Abstract
The lung is a key target of the cytokine storm that can be triggered by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), responsible for the widespread clinical syndrome known as coronavirus disease 2019 (COVID-19). Indeed, in some patients, SARS-CoV-2 promotes a dysfunctional immune response that dysregulates the cytokine secretory pattern. Hypercytokinemia underlies the hyperinflammatory state leading to injury of alveolar epithelial cells and vascular endothelial cells, as well as to lung infiltration sustained by neutrophils and macrophages. Within such a pathogenic context, interleukin-6 (IL-6) and other cytokines/chemokines play a pivotal pro-inflammatory role. Therefore, cytokines and their receptors, as well as cytokine-dependent intracellular signalling pathways can be targeted by potential therapies aimed to relieve the heavy burden of cytokine storm. In particular, the anti-IL-6-receptor monoclonal antibody tocilizumab is emerging as one of the most promising pharmacologic treatments.
The reviews of this paper are available via the supplemental material section.
Keywords: ARDS, COVID-19, cytokine storm, immunomodulatory drugs, SARS-CoV-2, pneumonia
Introduction
Immune responses, clinical presentations, and radiological patterns are quite heterogeneous among the multitude of people affected by the widespread COVID-19 syndrome.1 One of the worst scenarios is sustained by the so-called cytokine storm, historically labelled as secondary haemophagocytic lymphohistiocytosis (sHLH), which is a complication most commonly encountered in viral infections.2 Influenza viruses, Ebola virus, cytomegalovirus and, more recently, SARS-CoV-2 have been implicated in triggering the cytokine storm.3 This cytokine storm may provide the possible mechanism on why certain sub-populations are more likely to die of COVID-19 than others.
When the immune system mounts an effective adaptive response against SARS-CoV-2, the infection can be cleared and clinical manifestations are absent or prone to complete recovery. This successful result is mediated by an antiviral CD4+ T helper (Th) cell commitment, associated with activation of CD8+ cytotoxic T lymphocytes and with a B cell-driven response leading to the production of specific antibodies.4 However, when the human organism fails to develop an adequate adaptive immune reaction, viral persistence and the consequent prolongation and amplification of innate immune mechanisms, associated with dysfunctional adaptive responses, can cause a ‘hyperinflammatory’ state underlying the cytokine storm typical of acute respiratory distress syndrome (ARDS).5 In other words, the massive and continuous release of proinflammatory cytokines and chemokines is responsible for a severe, even deadly, attack against the lung. Besides the comorbidities (respiratory, cardiovascular, metabolic, oncologic, etc.) involved, the senescence of the immune system plays a role in the worst outcomes observed in the elderly.6,7 During childhood and adolescence, there are instead very high numbers of naïve T lymphocytes, ready to differentiate and to engage a successful fight against eventual new pathogens.5 Indeed, children are currently paying a very low death toll to SARS-CoV-2 infection.
On the basis of the above considerations, the present review will focus on the pathogenic mechanisms underpinning the lung damage induced by the cytokine storm triggered by SARS-CoV-2, as well as on potential therapies targeting such a very severe condition.
Pathobiology of SARS-CoV-2-induced cytokine storm
Mechanisms of viral infection
SARS-CoV-2 is an enveloped, positive-sense and single-stranded RNA β-coronavirus, similar to coronaviruses responsible for Severe Acute Respiratory Syndrome (SARS) and Middle East Respiratory Syndrome (MERS).8,9 Its nucleocapsid consists of genomic RNA and a phosphorylated N protein, which is embedded within phospholipid bilayers and surfaced by two spike proteins.10 The latter include the spike glycoprotein trimmer (S) and the hemagglutinin-esterase (NE), among which the type III transmembrane glycoprotein M and the envelope E protein are interposed.10 The S glycoprotein binds to the cell membrane receptor angiotensin-converting enzyme 2 (ACE2), expressed within the lower respiratory tract by type 2 alveolar epithelial cells.11,12 This key function of S glycoprotein is primed by TMPRSS2, a human type 2 transmembrane serine protease, which thus facilitates virus entry into host cells.11 The S glycoprotein comprises a S1 subunit mediating the cellular tropism of SARS-CoV-2, and a S2 subunit that is responsible for virus-cell membrane fusion.13 This fusion is followed by penetration of viral genomic RNA into the cytoplasm. Once inside target cells, single-stranded viral RNA is recognised by the intracellular Toll-like receptor 7 (TLR7) located in endosomes.14 As a consequence of this infectious process, SARS-CoV-2 RNA drives the translation and assembly of viral proteins inside the endoplasmic reticulum and Golgi apparatus. The newly formed vesicles, which contain viral particles, fuse with cell membrane thus releasing the virus.
Lymphopenia and lymphocytes exhaustion: a consequence of impaired adaptive immunity
Tissue destruction spreads throughout SARS-CoV-2-infected cells, which trigger innate immune responses mediated mostly by macrophages. Indeed, tight intercellular communications occur between ACE2-expressing lung epithelial cells and macrophages.15 Within the context of class I major histocompatibility complex (MHC-I), macrophages present viral antigens to T lymphocytes, thereby leading to T cell subset commitment and activation.16 The subsequent Th1-featured adaptive immune response should contribute to clear viral infection via the release of antiviral cytokines such as type I interferons (IFNs). However, it has been previously reported that severe infections caused by SARS coronavirus may be associated with low levels of IFN production.17 Therefore, this pathobiologic scenario could be characterised by polarisation towards an aberrant T cell lineage and a dysregulated cytokine secretory pattern.
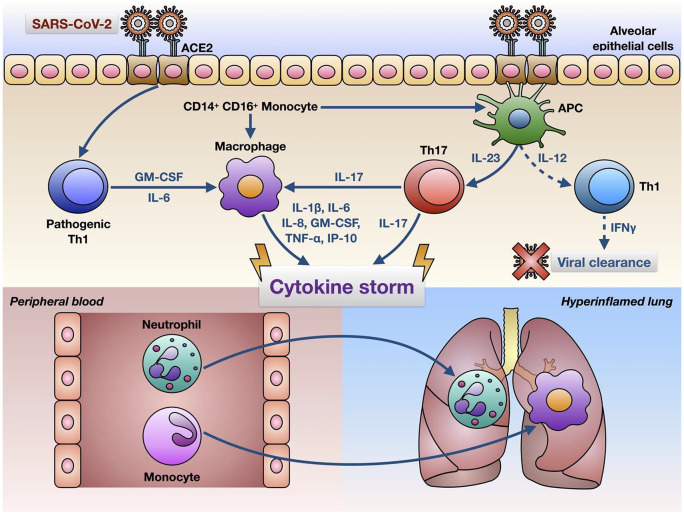
Indeed, it has been shown recently that SARS-CoV-2 infection can prime CD4+ T lymphocytes to differentiate into pathogenic Th1 cells, secreting high amounts of interleukin-6 (IL-6) and granulocyte macrophage-colony stimulating factor (GM-CSF) (Figure 1).18 Such a cytokine milieu promotes activation of CD14+ CD16+ monocytes, which in turn release IL-6 and may migrate from blood to lung, thus possibly becoming alveolar macrophages or dendritic cells (Figure 1).18 In addition, severely ill COVID-19 patients develop dysfunctional immunophenotypes of CD4+ and CD8+ T lymphocytes, characterised by a high co-expression of surface markers such as PD-1 (programmed cell death protein-1) and Tim-3 (T-cell immunoglobulin and mucin-domain containing-3), which predisposes to a rapid T cell exhaustion during viral infections.19–22 In fact, in patients with severe disease, innate immune mechanisms can fail to induce an effective virus-targeted cytotoxic response, normally implemented by activated CD8+ cells.23 Furthermore, the adaptive immune response induced by SARS-CoV-2 might be also shaped as a predominant Th17 profile.24
Figure 1.
Hypothetical mechanisms underlying the cytokine storm induced by SARS-CoV-2 in infected lungs. SARS-CoV-2 enters target cells (e.g. alveolar epithelial cells) via interaction with the ACE2 receptor, thus triggering a complex immune response characterised by activation of pathogenic Th1 cells, CD14+ CD16+ monocytes, alveolar macrophages and Th17 lymphocytes. These cells release high amounts of cytokines and chemokines, responsible for the cytokine storm sustaining a ‘hyperinflammatory’ environment featured by lung infiltration with neutrophils and macrophages. In critically ill COVID-19 patients with ARDS, the Th1-driven immune adaptive response leading to viral clearance appears to be defective (dashed lines).
ACE2, angiotensin-converting enzyme 2; APC, antigen presenting cells; ARDS, acute respiratory distress syndrome; COVID-19; coronavirus disease 2019; GM-CSF, granulocyte macrophage-colony stimulating factor; IFN, interferon; IL, interleukin; SARS-CoV-2, severe acute respiratory syndrome coronavirus-2; Th, T helper.
Hypercytokinemia in COVID-19
Cross-talking innate and adaptive immune pathways lead lung epithelial cells, activated monocytes/macrophages and T lymphocytes to massively release a broad array of proinflammatory cytokines and chemokines (cytokine storm), including interleukins-1β (IL-1β), 2 (IL-2), 6 (IL-6), 7 (IL-7), 8 (IL-8), 17 (IL-17), 18 (IL-18), 33 (IL-33), GM-CSF, interferon-γ-inducible protein 10 (IP-10), monocyte chemoattractant protein-1 (MCP-1), macrophage inflammatory protein-1α (MIP-1α), tumour necrosis factor-α (TNF-α) and transforming growth factor-β (TGF-β) (Figure 1).1,16
IL-6 plays a central role in the COVID-19 cytokine storm
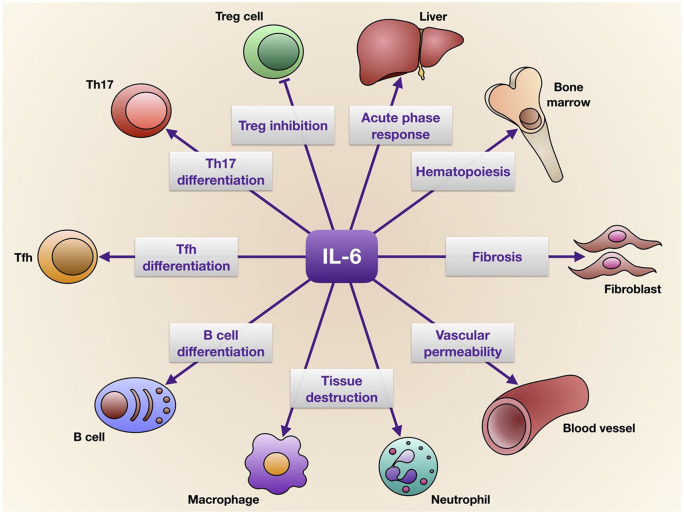
One of the most important cytokines produced as a consequence of SARS-CoV-2-induced TLR-7 signalling is IL-6, a pleiotropic proinflammatory mediator that promotes the proliferation of myeloid progenitor cells and the growth and activation of leukocytes, as well as induces pyrexia and the synthesis of acute phase proteins such as C reactive protein (CRP) (Figure 2).25 IL-6 plays a central role in immune responses by stimulating the differentiation of T follicular helper cells (Tfh) and contributing, together with TGF-β, to development of Th17 cells (Figure 2).5,24 Through activation of the SOCS-3 (suppressor of cytokine signalling-3) pathway, IL-6 can also suppress phosphorylation of signal transducer and activator of transcription-4 (STAT-4), thus impairing the activity of CD8+ cytotoxic and natural killer T cells.5,25 Furthermore, via up-regulation of IL-4 and down-regulation of IFN-γ, IL-6 inhibits antiviral Th1 cell commitment and favours Th2 cell differentiation.5 Elevated levels of IL-6 are associated with severe lung injury.26 IL-6 can suppress the functions of T lymphocytes, dendritic cells and macrophages aimed to eliminate coronaviruses, thereby dampening the ability of the immune system to clear such infections.27 Therefore, IL-6 overproduction can probably be induced by some viruses such as SARS-CoV-2, with the aim of escaping immune surveillance.
Figure 2.
Pleiotropic effects of IL-6, which exerts its biological actions on several cells, tissues and organs.
IL-6, interleukin-6; Th, T helper cells; Tfh, T follicular helper cells; Treg, regulatory T cells.
Role of other cytokines in COVID-19 cytokine storm
Active IL-1β and IL-18 originate from their inactive precursors via a cleavage catalysed by caspase-1, a proteolytic enzyme operating within the context of the multiprotein intracellular inflammasome complex,28 highly susceptible to activation induced by viral molecules. IL-1β and TNF-α, mostly generated by activated macrophages, are present in high concentrations in bronchoalveolar lavage fluid (BALF) from patients with ARDS and stimulate neutrophil functions.29–31 TNF-α also causes the apoptotic death of lung epithelial and endothelial cells.32
High IL-8 BALF concentrations have been also detected in subjects with ARDS.31 IL-8 is a powerful chemoattractant and activator of neutrophils, whose apoptosis is inhibited by this chemokine. Thus, upon release from monocytes/macrophages and alveolar epithelial cells, IL-8 plays a key role in stimulating neutrophil survival and recruitment to the lungs.31 IL-8 synthesis is effectively stimulated by IL-17A and IL-17F secreted by IL-6-dependent Th17 cells, which are likely involved in triggering the cytokine storm associated with lung neutrophilic infiltration.24 Indeed, high numbers of Th17 lymphocytes can be found in peripheral blood of patients with severe SARS-CoV-2 infection.33
With regard to inflammatory cell influx into SARS-CoV-2-infected lungs, a key pathologic function is also exerted by GM-CSF, which mediates relevant intercellular communications between pathogenic Th1 cells and CD14+ CD16+ monocytes (Figure 1).18 High numbers of CD14+ CD16+ monocytes are detectable in COVID-19 patients with severe involvement of lungs, where these cells actively participate in induction and amplification of tissue infiltration by macrophages.18
Another proinflammatory mediator potentially involved in the cytokine/chemokine storm characterising severe SARS-CoV-2 infection is the chemokine IP-10. In fact, it has been previously shown that IP-10 is up-regulated in bronchiolar and alveolar epithelial cells, as well as in T cells and monocytes/macrophages infiltrating the lungs of subjects with SARS.30 IP-10 exerts a strong chemotactic action on T lymphocytes, monocytes and natural killer cells.30 Moreover, high blood levels of IP-10 were detected in patients died of SARS.34 It is thus very likely that IP-10 significantly contributes to the recruitment of monocytes/macrophages into the lungs of SARS patients. Virus-induced production of IP-10 could also be responsible for a fast mobilisation, followed by a subsequent apoptosis of T cells30; this mechanism might be implicated in the impairment of T lymphocyte response against SARS-CoV-2.
Consequences of cytokine storm
Taken together, the above considerations suggest that, in critically ill COVID-19 patients, cytokine storm and cytokine dysregulation lead to remarkable pathological consequences. Indeed, the cytokine storm elicits immunological changes that can potentially weaken the immune response aimed to clear SARS-CoV-2 infection. T lymphocyte-dependent protective responses against SARS-CoV-2, mediated by both CD4+ and CD8+ T cells, may potentially fail because of the overproduction of IL-6 and TNF-α. In fact, these cytokines can inhibit T cell proliferation and activation, thereby contributing to the development of lymphopenia in severely injured SARS-CoV-2 infected patients.16,18 Consistent with such a speculation, it has been reported that high levels of both IL-6 and TNF-α coexist with relatively low counts of CD4+ and CD8+ T cells.21 It has been also hypothesised that a suppression of functionally exhausted Th1 lymphocytes might be concomitant with an immunological shift towards Th2-driven responses.1,16 All the above immunological changes likely occur more often in the elderly, because of a negative impact of aging on the protective efficiency afforded by the adaptive immune network against new viral infections.6
Cytokine/chemokine-mediated injury of lung endothelial and epithelial cells may impair the integrity of blood/air barrier, thereby promoting vascular permeability as well as alveolar oedema, infiltration by inflammatory cells (i.e. neutrophils and macrophages) and hypoxia.32 In addition, the presence within the cytokine storm context of fibrogenic factors such as TGF-β could favour tissue remodelling and lung fibrosis,30 thus further compromising gas exchange.
Diagnosis of cytokine storm
In clinical setting, there is an urgent need to detect the laboratory parameters which can be useful to diagnose cytokine storm. A recent meta-analysis of 21 studies globally including 3377 patients and 33 laboratory biomarkers suggests that elevated serum levels of IL-6 and ferritin, paralleled by high numbers of white blood cells and associated with low lymphocyte and platelet counts, could provide a valuable diagnostic platform for critical COVID-19 illness.35 Such data are partially consistent with those of a very recent explorative study carried out on 127 hospitalised COVID-19 patients, which showed that, when compared with the group of less severe subjects, more severe patients were characterised by high blood levels of IL-6, fibrinogen, sialic acid, CRP and neutrophils.36 In particular, neutrophil-to-lymphocyte ratio (NLR) resulted to be significantly higher in severely ill patients,36 thus suggesting that the decreased lymphocyte count might be indicative of the existing impairment of the immune system.
Therapeutic implications for SARS-CoV-2-induced cytokine storm
Several drugs, currently approved for treatment of various diseases and acting on different molecular targets, are potentially useful to lessen the strength of the cytokine storm triggered by SARS-CoV-2.37 However, their utilisation in COVID-19 patients is mostly empirical, because of lack of published controlled trials showing a reliable profile of efficacy and safety. Such drugs include IL-6 receptor antagonists, IL-1 receptor antagonists, JAK/STAT (Janus kinases/signal transducers and activators of transcription) inhibitors, corticosteroids, hydroxychloroquine and azithromycin.
IL-6 receptor antagonists
An effective inhibitor of IL-6 pathogenic action within the context of SARS-CoV-2-induced cytokine storm appears to be tocilizumab, a recombinant humanised monoclonal antibody targeting the IL-6 receptor, currently utilised for treatment of rheumatoid arthritis.38,39 A retrospective Chinese study, performed in 21 severely ill COVID-19 patients, showed that tocilizumab safely lowered fever and CRP, as well as improving hypoxemia and computed tomography (CT) scan lung lesions.40 On the basis of these positive results, a randomised controlled trial has been approved in China with the aim of testing tocilizumab in subjects with elevated IL-6 levels and interstitial pneumonia (ChiCTR2000029765).3 Tocilizumab is also undergoing clinical investigation in Italy (Trial TOCIVID-19 – NCT04317092). Current Chinese and Italian guidelines recommend the use of tocilizumab for treatment of COVID-19 infection.16 In particular, Italian guidelines suggest that tocilizumab should be utilised in patients with interstitial pneumonia and severe respiratory failure, characterised by high blood levels of IL-6 or CRP/D-dimer/fibrinogen/ferritin.16 A further phase III clinical trial has been approved by the United States Food and Drug Administration (FDA) to evaluate tocilizumab in hospitalised COVID-19 patients suffering from severe pneumonia [ClinicalTrials.gov identifier: NCT04320615]. However, the use of tocilizumab raises some concerns. Indeed, it is possible that the high viral load that drives the cytokine storm would be unsuppressed by the use of tocilizumab. Furthermore, although tocilizumab seems to be usually quite safe and well tolerated, it has been reported that the infusion of this drug can occasionally be associated with the occurrence of liver damage, thrombocytopenia, leukopenia, serious infections, gastrointestinal perforations, hypertension, skin reactions and anaphylaxis.41 Another IL-6 receptor antagonist is sarilumab, already licensed for treatment of rheumatoid arthritis and currently undergoing a phase II/III clinical trial enrolling severely ill hospitalised COVID-19 patients [ClinicalTrials.gov identifier: NCT04315298].42
IL-1 receptor antagonist
Anakinra is a recombinant antagonist of human IL-1 receptor that appears to be capable of improving the survival of septic patients with macrophage activation syndrome (MAS).43 In particular, in 43 septic patients with MAS and concomitant disseminated intravascular coagulation, hepatobiliary dysfunction, cytopenias and hyperferritinemia, when compared with placebo, anakinra treatment significantly improved the 28-day survival rate (anakinra: 65.4%; placebo: 35.3%).43
JAK/STAT inhibitors
Inhibitors of JAK/STAT (signal transducers and activators of transcription) signalling pathways such as baricitinib, a drug that is currently utilised to treat rheumatoid arthritis, can be potentially useful for treatment of cytokine storm. Indeed, by targeting the JAK/STAT signal transduction system, baricitinib interferes with the functions of adaptor-associated protein kinase 1 (AKK1) and cyclin G-associated kinase (GAK), which are implicated in viral endocytosis.44 Fedratinib is a specific JAK2 inhibitor able to reduce IL-17 expression, as well as to repress GM-CSF biological actions.24 Therefore, this drug could contribute to attenuate the cytokine storm associated with severe SARS-CoV-2 infection. Another JAK inhibitor is ruxolitinib, a drug that is currently utilised for treatment of myelofibrosis. Ruxolitinib has been shown to induce relevant clinical benefits in some COVID-19 patients treated in Southern Italy (unpublished observations). However, further studies are needed to corroborate and validate such positive preliminary data.
Corticosteroids
The use of systemic corticosteroids for treatment of COVID-19-associated cytokine storm could be of some value. In fact, by modulating cytokine production, these drugs might repress hyperinflammation associated with COVID-19-related ARDS.45 Some interesting observations suggest that in community-acquired pneumonia corticosteroids could increase the rate of therapeutic success, and also decrease the number of hospitalisation days and the time occurring to reach clinical stability.46 However, it has been reported that early use of hydrocortisone in subjects with MERS could delay viral clearance.47 Moreover, utilisation of methylprednisolone in SARS-CoV-2 infected patients with advanced ARDS and progressive disease appears to ameliorate respiratory symptoms and CT abnormalities, but does not seem to prolong overall survival.48,49 Nevertheless, because of the lack of control arms, such conclusions based on observational studies should be considered with extreme caution.
Hydroxychloroquine and azithromycin
Chloroquine, and especially its less toxic derivative hydroxychloroquine, could block SARS-CoV-2 entry inside target cells by interfering with glycosylation of ACE2 receptors.50 This drug also acts by suppressing TLR7 signalling and by inhibiting endosomal acidification, essential for viral replication.5 Moreover, hydroxychloroquine prevents endolysosomal fusion. The therapeutic action of hydroxychloroquine can be potentiated by azithromycin, capable of reducing the pro-inflammatory activity of IL-6 and TNF-α.51,52
Conclusion
The dramatic outbreak of SARS-CoV-2 infection is currently associated with an ongoing progress in the knowledge of underlying pathogenic mechanisms, which is shedding partial light on the immunophenotypic traits characterising infected patients more susceptible to the development of heavy lung damage caused by cytokine storm. Indeed, an impairment in anti-viral immune response and the concurrent aberrant hyperinflammatory reaction can facilitate, especially in elderly people with comorbid conditions, the occurrence of the most severe forms of COVID-19-related illness. A better understanding of cytokine storm pathobiology is also making it possible to explore the therapeutic efficacy of IL-6 receptor antagonists and JAK/STAT inhibitors, which, however, require to be carefully evaluated by definitely needed randomised controlled trials.
Supplemental Material
Supplemental material, Author_Response_1 for Lung under attack by COVID-19-induced cytokine storm: pathogenic mechanisms and therapeutic implications by Corrado Pelaia, Caterina Tinello, Alessandro Vatrella, Giovambattista De Sarro and Girolamo Pelaia in Therapeutic Advances in Respiratory Disease
Supplemental material, Reviewer_1_v.1 for Lung under attack by COVID-19-induced cytokine storm: pathogenic mechanisms and therapeutic implications by Corrado Pelaia, Caterina Tinello, Alessandro Vatrella, Giovambattista De Sarro and Girolamo Pelaia in Therapeutic Advances in Respiratory Disease
Supplemental material, Reviewer_2_v.1 for Lung under attack by COVID-19-induced cytokine storm: pathogenic mechanisms and therapeutic implications by Corrado Pelaia, Caterina Tinello, Alessandro Vatrella, Giovambattista De Sarro and Girolamo Pelaia in Therapeutic Advances in Respiratory Disease
Footnotes
Author contribution(s): Corrado Pelaia: Conceptualization; Methodology; Supervision; Writing-original draft; Writing-review & editing.
Caterina Tinello: Conceptualization; Methodology; Supervision; Writing-original draft; Writing-review & editing.
Alessandro Vatrella: Conceptualization; Methodology; Supervision; Writing-original draft; Writing-review & editing.
Giovambattista De Sarro: Conceptualization; Methodology; Supervision; Writing-original draft; Writing-review & editing.
Girolamo Pelaia: Conceptualization; Methodology; Supervision; Writing-original draft; Writing-review & editing.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Corrado Pelaia  https://orcid.org/0000-0002-4236-7367
https://orcid.org/0000-0002-4236-7367
Girolamo Pelaia  https://orcid.org/0000-0001-9288-8913
https://orcid.org/0000-0001-9288-8913
Supplemental material: The reviews of this paper are available via the supplemental material section.
Contributor Information
Corrado Pelaia, Department of Health Sciences, University ‘Magna Graecia’ of Catanzaro, Catanzaro, Calabria, Italy.
Caterina Tinello, Pediatrics Unit, Provincial Outpatient Center of Catanzaro, Catanzaro, Calabria, Italy.
Alessandro Vatrella, Department of Medicine, Surgery, and Dentistry, University of Salerno, Salerno, Campania, Italy.
Giovambattista De Sarro, Department of Health Sciences, University ‘Magna Graecia’ of Catanzaro, Catanzaro, Calabria, Italy.
Girolamo Pelaia, Campus Universitario ‘Salvatore Venuta’, Viale Europa – Località Germaneto, Catanzaro, 88100, Italy.
References
- 1. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020; 395: 497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ramos-Casals M, Brito-Zeron P, Lopez-Guillermo A, et al. Adult haemophagocytic syndrome. Lancet 2014; 383: 1503–1516. [DOI] [PubMed] [Google Scholar]
- 3. Mehta P, McAuley DF, Brown M, et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet 2020; 395: 1033–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yi Y, Lagniton PNP, Ye S, et al. COVID-19: what has been learned and to be learned about the novel coronavirus disease. Int J Biol Sci 2020; 16: 1753–1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ahmadpoor P, Rostaing L. Why the immune system fails to mount an adaptive immune response to a Covid-19 infection. Transpl Int. Epub ahead of print 1 April 2020. DOI: 10.1111/tri.13611. [DOI] [PubMed] [Google Scholar]
- 6. Weiskopf D, Weinberger B, Grubeck-Loebenstein B. The aging of the immune system. Transpl Int 2009; 22: 1041–1050. [DOI] [PubMed] [Google Scholar]
- 7. Onder G, Rezza G, Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA. Epub ahead of print 23 March 2020. DOI: 10.1001/jama.2020.4683. [DOI] [PubMed] [Google Scholar]
- 8. Guo YR, Cao QD, Hong ZS. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak – an update on the status. Mil Med Res 2020; 7: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhou P, Yang XL, Wang XG, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020; 579: 270–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Li G, Fan Y, Lai Y, et al. Coronavirus infections and immune responses. J Med Virol 2020; 92: 424–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically-proven protease inhibitor. Cell 2020; 181: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhao Y, Zhao Z, Wang Y, et al. Single-cell RNA expression profiling of ACE2, the putative receptor of Wuhan 2019-nCov. BioRxiv 2020; 202001.26919985. [Google Scholar]
- 13. Jin Y, Yang H, Ji W, et al. Virology, epidemiology, pathogenesis, and control of COVID-19. Viruses 2020; 12: 372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fung SY, Yuen KS, Ye ZW, et al. A tug-of-war between severe acute respiratory syndrome coronavirus 2 and host antiviral defense: lessons from other pathogenic viruses. Emerg Microbes Infect 2020; 9: 558–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Qi F, Qian S, Zhang S, et al. Single cell RNA sequencing of 13 human tissues identify cell types and receptors of human coronaviruses. Biochem Biophys Res Commun 2020; 526: 135–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sarzi-Puttini P, Giorgi V, Sirotti S, et al. COVID-19, cytokines and immunosuppression: what can we learn from severe acute respiratory syndrome? Clin Exp Rheumatol 2020; 38: 337–342. [PubMed] [Google Scholar]
- 17. Chen J, Subbarao K. The immunobiology of SARS. Annu Rev Immunol 2007; 25: 443–472. [DOI] [PubMed] [Google Scholar]
- 18. Zhou Y, Fu B, Zheng X, et al. Pathogenic T cells and inflammatory monocytes incite inflammatory storm in severe COVID-19 patients. Natl Sci Rev. Epub ahead of print 13 March 2020. DOI: 10.1093/nsr/nwaa041/5804736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Khaitan A, Unutmaz D. Revisiting immune exhaustion during HIV infection. Curr HIV/AIDS Rep 2011; 8: 4–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jin HT, Anderson AC, Tan WG, et al. Cooperation of Tim-3 and PD-1 in CD8 T-cell exhaustion during chronic viral infection. Proc Natl Acad Sci USA 2010; 107: 14733–14738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Diao B, Wang C, Tany Y, et al. Reduction and functional exhaustion of T cells in patients with Coronavirus disease 2019 (COVID-19). MedRxiv 2020; 202002.1820024364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zheng M, Gao Y, Wang G, et al. Functional exhaustion of antiviral lymphocytes in COVID-19 patients. Cell Mol Immunol 2020; 17: 533–535. [In Press; ]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Laderach D, Movassagh M, Johnson A, et al. 4-1BB co-stimulation enhances human CD8+ T cell priming by augmenting the proliferation and survival of effector CD8+ T cells. Int Immunol 2002; 14: 1155–1167. [DOI] [PubMed] [Google Scholar]
- 24. Wu D, Yang XO. TH17 responses in cytokine storm of COVID-19: an emerging target of JAK2 inhibitor fedratinib. J Microbiol Immunol Infect. Epub ahead of print 11 March 2020. DOI: 10.1016/j.jmii.2020.03.005, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Velazquez-Salinas L, Verdugo-Rodriguez A, Rodriguez LL, et al. The role of IL-6 during viral infections. Front Microbiol 2019; 10: 1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhang Y, Li J, Zhan Y, et al. Analysis of serum cytokines in patients with severe acute respiratory syndrome. Infect Immun 2004; 72: 4410–4415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yoshikawa T, Hill T, Li K, et al. Severe Acute Respiratory Syndrome (SARS) coronavirus-induced lung epithelial cytokines exacerbate SARS pathogenesis by modulating intrinsic functions of monocyte-derived macrophages and dendritic cells. J Virol 2009; 83: 3039–3048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pinkerton JW, Kim RY, Robertson AAB, et al. Inflammasomes in the lung. Mol Immunol 2017; 86: 44–55. [DOI] [PubMed] [Google Scholar]
- 29. Bhatia M, Moochhala S. Role of inflammatory mediators in the pathophysiology of acute respiratory distress syndrome. J Pathol 2004; 202: 145–156. [DOI] [PubMed] [Google Scholar]
- 30. Jiang Y, Xu J, Zhou C, et al. Characterization of cytokine/chemokine profiles of severe acute respiratory syndrome. Am J Respir Crit Care Med 2005; 171: 850–857. [DOI] [PubMed] [Google Scholar]
- 31. Butt Y, Kurdowska A, Allen TC. Acute lung injury: a clinical and molecular review. Arch Pathol Lab Med 2016; 140: 345–350. [DOI] [PubMed] [Google Scholar]
- 32. Channappanavar R, Perlman S. Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology. Semin Immunopathol 2017; 39: 529–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med 2020; 8: 420–422. [In Press]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Huang KJ, Su IJ, Theron M, et al. An interferon-γ-related cytokine storm in SARS patients. J Med Virol 2005; 75: 185–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Henry BM, de Oliveira MHS, Benoit S, et al. Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID-19): a meta-analysis. Clin Chem Lab Med. Epub ahead of print 10 April 2020. DOI: 10.1515/cclm-2020-0369. [DOI] [PubMed] [Google Scholar]
- 36. Zhu Z, Cai T, Fan L, et al. Clinical value of immune-inflammatory parameters to assess the severity of coronavirus disease 2019. Int J Infect Dis. Epub ahead of print 22 April 2020. DOI: 10.1016/j.ijid.2020.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Li G, De Clercq E. Therapeutic options for the 2019 novel coronavirus (2019-nCoV). Nat Rev Drug Discov 2020; 19: 149–150. [DOI] [PubMed] [Google Scholar]
- 38. Oldfield V, Dhillon S, Plosker GL. Tocilizumab: a review of its use in the management of rheumatoid arthritis. Drugs 2009; 69: 609–632. [DOI] [PubMed] [Google Scholar]
- 39. Lu CC, Chen MY, Chang YL. Potential therapeutic agents against COVID-19: what we know so far. J Chin Med Assoc. Epub ahead of print 1 April 2020. DOI: 10.1097/JCMA.0000000000000318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Xu X, Han M, Li T, et al. Effective treatment of severe COVID-19 patients with tocilizumab. ChinaXiv 2020; 20200300026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zhang S, Li L, Shen A, et al. Rationale use of tocilizumab in the treatment of novel coronavirus pneumonia. Clin Drug Invest. Epub ahead of print 26 April 2020. DOI: 10.1007/s40261-020-00917-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sanders JM, Monogue ML, Jodlowski TZ, et al. Pharmacologic treatments for coronavirus disease 2019 (COVID-19): a review. JAMA. Epub ahead of print 13 April 2020. DOI: 10.1001/jama.2020.6019. [DOI] [PubMed] [Google Scholar]
- 43. Shakoory B, Carcillo JA, Chatham WW, et al. Interleukin-1 receptor blockade is associated with reduced mortality in sepsis patients with features of macrophage activation syndrome: reanalysis of a prior phase III trial. Crit Care Med 2016; 44: 275–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Richardson P, Griffin I, Tucker C, et al. Baricitinib as potential treatment for 2019-nCoV acute respiratory disease. Lancet 2020; 395: e30–e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Matthay MA, Zemans RL, Zimmerman GA. Acute respiratory distress syndrome. Nat Rev Dis Prim 2019; 5: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wan YD, Sun TW, Liu ZQ, et al. Efficacy and safety of corticosteroids for community-acquired pneumonia: a systematic review and meta-analysis. Chest 2016; 149: 209–219. [DOI] [PubMed] [Google Scholar]
- 47. Hui DS. Systemic corticosteroid therapy may delay viral clearance in patients with middle east respiratory syndrome coronavirus infection. Am J Respir Crit Care Med 2018; 197: 700–701. [DOI] [PubMed] [Google Scholar]
- 48. Russell CD, Millar JE, Baillie JK. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet 2020; 395: 473–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zhou W, Liu Y, Tian D, et al. Potential benefits of precise corticosteroids therapy for severe 2019-nCoV pneumonia. Signal Transduct Target Ther 2020; 5: 17–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Vincent MJ, Bergeron E, Benjannet S, et al. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol J 2005; 2: 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Gautret P, Lagier JC, Parola P, et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-labeled non-randomized clinical trial. Int J Antimicrob Agents. Epub ahead of print 20 March 2020. DOI: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Schultz MJ. Macrolide activities beyond their antimicrobial effects: macrolides in diffuse panbronchiolitis and cystic fibrosis. J Antimicrob Chemother 2004; 54: 21–28. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Author_Response_1 for Lung under attack by COVID-19-induced cytokine storm: pathogenic mechanisms and therapeutic implications by Corrado Pelaia, Caterina Tinello, Alessandro Vatrella, Giovambattista De Sarro and Girolamo Pelaia in Therapeutic Advances in Respiratory Disease
Supplemental material, Reviewer_1_v.1 for Lung under attack by COVID-19-induced cytokine storm: pathogenic mechanisms and therapeutic implications by Corrado Pelaia, Caterina Tinello, Alessandro Vatrella, Giovambattista De Sarro and Girolamo Pelaia in Therapeutic Advances in Respiratory Disease
Supplemental material, Reviewer_2_v.1 for Lung under attack by COVID-19-induced cytokine storm: pathogenic mechanisms and therapeutic implications by Corrado Pelaia, Caterina Tinello, Alessandro Vatrella, Giovambattista De Sarro and Girolamo Pelaia in Therapeutic Advances in Respiratory Disease