Abstract
Dairy calves are born with a naïve immune system, making the pre-weaning phase a critical window for immune development. In the U.S., 40–60% of dairy farms feed milk replacer to pre-weaned calves, which are devoid of bioactive factors with immunological roles. Serotonin is a bioactive factor with immunoregulatory properties naturally produced by the calf and present in milk. Human and rodent immune cells express the serotonin machinery, but little is known about the role of serotonin in the bovine immune system. Supplementing milk replacer with 5-hydroxytryptophan (serotonin precursor) or fluoxetine (reuptake inhibitor) increases serotonin bioavailability. We hypothesized that increased serotonin bioavailability promotes serotonergic signaling and modulates the expression of immune related genes in peripheral leukocytes and immune-related tissues of dairy calves. The present experiment targeted candidate genes involved in serotonin production, metabolism, transport, signaling and immune regulation. We established that bovine peripheral leukocytes express all known serotonin receptors, and can synthesize, uptake and degrade serotonin due to the expression of serotonin metabolism-related genes. Indeed, we showed that increasing serotonin bioavailability alters gene expression of serotonin receptors and immune-related genes. Further research will determine whether manipulation of the serotonin pathway could be a feasible approach to bolster dairy calves’ immune system.
Subject terms: Physiology, Immunology
Introduction
Dairy calves are born with a naïve immune system. Feeding newborn calves high quality colostrum is a practice readily implemented on most U.S. dairy farms1,2. While colostrum is important for calf immune protection and survival, the remaining pre-weaning phase consists of a liquid diet of either whole milk or milk replacer. In the U.S., 40–60% of dairy farms feed milk replacers to pre-weaned dairy calves3. Dairy calves’ adaptive immune system develops gradually, and the pre-weaning phase has been shown to be critical for immune system development and maturation4. Emerging data demonstrate that milk not only delivers nutrients, but also primes the newborn’s growth and development through delivery of bioactive factors5,6. Although milk replacer formulation has improved over the years, it still lacks bioactive components naturally present in milk that could aid in the development of the dairy calf immune system. Therefore, there is a need to explore novel bioactive factors that when added to milk replacers can enhance dairy calf immune development.
Serotonin is a bioactive factor with immunoregulatory properties7–12 that is present in cow milk and is also endogenously synthesized by the calf13–15. However, little is known about the immunologic role of serotonin in milk or in cattle. Serotonin is derived from the conversion of L-tryptophan to 5-hydroxytryptophan (5-HTP) by the rate limiting enzyme tryptophan hydroxylase (TPH1, in peripheral tissues, and TPH2, in the brain), which is subsequently converted to serotonin by the aromatic amino acid decarboxylase enzyme (AADC/DDC)16. There are 7 serotonin receptor families with more than 10 G-protein coupled receptor (GPCR) subtypes and 3 ion-gated channel receptor subtypes17. Depending on which serotonin receptor subtype (Gs, q/11 or i/o) is activated, signaling cascades including adenylyl cyclase (AC), protein kinase C (PKC), inositol trisphosphate (IP3) and mitogen and extracellular signal regulated kinase (MERK) are activated18 to modulate the activity of proteins or to regulate gene transcription. Serotonin action is terminated by the serotonin transporter (SERT), which removes circulating serotonin from the extracellular space to be recycled or degraded by monoamine oxidase (MAO).
Peripheral serotonin (close to 95% of total serotonin in the body) is primarily synthesized by the enterochromaffin cells in the gut and is involved in the regulation of many physiological functions14,19–23, including immune modulation. Peripheral serotonin is mainly stored and transported by blood platelets24, the major source of serotonin for circulating immune cells and organs. Several studies support the immunomodulatory role of peripheral serotonin in the rodent and human model. For instance, platelet-derived serotonin in mice promotes neutrophil recruitment to inflammation sites by increasing L-selectin expression and enhancing endothelial interactions25. Research in humans and rodents show that different immune cells express one or multiple components of the serotonergic signaling pathway machinery (i.e., receptors, TPH1 and/or SERT, MAO8,9,26,27), indicating their capacity to synthesize, metabolize, respond to, and/or transport serotonin11,28,29. Dendritic cells (DCs) do not express TPH1, however upon activation they increase SERT expression which allows them to take up serotonin from circulation8. Activation of murine T lymphocytes increases TPH1 expression and hence, endogenous serotonin production. This serotonin then acts as an autocrine-paracrine cytokine to enhance T cell proliferation or is taken up by circulating cells (i.e., DCs and platelets)8,9. Furthermore, in mice, serotonin can attract mast cells, which express both TPH1 and SERT11, to inflammation sites30. Studies using TPH1 knockout mice (lacking peripheral serotonin) show reduced macrophage infiltration and lower proinflammatory cytokine production (i.e. IL-1β and -6) compared to wild type mice31. Dendritic cells of TPH1 knockout mice produce less IL-12 following a 24 h in vitro lipopolysaccharide (LPS) challenge compared to wild type mice32. It has also been shown that isolated monocytes incubated with LPS secrete more cytokines when serotonin is present26.
Serotonin has been shown to regulates physiological functions that are relevant to lactation performance including metabolic status, milk synthesis and calcium regulation15,23,33. However, studies exploring serotonin’s immunomodulatory role are limited in the bovine. One study showed that supplementation of 5-hydroxytryptophan to newborn calves for 15 days increased blood mRNA abundance of genes related to innate and adaptive immunity, including nuclear factor kappa beta, chemokine C-C motif ligand 5, cyclooxygenase-2 and interleukin 1 beta34. However, a more thorough characterization of the bovine serotonergic pathway and its ability to modulate immunity is lacking. Herein, we characterize the expression profile of genes involved in serotonin synthesis, metabolism and signaling, and its impact on cytokine expression in leukocytes and lymphoid tissues of dairy calves supplemented with 5-hydroxytryptophan, the serotonin precursor, or fluoxetine, a selective serotonin reuptake inhibitor (SSRI). We hypothesized that increased cell and tissue serotonin bioavailability will promote the expression of genes involved in serotonergic machinery and signaling, and positively modulate the expression of immune genes in peripheral leukocytes, spleen, thymus and popliteal lymph node of pre-weaned dairy calves.
Results
Effects of FLX and 5-HTP supplementation on white blood cells counts and subfractions
No differences were observed for total WBC (count/μL) among treatment groups before or after 10 days of FLX or 5-HTP supplementation (P > 0.19; Table 1). Likewise, WBC subfractions (count/μL) including neutrophils, eosinophils, basophils, monocytes and lymphocytes were not different among treatment groups before or after 10 days of supplementation (P > 0.47; Table 1).
Table 1.
Circulating white blood cells (neutrophils, lymphocytes, monocytes, eosinophils, and basophils) in dairy calves with increased serotonin bioavailability.
| WBC count | Treatmentsa | P-valueb | |||
|---|---|---|---|---|---|
| CON | 5-HTP | FLX | 5-HTP vs. CON | FLX vs. CON | |
| Neutrophils, 103/μL | 3.69 ± 0.62 | 3.96 ± 0.67 | 3.69 ± 0.62 | 0.78 | 0.99 |
| Monocytes, 103/μL | 1.08 ± 0.11 | 1.19 ± 0.12 | 0.97 ± 0.11 | 0.51 | 0.48 |
| Lymphocytes, 103/μL | 4.37 ± 0.23 | 4.79 ± 0.24 | 4.79 ± 0.23 | 0.22 | 0.48 |
| Eosinophils, 103/μL | 0.06 ± 0.02 | 0.06 ± 0.22 | 0.09 ± 0.02 | 0.96 | 0.42 |
| Basophils, 103/μL | 0.01 ± 0.02 | 0.05 ± 0.03 | 0.03 ± 0.02 | 0.26 | 0.71 |
aOral supplementation of milk replacer with saline (control; n = 8), fluoxetine (40 mg/d; n = 8) or 5-hydroxytryptophan (5-HTP, 90 mg/d; n = 8) to Holstein dairy calves for 10 consecutive days.
bStatistical significance declared at P-value ≤ 0.05 and tendencies at 0.05 < P ≤ 0.10.
Effects of 5-HTP on peripheral leukocyte gene expression
Serotonin synthesis, metabolism, and downstream pathways
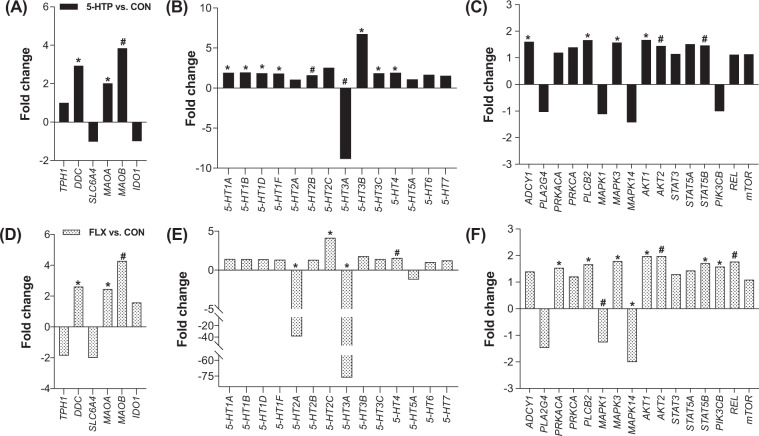
After 10 days of 5-HTP supplementation, peripheral leukocytes were isolated for gene expression analysis, reported as fold change relative to CON saline-supplemented group. Supplementation of 5-HTP upregulated or tended to upregulate genes involved in serotonin synthesis and metabolism, including DDC and MAOA (P < 0.03) and MAOB (P = 0.09), while TPH1, SLC6A4 and IDO1 gene expression was not affected (P > 0.94; Fig. 1A). Seven serotonin receptors were upregulated including 5-HT1A, -1B, -1D, -1F, -3B, -3C and -4 (P < 0.04) while 5-HT2B tended to be upregulated (P = 0.06; Fig. 1B). The 5-HT3A receptor subtype was downregulated more than 8-fold following 5-HTP supplementation (P = 0.06; Fig. 1B), whereas the expression of 5HT2A, -5A, -6 and -7 remained unchanged (P > 0.13). Four genes downstream of serotonin GPCR signaling were significantly upregulated by 5-HTP supplementation, including ADCY1, PLCB2, MAPK3 and AKT1 (P < 0.05); while AKT2 and STAT5B tended to be upregulated (P < 0.10; Fig. 1C).
Figure 1.
Gene expression in peripheral leukocytes of pre-weaned dairy calves after a 10-day oral supplementation of 5-hydroxytryptophan (5-HTP, 90 mg/d; n = 8), fluoxetine (FLX, 40 mg/d; n = 8) or saline (CON; n = 8). Gene expression is reported as fold change (2−ΔΔCt) relative to CON saline-supplemented group. Gene expression fold change of (A) genes related to serotonin synthesis and metabolism, (B) serotonin receptors, and (C) their downstream pathways after 10 days of 5-HTP oral supplementation. Gene expression fold change of (D) genes related to serotonin synthesis and metabolism, (E) serotonin receptors and (F) their downstream pathways. Black bars denote 5-HTP vs. CON gene expression fold change, and dotted bars denote FLX vs. CON gene expression fold change. The negative inverse of fold-change values <1 was calculated for visual representation of negative fold changes. (*) indicate significant differences (P ≤ 0.05) and (#) indicate tendencies (0.05 < P ≤ 0.10) between CON and FLX or CON and 5-HTP treatments.
Clusters of differentiation and immune related genes
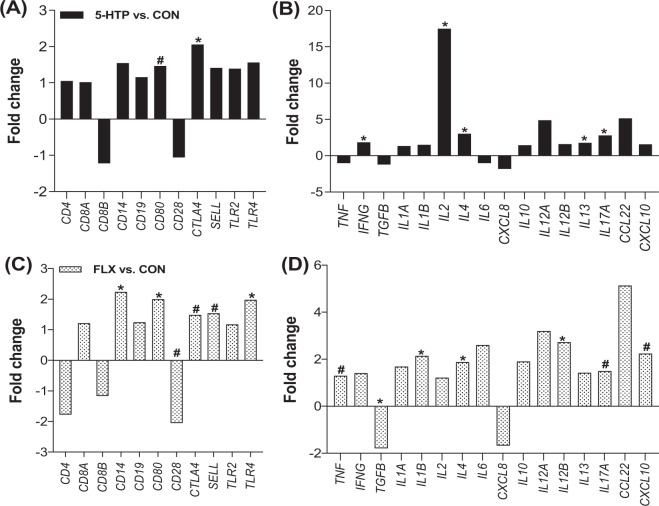
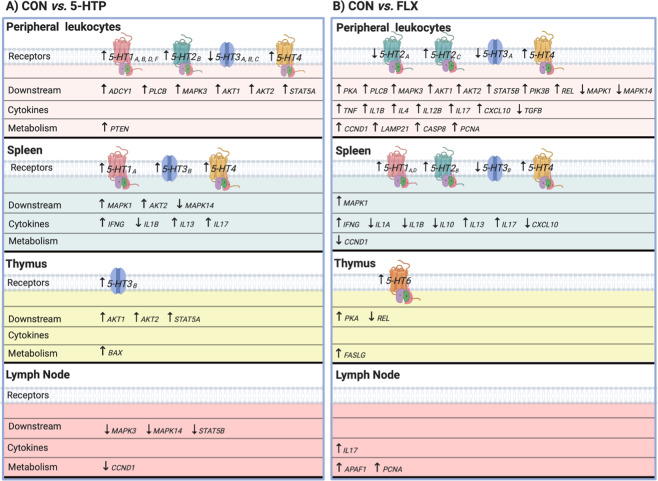
The expression of several immune related genes in peripheral leukocytes was modulated by 5-HTP supplementation. Specifically, CTLA4 was upregulated (P < 0.006), and CD80 tended to be upregulated compared to CON (P = 0.10; Fig. 2A). A significant upregulation of various cytokines including IFNG, IL2, IL4, IL13, and IL17A (P < 0.02) was observed, with IL2 having the highest fold-change of 17.5 when compared to CON (Fig. 2B). Differentially expressed genes in peripheral leukocytes are summarized in Fig. 3A.
Figure 2.
Gene expression in peripheral leukocytes of pre-weaned dairy calves after a 10-day oral supplementation of 5-hydroxytryptophan (5-HTP, 90 mg/d; n = 8), fluoxetine (FLX, 40 mg/d; n = 8) or control (CON; n = 8). Gene expression is reported as fold change (2−ΔΔCt) relative to CON saline-supplemented group. (A) Gene expression of immune surface markers and (B) cytokines after 10 days of 5-HTP oral supplementation. (C) Gene expression fold change of immune surface markers and (D) cytokines after 10 days of FLX oral supplementation. Black bars denote 5-HTP vs. CON gene expression fold change, and dotted bars denote FLX vs. CON gene expression fold change. The negative inverse of fold-change values <1 was calculated for visual representation of negative fold changes. (*) indicate significant differences (P ≤ 0.05) and (#) indicate tendencies (0.05 < P ≤ 0.10) between CON and FLX or CON and 5-HTP.
Figure 3.
Summary of serotonin receptors, intracellular downstream signaling, cytokines and metabolism genes that were differentially expressed at the mRNA level in peripheral leukocytes (n = 8 per treatment) and lymphoid tissues (spleen, thymus and lymph node, n = 4 per treatment) of pre-weaned dairy calves after (A) 10-d 5-hydroxytryptophan oral supplementation (5-HTP, 90 mg/d) vs. 10-d saline oral supplementation (CON), and (B) 10-d Fluoxetine oral supplementation (FLX, 40 mg/d) vs. 10-d saline oral supplementation (CON). Summarized genes were (P ≤ 0.05) or tended (0.05 < P ≤ 0.10) to be differentially expressed between 5-HTP vs. CON or FLX vs. CON groups.
Effects of FLX on peripheral leukocyte gene expression
Serotonin synthesis, metabolism and downstream pathways
After 10 days of FLX supplementation, peripheral leukocytes were isolated for gene expression analysis and reported as fold change relative to CON saline-supplemented group. Supplementation of FLX upregulated genes involved in serotonin synthesis and metabolism, including DDC and MAOA (P < 0.03) and tended to upregulate MAOB (P = 0.07), whereas TPH1, SERT and IDO1 were not differentially expressed (P > 0.12; Fig. 1D). Fluoxetine supplementation upregulated the expression of 5-HT2C (P = 0.03) and 5-HT4 (P = 0.08); while 5-HT2A and -3A were significantly downregulated (>30-fold, P < 0.006; Fig. 1E). All other serotonin receptors remained unchanged (P > 0.12). Eight genes downstream of serotonin receptor signaling were upregulated including PKA, PLCB2, MAPK3, AKT1, STAT5B and PIK3CB (P < 0.03) or tended to be upregulated such as AKT2 and REL (P < 0.10) by FLX supplementation (Fig. 1F). Meanwhile, MAPK14 was significantly downregulated (P = 0.008) and MAPK1 tended to be downregulated by FLX (P = 0.09, Fig. 1F).
Clusters of differentiation and immune related genes
The expression of various immune related genes in peripheral leukocytes was modulated by FLX supplementation. Specifically, FLX upregulated the gene expression of CD14, CD80, and TLR4 (P < 0.045), CTLA4, and SELL (P < 0.10), while it downregulated CD28 expression compared to CON (P < 0.10; Fig. 2C). Supplementation of FLX upregulated the gene expression of various cytokines, including IL1B, IL4 and IL12B (P < 0.03), TNF, IL17A and CXCL10 (P < 0.10; Fig. 2D). Only TGFB gene expression in peripheral leukocytes was significantly downregulated after the 10-d FLX supplementation (P = 0.04; Fig. 2D). Differentially expressed genes in peripheral leukocytes are summarized in Fig. 3B.
Effects of 5-HTP on Thymus, Spleen, and Lymph Node Gene Expression
Serotonin synthesis, metabolism and downstream pathways
Supplementation of 5-HTP for 10 days did not affect serotonin metabolism related genes in spleen tissue compared to CON (P > 0.15; Supplementary Fig. S1A), but upregulated serotonin receptors 5-HT1A and -4 (P < 0.01) and tended to upregulate 5-HT3B (P = 0.09; Supplementary Fig. S1B). In the spleen, MAPK1 and AKT2 tended to be upregulated, while MAPK14 tended to be downregulated (P < 0.07; Supplementary Fig. S2A). In the popliteal lymph node, serotonin metabolism related enzyme MAOB tended to be upregulated (P = 0.06; Supplementary Fig. S1A), but no serotonin receptors were differentially expressed by 5-HTP (P > 0.11; Supplementary Fig. S1B). Additionally, in the lymph node, MAPK3 and STAT5B tended to be downregulated (P > 0.08; Supplementary Fig. S2A). In the thymus, 5-HTP supplementation upregulated DDC (P = 0.04) and tended to downregulate TPH1 (P = 0.07; Supplementary Fig. S1A), while it significantly upregulated the serotonin receptor 5-HT3B (P = 0.02; Supplementary Fig. S1B). Thymus expression of genes downstream of serotonin receptors included upregulation of AKT1 and AKT2 (P < 0.043) and STAT5A (P = 0.06; Supplementary Fig. S2A). Differentially expressed genes in tissues are summarized in Fig. 3A.
Clusters of differentiation and immune related genes
The expression of several immune related genes in lymphoid tissues was altered by 5-HTP supplementation. In the spleen, 5-HTP supplementation upregulated the surface protein CTLA4 (P = 0.01) and tended to downregulate the T cell surface marker, CD8B (P = 0.07; Supplementary Fig. S3A). Spleen expression of IL17A was upregulated (P = 0.04) and IFNG and IL13 tended to be upregulated (P < 0.09) by 5-HTP supplementation; while IL1B cytokine was downregulated (P = 0.003; Supplementary Fig. S3B). In the popliteal lymph node, CD14 was downregulated (P = 0.04; Supplementary Fig. S3A) but cytokine gene expression was not affected by 5-HTP supplementation (P > 0.16; Supplementary Fig. S3B). In the thymus, the surface protein, CTLA4, gene expression was upregulated (P = 0.025; Supplementary Fig. S3A), however cytokines were not differentially expressed after 5-HTP supplementation (P > 0.11; Supplementary Fig. S3B).
Effects of FLX on Thymus, Spleen, and Lymph Node Gene Expression
Serotonin synthesis, metabolism and downstream pathways
In the spleen, FLX supplementation upregulated SLC6A4 (P = 0.02) and IDO1 (P = 0.07) and downregulated TPH1 (P = 0.06; Supplementary Fig. S1C). Additionally, serotonin receptors 5-HT1A and -4 were upregulated (P < 0.04), 5-HT1D and -2B tended to be upregulated (P < 0.08), while 5-HT3B tended to be downregulated (P = 0.054; Supplementary Fig. S1D). Only MAPK1 gene expression was upregulated by FLX (P = 0.03), while all other downstream serotonin receptor signaling genes remained unchanged in the spleen compared with CON calves (Supplementary Fig. S2B). In the popliteal lymph node, REL gene expression was downregulated following FLX supplementation (P = 0.002; Supplementary Fig. S2B). In the thymus, FLX tended to upregulate 5-HT6 receptor (P = 0.09) and the downstream PKA (P = 0.04), but the expression of genes related to serotonin metabolism or downstream pathways was similar to CON (P > 0.11; Supplementary Fig. S1D, S2B). Differentially expressed genes are summarized in Fig. 3B.
Clusters of differentiation and immune related genes
The expression of several immune related genes in lymphoid tissues was modulated by FLX supplementation. In the spleen, FLX upregulated the surface protein CTLA4 (P = 0.03) and tended to downregulate CD28 (P < 0.10; Supplementary Fig. S3C). The gene expression of IFNG and IL17A was upregulated (P < 0.04) and IL13 tended to be upregulated (P = 0.08; Supplementary Fig. S3D). Meanwhile, the expression of CXCL10 was downregulated (P = 0.001) and IL1A, IL1B and IL10 tended to be downregulated (P < 0.08; Supplementary Fig. S3D). Fluoxetine supplementation upregulated the expression of surface protein CTLA4 (P = 0.021; Supplementary Fig. S3C) in the thymus, however, cytokine genes were not differentially expressed (P > 0.11; Supplementary Fig. S3D). In the popliteal lymph node tissue, IL17A tended to be upregulated (P = 0.10; Supplementary Fig. S3D) but no other cytokine or surface markers genes were differentially expressed following FLX supplementation (P > 0.11; Supplementary Fig. S3C, S3D).
Effects of 5-HTP and FLX on Proliferation, Apoptosis, and Cell Metabolism Genes
Peripheral leukocytes
The expression of genes related to cell proliferation, apoptosis, cell metabolism and cell cycle in peripheral leukocytes after 10-d of 5-hydroxytryptophan or fluoxetine supplementation was evaluated. Supplementation of 5-HTP upregulated PTEN (P = 0.005), whereas FLX upregulated CCND1 (P = 0.02) and tended to upregulate LAMP2, CASP8 and PCNA (P < 0.10; Supplementary Table S1).
Secondary lymphoid tissues
In the spleen, 5-HTP supplementation had no effect on cell metabolism gene expression (P > 0.12) whereas FLX supplementation downregulated CCND1 (P = 0.04; data not shown). In the popliteal lymph node, 5-HTP supplementation tended to downregulate CCND1 expression (P = 0.07), while FLX supplementation upregulated APAF1 and CASP8 (P < 0.04; data not shown) and tended to upregulate FASLG and PCNA (P < 0.10; data not shown). In the thymus, 5-HTP upregulated BAX (P = 0.01), while FLX upregulated FASLG (P = 0.03; data not shown).
Discussion
The role of serotonin as an immunoregulatory molecule has been widely demonstrated in human and rodents7–11,25,27, however, evidence supporting its role in livestock species is lacking. We previously reported that increasing serotonin bioavailability in dairy calves is possible through the supplementation of 5-hydroxytryptophan or fluoxetine35. Herein, we report the effects of increased serotonin bioavailability on circulating WBC count and the gene expression of peripheral leukocytes and secondary lymphoid organs of dairy calves undergoing immune system maturation. To our knowledge, this is the first experiment to characterize how the serotonin axis regulates the bovine immune system.
In this experiment, WBC and WBC subfractions including neutrophil, monocyte, lymphocyte, eosinophil, and basophil counts were within the normal physiological ranges for growing dairy calves and similar among treatment groups before and after 10 days of treatment supplementation. This indicates that increasing serotonin bioavailability for 10 days did not significantly alter immune cell populations. Even though we did not see an increase in neutrophil counts, it is possible that serotonin is improving neutrophil function. For instance, human neutrophils cultured in vitro with high concentrations of serotonin have higher motility than neutrophils grown in low serotonin conditioned media36. Platelet expression of FcγRIIA, a receptor that recognizes immune complexes, plays a role in inflammation by activating neutrophils and enhancing endothelial vasodilatation37. Furthermore, neutrophils from wild type mice have been shown to have improved tissue infiltration compared to TPH1 knockout mice25. Thus, further in vitro experiments evaluating serotonin’s role in neutrophil motility and function (i.e. oxidative burst and phagocytic capacity) are needed in bovine.
For over 20 years, researchers have investigated the significance of serotonin receptors in human and murine immune cells and their possible implication in autoimmune diseases. In our experiment, the entire serotonergic machinery, including genes involved in serotonin synthesis, mechanism of action, and metabolism were expressed in the circulating leukocytes of all calves. This indicates that peripheral leukocytes of dairy calves can synthesize, metabolize, uptake and degrade serotonin. Supplementation of either 5-HTP or FLX increased serotonin bioavailability35 and upregulated several genes involved in serotonin machinery in peripheral leukocytes. The ubiquitous aromatic decarboxylase enzyme, DDC, that converts 5-HTP to serotonin, and monoamine oxidase enzyme, MAOA, that metabolizes serotonin, were significantly upregulated in the peripheral blood leukocytes of both 5-HTP and FLX fed calves. This suggests an overall increase of serotonin metabolism in immune cells of calves under supplementation.
Notably, supplementing 5-HTP upregulated the gene expression of 9 out of the 13 serotonin receptor subtypes evaluated in peripheral leukocytes compared to controls. Interestingly, all serotonin receptors from family 1 subtypes (5-HT1), including -1A, -1B, -1D, and -1F were significantly upregulated, suggesting a positive feedback loop to increase ligand binding. Serotonin receptor family 1 proteins couple mainly through Gi/o proteins to inhibit adenylyl cyclase in various cells and tissues and have high affinity towards serotonin18. Serotonin receptor 5-HT1 subtypes are expressed in various human and mouse immune cells including monocytes/macrophages, dendritic cells, neutrophils, mast cells, eosinophils, B cells and T cells7. Data in human and/or rodents shows that 5-HT1A receptor subtype modulates adhesion and chemotaxis of mast cells30 and enhances phagocytosis by murine macrophages38. Upregulation of serotonin receptors subtypes from family 1 in peripheral leukocytes after 5-HTP supplementation could support calves’ immune function by promoting adhesion and chemotaxis of mast cells, and/or enhancing phagocytosis. However, future functional studies targeting this specific receptor should be conducted in the bovine.
In this experiment, the 5-HT2B receptor subtype was upregulated following 5-HTP supplementation. Activation signals among 5-HT2 family receptor subtypes are different, but mainly couple through Gq/11 proteins. For instance, the 5-HT2A receptor subtype signals through the activation of PLC-β in tissues and cells, whereas the 45% homologous 5-HT2B receptor subtype signal through various phospholipases (i.e. PLC-β, PLA)18. This 5-HT2B receptor subtype has been widely studied in human dendritic cells (DCs) where it is reported to promote anti-inflammatory functions. Human monocytes cultured in the presence of serotonin, as well as IL-4 and granulocyte-macrophage colony-stimulating factor, differentiate into DCs with reduced expression of co-stimulatory molecules (i.e. CD86) which are needed for antigen-presenting cells (APC; i.e. DCs) and T cell cognate interactions39. Similarly, 5-HT2B receptor activation was found to downregulate monocyte derived DC expression of co-stimulatory molecules that activate naïve T cells, and possibly preventing inflammation by regulating both innate and adaptive immune systems40. Apart from 5-HT2B, 5-HT2A expression is upregulated on activated CD4+ and CD8+ T cells41. Furthermore, 5-HT2A antagonist treatment inhibits T cell activation and diminishes IL-2 and interferon gamma (IFN-γ) production in a dose dependent manner41. Thus, it appears that 5-HT2A acts as a proinflammatory serotonin receptor whereas 5-HT2B acts as an immunosuppressive serotonin receptor.
Herein, both IL2 and IFNG gene expression were upregulated even though 5-HT2A was not differentially expressed. Interleukin-2 is an immunoregulatory cytokine, mainly produced by CD4+ T cells, which enhances T cell proliferation, regulates T helper cell differentiation42, and limits immune responses by enhancing T-regulatory cells function43. Moreover, IL-2 induces the transcription of IFN-γ in T cells44. Interferon gamma is an important inflammatory cytokine that induces maturation and licensing of APC that in turn recruit and prime T cells, and increases the expression of major histocompatibility complex45. Translation of IFN-γ by T cells skews B cells to enhance antibody production and induces isotype switching from IgM to IgG2a46. Upregulation of IL2 and IFNG by 5-HTP supplementation, if translated to protein, could act to support adaptive immune responses. Further studies are warranted to confirm and determine the implications of these findings.
The serotonin receptors -3B and -3C, and -4 were upregulated following 5-HTP supplementation. The 5-HT3B receptor subtype is a ligand-gated ion channel47 and is 41% homologous to the 5-HT3A receptor48. This receptor has been linked to nausea in patients undergoing chemotherapy49. To our knowledge the function of 5-HT3B receptor subtype has not been linked to immunity, probably because 5-HT3B can be expressed as a heteromeric receptor, 5-HT3A/B, with properties differing from those of 5-HT3A receptor subtype50.
Gene expression of key signaling molecules downstream of serotonin receptors, including ADCY1, PLCB2, MAPK3 and AKT, was upregulated by 5-HTP supplementation. Adenyl cyclase (ADCY1) is a major downstream signaling gene for 5-HT4, -6 and -7 following Gs coupling activation18. Yet, in some cell types, activation of 5-HT1A receptor inhibits ADCY151. Since both 5-HT1 and -4 receptor families were upregulated in our experiment we cannot determine which specific serotonin receptor subtype might be upregulating the expression of ADCY1 intracellularly. Furthermore, both PLCB2 and 5-HT2B were differentially expressed and PLCB2 is known to be the major signaling downstream molecule for 5-HT2B52. Yet, further investigation is needed to characterize the effects of specific serotonin receptors and downstream pathways on specific circulating immune cells in the bovine.
The gene expression of clusters of differentiation CD4, CD8 and CD14 in peripheral leukocytes were not affected by 5-HTP supplementation, suggesting that increased serotonin bioavailability had no effect on immune cell concentrations. However, serotonin has been linked to the migration of specific immune cell types. For instance, Müller et al.53 reported that 5-HT1B receptor induces the migration of human immature DCs. Additionally, human DCs have been shown to secret IL-1 after activation of receptors 5-HT3, -4 and -754. Interestingly, in our experiment, peripheral blood leukocytes had greater expression of serotonin receptor subtypes 5-HT3B, -3C and -4, although upregulation of IL1B was not observed. Hernandez-Castellano et al.55 supplemented 5-HTP to newborn dairy calves for 15 days and reported an upregulation of both IL-1 and nuclear factor kappa beta (NF-) genes in blood but no differences in IgG production were observed. In our experiment, REL (NF- subunit) was not affected by 5-HTP supplementation, however discrepancies between Hernandez-Castellano’s and our results could be attributed primarily to calf age linked to different facets of immune system development.
To explore serotonin’s role on immune system activation, we also evaluated the gene expression of surface molecules and cytokines. Supplementation of 5-HTP for 10 days tended to upregulate CD80 gene expression and upregulated CTLA4, its preferential binding partner56. The cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) is a protein expressed by regulatory T cells and activated T cells that exerts negative feedback to diminish T cell responses, whereas CD80 is a costimulatory molecule expressed on APC57. As previously mentioned, 5-HTP supplementation upregulated IL2 gene expression, which plays an important role in T-helper cell proliferation and survival, and T-regulatory cell activation42. Our findings suggest that greater serotonin bioavailability might activate serotonin receptors on immune cells, promoting IL-2 production, T cell activation, and eventually CTLA4 expression to prevent excessive T cell activation42,43. Thus, we propose that the serotonin axis may play a role in balancing the immune system by promoting protective immune responses and preventing potentially dangerous inflammation.
Fluoxetine oral supplementation increases serotonin bioavailability by blocking SERT58. Fluoxetine supplementation downregulated 5-HT2A and -3A receptor subtypes by more than 20-fold in peripheral leukocytes. Contradictory findings have been reported linking the use of SSRIs to 5-HT2A downregulation in the rodent frontal cortex59. Nevertheless, the effects and implications of the downregulation of 5-HT2A receptor by SSRIs in the immune system remain unknown59–61. Monocytes and T cells express the 5-HT3A receptor subtype62, as well as naïve and activated B cells, predominantly by differentiating B cells at the germinal centers of lymph nodes63. Interestingly, the use of the 5-HT3A antagonist, tropisetron, inhibits T cell activation and production of IL-264. In 1994, Fan65 reported that 5-HT3 receptor is a target of fluoxetine, which in turn decreases serotonin influx into the cell. Moreover, fluoxetine blocks 5-HT3 receptors by interactions during both open and closed channel states, although the clinical relevance of this effect is still unknown66. Thus, further research is needed to understand 5-HT3B downregulation by fluoxetine supplementation.
The thymus is a central lymphoid organ where T cells develop, while the spleen and lymph nodes are important secondary lymphoid organs where immune responses are generated67. Therefore, we sought to explore the effects of increased serotonin bioavailability in these tissues. Similar to peripheral leukocytes, all tissues, independent of treatment supplementation, expressed the serotonergic machinery indicating that serotonin could be playing a role in the development and deployment of adaptive immune responses. We demonstrated that increased serotonin bioavailability exerts a less pronounced effect in these tissues compared to peripheral leukocytes, at least at the mRNA level. However, it is important to mention that tissue data was collected from a subset of animals (n = 4/treatment) euthanized after 10 days of treatment supplementation and that the statistical power to detect significant differences in tissues was 66%. Nevertheless, it is notable that we observed a significant downregulation of CD14 in the popliteal lymph node after 5-HTP supplementation and FLX tended to downregulate CD8B in spleen tissue, while both treatments upregulated CTLA4 in the thymus and spleen tissues. CD8 downregulation has been shown to occur when CD8 effector T cells are switching functions68. There is limited data exploring the effect of serotonin on thymus, lymph node, and/or spleen gene expression, thus, these results are novel and warrant deeper investigation.
The present experiment targeted candidate genes involved in serotonin production, metabolism, transport, signaling and immune regulation. We established that bovine peripheral blood leukocytes and immune tissues express components of the serotonin signaling pathway, including TPH1, SERT, DDC, MAO and serotonin receptors. This indicates that these cells and tissues have the potential to synthesize, transport, respond to and/or degrade serotonin. We demonstrated that at the mRNA level, increased serotonin bioavailability exerts a pronounced immunomodulatory response, particularly in peripheral leukocytes and spleen tissue. Indeed, specific serotonin receptors and cytokines were differentially expressed upon 5-HTP or FLX supplementation, which could potentially influence the developmental trajectory and maturation of immune cells in dairy calves at a young age. Differences in the modulatory effects of 5-HTP and FLX in the peripheral immune system could be attributed to intrinsic differences in their molecular mechanism of action. Indeed, we previously reported that 5-HTP calves had greater circulating serotonin concentrations when compared to fluoxetine calves35, thus we hypothesize that greater serotonin bioavailability exerts different effects. While promising correlations between serotonin and the immune system exist in other species, and now in the bovine at the transcriptional level, it is currently unknown whether promoting serotonin translates into both innate and adaptive immune system orchestration. Ultimately, the fact that 5-HTP is a biogenic modified amino acid whereas fluoxetine is a synthetic drug should be taken into consideration when developing strategies to enhance livestock health and development.
Methods
Animals and experimental design
All methods and procedures performed in this study were
carried out in accordance with relevant guidelines and regulations approved by the Institutional Animal Care and Use Committee at the University of Florida (protocol # 201709851). Experimental design and treatments are described in detail by Marrero et al.35. Briefly, Holstein bull calves (n = 24, 18 ± 2 d of age, 47 ± 3.2 kg) were assigned to one of three treatments in a complete randomized block design (8 pens, 2.3 m x 2.5 m; n = 3 per pen, one of each treatment). Calves received 4 L of milk replacer (Southeast Milk Inc, Okeechobee, FL) at 0700 h and 1700 h. Treatments were administered once daily (0700 h feeding) by supplementing milk replacer with 5-hydroxytryptophan (5-HTP, 90 mg/d, n = 8, Sigma, St. Louis, MO, USA; #H9772), fluoxetine (FLX, 40 mg/d, n = 8, Spectrum Chemical, Gardena, CA, USA; #F1200) or saline (CON, n = 8) for 10 consecutive days. Treatment was applied individually to each calf. Supplementing 5-HTP and FLX increases serotonin bioavailability by different mechanisms: exogenous 5-HTP bypasses the rate liming enzyme TPH1, allowing its conversion to serotonin by AADC69, whereas FLX binds to SERT inhibiting endogenous serotonin reuptake within the cell58,70.
Hematology analysis
Whole blood samples were collected from the jugular vein before (d0) and on the 10th day of treatment supplementation 4 h after 0700 h feeding in tubes containing K2 EDTA (BD, Franklin Lakes, NJ, USA, #368047). Within 2 h of collection, blood samples were analyzed for hematology parameters including white blood cells (WBC), neutrophil, lymphocyte, monocyte, eosinophil and basophil count/μL using the Idexx ProCyte Dx analyzer (IDEEX Laboratories Inc., Westbrook, ME).
Peripheral blood mononuclear cell isolation
Blood samples were collected from the jugular vein on the 10th day of treatment supplementation 4 h after the 0700 h feeding using heparin blood collection tubes (BD, Franklin Lakes, NJ, USA, # 366430) and kept on ice until laboratory arrival. Blood was centrifuged at 1,200 g for 18 min at 20 °C, plasma layer was discarded, and peripheral leukocytes (i.e., buffy coat) was transferred to a 15 mL conical tube. To lyse residual red blood cells, a hypotonic solution (lyse buffer:1.5 g Na2HPO4 (Fisher; #BP332-1) and 0.3 g NaH2PO4 (Fisher; #BP329-1) at pH 7.2) was used. To restore cells, restore buffer was used (27 g NaCl (Fisher cat. #BP358-1) added to the lyse buffer solution and adjusted to pH 7.2). To each sample, 8 mL of lyse buffer and 4 mL of restore buffer were added. Tubes were centrifuged at 650 g for 5 min at 4 °C to form a pellet. Pellets were resuspended in 200 µL of RNAlater (Invitrogen, Carlsbad, CA, USA; #AM7021) and stored at -80 °C until RNA extraction.
Euthanasia and tissue collection
After the 10th d of treatment supplementation, 4 calves per treatment (n = 4 pens) were euthanized at the University of Florida abattoir. Calves were sedated by intravenous administration of 0.2 mg/kg xylazine and euthanized using a captive bolt pistol followed by jugular exsanguination. Spleen, popliteal lymph node and thymus tissues (approximately 1 g each) were harvested, rinsed in sterile PBS, transferred to a cryotube containing RNAlater and stored at −80 °C until RNA extraction.
Peripheral Leukocyte and Tissue RNA Extraction
Peripheral leukocytes resuspended in RNAlater were centrifuged at 650 g for 10 min at 4 °C to reform the pellet and RNAlater was removed. For tissues, RNA was extracted from 60 mg each of spleen, popliteal lymph node and thymus. Each sample was placed in 1 mL of QIAzol Lysis reagent (Qiagen, cat. #79306) and homogenized using a tissue homogenizer (Tissue Master 125, Omni International, GA, USA). A commercial RNA extraction kit (RNeasy Plus Universal Mini Kit, Qiagen, cat. #73404) was used according to the manufacturer’s instructions. RNA concentration and quality were determined using a NanoDrop (NanoDrop Spectrophotometer, Thermo Scientific, USA; #ND-2000). RNA samples were stored at −80 °C until gene expression analysis.
Primer design, validation and, gene expression analysis
We quantified the expression of genes related to serotonin machinery and signaling (i.e., synthesis and metabolism, serotonin receptors, and downstream pathways), immune-related genes (i.e., cytokines), and genes involved in metabolic and cellular processes (i.e., apoptosis, cell cycle, among others) in peripheral leukocytes, spleen, thymus and popliteal lymph node tissues. For this, high-throughput Multiplex RT-qPCR BioMark Dynamic Array Integrated Fluidic Circuits (IFCs) was used (Fluidigm Corporation, South San Francisco, CA). Briefly, 96 primers targeting 91 genes of interest, 4 reference genes (ACTB, GADPH, RSP9 and HPRT1), and one reference structural gene were assayed (see Supplementary Table S1). An initial quantification run was performed for primer validation using an 8-point, two-fold dilution series (in triplicate) using RNA pools per tissue of interest. The linearity between RNA quantity and cycle threshold (Ct) was tested and efficiency of amplification was calculated. Primers were considered validated if they passed 5 points with an efficiency of 0.8–1.3 and an R2 ≥ 0.92. Specificity of amplification for each primer pair was evaluated by plotting the dissociation-characteristics of double-stranded DNA. A single peak following melt curve analysis indicated a pure, single amplicon. For gene expression of immune-related tissues and peripheral leukocytes, RNA was diluted to 5–15 ng/μL. All samples were normalized to 256 pg RNA and transferred to the IFC plate with the primer-probe sets. All nanoliter reactions were performed as per manufacturer’s recommendations using the following thermal protocol: 95 °C for 1 min, followed by 30 cycles of 96 °C for 5 s and 60 °C for 20 s. The software, Fluidigm Real-Time PCR Analysis, was used to calculate Ct values for all 96 genes for each sample. Non-detectable expression was set at a Ct of 26 for spleen tissue and 28 for WBC, popliteal lymph node, and thymus tissues. The geometric mean of the four reference genes was calculated for each sample and used to normalize Ct values of genes of interest. Normalized gene expression (ΔCt) was used for statistical analysis.
Statistical analysis
Data were analyzed using linear models in R programming 3.5.1 (R Foundation for Statistical Computing; Vienna, Austria). Variables analyzed were white blood cell counts and ΔCt of genes evaluated by qPCR. The model included pen and treatment (CON, 5-HTP or FLX) as fixed effects. For gene expression analysis, the estimates of the model (ΔΔCt) for each gene were used to calculate fold change relative to CON (i.e. 5-HTP vs. CON or FLX vs. CON), using the 2-ΔΔCt method71. The negative inverse of fold-change values <1 was calculated for visual representation of negative fold changes. Homogeneity of variance and normality of residuals were evaluated by plotting and influential points were detected using Cook’s distance test. Peripheral leukocyte gene expression ΔCt averages for CON, 5-HTP and FLX, and fold changes and P values for 5-HTP vs. CON and FLX vs. CON can be found in Supplementary Table S2. Statistical significance was declared at P ≤ 0.05 and tendencies at 0.05 < P ≤ 0.10.
Supplementary information
Acknowledgements
The authors would like to thank the undergraduate researchers for their assistance in animal care, Dr. Fiona Maunsell, Dr. Bahroz Ahmed and Dr. Myriam Jimenez for their assistance in tissue collection during euthanasia.
Author contributions
J.L. designed the experiment, obtained funding and supervised all aspects of the research. M.G.M., A.L.S., S.F., and B.D.-S. conducted the experiment. J.D. assisted with gene selection, data analysis and interpretation. M.G.M and J.L. wrote the initial draft. All authors read and approved the final manuscript.
Data availability
All data generated or analyzed during this study are included in this published article [and its supplementary figures and tables online].
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-66326-w.
References
- 1.Clover CK, Zarkower A. Immunologic responses in colostrum-fed and colostrum-deprived calves. Am. J. Vet. Res. 1980;41:1002–1007. [PubMed] [Google Scholar]
- 2.Denholm KS, McDougall S, Chambers G, Clough W. Factors associated with colostrum quality in individual cows from dairy herds in the Waikato region of New Zealand. N Z Vet J. 2018;66:115–120. doi: 10.1080/00480169.2017.1418684. [DOI] [PubMed] [Google Scholar]
- 3.Heinrichs AJ, Wells SJ, Losinger WC. A Study of the Use of Milk Replacers for Dairy Calves in the United States. J. Dairy Sci. 1995;8:2831–2837. doi: 10.3168/jds.S0022-0302(95)76913-2. [DOI] [PubMed] [Google Scholar]
- 4.Kampen AH, Olsen I, Tollersrud T, Storset AK, Lund A. Lymphocyte subpopulations and neutrophil function in calves during the first 6 months of life. Vet Immunol Immunopathol. 2006;113:53–63. doi: 10.1016/j.vetimm.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 5.Power ML. & Schulkin, J. Maternal regulation of offspring development in mammals is an ancient adaptation tied to lactation. Appl Transl Genom. 2013;2:55–63. doi: 10.1016/j.atg.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bagnell CA, Bartol FF. Relaxin and the ‘Milky Way’: The lactocrine hypothesis and maternal programming of development. Mol Cell Endocrinol. 2019;487:18–23. doi: 10.1016/j.mce.2019.01.003. [DOI] [PubMed] [Google Scholar]
- 7.Herr N, Bode C, Duerschmied D. The effects of serotonin in immune cells. Frontiers Cardiovasc Med. 2017;4:48–48. doi: 10.3389/fcvm.2017.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O’Connell PJ, et al. A novel form of immune signaling revealed by transmission of the inflammatory mediator serotonin between dendritic cells and T cells. Blood. 2006;107:1010–1017. doi: 10.1182/blood-2005-07-2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.León-Ponte M, Ahern GP, Connell PJ. Serotonin provides an accessory signal to enhance T-cell activation by signaling through the 5-HT7 receptor. Blood. 2007;109:3139–3146. doi: 10.1182/blood-2006-10-052787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berger M, Gray JA, Roth BL. The expanded biology of serotonin. Annu Rev Med. 2009;60:355–366. doi: 10.1146/annurev.med.60.042307.110802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kushnir-Sukhov NM, Brown JM, Wu Y, Kirshenbaum A, Metcalfe DD. Human mast cells are capable of serotonin synthesis and release. J Allergy Clin Immunol. 2007;119:498–499. doi: 10.1016/j.jaci.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 12.Shajib MS, Khan WI. The role of serotonin and its receptors in activation of immune responses and inflammation. Acta Physiol. 2015;213:561–574. doi: 10.1111/apha.12430. [DOI] [PubMed] [Google Scholar]
- 13.Laporta J, Gross JJ, Crenshaw TD, Bruckmaier RM, Hernandez LL. Short communication: timing of first milking affects serotonin (5-HT) concentrations. J Dairy Sci. 2014;97:2944–2948. doi: 10.3168/jds.2013-7336. [DOI] [PubMed] [Google Scholar]
- 14.Moore SAE, Laporta J, Crenshaw TD, Hernandez LL. Patterns of circulating serotonin and related metabolites in multiparous dairy cows in the peripartum period. J Dairy Sci. 2015;98:3754–3765. doi: 10.3168/jds.2014-8841. [DOI] [PubMed] [Google Scholar]
- 15.Kessler EC, Wall SK, Hernandez LL, Bruckmaier RM, Gross JJ. Short communication: Circulating serotonin is related to the metabolic status and lactational performance at the onset of lactation in dairy cows. J. Dairy Sci. 2018;101:11455–11460. doi: 10.3168/jds.2018-14626. [DOI] [PubMed] [Google Scholar]
- 16.Walther DJ, et al. Synthesis of Serotonin by a Second Tryptophan Hydroxylase Isoform. Science. 2003;299:76. doi: 10.1126/science.1078197. [DOI] [PubMed] [Google Scholar]
- 17.Hoyer D, et al. International Union of Pharmacology classification of receptors for 5-hydroxytryptamine (Serotonin) Pharmacol Rev. 1994;46:157–203. [PubMed] [Google Scholar]
- 18.Turner, J. H. et al. In The Serotonin Receptors: From Molecular Pharmacology to Human Therapeutics (ed. Bryan L. Roth) 143–206 (Humana Press, 2006).
- 19.Kaumann AJ, Levy FO. 5-Hydroxytryptamine receptors in the human cardiovascular system. Pharmacol Ther. 2006;111:674–706. doi: 10.1016/j.pharmthera.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 20.Lesurtel M, et al. Platelet-Derived Serotonin Mediates Liver Regeneration. Science. 2006;312:104–107. doi: 10.1126/science.1123842. [DOI] [PubMed] [Google Scholar]
- 21.Watanabe H, et al. Peripheral serotonin enhances lipid metabolism by accelerating bile acid turnover. Endocrinology. 2010;151:4776–4786. doi: 10.1210/en.2009-1349. [DOI] [PubMed] [Google Scholar]
- 22.Hernandez LL, Collier JL, Vomachka AJ, Collier RJ, Horseman ND. Suppression of lactation and acceleration of involution in the bovine mammary gland by a selective serotonin reuptake inhibitor. J Endocrinol. 2011;209:45–54. doi: 10.1530/JOE-10-0452. [DOI] [PubMed] [Google Scholar]
- 23.Laporta J, et al. Increasing serotonin concentrations alter calcium and energy metabolism in dairy cows. J Endocrinol. 2015;226:43–55. doi: 10.1530/JOE-14-0693. [DOI] [PubMed] [Google Scholar]
- 24.Cloutier N, et al. Platelets can enhance vascular permeability. Blood. 2012;120:1334–1343. doi: 10.1182/blood-2012-02-413047. [DOI] [PubMed] [Google Scholar]
- 25.Duerschmied D, et al. Platelet serotonin promotes the recruitment of neutrophils to sites of acute inflammation in mice. Blood. 2013;121:1008–1015. doi: 10.1182/blood-2012-06-437392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dürk T, et al. 5-Hydroxytryptamine modulates cytokine and chemokine production in LPS-primed human monocytes via stimulation of different 5-HTR subtypes. Int Immunol. 2005;17:599–606. doi: 10.1093/intimm/dxh242. [DOI] [PubMed] [Google Scholar]
- 27.Shajib MS, Khan W. The role of serotonin and its receptors in activation of immune responses and inflammation. Acta Physiol. 2014;213:561–574. doi: 10.1111/apha.12430. [DOI] [PubMed] [Google Scholar]
- 28.Ahern GP. 5-HT and the immune system. Curr Opin Pharmacol. 2011;11:29–33. doi: 10.1016/j.coph.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu H, Denna TH, Storkersen JN, Gerriets VA. Beyond a neurotransmitter: The role of serotonin in inflammation and immunity. Pharmacol Res. 2019;140:100–114. doi: 10.1016/j.phrs.2018.06.015. [DOI] [PubMed] [Google Scholar]
- 30.Kushnir-Sukhov NM, et al. 5-Hydroxytryptamine Induces Mast Cell Adhesion and Migration. The J Immunol. 2006;177:6422–6432. doi: 10.4049/jimmunol.177.9.6422. [DOI] [PubMed] [Google Scholar]
- 31.Ghia JE, et al. Serotonin Has a Key Role in Pathogenesis of Experimental Colitis. Gastroenterology. 2009;137:1649–1660. doi: 10.1053/j.gastro.2009.08.041. [DOI] [PubMed] [Google Scholar]
- 32.Li N, et al. Serotonin Activates Dendritic Cell Function in the Context of Gut Inflammation. The Am J Pathol. 2011;178:662–671. doi: 10.1016/j.ajpath.2010.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pai VP, Hernandez LL, Stull MA, Horseman ND. The type 7 serotonin receptor, 5-HT7, is essential in the mammary gland for regulation of mammary epithelial structure and function. Biomed Res Int. 2015;2015:364746–3647. doi: 10.1155/2015/364746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hernández-Castellano LE, Özçelik R, Hernandez LL, Bruckmaier RM. Short communication: Supplementation of colostrum and milk with 5-hydroxy-tryptophan affects immune factors but not growth performance in newborn calves. J. Dairy Sci. 2018;101:794–800. doi: 10.3168/jds.2017-13501. [DOI] [PubMed] [Google Scholar]
- 35.Marrero MG, et al. Increasing serotonin bioavailability in preweaned dairy calves impacts hematology, growth and behavior. Domest Anim Endocrinol. 2019;69:42–50. doi: 10.1016/j.domaniend.2019.04.007. [DOI] [PubMed] [Google Scholar]
- 36.Simonenkov AP, Fedorov VD, Galkin AA. Serotonin insufficiency of neutrophils. Bull Exp Biol Med. 1996;121:426–427. [PubMed] [Google Scholar]
- 37.Cloutier N, et al. Platelets release pathogenic serotonin and return to circulation after immune complex-mediated sequestration. Proc Natl Acad Sci USA. 2018;115:E1550–E1559. doi: 10.1073/pnas.1720553115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nakamura K, Sato T, Ohashi A, Tsurui H, Hasegawa H. Role of a serotonin precursor in development of gut microvilli. Am. J. Pathol. 2008;172:333–344. doi: 10.2353/ajpath.2008.070358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Katoh N, et al. Effect of serotonin on the differentiation of human monocytes into dendritic cells. Clin Exp Immunol. 2006;146:354–361. doi: 10.1111/j.1365-2249.2006.03197.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Szabo A, et al. Immunomodulatory capacity of the serotonin receptor 5-HT2B in a subset of human dendritic cells. Sci Rep. 2018;8:1765. doi: 10.1038/s41598-018-20173-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Inoue M, et al. Regulation of antigen-specific CTL and Th1 cell activation through 5-Hydroxytryptamine 2A receptor. Int Immunopharmacol. 2011;11:67–73. doi: 10.1016/j.intimp.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 42.Liao W, Lin J-X, Wang L, Li P, Leonard WJ. Modulation of cytokine receptors by IL-2 broadly regulates differentiation into helper T cell lineages. Nat immunol. 2011;12:551–559. doi: 10.1038/ni.2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nelson BH. IL-2, Regulatory T cells, and tolerance. J Immunol. 2004;172:3983–3988. doi: 10.4049/jimmunol.172.7.3983. [DOI] [PubMed] [Google Scholar]
- 44.Bream J, et al. A distal region in the interferon-γ gene is a site of epigenetic remodeling and transcriptional regulation by interleukin-2. J Biol Chem. 2004;279:41249–41257. doi: 10.1074/jbc.M401168200. [DOI] [PubMed] [Google Scholar]
- 45.Shirayoshi Y, Burke PA, Appella E, Ozato K. Interferon-induced transcription of a major histocompatibility class I gene accompanies binding of inducible nuclear factors to the interferon consensus sequence. Proc Natl Acad Sci USA. 1988;85:5884–5888. doi: 10.1073/pnas.85.16.5884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huang S, et al. Immune response in mice that lack the interferon-gamma receptor. Science. 1993;259:1742–1745. doi: 10.1126/science.8456301. [DOI] [PubMed] [Google Scholar]
- 47.Davies PA, et al. The 5-HT3B subunit is a major determinant of serotonin-receptor function. Nature. 1999;397:359–363. doi: 10.1038/16941. [DOI] [PubMed] [Google Scholar]
- 48.Barnes NM, Hales TG, Lummis SCR, Peters JA. The 5-HT3 receptor–the relationship between structure and function. Neuropharmacology. 2009;56:273–284. doi: 10.1016/j.neuropharm.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tremblay P-B, et al. Variations in the 5-hydroxytryptamine type 3B receptor gene as predictors of the efficacy of antiemetic treatment in cancer patients. J Clin Oncol. 2003;21:2147–2155. doi: 10.1200/JCO.2003.05.164. [DOI] [PubMed] [Google Scholar]
- 50.Morales M, Wang S-D. Differential composition of 5-hydroxytryptamine 3 receptors synthesized in the rat cns and peripheral nervous system. J Neurosci. 2002;22:6732–6741. doi: 10.1523/JNEUROSCI.22-15-06732.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Weiss S, Sebben M, Kemp DE, Bockaert J. Serotonin 5-HT1 receptors mediate inhibition of cyclic AMP production in neurons. Eur J Pharmacol. 1986;120:227–230. doi: 10.1016/0014-2999(86)90544-3. [DOI] [PubMed] [Google Scholar]
- 52.Kursar JD, Nelson DL, Wainscott DB, Baez M. Molecular cloning, functional expression, and mRNA tissue distribution of the human 5-hydroxytryptamine2B receptor. Mol Pharmacol. 1994;46:227–234. [PubMed] [Google Scholar]
- 53.Müller T, et al. 5-Hydroxytryptamine modulates migration, cytokine and chemokine release and t-cell priming capacity of dendritic cells in vitro and in vivo. PLoS One. 2009;4:e6453. doi: 10.1371/journal.pone.0006453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Idzko M, et al. The serotoninergic receptors of human dendritic cells: identification and coupling to cytokine release. J. Immunol. 2004;172:6011. doi: 10.4049/jimmunol.172.10.6011. [DOI] [PubMed] [Google Scholar]
- 55.Hernández-Castellano LE, Özçelik R, Hernandez LL, Bruckmaier RM. Short communication: Supplementation of colostrum and milk with 5-hydroxy-tryptophan affects immune factors but not growth performance in newborn calves. J Dairy Sci. 2018;101:794–800. doi: 10.3168/jds.2017-13501. [DOI] [PubMed] [Google Scholar]
- 56.Vandenborre K, et al. Interaction of CTLA-4 (CD152) with CD80 or CD86 inhibits human T-cell activation. Immunology. 1999;98:413–421. doi: 10.1046/j.1365-2567.1999.00888.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Park KT, et al. Phenotype and function of cd209+ bovine blood dendritic cells, monocyte-derived-dendritic cells and monocyte-derived macrophages. PloS one. 2016;11:e0165247–e0165247. doi: 10.1371/journal.pone.0165247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fuller RW, Wong DT. Inhibition of serotonin reuptake. Fed Proc. 1977;36:2154–2158. [PubMed] [Google Scholar]
- 59.Gray JA, Roth BL. Paradoxical trafficking and regulation of 5-HT2A receptors by agonists and antagonists. Brain Res Bull. 2001;56:441–451. doi: 10.1016/s0361-9230(01)00623-2. [DOI] [PubMed] [Google Scholar]
- 60.Butler MO, Morinobu S, Duman RS. Chronic Electroconvulsive Seizures Increase the Expression of Serotonin 2 Receptor mRNA in Rat Frontal Cortex. J Neurochem. 1993;61:1270–1276. doi: 10.1111/j.1471-4159.1993.tb13618.x. [DOI] [PubMed] [Google Scholar]
- 61.Stolz JF, Marsden CA, Middlemiss DN. Effect of chronic antidepressant treatment and subsequent withdrawal on [3H]-5-hydroxytryptamine and [3H]-spiperone binding in rat frontal cortex and serotonin receptor mediated behaviour. Psychopharmacology. 1983;80:150–155. doi: 10.1007/BF00427959. [DOI] [PubMed] [Google Scholar]
- 62.Fiebich BL, et al. Expression of 5‐HT3A receptors in cells of the immune system. Scand J Rheumatol Suppl. 2004;33:9–11. [PubMed] [Google Scholar]
- 63.Rinaldi A, et al. Serotonin receptor 3a expression in normal and neoplastic B cells. Pathobiology. 2010;77:129–135. doi: 10.1159/000292646. [DOI] [PubMed] [Google Scholar]
- 64.Vega L. d. l. et al. The 5-HT3 receptor antagonist tropisetron inhibits T cell activation by targeting the calcineurin pathway. Biochem Pharmacol. 2005;70:369–380. doi: 10.1016/j.bcp.2005.04.031. [DOI] [PubMed] [Google Scholar]
- 65.Fan P. Effects of antidepressants on the inward current mediated by 5-HT3 receptors in rat nodose ganglion neurones. Br. J. Pharmacol. 1994;112:741–744. doi: 10.1111/j.1476-5381.1994.tb13140.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Choi J-S, et al. Mechanism of block by fluoxetine of 5-hydroxytryptamine3 (5-HT3)-mediated currents in NCB-20 neuroblastoma cells. Biochem Pharmacol. 2003;66:2125–2132. doi: 10.1016/j.bcp.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 67.Murphy, K. & Weaver, C. Janeway’s Immunobiology 9th edition. (CRC Press, 2016).
- 68.Apte SH, Baz A, Groves P, Kelso A, Kienzle N. Interferon-γ and interleukin-4 reciprocally regulate CD8 expression in CD8+ T cells. Proc Natl Acad Sci USA. 2008;105:17475. doi: 10.1073/pnas.0809549105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Birdsall T. 5-Hydroxytryptophan: A Clinically-Effective Serotonin Precursor. Altern Med Rev. 1998;3:271–280. [PubMed] [Google Scholar]
- 70.Wong DT, Bymaster FP, Engleman EA. Prozac (fluoxetine, lilly 110140), the first selective serotonin uptake inhibitor and an antidepressant drug: Twenty years since its first publication. Life Sci. 1995;57:411–441. doi: 10.1016/0024-3205(95)00209-o. [DOI] [PubMed] [Google Scholar]
- 71.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative pcr and the 2−ΔΔCt method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article [and its supplementary figures and tables online].