Abstract
Background
Renal impairment is a risk factor for various adverse events, especially for death. In general, creatinine clearance (CrCl) is used for dose-adjustments of many drugs including oral anticoagulants, and estimated glomerular filtration rate (eGFR) is adopted for the diagnosis of chronic kidney disease. Predictive ability of CrCl versus eGFR for outcomes in patients with non-valvular atrial fibrillation (NVAF) remains controversial; therefore, this was compared using data from the J-RHYTHM Registry.
Methods
Out of 7406 outpatients with NVAF from 158 institutions, 6004 (age, 69.7 ± 9.9 years; men, 71.2%) having data of CrCl (mL/min, by the Cockcroft-Gault formula), eGFR (mL/min/1.73 m2, by the equations of the Japanese Society of Nephrology), and body surface area (BSA) were analyzed. C-statistics (area under the receiver-operating characteristic curve) of CrCl and eGFR for events were compared by DeLong's test.
Results
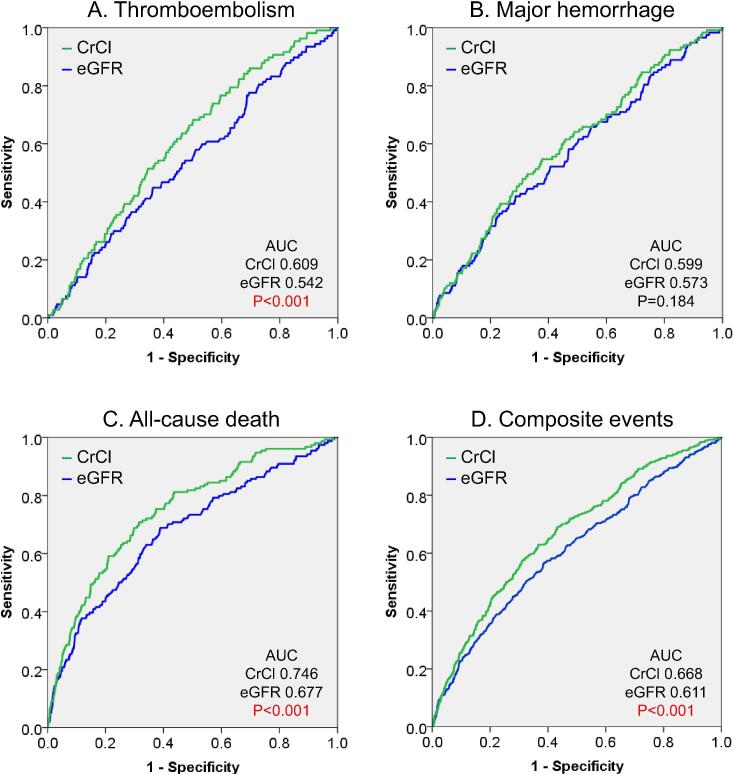
Thromboembolism, major hemorrhage, and all-cause death occurred in 107 (1.8%), 117 (1.9%), and 154 (2.6%) patients during the 2-year follow-up period. C-statistics of CrCl for each event were 0.609 (95% confidence interval, 0.559–0.658), 0.599 (0.548–0.657), and 0.746 (0.706–0.786); and those of eGFR were 0.542 (0.487–0.597), 0.573 (0.519–0.626), and 0.677 (0.631–0.723), respectively. C-statistics of CrCl for thromboembolism and all-cause death were significantly higher than those of eGFR (P < 0.001 for both). These results were consistent when BSA-unadjusted eGFR was used instead of eGFR (P = 0.002 for thromboembolism and P < 0.001 for all-cause death).
Conclusions
CrCl was superior to eGFR in the prediction of adverse outcomes, i.e., thromboembolism and all-cause death in Japanese patients with NVAF.
Keywords: Atrial fibrillation, Creatinine clearance, Estimated glomerular filtration rate, Thromboembolism, Mortality
1. Introduction
Renal impairment is a risk factor for various adverse events in patients with atrial fibrillation (AF) [1], [2], [3], [4], [5], [6] as well as in the general population [7], [8]. According to our previous reports in Japanese patients with non-valvular AF (NVAF), renal impairment is a stronger risk factor for all-cause death than for thromboembolism or major hemorrhage [9]; this was also true for low body mass index (BMI) [10] and low hemoglobin levels [11]. Although the estimated glomerular filtration rate (eGFR) is commonly adopted for the diagnosis of chronic kidney disease (CKD) [12] and is widely used for evaluation of renal function in a clinical practice, creatinine clearance (CrCl) calculated by the Cockcroft-Gault formula is typically used for dose-adjustments of many drugs including direct oral anticoagulants (DOACs) in patients with NVAF, as well as in other diseases [13]. However, the predictive ability of CrCl versus that of eGFR for outcomes in patients with NVAF remains controversial. Therefore, a post hoc analysis was performed using data of the J-RHYTHM Registry in order to compare the predictive ability for thromboembolism, major hemorrhage, and all-cause death between CrCl and eGFR in Japanese patients with NVAF. In addition, since body surface area (BSA)-unadjusted eGFR is often used to determine drug administration design in several clinical situations [14], the predictive ability of BSA-unadjusted eGFR for events was also evaluated in the present study.
2. Methods
2.1. Study design of the J-RHYTHM Registry
The J-RHYTHM Registry was conducted as a prospective observational study to evaluate the optimal anticoagulation therapy with warfarin in Japanese patients with AF [15]. The study design and baseline patient characteristics have been reported elsewhere [15], [16]. Briefly, the study protocol conformed to the Declaration of Helsinki and was approved by the ethics committee of each participating institution. A consecutive series of outpatients with AF of any type was enrolled from 158 institutions without any exclusion criterion regarding renal function. All participants gave written informed consent at the time of enrollment. All treatment strategies including antithrombotic therapy were determined at the discretion of the treating cardiologists. Patients with valvular AF (mechanical heart valve and mitral stenosis) were excluded from this subanalysis. Patients were followed up for 2 years or until the occurrence of an event, whichever occurred first. The following primary endpoints were assessed: thromboembolism, which included symptomatic ischemic stroke, transient ischemic attack (TIA), and systemic embolic events; major hemorrhage, which included intracranial hemorrhage, gastrointestinal hemorrhage, and other hemorrhages requiring hospitalization; and all-cause death. The composite of thromboembolism, major hemorrhage, and all-cause death, whichever occurred first for each patient, was also evaluated. The diagnostic criteria for each event have been described elsewhere [15], [16].
Anticoagulation intensity was determined using the prothrombin time international normalized ratio (PT-INR) in patients receiving warfarin, and the time in therapeutic range (TTR) was calculated by the method of Rosendaal [17]. The target PT-INR level was set at 1.6–2.6 for elderly patients aged ≥ 70 years and at 2.0–3.0 for patients aged < 70 years according to Japanese guidelines [18].
2.2. Evaluation of renal function and predictive ability for events
To estimate renal function, three different indices were calculated using the data for age, sex, body height (BH), body weight (BW), and serum creatinine concentration (sCr) at the time of enrollment. CrCl was calculated by the Cockcroft-Gault formula [19]: CrCl (mL/min) = (140 – age) × BW (kg)/72 × sCr (mg/dL) × (0.85 if female). eGFR was calculated by the equations of the Japanese Society of Nephrology [20]: eGFR (standard BSA-adjusted eGFR, mL/min/1.73 m2) = 194 × sCr (mg/dL)−1.094 × age−0.287 × (0.739 if female). BSA-unadjusted eGFR was calculated as follows: BSA-unadjusted eGFR (mL/min) = eGFR (mL/min/1.73 m2) × BSA (m2)/1.73. BSA was obtained from the DuBois formula [21]: BSA (m2) = BW (kg)0.425 × BH (cm)0.725 × 0.007184.
C-statistics of each renal function index for each outcome event were then obtained from the area under the receiver operating characteristic (ROC) curves (AUCs) as a marker of predictive ability for events. To confirm the consistency with results in the whole subjects, c-statistics for each event between CrCl and eGFR were compared in six subgroups of BSA (<1.73 vs. ≥ 1.73 m2), age (<70 vs. ≥ 70 years), sex (men vs. women), BMI (<25 vs. ≥ 25 kg/m2), BW (<60 vs. ≥ 60 kg), and eGFR (<60 vs. ≥ 60 mL/min/1.73 m2).
In addition, c-statistics of sCr, TTR, and established risk scores such as CHADS2 and CHA2DS2-VASc scores [22], [23] for each event were also calculated to compare with those of CrCl.
2.3. Statistical analysis
Data are presented as mean ± standard deviation. Relation between parameters was assessed by Pearson’s correlation coefficient analysis. AUCs for outcome events were compared between CrCl, eGFR, and BSA-unadjusted eGFR by DeLong's test [24]; those of CrCl were then compare with those of sCr, TTR, and clinical thromboembolic risk scores. Statistical analyses were performed with SPSS software version 23.0 (IBM Corporation, Armonk, NY, USA) and EZR version 1.40 [25] on R version 3.5.2 (The R Foundation for Statistical Computing, Vienna, Austria). Two-tailed P-values < 0.05 were considered statistically significant.
3. Results
Among the 7937 patients with AF who were enrolled in the J-RHYTHM Registry [16], 421 (5.3%) patients with valvular AF were excluded and 110 (1.5%) patients were lost to follow-up. Of the remaining 7406 patients with NVAF, 1402 patients were excluded due to missing BH, BW, and/or sCr data at the time of enrollment. Consequently, 6004 patients with all of CrCl, eGFR, and BSA values were included in this subanalysis.
3.1. Baseline patient characteristics and medications
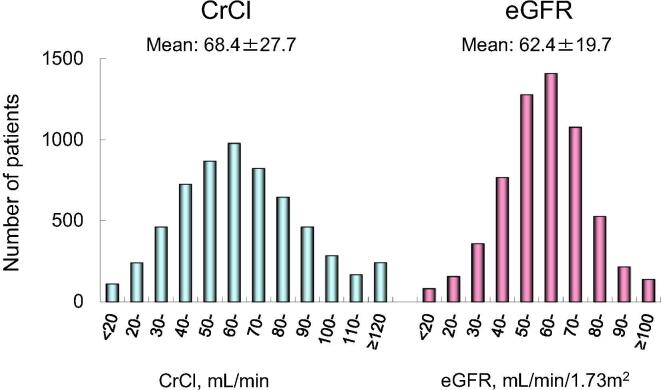
Baseline patient characteristics and medications are shown in Table 1. In 6004 patients (age, 69.7 ± 9.9 years; men, 71.2%), prevalence of heart failure, hypertension, age ≥ 75 years, diabetes mellitus, and a history of stroke or TIA was 28.1%, 60.1%, 34.4%, 19.2%, and 14.2%, respectively. Consequently, mean CHADS2 score [22] was 1.7 ± 1.2. Renal function indices of CrCl, eGFR, and BSA-unadjusted eGFR were 68.4 ± 27.7 mL/min, 62.4 ± 19.7 mL/min/1.73 m2, and 60.1 ± 20.4 mL/min, respectively. Distributions of CrCl and eGFR in this study population are shown in Fig. 1. These indices were significantly correlated with each other (CrCl and eGFR, r = 0.782, P < 0.001; CrCl and BSA-unadjusted eGFR, r = 0.922, P < 0.001; and eGFR and BSA-unadjusted eGFR, r = 0.935, P < 0.001). Anticoagulation therapy with warfarin was performed in 87.4% of patients and mean PT-INR was 1.90 ± 0.49 at the time of enrollment (Table 1). Baseline patient characteristics and medications in 1402 patients excluded from the present subanalysis are shown in Supplementary Table 1.
Table 1.
Baseline characteristics and medications.
| Number of patients | 6004 |
| Age, years | 69.7 ± 9.9 |
| Sex, male | 4275 (71.2) |
| Type of atrial fibrillation | |
| Paroxysmal | 2275 (37.9) |
| Persistent | 842 (14.0) |
| Permanent | 2887 (48.1) |
| Comorbidities | |
| Coronary artery disease | 673 (11.2) |
| Cardiomyopathy | 539 (9.0) |
| Congenital heart disease | 89 (1.5) |
| COPD | 114 (1.9) |
| Hyperthyroidism | 101 (1.7) |
| Risk factors for stroke | |
| Heart failure | 1727 (28.1) |
| Hypertension | 3655 (60.1) |
| Age (≥75 years) | 2063 (34.4) |
| Diabetes mellitus | 1154 (19.2) |
| Stroke or transient ischemic attack | 855 (14.2) |
| CHADS2 score | 1.7 ± 1.2 |
| CHA2DS2-VASc score | 2.8 ± 1.6 |
| Clinical parameters | |
| Body weight, kg | 62.3 ± 12.8 |
| BSA, m2 | 1.66 ± 0.19 |
| Body mass index, kg/m2 | 23.6 ± 4.0 |
| Serum creatinine, mg/dL | 0.97 ± 0.60 |
| Creatinine clearance, mL/min | 68.4 ± 27.7 |
| eGFR, mL/min/1.73 m2 | 62.4 ± 19.7 |
| BSA-unadjusted eGFR, mL/min | 60.1 ± 20.4 |
| Heart rate, /min | 72.5 ± 13.2 |
| Systolic blood pressure, mmHg | 126.0 ± 16.4 |
| Diastolic blood pressure, mmHg | 73.4 ± 15.5 |
| Medications | |
| Warfarin | 5248 (87.4) |
| Dosage, mg/day | 2.9 ± 1.2 |
| Baseline PT-INR | 1.90 ± 0.49 |
| TTR*, % | 59.3 ± 29.1 |
| Antiplatelet | 1601 (26.7) |
| Aspirin | 1385 (23.1) |
| Warfarin + antiplatelet | 1149 (19.1) |
Data are number of patients (%) or mean ± SD.
COPD, chronic obstructive pulmonary disease; CHADS2, congestive heart failure, hypertension, age ≥ 75 years, diabetes mellitus, and history of stroke or transient ischemic attack; CHA2DS2-VASc, CHADS2 components plus vascular disease (coronary artery disease), age 65–74 years, and female sex; eGFR, estimated glomerular filtration rate; BSA, body surface area; PT-INR, prothrombin time international normalized ratio; TTR, time in therapeutic range.
Target PT-INR was 2.0–3.0 (<70 years) or 1.6–2.6 (≥70 years).
Fig. 1.
Distributions of CrCl and eGFR, CrCl, creatinine clearance (mL/min); eGFR, estimated glomerular filtration rate (mL/min/1.73 m2).
3.2. Event rates and c-statistics of renal function indices for events
During the median follow-up period of 753 days, thromboembolism, major hemorrhage, and all-cause death occurred respectively in 107 (1.8%), 117 (1.9%), and 154 (2.6%) patients in 11,931 person-years; thus, incidence rates were 0.9, 1.0, and 1.3/100 person-years, respectively. These event rates were comparable with those in the excluded patients (Supplementary Table 2), although several clinical variables were significantly different between the included and the excluded patients (Supplementary Table 1).
C-statistics of CrCl, eGFR, and BSA-unadjusted eGFR for each event are summarized in Table 2. All c-statistics were statistically significant for events, except eGFR for thromboembolism (Table 2).
Table 2.
Predictive ability of renal function indices for events.
| CrCl |
eGFR |
BSA-unadjusted eGFR |
||||
|---|---|---|---|---|---|---|
| C-statistic (95% CI) | P value | C-statistic (95% CI) | P value | C-statistic (95% CI) | P value | |
| Thromboembolism | 0.609 (0.559–0.658) | <0.001 | 0.542 (0.487–0.597) | 0.134 | 0.574 (0.523–0.625) | 0.009 |
| Major hemorrhage | 0.599 (0.548–0.651) | <0.001 | 0.573 (0.519–0.626) | 0.007 | 0.567 (0.514–0.619) | 0.014 |
| All-cause death | 0.746 (0.706–0.786) | <0.001 | 0.677 (0.631–0.723) | <0.001 | 0.704 (0.660–0.749) | <0.001 |
| Composite events* | 0.668 (0.640–0.697) | <0.001 | 0.611 (0.580–0.642) | <0.001 | 0.630 (0.601–0.660) | <0.001 |
C-statistic, area under the receiver operating characteristic curve.
CrCl, creatinine clearance; eGFR, estimated glomerular filtration rate; BSA, body surface area; CI, confidence interval.
Thromboembolism, major hemorrhage, and all-cause death.
ROC curves of CrCl and eGFR for each event are shown in Fig. 2. Indices not adjusted for BSA (CrCl and BSA-unadjusted eGFR, mL/min) were superior to the index adjusted for BSA (eGFR, mL/min/1.73 m2) for prediction of outcome events, except for major hemorrhage (Table 3). For indices not adjusted for BSA, CrCl was superior to BSA-unadjusted eGFR for prediction of outcome events (Table 3).
Fig. 2.
ROC curves of CrCl and eGFR for thromboembolism (A), major hemorrhage (B), all-cause death (C), and composite events (D), ROC, receiver operating characteristic; AUC, area under the ROC curve; CrCl, creatinine clearance (mL/min); eGFR, estimated glomerular filtration rate (mL/min/1.73 m2). P values for comparison of AUCs between CrCl and eGFR by the DeLong’s test.
Table 3.
Comparison of predictive ability between renal function indices.
| Between CrCl and eGFR |
Between CrCl and BSA-unadjusted eGFR |
Between BSA-unadjusted eGFR and eGFR |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Difference of c-statistics | Z value | P value** | Difference of c-statistics | Z value | P value** | Difference of c-statistics | Z value | P value** | |
| Thromboembolism | 0.067 | 3.48 | <0.001 | 0.035 | 3.11 | 0.002 | 0.031 | 2.88 | 0.004 |
| Major hemorrhage | 0.027 | 1.33 | 0.184 | 0.033 | 2.90 | 0.004 | −0.006 | −0.51 | 0.611 |
| All-cause death | 0.069 | 4.85 | <0.001 | 0.041 | 4.36 | <0.001 | 0.027 | 3.48 | <0.001 |
| Composite events* | 0.057 | 5.59 | <0.001 | 0.038 | 6.21 | <0.001 | 0.019 | 3.15 | 0.002 |
C-statistic, area under the receiver operating characteristic curve.
CrCl, creatinine clearance; eGFR, estimated glomerular filtration rate; BSA, body surface area.
Thromboembolism, major hemorrhage, and all-cause death.
Comparison by the DeLong's test.
Additionally, c-statistics of sCr, TTR, and CHADS2 and CHA2DS2-VASc scores for each event are summarized in Supplementary Tables 3 and 4. None of them were significantly higher than those of CrCl.
3.3. Comparison of predictive ability between CrCl and eGFR in subgroups
Superiority of CrCl to eGFR for prediction of outcome events varied across subgroups based on clinical characteristics (Table 4). The superiority was observed for all outcome events in men, patients with BMI < 25 kg/m2, and those with eGFR ≥ 60 mL/min/1.73 m2. In women, the superiority was not seen for any event (Table 4).
Table 4.
Comparison of predictive ability between CrCl and eGFR in subgroups.
| A. C-statistics in subgroups by body surface area | ||||||
|---|---|---|---|---|---|---|
| BSA < 1.73 m2 (N = 3802) |
BSA ≥ 1.73 m2 (N = 2202) |
|||||
| CrCl | eGFR | P value** | CrCl | eGFR | P value** | |
| Thromboembolism | 0.548 | 0.492 | 0.337 | 0.631 | 0.607 | 0.455 |
| Major hemorrhage | 0.585 | 0.555 | 0.101 | 0.606 | 0.604 | 0.971 |
| All-cause death | 0.734 | 0.672 | <0.001 | 0.688 | 0.625 | 0.033 |
| Composite events* | 0.644 | 0.596 | <0.001 | 0.646 | 0.615 | 0.160 |
| B. C-statistics in subgroups by sex | ||||||
| Men (N=4275) |
Women (N=1729) |
|||||
| CrCl | eGFR | P value** | CrCl | eGFR | P value** | |
| Thromboembolism | 0.637 | 0.559 | 0.001 | 0.545 | 0.491 | 0.594 |
| Major hemorrhage | 0.629 | 0.576 | 0.017 | 0.567 | 0.598 | 0.480 |
| All-cause death | 0.750 | 0.667 | <0.001 | 0.781 | 0.727 | 0.068 |
| Composite events* | 0.689 | 0.614 | <0.001 | 0.648 | 0.622 | 0.200 |
| C. C-statistics in subgroups by age | ||||||
| Age<70 years (N=2717) |
Age≥70 years (N=3287) |
|||||
| CrCl | eGFR | P value** | CrCl | eGFR | P value** | |
| Thromboembolism | 0.610 | 0.462 | 0.144 | 0.533 | 0.509 | 0.691 |
| Major hemorrhage | 0.606 | 0.576 | 0.390 | 0.544 | 0.532 | 0.608 |
| All-cause death | 0.671 | 0.629 | 0.215 | 0.703 | 0.635 | <0.001 |
| Composite events* | 0.631 | 0.583 | 0.029 | 0.617 | 0.569 | <0.001 |
| D. C-statistics in subgroups by body mass index | ||||||
| BMI<25 kg/m2 (N=4100) |
BMI≥25 kg/m2 (N=1904) |
|||||
| CrCl | eGFR | P value** | CrCl | eGFR | P value** | |
| Thromboembolism | 0.593 | 0.531 | 0.004 | 0.642 | 0.568 | 0.019 |
| Major hemorrhage | 0.604 | 0.563 | 0.025 | 0.563 | 0.599 | 0.395 |
| All-cause death | 0.736 | 0.676 | <0.001 | 0.733 | 0.673 | 0.054 |
| Composite events* | 0.666 | 0.608 | <0.001 | 0.651 | 0.617 | 0.106 |
| E. C-statistics in subgroups by body weight | ||||||
| BW<60 kg (N = 2478) |
BW≥60 kg (N = 3526) |
|||||
| CrCl | eGFR | P value** | CrCl | eGFR | P value** | |
| Thromboembolism | 0.545 | 0.504 | 0.576 | 0.626 | 0.581 | 0.034 |
| Major hemorrhage | 0.606 | 0.558 | 0.005 | 0.585 | 0.582 | 0.912 |
| All-cause death | 0.720 | 0.663 | <0.001 | 0.703 | 0.657 | 0.016 |
| Composite events* | 0.649 | 0.594 | <0.001 | 0.643 | 0.612 | 0.018 |
| F. C-statistics in subgroups by eGFR | ||||||
| eGFR<60 mL/min/1.73 m2 (N=2640) |
eGFR≥60 mL/min/1.73 m2 (N=3364) |
|||||
| CrCl | eGFR | P value** | CrCl | eGFR | P value** | |
| Thromboembolism | 0.558 | 0.546 | 0.607 | 0.669 | 0.531 | <0.001 |
| Major hemorrhage | 0.571 | 0.583 | 0.669 | 0.633 | 0.545 | 0.016 |
| All-cause death | 0.713 | 0.631 | <0.001 | 0.668 | 0.497 | <0.001 |
| Composite events* | 0.645 | 0.603 | 0.005 | 0.661 | 0.527 | <0.001 |
C-statistic, area under the receiver operating characteristic curve.
CrCl, creatinine clearance; eGFR, estimated glomerular filtration rate; BSA, body surface area; BMI, body mass index; BW, body weight.
Thromboembolism, major hemorrhage, and all-cause death.
Comparison between CrCl and eGFR by the DeLong's test.
4. Discussion
The major findings of the present study were as follows. First, as a whole, the predictive ability of CrCl for outcome events was superior to that of eGFR, especially for thromboembolism and all-cause death. Second, the superiority of CrCl was consistent when BSA-unadjusted eGFR was used instead of eGFR. Finally, these trends were consistent across subgroups based on age, sex, body build, and renal function.
4.1. Renal impairment and adverse outcomes
Previous studies have reported that renal impairment is a potent risk factor for stroke [1], [2], [3], [4], bleeding complications [2], [26], [27], [28], [29], and all-cause and cardiovascular deaths [5], [6] in patients with AF. In our previous subanalysis of the J-RHYTHM Registry, renal impairment with CrCl < 30 mL/min was shown to be a stronger predictor of all-cause death than of thromboembolism or major hemorrhage in Japanese patients with NVAF [9]. Therefore, evaluation of renal function is crucial for predicting patient prognosis as well as for preventing thromboembolism and major hemorrhage in the total management of patients with NVAF.
4.2. Predictive ability of CrCl and eGFR for adverse events
CrCl has become an essential tool in patients with NVAF for the selection of oral anticoagulants and for the adjustment DOAC dosage. On the other hand, eGFR has been widely used for the diagnosis of CKD, the specific formula for which has been established for Japanese individuals [20]. Although both renal function indices are useful, it remains controversial as to which index is superior as a predictor of outcome events in patients with NVAF. Therefore, we compared the predictive ability between CrCl and eGFR for adverse outcomes in patients with NVAF in the present subanalysis.
Our results showed that c-statistics of CrCl for all outcome events were statistically significant (Table 2), indicating that CrCl can be used as a predictor of outcome events in patients with NVAF. In contrast, c-statistic of eGFR for thromboembolism was not significant (Table 2). In addition, CrCl was superior to eGFR for prediction of thromboembolism, all-cause death, and composite events (Table 3). This superiority of CrCl could be attributed to the inclusion of BW in the equation for calculating CrCl; eGFR is an estimated index of renal function without considering the body build of individual patients. Since low BW and low BMI are reportedly significant risk factors for adverse events, especially for all-cause death, in patients with NVAF [10], [30], it is reasonable to propose that predictive ability would improve when the equation includes BW in addition to age and sex.
In addition, c-statistics of sCr for thromboembolism, all cause death, and composite events were significantly lower than those of CrCl (Supplementary Table 3); a finding indicative of superiority of the predictive ability of CrCl for these events to that of simple sCr value.
4.3. Predictive ability of BSA-unadjusted eGFR for adverse events
If the above explanation were valid, what would the clinical significance of BSA-unadjusted eGFR be for predicting outcome events in NVAF patients?
In general, BSA-unadjusted eGFR is used to determine drug administration design in several clinical situations, especially for chemotherapy in patients with cancer [14]. eGFR estimates GFR for a standard BSA, but does not determine the actual GFR in individual patients. Therefore, eGFR often overestimates or underestimates the actual GFR depending on the patient’s body build [14]. Thus, the use of BSA-unadjusted eGFR (mL/min) is recommended for fixed-dose drugs (mg/day, BSA-independent), whereas eGFR (mL/min/1.73 m2) can be used for drugs for which the dose depends on BSA (mg/m2) or BW (mg/kg) [14]. Among several estimated renal function indices, BSA-unadjusted eGFR is reported to have the least bias along with the highest precision and accuracy against the reference GFR determined by the 99mTc-diethylene triamine penta-acetic acid plasma clearance method [31]. Therefore, we investigated the predictive ability of BSA-unadjusted eGFR for outcome events in addition to that of CrCl and eGFR in the present study.
As shown in Table 2, BSA-unadjusted eGFR can be used to predict outcome events in patients with NVAF. Moreover, BSA-unadjusted eGFR was superior to eGFR to predict these events (Table 3). When c-statistics were compared between CrCl and BSA-unadjusted eGFR for outcome events, CrCl was superior to BSA-unadjusted eGFR to predict all events (Table 3). Based on these results, among the three renal function indices, CrCl would be the most suitable for predicting adverse outcome events in patients with NVAF.
4.4. Predictive ability of TTR and established risk scores for adverse events
TTR and thromboembolic risk scores are useful for prediction of adverse outcome events in NVAF patients [22], [23], [32], [33], [34]. As shown in Supplementary Table 4, the predictive ability of CrCl was superior to that of TTR for all outcome events as well as to that of CHADS2 and CHA2DS2-VASc scores for all-cause death. Therefore, clinicians need to pay attention to renal function, especially CrCl, in addition to the quality of warfarin control or thromboembolic risk scores in the management of NVAF patients.
4.5. Implications of subgroup analysis
In general, CrCl calculated by the Cockcroft-Gault formula [19] overestimates GFR when sCr values are too low due to reduction of muscle mass caused by conditions such as sarcopenia, malnutrition, and emaciation, especially in elderly patients. Since these estimation errors might influence the predictive ability of renal function indices, we compared c-statistics between CrCl and eGFR for outcome events across subgroups by several clinical characteristics including BSA, sex, age, BMI, BW, and baseline eGFR. The results showed that c-statistics of CrCl for outcome events were mostly higher than those of eGFR, except for major hemorrhage in women, and in groups with BMI ≥ 25 kg/m2 and eGFR < 60 mL/min/1.73 m2 (Table 4). These findings indicated that the superiority of CrCl for prediction of outcome events would be universal in patients with NVAF. Notably, c-statistics of CrCl in men and in patients with BMI < 25 kg/m2 were significantly higher for all events (Table 4); thus, CrCl could be more useful than eGFR as a predictor of outcome events for such NVAF patients.
4.6. Limitation
The present study had several limitations. First, this study was a post hoc analysis of an observational study and was therefore, hypothesis-generating in nature. Warfarin was the only anticoagulant used at baseline in the present study; therefore, the results might not extrapolate to NVAF patients receiving DOACs. Second, the participants were recruited from only 158 institutions in Japan. Most participating physicians specialized in cardiology and in the management of cardiac arrhythmias. Therefore, these results cannot necessarily be extrapolated to the general Japanese population with NVAF. Third, since renal function indices were not directly measured using 24-h urine collection and were estimated by the equations based on sCr values [19], [20], they are not always equal to each other [35] or do not necessarily represent the actual GFR [31]. Renal function would change over time during the follow-up period. In the present analysis, only the baseline renal function indices were employed for analysis. Fourth, sCr values are generally measured using an enzymatic method in Japan, whereas the Cockcroft-Gault formula was originally determined based on the Jaffé method [19], in which sCr values are 0.2 mg/dL higher than those measured using an enzymatic method. In addition, eGFR is calculated by the equations of the Japanese Society of Nephrology [20] in Japan, whereas it is estimated by the equations of the Modification of Diet in Renal Disease (MDRD) [36] or the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) [37] worldwide. Therefore, present results cannot necessarily be extrapolated to patients in other countries. However, this study aimed to determine the predictive ability of renal function indices in a general clinical setting, but not to clarify the accuracy of the equations for CrCl and eGFR used in Japanese methods. Finally, owing to missing data, 1402 (18.9%) patients were excluded from the present analysis. However, event rates in the excluded patients were comparable with those that were included (Supplementary Table 2). Therefore, exclusion of 18.9% of patients might not affect the present results.
5. Conclusions
CrCl was superior to eGFR for the prediction of thromboembolism and all-cause death in Japanese NVAF patients. This was also true when BSA-unadjusted eGFR was used instead of eGFR.
6. Disclosures
Dr. Kodani received remuneration from Daiichi-Sankyo, Bristol-Myers Squibb, and Ono Pharmaceutical; Dr. Inoue received remuneration from Daiichi-Sankyo, Bayer Healthcare, Boehringer Ingelheim, and Bristol-Myers Squibb; Dr. Atarashi received remuneration from Daiichi-Sankyo; Dr. Tomita received research funding from Boehringer Ingelheim, Bayer, Daiichi-Sankyo, and Pfizer, and honorarium from Boehringer Ingelheim, Bayer, Daiichi-Sankyo, and Bristol-Myers Squibb; Dr. Okumura received research funding from Boehringer Ingelheim and Daiichi-Sankyo and remuneration from Boehringer Ingelheim, Bayer Healthcare, Daiichi-Sankyo, and Pfizer; Dr. Yamashita received research funding from Daiichi-Sankyo, Bayer Healthcare, and Bristol-Meyers Squibb and remuneration from Daiichi-Sankyo, Pfizer, Bayer Healthcare, Bristol-Myers Squibb, Toa Eiyo, and Ono Pharmaceutical; and Dr. Origasa received remuneration from Daiichi-Sankyo and Bayer Healthcare.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
We would like to thank all investigators of the J-RHYTHM Registry listed in references [15], [16].
Funding
The J-RHYTHM Registry is registered at the University Hospital Medicine Information Network (UMIN) Clinical Trials Registry (UMIN000001569) and was supported by a grant from the Japan Heart Foundation, Japan (12080025). This research was partially supported by the Practical Research Project for Life-Style related Diseases including Cardiovascular Diseases and Diabetes Mellitus from the Japan Agency for Medical Research and Development (AMED) (19ek0210082h0003).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijcha.2020.100559.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Go A.S., Fang M.C., Udaltsova N. Impact of proteinuria and glomerular filtration rate on risk of thromboembolism in atrial fibrillation: the anticoagulation and risk factors in atrial fibrillation (ATRIA) study. Circulation. 2009;119:1363–1369. doi: 10.1161/CIRCULATIONAHA.108.816082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Olesen J.B., Lip G.Y., Kamper A.L. Stroke and bleeding in atrial fibrillation with chronic kidney disease. N. Engl. J. Med. 2012;367:625–635. doi: 10.1056/NEJMoa1105594. [DOI] [PubMed] [Google Scholar]
- 3.Abumuaileq R.R., Abu-Assi E., Lopez-Lopez A. Renal function assessment in atrial fibrillation: Usefulness of chronic kidney disease epidemiology collaboration vs re-expressed 4 variable modification of diet in renal disease. World J. Cardiol. 2015;7:685–694. doi: 10.4330/wjc.v7.i10.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lau Y.C., Proietti M., Guiducci E., Blann A.D., Lip G.Y. Atrial fibrillation and thromboembolism in patients with chronic kidney disease. J. Am. Coll. Cardiol. 2016;68:1452–1464. doi: 10.1016/j.jacc.2016.06.057. [DOI] [PubMed] [Google Scholar]
- 5.Nakagawa K., Hirai T., Takashima S. Chronic kidney disease and CHADS(2) score independently predict cardiovascular events and mortality in patients with nonvalvular atrial fibrillation. Am. J. Cardiol. 2011;107:912–916. doi: 10.1016/j.amjcard.2010.10.074. [DOI] [PubMed] [Google Scholar]
- 6.Abe M., Ogawa H., Ishii M. Relation of stroke and major bleeding to creatinine clearance in patients with atrial fibrillation (from the Fushimi AF Registry) Am. J. Cardiol. 2017;119:1229–1237. doi: 10.1016/j.amjcard.2017.01.005. [DOI] [PubMed] [Google Scholar]
- 7.Go A.S., Chertow G.M., Fan D., McCulloch C.E., Hsu C.Y. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N. Engl. J. Med. 2004;351:1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 8.Nakayama M., Metoki H., Terawaki H. Kidney dysfunction as a risk factor for first symptomatic stroke events in a general Japanese population–the Ohasama study. Nephrol. Dial. Transplant. 2007;22:1910–1915. doi: 10.1093/ndt/gfm051. [DOI] [PubMed] [Google Scholar]
- 9.Kodani E., Atarashi H., Inoue H., Okumura K., Yamashita T., Origasa H. Impact of creatinine clearance on outcomes in patients with non-valvular atrial fibrillation: a subanalysis of the J-RHYTHM Registry. Eur. Heart J. Qual. Care Clin. Outcomes. 2018;4:59–68. doi: 10.1093/ehjqcco/qcx032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Inoue H., Kodani E., Atarashi H., Okumura K., Yamashita T., Origasa H. Impact of body mass index on the prognosis of Japanese patients with non-valvular atrial fibrillation. Am. J. Cardiol. 2016;118:215–221. doi: 10.1016/j.amjcard.2016.04.036. [DOI] [PubMed] [Google Scholar]
- 11.Kodani E., Inoue H., Atarashi H., Okumura K., Yamashita T., Origasa H. Impact of hemoglobin concentration and platelet count on outcomes of patients with non-valvular atrial fibrillation: a subanalysis of the J-RHYTHM Registry. Int. J. Cardiol. 2020;302:81–87. doi: 10.1016/j.ijcard.2019.11.127. [DOI] [PubMed] [Google Scholar]
- 12.Levey A.S., Eckardt K.U., Tsukamoto Y. Definition and classification of chronic kidney disease: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO) Kidney Int. 2005;67:2089–2100. doi: 10.1111/j.1523-1755.2005.00365.x. [DOI] [PubMed] [Google Scholar]
- 13.Heidbuchel H., Verhamme P., Alings M. Updated European Heart Rhythm Association Practical Guide on the use of non-vitamin K antagonist anticoagulants in patients with non-valvular atrial fibrillation. Europace. 2015;17:1467–1507. doi: 10.1093/europace/euv309. [DOI] [PubMed] [Google Scholar]
- 14.Horie S., Oya M., Nangaku M. Guidelines for treatment of renal injury during cancer chemotherapy 2016. Clin. Exp. Nephrol. 2018;22:210–244. doi: 10.1007/s10157-017-1448-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Atarashi H., Inoue H., Okumura K., Yamashita T., Origasa H. Investigation of optimal anticoagulation strategy for stroke prevention in Japanese patients with atrial fibrillation -The J-RHYTHM Registry study design. J. Cardiol. 2011;57:95–99. doi: 10.1016/j.jjcc.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 16.Atarashi H., Inoue H., Okumura K., Yamashita T., Kumagai N., Origasa H. Present status of anticoagulation treatment in Japanese patients with atrial fibrillation-a report from the J-RHYTHM Registry. Circ. J. 2011;75:1328–1333. doi: 10.1253/circj.cj-10-1119. [DOI] [PubMed] [Google Scholar]
- 17.Rosendaal F.R., Cannegieter S.C., van der Meer F.J., Briet E. A method to determine the optimal intensity of oral anticoagulant therapy. Thromb. Haemost. 1993;69:236–239. [PubMed] [Google Scholar]
- 18.JCS Joint Working Group: Guidelines for pharmacotherapy of atrial fibrillation (JCS 2013): Digest version, Circ. J. 78 (2014) 1997–2021. [DOI] [PubMed]
- 19.Cockcroft D.W., Gault M.H. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 20.Matsuo S., Imai E., Horio M. Revised equations for estimated GFR from serum creatinine in Japan. Am. J. Kidney Dis. 2009;53:982–992. doi: 10.1053/j.ajkd.2008.12.034. [DOI] [PubMed] [Google Scholar]
- 21.Du Bois D., Du Bois E.F. A formula to estimate the approximate surface area if height and weight be known. Arch. Intern. Med. 1916;17:863–871. [PubMed] [Google Scholar]
- 22.Gage B.F., Waterman A.D., Shannon W., Boechler M., Rich M.W., Radford M.J. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA. 2001;285:2864–2870. doi: 10.1001/jama.285.22.2864. [DOI] [PubMed] [Google Scholar]
- 23.Lip G.Y., Nieuwlaat R., Pisters R., Lane D.A., Crijns H.J. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest. 2010;137:263–272. doi: 10.1378/chest.09-1584. [DOI] [PubMed] [Google Scholar]
- 24.DeLong E.R., DeLong D.M., Clarke-Pearson D.L. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–845. [PubMed] [Google Scholar]
- 25.Kanda Y. Investigation of the freely available easy-to-use software 'EZR' for medical statistics. Bone Marrow Transplant. 2013;48:452–458. doi: 10.1038/bmt.2012.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wieloch M., Jonsson K.M., Sjalander A., Lip G.Y., Eriksson N., Svensson P.J. Estimated glomerular filtration rate is associated with major bleeding complications but not thromboembolic events, in anticoagulated patients taking warfarin. Thromb. Res. 2013;131:481–486. doi: 10.1016/j.thromres.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 27.Apostolakis S., Guo Y., Lane D.A., Buller H., Lip G.Y. Renal function and outcomes in anticoagulated patients with non-valvular atrial fibrillation: the AMADEUS trial. Eur. Heart J. 2013;34:3572–3579. doi: 10.1093/eurheartj/eht328. [DOI] [PubMed] [Google Scholar]
- 28.Jun M., James M.T., Manns B.J. The association between kidney function and major bleeding in older adults with atrial fibrillation starting warfarin treatment: population based observational study. BMJ. 2015;350 doi: 10.1136/bmj.h246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pisters R., Lane D.A., Nieuwlaat R., de Vos C.B., Crijns H.J., Lip G.Y. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest. 2010;138:1093–1100. doi: 10.1378/chest.10-0134. [DOI] [PubMed] [Google Scholar]
- 30.Hamatani Y., Ogawa H., Uozumi R. Low body weight is associated with the incidence of stroke in atrial fibrillation patients - Insight from the Fushimi AF Registry. Circ. J. 2015;79:1009–1017. doi: 10.1253/circj.CJ-14-1245. [DOI] [PubMed] [Google Scholar]
- 31.Chancharoenthana W., Wattanatorn S., Vadcharavivad S., Eiam-Ong S., Leelahavanichkul A. Agreement and precision analyses of various estimated glomerular filtration rate formulae in cancer patients. Sci. Rep. 2019;9:19356. doi: 10.1038/s41598-019-55833-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morgan C.L., McEwan P., Tukiendorf A., Robinson P.A., Clemens A., Plumb J.M. Warfarin treatment in patients with atrial fibrillation: observing outcomes associated with varying levels of INR control. Thromb. Res. 2009;124:37–41. doi: 10.1016/j.thromres.2008.09.016. [DOI] [PubMed] [Google Scholar]
- 33.Inoue H., Kodani E., Atarashi H., Okumura K., Yamashita T., Origasa H. Renal dysfunction affects anticoagulation control with warfarin and outcomes in Japanese elderly patients with non-valvular atrial fibrillation. Circ. J. 2018;82:2277–2283. doi: 10.1253/circj.CJ-18-0242. [DOI] [PubMed] [Google Scholar]
- 34.Tomita H., Okumura K., Inoue H. Validation of risk scoring system excluding female sex from CHA2DS2-VASc in Japanese patients with nonvalvular atrial fibrillation - subanalysis of the J-RHYTHM Registry. Circ. J. 2015;79:1719–1726. doi: 10.1253/circj.CJ-15-0095. [DOI] [PubMed] [Google Scholar]
- 35.Fernandez-Prado R., Castillo-Rodriguez E., Velez-Arribas F.J., Gracia-Iguacel C., Ortiz A. Creatinine clearance is not equal to glomerular filtration rate and Cockcroft-Gault equation is not equal to CKD-EPI Collaboration equation. Am. J. Med. 2016;129:1259–1263. doi: 10.1016/j.amjmed.2016.08.019. [DOI] [PubMed] [Google Scholar]
- 36.Levey A.S., Bosch J.P., Lewis J.B., Greene T., Rogers N., Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann. Intern. Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 37.Levey A.S., Stevens L.A., Schmid C.H. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.