Abstract
To establish an efficient, safe immunosuppressive regimen of adeno-associated vector (AAV)-mediated gene therapy for Duchenne muscular dystrophy (DMD), we evaluated the effect of tacrolimus (FK506) on skeletal muscle transduction with AAV8 and AAV9 vectors expressing the LacZ and microdystrophin (M3) genes labeled by FLAG. We utilized 3- to 4-year-old Macaca fascicularis, screened for neutralizing antibodies against AAV. 3 days before AAV injection and throughout the experiment, 0.06 mg/kg tacrolimus was intravenously administered. A viral suspension of 1 × 1013 viral genomes/muscle was intramuscularly injected bilaterally at the tibialis anterior and biceps brachii muscles, which were biopsied at 8, 16, 24, and 42 weeks after injection. Without tacrolimus, AAV8- and AAV9-mediated LacZ expression disappeared 8 and 16 weeks after transduction, respectively. With tacrolimus, AAV8/9-mediated LacZ expression persisted for at least 42 weeks after injection. At 42 weeks after AAV8CMVLacZ and AAV9CMVLacZ injection, nearly 50% and 17% of muscle fibers were positive for β-galactosidase, respectively. AAV8/9-mediated M3-FLAG expression lasted for up to 42 weeks using tacrolimus. No significant generalized toxicity was observed in any monkey. These results indicate that tacrolimus administration regulated the immune response to transgenes and truncated microdystrophin in normal primates and may enhance the benefits of AAV-mediated gene therapy for DMD.
Keywords: tacrolimus, muscular dystrophy, recombinant adeno-associated virus, gene therapy, microdystrophin
Graphical Abstract
It is very important to establish an efficient, safe immunosuppressive regimen of AAV-mediated gene therapy for muscular dystrophy. Tacrolimus administration regulated the immune response to transgenes and truncated microdystrophin in normal primates and may enhance the benefits of AAV-mediated gene therapy.
Introduction
Duchenne muscular dystrophy (DMD) is a hereditary disease that causes severe clinical muscle weakness, atrophy, degeneration, and necrosis of the skeletal muscle.1 No fundamental treatments have been developed, and thus an effective gene therapy is needed. We previously developed a recombinant adeno-associated virus (rAAV) capable of efficiently transferring genes to the skeletal muscle and examined its therapeutic potential using a miniaturized microdystrophin gene.2, 3, 4, 5 This vector is considered safe because it does not induce severe inflammation as observed with adenovirus6 and is not carcinogenic as in the case of lentivirus. In previous studies using mdx2, 3, 4 mice as a DMD model animal, good treatment results were observed following gene transfer into the skeletal and cardiac muscle. Additionally, local or systemic expression was achieved in dog5 models of muscular dystrophy, demonstrating the effectiveness of microdystrophin.1 However, the emergence of circulating dystrophin-specific T cells has been reported in patients with DMD following AAV-mediated gene therapy.7 Therefore, immunomodulation may be required to ensure successful gene therapy. Immunosuppression experiments should be performed in nonhuman primates whose immune systems are similar to that in humans. Therefore, we used Macaca fascicularis for this experiment.
When using viral vectors for gene therapy, immunomodulation may be an option if the case is diagnosed after birth or in adult patients, as is usual in muscular dystrophy. Gene transduction studies of muscular dystrophy models using immunosuppressive drugs such as cyclosporine, mycophenolate mofetil, anti-thymocyte globulin, and rituximab have been reported.8 Cramer et al.9 reported that prednisone reduced the extent of intramuscular T cell infiltrates in AAV-treated muscles in rhesus macaque, but the effects were insufficient. Thus, we used tacrolimus, also known as FK-506, which is an immunosuppressant discovered in Streptomyces tsukubaensis from the soil at Tsukuba Mountain and used mainly after allergenic organ transplant10 and to treat autoimmune diseases.11 The aim of this study was to establish an efficient and safe immunosuppressive regimen of AAV-mediated gene therapy for DMD. We evaluated the transduction of skeletal muscles of a primate model with AAV8 and AAV9 vectors expressing the LacZ and microdystrophin (M3)12 genes concurrently with tacrolimus treatment.
Results
Primate Colony Showed a High Prevalence of Antibodies against AAV
We estimated AAV-reacting antibodies by a cell-based assay13 using AAV2, AAV8, and AAV9 in a closed colony of 3- to 4-year-old M. fascicularis. 25 monkeys were examined. The neutralizing antibodies in the monkeys used in this study are summarized in Tables S1 and S2.
When an anti-AAV8 seropositive monkey was injected with AAV8CMVLacZ, no LacZ-positive fibers were observed at 4 weeks after inoculation (data not shown).
Short and Limited Expression after rAAV Transduction without Immunomodulation
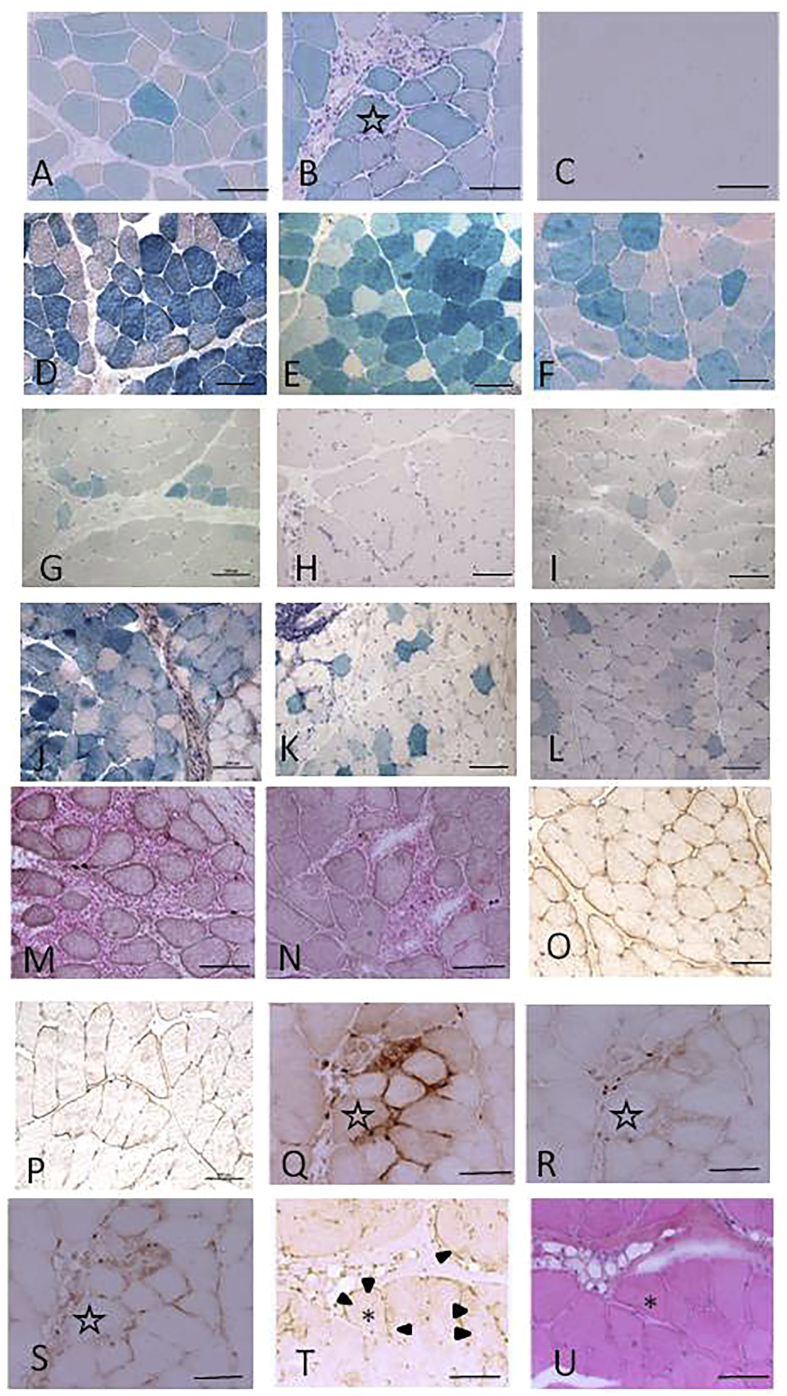
In the histochemical examination after muscle transduction with AAV8CMVLacZ, expression was confirmed after 4 weeks with small amounts of inflammatory cell infiltration, but it eventually disappeared by 8 weeks after transduction (Figures 1A–1C; Table 1; Table S1, No. 1). For muscles transduced with AAV9CMVLacZ, expression was observed 16 weeks after transduction (Figures 1G–1I; Table 1; Table S1, No. 3); however, the transduction efficiency was lower compared to that of AAV8CMVLacZ. AAV8CMVM3FLAG immunostaining-positive results were observed for up to 24 weeks, although cell infiltration was also detected in the section from 24 weeks after transduction and increased over time (Figures 1M and 1N; Table S1, No. 5). Infiltrated cells were identified as mostly CD68-positive macrophages, but CD4- and CD8-positive cells were also detected (Figures 1Q–1S; Table S1, No. 1).
Figure 1.
Improved rAAV-Mediated Gene Expression with Tacrolimus Treatment
(A–C) β-galactosidase staining after AAV8CMVLacZ injection without tacrolimus treatment (A, 2 weeks; B, 4 weeks; C, 8 weeks). (D–F) β-galactosidase staining after AAV8CMVLacZ injection with tacrolimus treatment (D, 8 weeks; E, 24 weeks; F, 42 weeks). AAV8CMVLacZ expression persisted for 16 weeks after transduction. Following tacrolimus co-treatment, AAV8CMVLacZ expression remained detectable for 42 weeks after transduction, and cell infiltration was observed. (G–I) β-galactosidase staining after AAV9CMVLacZ injection without tacrolimus treatment (G, 2 weeks; H, 8 weeks; I, 16 weeks). (J–L) β-galactosidase staining after AAV9CMVLacZ injection with tacrolimus treatment (J, 8 weeks; K, 24 weeks; L, 42 weeks). (M and N) FLAG staining after AAV8CMVM3FLAG injection without tacrolimus treatment (M, 16 weeks; N, 24 weeks). (O and P) FLAG staining after AAV8CMVM3FLAG injection with tacrolimus treatment (O, 24 weeks; P, 42 weeks). (Q–S) Immunostaining of inflammatory cells in serial section of B 4 weeks after AAV8CMVLacZ injection without tacrolimus treatment (Q, CD68 staining; R, CD4 staining; S, CD8 staining). (T and U) FLAG (T) and hematoxylin and eosin (U) staining 42 weeks after AAV9CMVM3FLAG injection with tacrolimus treatment. AAV9CMVM3FLAG expression remained detectable for 42 weeks after transduction. Cell infiltration was observed but to a lesser degree than that observed with the AAV8 vector. The same muscle fiber is indicated by an asterisk (∗) or star (☆). FLAG staining is marked with arrowheads. Scale bar, 100 μm. AAV, adeno associated virus; CMV, human cytomegalovirus immediate early enhancer and promoter; M3, microdystrophin; CD, cluster of differentiation.
Table 1.
Comparison of β-Galactosidase Expression after AAV Injection
| Animal (Vector) | Tacrolimus | 2 Weeks | 4 Weeks | 8 Weeks | 16 Weeks | 24 Weeks | 42 Weeks |
|---|---|---|---|---|---|---|---|
| No. 1 (AAV8CMVLacZ) | − | 82.2 ± 5.2 | 38.3 ± 3.6 | 0 | 0 | ND | ND |
| No. 2 (AAV8CMVLacZ) | + | ND | ND | 73.8 ± 2.8 | 45.3 ± 4.3 | 51.0 ± 11.6 | 54.5 ± 10.5 |
| No. 3 (AAV9CMVLacZ) | − | 18.6 ± 0.02 | 14.3 ± 0.02 | 0 | 0.2 ± 0.2 | ND | ND |
| No. 4 (AAV9CMVLacZ) | + | ND | ND | 39.1 ± 13.5 | 8.0 ± 3.2 | 8.4 ± 4.8 | 17.3 ± 9.2 |
Values represent percentage (%) of positive fibers ± SD. ND, no data.
Improved rAAV Expression with Immunomodulation
When transduction and tacrolimus treatment were performed simultaneously, AAV8CMVLacZ expression was observed for up to 42 weeks after transduction, although cell infiltration was present (Figures 1D–1F; Table 1; Table S1, No. 2). AAV9CMVLacZ expression continued for at least 42 weeks after transduction along with cell infiltration (Figures 1J–1L; Table 1; Table S1, No. 4). These results indicate that AAV8 transduction was more likely to be affected by tacrolimus treatment than by AAV9. Nearly 50% of muscle fibers were β-galactosidase-positive at 42 weeks after AAV8CMVLacZ injection, while only 17% of fibers were β-galactosidase-positive for AAV9CMVLacZ at 42 weeks (Table 1). When transduction was conducted with tacrolimus treatment, AAV8CMVM3FLAG expression remained visible for 42 weeks after transduction (Figures 1O and 1P; Table S1, No. 6). Cell infiltration was detected in the tissue, but to a lesser degree than that observed with the LacZ-containing vector. AAV9CMVM3FLAG immunostaining was detectable for up to 24 weeks with mononuclear cell infiltration without tacrolimus treatment (data not shown, Table S1, No. 7). When AAV9CMVM3FLAG was used together with tacrolimus treatment, expression was detected at 42 weeks after transduction (Figures 1T and 1U, Table S1, No. 8). Cell infiltration was even more limited compared to that observed with the AAV8 vector. CD4-, CD8-, and CD68-positive cells infiltrated the endomysium of AAV-injected muscles (data not shown).
Safety Evaluation
No adverse effects were observed for any treatments. The monkey injected with AAV8CMVLacZ showed a low degree of leukopenia; however, values remained within normal ranges. No laboratory abnormality or morbidity was identified in any of the monkeys during the observation period (Table S1).
Monitoring of Tacrolimus Concentrations
We found a close correlation between the levels of tacrolimus measured in the blood and spleen, and a wider difference when comparing the levels in the blood and liver. These results were expected because tacrolimus accumulates in red blood cells (Figure S1).
Changes in Immune Response Were Observed following Tacrolimus Treatment
When transduction was performed without tacrolimus treatment, immunoglobulin M (IgM) against LacZ was observed at 4 weeks after AAV9CMVLacZ injection. In contrast, no IgM for LacZ was produced after AAV9CMVLacZ was injected with tacrolimus (Figure S2).
Discussion
In this study, we found that tacrolimus administration regulated the immune response against LacZ and microdystrophin genes in a normal primate model, particularly with AAV8 administration. This may be because AAV8 was more immunogenic than AAV9 in this model, and thus the immunosuppressive effect of the agent was more easily detectable following AAV8 administration. This result also suggested that different immunosuppressive drugs may be required for each AAV serotype.
Chamberlain14 demonstrated that most fibers must accumulate at least approximately 20% of wild-type levels of dystrophin protein to significantly correct the pathology. Even if the expression efficiency of LacZ is maintained at over 50% after 42 weeks using tacrolimus, we did not confirm the expression level of transduced protein in this study. Therefore, to determine whether this expression level is sufficient for treating patients with muscular dystrophy, it is necessary to confirm the amount of dystrophin protein that can be produced using this approach.
The expression rate of AAV9-mediated LacZ was decreased at 16 weeks but recovered at 24 weeks. This may be because sustained LacZ expression and accumulation of β-galactosidase protein make detection easier in later stages, this will require further analysis for confirmation.
In this study, the most important factor identified to affect the gene transduction efficiency was the presence of neutralizing antibodies against AAV.12 To test the AAV8 and AAV9 vectors expressing the LacZ and microdystrophin (M3) genes and the efficiency of transduction of the primate skeletal muscles with tacrolimus treatment, we first screened the primates for the presence of antibodies against AAV. The prevalence of antibody-positive monkeys was higher than expected (64%). It has been reported that most healthy adults have neutralizing antibodies against AAV.15 Thus, the immune system status of our model was similar to that in the human population and is useful for predicting immune responses in humans. Greig et al.16 reported that a low titer of pre-existing AAV8 neutralizing antibodies did not affect the expression levels of a secreted transgene after intramuscular injection of the vector in R. macaques. We used M. fascicularis, and some studies reported that the immune response of this species differs from that of R. macaques.16 In human immunodeficiency virus (HIV), the response differs depending on the habitat of R. macaques.17 Therefore, the species of monkey used is very important for gene transfer experiments using viral vectors.
We also found that the products derived from the transduced gene were eliminated by cellular immunity, as evidenced by the CD4-, CD8-, and CD68-positive cells observed to have infiltrated the endomysium of AAV-injected muscles. Thus, immunosuppression targeting both B and T cells may be required for AAV gene therapy. Tacrolimus functions by blocking T cell proliferation in vitro by inhibiting the generation of several lymphokines. Crosstalk between T cells and macrophages is involved in the muscle pathology of DMD and may also be important in AAV-mediated gene transduction.18 Analysis of viral levels indicated that a certain amount of the viral genome is required for antibody production. Administration of an immunosuppressive agent may reduce the amount of virus necessary to elicit a therapeutic effect, and consequently the production of antibodies may be prevented. In this study, a ubiquitous promoter was used. The development of an effective muscle-specific promoter may also reduce the immune response and amount of viral vector required. This would also help to reduce the very high titers of AAV necessary for its systemic application, which is among the rate-limiting steps in gene therapy.
As described above, for patients with DMD treated with AAV-mediated gene therapy, T cells reactive against dystrophin are a known complication.7 In our study, IgM against LacZ was produced 4 weeks after AAV9CMVLacZ injection without tacrolimus. Production of antibodies against transduced proteins inhibits long-term gene expression, as well as expression after a second transduction. In this study, we showed that tacrolimus inhibited the immune response against the transduced gene product.
No significant generalized toxicity was observed in any of the tested monkeys. Side effects such as constant anorexia, continued weight loss, liver dysfunction, and hyperkalemia were not observed in any of the treated monkeys after tacrolimus administration, demonstrating that tacrolimus is a safe option as an immunosuppressive drug for gene therapy. Tacrolimus can also be easily monitored by determining its concentration in the blood. Another advantage is that tacrolimus can be delivered orally for human patients. Furthermore, tacrolimus is an easy-to-use immunosuppressant because there is no restriction for co-ingestion with substances such as cyclosporine, and the drug does not influence cell growth as everolimus and mycophenolate mofetil do. Additionally, because tacrolimus binds to erythrocytes, its concentration in the spleen can be evaluated.
We used normal monkeys in this experiment, as studies of the immune response in normal monkeys are important for advancing gene therapy. No natural DMD monkey model is available; however, a monkey DMD model using CRISPR/Cas9 has been reported.19 For future studies of gene therapy, the development of disease models in primates will eventually become necessary. It may be possible to produce these models by embryonic gene transfer20 or gene editing.
In conclusion, a tacrolimus-assisted transduction strategy can enhance the therapeutic benefits of rAAV-mediated gene therapy for muscular dystrophy in a safe and effective way.
Materials and Methods
Animals
All monkeys were treated in accordance with the rules for care and management of the Tsukuba Primate Research Center under the guiding Principles for Animal Experiments using Non-Human Primates formulated and enforced by the Primate Society of Japan. All experimental procedures were approved by the Animal Welfare and Animal Care Committee of the National Institute of Biomedical Innovation. Approval numbers of Tsukuba primate center review boad (DS25-15, DS29-24) and NCNP animal experiment committee (2011-004,2014-008). The monkeys were reared in the Tsukuba Primate Research Center, National Institute of Biomedical Innovation.
We estimated the AAV-neutralizing antibody titers in the colony of 3- to 4-year-old M. fascicularis and selected seronegative monkeys. We used 4 seronegative monkeys for the experiment without immunomodulation, and 3 seronegative monkeys and 1 seropositive monkey (No. 4 positive for anti-AAV8 neutralizing antibody) for the experiment including immunomodulation analysis (Table S1).
Blood was collected at 0, 8, 16, 24, and 42 weeks after injection and laboratory data were monitored, such as white and red blood cell counts, hemoglobin, platelet count, liver function, electrolytes, creatinine kinase, and tacrolimus concentration.
Viral Vectors
We utilized AAV2CMVLacZ, AAV8CMVLacZ, AAV9CMVLacZ, AAV8CMVM3FLAG, and AAVCMV9M3FLAG, as described previously.2, 3, 4, 5,12 The rAAV proviral plasmid harboring the luciferase gene with a CAG promoter was propagated as a marker. As a therapeutic gene for DMD, canine microdystrophin was placed under control of the CMV promoter. The vector genome was packaged into the pseudotyped rAAV9 capsid in HEK293 cells. A large-scale cell culture method with an active gassing system was used for transfection.13 The vector was produced by triple transfection of a proviral plasmid, rAAV helper plasmid pAAV2/9 (a gift from Dr. James M. Wilson), and adenovirus helper plasmid, pAdeno. rAAV particles were purified by a dual ion-exchange procedure with high-performance membrane absorption as previously described.1,5 Viral titers were determined by quantitative real-time PCR using the MyiQ single-color detection system (Bio-Rad, Hercules, CA, USA) and the following primer pairs: forward primer 5′-TCGAGGAACTGAAAAACCAGAAA-3′ and reverse primer 5′-CACTTCCGTACAGGCCTAGAAGT-3′ for rAAV-CMV-microdystrophin.
Direct Transduction of rAAV into Muscles
Because β-galactosidase was dose-dependently produced at 4 weeks after transduction of AAV2CMVLacZ, we used as a dosage 1 × 1013 viral genomes. AAV8CMVLacZ, AAV9CMVLacZ, AAV8CMVM3FLAG, and AAVCMV9M3FLAG were transduced each into a different monkey. A viral suspension of 1 × 1013 viral genomes/muscle was intramuscularly injected at the bilateral tibialis anterior and biceps brachii muscles using a 1-mL syringe with a 23-gauge needle (SS-01T, TERUMO, Tokyo, Japan).
Immunomodulation
3 days before AAV injection and each day throughout the experiment, 0.06 mg/kg of tacrolimus was administered intramuscularly. The concentration of tacrolimus in the blood was confirmed to be under 20 ng/mL during the experiment.
Muscle Biopsy and Histochemical Analysis
Transduced muscles were biopsied at 8, 16, and 24 weeks after injection. Biopsy was performed under anesthesia using ketamine and pentobarbital. Electrocardiogram, oxygen saturation, and body temperature were monitored. The individual muscle was divided into several pieces and immediately frozen in liquid nitrogen-cooled isopentane (166-00615, WAKO, Osaka, Japan). Six to eight blocks were sampled from the transduced muscle. We analyzed at least 30 sections from the blocks to observe the general trends.
At 42 weeks after injection, the monkeys were sacrificed for systemic evaluation and biopsy. Transverse cryosections (10 μm) from the rAAV-LacZ-injected muscles were stained with hematoxylin and eosin or 5-bromo-4-chloro-3-indolyl-β-D-galactopyranoside (029-07853, WAKO). Anti-FLAG, CD4, and CD8 (CBL131, Chemicon International, Temecula, CA, USA) immunostaining was also performed.
IgG and IgM Detection by Enzyme-Linked Immunosorbent Assay in Monkey Serum
A microtiter plate (MS-8596F, Sumitomo Bakelite, Tokyo, Japan) was precoated with promoter-deleted rAAV2, rAAV8, or rAAV9 (start 2 × 109 genomes/well and diluted serially) and blocked with blocking buffer (Block Ace, DS Pharma Biomedical, Osaka, Japan) overnight. The plate was incubated for 2 h at 25°C with sera from monkeys, followed by a 1:2,000 dilution of peroxidase-conjugated rabbit anti-monkey IgG or IgM (SAB3700764/SAB3700778, Sigma-Aldrich, St. Louis, MO, USA) for 1 h. Color was visualized using a peroxidase substrate system (TMBZ, ML-1120T, Sumitomo Bakelite). Reactivity was detected at a wavelength of 450 nm with a reference at 630 nm using the microplate reader model 680 (Bio-Rad).
Neutralizing Antibody Detection in Monkey Serum
HEK293 cells (1 × 105) were plated with sequentially diluted monkey serum in 96-well microtiter plates (MS-8596F, Sumitomo Bakelite).13 The plate was incubated for 72 h with rAAV2CMVLacZ, rAAV8CMVLacZ, or rAAV9CMVLacZ (2 × 109 genomes/well) at 37°C, 5% CO2, followed by β-galactosidase staining.
Author Contributions
Conceptualization, A.I., H.O., H.H.-K., and J.-H.S.; Methodology, A.I., H.O., H.H.-K., T.O., and S.T.; Investigation, A.I., H.O., H.H.-K., and J.-H.S.; Writing – Original Draft, A.I. and H.H.-K.; Writing – Review & Editing, A.I. and H.H.-K.; Funding Acquisition, A.I., A.T., T.O., and S.T.; Resources, A.I., H.O., H.H.-K., and J.-H.S.; Supervision, A.T., T.O., and S.T. All other authors have contributed to data collection and interpretation, and critically reviewed the manuscript. All authors approved the final version of the manuscript and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Conflicts of Interest
The authors declare no competing interests.
Acknowledgments
We are extremely grateful to Dr. Tadashi Sankai, Dr. Yumi Katakai, and Dr. Fumiko Ono at the Tsukuba Primate Research Center, National Institute of Biomedical Innovation for animal care. We would also like to thank Ms. Kinoshita, Dr. T. Chiyo, and Dr. A. Nishiyama for assistance with AAV production and purification. We would like to thank Dr. Posadas-Herrera Guillermo for English language editing. This research was supported by JSPS KAKENHI (grant number JP16K07081). This study was partly supported by Neurological and Psychiatric Disorders of the National Center of Neurology and Psychiatry intramural research (grant number 25-5 to S.T.), Neurological and Psychiatric Disorders of the National Center of Neurology and Psychiatry intramural research (grant number 28-6 to S.T.), and Japan Agency for Medical Research and Development (JP; grant number 16ek0109154h0002 to S.T.).
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.omtm.2020.05.012.
Contributor Information
Akiko Ishii, Email: a_ishii@md.tsukuba.ac.jp.
Takashi Okada, Email: t-okada@ims.u-tokyo.ac.jp.
Supplemental Information
References
- 1.Okada T., Takeda S. Current challenges and future directions in Recombinant AAV-mediated gene therapy of Duchenne muscular dystrophy. Pharmaceuticals (Basel) 2013;6:813–836. doi: 10.3390/ph6070813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yoshimura M., Sakamoto M., Ikemoto M., Mochizuki Y., Yuasa K., Miyagoe-Suzuki Y., Takeda S. AAV vector-mediated microdystrophin expression in a relatively small percentage of mdx myofibers improved the mdx phenotype. Mol. Ther. 2004;10:821–828. doi: 10.1016/j.ymthe.2004.07.025. [DOI] [PubMed] [Google Scholar]
- 3.Yuasa K., Sakamoto M., Miyagoe-Suzuki Y., Tanouchi A., Yamamoto H., Li J., Chamberlain J.S., Xiao X., Takeda S. Adeno-associated virus vector-mediated gene transfer into dystrophin-deficient skeletal muscles evokes enhanced immune response against the transgene product. Gene Ther. 2002;9:1576–1588. doi: 10.1038/sj.gt.3301829. [DOI] [PubMed] [Google Scholar]
- 4.Shin J.H., Nitahara-Kasahara Y., Hayashita-Kinoh H., Ohshima-Hosoyama S., Kinoshita K., Chiyo T., Okada H., Okada T., Takeda S. Improvement of cardiac fibrosis in dystrophic mice by rAAV9-mediated microdystrophin transduction. Gene Ther. 2011;18:910–919. doi: 10.1038/gt.2011.36. [DOI] [PubMed] [Google Scholar]
- 5.Ohshima S., Shin J.H., Yuasa K., Nishiyama A., Kira J., Okada T., Takeda S. Transduction efficiency and immune response associated with the administration of AAV8 vector into dog skeletal muscle. Mol. Ther. 2009;17:73–80. doi: 10.1038/mt.2008.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ishii A., Hagiwara Y., Saito Y., Yamamoto K., Yuasa K., Sato Y., Arahata K., Shoji S., Nonaka I., Saito I. Effective adenovirus-mediated gene expression in adult murine skeletal muscle. Muscle Nerve. 1999;22:592–599. doi: 10.1002/(sici)1097-4598(199905)22:5<592::aid-mus7>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 7.Mendell J.R., Campbell K., Rodino-Klapac L., Sahenk Z., Shilling C., Lewis S., Bowles D., Gray S., Li C., Galloway G. Dystrophin immunity in Duchenne’s muscular dystrophy. N. Engl. J. Med. 2010;363:1429–1437. doi: 10.1056/NEJMoa1000228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang Z., Kuhr C.S., Allen J.M., Blankinship M., Gregorevic P., Chamberlain J.S., Tapscott S.J., Storb R. Sustained AAV-mediated dystrophin expression in a canine model of Duchenne muscular dystrophy with a brief course of immunosuppression. Mol. Ther. 2007;15:1160–1166. doi: 10.1038/sj.mt.6300161. [DOI] [PubMed] [Google Scholar]
- 9.Cramer M.L., Shao G., Rodino-Klapac L.R., Chicoine L.G., Martin P.T. Induction of T-cell infiltration and programmed death ligand 2 expression by adeno-associated virus in rhesus macaque skeletal muscle and modulation by prednisone. Hum. Gene Ther. 2017;28:493–509. doi: 10.1089/hum.2016.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tanaka H., Kuroda A., Marusawa H., Hashimoto M., Hatanaka H., Kino T., Goto T., Okuhara M. Physicochemical properties of FK-506, a novel immunosuppressant isolated from Streptomyces tsukubaensis. Transplant. Proc. 1987;19(5, Suppl 6):11–16. [PubMed] [Google Scholar]
- 11.Sieb J.P. Myasthenia gravis: an update for the clinician. Clin. Exp. Immunol. 2014;175:408–418. doi: 10.1111/cei.12217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yamamoto K., Yuasa K., Miyagoe Y., Hosaka Y., Tsukita K., Yamamoto H., Nabeshima Y.I., Takeda S. Immune response to adenovirus-delivered antigens upregulates utrophin and results in mitigation of muscle pathology in mdx mice. Hum. Gene Ther. 2000;11:669–680. doi: 10.1089/10430340050015572. [DOI] [PubMed] [Google Scholar]
- 13.Ito T., Yamamoto S., Hayashi T., Kodera M., Mizukami H., Ozawa K., Muramatsu S. A convenient enzyme-linked immunosorbent assay for rapid screening of anti-adeno-associated virus neutralizing antibodies. Ann. Clin. Biochem. 2009;46:508–510. doi: 10.1258/acb.2009.009077. [DOI] [PubMed] [Google Scholar]
- 14.Chamberlin J.S. Dystrophin levels required for genetic correction of Duchenne muscular dystrophy. Basic Appl. Myol. 1997;7 257–255. [Google Scholar]
- 15.Blacklow N.R., Hoggan M.D., Sereno M.S., Brandt C.D., Kim H.W., Parrott R.H., Chanock R.M. A seroepidemiologic study of adenovirus-associated virus infection in infants and children. Am. J. Epidemiol. 1971;94:359–366. doi: 10.1093/oxfordjournals.aje.a121331. [DOI] [PubMed] [Google Scholar]
- 16.Greig J.A., Calcedo R., Grant R.L., Peng H., Medina-Jaszek C.A., Ahonkhai O., Qin Q., Roy S., Tretiakova A.P., Wilson J.M. Intramuscular administration of AAV overcomes pre-existing neutralizing antibodies in rhesus macaques. Vaccine. 2016;34:6323–6329. doi: 10.1016/j.vaccine.2016.10.053. [DOI] [PubMed] [Google Scholar]
- 17.Kono K., Song H., Shingai Y., Shioda T., Nakayama E.E. Comparison of anti-viral activity of rhesus monkey and cynomolgus monkey TRIM5alphas against human immunodeficiency virus type 2 infection. Virology. 2008;373:447–456. doi: 10.1016/j.virol.2007.12.022. [DOI] [PubMed] [Google Scholar]
- 18.Boisgerault F., Mingozzi F. The skeletal muscle environment and its role in immunity and tolerance to AAV vector-mediated gene transfer. Curr. Gene Ther. 2015;15:381–394. doi: 10.2174/1566523215666150630121750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen Y., Zheng Y., Kang Y., Yang W., Niu Y., Guo X., Tu Z., Si C., Wang H., Xing R. Functional disruption of the dystrophin gene in rhesus monkey using CRISPR/Cas9. Hum. Mol. Genet. 2015;24:3764–3774. doi: 10.1093/hmg/ddv120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ishibashi H., Motohashi H.H., Kumon M., Yamamoto K., Okada H., Okada T., Seki K. Efficient embryo transfer in the common marmoset monkey (Callithrix jacchus) with a reduced transfer volume: a non-surgical approach with cryopreserved late-stage embryos. Biol. Reprod. 2013;88:115. doi: 10.1095/biolreprod.113.109165. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.