Abstract
Background
In this study, we aimed to present our experience with interventional bronchoscopy in the treatment of endobronchial lesions in our clinic.
Methods
Between January 2010 and December 2018, a total of 18 patients (11 males, 7 females; mean age 55.1 years; range, 17 to 82 years) who were diagnosed with an endobronchial lesion using bronchoscopy in our clinic were retrospectively analyzed. Demographic characteristics, presenting symptoms, bronchoscopic procedure, location of the lesion, pathological diagnosis, treatment approaches, success of the bronchoscopic treatment, and follow-up outcomes of the patients were evaluated.
Results
Control bronchoscopy was performed in 14 patients and a second control bronchoscopy was performed in eight patients. The lesions were located in the right bronchial system in nine (50%), in the left bronchial system in six (33%), and in the trachea in three patients (17%). Except for one pregnant patient, all interventional procedures were performed with a rigid bronchoscope under general anesthesia. Distal areas which were unable to be reached with the rigid bronchoscope were evaluated by a flexible bronchoscope. There were no complications in any of the patients. At the end of the study, the final control biopsies of all patients were found to be normal. The success rate of interventional bronchoscopic methods was 100%.
Conclusion
Interventional bronchoscopic methods are the most effective procedures in the diagnosis and treatment of bronchial lesions with a high success rate. Based on our study findings, we suggest that bronchoscopic methods should be preferred as the first-line treatment of benign and selected some malignant endobronchial lesions.
Introduction
Primary tracheobronchial tree tumors can be either malignant or benign. For lung carcinomas, treatment process includes surgery, chemotherapy, and radiotherapy, if necessary. However, pure endobronchial neoplasms are rare and their treatment methods are still controversial.[1] The malignant forms are as follows: adenocarcinoma, squamous cell carcinoma, carcinoid tumor, mucoepidermoid carcinoma, and adenoid cystic carcinoma. Benign forms include hamartomas, leiomyoma, lipoma, squamous cell papilloma, pleomorphic adenoma, granular cell tumors, hemangiomas, fibroma, neurogenic tumors, or inflammatory myofibroblastic tumor.[2] Benign endobronchial tumors are rare primary tumors of the tracheobronchial system. These are about 1 to 10% of bronchopulmonary neoplasms. They usually settle toward the lumen through the trachea and the main airways and may cause obstruction. This may result in life-threatening complications such as respiratory insufficiency, bleeding, and infection.[3] While the most common lesions are bronchial adenomas and hamartomas, other benign lesions include leiomyoma, hemangioma, lipoma, papilloma, chondroma, fibroma, endometriosis, teratoma, and pseudolymphoma. It is recommended to use bronchoscopic resection for the diagnosis, palliative care, and treatment of both benign and malignant endobronchial tumors.[4] In benign endobronchial tumors, malignancy should first be excluded and treated before the development of distal airway diseases (obstruction, pneumonia). Endoscopic methods particularly in benign and suitable malignant neoplasms are superior to surgical methods in terms of protecting the lung parenchyma.[5]
The literature on this topic is lacking in terms of adequate information about the treatment of endobronchial lesions. The majority of data are drawn from case reports or experiences from small series. This situation constitutes considerable confusion in the approach to treatment. In this study, we aimed to present our experience with interventional bronchoscopy in our clinic and to identify the characteristics and findings of endobronchial lesions treated with these methods.
Patients and Methods
In this single-center study, we retrospectively evaluated all patients who were diagnosed with an endobronchial lesion at Gaziantep University Hospital, Department of Thoracic Surgery between January 2010 and December 2018. We only included the cases in which the endobronchial lesion was thoroughly assessed with bronchoscopy and was not found to be related with histopathologically another tumor. Those with endobronchial metastasis due to solid tumors were excluded from the study. Finally, a total of 18 patients (11 males, 7 females; mean age 55.1 years; range, 17 to 82 years) were included. A written informed consent was obtained from each patient. The study protocol was approved by the Gaziantep University Faculty of Medicine Ethics Committee. The study was conducted in accordance with the principles of the Declaration of Helsinki.
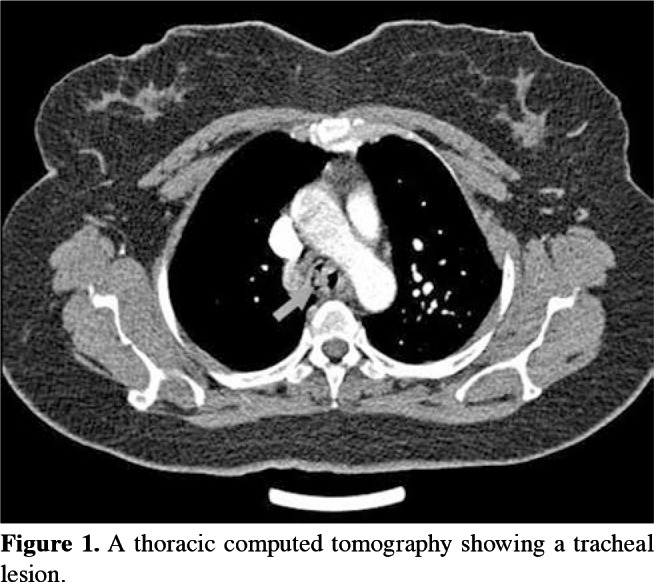
Demographic characteristics, initial complaints, the bronchoscopic procedure and number of interventions, localization, type of lesion, pathological diagnosis, treatment approach, success of the bronchoscopic treatment, and follow-up period of all patients were recorded. Preoperative examinations included complete blood count, biochemistry work-up, pulmonary function tests, electrocardiogram, and imaging studies consisting of direct X-ray of the lung, and cervical and thoracic computed tomography (Figure 1). Interventional procedures were performed with a rigid bronchoscope under general anesthesia. Only a 27-week pregnant woman received deep sedation. However, a flexible bronchoscope was used for distal areas which could not be accessed with the rigid bronchoscope, where necessary. There were no complications during and after any of the bronchoscopic procedures.
Figure 1. A thoracic computed tomography showing a tracheal lesion.
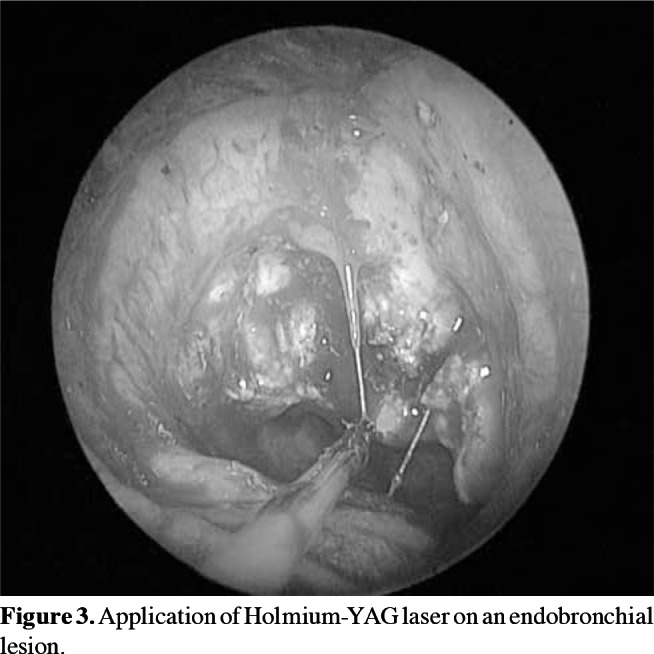
In the bronchoscopic treatment of endobronchial lesions, methods such as mechanical debridement by forceps, laser, and cautery were used and total excision was performed (Figure 2). Laser cautery was performed with a Holmium-YAG laser system (StoneLight, American Medical Systems, San Jose, California, USA) (Figure 3). After the initial treatment, further excisions were performed on suspected sites with control bronchoscopy (scheduled three weeks after the initial intervention) via laser or cautery, and biopsies were obtained, when required. Control pathological study was performed in suspicious cases.
Figure 2. Application of bipolar cautery on an endobronchial lesion.
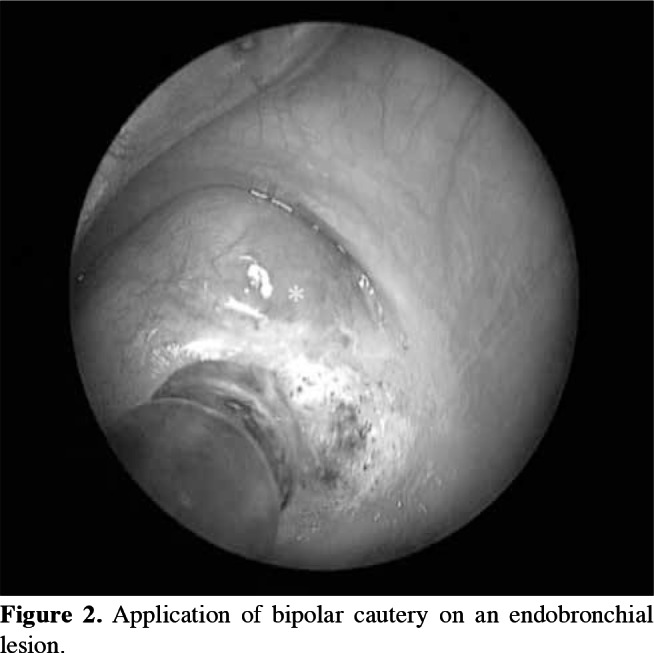
Figure 3. Application of Holmium-YAG laser on an endobronchial lesion.
Statistical analysis
Statistical analysis was performed using IBM SPSS Statistics version 23.0 software (IBM Corp., Armonk, NY, USA). Descriptive data were expressed in mean ± standard deviation (SD), median (min-max) or number and frequency.
Results
Presenting symptoms were hemoptysis (n=2), cough (n=5), productive cough (n=3), and dyspnea (n=13). The lesions were located in the right bronchial system in nine (50%), in the left bronchial system in six (33%), and in the trachea in three cases (17%). Six lesions were diagnosed as hamartomas, four as carcinoid tumors, one as a Ewing sarcoma, one as focal squamous metaplasia (FSM), one as a lipoma, one as a schwannoma, two as a benign bronchial polyp, one as an intraepithelial carcinoma, and one as a mucoepidermoid carcinoma upon pathological examination. Demographic and clinical characteristics of the patients are shown in Table 1.
Table 1. Demographic and clinical characteristics of patients.
| No | Age/ | Initial | Tumor type | Location | Resection type | Control (repeat) | Control | Control (repeat) | Control | Follow-up | Survival |
| Gender | symptoms | Bronchoscopy 1 | Bronchoscopy 1 | Bronchoscopy 2 | Bronchoscopy 2 | periods | |||||
| 1 | 42/F | H | Carcinoid | Right intermediate bronchus |
Total with cautery and laser |
Bulging at lesion site Cauterized |
N/A | Normal | N/A | 21 | Alive |
| 2 | 82/F | D | FSM | Left main bronchus | Total with forceps and laser |
Normal | N/A | N/A | N/A | 88 | Alive |
| 3 | 76/M | D | Ewing | Right upper lobe entrance |
Total with laser | Bulging at lesion site Biopsy taken |
Ewing sarcoma/ PNET |
Bulging at lesion site Biopsy taken |
Benign | 6 | Exitus |
| 4 | 23/F | C, H | Carcinoid | Right main bron- chus entrance |
Total with laser | Bulging at lesion site Cauterized |
N/A | Normal | N/A | 53 | Alive |
| 5 | 50/M | D | Hamartoma | Right upper lobe entrance |
Total with laser | Normal | N/A | Normal | N/A | 41 | Alive |
| 6 | 77/M | D, C | Lipoma | Right middle-lower lobe carina |
Total with forceps | Normal | N/A | N/A | N/A | 24 | Alive |
| 7 | 45/F | D | Schwannoma | Left main bronchus | Total with laser | Normal | N/A | N/A | N/A | 53 | Alive |
| 8 | 77/F | PC | Hamartoma | Right upper lobe entrance |
Total with laser | N/A | N/A | N/A | N/A | 14 | Exitus |
| 9 | 24/M | PC | Carcinoid | Right upper lobe entrance |
Total with cautery and laser |
Bulging at lesion site Total excision performed with laser and forceps |
Carcinoid | Normal | Normal | 44 | Alive |
| 11 | 68/M | D | Hamartoma | Trachea | Total with cautery and forceps |
N/A | N/A | N/A | N/A | 41 | Alive |
| 12 | 74/M | D | Chondroid Hamartoma |
Left upper lobe entrance |
Total with forceps and laser |
Irregular left main bronchus wall |
Chondroid Hamartoma |
Bulging at lesion site Excised with forceps |
N/A | 75 | Alive |
| Total excision with forceps |
|||||||||||
| 13 | 17/M | S | Lipomatous | Trachea | Total with laser | Same site cauterized. | N/A | Normal | N/A | 21 | Alive |
| Hamartoma | |||||||||||
| 14 | 72/M | D, C | Polypoid lesion |
Left upper lobe entrance |
Total with laser and forceps |
N/A | N/A | N/A | N/A | 8 | Exitus |
| 10 | 68/M | D | Polypoid lesion |
Left main bronchus | Total with laser | N/A | N/A | N/A | N/A | 19 | Alive |
| 15 | 35/M | D, C | Hamartoma | Right intermediate bronchus |
Total with forceps and cautery |
Residue at initial site | N/A | N/A | N/A | 41 | Alive |
| 16 | 47/F | D, PC | Carcinoid | Trachea | Total with laser | Residue at initial site | Benign | N/A | N/A | 51 | Alive |
| Biopsy taken | |||||||||||
| 17 | 53/M | D, C | Mucoepidermoid Carcinoma |
Left lower lobe entrance |
Total with laser | No residue Biopsy taken for con- trol evaluation |
Benign | Normal | Benign | 44 | Alive |
| 18 | 63/F | D | Intraepithelial Carcinoma |
Right intermediate bronchus |
Total with forceps and laser |
Normal | Benign | Normal | - | 8 | Alive |
| Bx: Biopsy; F: Female; H: Hemoptysis; N/A: not applicable; D: Dyspnea; FSM: Focal Squamous Metaplasia; M: Male; PNET: Primitive neuroectodermal tumor; C: Cough; PC: Productive cough; S: Stridor. | |||||||||||
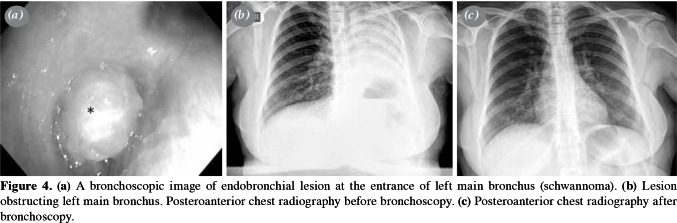
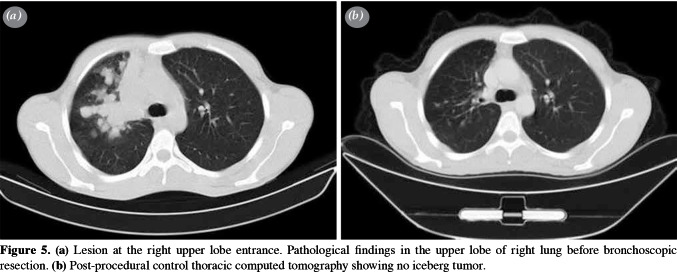
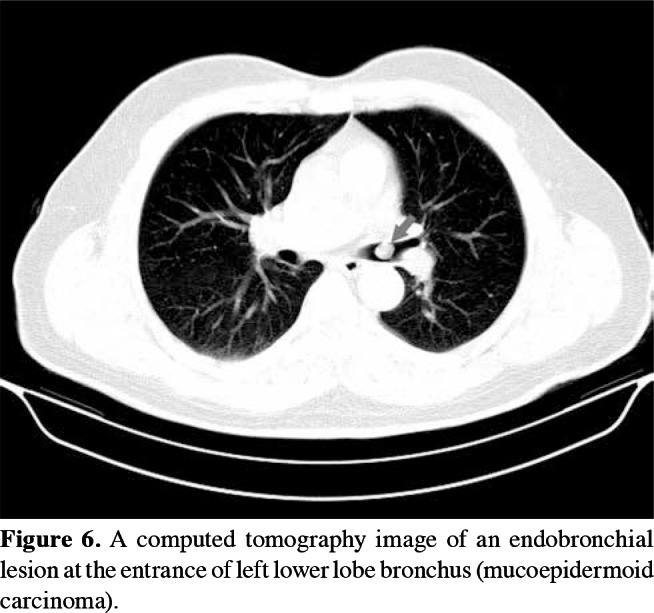
Control bronchoscopy was performed in 14 patients, and eight of them underwent a second control bronchoscopy. At the end of the study, the final control biopsies of all patients were found to be normal. No surgical intervention was required after bronchoscopic excision in any of the patients, except for two similar cases. The lesion of the first case (a 53-year-old male) was located in the left lower lobe of the lung. Total laser excision was performed, and the diagnosis was initially reported as an adenocarcinoma. In the second case (a 63-year-old female), the lesion was located in the right intermediate bronchus; excision was performed with forceps and a Holmium- YAG laser system and pathological examination result was found to be malignant. Both patients underwent bronchial sleeve resection and biopsy samples were obtained. The results of these biopsies were reported to have no evidence of disease in the resected tissue, indicating that the first bronchoscopic intervention was sufficient to treat the lesion in both cases. The single schwannoma case was also treated with laser (Figure 4a-c). Total excision of the lesion was achieved, and control bronchoscopy revealed no abnormal findings. At the end of the study, the final control biopsies of all patients were found to be normal (Figure 5a, b). Therefore, interventional bronchoscopic methods were considered successful in 18 patients (100%) cases. The pathological sample of the first patient (initially reported as an adenocarcinoma) was decided to be a mucoepidermoid carcinoma after re-consultation with another pathology department of an external institute, while the second patient was diagnosed with an intraepithelial carcinoma (Figure 6).
Figure 4. (a) A bronchoscopic image of endobronchial lesion at the entrance of left main bronchus (schwannoma). (b) Lesion obstructing left main bronchus. Posteroanterior chest radiography before bronchoscopy. (c) Posteroanterior chest radiography after bronchoscopy.
Figure 5. (a) Lesion at the right upper lobe entrance. Pathological findings in the upper lobe of right lung before bronchoscopic resection. (b) Post-procedural control thoracic computed tomography showing no iceberg tumor.
Figure 6. A computed tomography image of an endobronchial lesion at the entrance of left lower lobe bronchus (mucoepidermoid carcinoma).
There were no serious complications due to bronchoscopy in any of our patients. Three patients died during follow-up: one from comorbidities of chemotherapy, one from a cerebrovascular event, and one from gastrointestinal tract problems.
Discussion
Bronchoscopy is an invasive procedure utilized for the diagnosis and treatment of lung and trachea disorders.[6] Currently, endobronchial tumor destruction can be accomplished with several techniques via bronchoscopy including heat therapy (i.e., laser therapy, electrocautery, or argon plasma coagulation), photodynamic therapy, cryotherapy, mechanical debridement by forceps, or radiotherapy (brachytherapy).[7-11] To date, no consensus or clear recommendation has been established regarding the method for the bronchoscopic treatment of benign endobronchial tumors. There are two main reasons for this: firstly, all of these methods have been shown to have advantages and disadvantages leading to different suggestions by the authors, and, secondly, several studies have reported success with hybrid use of methods, suggesting that an optimal method has not been put forth to date.[12,13] In some clinics, such endobronchial lesions are still removed by lung or trachea or bronchial resections. However, this situation increases mortality particularly in elderly patients and patients with poor general condition. Therefore, although there is no decrease in the respiratory capacity of the patients during such endobronchial procedures, it significantly reduces mortality and morbidity.
Lung neuroendocrine tumors (NETs) are another group of pulmonary neoplasms which also include bronchial carcinoid tumors. In these tumors, surgical resection is recommended for definitive treatment such as endobronchial resection, although it has suboptimal results.[14] However, in patients with a low pulmonary reserve and those which have low-grade localized tumors, bronchoscopic resection can be utilized as an alternative in the treatment of endobronchial carcinoids.[15,16] In our study, we performed total excision by laser for two carcinoid tumor cases and total excision by laser with cautery for the other two carcinoid tumor cases. In these cases, radiological examination revealed only endobronchial presence and no iceberg tumor requiring sleeve bronchial and/or lung resection. Control bronchoscopy revealed that one patient had residue at the initial site, while the other three patients only had mild bulging due to scar tissue. Control biopsy results of one of these patients were consistent with a carcinoid and the lesion was excised with laser and forceps. The final bronchoscopy findings of all patients were normal. All patients were continued to attend follow-up visits and were found to be disease-free at 21, 41, and 49 months.
Schwannomas are very rare tumors of the intrapulmonary nerve sheath.[17] Schwannomas and neurofibromas are benign lesions accounting for 90% of adult neurogenic tumors.[18] In the literature, noninvasive methods are recommended in asymptomatic cases, and resection and laser are recommended in symptomatic cases or in patients who do not respond to conservative treatment.[19,20] In our study, the single schwannoma case was treated with laser. Total excision of the lesion was achieved, and control bronchoscopy revealed no abnormal findings.
Endobronchial polyp cases are reported to be diagnosed by bronchoscopy and treatment approach is endoscopic.[21-23] In our study, the single patient with an endobronchial polyp presented with dyspnea. Treatment was performed with laser. Total resection was achieved, and the patient was scheduled for further evaluation and control bronchoscopy; however, we were unable to reach the patient by any means and control studies were unable to be performed.
The diagnoses of the remaining six patients were as follows: FSM, Ewing sarcoma, lipoma, benign polypoid lesion, mucoepidermoid carcinoma, and intraepithelial carcinoma. The FSM case was treated via total excision with forceps and control bronchoscopy was normal. The patient with primary a tracheal Ewing sarcoma without metastasis was treated via total excision with laser and their control biopsy, taken during repeat bronchoscopy, was identified as an Ewing sarcoma/primitive NET and another control bronchoscopy was scheduled for biopsy which was normal; however, the patient died due to comorbid conditions after four months of disease-free survival. In the lipoma case, forceps was used for total excision and control bronchoscopy was normal. Forceps excision was also used in the treatment of the patient with a benign polypoid lesion and total excision was achieved; however, residual tissue was observed during control bronchoscopy. The patient died due to gastrointestinal tract disease after eight months of disease-free survival. Another patient with a mucoepidermoid carcinoma (initially diagnosed as an adenocarcinoma) was treated with total excision via laser and control bronchoscopy showed no abnormal findings; however, biopsy was obtained to confirm the results. The patient has been symptom-free for 44 months and is still attending to his regular follow-up. Finally, the patient with an intraepithelial carcinoma was treated with total excision via forceps and laser, and the initial and second control biopsy results were normal.
Our study has several limitations such as a small sample size and retrospective, single-center study design. However, most studies in this field have similar limitations due to the rareness of these cases. Of note, long-term follow-up is the main strength of our study. Nonetheless, to make more precise judgments about the characteristics of patients and to determine optimal treatment approach, further large-scale, prospectivecontrolled studies are warranted.
In conclusion, despite the low incidence of benign endobronchial tumors, early diagnosis and treatment are of utmost importance due to the possibility of serious complications. Our study results suggest that bronchoscopic procedures play an important role in the evaluation and treatment of endobronchial lesions, although repeat procedures may be required, with a success rate of 100% and without any complications in patients with miscellaneous diagnoses. Nevertheless, due to the fact that most studies have small sample sizes, it is difficult to determine the impact of bronchoscopic techniques on survival. It is rather clear that more researches are needed to address the characteristics of interventional approaches and to compare their results.
Footnotes
Conflict of Interest: The authors declared no conflicts of interest with respect to the authorship and/or publication of this article.
Financial Disclosure: The authors received no financial support for the research and/or authorship of this article.
References
- 1.Gasparini S, Bonifazi M. Management of endobronchial tumors. Curr Opin Pulm Med. 2016;22:245–251. doi: 10.1097/MCP.0000000000000259. [DOI] [PubMed] [Google Scholar]
- 2.Weerakkody Y. Tracheal and endobronchial lesions. Available at: https://radiopaedia.org/articles/tracheal-andendobronchial-lesions [Accessed: October, 2018]. [Google Scholar]
- 3.Comert SS. Benign endobronchial tumors. In: Dalar L, Yılmaz A, editors. Diagnostic and Therapeutic Bronchoscopy Reconciliation Report. İstanbul: TÜSAD Eğitim Kitapları Serisi; 2017. pp. 159–169. [Google Scholar]
- 4.Madan K, Agarwal R, Bal A, Gupta D. Bronchoscopic management of a rare benign endobronchial tumor. Rev Port Pneumol. 2012;18:251–254. doi: 10.1016/j.rppneu.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 5.Moorjani N, Beeson JE, Evans JM, Maiwand MO. Cryosurgery for the treatment of benign tracheo-bronchial lesions. Interact Cardiovasc Thorac Surg. 2004;3:547–550. doi: 10.1016/j.icvts.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 6.Ernst A, Silvestri GA, Johnstone D, American College of Chest Physicians Interventional pulmonary procedures: Guidelines from the American College of Chest Physicians. Chest. 2003;123:1693–1717. doi: 10.1378/chest.123.5.1693. [DOI] [PubMed] [Google Scholar]
- 7.Coulter TD, Mehta AC. The heat is on: impact of endobronchial electrosurgery on the need for Nd-YAG laser photoresection. Chest. 2000;118:516–521. doi: 10.1378/chest.118.2.516. [DOI] [PubMed] [Google Scholar]
- 8.Morice RC, Ece T, Ece F, Keus L. Endobronchial argon plasma coagulation for treatment of hemoptysis and neoplastic airway obstruction. Chest. 2001;119:781–787. doi: 10.1378/chest.119.3.781. [DOI] [PubMed] [Google Scholar]
- 9.Cavaliere S, Venuta F, Foccoli P, Toninelli C, La Face B. Endoscopic treatment of malignant airway obstructions in 2,008 patients. Chest. 1996;110:1536–1542. doi: 10.1378/chest.110.6.1536. [DOI] [PubMed] [Google Scholar]
- 10.Diaz-Jiménez JP, Martínez-Ballarín JE, Llunell A, Farrero E, Rodríguez A, Castro MJ. Efficacy and safety of photodynamic therapy versus Nd-YAG laser resection in NSCLC with airway obstruction. Eur Respir J. 1999;14:800–805. doi: 10.1034/j.1399-3003.1999.14d13.x. [DOI] [PubMed] [Google Scholar]
- 11.Mathur PN, Wolf KM, Busk MF, Briete WM, Datzman M. Fiberoptic bronchoscopic cryotherapy in the management of tracheobronchial obstruction. Chest. 1996;110:718–723. doi: 10.1378/chest.110.3.718. [DOI] [PubMed] [Google Scholar]
- 12.Hoca NT, Günay E, Aktaş Z. Endobronşiyal tedavide argon plazma koagülasyon. Solunum Hastalıkları. 2010;21:32–38. [Google Scholar]
- 13.Bolliger CT, Sutedja TG, Strausz J, Freitag L. Therapeutic bronchoscopy with immediate effect: laser, electrocautery, argon plasma coagulation and stents. Eur Respir J. 2006;27:1258–1271. doi: 10.1183/09031936.06.00013906. [DOI] [PubMed] [Google Scholar]
- 14.Thomas CF, Jet JR, Strosberg JR. Lung neuroendocrine (carcinoid) tumors: Epidemiology, risk factors, classification, histology, diagnosis, and staging. www.uptodate.com. Available at: www.uptodate.com. [Accessed: October, 2018]. [Google Scholar]
- 15.Karasulu L, Altın S, Dalar L, Sökücü S, Şimşek N. Endobronşiyal yolla tedavi edilen iki tipik karsinoid tümör olgusu. Tüberküloz ve Toraks Dergisi. 2009;57:212–217. [PubMed] [Google Scholar]
- 16.Cardillo G, Sera F, Di Martino M, Graziano P, Giunti R, Carbone L, et al. Bronchial carcinoid tumors: nodal status and long-term survival after resection. Ann Thorac Surg. 2004;77:1781–1785. doi: 10.1016/j.athoracsur.2003.10.089. [DOI] [PubMed] [Google Scholar]
- 17.Kasahara K, Fukuoka K, Konishi M, Hamada K, Maeda K, Mikasa K, et al. Two cases of endobronchial neurilemmoma and review of the literature in Japan. Intern Med. 2003;42:1215–1218. doi: 10.2169/internalmedicine.42.1215. [DOI] [PubMed] [Google Scholar]
- 18.Berry MF, Friedberg JS, Midthun DE, Vora SR. Approach to the adult patient with a mediastinal mass. UpToDate, Waltham, MA: Available at: https://www.uptodate.com/contents/approach-to-the-adult-patient-with-a-mediastinalmass [Accessed: April 22, 2017]. [Google Scholar]
- 19.Mizobuchi T, Iizasa T, Iyoda A, Satoh S, Anayama T, Hiroshima K, et al. A strategy of sequential therapy with a bronchoscopic excision and thoracotomy for intra- and extrabronchial wall schwannoma: report of a case. Surg Today. 2005;35:778–781. doi: 10.1007/s00595-005-3025-4. [DOI] [PubMed] [Google Scholar]
- 20.Nasiri H, Zeki AA, Albertson E. A rare diagnosis: Endobronchial schwannoma. J Respir Dis 2010. Available at: https://www.patientcareonline.com/journal-respiratorydiseases/rare-diagnosis-endobronchial-schwannoma. [Google Scholar]
- 21.Dinçer I, Demir A, Akin H, Melek H, Altin S. A giant endobronchial inflammatory polyp. Ann Thorac Surg. 2005;80:2353–2356. doi: 10.1016/j.athoracsur.2004.07.038. [DOI] [PubMed] [Google Scholar]
- 22.Komatsu Y, Koizumi T, Ideura G, Wakamatsu T, Tsushima K, Urushihata K. A case of bronchial fibroepithelial polyp. J Jpn Soc Resp Endoscopy. 2006;28:310–313. [Google Scholar]
- 23.Arısoy A, Ekin S, Özbay B, Çetinkaya E, Özgül A. Endobronşial Polip. Respir Case Rep. 2014;3:153–155. [Google Scholar]