Abstract
Purpose
The purpose of this study was to evaluate the results of the reconstruction using the second free flap following resection of recurrent oral squamous cell carcinoma(OSCC).
Patients and methods
A total of 25 patients from 2005 to 2018 who had undergone salvage surgery and reconstruction using the second free flap for recurrent OSCC were included in this study. Medical records were reviewed to obtain demographic data, stages of the primary cancer, region of recurrent OSCC, the period until recurrence, type of the second reconstruction flaps, recipient vessels, survival rate of the flaps and 2- and 5-year survival rates.
Results
The patients were 13 males and 12 females. The average age was 64.1 years. Of the stages of primary cancer, stage IV patients accounted for the largest number with 13 patients (52.0%). The region of recurrent OSCC was the largest in the mandible with 13 patients, followed by 5 patients in the tongue and 4 patients in the buccal mucosa and maxilla. The mean time interval between the first and second reconstruction was about 34.1 months. Latissimus dorsi free flap and radial forearm free flap used in the second reconstruction were most frequently used in 11 patients (35.5%), followed by fibular composite free flap with 6 patients (19.4%). Facial artery in the recipient arteries of the second reconstruction was most frequently used with 13 cases (43.3%), followed by superior thyroid artery with 8 cases (26.7%) and lingual artery with 7 cases (23.3%). In the second free flap reconstruction, survival rate of the flaps was 96.8%. The 2- and 5-year survival rates in the patients were 70.0% and 62.5%, respectively.
Conclusion
The study showed that the second free flap reconstruction with salvage surgery in resectable recurrent OSCC is a safe and reliable method with a high success rate of the flap and improvement of the 5-year survival rate.
Keywords: Pathology, Cancer surgery, Dental surgery, Rehabilitation, Oncology, Recurrent oral squamous cell carcinoma, Second free flap reconstruction
Pathology; Cancer Surgery; Dental Surgery; Rehabilitation; Oncology; Recurrent oral squamous cell carcinoma, Second free flap reconstruction.
1. Introduction
Oral squamous cell carcinoma(OSCC) is defined as a malignant tumor that occurs in the oral mucosa, tongue, lip, or other areas of the oral cavity. SCC accounts for nearly 90% of all head and neck cancers, and of all the anatomical cancers in the head and neck region, SCC occurs mostly within the oral cavity [1, 2]. In 2003, OSCC was the eighth most common cancer in the world and became the sixth most common cancer in the world in 2016 [3, 4].
Primary surgical management of oral cavity carcinomas is the standard of care in the most circumstances and is associated with excellent oncologic control of early-stage tumors with acceptable functional results. Advanced-stage oral cavity malignancies require combined modality therapy, and patients typically undergo surgical resection followed by adjuvant radiation with or without chemotherapy [5]. The surgical approach to the oral cavity primary site is dictated by the size and location of the tumor and the anatomic region involved relative to the tumor's presentation.
Despite appropriate treatment, local and regional recurrence may occur in the long-term follow-up of patients with oral carcinoma. Up to 30% of patients who undergo definitive treatment for advanced head and neck cancer may experience local and/or regional recurrence [6]. In the patients with unresectable recurrent or metastatic SCC of the head and neck, median survival with supportive care and chemotherapy alone is less than one year, even with the current combinations of platinum agents and epidermal growth factor receptor inhibitors [7]. Recent trials with immunotherapy exhibited only a modest improvement in overall survival over standard chemotherapy [8]. Re-irradiation is an option in selected patients and may result in long-term survival. However, oncologic control with salvage surgery may be higher in resectable recurrent SCC of the head and neck, and re-irradiation can lead to severe late toxicity including treatment-related deaths in up to 10% of patients [9]. For these reasons, surgery is the current mainstay of management for resectable recurrent SCC even though the long-term survival rate after surgical salvage is generally less than 40% [10].
The purpose of this study was to investigate the results of the reconstruction using the second free flap after resection in recurrent OSCC patients. Through this retrospective study, we evaluated the usefulness of surgical treatment for recurrent oral cancer by investigating the curative effect of surgical resection with the second free flap and the survival rate of recurrent OSCC patients.
2. Patients and methods
This study followed all the guidelines and tenets of the Helsinki Declaration and was granted an exemption in writing by the Seoul National University Dental Hospital IRB(ERI19014) due to its retrospective nature. From 2005 to 2018, patients with recurrent OSCC who were treated with salvage surgery and reconstruction using the second free flaps at the Department of Oral and Maxillofacial Surgery in the Seoul National University Dental Hospital were included in this study. Patients with recurrent OSCC who did not undergo reconstruction using by free flaps in primary surgery were excluded.
This retrospective study included patients who underwent salvage surgery and reconstruction using the second free flaps to treat recurrent OSCC. The medical records were reviewed to obtain demographic data, the stages of the primary cancer, sites of the primary and recurrent cancers, the period until recurrence, types of the primary and secondary reconstruction flaps, recipient vessels, survival rate and complications of flaps and 2- and 5-year survival rates. Adjuctive therapies such as radiotherapy and chemotherapy were also included in the review. Statistical analysis with Kaplan-Meier method was performed using MedCalc Statistical Software version 18.11.6 (MedCalc Software bvba, Ostend, Belgium).
3. Results
A total of 25 patients with recurrent OSCC who underwent salvage and reconstructive surgeries using the second free flaps were evaluated. They were 13 males and 12 females. Age standards were based on the age at the time of the salvage and reconstructive surgery using the second free flap, and the average age was 64.1 years. By age group, the patients in their 60s accounted for the largest number with 11 patients, followed by 5 patients in their 50s and 4 patients in their 70s. Of the stages of the primary cancer, stage IV patients accounted for the largest number with 13 patients (52.0%) (Table 1).
Table 1.
Demographic characteristic in patients with recurrent oral squamous cell carcinoma treated by salvage surgery and reconstruction using second free flaps.
| Characteristic | Number of patients | Percentage |
| Sex | ||
| Male | 13 | 52.0 |
| Female |
12 |
48.0 |
| Age | ||
| Mean | 64.1 | |
| Range |
43–83 |
|
| Stage | ||
| Stage I | 1 | 4.0 |
| Stage II | 6 | 24.0 |
| Stage III | 2 | 8.0 |
| Stage IVa | 10 | 40.0 |
| Stage IVb | 3 | 12.0 |
| Unknown | 3 | 12.0 |
| Total | 25 | 100 |
The area of the primary cancer largely involved the mandible (13 cases), followed by 8 cases in the tongue area and 2 cases in the buccal mucosa. The mandible was the most frequent area of the recurrent cancer with 13 patients, followed by the tongue with 5 patients and the buccal mucosa with 4 patients (Table 2). The average period from resection with the first reconstruction to salvage surgery with the second free flap was approximately 34.1 months.
Table 2.
Sites of primary and recurred tumor in patients with recurrent oral squamous cell carcinoma.
| Site | Primary sites, No.(%) | Recurred sites, No.(%) |
|---|---|---|
| Tongue | 8(32.0) | 5(17.9) |
| Maxilla | 1(4.0) | 4(14.3) |
| Mandible | 13(52.0) | 13(46.4) |
| Buccal mucosa | 2(10.3) | 4(14.3) |
| Lip | 1(3.4) | 1(3.6) |
| Mouth corner | 1(3.6) | |
| Total | 25(100) | 28(100) |
In the first reconstruction, radial forearm free flaps were used most frequently in 14 patients (56.0%), followed by fibular composite free flaps in 7 patients (28.0%) and latissimus dorsi free flaps in 2 patients (8.0%) (Table 3). In the second reconstruction, latissimus dorsi free flaps and radial forearm free flaps were used most frequently in 11 patients (35.5%), followed by fibular composite free flaps in 6 patients (20.0%) (Table 3). In the second reconstruction, double-flaps were used in 5 patients, and when flap necrosis was observed during hospitalization, an additional flap was used. A total of 31 flaps were used in the study.
Table 3.
Reconstruction flaps in patients with recurrent oral squamous cell carcinoma.
| Flap | 1st reconstruction, No.(%) | 2nd reconstruction, No(%) |
|---|---|---|
| Radial forearm | 14(56.0) | 11(35.5) |
| Fibular | 7(28.0) | 6(19.4) |
| Latissimus dorsi | 2(8.0) | 11(35.5) |
| Rectus abdominis | 1(4.0) | |
| Anterolateral thigh | 1(4.0) | |
| Peroneal artery perforator | 1(3.2) | |
| Dorsalis pedis artery | 2(6.5) | |
| Total | 25(100) | 31(100) |
In the recipient artery for the first reconstruction, the superior thyroid artery was most frequently used in 12 cases (48.0%), followed by the facial artery in 4 cases (16.0%) and lingual artery in 3 cases (12.0%) (Table 4). In the recipient vein for the first reconstruction, the internal jugular vein was most frequently used in 21 cases (70.0%), followed by the anterior jugular vein in 3 cases (10.0%) and the superior thyroid vein in 3 cases (10.0%) (Table 4). In the recipient artery for the second reconstruction, the facial artery was the most frequently used in 13 cases (43.3%), followed by the superior thyroid artery in 8 cases (26.7%) and the lingual artery in 7 cases (23.3%) (Table 4). In the recipient vein for the second reconstruction, the internal jugular vein was the most frequently used in 19 cases (51.4%), followed by the external jugular vein in 5 cases (13.5%) and the facial vein in 5 cases (13.5%) (Table 4). In the second reconstruction with salvage surgery, double flaps were used in five patients, and the overall number of arteries and veins used in the second reconstruction was higher than that in the first reconstruction (Table 4).
Table 4.
Recipient vessels in 1ST and 2ND reconstruction in patients with recurrent oral squamous cell carcinoma.
| Vessel | 1st reconstruction, No.(%) | 2nd reconstruction, No.(%) |
|---|---|---|
| Artery | ||
| Sup. thyroid a. | 12(48.0) | 8(26.7) |
| Lingual a. | 3(12.0) | 7(23.3) |
| Facial a. | 4(16.0) | 13(43.3) |
| Transverse cervical a. | 1(4.0) | 2(6.7) |
| Not reported | 5(20.0) | |
| Total |
25(100) |
30(100) |
| Vein | ||
| Internal jugular v. | 21(70.0) | 19(51.4) |
| Ant. Jugular v. | 3(10.0) | |
| Sup. thyroid v. | 3(10.0) | 4(10.8) |
| External jugular v. | 5(13.5) | |
| Facial v. | 1(3.3) | 5(13.5) |
| Retromandibular v. | 1(3.3) | 1(2.7) |
| Sup. Laryngeal v. | 1(3.3) | |
| Transverse cervical v. | 2(5.4) | |
| Thyrolinguofacial trunk v. | 1(2.7) | |
| Total | 30(100) | 37(100) |
The survival rate of the flaps in the second free flap reconstruction was 96.8%, with a failure of one in the 31 flaps. Complications after the second free flap reconstruction occurred in 6 cases with wound dehiscence and 1 case with vessel compromise.
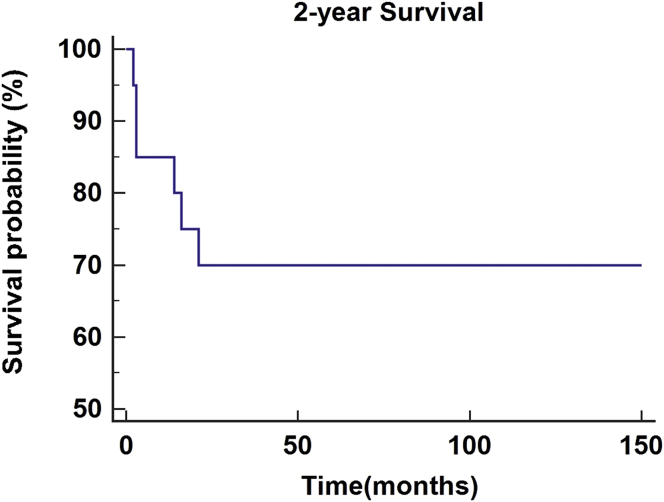
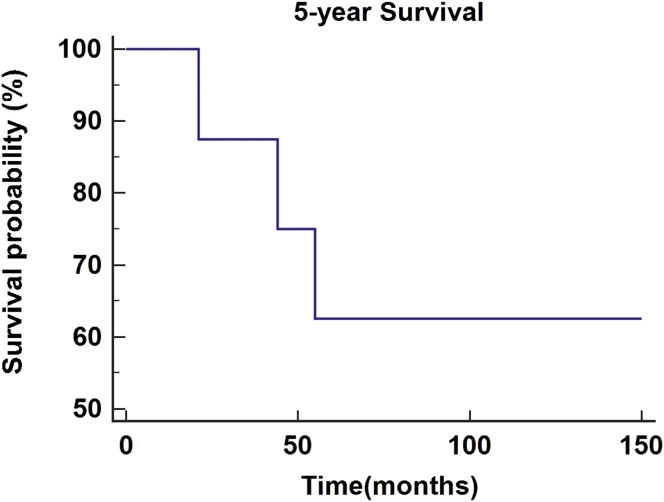
As of February 28, 2019, the 2- and 5-year survival rates were investigated for patients who underwent salvage surgery with the second free flap reconstruction. Survival period in each patient was investigated from the second reconstruction to the day of the last follow-up observation. A total of 20 patients had a follow-up period of more than two years, with an average observation period of 52.6 months. Fourteen of the patients survived with a survival rate of 70.0% (Figure 1). A total of eight patients had a follow-up period of more than five years, with an average observation period of 88.3 months. Five of the patients survived with a survival rate of 62.5% (Figure 2).
Figure 1.
2-year survival rate in patients with recurrent oral squamous cell carcinoma treated by salvage surgery and reconstruction using second free flaps.
Figure 2.
5-year survival rate in patients with recurrent oral squamous cell carcinoma treated by salvage surgery and reconstruction using second free flaps.
The adjunctive therapies were radiotherapy, chemo-radio therapy, and chemotherapy. Eleven patients (44.0%) received radiotherapy after the first reconstruction, followed by 10 patients (40.0%) who did not receive adjunctive therapy and 2 patients (8.0%) who received chemo-radiotherapy. Thirteen patients (52.0%) did not receive adjunctive therapy after the second reconstruction, followed by 8 patients (32.0%) who received radiotherapy and 2 patients (8.0%) who received chemo-radiotherapy.
4. Discussion
Surgical resection, the main treatment for oral cancer, is the standard treatment in the most cases and can be associated with superior oncological treatment with desirable functional outcomes for early cancer [5]. In addition, recovery of defects, function, and aesthetics can be achieved through reconstruction using local or free flaps for surgical defects. However, despite these appropriate treatments, long-term observations of oral cancer patients may show local and regional recurrence. Up to 30% of patients who have experienced clear treatment for advanced head and neck cancer can experience local and regional recurrence [6]. For treatment of these recurrent oral cancers, surgical resection could be selected for a treatment if the recurrent cancers are resectable. After surgical resection, radiotherapy, chemo-radiotherapy, and chemotherapy could be used as adjunctive therapies.
Surgical resection of resectable recurrent cancer requires consideration of the reconstruction of postoperative defects. In many cases, recurrent cancer patients are often subjected to surgical resection in the primary treatment with local or free flap reconstruction. In these cases, resection of recurrent cancer could result in increased defects compared to the primary treatment, and secondary reconstruction of the defect is required. Due to advances in micro-surgical technology, the success rate of the primary free flap reconstruction is very high, as is that of the secondary free flap reconstruction for recurrent cancer. Demirkan et al [11] reported the use of the second free flap in the same patient for microsurgery reconstruction in recurrent oral cancer. They reported 35 oral cancer patients who underwent successful tumor resections and free flap reconstructions. In this study, a total of 75 free tissue transplantations was performed for the first and second reconstructions. After the first tumor resection, 28 radial forearm fasciocutaneous flaps, 7 fibula osteoseptocutaneous flaps, 1 iliac osteomyocutaneous flap, and 2 rectus abdominis myocutaneous flaps were used. For the reconstruction after recurrence, 17 radial forearm fasciocutaneous flaps, 13 fibula osteoseptocutaneous flaps, 3 rectus abdominis myocutaneous flaps, 2 anterolateral thigh flaps, 1 jejunum flap, and 1 tensor fasciae latae flap were used. More vascularized bone transfers were performed during the second reconstruction since the excision for recurrent cancer frequently required segmental mandibulectomy. The overall survival rate of the flaps was 97.3% and 94.6% respectively. Based on these results, it is suggested that free flap reconstruction is an important option for reconstruction in patients with recurrent oral cancer under salvage surgery. If the flaps are adaptive, they are as safe and effective as in the early stages of treatment [11].
Knoetgen et al. [12] reported a research on reconstruction of the head and neck region using the second free flaps after resection of recurrent malignancy. They conducted a retrospective analysis of 12 patients treated by tumor ablation and the second free flaps. The Rochester Mayo Clinic examined the 15-year experience of 12 patients (five males and seven females) who underwent 25 cases of free flap reconstruction from 1988 to 2003. The overall survival rate of the flaps was 92%, with 100% survival rate in the primary free flap transplantations and 85% survival rate in the secondary free flap transplantations. Among the secondary free flaps, one complication was mild (8%) and two were critical (15%). Overall, 10 (77%) of the 13 secondary free flaps were anastomosed in the blood vessels in the ipsilateral neck area. Moreover, five of 13 cases (38%) used the same artery, while seven (54%) used the same vein for both primary and secondary free flaps. Based on these results, they reported the safety and efficacy of the second free flaps on reconstructing the head and neck region of patients with recurrent tumors and stated that the existing recipient blood vessels can often be used for the secondary reconstruction [12]. McCarn et al [13] reported a study for the secondary free tissue transplantation in reconstruction of the head and neck. In the study, 65 patients received the secondary free tissue transplantation, apart from the primary flap. The most common reason for the second flap was tumor recurrence, and radial forearm free flaps and fibula free flaps were most commonly used in the primary and secondary reconstructions. Larger flaps were used for the secondary reconstructions. Flap survival rate was 97%, with 13% of the second flaps required additional surgery for complications, and eight patients needed the third free flap. Based on these results, they stated that the secondary free tissue transplantation was a viable resource for reconstruction of the head and neck and exhibited acceptable flap survival and complications [13]. In addition, Baek et al [14] reported the results of free flap reconstruction in the primary ablation and salvage surgery. Of the total 225 patients, 56 underwent the primary resection and 169 underwent salvage surgery. Flap-related complications occurred in 22.2% of patients, followed by three (5.4%) in the primary ablation group and seven (4.1%) in the salvage surgery group. The success rate of free flaps accompanying salvage surgery was 95.9%. They suggested that the free flap reconstruction for defects of the head and neck is a safe and reliable method.
According to these studies, the use of the second free flaps accompanying resection of recurrent cancer is a safe and effective treatment. In this study, the success rate of free flap reconstruction was similar to the aforementioned studies. In this study, a total of 25 recurrent OSCC patients who also had undergone free flap reconstructions in the primary treatments were treated by salvage surgery with the second free flap reconstruction. A total of 31 free flaps was used in the second free flap reconstruction, with 30 of those flaps survived, the survival rate was 96.8%. In this study, flap-related minor complications were also observed, with 6 cases of wound dehiscence and 1 case of vessel compromise; all complications were resolved with local treatment or observation. One patient who had undergone a dorsalis pedis artery flap experienced flap necrosis as a major complication, which was eventually reconstructed and resolved with a radial forearm free flap through additional surgery. Although complications occurred, the overall survival rate of the flaps was 96.8%, exhibiting a safe and high success rate.
However, the second free flap reconstructions require consideration of several factors that could limit the success rate. These limiting factors include the presence of hard fibrous tissue from radiation, scar tissue associated with previous surgery, delay in diagnosis associated with an increase in number of lymph nodes affected by extracapsular spread, inadequate recipient vessels for microvascular anastomosis by previous primary flap or radiotherapy, wider complex defects resulting from resection of recurrent cancer, insufficient soft tissue volume, lack of bone support due to tumor resection, altered anatomical relations, and difficulty of the flap selection. Proper consideration of these limiting factors will increase the success rate of the second free flap reconstruction.
Though it is more important to reduce recurrence through proper control of the primary tumors, the success of reconstruction for recovery of defects is also important. Despite high success rates of treatment of recurrent tumors and reconstruction using the second free flaps, patients could experience additional recurrence, which could lead to a decrease in survival. Survival results are affected by a number of patient and treatment factors, anatomical boundaries of the head and neck, and by the intrinsic pathology of the recurrent tumor [15]. Kostrzewa et al [16] reported the results of salvage surgery through reconstruction of free flaps for recurrent oral and oropharyngeal cancers. From January 2001 to January 2008, all patients who underwent salvage surgery with free flap reconstructions for oropharyngeal (n = 36) or oral (n = 36) SCC were included. Previous chemo-radiotherapy was used in 40% of the total patients, and radiotherapy alone was used in 60%. The overall average survival period after salvage surgery for recurrent head and neck SCC was 44.8 months in oral cancer and 53.8 months in oropharyngeal cancer. Overall, the observed survival rates for one year, two years, and five years were 98%, 77.2%, and 43.7%, respectively. Based on these findings, salvage surgery accompanied by free flap reconstruction for oral and oropharyngeal tumors after chemo-radiotherapy had an appropriate morbidity rate and similar treatment rate to treatment performed after radiotherapy without chemotherapy [16]. Other studies presented the results of patients who underwent salvage surgery after previous surgery and adjunctive therapies and their overall survival estimates over five years, ranging from 10% to 40% [17, 18, 19]. Kao et al [20] conducted a systematic review of the results of survival after salvage surgery of oropharyngeal SCC. Various electronic databases were retrieved comprehensively, and studies included patients with recurrent or residual oropharyngeal SCC treated by salvage surgery. The main result was the survival rate after salvage surgery. A total of 18 articles were included and the 2- and 5-year survival rates of the patients were 52% and 30%, respectively. They concluded that the improvements in treatment methods for recurrent oropharyngeal SCC were associated with improvements in 2-year overall survival rates, with minimal change to 5-year overall survival rates [20]. As in many of the aforementioned studies, this study examined the survival results of salvage surgery with the second free flap reconstruction. In this study, the survival rates for patients at two and five years or more since treatment were investigated. Patients with more than two years of survival had an average observation period of 52.6 months, with 14 of 20 patients survived, the survival rate was 70.0%. Patients who survived more than five years had an average observation period of 88.3 months, with 5 of 8 patients survived, the survival rate was 62.5%. In the study conducted by Kostrzewa et al [16], the 2-year survival rate was 77.2%, and the 5-year survival rate was 43.7%. Compared with our study, the 2-year survival rate was slightly higher and the 5-year survival rate was lower than our study. The 5-year survival rates of the aforementioned studies were 10%–40% and 30% [17, 18, 19, 20], and the 5-year survival rate of this study was higher.
In conclusion, this study showed that the second free flap reconstruction with salvage surgery in resectable recurrent OSCC patients is a safe and reliable method with a high flap success rate. Salvage surgery accompanied by free flap reconstruction with high success rates could improve the 5-year survival of recurrent OSCC patients.
Declarations
Author contribution statement
J. Lee: Conceived and designed the experiments; Performed the experiments.
S. Kim and S. Park: Performed the experiments.
K. Sung: Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
T. Jung Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This work was supported by a grant from Research year of Inje University in 20180011 and the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number : HI18C1224).
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Marur S., Forastiere A.A. Head and neck cancer: changing epidemiology, diagnosis, and treatment. Mayo Clin. Proc. 2008;83:489. doi: 10.4065/83.4.489. [DOI] [PubMed] [Google Scholar]
- 2.Brands M.T., Brennan P.A., Verbeek A.L.M. Follow-up after curative treatment for oral squamous cell carcinoma. A critical appraisal of the guidelines and a review of the literature. Eur. J. Surg. Oncol. 2018;44:559. doi: 10.1016/j.ejso.2018.01.004. [DOI] [PubMed] [Google Scholar]
- 3.Petersen P.E. The world oral health report 2003: continuous improvement of oral health in the 21st century--the approach of the WHO global oral health programme. Community Dent. Oral Epidemiol. 2003;31(3) doi: 10.1046/j..2003.com122.x. [DOI] [PubMed] [Google Scholar]
- 4.Siegel R.L., Miller K.D., Jemal A. Cancer statistics. CA A Cancer J. Clin. 2016;66(7) doi: 10.3322/caac.21332. 2016. [DOI] [PubMed] [Google Scholar]
- 5.Gourin C.G., Johnson J.T. A contemporary review of indications for primary surgical care of patients with squamous cell carcinoma of the head and neck. Laryngoscope. 2009;119:2124. doi: 10.1002/lary.20619. [DOI] [PubMed] [Google Scholar]
- 6.Gañán L., López M., García J. Management of recurrent head and neck cancer: variables related to salvage surgery. Eur. Arch. Oto-Rhino-Laryngol. 2016;273:4417. doi: 10.1007/s00405-016-4093-3. [DOI] [PubMed] [Google Scholar]
- 7.Vermorken J.B., Mesia R., Rivera F. Platinum-based chemotherapy plus cetuximab in head and neck cancer. N. Engl. J. Med. 2008;359:1116. doi: 10.1056/NEJMoa0802656. [DOI] [PubMed] [Google Scholar]
- 8.Ferris R.L., Blumenschein G., Fayette J. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N. Engl. J. Med. 2016;375:1856. doi: 10.1056/NEJMoa1602252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Strojan P., Corry J., Eisbruch A. Recurrent and second primary squamous cell carcinoma of the head and neck: when and how to reirradiate. Head Neck. 2015;37:134. doi: 10.1002/hed.23542. [DOI] [PubMed] [Google Scholar]
- 10.Goodwin W.J. Salvage surgery for patients with recurrent squamous cell carcinoma of the upper aerodigestive tract: when do the ends justify the means? Laryngoscope. 2000;110(1) doi: 10.1097/00005537-200003001-00001. [DOI] [PubMed] [Google Scholar]
- 11.Demirkan F., Wei F.C., Chen H.C., Chen I.H., Hau S.P., Liau C.T. Microsurgical reconstruction in recurrent oral cancer: use of a second free flap in the same patient. Plast. Reconstr. Surg. 1999;103:829. doi: 10.1097/00006534-199903000-00008. [DOI] [PubMed] [Google Scholar]
- 12.Knoetgen J., Choudry U., Finical S.J., Johnson C.H. Head and neck reconstruction with a second free flap following resection of a recurrent malignancy. Ann. Plast. Surg. 2005;55:378. doi: 10.1097/01.sap.0000178812.87583.7a. [DOI] [PubMed] [Google Scholar]
- 13.McCarn K.E., Ghanem T., Tartaglia J., Gross N., Andersen P., Wax M.K. Second free tissue transfers in head and neck reconstruction. Otolaryngol. Head Neck Surg. 2008;139:525. doi: 10.1016/j.otohns.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 14.Baek C.H., Park W.R., Choi N.Y., Gu S.H., Sohn I.S., Chung M.K. Free flap outcome of salvage surgery compared to primary surgery for head and neck defects: a propensity score analysis. Oral Oncol. 2016;62:85. doi: 10.1016/j.oraloncology.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 15.Zengaa J., Grossb J., Fowlerc S. Salvage of recurrence after surgery and adjuvant therapy: a systematic Review. Am. J. Otolaryngol. 2018;39:223. doi: 10.1016/j.amjoto.2018.01.009. [DOI] [PubMed] [Google Scholar]
- 16.Kostrzewa J.P., Lancaster W.P., Iseli T.A., Desmond R.A., Carroll W.R., Rosenthal E.L. Outcomes of salvage surgery with free flap reconstruction for recurrent oral and oropharyngeal. Cancer Laryngoscope. 2010;120:267. doi: 10.1002/lary.20743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Agra I.M., Carvalho A.L., Ulbrich F.S. Prognostic factors in salvage surgery for recurrent oral and oropharyngeal cancer. Head Neck. 2006;28:107. doi: 10.1002/hed.20309. [DOI] [PubMed] [Google Scholar]
- 18.Röösli C., Studer G., Stoeckli S.J. Salvage treatment for recurrent oropharyngeal squamous cell carcinoma. Head Neck. 2010;32:989. doi: 10.1002/hed.21273. [DOI] [PubMed] [Google Scholar]
- 19.Tam S., Araslanova R., Low T.H. Estimating survival after salvage surgery for recurrent oral cavity cancer. JAMA Otolaryngol Head Neck Surg. 2017;143:685. doi: 10.1001/jamaoto.2017.0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kao S.S., Ooi E.H. Survival outcomes following salvage surgery for oropharyngeal squamous cell carcinoma: systematic review. J. Laryngol. Otol. 2018;132:299. doi: 10.1017/S0022215117000998. [DOI] [PubMed] [Google Scholar]