Abstract
The protozoan Trypanosoma cruzi is responsible for triggering a damage immune response in the host cardiovascular system. This parasite has a high affinity for host lipoproteins and uses the low-density lipoprotein (LDL) receptor for its invasion. Assuming that the presence of LDL cholesterol in tissues could facilitate T. cruzi proliferation, dietary composition may affect the parasite-host relationship. Therefore, the aim of this study was to evaluate myocarditis in T. cruzi-infected C57BL/6 mice—acute phase—fed a high-fat diet and treated with simvastatin, a lipid-lowering medication. Animals (n = 10) were infected with 5 × 103 cells of the VL-10 strain of T. cruzi and treated or untreated daily with 20 mg/kg simvastatin, starting 24 h after infection and fed with a normolipidic or high-fat diet. Also, uninfected mice, treated or not with simvastatin and fed with normolipidic or high-fat diet, were evaluated as control groups. Analyses to measure the production of chemokine (C-C motif) ligand 2 (CCL2), interferon- (IFN-) γ, interleukin- (IL-) 10, and tumor necrosis factor (TNF); total hepatic lipid dosage; cholesterol; and fractions, as well as histopathological analysis, were performed on day 30 using cardiac and fat tissues. Our results showed that the high-fat diet increased (i) parasite replication, (ii) fat accumulation in the liver, (iii) total cholesterol and LDL levels, and (iv) the host inflammatory state through the production of the cytokine TNF. However, simvastatin only reduced the production of CCL2 but not that of other inflammatory mediators or biochemical parameters. Together, our data suggest that the high-fat diet may have worsened the biochemical parameters of the uninfected and T. cruzi-infected animals, as well as favored the survival of circulating parasites.
1. Introduction
Trypanosoma cruzi, an intracellular protozoan parasite, is the causative agent of Chagas disease, which affects nearly 8 million people worldwide [1]. Multiple factors contribute to the clinical course of this infection, but the host immune response appears to be essential in coordinating the parasite replication and cardiac pathogenesis [2–4]. In this context, the production of cytokines and chemokines has been shown to essentially regulate leukocyte recruitment during T. cruzi infection, which could eliminate the parasite but also worsen the clinical condition [5–7].
Extrinsic factors such as elements present in the host diet or even medication might also contribute to the pathogenesis induced by T. cruzi. For instance, host lipid metabolism has been shown to have an important role in the host immune response and during infection by T. cruzi. This protozoan interacts with distinct components of the host metabolism to increase the lipid bodies in activated macrophages [8] and uses adipocytes as a chronic reservoir that can contribute to the up- or downregulation of inflammatory cytokines [9]. In addition, T. cruzi downregulates adiponectin secretion via peroxisome proliferator-activated receptor (PPAR) expression [10] and its in vivo invasion in animals fed a high-fat diet who present with high circulating levels of intracellular cholesterol is higher than it is in animals fed normal diets [11, 12]. Moreover, in vitro, the high-density lipoprotein (HDL) cholesterol of the host inhibits T. cruzi transsialidase activities by increasing parasitic infection and downregulating adiponectin release via PPAR expression [13].
Statins are an effective class of low-density lipoprotein (LDL) cholesterol-lowering agents, which act by inhibiting 3-hydroxy-3-methylglutaryl coenzyme A reductase [14]. Statins (e.g., simvastatin) also exert a cholesterol-independent immunomodulatory effect, which is likely mediated by preventing the production of isoprenoids, which act as critical lipid attachments for the posttranslational alterations of essential intracellular signaling proteins [15, 16]. We previously showed that mice and dogs treated with simvastatin exhibited reduced circulating inflammatory mediators and cell recruitment into T. cruzi-infected tissues [17, 18].
We assumed that some lipids and statins independently interfere with T. cruzi infection progression and the host immune response. Therefore, we performed experiments to prove our hypothesis that both extrinsic factors interact in C57BL/6 mice who were fed a high-fat diet (60% lipids), infected with the VL-10 of T. cruzi, and underwent simvastatin therapy (20 mg/day) for 30 days.
2. Material and Methods
2.1. Experimental Model, Parasite Infection, and Diets
Female C57BL/6 mice [19], 3-week-old age, were maintained at the Animal Facility of the Universidade Federal de Ouro Preto (UFOP) on a 12 h light/dark cycle with controlled temperature (22.0°C ± 2) and free access to water and food. Animals were fed a normolipidic diet (American Institute of Nutrition-93M) as the control diet and a modified high-fat diet containing 60% of calories obtained by lipids (PragSoluções Ltd., Sao Paulo-SP). The animals were fed for 2 months, and on day 60, they were infected with 5 × 103 trypomastigotes of the VL-10 T. cruzi strain. The infection was confirmed by determining the daily parasitemia level by counting the parasites in 5 μL blood samples obtained from the tail vein, according to the method of Brener (1962).
Before and after the infection, the animals were grouped (n = 10) as follows: uninfected, fed a normolipidic diet; uninfected, fed a high-fat diet; uninfected, fed a normolipidic diet and treated with simvastatin; and uninfected, fed a high-fat diet treated with simvastatin. The other infected groups were T. cruzi-infected and fed a normolipidic diet; T. cruzi-infected and fed a high-fat diet; T. cruzi-infected, fed a normolipidic diet, and treated with simvastatin, and T. cruzi-infected, fed a high-fat diet, and treated with simvastatin. See nutritional information on Table 1. Dietary intake was measured daily, and body mass gain was assessed weekly.
Table 1.
Nutritional composition in 1 kilogram of diet.
| Ingredients | Normolipidic diet (grams) | High-fat diet (grams) |
|---|---|---|
| Starch | 619.4 | 249.4 |
| Lard | - | 320.0 |
| Casein | 14.0 | 190.0 |
| Coline | 2.5 | 2.5 |
| Fiber | 50.0 | 50.0 |
| L-Cystine | 3.0 | 3.0 |
| Mineral mix | 35.0 | 35.0 |
| Vitamin mix | 10.0 | 10.0 |
| Soy oil | 40.0 | 40.0 |
| Sucrose | 100.0 | 100.0 |
| Total caloric value (kilocalories-kcal) | 4,447.4 | 5,917.5 |
(I) Mineral mix (expressed in g/kg of the mixture): NaCl-139.3/KI-0.79/MgSO4·7H2O-57.3/CaCO3-381.4/MnSO4·H2O-4.01/FeSO4 ·7H2O–0.548/CuSO4·5H2O–0.477/CoCl2·6H2O–0.023/KH2PO4–389.0. (II) Vitamin mix (expressed in mg/kg of the mixture): retinol acetate–690.0/cholecalciferol-5,-/p-amino benzoic acid-10.0/inositol-10.0/niacin-4000.0/riboflavin-800.0/thiamine HCL-500.0; folic acid- 200.0/biotin-40.0/cyanocobalamin-3.0/dl-α-tocopherol-6.7/sucrose -q.s.p.1000.0. (III) BHT (Tert-Butylhydroquinone)-0.008 g to both diets/Kg ∗Added 1% of SIGMA® cholesterol conversion factors: proteins 4.0 kcal/g, lipids 9,0 kcal/g, sugars 4,0 kcal/g.
2.2. Treatment with Simvastatin
Simvastatin was administered at 20 mg/kg as previously standardized by our research group [18] and administered daily by gavage. Briefly, the treatment was started 24 h after infection and lasted for 30 days. The untreated animals received 0.05% carboxymethyl cellulose diluted in phosphate buffer solution by gavage while treated mice were administered simvastatin in the morning (7 : 30–8 : 00 a.m.) to coincide with the animals' cortisol peak.
2.3. Euthanasia and Tissue Collection
On day 90 after the infection, animals in their proestrus cycle were euthanized by an intraperitoneal overdose of ketamine (80 mg/kg) and xylazine (7 mg/kg) anesthetic and their blood and organs (the heart, liver, and abdominal fat tissue) were removed and one aliquot was stored in 10% formalin and another aliquot in a freezer at -80°C. The experimental protocol for this study was previously approved by the Ethical Committee for the use of Experimental Animals (CEUA) of the UFOP (Protocol #2012/42).
2.4. Total Cholesterol, HDL, LDL, and Triglycerides
The total cholesterol, HDL, and triglycerides were determined in plasma samples, using an enzymatic colorimetric kit for cholesterol, triglycerides, and HDL liquiform, from Labtest Diagnostica SA (Lagoa Santa, MG, BR). The LDL values were calculated using the following formula of Friedewald et al. [20], which is considered a reference by the Center for Disease, Control and Prevention (CDC): LDL cholesterol = total cholesterol − HDL cholesterol triglycerides/5.
2.5. Immunoassays
The circulating levels of CCL2, IFN-γ, IL-10, and TNF were quantified in the plasma and in homogenates of 50 mg of adipose or cardiac tissues, using an enzyme-linked immunosorbent assay (ELISA, PeproTech, Rocky Hill, NJ, USA). Briefly, 96-well microtiter plates were coated with the monoclonal capture antibodies at 100 μL/well and incubated overnight at 25°C. After rinsing with the buffer (phosphate-buffered saline (PBS), 0.05% Tween-20), the nonspecific binding sites were blocked with 300 μL/well of the blocking buffer (1% bovine serum albumin in PBS) for 1 h.
The samples were added (100 μL/well), incubated for 2 h at room temperature, and then 100 μL/well of the appropriate biotinylated detection antibodies diluted in blocking buffer containing 0.05% Tween-20 was added, followed by a 2 h incubation at room temperature. Avidin was added (100 μL/well), and the plates were incubated for 30 min at room temperature. Finally, 100 μL/well of the chromogen substrate 2,2′-alzino-bis (3-ethylbenzothiazoline-6-sulfonic acid (ABTS, Sigma-Aldrich Corp., St. Louis, MO, USA) was added, and the plates were incubated in the dark for 30 min at room temperature. Triplicate samples were read at 405 nm with wavelength correction set at 650 nm using a spectrophotometer (EMax Molecular Devices).
2.6. Total Hepatic Lipid Levels
The total hepatic lipids were extracted according to the method of Folch et al. [21]. In brief, 400 mg of the liver tissue was weighed into previously labeled plastic tubes, triturated with 8 mL of a chloroform-methanol (2 : 1, v/v) solution, and homogenized for 3 min at a speed of 10,000 rpm. The supernatants were transferred to new glass tubes of known weight, 2 mL 0.73% sodium chloride (NaCl) solution was added, the mixtures were homogenized and centrifuged again for 10 min at 3000 rpm, and the upper phase was discarded. The wall of each tube was washed three times with 1 mL Folch solution (3% chloroform solution, 48% methanol, 47% distilled water, and 2% NaCl), and the extracted lipids were oven-dried overnight at 60°C. After ensuring that the tubes were completely dry, they were weighed, and the amount of lipid extracted was calculated as the weight difference of the glass tubes before and after drying.
2.7. Histopathological Analysis
Fragments of the heart and inguinal adipose tissue were fixed in a methanol-dimethyl sulfoxide (DSMO) solution and embedded in Paraplast. Blocks containing these fragments were cut into 4 mm thick sections and stained with hematoxylin and eosin (H&E) for the quantification of inflammatory leukocytes. The stained sections were randomly evaluated at 40x magnification, in a total area of 74985 μm2, the equivalent of 50 fields of analyzed myocardium and adipose tissue. Images were obtained using a Leica DM5000 B microchamber (Leica Application Suite, UK, version 2.4.0 R1) and processed using the Leica QWin (V3) image analyzer. For the myocardium, the inflammation was quantified by counting the number of cardiac cellular nuclei in the infected heart tissue compared with that in the uninfected mouse tissue.
2.8. Statistical Analysis
The data are expressed as the mean ± standard error of the mean (SEM) and were analyzed using the Kolmogorov-Smirnov normality test and Anova two-way followed by Tukey's posttest. All the analyses were performed using the PRISM 5.01 software (GraphPad, San Diego, CA, USA), and the level of significance was accepted at p < 0.05.
3. Results
In this present study, we monitored food intake and body mass gain and determined the total hepatic lipid levels of T. cruzi-infected animals receiving normolipidic and high-fat diets with or without simvastatin treatment, and the results are presented in Table 2. According to the data obtained, there was no difference in feed consumption and weight gain of the animals throughout the study. The animals fed with the normolipidic diet presented lower liver mass when compared to the animals treated with simvastatin. In this same group, the infection increased liver mass when compared to uninfected animals. In the group that received the normolipidic diet, there was also an increase in the liver mass in the association of infection with the treatment with simvastatin. When the animals were fed the high-fat diet, the infection also increased the body mass, and the same occurred in the infected animals treated with simvastatin. On the other hand, liver fat analysis showed an increase in fat deposition in this organ only among animals fed a high-fat diet, infected, and treated with simvastatin when compared to the same situation of the animals fed with the normolipidic diet.
Table 2.
The body parameters.
| Parameters | Groups (mean ± std error) | |||||||
|---|---|---|---|---|---|---|---|---|
| Uninfected | T. cruzi | |||||||
| NLD | NLD+Simvas | HFD | HFD+Simvas | NLD | NLD+Simvas | HFD | HFD+Simvas | |
| Food intake (g) | 9.83 ± 0.18 | 9.65 ± 0.18 | 9.70 ± 0.53 | 9.66 ± 0.50 | 9.25 ± 0.39 | 9.46 ± 0.40 | 8.78 ± 0.27 | 9.10 ± 0.49 |
| Body mass (g) | 18.28 ± 0.72 | 17.63 ± 0.05 | 19.26 ± 0.56 | 19.83 ± 0.46 | 18.35 ± 0.51 | 19.09 ± 0.54 | 20.24 ± 0.57 | 20.17 ± 0.55 |
| Liver relative mass (g) | 0.041 ± 0.00a, b, c | 0.045 ± 0.00b, d | 0.048 ± 0.00a, e | 0.045 ± 0.00f | 0.058 ± 0.00c | 0.060 ± 0.00d | 0.061 ± 0.00e | 0.061 ± 0.00f |
| Liver fat (g) | 0.004 ± 0.0 | 0.002 ± 0.00 | 0.005 ± 0.002 | 0.004 ± 0.001 | 0.005 ± 0.001 | 0.003 ± 0.001a | 0.008 ± 0.001 | 0.007 ± 0.001 a |
Equal letters indicate difference between groups using Anova two-way and Tukey posttest (p < 0.05). NLD: normolipidic diet; HFD: high-fat diet. Simvas: Simvastatin.
T. cruzi increased the total cholesterol (Figure 1(a)), the LDL cholesterol (Figure 1(c)), and triglycerides (Figure 1(d)) in mice fed with the normolipidic diet. In this context, simvastatin treatment was able to convert the pattern of total cholesterol and LDL cholesterol. In these T. cruzi-infected animals, the high-fat diet increased the total cholesterol and LDL cholesterol but neither HDL cholesterol (Figure 1(b)) nor triglycerides. Simvastatin failed in reducing the cholesterol and triglyceride values in the presence of T. cruzi.
Figure 1.
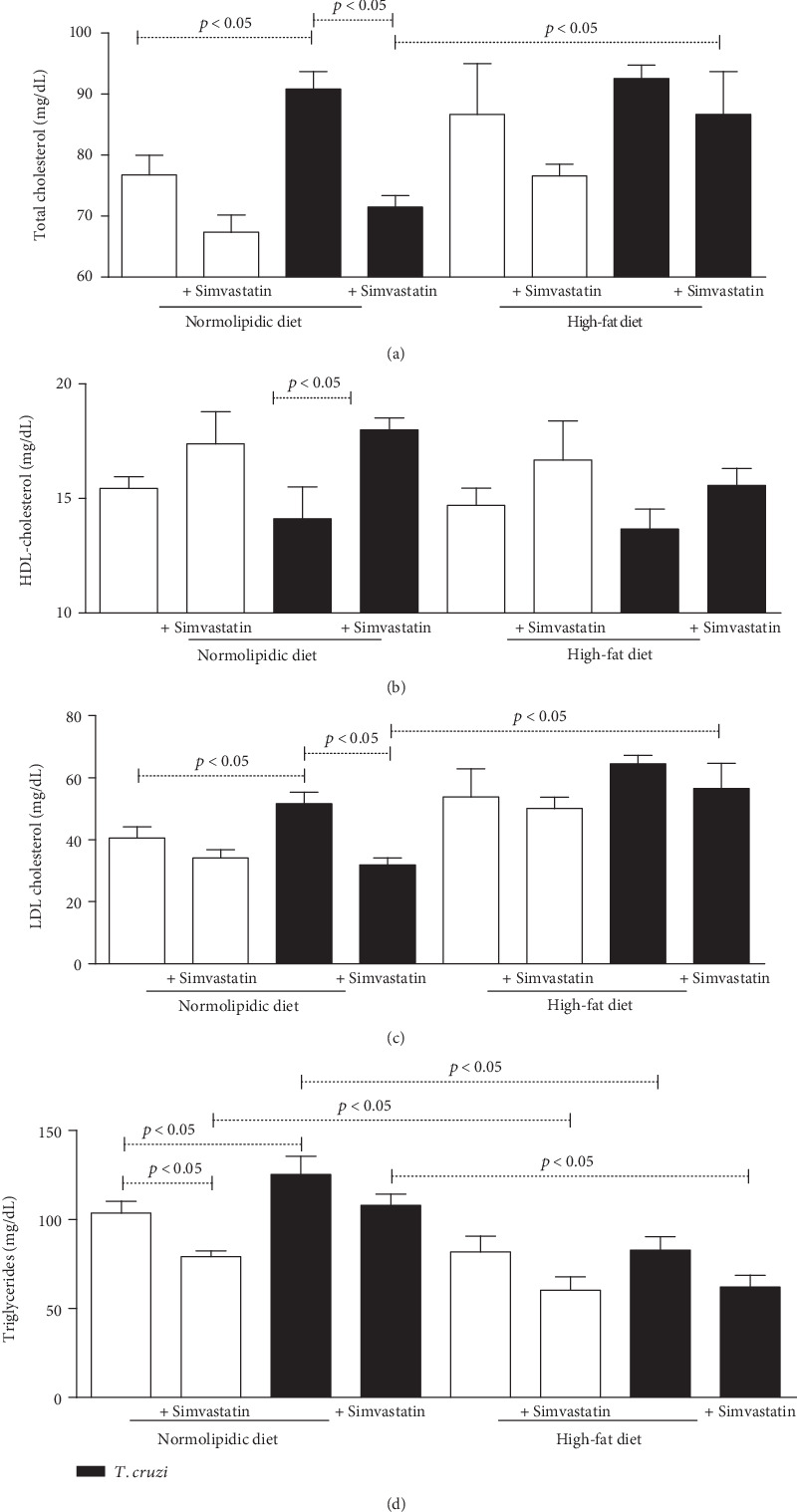
Trypanosoma cruzi increases total cholesterol, LDL, and triglycerides during acute phase of experimental infection. C5BL/6 mice were infected with VL-10 of T. cruzi and plasma used to measure total cholesterol (a), HDL cholesterol (b), LDL cholesterol (c), and triglycerides (d) using colorimetric enzymatic kits. Animals were fed with normolipid or high-fat diets and treated or not with simvastatin. White bars: uninfected animals; black bars: animals infected with T. cruzi. The data are mean ± standard error of the mean (SEM).
The parasitemia curve (Figure 2(a)) and area under the curve analysis (Figure 2(b)) revealed that the high-fat diet contributed to a higher parasite amount in the blood of animals fed a normolipidic diet. In addition, simvastatin reduced the number of parasites in blood in the group fed a high-fat diet (50.40%), while in the group fed the normolipidic diet, no difference was observed.
Figure 2.
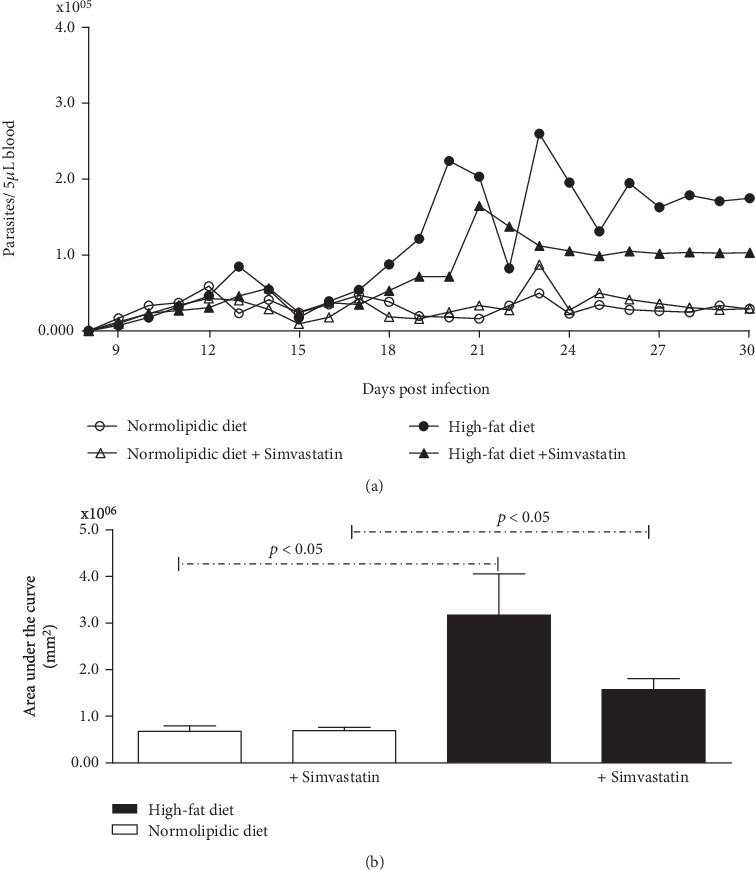
The high-fat diet increases the parasitemia curve. (a) Number of circulating parasites. White symbols: animals fed with normolipidic diet with and without simvastatin treatment; black symbols: animals fed with high-fat diet with and without simvastatin treatment. (b) Area under the parasitemia curve during 30 days of Trypanosoma cruzi infection in C57BL/6 mice. White bars: animals fed with normolipidic diet with and without simvastatin treatment; black bars: animals fed with high-fat diet with and without simvastatin treatment. Data are mean ± standard error of the mean (SEM).
We also observed an increase in the levels of plasma TNF in infected animals, and infected animals fed a high-fat diet had higher values of this cytokine when compared to infected animals fed a normolipidic diet (Figure 3(a)). Similarly, the infection elevated plasma CCL2 levels, and simvastatin worked by reducing this chemokine only in the group of infected animals fed a high-fat diet (Figure 3(b)). On the other hand, plasma IL-10 (Figure 3(c)) was higher in animals fed a normolipidic diet when compared to the high-fat diet, and treatment with simvastatin, as well as infection, increased levels of this interleukin in animals treated with a high-fat diet.
Figure 3.
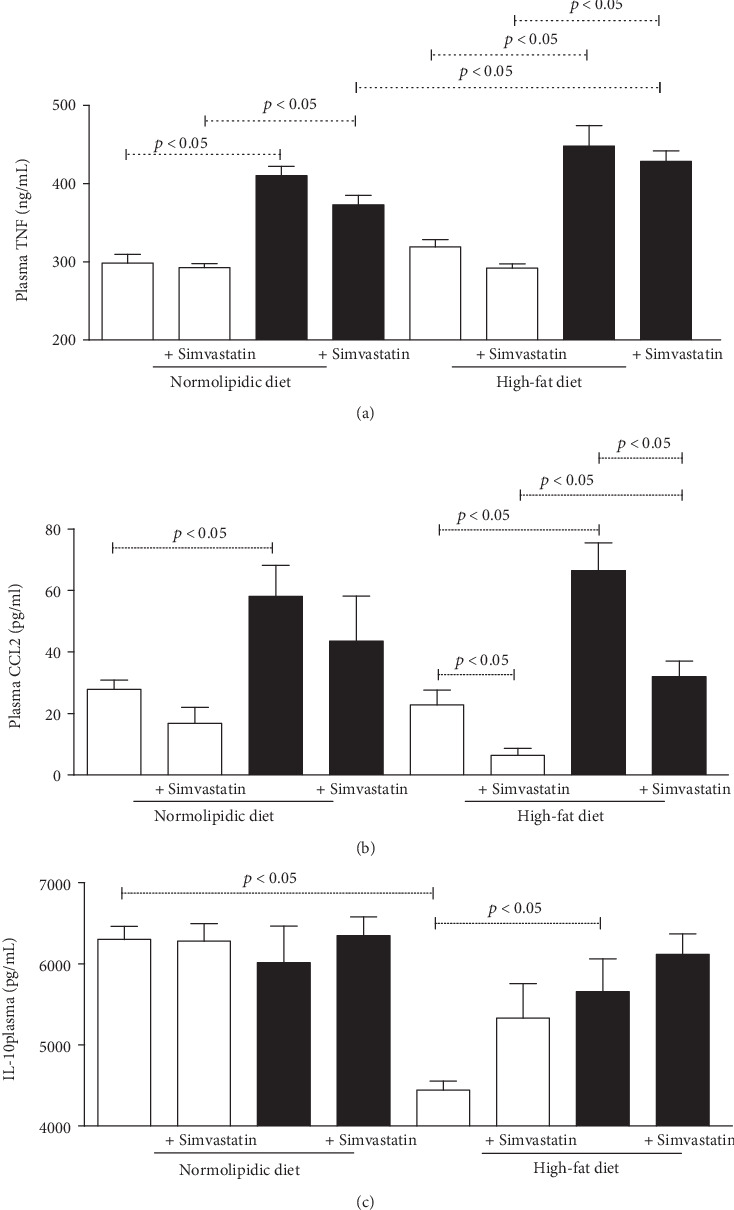
High-fat diet increases plasma TNF and CCL2 during the acute phase of experimental T. cruzi infection. TNF (a), CCL2 (b), and IL-10 (c) were measured by immunoassays in the plasma of C57BL/6 mice at 30 days of T. cruzi infection and after receiving normolipidic or high-fat diets. White bars: noninfected animals with and without simvastatin treatment; black bars: T. cruzi-infected mice with and without simvastatin treatment. Data are mean ± standard error of the mean (SEM).
The cardiac tissue analysis showed higher TNF cytokine production among the infected and the high-fat diet mice when compared to the respective control group (Figure 4(a)). In the assessment of TNF in adipose tissue showed that regardless of infection, the high-fat diet stimulated high production of this cytokine (Figure 4(b)). On the other hand, there was no difference in the production of IFN-gamma in cardiac tissue (Figure 4(c)), but in adipose tissue, this cytokine had higher production in the groups fed with a high-fat diet when compared to the groups fed the normolipidic diet (Figure 4(d)). The production of IL-10 in cardiac (Figure 4(e)) and adipose (Figure 4(f)) tissues was higher in the groups fed the normolipidic diet, infected or not, when compared to the animals fed a high-fat diet. The animals fed the high-fat diet also had higher values of this cytokine when compared to the infected control.
Figure 4.
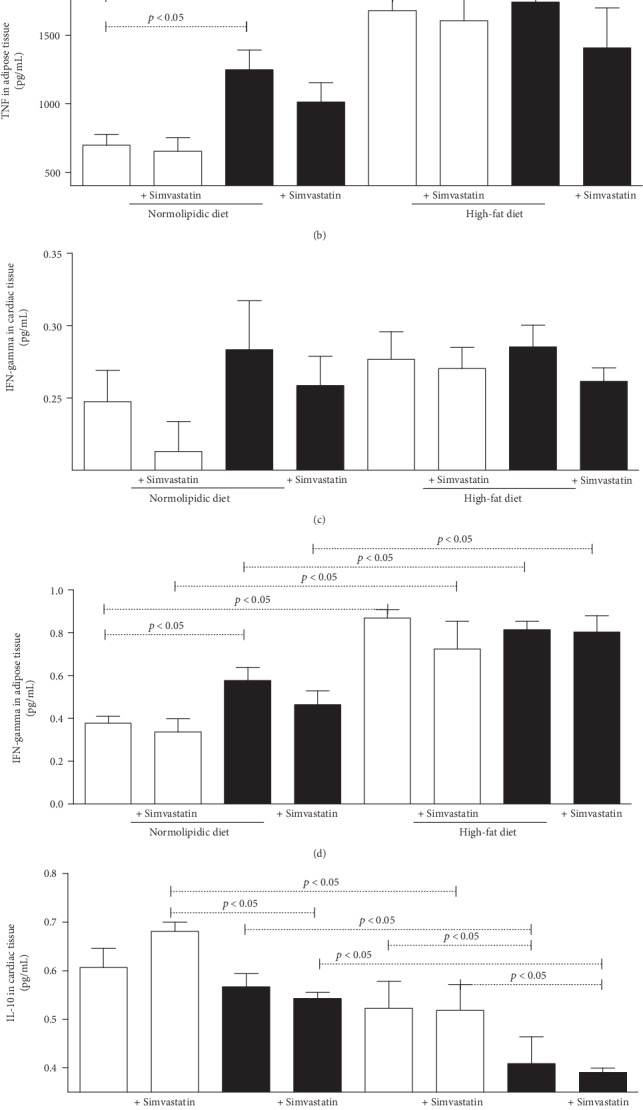
Biomarkers in cardiac or adipose tissues from C57BL/6 mice infected with the Trypanosoma cruzi. Uninfected (white bars) and T. cruzi-infected (black bars) mice were fed with normolipidic and high-fat diets and with and without simvastatin treatment. TNF (a, b), IFN-gamma (c, d), and IL-10 (e, f) were measured by immunoassay in 50 mg of cardiac or adipose tissues, respectively, after 30 days of infection. Data are mean ± standard error of the mean (SEM).
Regardless of the diet (normolipidic control or high fat), the heart and adipose tissues of uninfected animals showed a normal histological appearance (Figure 5). Infected animals fed the normolipidic diet demonstrated a multifocal inflammatory process with moderate characteristics in the heart and discrete features in the adipose tissue that consisted mainly of mononuclear cells. Additionally, the infected animals fed the high-fat diet showed an increase in the inflammatory process compared with the infected animals fed the normolipidic diet that showed moderate to intense characteristics in the heart and discrete to moderate features in the adipose tissue.
Figure 5.
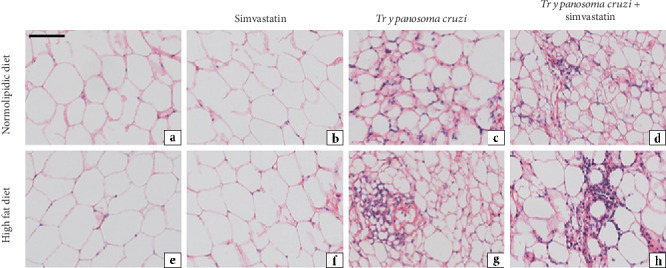
Representative photomicrographs of histological sections of adipose tissue from uninfected and infected C57BL/6 mice with T. cruzi (VL-10 strain). The acute inflammatory process was evaluated in the adipose tissue of mice infected with T. cruzi and fed with high-fat diet, under simvastatin treatment or not, as indicated in the figure. Tissues were stained with hematoxylin and eosin (H&E) at 30 days after infection. Scale bar = 50 mm.
In the heart (Figure 6), the atrial region showed higher concentrations of the inflammatory infiltrate while in the adipose tissue, areas with multilocular adipose cells were larger in animals fed the high-fat diet with or without the infection than they were in animals fed the normolipidic diet.
Figure 6.
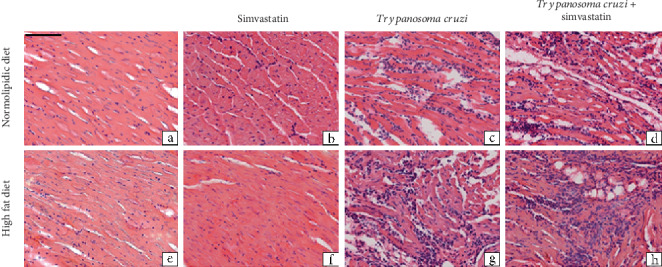
Representative photomicrographs of histological sections of heart tissue of mice infected with Trypanosoma cruzi. C57BL/6 mice were infected or not with the VL-10 strain of T. cruzi and fed, or not, with high-fat diet. In addition, some animals were treated with simvastatin and cardiac tissue extracted at 30 days after infection to processing and hematoxylin and eosin (H&E) staining. Scale bar = 50 mm.
4. Discussion
Over the last few years, the role of adipose tissue has been highlighted in innate and adaptive immune responses as a modulator of the production of adipokines and other lipid mediators under infectious disease conditions [22, 23]. It is well-established that the inflammation caused by T. cruzi infection persists in distinct tissues and is highly dependent on the genetic background of the parasite and the immune response of the host, which determines the chronic pathogeny [24]. Thus, in T. cruzi infection research, adipose tissue and circulating fats have emerged as factors capable of controlling the adaptation of these protozoans and defining the course of the pathogenesis. In mammalian hosts, the adipose tissue also acts as a reservoir for protozoans in chronic stages of the infection. This phenomenon could be critical to the recrudescence of the infection under immunosuppressed conditions or may interfere with the development of cardiac and other associated metabolic disturbances [25, 26]. This present study provides key information that a high-fat diet affects the course of experimental T. cruzi infection by increasing the replication of the parasites; accumulation of fat in the liver, total cholesterol, and LDL levels; and circulating levels of inflammatory mediators. In addition and based on our previous experience where simvastatin exerted an immunomodulatory effect during the T. cruzi infection using the Y [17] and Colombiana [18] strains, this study also suggests a pharmacological action of simvastatin modulating the VL-10 strain infection of T. cruzi.
Generally, body lipids can be maintained at healthy levels by low-fat diet consumption, physical exercises, or pharmacological interventions such as statins, which also have an immunomodulatory property [27]. Therefore, an increase in the cholesterol levels and adipose tissue in association with the T. cruzi infection is thought to (i) benefit the parasites by providing the cholesterol and other lipids they require to maintain their metabolism, (ii) provide a dynamic cellular environment where parasites can hide, and (ii) elevate adipokines, which could somehow contribute to controlling the parasite by enhancing systemic and local inflammatory responses. We previously used simvastatin in experimental T. cruzi infection, but our focus was exclusively on the anti-inflammatory actions and potential amelioration of the clinical features. In these studied populations of T. cruzi, Y [17] and Colombiana [18] strains, they were able to induce high inflammatory responses in murine and dog models and, in these models, we demonstrated that simvastatin reduced the TNF, CCL2, and CCL5 levels, as well as the cardiac leukocyte infiltration and amastigote nests, which improved the echocardiographic parameters. In addition, a previous study showed simvastatin in association with benznidazole prevented cell adhesion molecule expression on endothelial cells during T. cruzi infection [28]. In this study, the simvastatin-induced 15-epi-lipoxin A4 prevented the ECAM expression on T. cruzi-infected EA.hy926 cells and blocked NF-κB activation. It is worth stressing that T. cruzi presents high genetic variability which reflects characteristics concerning the natural history of infection, the biological behavior, the epidemiological patterns, and issues related to the diagnosis and treatment of this infection.
However, in our current study, we detected the simvastatin immunomodulatory effect on CCL2 plasma levels but not on other immunological parameters. Beyond lipid-lowering properties, simvastatin is known as an anti-inflammatory drug mainly through the inactivation of NF-κB, a key activator of cytokine transcription and through the resulting decrease in the expression levels of reactive oxygen species, inhibition of matrix metalloproteinases, and decrease of proinflammatory cytokines including TNF. IFN-gamma and TNF are key markers released during the initial phase of T. cruzi infection to promote elimination and/or control of the parasite [5, 14, 15, 18]. The inflammatory response elicited by T. cruzi infection is accompanied by elevation of IL-10, which was not observed in the present study. A plausible explanation is based on the biological characteristic of the VL-10 strain. The VL-10 strain induces a moderate systemic and cardiac inflammation with a higher host survival than other strains, as previously demonstrated [29].
In this study, mice without simvastatin intervention fed a high-fat diet presented with alterations in parasitological and immunological parameters, which were beneficial to the circulating parasites. Specifically, this condition induced the accumulation of circulating total cholesterol and LDL and of fat in the liver. It has been demonstrated that a high-fat diet modified the immune response in uninfected mice by suppressing the cAMP response element-binding protein- (CREB-) PPAR-y signaling pathway, which could be associated with liver steatosis events [30]. In the presence or absence of infection, a high-fat diet was also shown to increase the detection of macrophages in fat tissue, production of reactive oxygen species, and mortality [31].
High-fat diet has been previously used in other studies of T. cruzi infection, but these involved different genetic variations of the parasite [9, 25]. It is worth noting that during the experimental T. cruzi infection, all variables of the different groups must be carefully considered to enhance the comprehension of the peculiarities and complexity of this infection. A hypothesis of the method by which parasites establish a close interaction with the adipose tissue is based on the metabolic characteristic of the adipocytes and their long lifespan (6–12 months) in the mammalian body. In the current study, we observed that the increase in LDL and total cholesterol levels related to the high-fat diet favored the survival of this protozoan. LDL has been shown to inhibit T. cruzi transsialidase in cultures of human fibroblasts in a concentration-dependent manner, which means that higher infection rate was observed with the addition of the LDL than without the LDL [32]. This observation could be a plausible explanation for the increased parasitemia in the experimental T. cruzi infection associated with the high-fat diet [33]. Furthermore, adipose tissue provides a suitable environment for replication of parasite [26].
Finally, unhealthy dietary habits have been strongly associated with body fat accumulation, cardiovascular disturbances, and untimely death in individuals from industrialized countries. The focus on the high-fat diets has now been extended to immune interventions, mainly associated with some tropical parasitic diseases such as T. cruzi infection. In summary, a high-fat diet could ameliorate the environmental for the T. cruzi parasite and worsen the biochemical and immunological responses in mammalian hosts. This fact can be illustrated with the present study, from the dosages of TNF, IFN, and IL-10 performed. In contrast, simvastatin is able to modulate the infection, since it acts on the low-density lipoprotein mechanisms, reducing the parasitemia and providing a protective heart effect. Therefore, further investigations would be necessary to enhance the understanding of how different populations of T. cruzi interact with elevated circulating fat levels in mammals and induce pathological alterations and how simvastatin contributes its protective effect.
Acknowledgments
The present study was funded by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior/CAPES, Conselho Nacional de Desenvolvimento Científico e Tecnológico/CNPq, Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG), and Universidade Federal de Ouro Preto (UFOP). AT (Process # 305634/2017-8) was supported by the CNPq with the Fellowship of Research Productivity.
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Authors' Contributions
Débora Maria Soares Souza is responsible for conceptualization, investigation, and writing; Guilherme de Paula Costa, Ana Luiza Junqueira Leite, Daniela Silva de Oliveira, Natália Figueiroa Simões, and Nívia Carolina Nogueira de Paiva are in charge of the investigation in the study; Kelerson Mauro de Castro Pinto is in charge of formal analysis: Paula Melo de Abreu Vieira is in charge of formal analysis; Camilo Adalton Mariano da Silva is in charge of formal analysis; Vivian Paulino Figueiredo performed supervision and investigation; Ana Paula de Jesus Menezes is in charged of reviewing and editing; Andre Talvani is responsible for conceptualization, writing, project administration, and funding acquisition.
References
- 1.WHO. Chagas disease (American trypanosomiasis) Fact sheet N 340. Geneva: World Health Organization; 2017. [Google Scholar]
- 2.Dutra W. O., Menezes C. A. S., Magalhães L. M. D., Gollob K. J. Immunoregulatory networks in human Chagas disease. Parasite Immunology. 2014;36(8):377–387. doi: 10.1111/pim.12107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Geiger A., Bossard G., Sereno D., et al. Escaping deleterious immune response in their hosts: lessons from trypanosomatids. Frontiers in Immunology. 2016;7 doi: 10.3389/fimmu.2016.00212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sueth-Santiago V., Moraes J. . B. B., Sobral Alves E. S., et al. The effectiveness of natural diarylheptanoids against Trypanosoma cruzi: cytotoxicity, ultrastructural alterations and molecular modeling studies. PLoS One. 2016;11(9, article e0162926) doi: 10.1371/journal.pone.0162926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Talvani A., Ribeiro C. S., Aliberti J. C. S., et al. Kinetics of cytokine gene expression in experimental chagasic cardiomyopathy: tissue parasitism and endogenous IFN-γ as important determinants of chemokine mRNA expression during infection with Trypanosoma cruzi. Microbes and Infection. 2000;2(8):851–866. doi: 10.1016/S1286-4579(00)00388-9. [DOI] [PubMed] [Google Scholar]
- 6.Brener Z., Gazzinelli R. T. Immnunological control of Trypanosoma cruzi infection and pathogenesis of Chagas’ disease. International Archives of Allergy and Immunology. 2004;114(2):103–110. doi: 10.1159/000237653. [DOI] [PubMed] [Google Scholar]
- 7.Esper L., Talvani A., Pimentel P., Teixeira M. M., Machado F. S. Molecular mechanisms of myocarditis caused by Trypanosoma cruzi. Current Opinion in Infectious Diseases. 2015;28(3):246–252. doi: 10.1097/QCO.0000000000000157. [DOI] [PubMed] [Google Scholar]
- 8.Melo R. C. N. Depletion of immune effector cells induces myocardial damage in the acute experimental Trypanosoma cruzi infection: ultrastructural study in rats. Tissue & Cell. 1999;31(3):281–290. doi: 10.1054/tice.1999.0040. [DOI] [PubMed] [Google Scholar]
- 9.Ferreira A. V. M., Segatto M., Menezes Z., et al. Evidence for Trypanosoma cruzi in adipose tissue in human chronic Chagas disease. Microbes and Infection. 2011;13(12-13):1002–1005. doi: 10.1016/j.micinf.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hovsepian E., Penas F., Mirkin G. A., Goren N. B. Role of PPARs in Trypanosoma cruzi infection: implications for Chagas disease therapy. PPAR Research. 2012;2012:8. doi: 10.1155/2012/528435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johndrow C., Nelson R., Tanowitz H., Weiss L. M., Nagajyothi F. Trypanosoma cruzi infection results in an increase in intracellular cholesterol. Microbes and Infection. 2014;16(4):337–344. doi: 10.1016/j.micinf.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nagajyothi F., Weiss L. M., Silver D. L., et al. Trypanosoma cruzi utilizes the host low density lipoprotein receptor in invasion. PLoS Neglected Tropical Diseases. 2011;5(2, article e953) doi: 10.1371/journal.pntd.0000953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Penas F. N., Cevey Á. C., Siffo S., Mirkin G. A., Goren N. B. Hepatic injury associated with Trypanosoma cruzi infection is attenuated by treatment with 15-deoxy-Δ12,14 prostaglandin J2. Experimental Parasitology. 2016;170:100–108. doi: 10.1016/j.exppara.2016.09.015. [DOI] [PubMed] [Google Scholar]
- 14.Fukuda K., Matsumura T., Senokuchi T., et al. Statins meditate anti-atherosclerotic action in smooth muscle cells by peroxisome proliferator-activated receptor-γ activation. Biochemical and Biophysical Research Communications. 2015;457(1):23–30. doi: 10.1016/j.bbrc.2014.12.063. [DOI] [PubMed] [Google Scholar]
- 15.Rodríguez-Perea A. L., Montoya C. J., Olek S., Chougnet C. A., Velilla P. A. Statins increase the frequency of circulating CD4+FOXP3+regulatory T cells in healthy individuals. Journal of Immunology Research. 2015;2015:8. doi: 10.1155/2015/762506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Côté-Daigneault J., Mehandru S., Ungaro R., Atreja A., Colombel J. F. Potential immunomodulatory effects of statins in inflammatory bowel disease. Inflammatory Bowel Diseases. 2016;22(3):724–732. doi: 10.1097/MIB.0000000000000640. [DOI] [PubMed] [Google Scholar]
- 17.Melo L., Figueiredo V. P., Azevedo M. A., et al. Low doses of simvastatin therapy ameliorate cardiac inflammatory remodeling in Trypanosoma cruzi-infected dogs. The American Journal of Tropical Medicine and Hygiene. 2011;84(2):325–331. doi: 10.4269/ajtmh.2011.10-0451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Silva R. R., Shrestha-Bajracharya D., Almeida-Leite C. M., Leite R., Bahia M. T., Talvani A. Short-term therapy with simvastatin reduces inflammatory mediators and heart inflammation during the acute phase of experimental Chagas disease. Memórias do Instituto Oswaldo Cruz. 2012;107(4):513–521. doi: 10.1590/S0074-02762012000400012. [DOI] [PubMed] [Google Scholar]
- 19.Horii Y., Nagasawa T., Sakakibara H., et al. Hierarchy in the home cage affects behaviour and gene expression in group- housed C57BL/6 male mice. Scientific Reports. 2017;7(1):p. 6991. doi: 10.1038/s41598-017-07233-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Friedewald W. T., Levy R. I., Fredrickson D. S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clinical Chemistry. 1972;18(6):499–502. doi: 10.1093/clinchem/18.6.499. [DOI] [PubMed] [Google Scholar]
- 21.FOLCH J., LEES M., STANLEY G. H. S. L. O. A. N. E. A simple method for the isolation and purification of total lipides from animal tissues. Journal of Biological Chemistry. 1957;226(1):497–509. [PubMed] [Google Scholar]
- 22.Baker R., Dauner J. G., Rodriguez A. C., et al. Increased plasma levels of adipokines and inflammatory markers in older women with persistent HPV infection. Cytokine. 2011;53(3):282–285. doi: 10.1016/j.cyto.2010.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu S. H., Lee L. T., Hu N. Y., et al. Effects of alpha-mangostin on the expression of anti-inflammatory genes in U937 cells. Chinese Medicine. 2012;7(1):p. 19. doi: 10.1186/1749-8546-7-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bellini M. F., Silistino-Souza R., Varella-Garcia M., de Azeredo-Oliveira M. T. V., Silva A. E. Biologic and genetics aspects of chagas disease at endemic areas. Journal of Tropical Medicine. 2012;2012:11. doi: 10.1155/2012/357948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brima W., Eden D. J., Mehdi S. F., et al. The brighter (and evolutionarily older) face of the metabolic syndrome: evidence from Trypanosoma cruzi infection in CD-1 mice. Diabetes/Metabolism Research and Reviews. 2015;31(4):346–359. doi: 10.1002/dmrr.2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tanowitz H. B., Scherer P. E., Mota M. M., Figueiredo L. M. Adipose tissue: a safe haven for parasites? Trends in Parasitology. 2017;33(4):276–284. doi: 10.1016/j.pt.2016.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Taylor B. A., Ng J., Stone A., Thompson P. D., Papasavas P. K., Tishler D. S. Effects of statin therapy on weight loss and diabetes in bariatric patients. Surgery for Obesity and Related Diseases. 2017;13(4):674–680. doi: 10.1016/j.soard.2016.11.018. [DOI] [PubMed] [Google Scholar]
- 28.Campos-Estrada C., Liempi A., González-Herrera F., et al. Simvastatin and benznidazole-mediated prevention of Trypanosoma cruzi-induced endothelial activation: role of 15-epi-lipoxin A4 in the action of simvastatin. PLoS Neglected Tropical Diseases. 2015;9(5, article e0003770) doi: 10.1371/journal.pntd.0003770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leite A. L. J., Paula Costa G. ., Lopes L. R., Reis Mota L. W. ., Vieira P. M. . A., Talvani A. The immunomodulatory effects of the Enalapril in combination with Benznidazole during acute and chronic phases of the experimental infection with Trypanosoma cruzi. Acta Tropica. 2017;174:136–145. doi: 10.1016/j.actatropica.2017.07.005. [DOI] [PubMed] [Google Scholar]
- 30.Inoue M., Ohtake T., Motomura W., et al. Increased expression of PPARgamma in high fat diet-induced liver steatosis in mice. Biochemical and Biophysical Research Communications. 2005;336(1):215–222. doi: 10.1016/j.bbrc.2005.08.070. [DOI] [PubMed] [Google Scholar]
- 31.Strandberg L., Verdrengh M., Enge M., et al. Mice chronically fed high-fat diet have increased mortality and disturbed immune response in sepsis. PLoS One. 2009;4(10, article e7605) doi: 10.1371/journal.pone.0007605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Prioli R. P., Rosenberg I., Shivakumar S., Pereira M. E. A. Specific binding of human plasma high density lipoprotein (cruzin) to Trypanosoma cruzi. Molecular and Biochemical Parasitology. 1988;28(3):257–263. doi: 10.1016/0166-6851(88)90010-2. [DOI] [PubMed] [Google Scholar]
- 33.Nagajyothi F., Weiss L. M., Zhao D., et al. High fat diet modulates Trypanosoma cruzi infection associated myocarditis. PLoS Neglected Tropical Diseases. 2014;8(10, article e3118) doi: 10.1371/journal.pntd.0003118. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.


