 2014;25:947–958. doi: 10.1093/annonc/mdt551. [DOI] [PubMed] [Google Scholar]
- 75.Mijwel S, et al. Exercise training during chemotherapy preserves skeletal muscle fiber area, capillarization, and mitochondrial content in patients with breast cancer. FASEB J. 2018;32:5495–5505. doi: 10.1096/fj.201700968R. [DOI] [PubMed] [Google Scholar]
- 76.Spencer JC, Wheeler SB. A systematic review of Motivational Interviewing interventions in cancer patients and survivors. Patient Educ. Couns. 2016;99:1099–1105. doi: 10.1016/j.pec.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 77.Bandura A. Health promotion by social cognitive means. Health Educ. behavior: Off. Publ. Soc. Public. Health Educ. 2004;31:143–164. doi: 10.1177/1090198104263660. [DOI] [PubMed] [Google Scholar]
- 78.Marcus BH, Simkin LR. The transtheoretical model: applications to exercise behavior. Med. Sci. Sports Exerc. 1994;26:1400–1404. doi: 10.1249/00005768-199411000-00016. [DOI] [PubMed] [Google Scholar]
- 79.Sasso JP, et al. A framework for prescription in exercise-oncology research. J. cachexia, sarcopenia muscle. 2015;6:115–124. doi: 10.1002/jcsm.12042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tweed EJ, Allardice GM, McLoone P, Morrison DS. Socio-economic inequalities in the incidence of four common cancers: a population-based registry study. Public. health. 2018;154:1–10. doi: 10.1016/j.puhe.2017.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dano H, Andersen O, Ewertz M, Petersen JH, Lynge E. Socioeconomic status and breast cancer in Denmark. Int. J. Epidemiol. 2003;32:218–224. doi: 10.1093/ije/dyg049. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Anonymous data are available upon from the corresponding author (TM) upon reasonable request. The datasets generated and/or analysed during the current study are available from the corresponding author upon reasonable request.