Abstract
Chronic kidney disease (CKD) is a relentlessly progressive disease with a very high mortality mainly due to cardiovascular complications. Endothelial dysfunction is well documented in CKD and permanent loss of endothelial homeostasis leads to progressive organ damage. Most of the vast endothelial surface area is part of the microcirculation, but most research in CKD-related cardiovascular disease (CVD) has been devoted to macrovascular complications. We have reviewed all publications evaluating structure and function of the microcirculation in humans with CKD and animals with experimental CKD. Microvascular rarefaction, defined as a loss of perfused microvessels resulting in a significant decrease in microvascular density, is a quintessential finding in these studies. The median microvascular density was reduced by 29% in skeletal muscle and 24% in the heart in animal models of CKD and by 32% in human biopsy, autopsy and imaging studies. CKD induces rarefaction due to the loss of coherent vessel systems distal to the level of smaller arterioles, generating a typical heterogeneous pattern with avascular patches, resulting in a dysfunctional endothelium with diminished perfusion, shunting and tissue hypoxia. Endothelial cell apoptosis, hypertension, multiple metabolic, endocrine and immune disturbances of the uremic milieu and specifically, a dysregulated angiogenesis, all contribute to the multifactorial pathogenesis. By setting the stage for the development of tissue fibrosis and end organ failure, microvascular rarefaction is a principal pathogenic factor in the development of severe organ dysfunction in CKD patients, especially CVD, cerebrovascular dysfunction, muscular atrophy, cachexia, and progression of kidney disease. Treatment strategies for microvascular disease are urgently needed.
Keywords: capillary, cardiovascular disease, chronic kidney disease, endothelial dysfunction, hypertension, Microcirculation
Chronic kidney disease (CKD)-specific mechanisms explaining the link between CKD and cardiovascular disease (CVD) are still incompletely understood. Accumulating evidence indicates that the earliest manifestations of CVD occur at the level of the microcirculation [1]. Endothelial dysfunction has been consistently observed in patients of all age groups, even at the earliest stages of CKD [2]. Here we review studies in animal models and humans with CKD on the morphology of the microcirculation, which provides the structural basis of endothelial function. Almost all of these studies have found rarefaction, defined as a loss of perfused microvessels, resulting in a significant decrease in microvascular density. Furthermore, we review mechanisms involved in the pathogenesis of rarefaction and the overall and organ-specific clinical relevance of microvascular disease in patients with CKD.
Introduction: the microcirculatory network
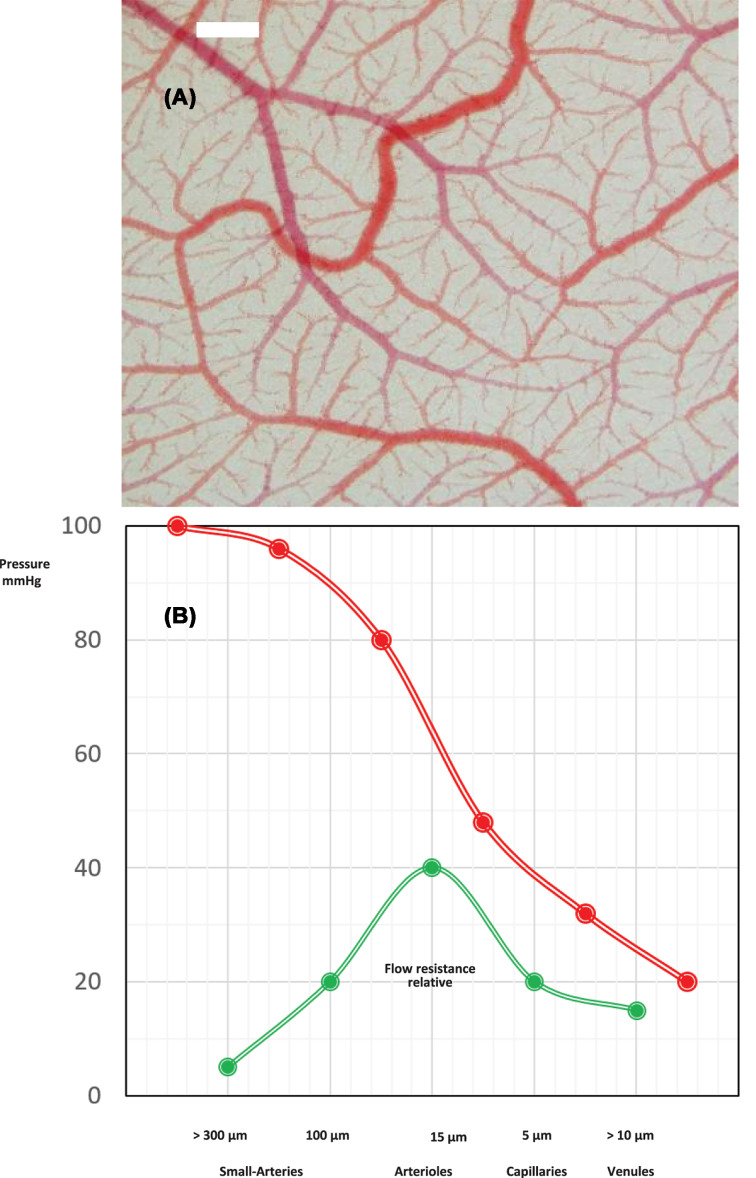
The human microcirculation is formed by networks of arterioles, capillaries and venules (Figure 1) with a diameter less than ∼300 μm and includes small arteries (diameter 100–300 mm), arterioles (diameter < 100 mm) and capillaries (diameter ∼7 mm) [3,4]. With an estimated area of 350 m2, it provides an enormous endothelial interface between blood and tissues for solute and gas exchange and is actively involved in essential physiological functions including regulation of perfusion, hemostasis and coagulation, inflammatory responses, vasculogenesis and angiogenesis [5]. Permanent loss of endothelial homeostasis results in progressive target organ damage [6].
Figure 1. The microcirculatiory network.
(A) Vascular network of the chorioallantoic membrane. In-vivo microphotography, bar = 100 μm. (B) Functional anatomy: pressure and relative flow resistance for coronary vessels of different sizes. The main flow resistance and pressure decrease is located in the arteriolar section of the coronary tree (modified from [190]).
First encounter: hypertension is associated with rarefaction
Rarefaction of the microvasculature was initially described in the context of hypertension research [7]. In studies dating back to the 1970s, microvascular rarefaction was extensively documented in experimental work exploring the origins of increased vascular resistance in animal models of arterial hypertension. Microvascular rarefaction was first shown in the spontaneously hypertensive rats (SHRs) [8,9], later in other models of renal hypertension [10] and in human hypertension [11,12]. These studies demonstrated that in experimental hypertension, functional rarefaction, i.e. active closure of arterioles with diminished perfusion, is an early event, followed by loss of terminal arterioles (third and fourth orders) and capillary loss, i.e. structural rarefaction, in the chronic stage [10]. Today, it is generally accepted that human hypertension is associated with rarefaction, and that tissue perfusion and oxygenation are affected by the extent of rarefaction, which is thus contributing to target organ damage [4].
Studies of the microcirculation in CKD
Animal studies
Skeletal muscle
In the 1990s, the group of Lombard et al. were the first to systematically study the microcirculation in animals (i.e. rats) with surgically induced CKD (Table 1) in the standard cremaster muscle preparation for in-vivo microscopy [10,13,14]. A 75% reduction in renal parenchyma and salt loading resulted in structural microvascular rarefaction in the M. cremaster, mediated by atrophy and structural degeneration as shown by electron microscopy [10]. The reduction in renal mass was used as a model for chronic hypertension and consequently, changes in microvascular density were attributed to hypertension. These authors also noted the marked heterogeneity of microvascular rarefaction in the animals with CKD. Reduced microvascular density was attributed to acute suppression of angiotensin II levels after salt loading [14], implying a role of the renin–angiotensin system (RAS) in microvascular homeostasis, which is supported by earlier observations of arteriolar rarefaction in the M. cremaster after captopril treatment in one-kidney, one-clip hypertensive rats [15]. Hernandez and Greene studied the development of microvascular rarefaction prospectively for 28 days using a plastic skin window implanted over the M. biceps femoralis in rats with reduced renal mass and a high salt diet. Compared with sham-operated controls, microvascular density decreased significantly (−25% by day 10). These experiments also confirmed that microvascular rarefaction contributed significantly (up to an estimated 40%) to increased peripheral vascular resistance [16].
Table 1. Studies of the microcirculation in animal models of CKD: skeletal muscle.
| Author | Year | Species (strain); organ examined | CKD model; CKD duration |
Main findings | Rarefaction [%] Compared with controls (method) |
Ref. |
|---|---|---|---|---|---|---|
| Lombard et al. | 1989 | Rat (Sprague–Dawley); M. cremaster |
¾ NX; short-term HTN (NaCl infusion, 36 h); long-term HTN (4% NaCl in diet 5–6 weeks) | Arterioles were constricted (35–50%) in rats with short term (36 h), but not chronic (5–6 weeks) CKD + hypertension (HTN) | 15% in long-term HTN (Microfil sections) |
[13] |
| Hansen-Smith et al. | 1990 | Rat (Sprague–Dawley); M. cremaster |
¾ NX, HTN (4% NaCl in diet); 4 weeks | Degenerative changes in small- and medium-sized arterioles with loss of endothelial and smooth muscle cells | Proof of anatomic rarefaction (light and electron microscopy) |
[10] |
| Hernandez and Greene | 1995 | Rat (Sprague–Dawley); M. biceps femoralis |
¾ NX; after 10 days, switch from a low-salt to a high-salt diet | Progressive HTN and vascular resistance, decreasing tissue blood flow and MV density during observation (5–28 days) | 25%; (day 10) (videomicroscopy fluorescence) |
[16] |
| Hansen-Smith et al. | 1996 | Rat (Sprague–Dawley); M. cremaster |
¾ NX; short-term HTN (3 days, 4% NaCl in diet) | MV rarefaction in both CKD and sham-op. controls after salt loading | 22–24%, staining of third and fourth orders’ arterioles (lectin staining) | [14] |
| Amann et al. | 1997 | Rat (Sprague–Dawley) M. psoas and heart |
5/6 NX; CKD for 8 weeks | HTN; myocyte cross-sectional area and interstitial tissue increased, MV density reduced in the heart but unchanged in M. psoas | 23% in heart, none in M. psoas (stereological evaluation) |
[24] |
| Jacobi et al. | 2006 | Rat (Sprague–Dawley) M. gastrocnemius (locomotor) M. soleus (locomotor) |
5/6 NX + hindlimb ischemia CKD for 12 weeks |
No HTN; MV unchanged compared with controls at baseline in MG and MS, but increase after ischemia diminished in 5/6 NX rats | No rarefaction, but less increase in MV density after ischemia in CKD animals (CD31 IF) |
[22] |
| Flisinski et al. | 2008 | Rat (Wistar) M. gastrocnemius (MG; locomotor), M. longissimus thoracis (ML; statomotor) |
½ NX or 5/6 NX; CKD for 4 weeks |
½ NX normotensive; 5/6 NX had HTN Significant rarefaction in both stages independent of HTN, similar decrease in capillary/fiber ratio; changes differ in muscles (MG >> ML) |
MG: 56% (½ NX), 48% (5/6 NX) ML: 33% (½ NX), 11% (5/6 NX) (Staining, alkaline phosphatase) |
[17] |
| Flisinski et al. | 2012 | Rat (Wistar) M. gastrocnemius (MG; locomotor), M. longissimus thoracis (ML; statomotor) |
½ NX or 5/6 NX; CKD for 6 weeks |
Decreased expression of HIF-1α, VEGF, VEGF-R1,2 only in MG. Increased HIF-1α protein, iNOS in ML | Not examined | [21] |
| Schellinger et al. | 2017 | Rat (Sprague–Dawley) M. gastrocnemius |
5/6 NX CKD for 8 weeks + hindlimb ischemia for 2 weeks + HIF-1α stabilization by carbon monoxide or prolyl-hydroxylase inhibitor |
MV density decreased after ischemia, but not at baseline compared with sham-op. Post-ischemic MV sprouting impaired in CKD, restored by HIF-1α stabilization |
No rarefaction, but no increase in MV density after ischemia in CKD animals | [23] |
| Prommer and Maurer et al. | 2018 | Mouse (BALB/c) M. cremaster Heart |
5/6 NX; adenine feeding (0.2%) CKD for 4 months (5/6 NX) or for 4 weeks (adenine) |
No HTN; loss of coherent MV networks, large avascular areas, diminished bloodflow velocity, vascular tone, oxygen uptake, MV rarefaction in the cremaster muscle paralleled rarefaction in the myocardium. Decrease in mRNA levels of HIF-1α, Angpt-2, TIE-1 and TIE-2, Flkt-1 and MMP-9 in the heart |
Progressive rarefaction with increasing severity of CKD (serum urea levels). Mean: 34% (5/6 NX); 43% (adenine) | [18] |
Abbreviations: ACEi, angiotensin-converting enzyme inhibitor; HIF, hypoxia-inducible factor; HTN, hypertension; IF, immunofluorescence; iNOS, inducible NO synthase; MV, microvascular; NX, nephrectomy; sham-op., sham operated.
In a study by Flisinki et al. comparing the M. gastrocnemius (locomotor) and the M. longissimus thoracis (statomotor) in rats with uni- or 5/6 nephrectomy (5/6 NX), microvascular density was reduced to a greater degree in the locomotor muscle, corresponding with a greater decrease in fiber, but not with the degree of renal impairment [21]. The authors concluded that locomotor muscles might be more affected by CKD-induced microvascular rarefaction than postural muscles [17]. These data also suggested that muscular atrophy could be a major factor in the pathogenesis of microvascular rarefaction in muscle. In a follow-up study using the identical design, Flisinski et al. found significantly reduced expression of hypoxia-inducible factor (HIF)-1α (HIF-1α) mRNA and protein, and reduced expression of VEGF-A, VEGFR-1 and VEGFR-2 mRNA in the M. gastrocnemius in 5/6 NX animals [21]. In contrast, in the M. longissimus thoracis mRNA levels of VEGF-A, VEGFR-1, VEGFR-2 and HIF-1α did not differ compared with controls, while HIF-1α protein levels and the inducible NO synthase (iNOS) were increased. The authors concluded that in CKD, postural skeletal muscle (but not locomotor muscle) with more oxidative fibers might be protected from rarefaction by the increased expression of iNOS and NO-dependent HIF-1α stabilization. These findings provided important insight into the involvement of HIF-1α, the transcriptional regulator of the compensatory response to hypoxia, in the pathogenesis of microvascular disease. Whether stabilization or up-regulation of HIF-1α can ameliorate CKD-induced microvascular disease, has been more closely examined in studies of post-ischemic muscle in animals with CKD (see below).
We have recently performed a comprehensive in-vivo analysis of microcirculatory architecture and function in the murine cremaster muscle, using two different models of experimental uremia in mice, 5/6 NX and adenine feeding [18]. There was considerable variation in the degree of uremia produced by both experimental protocols, permitting the investigation of the effects of CKD of different severities. Microvascular density was strongly correlated with renal dysfunction as assessed by urea levels, independent of the experimental model and other CKD-associated conditions such as hypertension, anemia, weight loss and inflammation. In an incremental manner, CKD was associated with distinct structural changes involving loss of coherent networks of microvessels. These included not only capillaries (caliber 8–16 μm), but also small arterioles and venules with caliber classes up to 64 μm resulting in a highly heterogeneous pattern of focal microvascular rarefaction and large avascular areas. The calculated impairment of oxygen uptake was 25 and 63% in mildly and severely uremic mice, respectively, for microvessels with a diameter of 64–128 μm. This was accompanied by reduced blood flow (due to rarefaction), a lower hematocrit (due to renal anemia) and a diminished avDO2 (due to rarefaction and functional shunting). In addition, blood flow velocity was decreased and vascular tone was diminished. Microvascular density in cardiac and cremaster muscle of identical animals declined in parallel, strongly suggesting a systemic ‘toxic’ effect of uremia on the microcirculation. In the myocardium, there was a decrease in transcription levels of not only HIF-1α, but also of its target genes Angpt-2, TIE-1 and TIE-2, Flkt-1 and MMP-9, thus demonstrating lack of a core response to hypoxia.
Skeletal muscle after hindlimb ischemia
Angiogenesis is severely impaired in all forms of experimental renal failure, including acute kidney injury (AKI), chronic (CKD) or progressive (AKI-to-CKD transition) disease [19]. The physiological response to hypoxia is mainly driven by hypoxia-induced factors such as HIF-1α and a network of target genes in a coordinated fashion [20]. However, there is evidence for paradoxical down-regulation of HIF-1α and its target genes in murine models of CKD [18,21] and in a rat model of hindlimb ischemia [22,23]. Schellinger et al. could demonstrate that stabilization of HIF-1α by either carbon monoxide administration or prolyl-hydroxylase inhibition rescued angiogenic gene expression and induced post-ischemic capillary sprouting in 5/6 NX rats [23].
In summary, most, but not all, studies found microvascular rarefaction in muscle tissue of animals with experimental CKD. In the study by Prommer et al., rarefaction in the cremaster muscle paralleled rarefaction in the myocardium, suggesting a systemic effect of CKD on the microcirculation [18]. However, some studies found no rarefaction in locomotor muscles [22–24], and statomotor muscles may even be protected from rarefaction by HIF-1α stabilization, as shown by Flisinski et al. [21]. At present, it remains unclear whether these conflicting findings are due to differences in experimental technique or the severity of uremia, which seems to be rather closely correlated to the degree of rarefaction [18]. The 5/6 NX procedure results in relatively mild CKD which can differ in severity between animals in the same experiment [18]. In addition, it is unclear to what extent hypertension contributed to rarefaction in these studies; blood pressure is highly variable in different animal models of CKD [25], while CKD-induced microvascular rarefaction has been observed in the absence of hypertension (Tables 1 and 2).
Table 2. Studies of the microcirculation in animal models of CKD: heart.
| First author | Year | Species (strain) | CKD model; CKD duration treatment | Main findings | Rarefaction [%] Compared with controls (method) | Ref. |
|---|---|---|---|---|---|---|
| Amann | 1992 | Rat (Sprague–Dawley) | 5/6 NX; CKD for 14 months | HTN, left ventricular hypertrophy and MV rarefaction | 25% (stereological evaluation) | [26] |
| Törnig | 1996 | Rat (Sprague–Dawley) | 5/6 NX; CKD for 8 weeks + antihypertensive treatment | No HTN; reduction in MV density was completely prevented by Moxonidine and in part by Ramipril | 22% (stereological evaluation) | [27] |
| Amann | 1997 | Rat (Sprague–Dawley) heart, M. psoas |
5/6 NX; CKD for 8 weeks | HTN; myocyte cross-sectional area and interstitial tissue increased, MV density reduced in the heart but unchanged in M. psoas | 23% in heart, none in M. psoas (stereological evaluation) | [24] |
| Amann | 2000 | Rat (Sprague–Dawley) | 5/6 NX; CKD for 8 weeks ± Epo or Epo + antihypertensives (hydralazine+furosemide) | HTN; capillary rarefaction unchanged with Epo or Epo +antihypertensive treatment | 25% (stereological evaluation) | [34] |
| Amann | 2000 | Rat (Sprague–Dawley) | 5/6 NX; CKD for 15 weeks ± endothelin receptor antagonist or ACEi | HTN; MV density normalized with endothelin receptor antagonist, but not with ACEi | 17% (stereological evaluation) | [35] |
| Amann | 2000 | Rat (Sprague–Dawley) | 5/6 NX; CKD for 8 weeks ± ACEi or bradykinin receptor antagonist or both | No HTN; ACEi, but not bradykinin receptor antagonist abrogated MV rarefaction | 29% (stereological evaluation) | [36] |
| Amann | 2002 | Rat (Sprague–Dawley) | 5/6 NX; CKD for12 weeks ± α-Tocopherol (Vitamin E) | HTN; α-Tocopherol significantly attenuated MV rarefaction and interstitial fibrosis | 24% (stereological evaluation) | [37] |
| Ogata | 2003 | Rat (Sprague–Dawley) | 5/6 NX; CKD for 8 weeks ± PTX or calcimimetic | HTN; treatment significantly attenuated MV rarefaction and interstitial fibrosis | 28% (stereological evaluation) | [39] |
| Gross | 2005 | Spontaneously hypertensive stroke-prone rat (Wistar–Kyoto) | ½ NX; CKD for 12 weeks ± ovariectomy | HTN; significant improvement in MV density in ovariectomized rats treated with estrogens | n.a. (no controls without CKD) | [152] |
| Koleganova | 2009 | Rat (Sprague–Dawley) | 5/6 NX; CKD for 12 weeks (or 4 weeks) ± calcimimetic | HTN; treatment with a calcimimetic attenuated rarefaction and interstitial fibrosis | Approx. 28% (15% after 4 weeks) (stereological evaluation) | [38] |
| Koleganova | 2009 | Rat (Sprague–Dawley) | 5/6 NX; CKD for 12 weeks ± calcitriol | HTN; treatment with calcitriol ameliorated rarefaction and fibrosis | Approx. 25% (stereological evaluation) | [40] |
| Tyralla | 2011 | Rat (Sprague–Dawley) | 5/6 NX; CKD for 8 weeks + 4 weeks antihypertensive treatment | HTN; treatment with ACEi, (but not with furosemide/hydralazine) improved myocardial fibrosis but not rarefaction | 10%; unchanged by antihypertensive treatment (stereological evaluation) | [41] |
| Amann | 2011 | Rat (Sprague–Dawley) | (1) 5/6 NX; CKD for 8 weeks(2) 5/6 NX; CKD for 10 days ± renal denervation | No HTN; MV rarefaction prevented by renal denervation (after 10 days) | (1)18%;(2) 24% (stereological evaluation) | [33] |
| DiMarco | 2011 | Rat (Sprague–Dawley) | 5/6 NX; CKD for 14 days ± calcineurin inhibitor, hydralazine | HTN; treatment with calcineurin inhibitors, but not hydralazine, normalized MV density | ∼20% (lectin staining) | [42] |
| Gut | 2013 | Rat (Sprague–Dawley) | 5/6 NX CKD for 16 weeks ± Epo + enalapril | HTN; treatment normalized MV density, ameliorated myocardial fibrosis in 5/6 NX rats | −15% in 5/6 NX (methylene blue/basic fuchsin staining) | [47] |
| Ali | 2014 | Rat (Wistar) | Adenine feeding (0.75%), 4 weeks ± gum acacia | HTN, myocardial hypertrophy, MV rarefaction ameliorated by treatment | Significantly decreased (with adenine; HE and PAS staining) | [50] |
| Di Marco | 2015 | Rat (Spague–Dawley) | 5/6 NX ± sFlt-1 or VEGF121 infusion for 14 days (starting after NX) | HTN, MV density −15% in 5/6 NX treated with sFlt-1 and unchanged compared with sham-op. if treated with VEGF121 | (lectin staining) | [48] |
| Golle | 2017 | Rat (Sprague–Dawley) | 5/6 NX CKD for 14 days, ± bone marrow-derived cells or their conditioned medium | MV density significantly decreased, restored by treatment | −20% (lectin staining) | [44] |
| Prommer and Maurer | 2018 | Mouse (BALB/c) heart, M. cremaster | 5/6 NX; adenine feeding (0.2%) CKD for 4 months (5/6 NX) or for 4 weeks (adenine) | No HTN; loss of coherent MV networks, large avascular areas, diminished blood flow velocity, vascular tone, oxygen uptake. Rarefaction in the cremaster muscle paralleled rarefaction in the myocardium. Decrease in mRNA levels of HIF-1α, Angpt-2, TIE-1 and TIE-2, Flkt-1 and MMP-9 in the heart | Progressive rarefaction with increasing severity of CKD (serum urea levels). Mean: 34% (5/6 NX); 43% (adenine) | [18] |
| Uchida | 2020 | Rat (Sprague−Dawley) | 5/6 NX CKD for 6 weeks + N-nitro-l-arginine (nitric oxide synthase inhibitor), ± enarodustat (prolyl-hydroxylase inhibitor,) | HTN; enarodustat restored capillary density in heart and kidneys, ameliorated myocardial fibrosis without change in HTN | Significantly decreased (mouse anti-aminopeptidase P monoclonal antibody) | [151] |
Heart
The hearts of uremic rats (5/6 NX) were first examined by Amann et al. (Table 2), who noted hypertension, cardiac hypertrophy with increased interstitial fibrosis and decreased capillary supply in the myocardium of these animals with long-standing (14 months) CKD [26]. In a follow-up study with CKD duration of 8 weeks, these researchers confirmed the findings in the heart, but could not find a reduced microvascular density in the M. psoas [24].
In another study by this group, 5/6 NX rats were normotensive after 8 weeks and systolic blood pressure was further lowered by antihypertensive treatment. Significant microvascular rarefaction was found in normotensive CKD rats, which could be completely prevented by administration of moxonidine, a central sympathicolytic agent, but not by the ACE inhibitor ramipril or the calcium antagonist nifedipine [27]. This study clearly showed that microvascular rarefaction in the 5/6 NX model of CKD was not dependent on the presence of hypertension. Furthermore, the observed protective effect of moxonidine in this study confirmed a modulating effect of sympathetic nerve trafficking on microvascular proliferation in the myocardium in experimental CKD, which had been previously described in hypertensive rabbits and rats after sympathectomy [28–30]. It has long been known that sympathetic nerve activity, a chief regulator of blood pressure, is elevated in early stages of CKD and increases with progression of CKD; even in the absence of hypertension, sympathetic overactivity has deleterious effects on arterial compliance and endothelial function (flow-mediated dilation) (reviewed in [31]). In line with these findings, suppressing sympathetic hyperactivity by renal denervation in SHRs has been shown to result in preservation of left ventricular systolic and vascular endothelial function, prevention of stroke and brain injury; this could not be achieved with similar lowering of the blood pressure with hydralazine, indicating blood pressure-independent beneficial effects of renal denervation on endothelial function [32]. Furthermore, sympathetic down-regulation by interruption of afferent pathways, i.e. selective renal denervation, prevented microvascular rarefaction in rats with 5/6 NX in the absence of hypertension, and normalized the expression of the main angiogenic factor VEGF in the myocardium [33]. Taken together, these studies indicate that sympathetic overactivity, independent of blood pressure, is a major contributor to microvascular disease in the heart of animals with experimental CKD.
In several follow-up studies the group of Amann and Ritz investigated how the myocardial microvascular supply and myocardial fibrosis can be ameliorated by pharmacological interventions in experimental CKD (5/6 NX rats). First, it could be shown that neither treatment with erythropoietin alone or in combination with antihypertensive treatment (hydralazine + furosemide) could ameliorate microvascular rarefaction in this model, confirming that the development of microvascular disease was independent of anemia and hypertension [34]. In contrast, treatment with an endothelin receptor antagonist, but not with and ACE inhibitor (trandolapril), normalized microvascular density [35]. However in another study, treatment with a different ACE inhibitor (ramipril) normalized microvascular density, whereas treatment with a bradykinin receptor antagonist had no effect [36]. High-dose antioxidant treatment with vitamin E abrogated microvascular rarefaction and interstitial fibrosis [37]. Treatment of secondary hyperparathyroidism with parathyroidectomy or with a calcimimetic drug attenuated the development of microvascular rarefaction and interstitial in the myocardium without affecting hypertension [38,39]. Similar findings were made if 5/6 NX rats were treated with calcitriol [40]. When treated either with an ACE inhibitor (high-dose enalapril) or with furosemide/hydralazine, myocardial fibrosis was significantly reduced by the ACE inhibitor, but microvascular rarefaction was unchanged by either treatment [41]
Di Marco et al. could demonstrate in 5/6 NX rats that treatment with the calcineurin inhibitors cyclosporin A and tacrolimus normalized microvascular density and prevented cardiac remodeling, without affecting blood pressure [42]. The calcineurin signaling pathway was found to be strongly up-regulated in CKD rats, and inhibition was accompanied by a significant increase in gene expression and protein abundance of angiogenic and stem cell-related genes; the highest increase in expression levels was measured for VEGFR2, survivin, cKit-1 and stem cell factor. These results indicated that calcineurin inhibition enhances angiogenesis via a VEGF-dependent mechanism including mobilization and homing of stem/progenitor cells. These data are in line with a study by Wang et al., who could show that cyclosporin A treatment in C57BL/6 mice (without CKD) improved perfusion of ischemic hindlimbs by mobilization of endothelial progenitor cells (EPCs) from bone marrow [43].
Furthermore, Di Marco et al. could show that VEGF inhibition by sFlt-1, a specific inhibitor of the Flt-1 VEGF receptor, abolished the beneficial effect of calcineurin inhibition on myocardial microvascular density but not on cardiac hypertrophy [42]. This study showed that microvascular rarefaction in the myocardium of 5/6 NX animals is associated with up-regulation of calcineurin signaling, a diminished angiogenic response to VEGF (which could be overcome by calcineurin inhibition) and a decreased number of circulating progenitor cells.
In a study by the same group, Golle et al. could show that treatment with bone marrow-derived cells (a pool of pluripotent stem and progenitor cells) or their conditioned medium was able to restore microvascular density in the heart of rats when given after 14 days after subtotal nephrectomy [44]. These data are in line with earlier human studies by Choi et al. who found a diminished number and an impaired angiogenic function of circulating EPCs in hemodialysis patients [45]. EPCs maintain vascular integrity by promoting angiogenesis, thus restoring tissue vascularization after endothelial injury [46].
Gut et al. investigated the effect of erythropoietin and ACE inhibition with enalapril on myocardial remodeling and microvascular density in 5/6 NX rats [47]. They could demonstrate the presence of hypoxia, oxidative stress, apoptosis and fibrosis in the myocardium of CKD rats. Treatment with the combination of erythropoietin and enalapril (but not with either drug alone) prevented microvascular rarefaction and oxidative stress and reduced apoptotic signaling and fibrosis [47]. In the 5/6 NX rats with microvascular rarefaction, VEGF was significantly increased, indicating VEGF resistance, while Flk-1 (the VEGF receptor 2) was significantly decreased and angiopoietin receptors TIE-1 and TIE-2 unchanged; combination treatment normalized VEGF and Flk-1 expression.
Di Marco et al. showed that serum levels of Flk-1 (sFlk-1) are increased in CKD patients and associated with eGFR and clinical signs of heart failure [48]. Treatment of rats with sFlk-1 led to a significant reduction in heart capillary density and cardiac output, which could be prevented by treatment with the sFlk-1 antagonist VEGF121. This study could thus demonstrate a link between microvascular rarefaction, sFlt-1 and heart failure in CKD.
In the model of adenine-induced CKD in rats, administration of gum acacia, a dietary polysaccharide with putative anti-inflammatory and antioxidative properties [49] was shown to achieve amelioration of left myocardial hypertrophy and microvascular rarefaction. However, gum acacia treatment also reversed hypertension, ameliorated proteinuria, hyperphosphatemia and decreased serum levels of the uremic toxin indoxyl-sulfate, all of which may have contributed to the beneficial cardiac effects [50]. It remains thus unknown whether gum acacia has a specific direct protective effect on the endothelium.
In summary, CKD-induced microvascular disease in target organs has been most frequently examined in the myocardium. Significant microvascular rarefaction in the myocardium was found in all studies examining the heart in rodents with experimental CKD. Microvascular rarefaction in the myocardium is accompanied by interstitial fibrosis. Hypertension is a chief contributor to microvascular rarefaction in CKD, but the effects of antihypertensive treatment have not been consistent. Importantly, these studies have uncovered several other pathogenetic mechanisms and shown potential avenues of treatment: sympathetic hyperactivity, up-regulation of calcineurin signaling, hypoxia, oxidative stress, apoptosis, a decrease in EPCs and a suppressed angiogenesis.
Kidney
Ehling et al. studied progressive changes in the renal microcirculation by in-vivo micro CT in three different murine models of CKD: ischemia–reperfusion (I/R, days 1–56), unilateral ureteral obstruction (UUO, days 1–10) and Col4a3-deficient (Alport) mice (6–8 weeks old) [51]. First, these researchers could demonstrate a progressive decline in renal blood volume ranging from 20% in earlier to 61% in later CKD stages. Second, in parallel to this reduction, immunohistochemistry showed a progressive and continuous rarefaction of peritubular capillaries in parallel with the development of, but preceding, fibrosis. Furthermore, ex-vivo Microfil perfusion studies could show distinct alterations of microvascular networks: a reduction in vessel diameter and in vessel branching, and increased vessel tortuosity. These microvascular alterations were strikingly similar in all three models, resulting in pronounced rarefaction of small-caliber arteries (from Aa interlobulares downward), but not larger arteries. In a follow-up study, this group could demonstrate progressive ultrastructural and functional alterations (protein leakage) in peritubular capillaries indicating microvascular dysfunction regardless of the etiology of CKD [52]. Taken together, these studies show progressive microvascular rarefaction in the kidney in CKD involving capillaries and small arteries with a diameter up to 30–90 μm. We obtained similar data in the rat cremaster muscle [18]. These data suggest that CKD induces ‘dose-dependent’ loss of coherent microvascular networks including small arteries, capillaries and venules. Importantly, these small-sized vessels make up the bulk of the microcirculation and provide the vital physiological functions of the endothelium.
Human studies
Autopsy, biopsy and nailfold capillaroscopy studies
Amann et al. studied myocardial capillary density in an autopsy study including nine patients on dialysis, nine hypertensive patients without CKD and ten controls (Table 3) [53]. In the dialyzed patients, capillary density was reduced by 50% compared with controls and by 35% compared with hypertensive patients. In addition, myocyte diameter and volume density of myocardial interstitial tissue were significantly increased in uremic patients compared with patients with hypertension and control patients, indicating mismatch between myocytes and microvascular supply in CKD.
Table 3. Microvascular density in humans with CKD: autopsy, biopsy and nailfold capillaroscopy studies.
| First author (type of study) | Year | N patients/controls CKD stage | Organ examined | Main findings | Method | Ref. |
|---|---|---|---|---|---|---|
| Lewis (Biopsy) | 1985 | 60 on dialysis/21 controls | M. vastus lateralis | Capillary/fiber ratio decreased (−34%) in dialysis patients | CD31 antibody staining | [191] |
| Amann (Autopsy) | 1998 | 9 on dialysis/9 with essential HTN/10 controls | Heart | MV density significantly lower compared with patients with HTN (−21%) or normotensive controls (−49%). Interstitial tissue significantly increased in dialyzed patients | Lectin staining | [53] |
| Sakkas (Biopsy) | 2003 | 22 with stage 5 CKD, pre-dialysis/20 controls | M. rectus abdominis (statomotor) | Capillary/fiber ratio −20%, general muscle fiber atrophy (−20%) in CKD pts | α-amylase PAS stain | [56] |
| Sakkas (Biopsy) | 2003 | 24 on HD | M. gastrocnemius (locomotor) before and after 6 months aerobic exercise | Increase in cross-sectional fiber area by 46% and capillary contact per fiber by 24% after exercise training | α-amylase PAS stain | [57] |
| Charytan (Autopsy) | 2014 | 17 with stage 3–4 CKD, 7 on dialysis/21 controls | Heart | MV density decreased by 12% in CKD and 16% in dialysis patients. Interstitial fibrosis increased by 12% in CKD and 77% in dialysis patients. EndMT increased by 17% in CKD and dialysis patients | CD31 antibody staining | [54] |
| Nissel (Capillaroscopy) | 2009 | 6 with CKD stage 3–4, 9 on dialysis/15 controls | Nailfold | Capillary density decreased by 26% at baseline | Nailfold capillary microscopy | [60] |
| Thang (Capillaroscopy) | 2011 | 19 with stage 5 CKD (predialysis), 20 HD, 15 PD; 19 controls | Nailfold | Capillary density reduced in CKD groups at baseline (–25, 20%), during reactive hyperemia and during venous occlusion | Nailfold capillary microscopy | [58] |
| Edwards-Richards (Capillaroscopy) | 2014 | 19 on HD/20 controls | Nailfold | Capillary density reduced by 24% at baseline and 31% after 6 months in HD patients | Nailfold capillary microscopy | [59] |
| Burkhardt (Biopsy) | 2016 | 23 children stage 5 CKD predialysis/32 controls | Omentum | MV surface area 36% (manual imaging) and 51% (automated imaging) lower vs. controls | CD31 antibody staining | [61] |
| Von Stillfried (CT angiography postmortem/in patients) | 2016 | 9 CKD (stage 3 and 4) 8 controls | Kidney | Capillary density reduced by 39%/59% in cortex and 32%/49% in medulla | CD31 antibody staining | [62] |
In an autopsy study including 24 patients with stages 2–5 CKD, Charytan et al. could demonstrate microvascular rarefaction and fibrosis in the myocardium, accompanied by an increased rate of endothelial to mesenchymal transition (EndMT) with decreasing GFR [54]. In a cohort of 162 patients with stages 2–5 CKD, these authors showed high serum concentrations of the angiogenesis inhibitors asymmetric dimethylarginine (ADMA), endostatin (END), angiopoietin-2 (ANG) and thrombospondin-2 (TSP), which were negatively correlated with the calculated GFR of these patients. In vitro, treatment with angiogenesis inhibitors dose-dependently caused endothelial apoptosis, proliferation and EndMT in in human coronary endothelial cells [54].
Since cachexia, muscle weakness and wasting are well-known comorbid conditions in CKD, skeletal muscle morphology and function has been extensively studied in CKD patients (reviewed in [55], but only few studies have analyzed changes in the microcirculation in muscle biopsies. Of note, physical activity and muscle function (e.g. statomotor vs. locomotor) determines muscle fiber structure and vascularization; microvascular density should be assessed in relation to fiber, expressed as capillary/fiber ratio. Sakkas et al. could document a diminished capillary-fiber ratio (−20%) in patients with stage 5 (predialysis) CKD and an improvement in microvascular supply after 6 months of aerobic exercise in hemodialysis patients [56,57].
The cutaneous microcirculation, increasingly used as a representative vascular bed to study systemic microvascular structure and function, can be easily assessed in vivo by nailfold capillary microscopy. Thang et al. observed significantly reduced capillary density in stage 5 CKD patients at baseline and during reactive hyperemia after arterial compression [58]. Venous occlusion, exposing the maximal number of perfused capillaries, resulted in reduction in capillary density of 23%. Density at baseline and after venous occlusion was significantly associated with serum phosphorus and bicarbonate concentrations [58]. In pediatric hemodialysis patients, nailfold microvascular density was significantly decreased at baseline (non-stimulated), after post-occlusive hyperemia (recruitment) and post-venous occlusion, while functional measurements (recruitment and perfusion) were similar to control subjects [59]. Maximal capillary density correlated significantly with serum calcium, parathyroid hormone (PTH) and 25-hydroxyvitamin D concentrations in this study. Nissel et al. found significantly reduced nailfold capillary density in CKD patients, associated with diminished post-ischemic red cell blood flow, which interestingly could be increased by short-term treatment with growth hormone [60].
We have studied microvascular density in the omentum of children with stage 5 predialysis CKD and age-matched controls undergoing abdominal surgery. As shown by manual and automated imaging, microvascular density was significantly reduced (by 36 and 51%, respectively). There was no evidence for apoptosis or autophagy in the omental biopsy sections by immunohistochemistry; VEGF-A, Flk-1 and Angpt1 staining was not different, but Angpt2 staining was significantly lower in biopsies of CKD children [61].
Microvascular rarefaction in the kidney (with a corresponding reduction in cortical renal blood volume) was confirmed in a small cohort of CKD patients by CT angiography or postmortem virtual biopsy [62]; overall, the pattern of arteriolar and capillary reduction was similar to findings by micro-CT imaging in murine models of CKD as characterized by the same group [51].
Retina
The retina has been termed as the ‘window to the microcirculation’. In patients with CKD, however, the retina may be involved in inherited renal diseases, such as Fabry disease, Alport syndrome, cystinosis or oxalosis. Abnormalities specifically associated with uremia may not only consist of microcirculatory changes but also include diabetic or hypertensive retinopathy, macular degeneration, hemorrhage and calcification [63]. Therefore, it may be difficult to attribute microcirculatory changes to uremia per se, and many adult patients have further unspecific risk confounding factors such as old age or smoking. Taking these confounding factors in consideration, several case–control studies have evaluated retinal abnormalities in CKD patients (Table 4).
Table 4. Case–control studies of the retinal vessels in humans with CKD.
| First author (type of study) | Year | N patients/controls CKD stage | Main findings | Method | Ref. |
|---|---|---|---|---|---|
| Baumann | 2009 | 34 non-diabetic CKD, 33 controls | Ratio of arteriolar and venular lumen diameters lower in CKD | Vascular lumen measurement | [64] |
| Sabanayagam | 2009 | 185 CKD, identified from a multiethnic population-based study (n=3602) | Hypertension and retinal arteriolar narrowing independently associated with CKD | Vascular caliber measurement | [66] |
| Sng | 2010 | 251 CKD, 633 controls (matched from a population-based study) | CKD associated with abnormally low or high fractal dimension, independent of other risk factors | Fractal analysis of fundus photographs | [67] |
| Deva | 2011 | 150 CKD 3–5 vs. 150 CKD 1–2 | Prevalence of retinal abnormalities significantly higher in stage 3–5 CKD | Retinal photography | [63] |
| Ooi | 2011 | 126 CKD 3–5 vs. 126 matched patients with CKD 1–2 | CKD and hypertension independent determinants of arteriolar narrowing | Retinal photography | [65] |
| Liew | 2012 | 1360 CKD, identified from a population based study (n=2971) | Retinopathy and venular dilation significantly associated with CKD independent of diabetes | Retinal photography | [68] |
| Bao | 2015 | 892 CKD, identified from a population based study (n=5158) | Retinopathy significantly associated with CKD, albuminuria | Retinal photography | [69] |
| Mc Gowan | 2015 | 623 CKD, identified from a population based study (n=1122) | No significant associations between retinal vascular parameters and CKD | Retinal photography | [70] |
| Bosch | 2018 | 76 patients with CKD stage 3+ or proteinuria, 53 controls | increased arteriolar wall-to-lumen ratio and capillary rarefaction in CKD patients | Scanning Laser Doppler Flowmetry | [74] |
| Yeung | 2019 | 200 CKD stage 3–5 50 matched controls | Patients with CKD had rarefaction of retinal microvasculature in superficial and deep vascular plexus (5–6%) | Optical coherence tomography angiography | [75] |
| Vadala | 2019 | 120 non diabetic hypertensive patients; 53 CKD vs, 67 non-CKD | Parafoveal vascular density signifiantly lower in CKD patients (1–2%) | Optical coherence tomography angiography | [76] |
Baumann et al. found that the ratio of the arteriolar and venular lumen diameters was significantly decreased in a group of non-diabetic CKD patients compared with controls and correlated with eGFR [64]. In a study of 300 patients with CKD, Deva et al. found that 2% of patients had retinal abnormalities due to inherited renal diseases, 19% had diabetic retinopathy, 35% had macular degeneration and 66% had microvascular retinopathy [63]. Evaluation of the digitized retinal images in this cohort showed arteriolar narrowing that increased progressively with each stage of CKD; multivariate analysis showed that arteriolar caliber was independently determined by eGFR and hypertension, whereas the venular caliber was only dependent on eGFR [65].
Other studies have used a nested case–control design in large population-based studies to evaluate the associations of retinal abnormalities with CKD (eGFR < 60 ml/min/1.73 m2). In a multiethnic study in Singapore, Sabanayagam et al. found that in CKD patients (5.1% of the population) both hypertension and retinal arteriolar narrowing were independently associated with CKD; this finding could be replicated in an independent control population [66]. In a separate analysis of these data, Sng et al. compared patients with CKD with matched healthy controls by studying fractal analysis (reflecting geometic complexity) of retinal vessels from digital fundus images [67]. Fractal dimensions of the highest and lowest quintile were independently associated with CKD, even in participants without diabetes or hypertension, suggesting that abnormal vascular geometry of the retina is associated with CKD. Liew et al. analyzed retinal photographs of participants in a large population based study in Australia [68]. CKD was common (45.8%) in this elderly population (mean age of individuals with CKD: 71.2 years) and significantly associated with retinopathy and venular dilation in diabetic and non-diabetic participants [68]. In a very large study in rural China, retinopathy was significantly associated with the presence of CKD (prevalence 17.3%) and of albuminuria [69]. In contrast with these studies, a population-based study in elderly white Irish nuns (mean age: 76 years) with a high prevalence of CKD (58.7%) found arteriolar narrowing in hypertensive individuals, but no significant associations between retinal vascular parameters and CKD in this cohort [70].
It should be kept in mind that the use of serum creatinine-based equations in studies of elderly populations is problematic, since levels are influenced by muscle mass, chronic conditions and dietary habits [71]. Moreover, eGFR reference values decrease with older age and there is ongoing discussion whether elderly subjects may be classified as having CKD if a cutoff of 60 ml/min/1.73 m2 is applied [72]. This may at least in part explain the reported high prevalence of CKD in studies of elderly populations [68,70] and results of these studies should be viewed with caution. Finally, in a study of CKD patients without controls, Mehta et al. performed a cross-sectional study of fundoscopic images of 1800 patients with moderate to severe CKD participating in the Chronic Renal Insufficiency Cohort study. Higher serum phosphate, but not FGF23, was independently associated with more severe retinopathy and microvascular retinal venous dilatation [73].
While earlier studies have mainly relied on digital fundus photography analysis, recent case–control studies have applied newer non-invasive high-resolution imaging, allowing detailed observations of retinal structures and measurement of retinal capillary density. Bosch et al. could demonstrate an increase in retinal arteriolar wall-to-lumen ratio and capillary rarefaction in patients with stage 3 CKD using Scanning Laser Doppler Flowmetry [74]. Two studies applying optical coherence tomography or optical coherence tomography angiography have shown significant rarefaction of the retinal microvessels in CKD patients in both the superficial and deep vascular plexus [75,76]. The study by Vadala et al. found retinal and choroidal thinning in CKD patients and an association of decreasing renal function with progressive reduction in choroidal and retinal vascular density [76]. In the study by Yeung et al. in 200 patients with CKD stages 3–5, the presence of CKD was independently associated with capillary density [75]. In a study of hypertensive patients without CKD using optical coherence tomography angiography, eGFR was independently correlated with capillary density [77].
In summary, regardless of the chosen design, almost all studies of retinal abnormalities found a high prevalence of retinopathy and to a varying extent, of arteriolar narrowing and venular dilation in association with CKD and/or eGFR. High-resolution imaging by newer imaging methods has confirmed the heterogeneous pattern of microvascular rarefaction in CKD patients. Optical coherence tomography angiography and Scanning Laser Doppler Flowmetry provide razor-sharp images of retinal vessels and might be of great diagnostic and predictive value in future studies of patients with CKD [78].
Clinical relevance of microvascular disease
Vascular disease
The presence of microvascular rarefaction has been confirmed in almost all animal and human studies and several different tissues, suggesting a systemic microvascular disease induced by CKD. However, in contrast with macrovascular complications, the implications of these findings have not been truly appreciated [79]; the PubMed database holds 23 entries for ‘microvascular rarefaction in CKD’, but 1178 for ‘vascular calcification in CKD’. The studies in animal models of CKD reviewed here show microvascular rarefaction with a median of 29% in skeletal muscle and 24% in the heart; studies in human tissue show rarefaction with a median of 32% in human biopsy, autopsy and capillaroscopy studies. Regional differences may occur such as in locomotor vs. statomotor muscles or in the retina (microvascular density of the human retina was only quantified in two studies of small CKD cohorts, showing only 3–4% rarefaction in distinct capillary plexus). However, all vascular beds are lined by one contiguous endothelial layer, and the loss of 20–30% of the total surface area (as found in skeletal muscle and heart) amounts to a massive loss of tissue perfusion.
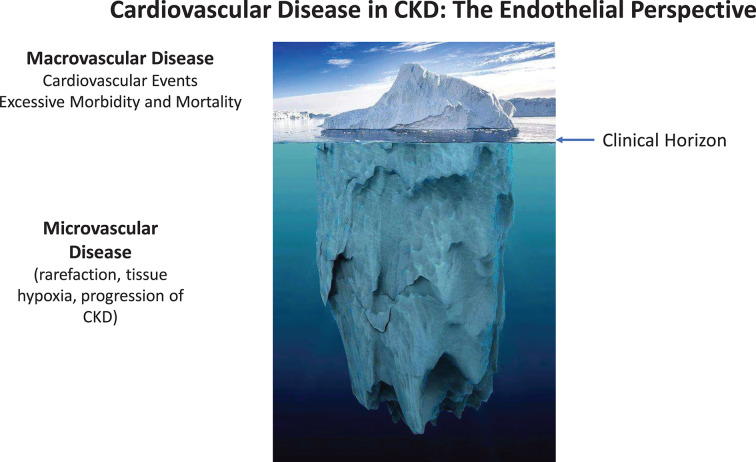
Endothelial dysfunction, with the hallmark of reduced NO bioavailability, is well documented in CKD [2,80], apparently progressive with deterioration of renal function [81], and—as measured by non-invasive surrogate parameters—an independent predictor of cardiovascular events in several studies [82–84]. While endothelial dysfunction has been found associated with many other factors (oxidative stress, inflammatory cytokines, gut-derived uremic toxins etc.) in clinical studies, we here illustrate the need to acknowledge the scope of the underlying structural problem of microvascular rarefaction. Endothelial dysfunction in patients with CKD merely reflects the remaining function of a damaged microcirculation. Thus, considering the entire vasculature from an ‘endothelial perspective’, CKD affects first and foremost the vast area of the microcirculation, in a silent, subclinical process initiated at early CKD stages, preceding macrovascular alterations and progressing with further loss of GFR. When macrovascular involvement, and CVD events appear on the clinical horizon, these late events can be viewed as the tip of the iceberg of CKD-induced vascular disease (Figure 2).
Figure 2. The ‘iceberg delusion’ may prevent appreciation of the magnitude of CVD in CKD patients.
Most of the vast endothelial surface area is part of the microcirculation. Considering the entire vasculature, CKD affects first and foremost the microcirculation in a silent, subclinical process. When macrovascular involvement and CVD events appear on the clinical horizon, these late events can be viewed as the tip of the iceberg of CKD-induced vascular disease.
Cardiac disease
Sudden cardiac death is the leading cause of CVD-associated mortality in adult and pediatric patients with CKD and more prevalent than myocardial infarction [85]. Left ventricular hypertrophy (LVH), progressing with each stage of CKD, has been observed in adult [86] and pediatric cohorts [87] and is strongly associated with survival [88]. LVH reflects structural remodeling of the myocardium, which—as shown by human and animal studies—includes microvascular rarefaction, interstitial fibrosis and myocyte hypertrophy, resulting in increased intercapillary distance and decreased oxygen delivery [18,34], thus predisposing to ventricular arrythmias and sudden cardiac death [85]. Endothelial dysfunction measured by brachial artery flow-mediated dilatation was found associated with LVH [89]. The contribution of microvascular rarefaction to these events is further illustrated by the incremental predictive value of coronary flow reserve for cardiovascular survival in CKD patients of all CKD stages [90–92]. Furthermore, microvascular dysfunction is believed to be the main reason for silent myocardial ischemia, which is found with increased prevalence in CKD patients and not explained by renal anemia [93,94]. In dialysis patients, diminished myocardial oxygen delivery due to cardiac remodeling contributes to intradialytic subclinical myocardial ischemia and myocardial stunning [95].
Cerebrovascular dysfunction
Although no systematic studies of the cerebral microcirculation in CKD have been performed, cerebral ischemic small vessel disease is an essential contributor to cognitive impairment and dementia [96]. In the general population, the presence of even mild CKD is associated with cognitive impairment which is not explained by other established risk factors [97]. In large cohorts of elderly subjects, moderate-to-severe CKD was incrementally associated with incident cognitive impairment at follow-up [98]. In a population-based cohort of 7839 subjects over 65 years, faster eGFR decline was associated with global cognitive decline and incident dementia over 7 years of follow-up [99]. In an elderly population with CKD (eGFR < 45 ml/min/1.73 m2), 48% had mild or severe cognitive impairment [100]. A poorer neurocognitive performance was associated with decreasing eGFR in children and adolescents with CKD compared with age-matched controls [101]. Cognitive impairment has shown close associations with retinal microvascular disease in several CKD cohort studies (reviewed in [102]). Taken together, there is strong clinical and epidemiological evidence implicating microvascular disease in the pathogenesis of cognitive impairment and dementia. Cerebral small vessel disease may also be the main pathogenetic factor for the increased incidence of cerebral microbleeds, white matter lesions and silent cerebral infarcts in CKD patients [102]. Furthermore, the burden of cerebral small vessel disease was associated with ischemic attacks and stroke in younger (<60 years) patients with CKD [103,104].
Kidney disease
Many patients with AKI go on to develop CKD, and much recent research has been devoted to the underlying maladaptive repair process. Animal studies, mostly using unilateral I/R have consistently shown that peritubular capillary rarefaction is an essential component in the pathophysiology of progression of AKI-to-CKD (reviewed in [105]).
In established CKD, systemic microvascular disease severely affects the kidneys in a deleterious feedback loop. Animal models of CKD have provided strong evidence that peritubular capillary loss and subsequent renal fibrosis are a common pathway in CKD progression. As clearly shown in the remnant kidney model, angiogenesis is severely impaired after 5/6 NX in rats [106]. Kang et al. have shown that CKD-induced capillary rarefaction in the remaining kidney tissue is a key pathogenetic factor in the progression of CKD [107]; however, MV rarefaction also occurs in other models of progressive renal disease such as the aging kidney and chronic cyclosporine A nephropathy, and correlates directly with the development of glomerular and tubulointerstitial scarring [108]. Thus, progressive loss of the microvasculature in the kidney is an essential contributor to CKD progression, as well as to the aging-associated decline in renal function.
Recent studies have provided evidence for an involvement of several antiangiogenic factors in the pathogenesis of CKD (reviewed in [109]). Serum levels of antiangiogenic factors such as endostatin and vasohibin-1 were found predictive of incident CKD and/or loss of GFR [110,111]. Antiangiogenesis therefore might be a link between endothelial dysfunction (induced by CKD itself or associated with other conditions such as aging or hypertension) and progressive loss of renal function. At present, while serum levels of antiangiogenic factors appear to be promising biomarkers that parallel kidney damage and dysfunction [109], further prospective studies are needed to determine whether antiangiogenic factors are independent risk factors for CKD.
Finally, it is of interest that several large prospective cohort studies in the general population, which carefully controlled for comorbidities such as hypertension and diabetes, strongly suggest that retinal abnormalities have independent predictive value for the development of renal function. The Cardiovascular Health Study [112], the Multi-Ethnic Study of Atherosclerosis [113], the Atherosclerosis Risk in Communities Study [114] and the Singapore Malay Eye Study [115] unanimously found that microvascular changes involving the retinal arterioles and venules was an independent predictor of incident CKD and/or a significant decline in GFR during follow-up. These studies collectively suggest that the kidney may not only be the villain but also the victim of microvascular disease.
Muscle wasting and cachexia
Patients with CKD suffer from reduced muscle mass, exercise capacity and fitness [116]. In dialysis patients, oxygen transport is impaired in skeletal muscle [117] and oxygen uptake during exercise is reduced, even after normalization of hemoglobin levels by erythropoietin therapy [118,119]. Muscle biopsy specimens showed altered capillary structure on electron microscopy, with an increased thickness of the basement membrane and the capillary endothelium [119]. Muscle capillary density in hemodialysis patients could be ameliorated by exercise training in two studies [56,120], but not by normalization of hematocrit and exercise in a recent study [119]. Thus, microvascular rarefaction is an important component of diminished exercise capacity in CKD.
Muscle wasting and cachexia correlate with quality of life estimates and mortality in maintenance hemodialysis patients and may be related to insulin resistance and vitamin D insufficiency [121]. In a mouse model of nephropathic cystinosis, we recently showed that vitamin D insufficiency was associated with cachexia and muscle weakness and that vitamin D repletion ameliorated muscle wasting and function [122]. Vitamin D insufficiency was also found associated with reduced renal capillary density, fibrosis and inflammation in rats with AKI [123]. A beneficial effect of vitamin D compounds on endothelial function as measured by flow-mediated dilation was described in a small meta-analysis of four randomized trials in CKD patients [124]. Besides Vitamin D insufficiency, increased serum levels of myostatin in CKD patients have been found associated with sarcopenia [125,126]. Myostatin is an inhibitor of skeletal muscle growth and pharmacological inhibition of myostatin prevents loss of muscle mass in mice with CKD [127,128]. Myostatin KO mice have significantly increased lean muscle mass and muscle‐specific increases in endothelium‐dependent vasodilation [129]. However, blocking of myostatin (with the soluble activin receptor IIb) in mice without CKD was shown to result in a decrease in capillary density and proangiogenic signaling [130]. Altogether, current evidence implicates vitamin D deficiency and overexpression of myostatin as contributing factors in the pathogenesis of muscle wasting and cachexia in CKD, but their role in the development of microvascular rarefaction in skeletal muscle remains to be clarified.
Skin
Skin changes are highly prevalent in patients with CKD and pallor and xerosis are among the most frequent symptoms [131], while wound healing is delayed [132], all suggesting poor tissue perfusion. Indeed, studies in patients with all stages of CKD have detected microangiopathic changes in normal-looking skin by skin biopsy, including basement membrane changes and necrosis of endothelial cells of capillaries, arterioles and venules [133,134]; changes increased in severity with time on dialysis [135].
Erectile dysfunction
Endothelial dysfunction with decreased NO synthesis is central in the pathophysiology of erectile dysfunction, which has a reported prevalence of approximately 80% in CKD patients [136].
Mechanisms of CKD-induced rarefaction
Apoptosis
An important role for apoptosis, involving both tubular cells and peritubular capillaries, is established in AKI and its progression to CKD [137–139]. In the setting of I/R injury, peritubular rarefaction is regulated by caspase-3; mice deficient in this caspase had less rarefaction and fibrosis after 3 weeks of follow-up [139]. Apoptosis is also a cause of capillary rarefaction in animal models of hypertension [140].
In established CKD however, it is currently less clear whether the massive loss of endothelium is initiated or accompanied by widespread apoptosis of microvascular cells (endothelial cells, smooth muscle cells, pericytes). Under physiological conditions, the trimming of vascular networks involves either a subset of microvessels (‘pruning’) or complete regression. It is a matter of debate whether this is stimulated by active processes (such as apoptosis) or by withdrawal of survival factors (such as VEGF) or both [141,142]. VEGF levels have been reported as normal or low in CKD, whereas endostatin and several other antiangiogenic peptides accumulate and are capable of inducing apoptosis in vitro [54,143]. Of note, serum from CKD patients induces apoptosis in cultured human endothelial cells [54]. Apoptosis has not been reported in most studies and was absent from some [18,61]. However, the study by Di Marco et al. could show that sFlk-1 accumulates in CKD and that administration of sFlk-1 in rats induced apoptosis and capillary rarefaction in the myocardium; this provides further indirect evidence for a role of apoptosis in microvascular rarefaction in CKD [48].
Dysregulated angiogenesis
Microvascular structure and function is critically dependent on a balance of angiogenesis-regulating factors. This balance is severely disturbed in CKD resulting in an inadequate response to ischemia. HIF-1α, the transcriptional regulator of this response and its pro-angiogenic target genes were shown to be down-regulated in several murine CKD models in muscle [21,23] and heart [18,144]. In the hypoxic tissue of the kidney, HIF-1α accumulates in the interstitium, where it promotes fibrosis but fails to stimulate angiogenesis [19]. Several factors impair the transcriptional response to HIF-1α including hypoxia and indoxylsulfate, a gut-derived uremic toxin, which accumulates in CKD [145]. In addition, high circulating concentrations of antiangiogenic factors such as endostatin have been reported in patients with CKD [54,146]. Gu et al. could show that tissue levels of endostatin in normal rats correlate inversely with microvascular density in heart and skeletal muscle [147]. Furthermore, the number of circulating stem and progenitor cells are decreased and bone marrow-derived stromal cells and EPCs are dysfunctional in CKD [44,148,149]. In summary, angiogenesis is impaired on several levels in CKD: by a deficient core response to hypoxia, by an increase in antiangiogenic factors and by insufficient repair mechanisms.
The central role of angiogenesis suppression in the pathogenesis of microvascular rarefaction is also illustrated by several successful angiogenesis-stimulating experimental strategies in animal models of CKD: (1) systemic or subcutaneous injection of VEGF stabilized renal function and attenuated disease progression in animal models of AKI [150]. (2) Stabilization of HIF-1α by carbon monoxide or inhibition of degradation (by a prolyl hydroxylation inhibitor) restored diminished post-ischemic sprouting in muscle after hindlimb ischemia [23] and restored capillary density in heart and kidneys of 5/6 NX rats [151]. (3) Rescuing the defective repair mechanism by treatment with bone marrow-derived cells or their conditioned medium restored diminished capillary density in the myocardium [44]. (4) Amelioration of microvascular rarefaction was associated with normalized or increased expression of VEGF and/or Flk-1 following different experimental approaches, e.g. treatment with estrogen [152], a calcimimetic [144], calcitriol [40], renal denervation [33], a calcineurin inhibitor [42], the combination of epo and enalapril [47] and the sFlk-1 antagonist VEGF121 [48,109].
Hypertension
Hypertension promotes remodeling of resistance vessels and microvascular rarefaction, although the cause-and-effect relationships of rarefaction and hypertension are still debated [7,153]. The presence of CKD alone is sufficient to induce microvascular rarefaction in animal experiments (Tables 1 and 2), but it can be assumed that rarefaction and end organ damage are further aggravated by hypertension. This notion is supported by studies in humans; autopsy/biopsy and retinal imaging data (Tables 3 and 4) show the presence of MV rarefaction in normotensive CKD patients [61] and independent effects of hypertension and CKD on MV density [53,54,65]. Hypertension is highly prevalent in CKD patients, and generated by multiple mechanisms including an increased activity of the RAS, sympathetic hyperactivity, sodium retention and more (reviewed in [154]). Animal studies could show amelioration or even prevention of rarefaction by pharmacological blockade of the RAAS or of sympathetic hyperactivity (Table 2), demonstrating the important impact of these regulatory networks on the pathogenesis of MV rarefaction. However, data from these studies also show the presence of several other mechanisms contributing to microvascular disease in CKD.
Hypertension is also a principal factor for progression of CKD, thus increasing the impact of CKD on the microcirculation. Both, CKD and hypertension, share dysfunction of common pathways regulating angiogenesis, including an activated RAS [155], sympathetic hyperactivity [156], endothelial cell senescence and macro- and microvascular aging [157,158], and increased levels of circulating antiangiogenic factors [109]; all of these are likely to amplify their respective antiangiogenic effects, aggravating microvascular disease.
Uremic milieu
CKD generates a plethora of metabolic, endocrine and immune disturbances with a deleterious impact on the endothelium. Oxidative stress, carbonyl stress, chronic inflammation, urea and gut-rived uremic toxins and countless altered signaling pathways have been shown associated with endothelial dysfunction [159,160]. While a detailed description is beyond the scope of this review, it should be noted that the sheer multitude of contributing factors underscore the multifactorial impact of uremia in the pathogenesis of microvascular disease. Furthermore, it suggests that therapeutic measures focusing on one particular pathway may be ineffective.
Diabetes is the leading cause of CKD in many countries, and the collective impact of diabetes and CKD creates the highest risk for cardiovascular events and death compared with diabetes or CKD alone [161]. Several studies in diabetic patients have shown microvascular structural abnormalities and rarefaction in the retina at the earliest stage of diabetic microangiopathy (before neovascularization and proliferative changes) [162–164]. Thus, similar to CKD, microvascular disease and endothelial dysfunction is present at early stages of diabetes, preceding macrovascular complications [165]. Driven mainly by hyperglycemia and oxidative stress, diabetic microangiopathy is characterized by thickening of the capillary basement membrane, abnormal growth and permeability of microcirculatory vessels with disordered capillarization [166], and at later stages, microvascular dropout in most organs, [167] including the heart [168] and skeletal muscle [169,162,170].
In long-term prospective studies of diabetic patients, the presence of retinopathy was a significant predictor for the development of albuminuria/nephropathy [170]. In patients with type 2 diabetes, the presence of retinopathy or a reduced GFR or albuminuria dramatically enhances the risk for cardiovascular events [171,172], and in diabetic patients with CKD, the risk for end-stage disease and mortality is closely associated with glycemic control [161]. Although systematic studies are lacking, it can be assumed that CKD-related microvascular rarefaction if further aggraved by diabetic microangiopathy, which might at least in part explain the deleterious impact of the combination of CKD and diabetes on morbidity and mortality.
The phenotype: uremic microangiopathy
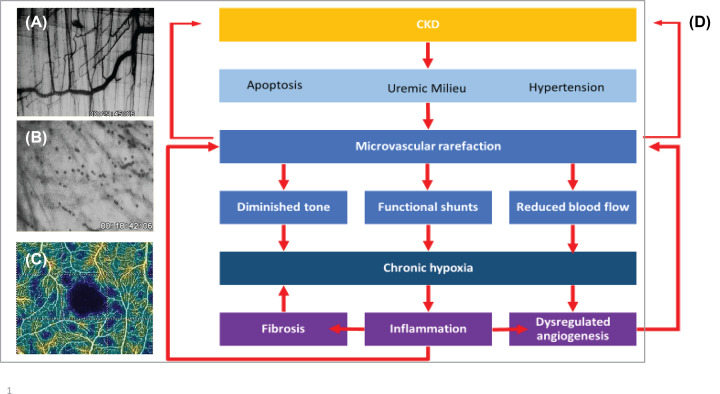
The studies reviewed here have provided evidence for a distinct phenotype of CKD-induced microvascular disease. In three different murine models of CKD, Ehling et al. could demonstrate that microvascular rarefaction in the kidney consistently involves capillaries and small arteries ranging in size from Aa. interlobulares (diameter 30–90 μm in rats) downward [51]. Similarly, loss of microvascular with a diameter below 64 μm was found in the cremaster muscle of mice with 5/6 NX and with adenine-induced CKD [18]. Thus, CKD seems to generate the loss of coherent vessel systems distal to the level of smaller arterioles, i.e. with a cut-off level ∼60 μm in experimental animals. It is conceivable that this process originates in the supplying arteriole [14]. These and other studies in heart and muscle have noted the marked heterogeneity of rarefaction resulting in long intercapillary distances and large avascular areas, where hypoxic conditions prevail [14,18]. Similarly, in a study of CKD patient with optical coherence tomography angiography, the pattern of rarefaction was very heterogeneous with localized non-perfusion areas [75] (Figure 3). Endothelial dysfunction of the uremic microvasculature was evident in a study showing reduced blood flow, diminished vascular tone, shunting and a decrease in calculated oxygen uptake in uremic mice [18]. Similarly, tissue oxygen uptake and transport measured in skeletal muscle was diminished in CKD patients [117].
Figure 3. Microvascular disease in CKD.
(A,B,C) Microvascular rarefaction in-vivo. In-vivo microscopic view (40×) of the microvasculature of an area (350 × 250 μm) of the murine M. cremaster. Panel (A) shows microvessels with a normal density of a control mouse, panel (B) the avascular area of a mouse with severe adenine-induced uremia. (C) Optical coherence tomography angiography, vessel density map of the superior vascular plexus of the retina in a patient with CKD stage 5. Avascular zone of the fovea shown in black, patchy distribution of areas of capillary rarefaction shown in blue (taken from Yeung et al. [75]). (D) A simplified scheme of the pathogenesis of microvascular disease in CKD.
Treatment
Various experimental approaches have been successful in ameliorating or restoring microvascular rarefaction in animal models of CKD (Tables 1 and 2). Improved angiogenic signaling is the hallmark of these studies and has raised expectations of the angiogenic potential of prolyl-hydroxylase inhibitors, which were developed to stimulate endogenous erythropoietin production by stabilizing the HIF complex [173]. Clinical trials have shown therapeutic efficacy of prolyl-hydroxylase inhibition in correcting renal anemia [174,175]. Prolyl-hydroxylase domain proteins mediate oxygen-dependent degradation of HIF-α subunits, and inhibition of these proteins promotes angiogenesis in mice [176]. In a recent study in rats with 5/6 NX, treatment with a prolyl-hydroxylase inhibitor restored capillary density in the heart and the kidneys without affecting blood pressure, ameliorated cardiac hypertrophy and fibrosis, reduced apoptosis and up-regulated angiogenic factors in the myocardium [151]. However, at present, concerns remain that long-term HIF stabilization-induced angiogenesis in humans will increase the risk for tumor growth and diabetic retinopathy [177]. Whether use of these agents can ameliorate CKD [173] or have beneficial effects on cardiovascular events remains to be shown.
While systematic studies targeting the microcirculation in humans are lacking, it could be shown that commonly used drugs have beneficial effects on the microcirculation, which contribute to their protective effects in CKD patients. Pharmacological blockade of the RAS with angiotensin-converting enzyme inhibitors (ACEi’s) or angiotensin resceptor antagonists (ARBs) is protective against end organ damage due to hypertension or diabetes. Independent of blood pressure lowering, the protective effect of these drugs is due to suppressing the activation of the tissue RAS (with local autocrine angiotensin synthesis), which is involved in the pathogenesis of end organ damaging factors including apoptosis, inflammation and fibrosis [178]. In addition, ACEi’s and ARBs have angiogenic effects in experimental animal and in-vitro models of hypertension (reviewed in [179]). In diabetes, the RAS is involved in the pathogenesis of hyperglycemic cell injury and RAS blockade has been shown to protect against microvascular complications of diabetic kidney nephropathy [178]. Altogether, the protective effect of RAS inhibitors on end organ damage is at least in part mediated by protective effects on the microcirculation [179].
RAAS inhibition is also the first line of treatment in diabetic patients with micro- or macroalbuminuria. In addition, the sodium-glucose cotransporter 2 inhibitors (SGLT2i’s) or gliflozines, have pleiotropic effects beyond blood gluose control. A strong reduction in cardiovascular mortality [180,181] and slower progression of CKD [182] has been found in clinical trials of T2DM patients with empagliflozin. The beneficial effect of SGLT2i’s on cardiovascular mortality are believed to be mediated by change in hematocrit, fasting glucose, uric acid and urine albumin:creatinine ratio [183], and their renoprotective effects have been attributed to lowering of blood pressure and intraglomerular pressure and hyperfiltration, modification of inflammatory processes, reduction in ischemia-related renal injury and increases in glucagon levels [184]. However, there is emerging evidence of beneficial effects of inhibitors on the microcirculation, including endothelial function [185], capillary blood flow and arteriolar remodeling of retinal microvessels [186] and projected microvascular complications in patients with T2DM [187]. Besides the SGLT2i’s, the antidiabetic drugs metformin and the glucagon-like peptide-1 (GLP-1) agonists were shown to have cardioprotective effects and beneficial effects on the endothelium (reviewed in [188]).
Summary
Taken together, collective evidence from animal and human studies shows that CKD induces systemic microvascular rarefaction. The phenotype of this ‘uremic angiopathy’ is characterized by loss of coherent microvascular networks in a heterogeneous pattern with avascular patches, resulting in a dysfunctional microcirculation with diminished perfusion, shunting and tissue hypoxia. In different animal models of CKD, microvascular loss uniformly precedes fibrosis, and the progression of rarefaction and fibrosis are closely correlated [51]. Thus, uremic microangiopathy, in concert with chronic inflammation and other metabolic disturbances, sets the stage for the development of tissue fibrosis and end organ failure [189] (Figure 3). Microvascular disease is a principal pathogenic factor in the progression of CKD and the development of widespread severe organ dysfunction and multimorbidity in CKD patients, especially in advanced stages [85,86]. Substantial loss of endothelium is already apparent in early stages of CKD, but in view of the vital importance of the microcirculation, the clinical relevance of microvascular rarefaction has been widely underestimated (‘iceberg delusion’). Studies exploring pro-angiogenic therapeutic modalities may be of key importance to improve quality of life and survival of CKD patients.
Acknowledgements
We wish to thank Dr. Geert W. Schmid-Schoenbein, Jacobs School of Engineering, UC San Diego, for helpful discussions and advice.
Abbreviations
- ACEi
angiotensin-converting enzyme inhibitor
- AKI
acute kidney injury
- ARB
angiotensin resceptor antagonist
- CKD
chronic kidney disease
- CVD
cardiovascular disease
- eGFR
estimated glomerular filtration rate
- EndMT
endothelial to mesenchymal transition
- EPC
endothelial progenitor cell
- FGF23
fibroblast growth factor 23
- HIF
hypoxia-inducible factor
- HTN
hypertension
- iNOS
inducible NO synthase
- I/R
ischemia–reperfusion
- LVH
left ventricular hypertrophy
- MV
microvessel/microvascular
- RAS
renin–angiotensin system
- sFlk-1
serum level of Flk-1
- SGLT2i
sodium-glucose cotransporter 2 inhibitor
- SHR
spontaneously hypertensive rat
- VEGF
vascular endothelial growth factor
- 5/6 NX
5/6 nephrectomy
Competing Interests
The authors declare that there are no competing interests associated with the manuscript.
References
- 1.Bonetti P.O., Lerman L.O. and Lerman A. (2003) Endothelial dysfunction: a marker of atherosclerotic risk. Arterioscler. Thromb. Vasc. Biol. 23, 168–175 10.1161/01.ATV.0000051384.43104.FC [DOI] [PubMed] [Google Scholar]
- 2.Fliser D. et al. (2011) The dysfunctional endothelium in CKD and in cardiovascular disease: mapping the origin(s) of cardiovascular problems in CKD and of kidney disease in cardiovascular conditions for a research agenda. Kidney Int. Suppl. 1, 6–9 10.1038/kisup.2011.6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Levy B.I., Ambrosio G., Pries A.R. and Struijker-Boudier H.A. (2001) Microcirculation in hypertension: a new target for treatment? Circulation 104, 735–740 10.1161/hc3101.091158 [DOI] [PubMed] [Google Scholar]
- 4.Virdis A. et al. (2014) Evaluation of microvascular structure in humans: a ‘state-of-the-art’ document of the Working Group on Macrovascular and Microvascular Alterations of the Italian Society of Arterial Hypertension. J. Hypertens. 32, 2120–2129, 10.1097/HJH.0000000000000322 [DOI] [PubMed] [Google Scholar]
- 5.Pries A.R., Secomb T.W. and Gaehtgens P. (2000) The endothelial surface layer. Pflug. Arch. 440, 653–666 10.1007/s004240000307 [DOI] [PubMed] [Google Scholar]
- 6.Lockhart C.J., Hamilton P.K., Quinn C.E. and McVeigh G.E. (2009) End-organ dysfunction and cardiovascular outcomes: the role of the microcirculation. Clin. Sci. (Lond.) 116, 175–190 10.1042/CS20080069 [DOI] [PubMed] [Google Scholar]
- 7.Feihl F., Liaudet L., Waeber B. and Levy B.I. (2006) Hypertension: a disease of the microcirculation? Hypertension 48, 1012–1017 10.1161/01.HYP.0000249510.20326.72 [DOI] [PubMed] [Google Scholar]
- 8.Hutchins P.M. (1974) Observation of a decreased number of small arterioles in spontaneously hypertensive rats. Circ. Res. 34/35, I–161-I-165 [Google Scholar]
- 9.Prewitt R.L., Chen I.I. and Dowell R. (1982) Development of microvascular rarefaction in the spontaneously hypertensive rat. Am. J. Physiol. 243, H243–H251 [DOI] [PubMed] [Google Scholar]
- 10.Hansen-Smith F., Greene A.S., Cowley A.W. Jr and Lombard J.H. (1990) Structural changes during microvascular rarefaction in chronic hypertension. Hypertension 15, 922–928 10.1161/01.HYP.15.6.922 [DOI] [PubMed] [Google Scholar]
- 11.Struijker Boudier H.A. et al. (1992) The microcirculation and hypertension. J. Hypertens. Suppl. 10, S147–S156 [PubMed] [Google Scholar]
- 12.Serne E.H. et al. (2001) Impaired skin capillary recruitment in essential hypertension is caused by both functional and structural capillary rarefaction. Hypertension 38, 238–242 10.1161/01.HYP.38.2.238 [DOI] [PubMed] [Google Scholar]
- 13.Lombard J.H., Hinojosa-Laborde C. and Cowley A.W. Jr (1989) Hemodynamics and microcirculatory alterations in reduced renal mass hypertension. Hypertension 13, 128–138 10.1161/01.HYP.13.2.128 [DOI] [PubMed] [Google Scholar]
- 14.Hansen-Smith F.M., Morris L.W., Greene A.S. and Lombard J.H. (1996) Rapid microvessel rarefaction with elevated salt intake and reduced renal mass hypertension in rats. Circ. Res. 79, 324–330 10.1161/01.RES.79.2.324 [DOI] [PubMed] [Google Scholar]
- 15.Wang D.H. and Prewitt R.L. (1990) Captopril reduces aortic and microvascular growth in hypertensive and normotensive rats. Hypertension 15, 68–77 10.1161/01.HYP.15.1.68 [DOI] [PubMed] [Google Scholar]
- 16.Hernandez I. and Greene A.S. (1995) Hemodynamic and microcirculatory changes during development of renal hypertension. Am. J. Physiol. 268, H33–H38 [DOI] [PubMed] [Google Scholar]
- 17.Flisinski M. et al. (2008) Influence of different stages of experimental chronic kidney disease on rats locomotor and postural skeletal muscles microcirculation. Ren. Fail. 30, 443–451 10.1080/08860220801985694 [DOI] [PubMed] [Google Scholar]
- 18.Prommer H.U. et al. (2018) Chronic kidney disease induces a systemic microangiopathy, tissue hypoxia and dysfunctional angiogenesis. Sci. Rep. 8, 5317 10.1038/s41598-018-23663-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tanaka S., Tanaka T. and Nangaku M. (2015) Hypoxia and dysregulated angiogenesis in kidney disease. Kidney Dis. (Basel) 1, 80–89 10.1159/000381515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heiss R.U. et al. (2017) Blunted transcriptional response to skeletal muscle ischemia in rats with chronic kidney disease: potential role for impaired ischemia-induced angiogenesis. Physiol. Genomics 49, 230–237 10.1152/physiolgenomics.00124.2016 [DOI] [PubMed] [Google Scholar]
- 21.Flisinski M. et al. (2012) Decreased hypoxia-inducible factor-1alpha in gastrocnemius muscle in rats with chronic kidney disease. Kidney Blood Press. Res. 35, 608–618 10.1159/000339706 [DOI] [PubMed] [Google Scholar]
- 22.Jacobi J. et al. (2006) Subtotal nephrectomy impairs ischemia-induced angiogenesis and hindlimb re-perfusion in rats. Kidney Int. 69, 2013–2021 10.1038/sj.ki.5000448 [DOI] [PubMed] [Google Scholar]
- 23.Schellinger I.N. et al. (2017) Hypoxia inducible factor stabilization improves defective ischemia-induced angiogenesis in a rodent model of chronic kidney disease. Kidney Int. 91, 616–627 10.1016/j.kint.2016.09.028 [DOI] [PubMed] [Google Scholar]
- 24.Amann K. et al. (1997) Rats with moderate renal failure show capillary deficit in heart but not skeletal muscle. Am. J. Kidney Dis. 30, 382–388, 10.1016/S0272-6386(97)90283-3 [DOI] [PubMed] [Google Scholar]
- 25.Bongartz L.G. et al. (2012) Target organ cross talk in cardiorenal syndrome: animal models. Am. J. Physiol. Renal Physiol. 303, F1253–F1263 10.1152/ajprenal.00392.2012 [DOI] [PubMed] [Google Scholar]
- 26.Amann K. et al. (1992) Reduced capillary density in the myocardium of uremic rats–a stereological study. Kidney Int. 42, 1079–1085 10.1038/ki.1992.390 [DOI] [PubMed] [Google Scholar]
- 27.Tornig J. et al. (1996) Arteriolar wall thickening, capillary rarefaction and interstitial fibrosis in the heart of rats with renal failure:the effects of ramipril, nifedipine and moxonidine. J. Am. Soc. Nephrol. 7, 667–675 [DOI] [PubMed] [Google Scholar]
- 28.Torry R.J., Connell P.M., O’Brien D.M., Chilian W.M. and Tomanek R.J. (1991) Sympathectomy stimulates capillary but not precapillary growth in hypertrophic hearts. Am. J. Physiol. 260, H1515–H1521 [DOI] [PubMed] [Google Scholar]
- 29.Tomanek R.J. (1989) Sympathetic nerves modify mitochondrial and capillary growth in normotensive and hypertensive rats. J. Mol. Cell Cardiol. 21, 755–764 10.1016/0022-2828(89)90714-1 [DOI] [PubMed] [Google Scholar]
- 30.Amann K. et al. (1992) Effects of nifedipine and moxonidine on cardiac structure in spontaneously hypertensive rats. Stereological studies on myocytes, capillaries, arteries, and cardiac interstitium. Am. J. Hypertens. 5, 76–83 10.1093/ajh/5.2.76 [DOI] [PubMed] [Google Scholar]
- 31.Kaur J., Young B.E. and Fadel P.J. (2017) Sympathetic overactivity in chronic kidney disease: consequences and mechanisms. Int. J. Mol. Sci. 18, 1682 10.3390/ijms18081682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nakagawa T. et al. (2013) Renal denervation prevents stroke and brain injury via attenuation of oxidative stress in hypertensive rats. J. Am. Heart Assoc. 2, e000375 10.1161/JAHA.113.000375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Amann K. et al. (2011) Sympathetic blockade prevents the decrease in cardiac VEGF expression and capillary supply in experimental renal failure. Am. J. Physiol. Renal Physiol. 300, F105–F112 10.1152/ajprenal.00363.2010 [DOI] [PubMed] [Google Scholar]
- 34.Amann K. et al. (2000) Capillary/myocyte mismatch in the heart in renal failure–a role for erythropoietin? Nephrol. Dial. Transplant. 15, 964–969 10.1093/ndt/15.7.964 [DOI] [PubMed] [Google Scholar]
- 35.Amann K. et al. (2000) Endothelin A receptor blockade prevents capillary/myocyte mismatch in the heart of uremic animals. J. Am. Soc. Nephrol. 11, 1702–1711 [DOI] [PubMed] [Google Scholar]
- 36.Amann K. et al. (2000) Effects of ACE inhibition and bradykinin antagonism on cardiovascular changes in uremic rats. Kidney Int. 58, 153–161 10.1046/j.1523-1755.2000.00163.x [DOI] [PubMed] [Google Scholar]
- 37.Amann K. et al. (2002) Effect of antioxidant therapy with dl-alpha-tocopherol on cardiovascular structure in experimental renal failure. Kidney Int. 62, 877–884 10.1046/j.1523-1755.2002.00518.x [DOI] [PubMed] [Google Scholar]
- 38.Koleganova N. et al. (2009) Arterial calcification in patients with chronic kidney disease. Nephrol. Dial. Transplant. 24, 2488–2496, 10.1093/ndt/gfp137 [DOI] [PubMed] [Google Scholar]
- 39.Ogata H., Ritz E., Odoni G., Amann K. and Orth S.R. (2003) Beneficial effects of calcimimetics on progression of renal failure and cardiovascular risk factors. J. Am. Soc. Nephrol. 14, 959–967 10.1097/01.ASN.0000056188.23717.E5 [DOI] [PubMed] [Google Scholar]
- 40.Koleganova N., Piecha G., Ritz E. and Gross M.L. (2009) Calcitriol ameliorates capillary deficit and fibrosis of the heart in subtotally nephrectomized rats. Nephrol. Dial. Transplant. 24, 778–787 10.1093/ndt/gfn549 [DOI] [PubMed] [Google Scholar]
- 41.Tyralla K. et al. (2011) High-dose enalapril treatment reverses myocardial fibrosis in experimental uremic cardiomyopathy. PLoS ONE 6, e15287 10.1371/journal.pone.0015287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Di Marco G.S. et al. (2011) Cardioprotective effect of calcineurin inhibition in an animal model of renal disease. Eur. Heart J. 32, 1935–1945 10.1093/eurheartj/ehq436 [DOI] [PubMed] [Google Scholar]
- 43.Wang C.H. et al. (2008) Cyclosporine increases ischemia-induced endothelial progenitor cell mobilization through manipulation of the CD26 system. Am. J. Physiol. Regul. Integr. Comp. Physiol. 294, R811–R818 10.1152/ajpregu.00543.2007 [DOI] [PubMed] [Google Scholar]
- 44.Golle L. et al. (2017) Bone marrow-derived cells and their conditioned medium induce microvascular repair in uremic rats by stimulation of endogenous repair mechanisms. Sci. Rep. 7, 9444 10.1038/s41598-017-09883-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Choi J.H. et al. (2004) Decreased number and impaired angiogenic function of endothelial progenitor cells in patients with chronic renal failure. Arterioscler. Thromb. Vasc. Biol. 24, 1246–1252 10.1161/01.ATV.0000133488.56221.4a [DOI] [PubMed] [Google Scholar]
- 46.Rafii S. and Lyden D. (2003) Therapeutic stem and progenitor cell transplantation for organ vascularization and regeneration. Nat. Med. 9, 702–712 10.1038/nm0603-702 [DOI] [PubMed] [Google Scholar]
- 47.Gut N. et al. (2013) Erythropoietin combined with ACE inhibitor prevents heart remodeling in 5/6 nephrectomized rats independently of blood pressure and kidney function. Am. J. Nephrol. 38, 124–135 10.1159/000353106 [DOI] [PubMed] [Google Scholar]
- 48.Di Marco G.S. et al. (2015) Soluble Flt-1 links microvascular disease with heart failure in CKD. Basic Res. Cardiol. 110, 30 10.1007/s00395-015-0487-4 [DOI] [PubMed] [Google Scholar]
- 49.Ali B.H. et al. (2010) Effects of Gum Arabic in rats with adenine-induced chronic renal failure. Exp. Biol. Med. (Maywood) 235, 373–382 10.1258/ebm.2009.009214 [DOI] [PubMed] [Google Scholar]
- 50.Ali B.H. et al. (2014) Renal and myocardial histopathology and morphometry in rats with adenine - induced chronic renal failure: influence of gum acacia. Cell. Physiol. Biochem. 34, 818–828 10.1159/000363045 [DOI] [PubMed] [Google Scholar]
- 51.Ehling J. et al. (2016) Quantitative micro-computed tomography imaging of vascular dysfunction in progressive kidney diseases. J. Am. Soc. Nephrol. 27, 520–532 10.1681/ASN.2015020204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Babickova J. et al. (2017) Regardless of etiology, progressive renal disease causes ultrastructural and functional alterations of peritubular capillaries. Kidney Int. 91, 70–85 10.1016/j.kint.2016.07.038 [DOI] [PubMed] [Google Scholar]
- 53.Amann K., Breitbach M., Ritz E. and Mall G. (1998) Myocyte/capillary mismatch in the heart of uremic patients. J. Am. Soc. Nephrol. 9, 1018–1022 [DOI] [PubMed] [Google Scholar]
- 54.Charytan D.M. et al. (2014) Increased concentration of circulating angiogenesis and nitric oxide inhibitors induces endothelial to mesenchymal transition and myocardial fibrosis in patients with chronic kidney disease. Int. J. Cardiol. 176, 99–109 10.1016/j.ijcard.2014.06.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sawant A., Garland S.J., House A.A. and Overend T.J. (2011) Morphological, electrophysiological, and metabolic characteristics of skeletal muscle in people with end-stage renal disease: a critical review. Physiother. Can. 63, 355–376 10.3138/ptc.2010-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sakkas G.K. et al. (2003) Atrophy of non-locomotor muscle in patients with end-stage renal failure. Nephrol. Dial. Transplant. 18, 2074–2081 10.1093/ndt/gfg325 [DOI] [PubMed] [Google Scholar]
- 57.Sakkas G.K. et al. (2003) Changes in muscle morphology in dialysis patients after 6 months of aerobic exercise training. Nephrol. Dial. Transplant. 18, 1854–1861 10.1093/ndt/gfg237 [DOI] [PubMed] [Google Scholar]
- 58.Thang O.H. et al. (2011) Capillary rarefaction in advanced chronic kidney disease is associated with high phosphorus and bicarbonate levels. Nephrol. Dial. Transplant. 26, 3529–3536 10.1093/ndt/gfr089 [DOI] [PubMed] [Google Scholar]
- 59.Edwards-Richards A. et al. (2014) Capillary rarefaction: an early marker of microvascular disease in young hemodialysis patients. Clin. Kidney J. 7, 569–574 10.1093/ckj/sfu106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nissel R. et al. (2009) Short-term growth hormone treatment and microcirculation: effects in patients with chronic kidney disease. Microvasc. Res. 78, 246–252 10.1016/j.mvr.2009.05.006 [DOI] [PubMed] [Google Scholar]
- 61.Burkhardt D. et al. (2016) Reduced microvascular density in omental biopsies of children with chronic kidney disease. PLoS ONE 11, e0166050 10.1371/journal.pone.0166050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.von Stillfried S. et al. (2016) Contrast-enhanced CT imaging in patients with chronic kidney disease. Angiogenesis 19, 525–535 10.1007/s10456-016-9524-7 [DOI] [PubMed] [Google Scholar]
- 63.Deva R. et al. (2011) Vision-threatening retinal abnormalities in chronic kidney disease stages 3 to 5. Clin. J. Am. Soc. Nephrol. 6, 1866–1871 10.2215/CJN.10321110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Baumann M. et al. (2009) Non-diabetic chronic kidney disease influences retinal microvasculature. Kidney Blood Press. Res. 32, 428–433 10.1159/000264650 [DOI] [PubMed] [Google Scholar]
- 65.Ooi Q.L. et al. (2011) The microvasculature in chronic kidney disease. Clin. J. Am. Soc. Nephrol. 6, 1872–1878 10.2215/CJN.10291110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sabanayagam C. et al. (2009) Retinal arteriolar narrowing increases the likelihood of chronic kidney disease in hypertension. J. Hypertens. 27, 2209–2217 10.1097/HJH.0b013e328330141d [DOI] [PubMed] [Google Scholar]
- 67.Sng C.C. et al. (2010) Fractal analysis of the retinal vasculature and chronic kidney disease. Nephrol. Dial. Transplant. 25, 2252–2258 10.1093/ndt/gfq007 [DOI] [PubMed] [Google Scholar]
- 68.Liew G., Mitchell P., Wong T.Y. and Wang J.J. (2012) Retinal microvascular signs are associated with chronic kidney disease in persons with and without diabetes. Kidney Blood Press. Res. 35, 589–594 10.1159/000339173 [DOI] [PubMed] [Google Scholar]
- 69.Bao S. et al. (2015) Retinal vessel diameter and chronic kidney disease in rural china: a cross-sectional study. Medicine (Baltimore) 94, e2076 10.1097/MD.0000000000002076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.McGowan A. et al. (2015) Evaluation of the retinal vasculature in hypertension and chronic kidney disease in an elderly population of Irish nuns. PLoS ONE 10, e0136434 10.1371/journal.pone.0136434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schaeffner E.S. et al. (2012) Two novel equations to estimate kidney function in persons aged 70 years or older. Ann. Intern. Med. 157, 471–481 10.7326/0003-4819-157-7-201210020-00003 [DOI] [PubMed] [Google Scholar]
- 72.Glassock R.J. and Winearls C. (2009) Ageing and the glomerular filtration rate: truths and consequences. Trans. Am. Clin. Climatol. Assoc. 120, 419–428 [PMC free article] [PubMed] [Google Scholar]
- 73.Mehta R. et al. (2015) Phosphate, fibroblast growth factor 23 and retinopathy in chronic kidney disease: the Chronic Renal Insufficiency Cohort Study. Nephrol. Dial. Transplant. 30, 1534–1541 10.1093/ndt/gfv123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bosch A. et al. (2018) Retinal capillary and arteriolar changes in patients with chronic kidney disease. Microvasc. Res. 118, 121–127 10.1016/j.mvr.2018.03.008 [DOI] [PubMed] [Google Scholar]
- 75.Yeung L. et al. (2019) Early retinal microvascular abnormalities in patients with chronic kidney disease. Microcirculation, e12555 10.1111/micc.12555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Vadala M. et al. (2019) Retinal and choroidal vasculature changes associated with chronic kidney disease. Graef. Arch. Clin. Exp. Ophthalmol. 257, 1687–1698 10.1007/s00417-019-04358-3 [DOI] [PubMed] [Google Scholar]
- 77.Chua J. et al. (2019) Impact of hypertension on retinal capillary microvasculature using optical coherence tomographic angiography. J. Hypertens. 37, 572–580 10.1097/HJH.0000000000001916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ang M. et al. (2018) Optical coherence tomography angiography: a review of current and future clinical applications. Graef. Arch. Clin. Exp. Ophthalmol. 256, 237–245 10.1007/s00417-017-3896-2 [DOI] [PubMed] [Google Scholar]
- 79.Amann K. and Ritz E. (2000) Microvascular disease–the Cinderella of uraemic heart disease. Nephrol. Dial. Transplant. 15, 1493–1503 10.1093/ndt/15.10.1493 [DOI] [PubMed] [Google Scholar]
- 80.Kopel T. et al. (2017) Endothelium-dependent and -independent vascular function in advanced chronic kidney disease. Clin. J. Am. Soc. Nephrol. 12, 1588–1594 10.2215/CJN.12811216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yilmaz M.I. et al. (2011) Vascular health, systemic inflammation and progressive reduction in kidney function; clinical determinants and impact on cardiovascular outcomes. Nephrol. Dial. Transplant. 26, 3537–3543 10.1093/ndt/gfr081 [DOI] [PubMed] [Google Scholar]
- 82.Pannier B. et al. (2000) Postischemic vasodilation, endothelial activation, and cardiovascular remodeling in end-stage renal disease. Kidney Int. 57, 1091–1099 10.1046/j.1523-1755.2000.00936.x [DOI] [PubMed] [Google Scholar]
- 83.London G.M. et al. (2004) Forearm reactive hyperemia and mortality in end-stage renal disease. Kidney Int. 65, 700–704 10.1111/j.1523-1755.2004.00434.x [DOI] [PubMed] [Google Scholar]
- 84.Hirata Y. et al. (2014) Endothelial function and cardiovascular events in chronic kidney disease. Int. J. Cardiol. 173, 481–486 10.1016/j.ijcard.2014.03.085 [DOI] [PubMed] [Google Scholar]
- 85.Whitman I.R., Feldman H.I. and Deo R. (2012) CKD and sudden cardiac death: epidemiology, mechanisms, and therapeutic approaches. J. Am. Soc. Nephrol. 23, 1929–1939 10.1681/ASN.2012010037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cioffi G. et al. (2011) Chronic kidney disease elicits excessive increase in left ventricular mass growth in patients at increased risk for cardiovascular events. J. Hypertens. 29, 565–573 10.1097/HJH.0b013e3283424188 [DOI] [PubMed] [Google Scholar]
- 87.Schaefer F. et al. (2017) Cardiovascular phenotypes in children with CKD: The 4C Study. Clin. J. Am. Soc. Nephrol. 12, 19–28 10.2215/CJN.01090216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Paoletti E. et al. (2016) Associations of left ventricular hypertrophy and geometry with adverse outcomes in patients with CKD and hypertension. Clin. J. Am. Soc. Nephrol. 11, 271–279 10.2215/CJN.06980615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Poulikakos D. et al. (2014) Left ventricular hypertrophy and endothelial dysfunction in chronic kidney disease. Eur. Heart J. Cardiovasc. Imaging 15, 56–61 10.1093/ehjci/jet120 [DOI] [PubMed] [Google Scholar]
- 90.Murthy V.L. et al. (2012) Coronary vascular dysfunction and prognosis in patients with chronic kidney disease. JACC Cardiovasc. Imaging 5, 1025–1034 10.1016/j.jcmg.2012.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nakanishi K. et al. (2013) Prognostic value of coronary flow reserve on long-term cardiovascular outcomes in patients with chronic kidney disease. Am. J. Cardiol. 112, 928–932 10.1016/j.amjcard.2013.05.025 [DOI] [PubMed] [Google Scholar]
- 92.Charytan D.M. et al. (2018) Coronary flow reserve is predictive of the risk of cardiovascular death regardless of chronic kidney disease stage. Kidney Int. 93, 501–509 10.1016/j.kint.2017.07.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Conlon P.J., Kovalik E., Schumm D., Minda S. and Schwab S.J. (2000) Normalization of hematocrit in hemodialysis patients does not affect silent ischemia. Ren. Fail. 22, 205–211 10.1081/JDI-100100864 [DOI] [PubMed] [Google Scholar]
- 94.Das M., Aronow W.S., McClung J.A. and Belkin R.N. (2006) Increased prevalence of coronary artery disease, silent myocardial ischemia, complex ventricular arrhythmias, atrial fibrillation, left ventricular hypertrophy, mitral annular calcium, and aortic valve calcium in patients with chronic renal insufficiency. Cardiol. Rev. 14, 14–17 10.1097/01.crd.0000148162.88296.9f [DOI] [PubMed] [Google Scholar]
- 95.McIntyre C.W. et al. (2008) Hemodialysis-induced cardiac dysfunction is associated with an acute reduction in global and segmental myocardial blood flow. Clin. J. Am. Soc. Nephrol. 3, 19–26 10.2215/CJN.03170707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gorelick P.B. et al. (2011) Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 42, 2672–2713 10.1161/STR.0b013e3182299496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kurella M. et al. (2005) Chronic kidney disease and cognitive impairment in the elderly: the health, aging, and body composition study. J. Am. Soc. Nephrol. 16, 2127–2133 10.1681/ASN.2005010005 [DOI] [PubMed] [Google Scholar]
- 98.Etgen T. et al. (2009) Chronic kidney disease is associated with incident cognitive impairment in the elderly: the INVADE study. Nephrol. Dial. Transplant. 24, 3144–3150 10.1093/ndt/gfp230 [DOI] [PubMed] [Google Scholar]
- 99.Helmer C. et al. (2011) Chronic kidney disease, cognitive decline, and incident dementia: the 3C Study. Neurology 77, 2043–2051 10.1212/WNL.0b013e31823b4765 [DOI] [PubMed] [Google Scholar]
- 100.Burns C.M. et al. (2018) Prevalence and risk of severe cognitive impairment in advanced chronic kidney disease. J. Gerontol. A Biol. Sci. Med. Sci. 73, 393–399 10.1093/gerona/glx241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ruebner R.L. et al. (2016) Neurocognitive dysfunction in children, adolescents, and young adults with CKD. Am. J. Kidney Dis. 67, 567–575 [DOI] [PubMed] [Google Scholar]
- 102.Toyoda K. (2015) Cerebral small vessel disease and chronic kidney disease. J. Stroke 17, 31–37 10.5853/jos.2015.17.1.31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Liu B. et al. (2018) Age-specific associations of renal impairment with magnetic resonance imaging markers of cerebral small vessel disease in transient ischemic attack and stroke. Stroke 49, 899–904 10.1161/STROKEAHA.117.019650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lau K.K. et al. (2019) Age-specific associations of renal impairment and cerebral small vessel disease burden in chinese with ischaemic stroke. J. Stroke Cerebrovasc. Dis. 28, 1274–1280 10.1016/j.jstrokecerebrovasdis.2019.01.018 [DOI] [PubMed] [Google Scholar]
- 105.Basile D.P. et al. (2016) Progression after AKI: understanding maladaptive repair processes to predict and identify therapeutic treatments. J. Am. Soc. Nephrol. 27, 687–697 10.1681/ASN.2015030309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kang D.H. et al. (2001) Impaired angiogenesis in the remnant kidney model: I. Potential role of vascular endothelial growth factor and thrombospondin-1. J. Am. Soc. Nephrol. 12, 1434–1447 [DOI] [PubMed] [Google Scholar]
- 107.Kang D.H., Hughes J., Mazzali M., Schreiner G.F. and Johnson R.J. (2001) Impaired angiogenesis in the remnant kidney model: II. Vascular endothelial growth factor administration reduces renal fibrosis and stabilizes renal function. J. Am. Soc. Nephrol. 12, 1448–1457 [DOI] [PubMed] [Google Scholar]
- 108.Kang D.H. et al. (2002) Role of the microvascular endothelium in progressive renal disease. J. Am. Soc. Nephrol. 13, 806–816 10.1097/01.ASN.0000034910.58454.FD [DOI] [PubMed] [Google Scholar]
- 109.Tanabe K., Sato Y. and Wada J. (2018) Endogenous antiangiogenic factors in chronic kidney disease: potential biomarkers of progression. Int. J. Mol. Sci. 19, 1859 10.3390/ijms19071859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ruge T. et al. (2014) Endostatin level is associated with kidney injury in the elderly: findings from two community-based cohorts. Am. J. Nephrol. 40, 417–424 [DOI] [PubMed] [Google Scholar]
- 111.Hinamoto N. et al. (2014) Urinary and plasma levels of vasohibin-1 can predict renal functional deterioration in patients with renal disorders. PLoS ONE 9, e96932 10.1371/journal.pone.0096932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Edwards M.S. et al. (2005) Associations between retinal microvascular abnormalities and declining renal function in the elderly population: the Cardiovascular Health Study. Am. J. Kidney Dis. 46, 214–224 10.1053/j.ajkd.2005.05.005 [DOI] [PubMed] [Google Scholar]
- 113.Yau J.W. et al. (2011) Retinal arteriolar narrowing and subsequent development of CKD Stage 3: the Multi-Ethnic Study of Atherosclerosis (MESA). Am. J. Kidney Dis. 58, 39–46 10.1053/j.ajkd.2011.02.382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wong T.Y. et al. (2004) Retinal microvascular abnormalities and renal dysfunction: the atherosclerosis risk in communities study. J. Am. Soc. Nephrol. 15, 2469–2476 10.1097/01.ASN.0000136133.28194.E4 [DOI] [PubMed] [Google Scholar]
- 115.Yip W. et al. (2017) Retinal vascular imaging markers and incident chronic kidney disease: a prospective cohort study. Sci. Rep. 7, 9374 10.1038/s41598-017-09204-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Roshanravan B., Gamboa J. and Wilund K. (2017) Exercise and CKD: skeletal muscle dysfunction and practical application of exercise to prevent and treat physical impairments in CKD. Am. J. Kidney Dis. 69, 837–852 10.1053/j.ajkd.2017.01.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sala E. et al. (2001) Impaired muscle oxygen transfer in patients with chronic renal failure. Am. J. Physiol. Regul. Integr. Comp. Physiol. 280, R1240–R1248 10.1152/ajpregu.2001.280.4.R1240 [DOI] [PubMed] [Google Scholar]
- 118.Marrades R.M. et al. (1996) Effects of erythropoietin on muscle O2 transport during exercise in patients with chronic renal failure. J. Clin. Invest. 97, 2092–2100 10.1172/JCI118646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Stray-Gundersen J., Howden E.J., Parsons D.B. and Thompson J.R. (2016) Neither hematocrit normalization nor exercise training restores oxygen consumption to normal levels in hemodialysis patients. J. Am. Soc. Nephrol. 27, 3769–3779 10.1681/ASN.2015091034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lewis M.I. et al. (2015) Effect of endurance and/or strength training on muscle fiber size, oxidative capacity, and capillarity in hemodialysis patients. J. Appl. Physiol. (1985) 119, 865–871 10.1152/japplphysiol.01084.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Mak R.H. et al. (2011) Wasting in chronic kidney disease. J. Cachexia Sarcopenia Muscle 2, 9–25 10.1007/s13539-011-0019-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Cheung W.W. et al. (2020) Vitamin D repletion ameliorates adipose tissue browning and muscle wasting in infantile nephropathic cystinosis-associated cachexia. J. Cachexia Sarcopenia Muscle 11, 120–134 10.1002/jcsm.12497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.de Braganca A.C., Volpini R.A., Mehrotra P., Andrade L. and Basile D.P. (2016) Vitamin D deficiency contributes to vascular damage in sustained ischemic acute kidney injury. Physiol. Rep. 4, e12829 10.14814/phy2.12829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lundwall K., Jacobson S.H., Jorneskog G. and Spaak J. (2018) Treating endothelial dysfunction with vitamin D in chronic kidney disease: a meta-analysis. BMC Nephrol. 19, 247 10.1186/s12882-018-1042-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Mak R.H. (2011) Serum myostatin in patients on maintenance haemodialysis: a useful biomarker of muscle wasting? Clin. Endocrinol. (Oxf.) 75, 738–739 10.1111/j.1365-2265.2011.04225.x [DOI] [PubMed] [Google Scholar]
- 126.Yano S. et al. (2015) Relationship between blood myostatin levels and kidney function: Shimane CoHRE Study. PLoS ONE 10, e0141035 10.1371/journal.pone.0141035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zhang L. et al. (2011) Pharmacological inhibition of myostatin suppresses systemic inflammation and muscle atrophy in mice with chronic kidney disease. FASEB J. 25, 1653–1663 10.1096/fj.10-176917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wang B. et al. (2017) MicroRNA-23a and MicroRNA-27a mimic exercise by ameliorating CKD-induced muscle atrophy. J. Am. Soc. Nephrol. 28, 2632–2641 10.1681/ASN.2016111213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Butcher J.T. et al. (2017) Effect of myostatin deletion on cardiac and microvascular function. Physiol. Rep. 5, 10.14814/phy2.13525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hulmi J.J. et al. (2013) Muscle protein synthesis, mTORC1/MAPK/Hippo signaling, and capillary density are altered by blocking of myostatin and activins. Am. J. Physiol. Endocrinol. Metab. 304, E41–E50 10.1152/ajpendo.00389.2012 [DOI] [PubMed] [Google Scholar]
- 131.Solak B., Acikgoz S.B., Sipahi S. and Erdem T. (2016) Cutaneuos findings in patients with predialysis chronic kidney disease. J. Eur. Acad. Dermatol. Venereol. 30, 1609–1613 10.1111/jdv.13643 [DOI] [PubMed] [Google Scholar]
- 132.Maroz N. and Simman R. (2013) Wound healing in patients with impaired kidney function. J. Am. Coll. Clin. Wound Spec. 5, 2–7 10.1016/j.jccw.2014.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Gilchrest B.A., Rowe J.W. and Mihm M.C. Jr (1980) Clinical and histological skin changes in chronic renal failure: evidence for a dialysis-resistant, transplant-responsive microangiopathy. Lancet 2, 1271–1275 10.1016/S0140-6736(80)92337-5 [DOI] [PubMed] [Google Scholar]
- 134.Ichimaru K. and Horie A. (1987) Microangiopathic changes of subepidermal capillaries in end-stage renal failure. Nephron 46, 144–149 10.1159/000184330 [DOI] [PubMed] [Google Scholar]
- 135.Lundin A.P., Fani K., Berlyne G.M. and Friedman E.A. (1995) Dermal angiopathy in hemodialysis patients: the effect of time. Kidney Int. 47, 1775–1780 10.1038/ki.1995.245 [DOI] [PubMed] [Google Scholar]
- 136.Suzuki E., Nishimatsu H., Oba S., Takahashi M. and Homma Y. (2014) Chronic kidney disease and erectile dysfunction. World J. Nephrol. 3, 220–229 10.5527/wjn.v3.i4.220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Havasi A. and Borkan S.C. (2011) Apoptosis and acute kidney injury. Kidney Int. 80, 29–40 10.1038/ki.2011.120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Horbelt M. et al. (2007) Acute and chronic microvascular alterations in a mouse model of ischemic acute kidney injury. Am. J. Physiol. Renal Physiol. 293, F688–F695 10.1152/ajprenal.00452.2006 [DOI] [PubMed] [Google Scholar]
- 139.Yang B. et al. (2018) Caspase-3 is a pivotal regulator of microvascular rarefaction and renal fibrosis after ischemia-reperfusion injury. J. Am. Soc. Nephrol. 29, 1900–1916 10.1681/ASN.2017050581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kobayashi N., DeLano F.A. and Schmid-Schonbein G.W. (2005) Oxidative stress promotes endothelial cell apoptosis and loss of microvessels in the spontaneously hypertensive rats. Arterioscler. Thromb. Vasc. Biol. 25, 2114–2121 10.1161/01.ATV.0000178993.13222.f2 [DOI] [PubMed] [Google Scholar]
- 141.Hlushchuk R. et al. (2011) Decrease in VEGF expression induces intussusceptive vascular pruning. Arterioscler. Thromb. Vasc. Biol. 31, 2836–2844 10.1161/ATVBAHA.111.231811 [DOI] [PubMed] [Google Scholar]
- 142.Korn C. and Augustin H.G. (2015) Mechanisms of vessel pruning and regression. Dev. Cell 34, 5–17 10.1016/j.devcel.2015.06.004 [DOI] [PubMed] [Google Scholar]
- 143.Walia A. et al. (2015) Endostatin’s emerging roles in angiogenesis, lymphangiogenesis, disease, and clinical applications. Biochim. Biophys. Acta 1850, 2422–2438 10.1016/j.bbagen.2015.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Koleganova N. et al. (2009) Interstitial fibrosis and microvascular disease of the heart in uremia: amelioration by a calcimimetic. Lab. Invest. 89, 520–530 10.1038/labinvest.2009.7 [DOI] [PubMed] [Google Scholar]
- 145.Tanaka T., Yamaguchi J., Higashijima Y. and Nangaku M. (2013) Indoxyl sulfate signals for rapid mRNA stabilization of Cbp/p300-interacting transactivator with Glu/Asp-rich carboxy-terminal domain 2 (CITED2) and suppresses the expression of hypoxia-inducible genes in experimental CKD and uremia. FASEB J. 27, 4059–4075 10.1096/fj.13-231837 [DOI] [PubMed] [Google Scholar]
- 146.Futrakul N., Butthep P., Laohareungpanya N., Chaisuriya P. and Ratanabanangkoon K. (2008) A defective angiogenesis in chronic kidney disease. Ren. Fail. 30, 215–217 10.1080/08860220701813335 [DOI] [PubMed] [Google Scholar]
- 147.Gu J.W., Shparago M., Tan W. and Bailey A.P. (2006) Tissue endostatin correlates inversely with capillary network in rat heart and skeletal muscles. Angiogenesis 9, 93–99 10.1007/s10456-006-9035-z [DOI] [PubMed] [Google Scholar]
- 148.Noh H. et al. (2012) Uremia induces functional incompetence of bone marrow-derived stromal cells. Nephrol. Dial. Transplant. 27, 218–225 10.1093/ndt/gfr267 [DOI] [PubMed] [Google Scholar]
- 149.Goligorsky M.S., Yasuda K. and Ratliff B. (2010) Dysfunctional endothelial progenitor cells in chronic kidney disease. J. Am. Soc. Nephrol. 21, 911–919 10.1681/ASN.2009111119 [DOI] [PubMed] [Google Scholar]
- 150.Guise E. and Chade A.R. (2018) VEGF therapy for the kidney: emerging strategies. Am. J. Physiol. Renal Physiol. 315, F747–F751 10.1152/ajprenal.00617.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Uchida L. et al. (2020) Effects of a prolyl hydroxylase inhibitor on kidney and cardiovascular complications in a rat model of chronic kidney disease. Am. J. Physiol. Renal Physiol. 318, F388–F401 10.1152/ajprenal.00419.2019 [DOI] [PubMed] [Google Scholar]
- 152.Gross M.L. et al. (2005) Effects of estrogens on cardiovascular structure in uninephrectomized SHRsp rats. Kidney Int. 67, 849–857 10.1111/j.1523-1755.2005.00149.x [DOI] [PubMed] [Google Scholar]
- 153.Feihl F., Liaudet L., Levy B.I. and Waeber B. (2008) Hypertension and microvascular remodelling. Cardiovasc. Res. 78, 274–285 10.1093/cvr/cvn022 [DOI] [PubMed] [Google Scholar]
- 154.Ku E., Lee B.J., Wei J. and Weir M.R. (2019) Hypertension in CKD: Core Curriculum 2019. Am. J. Kidney Dis. 74, 120–131 10.1053/j.ajkd.2018.12.044 [DOI] [PubMed] [Google Scholar]
- 155.Greene A.S. and Amaral S.L. (2002) Microvascular angiogenesis and the renin-angiotensin system. Curr. Hypertens. Rep. 4, 56–62 10.1007/s11906-002-0054-x [DOI] [PubMed] [Google Scholar]
- 156.Pan L., Tang J., Liu H. and Cheng B. (2016) Sympathetic nerves: How do they affect angiogenesis, particularly during wound healing of soft tissues? Clin. Hemorheol. Microcirc. 62, 181–191 10.3233/CH-152019 [DOI] [PubMed] [Google Scholar]
- 157.Harvey A., Montezano A.C. and Touyz R.M. (2015) Vascular biology of ageing - implications in hypertension. J. Mol. Cell Cardiol. 83, 112–121 10.1016/j.yjmcc.2015.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Tian X.L. and Li Y. (2014) Endothelial cell senescence and age-related vascular diseases. J. Genet. Genomics 41, 485–495 10.1016/j.jgg.2014.08.001 [DOI] [PubMed] [Google Scholar]
- 159.Malyszko J. (2010) Mechanism of endothelial dysfunction in chronic kidney disease. Clin. Chim. Acta 411, 1412–1420 10.1016/j.cca.2010.06.019 [DOI] [PubMed] [Google Scholar]
- 160.Lipphardt M. et al. (2017) The third path of tubulointerstitial fibrosis: aberrant endothelial secretome. Kidney Int. 92, 558–568 10.1016/j.kint.2017.02.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Navaneethan S.D. et al. (2017) Diabetes control and the risks of ESRD and mortality in patients with CKD. Am. J. Kidney Dis. 70, 191–198 10.1053/j.ajkd.2016.11.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Klein R., Myers C.E., Lee K.E., Gangnon R. and Klein B.E. (2012) Changes in retinal vessel diameter and incidence and progression of diabetic retinopathy. Arch. Ophthalmol. 130, 749–755 10.1001/archophthalmol.2011.2560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Kannenkeril D. et al. (2018) Early vascular parameters in the micro- and macrocirculation in type 2 diabetes. Cardiovasc. Diabetol. 17, 128 10.1186/s12933-018-0770-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Simonett J.M. et al. (2017) Early microvascular retinal changes in optical coherence tomography angiography in patients with type 1 diabetes mellitus. Acta Ophthalmol. 95, e751–e755 10.1111/aos.13404 [DOI] [PubMed] [Google Scholar]
- 165.Sorensen B.M. et al. (2016) Prediabetes and type 2 diabetes are associated with generalized microvascular dysfunction: The Maastricht Study. Circulation 134, 1339–1352 10.1161/CIRCULATIONAHA.116.023446 [DOI] [PubMed] [Google Scholar]
- 166.Madonna R., Balistreri C.R., Geng Y.J. and De Caterina R. (2017) Diabetic microangiopathy: pathogenetic insights and novel therapeutic approaches. Vascul. Pharmacol. 90, 1–7 10.1016/j.vph.2017.01.004 [DOI] [PubMed] [Google Scholar]
- 167.Orasanu G. and Plutzky J. (2009) The pathologic continuum of diabetic vascular disease. J. Am. Coll. Cardiol. 53, S35–S42 10.1016/j.jacc.2008.09.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Hinkel R. et al. (2017) Diabetes mellitus-induced microvascular destabilization in the myocardium. J. Am. Coll. Cardiol. 69, 131–143 10.1016/j.jacc.2016.10.058 [DOI] [PubMed] [Google Scholar]
- 169.Olver T.D. and Laughlin M.H. (2016) Endurance, interval sprint, and resistance exercise training: impact on microvascular dysfunction in type 2 diabetes. Am. J. Physiol. Heart Circ. Physiol. 310, H337–H350 10.1152/ajpheart.00440.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Girach A. and Vignati L. (2006) Diabetic microvascular complications–can the presence of one predict the development of another? J. Diabetes Complications 20, 228–237 10.1016/j.jdiacomp.2006.03.001 [DOI] [PubMed] [Google Scholar]
- 171.Kramer C.K., Rodrigues T.C., Canani L.H., Gross J.L. and Azevedo M.J. (2011) Diabetic retinopathy predicts all-cause mortality and cardiovascular events in both type 1 and 2 diabetes: meta-analysis of observational studies. Diabetes Care 34, 1238–1244 10.2337/dc11-0079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Ninomiya T. et al. (2009) Albuminuria and kidney function independently predict cardiovascular and renal outcomes in diabetes. J. Am. Soc. Nephrol. 20, 1813–1821 10.1681/ASN.2008121270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Tanaka T. and Nangaku M. (2009) Drug discovery for overcoming chronic kidney disease (CKD): prolyl-hydroxylase inhibitors to activate hypoxia-inducible factor (HIF) as a novel therapeutic approach in CKD. J. Pharmacol. Sci. 109, 24–31 10.1254/jphs.08R09FM [DOI] [PubMed] [Google Scholar]
- 174.Provenzano R. et al. (2016) Oral hypoxia-inducible factor prolyl hydroxylase inhibitor Roxadustat (FG-4592) for the treatment of anemia in patients with CKD. Clin. J. Am. Soc. Nephrol. 11, 982–991 10.2215/CJN.06890615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Chen N. et al. (2019) Roxadustat for anemia in patients with kidney disease not receiving dialysis. N. Engl. J. Med. 381, 1001–1010 10.1056/NEJMoa1813599 [DOI] [PubMed] [Google Scholar]
- 176.Loinard C. et al. (2009) Inhibition of prolyl hydroxylase domain proteins promotes therapeutic revascularization. Circulation 120, 50–59 10.1161/CIRCULATIONAHA.108.813303 [DOI] [PubMed] [Google Scholar]
- 177.Gupta N. and Wish J.B. (2017) Hypoxia-inducible factor prolyl hydroxylase inhibitors: a potential new treatment for anemia in patients with CKD. Am. J. Kidney Dis. 69, 815–826 10.1053/j.ajkd.2016.12.011 [DOI] [PubMed] [Google Scholar]
- 178.Steckelings U.M., Rompe F., Kaschina E. and Unger T. (2009) The evolving story of the RAAS in hypertension, diabetes and CV disease: moving from macrovascular to microvascular targets. Fundam. Clin. Pharmacol. 23, 693–703 10.1111/j.1472-8206.2009.00780.x [DOI] [PubMed] [Google Scholar]
- 179.Humar R., Zimmerli L. and Battegay E. (2009) Angiogenesis and hypertension: an update. J. Hum. Hypertens. 23, 773–782 10.1038/jhh.2009.63 [DOI] [PubMed] [Google Scholar]
- 180.Zinman B. et al. (2015) Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N. Engl. J. Med. 373, 2117–2128 10.1056/NEJMoa1504720 [DOI] [PubMed] [Google Scholar]
- 181.Wanner C. et al. (2018) Empagliflozin and clinical outcomes in patients with type 2 diabetes mellitus, established cardiovascular disease, and chronic kidney disease. Circulation 137, 119–129 10.1161/CIRCULATIONAHA.117.028268 [DOI] [PubMed] [Google Scholar]
- 182.Wanner C., Inzucchi S.E. and Zinman B. (2016) Empagliflozin and progression of kidney disease in type 2 diabetes. N. Engl. J. Med. 375, 1801–1802 10.1056/NEJMoa1515920 [DOI] [PubMed] [Google Scholar]
- 183.Inzucchi S.E. et al. (2018) How does empagliflozin reduce cardiovascular mortality? Insights from a mediation analysis of the EMPA-REG OUTCOME Trial Diabetes Care 41, 356–363 10.2337/dc17-1096 [DOI] [PubMed] [Google Scholar]
- 184.Davidson J.A. (2019) SGLT2 inhibitors in patients with type 2 diabetes and renal disease: overview of current evidence. Postgrad. Med. 131, 251–260 10.1080/00325481.2019.1601404 [DOI] [PubMed] [Google Scholar]
- 185.Shigiyama F. et al. (2017) Effectiveness of dapagliflozin on vascular endothelial function and glycemic control in patients with early-stage type 2 diabetes mellitus: DEFENCE study. Cardiovasc. Diabetol. 16, 84 10.1186/s12933-017-0564-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Ott C. et al. (2017) A randomised study of the impact of the SGLT2 inhibitor dapagliflozin on microvascular and macrovascular circulation. Cardiovasc. Diabetol. 16, 26 10.1186/s12933-017-0510-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Dziuba J. et al. (2014) Modeling effects of SGLT-2 inhibitor dapagliflozin treatment versus standard diabetes therapy on cardiovascular and microvascular outcomes. Diabetes Obes. Metab. 16, 628–635 10.1111/dom.12261 [DOI] [PubMed] [Google Scholar]
- 188.Triggle C.R., Ding H., Marei I., Anderson T.J. and Hollenberg M.D. (2020) Why the endothelium? The endothelium as a target to reduce diabetes-associated vascular disease Can. J. Physiol. Pharmacol. 10.1139/cjpp-2019-0677 [DOI] [PubMed] [Google Scholar]
- 189.Rockey D.C., Bell P.D. and Hill J.A. (2015) Fibrosis–a common pathway to organ injury and failure. N. Engl. J. Med. 372, 1138–1149 10.1056/NEJMra1300575 [DOI] [PubMed] [Google Scholar]
- 190.Pries A.R. et al. (2015) Coronary vascular regulation, remodelling, and collateralization: mechanisms and clinical implications on behalf of the working group on coronary pathophysiology and microcirculation. Eur. Heart J. 36, 3134–3146 10.1093/eurheartj/ehv100 [DOI] [PubMed] [Google Scholar]
- 191.Lewis M.I. et al. (2012) Metabolic and morphometric profile of muscle fibers in chronic hemodialysis patients. J. Appl. Physiol. (1985) 112, 72–78 10.1152/japplphysiol.00556.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]