Abstract
Purpose
Real-time polymerase chain reaction (RT-PCR) detection of severe acute respiratory syndrome coronavirus (SARS-CoV-2) is required for diagnosis of coronavirus disease 2019 (COVID-19). Sensitivity of RT-PCR nasopharyngeal (NP) testing is presumed to be high, but there is no gold standard against which this has been determined. The objective was to determine whether lower respiratory tract infection (LRTI), detected in bronchoalveolar lavage fluid (BALF), occurs in the absence of upper respiratory tract infection with clinical testing of both specimen types.
Methods
Between March 26, 2020 and April 17, 2020 at the University of Washington Medical Center all patients with BALF specimens clinically tested for SARS-CoV-2 were identified. We assessed the proportion of patients with positive RT-PCR for SARS-CoV-2 in BALF after negative NP testing. We describe 3 cases with positive testing in BALF.
Results
Among 16 patients with BALF samples, 3 cases (19%) had SARS-CoV-2 detected in BALF. In Case 1, negative NP testing occurred early in the infection and respiratory symptoms may have been missed due to neurologic injury. In Case 2, outpatient diagnosis was aspiration pneumonia, but clinical suspicion remained high for COVID-19 at hospitalization based on epidemiological and clinical features. All 3 cases involved older adults (age >65 years), one of whom was immunosuppressed in the setting of lung transplantation (Case 3).
Conclusions
These data demonstrate that SARS-CoV-2 LRTI occurs in the presence of negative NP testing. NP testing may underestimate the prevalence of COVID-19 and has implications for spread of SARS-CoV2 in the community and healthcare setting.
Highlights
-
•
Negative nasopharyngeal testing may underestimate the true prevalence of COVID-19.
-
•
COVID-19 lower respiratory tract infection occurs despite negative NP testing.
-
•
False negative nasopharyngeal testing has implications for the spread of COVID-19.
1. Introduction
Diagnosis of coronavirus disease 2019 (COVID-19) relies on RT-PCR detection of severe acute respiratory syndrome coronavirus (SARS-CoV-2) [[1], [2], [3]]. Test sensitivity is presumed to be high, but prior studies demonstrated variability of SARS-CoV-2 detection depending on stage of illness [4] or sample source among patients with confirmed disease [5]. Upper respiratory tract (URT) infection (URTI) is the presumed primary source for viral transmission, preferred specimen type for testing, and basis for determining infection precautions [[6], [7], [8]]. Whether lower respiratory tract (LRT) infection (LRTI) occurs in the absence of detectable URTI is uncertain because of limited data on simultaneous testing of both specimen types [5]. While negative nasopharyngeal (NP) testing is often used to “rule out” SARS-CoV-2 disease and infectivity, rates of discordance between URT and LRT samples are unknown and can only be assessed by testing specimens concurrently.
This study aimed to determine whether LRTI, detected in bronchoalveolar lavage fluid (BALF), occurs in the absence of URTI with clinical testing of both specimen types.
2. Methods
University of Washington (UW) protocol during the study period (COVID-19 pandemic) limited bronchoscopy to urgent cases and was avoided in patients with positive SARS-CoV-2 NP testing. Testing was performed on 200μL of BALF using our Washington State emergency use authorization (EUA), CDC- developed test incorporating the N1/N2 primer sets and an internal control transcript spiked into every specimen [1]. SARS-CoV-2 detection is defined at a cycle threshold (Ct) < 40 and “positive” if both N1 and N2 targets, “low-positive” if 1 out of 2 targets, and “negative” if 0 targets are identified [1]. Clinical records were reviewed for patients who underwent BALF testing for SARS-CoV-2. The UW IRB approved this study (#00009734).
3. Results
Between March 26 - April 17, 2020, 16 patients underwent bronchoscopy after negative NP testing for SARS-CoV-2. Outside hospitals submitted 5 (31%) of samples tested at UW. Mean age was 59 years (SD 14 years), 8 (50%) were male. Three cases (19%) of COVID-19 were identified. Among 11 UW patients, all had at least one negative NP test within 4 days of bronchoscopy and 55% had ≥1 negative NP test within 24 hours of bronchoscopy. Of patients with positive BALF, two (67%) had two negative NP tests prior to bronchoscopy (Fig. 1).
Fig. 1.
Timeline for negative nasopharyngeal (NP) testing prior to positive bronchoalveolar lavage (BAL) fluid RT-PCR testing for SARS-CoV-2.
3.1. Case 1
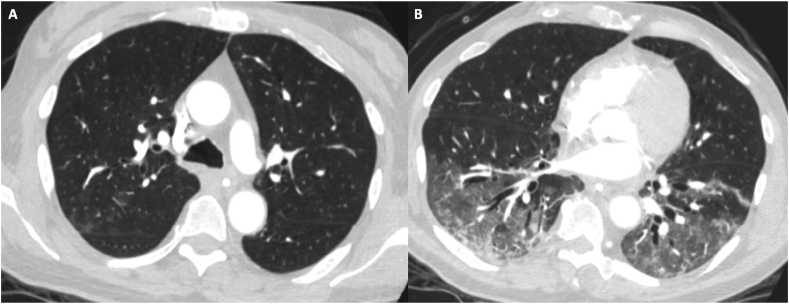
A 67-year-old man was admitted with intracerebral hemorrhage and fever from a nursing facility where residents had tested positive for COVID-19. SARS-CoV-2 NP testing was negative and chest radiograph unremarkable on hospital day (HD) 1. Droplet precautions were discontinued. On HD 4, he was persistently febrile and diagnosed with Klebsiella ventilator-associated pneumonia by bronchoscopy, not performed under Airborne Infection Isolation Room (AIIR) precautions. SARS-CoV-2 testing was not repeated due to previously negative result and presumptive alternate explanation for fever. He developed increasing oxygen and pressor requirements on HD 6. Chest CT showed bilateral ground glass opacities (Fig. 2). Repeat NP testing was SARS-CoV-2 positive. He developed progressive lung injury, shock, cerebral herniation, then brain death. Retrospective testing of BALF from HD 4 was positive for SARS-CoV-2 with Ct of 11 for N1 and 12 for N2, indicating high viral load.
Fig. 2.
Case 1: CT Chest imaging shows (A) faint patchy upper lobe ground glass opacities, (B) bibasilar ground glass opacities and patchy consolidation.
3.2. Case 2
An 81-year-old woman with Alzheimer's Dementia was admitted from a nursing facility with pneumonia. NP testing for SARS-CoV-2 was negative 4 days earlier. She underwent outpatient treatment for presumed aspiration pneumonia. In the ED, she was hypoxemic, encephalopathic and was intubated; chest radiograph revealed patchy opacities. Repeat NP testing was negative, but ongoing concern for COVID-19 prompted bronchoscopy under AIIR precautions. BALF returned low-positive for SARS-CoV-2 with Ct 37.1 for N1 and undetectable for N2, indicating low viral load. BALF bacterial cultures were negative. On HD 6, she died from respiratory failure.
3.3. Case 3
A 68-year-old man 3 years post bilateral lung transplantation with recent episodes of acute cellular rejection presented in mid-April 2020 with declining home spirometry over 6–8 weeks. He denied fever, cough, or sick contacts, and had self-quarantined since early March to avoid exposure to SARS-CoV-2. Chest CT revealed apical consolidations. Bronchoscopy was performed to evaluate for persistent acute rejection. NP testing for SARS-CoV-2 two days prior and the morning of bronchoscopy were negative. BALF returned low-positive for SARS-CoV-2 with Ct of undetected for N1 and 39.8 for N2, indicating a viral load near the lower limit of detection. Transbronchial biopsies for rejection, BALF for other infections, and testing for donor specific antibodies were negative.
4. Discussion
These data highlight the need to consider SARS-CoV-2 infection despite negative NP testing and limitations in current testing algorithms that rely solely on URT PCR testing to guide infection precautions in the COVID-19 era. PCR detection of SARS-CoV-2 is considered the “gold standard” for the case definition of COVID-19 regardless of symptoms [2]. However, as these cases demonstrate, LRT samples may yield positive results when NP samples are negative. Negative NP testing may occur during a pre-symptomatic/early stage, while symptomatic with LRTI, or following infection. Older adults, those with underlying lung disease, and immunocompromised patients may represent particularly important groups in whom to maintain suspicion for SARS-CoV-2 LRTI despite negative URT testing. Discordance between PCR-based testing for common viruses from URT and LRT specimens has been recognized, particularly in immunosuppressed populations [9].
Many institutions require NP testing for SARS-CoV-2 prior to aerosol-generating procedures and consider COVID-19 “ruled out” after negative NP testing. This approach presumes LRTI is unlikely enough in persons with negative NP swabs that contact/droplet precautions are adequate. Our study provides evidence for the use of AIIR precautions, despite negative NP testing, for symptomatic patients (e.g. any concern for LRTI, decreased spirometry, pulmonary symptoms) undergoing bronchoscopy in areas with community spread of COVID-19 [10]. The widely divergent viral loads in these cases illustrate the heterogeneous nature of SARS-CoV-2 LRTI. Access to BALF SARS-CoV-2 testing is not universal, but testing is crucial to understanding diagnosis of LRTI.
5. Conclusions
NP testing for SARS-CoV-2 is the most widely used specimen for diagnosis, but false negative testing may underestimate the true prevalence of COVID-19. We identified SARS-CoV-2 LRTI in individuals with negative NP testing, which has implications for unintended healthcare worker exposure and spread of COVID-19 in the community.
Declaration of competing interest
All authors report no significant financial conflicts of interest related to this manuscript. KJR receives funding from the National Institutes of Health (K23HL138154-01) and the Cystic Fibrosis Foundation (RAMOS17A0, LEASE16A3).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.rmcr.2020.101120.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Nalla A.K., Casto A.M., Huang M.-L.W., Perchetti G.A., Sampoleo R., Shrestha L., Wei Y., Zhu H., Jerome K.R., Greninger A.L. Comparative performance of SARS-CoV-2 detection assays using seven different primer/probe sets and one assay kit. J. Clin. Microbiol. 2020;58(6) doi: 10.1128/JCM.00557-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Organization W.H. World Health Organization; 2020. Global Surveillance for COVID-19 Caused by Human Infection with COVID-19 Virus: Interim Guidance. 20 March 2020. [Google Scholar]
- 3.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wölfel R., Corman V.M., Guggemos W., Seilmaier M., Zange S., Müller M.A., Niemeyer D., Jones T.C., Vollmar P., Rothe C. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020:1–10. doi: 10.1038/s41586-020-2196-x. [DOI] [PubMed] [Google Scholar]
- 5.Wang W., Xu Y., Gao R., Lu R., Han K., Wu G., Tan W. Detection of SARS-CoV-2 in different types of clinical specimens. Jama. 2020;323(18) doi: 10.1001/jama.2020.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kimball A. Asymptomatic and presymptomatic SARS-CoV-2 infections in residents of a long-term care skilled nursing facility—king County, Washington, March 2020. MMWR Morb. Mortal. Wkly. Rep. 2020;69 doi: 10.15585/mmwr.mm6913e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.He X., Lau E.H., Wu P., Deng X., Wang J., Hao X., Lau Y.C., Wong J.Y., Guan Y., Tan X. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat. Med. 2020:1–4. doi: 10.1038/s41591-020-0869-5. [DOI] [PubMed] [Google Scholar]
- 8.Wei W.E., Li Z., Chiew C.J., Yong S.E., Toh M.P., Lee V.J. Presymptomatic transmission of SARS-CoV-2—Singapore, january 23–march 16, 2020. MMWR (Morb. Mortal. Wkly. Rep.) 2020;69(14):411. doi: 10.15585/mmwr.mm6914e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boonyaratanakornkit J., Vivek M., Xie H., Pergam S.A., Cheng G.-S., Mielcarek M., Hill J.A., Jerome K.R., Limaye A.P., Leisenring W. Predictive value of respiratory viral detection in the upper respiratory tract for infection of the lower respiratory tract with hematopoietic stem cell transplantation. J. Infect. Dis. 2020;221(3):379–388. doi: 10.1093/infdis/jiz470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wahidi M.M.S.S., Lamb C.R., Ost D., Maldonado F., Eapen G., Caroff D.A.S.M., Ouellette D.R., Lilly C., Gardner D.D., Glisinski K., Pennington K., Alalawi R. The use of bronchoscopy during the COVID-19 pandemic: CHEST/AABIP guideline and expert panel report. Chest. 2020;3692(20):30850–30853. doi: 10.1016/j.chest.2020.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.