Summary
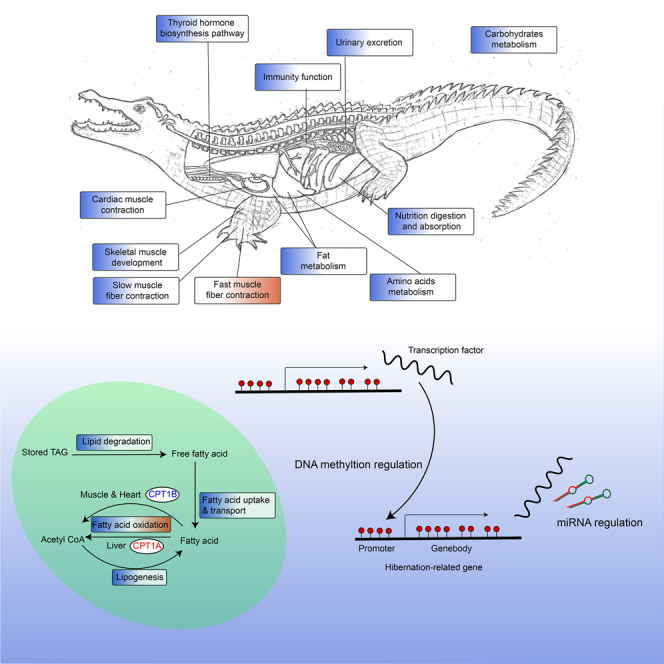
Many ectotherms hibernate in face of the harsh winter conditions to improve their survival rate. However, the molecular mechanism underlying this process remains unclear. Here, we explored the hibernation mechanism of Chinese alligator using integrative multi-omics analysis. We revealed that (1) the thyroid hormone biosynthesis, nutrition absorption and metabolism, muscle contraction, urinary excretion and immunity function pathways are overall downregulated during hibernation; (2) the fat catabolism is completely suppressed, contrasting with the upregulation of hepatic fatty-acid-transporter CPT1A, suggesting a unique energy-saving strategy that differs from that in hibernating mammals; (3) the hibernation-related genes are not only directly regulated by DNA methylation but also controlled by methylation-dependent transcription networks. In addition, we identified and compared tissue-specific, species-specific, and conserved season-biased miRNAs, demonstrating complex post-transcriptional regulation during hibernation. Our study revealed the genetic and epigenetic mechanisms underlying hibernation in the Chinese alligator and provided molecular insights into the evolution of hibernation regulation.
Subject Areas: Biological Sciences, Evolutionary Ecology, Transcriptomics
Graphical Abstract
Highlights
-
•
Metabolic and physiological pathways are overall suppressed during hibernation
-
•
Suppressed fat catabolism with active CPT1A suggests a unique energy-saving strategy
-
•
Hibernation-related genes are controlled by methylation-dependent transcription network
-
•
miRNAs play complex post-transcriptional regulation roles during hibernation
Biological Sciences; Evolutionary Ecology; Transcriptomics
Introduction
Many reptiles and amphibians survive winter in refuges where they enter a state of dormancy, which allows them to substantially save energy. This is often referred to as hibernation, although the process itself is very different from that in mammals (Staples, 2016), as the body temperature of ectotherms is cooled to an ambient temperature by the Q10 effect, rather than through blocking thermoregulatory heat production (Grigg and Kirshner, 2015). Nonetheless, akin to mammals, the metabolic rates of hibernating ectotherms are strongly suppressed and their physical states and cardiovascular functions are dramatically altered (Herbert and Jackson, 1985, Storey, 1996) to a level that exceeds the passive effects of cooling, processes that are critical for their long-term survival during hibernation.
The Chinese alligator (Alligator sinensis) is a critically endangered freshwater crocodilian endemic to China (Wan et al., 2013) that diverged from the American alligator (Alligator mississippiensis) 31–58 million years ago (Oaks, 2011). Both species of the Alligator genus live at higher, thus cooler, latitudes than other crocodilian species and enter hibernation so as to survive the cold winter (Chen et al., 2003, Grigg and Kirshner, 2015). Typically, the Chinese alligator stops eating and goes into hibernation when temperatures drop in late October until late March (Chen et al., 2003, Fang et al., 2015). During this time, their metabolism is strongly suppressed and the animals sleep continuously unless they are disturbed (Fang et al., 2015). The quality of this hibernation period (as defined by undisturbed sleep in a temperature-appropriate environment) is known to exert a crucial impact on their health (Chen et al., 2003, Xia et al., 2006, Zhang et al., 2003). In addition to human hunting and habitat disruption, global climate change is becoming a critical threat to Chinese alligators, as indeed it is for many other hibernators (Humphries et al., 2002, Inouye et al., 2000). Therefore, exploring the gene regulatory network underlying seasonal physiological changes is not only important for revealing how hibernating ectotherms overcome the cold and foodless winter in their habitat but may also ultimately aid their conservation in the future.
Various studies over the past half century have investigated the molecular mechanisms underlying hibernation, and numerous associated genes and pathways have been identified (Storey, 2006, Storey and Storey, 2007, Storey and Storey, 2013). With the development of high-throughput sequencing, mRNA-sequencing (mRNA-seq) has been used to explore the molecular and genetic bases of hibernation. However, these studies generally focused on mammalian hibernators (Cooper et al., 2016, Faherty et al., 2016, Faherty et al., 2018, Hampton et al., 2013, Lei et al., 2014, Luan et al., 2018, Nespolo et al., 2018), with only a few studies in reptiles (Capraro et al., 2019, Sun et al., 2018). As the two transcriptome studies in reptile hibernators focused on only three tissues (heart, skeletal muscle, and kidneys/brain), there is a clear interest in characterizing genome-wide regulatory networks underlying hibernation in the Chinese alligator using more tissues, especially those in charge of metabolism.
Epigenetic mechanisms mediate gene-environment interactions in various biological processes. DNA methylation is an ancient epigenetic modification in eukaryotic genomes that plays essential roles in various biological processes, including the regulation of gene expression, development, and stress responses (Breiling and Lyko, 2015, Pelizzola and Ecker, 2011, Smith and Meissner, 2013, Su et al., 2011). The level and pattern of DNA methylation typically varies among species and cell types. Usually, promoter methylation is negatively correlated with gene transcription levels, whereas methylation in the gene body is associated with active transcription (Jones, 2012). DNA methylation reportedly plays an important role in the regulation of gene expression associated with mammal hibernation (Alvarado et al., 2015, Biggar and Storey, 2014b, Fujii et al., 2006); however, previous studies largely focused on changes in overall DNA methylation levels, or methylation of specific genes. MicroRNAs (miRNAs) are another epigenetic mechanism involved in hibernation (Arfat et al., 2018, Biggar and Storey, 2015, Lyons et al., 2013). DNA methylation is associated with gene transcription potential, whereas miRNAs participate in post-transcriptional regulation. Although animal miRNAs are phylogenetically conserved (Ambros, 2004), seasonal expression changes vary among species and even tissues (Arfat et al., 2018, Biggar and Storey, 2015, Lyons et al., 2013).
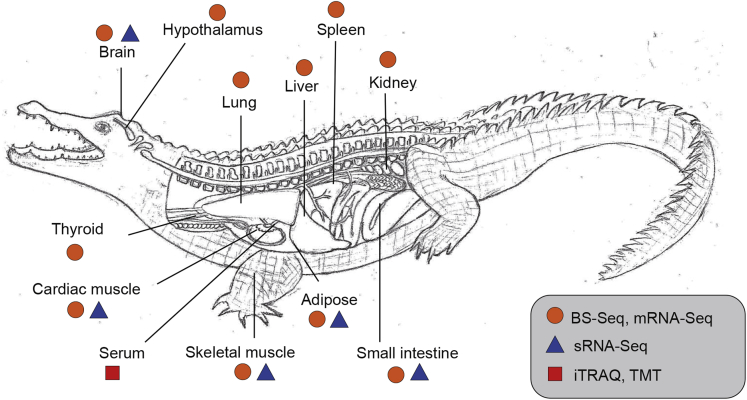
We collected tissues and serum samples from adult Chinese alligators in winter (the coldest time of the year in the Chinese alligator habitat) and summer (the active season of the Chinese alligator) and analyzed them using mRNA-seq, bisulfite sequencing (BS-seq), small RNA sequencing (sRNA-seq), and iTRAQ/TMT protein analysis to comprehensively explore the genetic and epigenetic mechanisms underlying reptile hibernation (Figure 1, Table S1, Supplementary Text).
Figure 1.
Chinese Alligator Tissue Samples Used in this Study
See also Table S1.
Results
Energy Metabolism Is Suppressed during Hibernation
To gain insights into the molecular mechanisms that enable the Chinese alligator to substantially save energy during hibernation, we compared the transcriptomes of tissues and proteomes of serum collected during winter and summer periods. The seasonal transcriptome variations were matched to the biological functions of different tissues. In order to correlate this with the BS-seq and sRNA-seq data, we focused on the transcriptomes of the female alligator in most of the subsequent analyses. However, we note that gene expression patterns were largely similar in tissues from male and female animals.
Thyroid Hormone Biosynthesis and Signaling
The hypothalamus-pituitary-thyroid axis is essential for metabolism regulation. Our results revealed that both the thyrotropin-releasing hormone gene (TRH) in the hypothalamus and the thyroid hormone biosynthesis pathway in the thyroid glands are downregulated during hibernation. KEGG pathway analysis revealed that the “thyroid hormone biosynthesis pathway” was enriched in downregulated differentially expressed genes (DEGs) during hibernation (winter-suppressed DEGs) in the thyroid gland (q < 0.05) (Table S2). Various genes involved in thyroid hormone biosynthesis, including TSHR, TG, and TPO, are suppressed in winter (Figure 2A). Correspondingly, serum levels of thyroid hormones (T3, T4, free T3, and free T4), TG antibody, and TPO antibody are substantially lower in winter (Table S3). iTRAQ/TMT analysis of serum samples indicated that von Willebrand factor (VWF), cartilage oligomeric matrix protein (COMP), and actin 5 (ACT5) in the “thyroid hormone signaling pathway” are significantly downregulated in winter (Table S4). We assumed that fuel use and physiological states would be altered significantly during hibernation (see below) owing to the downregulation of the thyroid hormone.
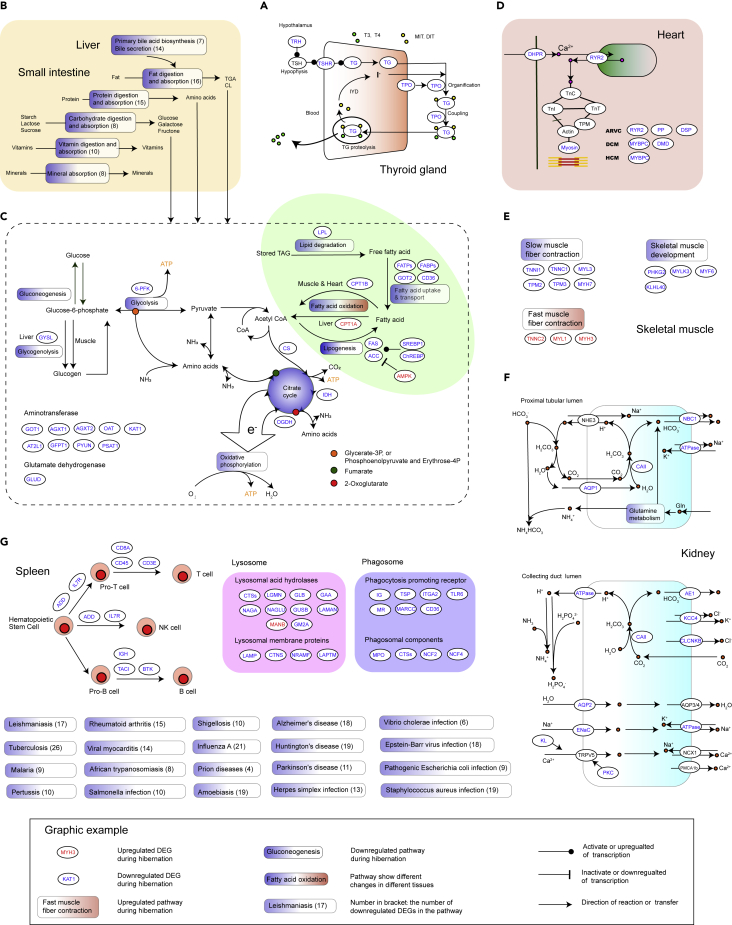
Figure 2.
Changes in Gene Expression in Pathways Participating in Energy Metabolism in Multiple Tissues during Hibernation in the Chinese Alligator
(A)Thyroid hormone synthesis pathway in the thyroid gland.
(B) Pathways involved in nutrient digestion and absorption in the liver and small intestine in the female alligator and corresponding numbers of winter-repressed DEGs (numbers in the brackets).
(C) Gene expression patterns in pathways participating in lipid metabolism (highlighted by green background), carbohydrate metabolism, and amino acid metabolism.
(D) DEGs involved in cardiac contraction and heart disease.
(E) DEGs involved in skeletal muscle contraction and development.
(F) Gene expression pattern in the pathways participating in renal function.
(G) Pathways involved in immunity and disease in the spleen.
See also Figures S1–S3 and Tables S2, S3, and S4.
Digestion and Absorption
Food digestion and absorption largely take place in the small intestine, and fat is digested with the aid of bile acid synthesized by the liver. KEGG pathway analysis revealed that “primary bile acid biosynthesis” (liver), “fat digestion and absorption,” “vitamin digestion and absorption,” and “mineral absorption” (small intestine) were significantly enriched (q < 0.05) in winter-suppressed DEGs (Figures 2B and S1A, Table S2). Significantly more downregulated than upregulated DEGs (Wilcoxon signed-rank test, q < 0.05) were observed in digestion- and absorption-related pathways (Figure S1). These results suggested that digestion and absorption in the small intestine and bile acid biosynthesis and secretion in the liver are suppressed during hibernation, which is consistent with the alligator's fasting state in winter.
Nutrient Metabolism
Carbohydrates are the primary energy source in animals and are degraded for ATP production through glycolysis/gluconeogenesis, the citrate cycle (tricarboxylic acid cycle), and oxidative phosphorylation. Significantly more winter-suppressed than winter-activated DEGs (Wilcoxon signed-rank test, q < 0.05) were observed in these three pathways (Figures 2C and S2A). Particularly, genes encoding rate-limiting enzymes of glycolysis (6-PFK) and the citrate cycle (CS, IDH, and OGDH) were significantly downregulated during hibernation in most tissues (Figure 2C). Pyruvate kinase genes (KPM and KPLR) showed limited seasonal differences in most tissues, whereas KPM and KPLR proteins in the serum were significantly downregulated during hibernation (Table S3), suggesting post-transcriptional regulation of these two factors. Glycogen serves as a form of energy storage mainly in the liver and muscles. Glycogen serves as a form of energy storage, mainly in the liver and muscles. The expression of the liver glycogen synthase gene GYSL, but not that of the muscle glycogen synthase gene GYSM or glycogen phosphorylase genes (GYPL, GYPM, and GYPB), was significantly downregulated during hibernation (Figure 2C). Conversely, the expression of the muscle glycogen phosphorylase gene GYPM was considerably upregulated, although not statistically significantly (q = 6.85 × 10−36, fold change = 1.48). These results suggested that fasting during hibernation leads to suppression of carbohydrate catabolism and hepatic glycogen synthesis, but stored glycogen may be, at least partly, a reserve energy source.
Fat is another form of energy storage. Unlike mammals, the fat catabolism pathway was not upregulated during hibernation; instead, the expression of lipid metabolic pathway genes was generally downregulated in the hibernating Chinese alligator. In adipose and liver tissues of female alligators, the “PPAR signaling pathway,” which is involved in lipid metabolism regulation, was significantly enriched (q < 0.05) in case of DEGs suppressed during the winter (Table S2). Lipoprotein lipase (LPL) hydrolyzes triacylglycerols in chylomicrons in adipose tissue, the heart, and skeletal muscle into fatty acid (FA) and glycerin, whereas hormone-sensitive lipase (HSL) hydrolyzes dietary fat. During hibernation, LPL expression was suppressed in adipose tissue, the heart, and skeletal muscle, whereas HSL expression was not significantly changed (Figures 2C and S2B). Genes involved in fatty acid transport and uptake, including the FATP family, FABP family, GOT2, and CD36, were also downregulated in winter (Figures 2C and S2B). FA oxidation is regulated by the rate-limiting enzymes CPT1A (liver) and CPT1B (heart and skeletal muscle) (Britton et al., 1997). During hibernation, CPT1A2 was upregulated, whereas CPT1B was downregulated (Figures 2C and S2B). These results suggested that, although utilization of stored fat decreased during hibernation, the Chinese alligator makes greater use of FAs in the liver to maintain its normal function and metabolic activity but saves energy by using less fat in the heart and skeletal muscle. This is a unique energy-saving strategy different from that in hibernating mammals, which extensively upregulate fat catabolism-related genes and fully utilize fat. Fatty acid biosynthesis takes place mainly in the liver. The KEGG pathways “biosynthesis of unsaturated fatty acids” and “fatty acid biosynthesis” were significantly enriched (q < 0.05) in winter-suppressed DEGs in the livers of female alligators (Table S2). In particular, key genes ACC and FAS, and their enhancing factors SREBP1 and ChREBP, were significantly downregulated, whereas ACC inhibitor AMPK was upregulated during hibernation (Figures 2C and S2B). This is an energy-saving strategy in the fasting alligator.
Amino acids are metabolized mainly in the liver. Amino acid metabolic genes were generally downregulated in the hibernating alligator. There were substantially more winter-suppressed than winter-activated DEGs in the amino acid metabolism pathways (Wilcoxon signed rank test, q < 0.05) (Figure S2C). Winter-suppressed DEGs were enriched for “glycine, serine, and threonine metabolism,” “tryptophan metabolism,” and “cysteine and methionine metabolism” (Table S2) and included aminotransferase genes (GOT1, AGXT1, AGXT2, OAT, KAT1, AT2L1, GFPT1, PYUN, and PSAT1) and a glutamate dehydrogenase gene (GLUD) (Figures 2C and S2D). These results suggested that amino acid metabolism is suppressed during hibernation.
Cardiac Muscle and Skeletal Muscle Contraction
During hibernation, the heart beats slower. Cardiac muscle contraction is a complex process initiated by Ca2+ influx. Seven voltage-dependent calcium channel genes (CACNA1C-1, CACNA1C-2, CACNA2D2-1, CACNA2D2-2, CACNA2D1, CACNB4, and CACNG2) were downregulated in the hibernating alligator (Figures 2D and S3A). Interestingly, six pathogenicity genes involved in heart failure, i.e., RYR2, DSP, and PKP2 for arrhythmogenic right-ventricular cardiomyopathy, DMD and MYBPC3 for dilated cardiomyopathy, and MYBPC3 for hypertrophic cardiomyopathy, were suppressed during hibernation (Figures 2D and S3A). The downregulation of genes involved in cardiac muscle contraction provides a molecular mechanism underlying the alligator's low heart rate during hibernation.
We also identified various season-biased DEGs involved in skeletal muscle function and development. PHKG2, MYLK3, KLHL40, and MYF6, involved in skeletal muscle development, were downregulated during hibernation (Figures 2E and S3B). TNNI1, TNNC1, TPM2, TPM3, MYL3, and MYH7, encoding slow-twitch skeletal muscle components, were also suppressed, whereas TNNC2, MYL1, and MYH3, encoding fast-twitch skeletal muscle components, were upregulated during hibernation (Figures 2E and S3B). These gene expression patterns are consistent with our previous finding that alligators show self-defense behavior when they are disturbed and awakened from hibernation but soon calm down and fall asleep again (Fang et al., 2015).
Urinary Excretion
With the significant decrease in metabolic gene expression, renal function-related gene expression was also expected to be suppressed during hibernation. Indeed, the KEGG pathways “collecting duct acid secretion” and “proximal tubule bicarbonate reclamation” were significantly enriched (q < 0.05) in winter-suppressed DEGs in the kidneys (Table S2). These DEGs included genes encoding CA2, which catalyzes the reversible hydration of carbon dioxide; SNAT3, GLS, and GLUD, which transport and catalyze glutamine to produce NH4+; and many transmembrane transporters in proximal tubular cells (NBC1, ATP1, ATP1B, and AQP1) and collecting duct intercalated cells (AE1, KCC4, CLCNKB, and seven ATPases), which transport ions across urine, cytoplasm, and blood. Various genes involved in the reabsorption of water, calcium, and sodium were downregulated, including AQP2 and ENACA, ENACB and ENACG, as well as VDR, PTHR, and KL, the protein products of which regulate Ca2+ channel expression and apical abundance (Figures 2F and S3C). These results indicated that kidney function is suppressed in the hibernating alligator.
Immunity
The spleen is the largest immune organ and is a reservoir of macrophages and lymphocytes, which act as scavengers and in immune defense against pathogens, respectively. Winter-suppressed DEGs in the spleen were enriched in KEGG pathways involved in immunity and hematopoiesis (Table S2). In particular, the downregulated DEGs included genes crucial for lymphocyte production, including ADD, IL7R (NK-cell and pro-T-cell production), CD3E, CD45, CD8A (T cell production), IGH, BTK, and TACI (B-cell production) (Figure 2G), suggesting that lymphocyte proliferation is suppressed during hibernation. Various genes encoding lysosomal acid hydrolases and lysosomal membrane proteins were significantly downregulated. Genes encoding phagosomal components, including MPO, NCF2, NCF4, and CTL, were downregulated, indicating that phagocytosis is suppressed during hibernation (Figure 2G). In addition, various winter-suppressed DEGs are involved in infectious diseases (leishmaniasis, malaria, asthma, tuberculosis, pathogenic Escherichia coli infection, etc.) (Figure 2G, Table S2). These results suggested that, as the hibernating Chinese alligator stays in a refuge, where less exogenous pathogens are present, immunity and hemopoiesis are suppressed to save energy.
Factors Actively Upregulated during Hibernation
Several factors actively upregulated during hibernation were identified in the Chinese alligator. The expression of cold-inducible RNA-binding protein genes (CIRBP) can be induced simply by cold stress (Saito et al., 2000, Sugimoto and Jiang, 2008). In nearly all tissues, the transcription of CIRBP was actively upregulated, especially in the brain, thyroid, liver, small intestine, adipose tissues, kidneys, heart, and skeletal muscle (Figure S3D). These results suggested that CIRBP plays a critical, although not fully elucidated, role in the ectotherm hibernator. Unexpectedly, c-FOS, a neural activity marker gene (Mateju et al., 2009), was also overexpressed during hibernation in most central and peripheral organs, except the thyroid gland (Figure S3E), suggesting active neural states in the hibernating alligator and its positive regulation during hibernation. Furthermore, general transcription factor (GTF) genes were generally activated during winter (Wilcoxon signed-rank test, q = 7.896E-005) (Figure S3F). Thus, the downregulation of genes participating in thyroid hormone biosynthesis, nutrient absorption and metabolism, urinary excretion, and immunity during hibernation is likely not simply regulated via GTF gene repression but by more specific and complex pathways, such as DNA methylation and miRNAs.
DNA Methylation Landscapes in the Hibernating Chinese Alligator
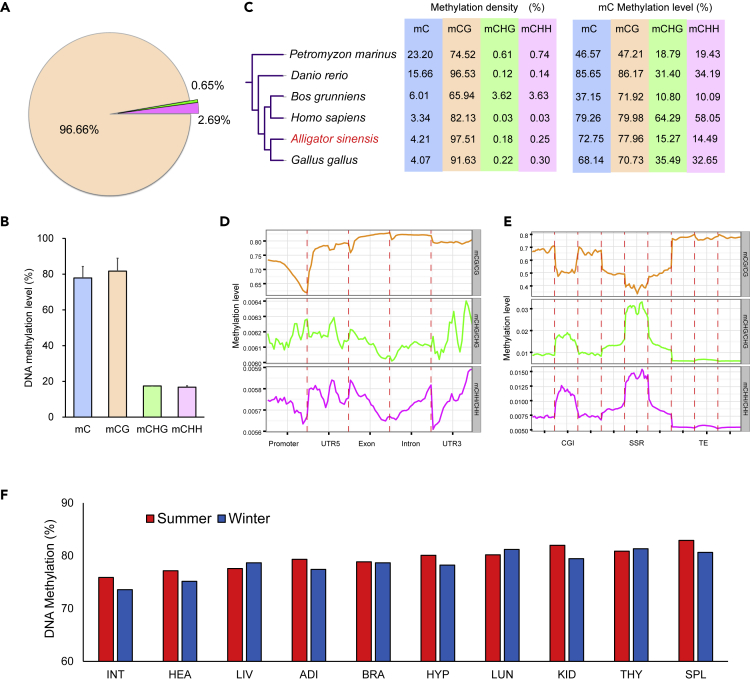
An average of 24.43 M methylated cytosines (mCs) were identified by BS-seq in each tissue sample, accounting for 2.49% of cytosines (Cs) in the Chinese alligator. Most mCs (96.66%) were in CpG context (Figure 3A), whereas mCs in CHG and CHH contexts were rare and were substantially less methylated (Figures 3A and 3B), consistent with findings in other vertebrates (Figure 3C). Therefore, we focused on CpG sites in most of the subsequent analyses.
Figure 3.
DNA Methylation Patterns in the Hibernating Chinese Alligator
(A) Percentage of methylated cytosines (mC) identified in each sequence context
(B) Average mC level in each indicated sequence context.
(C) mC densities and levels in six chordate species.
(D) DNA methylation levels in different gene features in the Chinese alligator. Promoters encompass 2 kb upstream of the transcription start site.
(E) DNA methylation levels in CG island (CGI), simple-sequence repeat (SSR), and transposable element (TE) regions and 2 kb upstream and downstream.
(F) DNA methylation level in the CG context of each tissue in inactive (winter) and active (summer) periods.
See also Figure S4.
DNA methylation patterns varied among genome regions. In transcribed regions and ∼2 kb upstream and downstream of these regions, CG methylation levels were lowest in the promoter, where the methylation level gradually declined to a minimum at the transcription start site (TSS) and then increased again in the 5′ UTR. CG methylation levels were highest in the exon and intron regions but slightly decreased in the 3′ UTR (Figure 3D). These results suggested that DNA methylation may participate in the regulation of transcription initiation. We also evaluated methylation levels in GC islands, microsatellites, transposable elements, and their adjacent regions. The relatively higher CG methylation levels within transposable elements suggested suppression of active transposons (Figure 3E). No significant difference was found in global DNA methylation patterns between winter and summer samples in terms of methylation broadness and deepness levels (p > 0.05), suggesting that the regulation of DNA methylation in hibernation is not simply through overall hyper- or hypomethylation (Figure 3F, Figure S4).
Correlation between DNA Methylation Status and Gene Expression
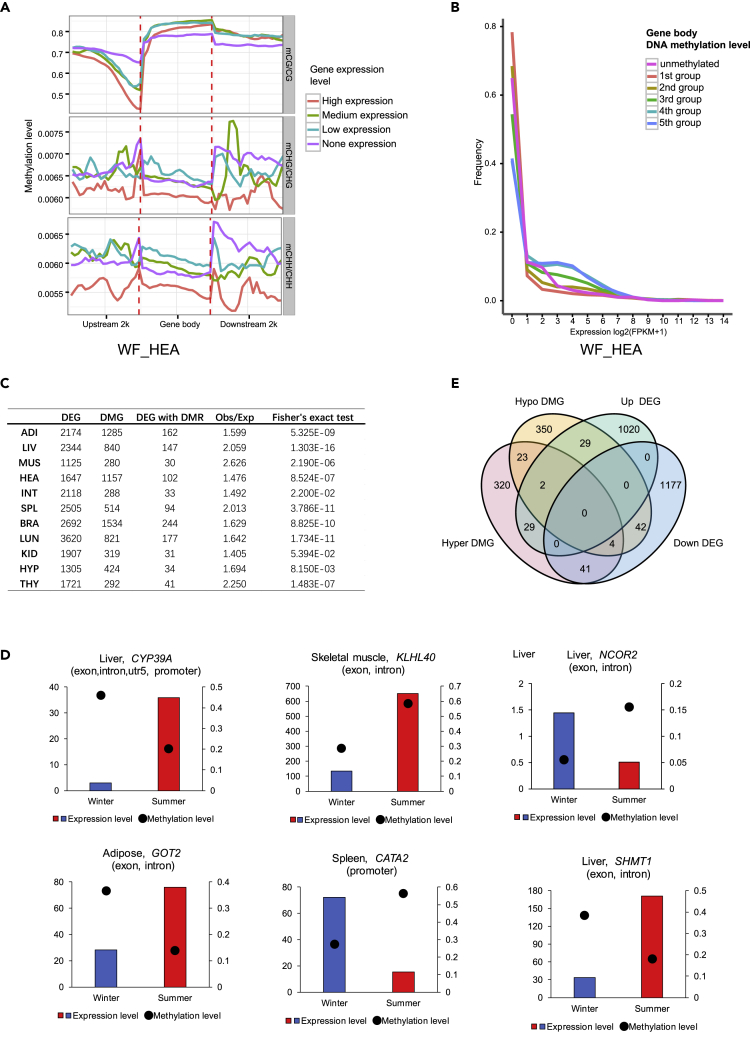
To explore potential regulatory roles of DNA methylation in gene expression in the hibernating Chinese alligator, we correlated mRNA-seq data with BS-seq data obtained from the same tissues. Promoter CG methylation was negatively correlated with gene expression. As for CG methylation in the gene body and downstream thereof, non-expressed genes had the lowest DNA methylation levels, whereas genes with intermediate expression demonstrated the highest DNA methylation levels (Figure 4A). Genes with the highest gene-body methylation levels (fourth and fifth groups in Figure 4B) tended to be expressed at intermediate levels. These results suggested that promoter hypermethylation is associated with transcriptional repression, whereas gene-body methylation plays a role in the normalization of gene overexpression.
Figure 4.
DNA Methylation Regulation of Gene Expression during Chinese Alligator Hibernation
(A) Distributions of methylation levels within gene bodies and 2-kb upstream and downstream regions by different expression levels. Based on the expression level, protein-coding genes were divided into two groups: non-expressed genes (FPKM <1) and expressed genes. The latter were further divided into three groups: low-expression genes (FPKM < lower quartile), intermediate-expression genes (upper quartile < FPKM < upper quartile), and high-expression genes (FPKM > upper quartile).
(B) Expression profiles of methylated and unmethylated genes. Methylated genes were further divided into five groups based on the methylation level in their gene body (20% quintiles).
(C) Correlations between season-biased differentially expressed genes (DEGs) and differentially methylated regions (DMRs).
(D) Venn diagram of season-biased DMGs and DEGs in the liver.
(E) Expression levels of DEGs CYP39A (liver), KLHL40 (skeletal muscle), NCOR2 (liver), GOT2 (adipose), CATA2 (spleen), and SHMT1 (liver) and methylation levels in the corresponding DMRs.
To explore DNA methylome alterations in the hibernating Chinese alligator, we identified differentially methylated genes (DMGs) between winter and summer samples. DEGs were significantly enriched among DMGs in most tissues (q < 0.05), except the kidneys (q = 0.054) (Figure 4C), suggesting that DNA methylation does regulate transcription. Some genes involved in physiological function regulation in hibernation exhibited altered methylation. For example, CYP39A transcription was significantly suppressed in the liver during hibernation, with a hyper differentially methylated region (DMR) from promoter to gene body (Figure 4D). Both HLHL40 and NCOR2 were hypermethylated in the gene body, but HLHL40 was downregulated in hibernating skeletal muscle, whereas NCOR2 was upregulated in the liver (Figure 4D). These results indicated that DNA methylation is, at least in part, responsible for adaptive transcriptional changes during hibernation, but its roles are much more complicated than previously realized. Indeed, the correlations between hyper- and hypo-DMGs, and down- and upregulated DEGs, were substantially more complex than anticipated. For example, 45 genes were hypermethylated and downregulated, whereas 31 genes were hypomethylated and upregulated in the liver during hibernation. However, 31 hyper-DMGs and 46 hypo-DMGs were up- and downregulated, respectively (Figure 4E).
Furthermore, a large number of DEGs (2,197) did not overlap with DMGs (Figure 4E), suggesting that seasonal changes in the expression of these genes may not be directly regulated by DNA methylation but rather result from methylation-dependent alterations in transcription networks.
DNA Methylation Alterations in TFs and Their Regulatory Networks during Hibernation
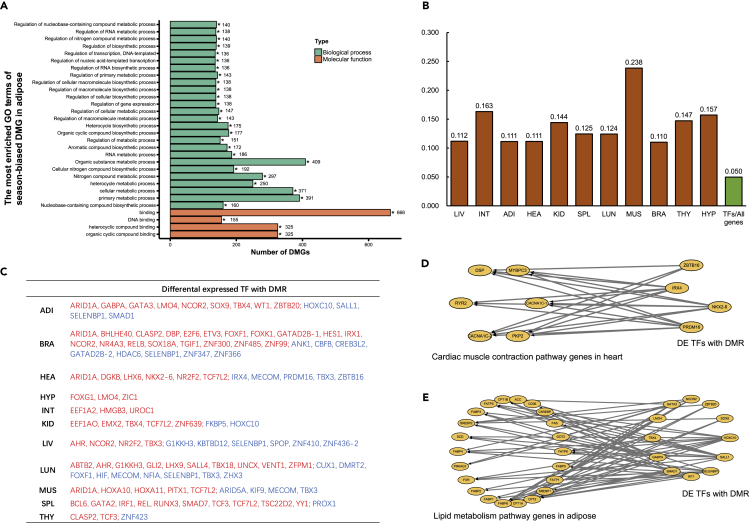
To investigate the role of methylation alterations in gene regulatory networks during hibernation, we subjected the season-biased DMGs to GO analysis, which revealed that, in nearly all tissues, GO terms referring to regulation of gene expression and metabolic process were significantly enriched (q < 0.05) in season-biased DMGs (Figure 5A). We identified 1,370 transcription factor (TF) genes in the Chinese alligator genome and analyzed them in relation to seasonal DMGs in each tissue. The observed ratio of differentially methylated TFs versus DMGs was significantly higher than expected (1,370/27,500) (Fisher's exact test, q < 0.05) (Figure 5B), suggesting that genes involved in gene expression regulation, especially TF genes, are more likely to be differentially methylated. To explore methylation-dependent regulatory networks in the hibernating Chinese alligator, we identified differentially expressed TFs with DMRs in each tissue (Figure 5C). Furthermore, we performed weighted gene co-expression network analysis (WGCNA) based on transcriptome data and constructed methylation-dependent regulatory networks for each tissue (Figures 5D, 5E, and S5). Most of the season-biased DEGs were regulated by the differentially methylated TFs in the transcription networks.
Figure 5.
Regulation of Differentially Expressed and Differentially Methylated Transcription Factor (TF) Genes
(A) Thirty most enriched GO terms in season-biased differentially methylated genes (DMGs) in adipose tissues.
(B) Ratio of differentially methylated TF genes to DMGs.
(C) Correlation between differentially expressed TFs and DMRs.
(D and E) Associations between differentially expressed and differentially methylated TFs and cardiac muscle contraction pathway DEGs in the heart (D) and lipid metabolic pathway DEGs in adipose tissues (E).
See also Figure S5.
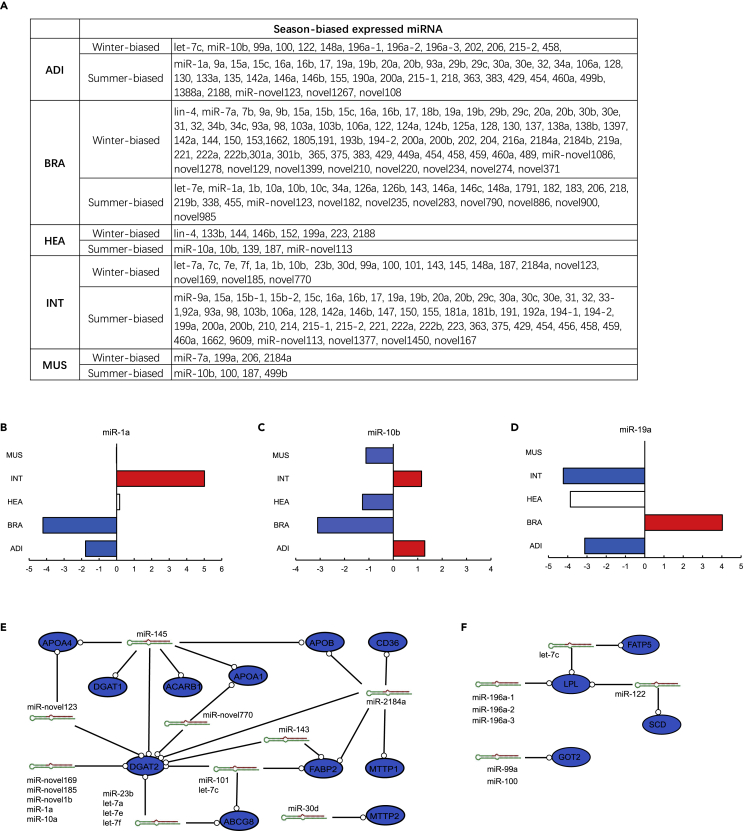
miRNA Regulation during Hibernation
miRNAs reportedly play a crucial role in gene expression regulation during hibernation (Arfat et al., 2018, Biggar and Storey, 2015, Lyons et al., 2013). Thus, we carried out sRNA-seq using adipose, brain, heart, small intestine, muscle, and gonad tissues to identify season-biased differentially expressed miRNAs (DEmiRs, q < 0.05). Some hibernation-related miRNAs are conserved and play roles in other hibernators. For example, miR-103, miR-124 (brain), and miR-206 (skeletal muscle) were upregulated in the hibernating alligator (Figure 6A), and these expression changes were also reported in corresponding tissues of the hibernating little brown bat (Myotis lucifugus) (Biggar and Storey, 2014a, Kornfeld et al., 2012). We also discovered new hibernation-related miRNAs, some of which are specific to the Chinese alligator (Figure 6A). The roles of miRNAs in gene expression regulation were found to be tissue specific. Their expression levels varied in different tissues during hibernation (Figures 6B–6D). For example, miR-10b was upregulated in the small intestine and adipose tissues during hibernation but downregulated in the brain, heart, muscle, and ovaries (Figure 6C). Similar patterns were observed for miR-1a and miR-19a (Figures 6B and 6D). Furthermore, the expression patterns suggested that the roles of some miRNAs are species specific. MiR-200a was downregulated in the adipose tissues of the hibernating Chinese alligator (Figure 6A) but reportedly is upregulated in hibernating thirteen-lined ground squirrels (Ictidomys tridecemlineatus) (Wu et al., 2014). MiR-1a is upregulated in the muscle in several hibernators (Arfat et al., 2018, Biggar and Storey, 2015) but not in the Chinese alligator.
Figure 6.
MiRNA Regulation of Gene Expression during Chinese Alligator Hibernation
(A) Functional key genes targeted by season-biased differentially expressed miRNAs (DEmiRs) in each tissue.
(B–D) Seasonal expression changes for miR-1a (B), miR-10b (C), and miR-19a (D).
(E and F) Relations between DEmiRs and differentially expressed genes (DEGs) in fat digestion and absorption in the small intestine (E) and lipid metabolic pathway genes in adipose tissues (F).
See also Table S5.
To explore the regulatory roles of annotated miRNAs during hibernation in the Chinese alligator, we predicted target genes of season-biased DEmiRs. Various genes involved in functional regulation during hibernation in each tissue were targeted by miRNAs (Table S5), including DGAT1, DGAT2, APOA1, APOA4, APOB, ABCG8, CD36, MTTP1, MTTP2, FABP2 and SCARB1, which are related to fat digestion and absorption and were downregulated in the small intestine (Figure 6E), and LPL, FATP6, SCD, and GOT2, which are related to lipid metabolism and were downregulated in adipose tissues during hibernation (Figure 6F). One gene could be regulated by multiple miRNAs and, conversely, one miRNA could target multiple genes, thus forming a complex regulatory network (Figures 6E and 6F).
Discussion
Although hibernation in ectotherms seems quite similar to that in endotherms, they are quite different in behavioral, physiological, and biochemical traits (Grigg and Kirshner, 2015, Staples, 2016). In hibernating mammals, the metabolic rate is suppressed during winter, along with a decrease in the core body temperature, which results in substantial energy saving (Hampton et al., 2013, Staples, 2016); however, periodic interbout arousals during hibernation still consume much energy (Karpovich et al., 2009). On the other hand, ectotherm hibernators exhibit continuous metabolic suppression exceeding the passive thermal effects due to a decrease in the environmental temperature in winter (Grigg and Kirshner, 2015, Staples, 2016).
In mammals, the entering of hibernation is accompanied by a switch of fuel use from carbohydrates to lipids, which provide the most energy-dense metabolic substrate (Sheriff et al., 2013). Previous transcriptome studies in hibernating mammals have provided molecular evidences for this switch. For example, in the white adipose tissue of hibernating free-ranging dwarf lemurs in a wild population, several genes involved in lipid catabolism (e.g., PLPP1, APOC2, SCD, FASN, and ELOVL6) and carbohydrate oxidation repression (PDK4) were induced, whereas several genes involved in carbohydrate catabolism (e.g., PDHA1, PDHB, DLAT, and DLD) were downregulated (Faherty et al., 2018). In the brown adipose tissue of hibernating thirteen-lined ground squirrel, many genes participating in lipolysis (e.g., PNPLA2, PLIN2, PLIN4, and PLIN5) and lipid transport (e.g., OBP2B, FABP3, SLC25A20, CPT1A, and CPT2), as well as PDK4 are upregulated (Hampton et al., 2013). In addition, in the past three decades, many studies on gene expression, epigenetic regulation, proteins, enzymes, and posttranslational modification have provided evidence supporting the switch of fuel use in mammalian hibernators (Carey et al., 2003, Staples, 2016).
The main energy source and the energy-saving strategy of hibernating ectotherms are still unclear. Similar to mammal hibernators, the common lizard (Lacerta vivipara) and gecko (Phyllodactylus marmoratus) reportedly rely on fat stores during hibernation (Avery, 1970, Daniels, 1984), whereas another study suggested that glycogen, not lipids, limits winter survival of side-blotched lizards (Uta stansburiana) (Zani et al., 2012). Both liver- and muscle-stored glycogens are substantially consumed during hibernation and considerably account for the winter energy budget of several lizard and snake species (Costanzo, 1985, Dessauer, 1953, Zani et al., 2012). Transcriptomic studies on the heart, skeletal muscle, and kidneys/brain of Chinese alligator and Australian central bearded dragon have identified candidate genes and pathways that are involved in seasonal adaption and tissue-specific function maintenance and have provided valuable insights into the molecular regulatory mechanism underlaying reptile hibernation (Capraro et al., 2019, Sun et al., 2018). In our study, we extend the analysis to other important tissues, including the brain, hypothalamus (central control), thyroid gland (metabolism regulation), small intestine (nutrient digestion and absorption), liver (metabolism), adipose (energy storage), lung (gas exchange), heart (blood supply), skeletal muscle (movement), and spleen (immunity), to provide a comprehensive transcriptome profile of the hibernating reptile.
Our data revealed a unique energy-saving strategy in the hibernating Chinese alligator during hibernation. Adapting to the fasting state, the hibernating alligator suppressed pathways related to nutrition absorption and metabolism. Overall, genes in fat catabolism pathways were dramatically downregulated in the hibernating Chinese alligator, except for liver CPT1A, which was significantly upregulated, suggesting that the fat metabolism pathways are generally suppressed instead of activated during alligator hibernation. However, β-oxidation of FA in the liver, but not in the muscle, was activated to use the limited FAs, ensuring the energy demands of the liver as the metabolic center. This is supported by our finding during tissue collection that the amount of adipose tissue did not differ significantly between winter and summer. In addition, glycogen phosphorylase genes were not downregulated like other genes in carbohydrate metabolism; instead, the muscle glycogen phosphorylase gene GYPM was considerably upregulated (q = 6.85 × 10−36, fold change = 1.48), suggesting that glycogen, especially that stored in muscle, may be another reserve energy source of the hibernating Chinese alligator. Our results provide molecular evidence that glycogenolysis in muscle and β-oxidation of hepatic FA supply scarce energy for the hibernating Chinese alligator with suppressed carbohydrate and fat catabolism. Through downregulation of thyrotropin-releasing hormone gene and its downstream thyroid hormone biosynthesis pathway, nutrition absorption and metabolism, cardiac and show skeletal muscle contraction, and urinary excretion and immunity function pathways were also generally downregulated during hibernation, reflecting a coordinated suppression of the metabolic rate and physiological states. However, a few upregulated genes in these pathways, for example, CPT1A and AMPK, which, respectively, catalyze FA catabolism and inhibit FA synthesis in the liver, as well as upregulated genes involved in fast muscle fiber contraction (TNNC2, MYL1, and MYH3), reveal the ingenious energy utilization and survival strategies in this species.
In Australian central bearded dragon, the enrichment of “lipid catabolic processes” and “carbohydrate catabolic processes” GO terms in downregulated genes suggest an overall suppression of lipid and carbohydrate catabolism, but the upregulation of carbohydrate metabolism genes, such as PFKFB3, GSK3A, and FBP1, which are important in glycolysis, glycogen synthesis, and gluconeogenesis, respectively, indicates a different strategy of fuel use and energy saving (Capraro et al., 2019). Using seasonal transcriptome data from the kidneys, skeletal muscle, and heart in the Chinese alligator reported by another research group (Sun et al., 2018), we were able to explore whether the adaptive mechanisms are similar between the only two Chinese alligator populations. We identified five DEGs (62.5%) among the eight seasonal DEGs reported in their study, including CSRP3, AT1A1, PCKGC, KCRB, and CIRBP. In addition, the two datasets share many of the KEGG pathways enriched in seasonal DEGs. These results support the repeatability and universality of our data.
DNA methylation reportedly plays an important role in the regulation of gene expression associated with mammalian hibernation (Alvarado et al., 2015, Biggar and Storey, 2014b, Fujii et al., 2006). TFs read DNA methylation and translate the information into certain gene expression patterns (Buck-Koehntop and Defossez, 2013, Zhu et al., 2016). TF genes themselves are also DNA methylation targets in various biological processes (Ivascu et al., 2007, Zinger et al., 2019). In thirteen-lined ground squirrels, the global DNA methylation level was increased in brown adipose tissue (Biggar and Storey, 2014b) but decreased in skeletal muscle (Alvarado et al., 2015). The CpG methylation level of the MEF2C promoter region correlated with the downregulation of gene expression in skeletal muscle of thirteen-lined ground squirrels (Alvarado et al., 2015). In chipmunk, CpG methylation in the USF-binding site is crucial for liver-specific transcription of the hibernation-specific gene, HP-27 (Fujii et al., 2006). Although these studies provided a glance into the DNA regulation in hibernation, they largely focused on changes in overall DNA methylation levels or the methylation of specific genes. In this study, we carried out BS-seq, the gold standard for DNA methylation profiling with high resolution, in active and hibernating states. By combining transcriptome and methylome data, we found that cis- and trans-regulation of DNA methylation participates in gene transcription changes during hibernation. Although DMGs are likely to be differentially expressed, most DEGs are not directly regulated by DNA methylation changes but by differentially expressed TFs with DMRs. In this economical and ingenious strategy, reversible drastic transcriptome changes in the Chinese alligator are regulated by modulating the transcription of TF genes. These results are consistent with observations in other hibernators, where numerous TFs play important roles during hibernation, such as HNF-1 in the chipmunk (Tamias asiaticus) (Ono et al., 2001), ATF4 and NFAT in the thirteen-lined ground squirrel (Mamady and Storey, 2008, Zhang and Storey, 2016), ZBED1 in the greater horseshoe bat (Rhinolophus ferrumequinum) (Xiao et al., 2016), and HSF in the red-eared slider turtle (Trachemys scripta elegans) (Krivoruchko and Storey, 2010).
Overall, our results provide insights into the genetic and epigenetic mechanisms underlying hibernation in the Chinese alligator and are expected to facilitate the development of scientific programs for successful conservation of this endangered species.
Limitations of the Study
This study revealed the suppression of metabolic rate and physiological states of hibernating Chinese alligator and suggested a unique energy-saving strategy that differs from that in hibernating mammals. Although we found that the downregulation of thyroid hormone biosynthesis plays an important regulatory role during this process, more investigations are needed to identify the core “hibernation factor,” which triggers and turns off the hibernation state. In addition, similar omics study should be done on more hibernating ectotherms to further explore the evolution of hibernation regulation in hibernating animals including reptiles as well as other ectotherms.
Resource Availability
Lead Contact
Further information and requests for resources should be directed to and will be fulfilled by the Lead Contact, Sheng-Guo Fang (sgfanglab@zju.edu.cn).
Materials Availability
The study did not generate new unique reagents.
Data and Code Availability
The Chinese alligator reference genome is available in the NCBI with the assembly accession number GCA_000455745.1. The BS-seq, mRNA-seq, and sRNA-seq data generated in this work have been deposited in the SRA database under NCBI BioProjects: PRJNA556094, PRJNA556093, and PRJNA556092, respectively. The iTRAQ and TMT data have been deposited in the ProteomeXchange with identifier PXD019278 and PXD019277, respectively. The DNA methylome data of Petromyzon marinus, Danio rerio, Bos grunniens, Homo sapiens, and Gallus gallus were downloaded from NCBI SRA database: SRR2457525, SRR800080, SRR8834688, SRR3427332, and SRR5003428, respectively.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
We would like to thank Prof. M. Thomas P. Gilbert (the University of Copenhagen) for his help modifying the manuscript. We would also like to thank Xuan-Min Guang (Zhejiang University) for his help with data analysis. We would like to thank Li-Ming Fang, Zhen-Wei Wang, Wei-Qiang Zou, Da-Bin Ren, and Ju-Min Xu (Changxing Yinjiabian Chinese Alligator Nature Reserve) for their help in sample collection. This work was supported by the Key Program of the National Natural Science Foundation of China (31530087) and the National Key Program (2016YFC0503200) from the Ministry of Science and Technology of China.
Author Contributions
Conceptualization, S.-G.F. and Q.-H.W.; Methodology, Q.-H.W. and J.-Q.L.; Investigation and Formal Analysis, J.-Q.L., Y.-Y.H., M.-Y.B., Q.-H.W., and S.-G.F.; Resources, S.-G.F.; Writing – Original Draft Preparation, J.-Q.L, M.-Y.B, Q.-H.W., and S.-G.F.; Writing – Review & Editing, J.-Q.L., Y.-Y.H., Q.-H.W., and S.-G.F.; Supervision, S.-G.F. and Q.-H.W.; Project Administration and Funding Acquisition, S.-G.F.
Declaration of Interests
The authors declare no competing interests.
Published: June 26, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2020.101202.
Supplemental Information
References
- Alvarado S., Mak T., Liu S., Storey K.B., Szyf M. Dynamic changes in global and gene-specific DNA methylation during hibernation in adult thirteen-lined ground squirrels, Ictidomys tridecemlineatus. J. Exp. Biol. 2015;218:1787–1795. doi: 10.1242/jeb.116046. [DOI] [PubMed] [Google Scholar]
- Ambros V. The functions of animal microRNAs. Nature. 2004;431:350–355. doi: 10.1038/nature02871. [DOI] [PubMed] [Google Scholar]
- Arfat Y., Chang H., Gao Y. Stress-responsive microRNAs are involved in re-programming of metabolic functions in hibernators. J. Cell Physiol. 2018;233:2695–2704. doi: 10.1002/jcp.26034. [DOI] [PubMed] [Google Scholar]
- Avery R.A. Utilization of caudal fat by hibernating common lizards, LacertaVivipara. Comp. Biochem. Physiol. 1970;37:119–121. [Google Scholar]
- Biggar K.K., Storey K.B. Identification and expression of microRNA in the brain of hibernating bats, Myotis lucifugus. Gene. 2014;544:67–74. doi: 10.1016/j.gene.2014.04.048. [DOI] [PubMed] [Google Scholar]
- Biggar K.K., Storey K.B. Insight into post-transcriptional gene regulation: stress-responsive microRNAs and their role in the environmental stress survival of tolerant animals. J. Exp. Biol. 2015;218:1281–1289. doi: 10.1242/jeb.104828. [DOI] [PubMed] [Google Scholar]
- Biggar Y., Storey K.B. Global DNA modifications suppress transcription in brown adipose tissue during hibernation. Cryobiology. 2014;69:333–338. doi: 10.1016/j.cryobiol.2014.08.008. [DOI] [PubMed] [Google Scholar]
- Breiling A., Lyko F. Epigenetic regulatory functions of DNA modifications: 5-methylcytosine and beyond. Epigenetics Chromatin. 2015;8:24. doi: 10.1186/s13072-015-0016-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britton C.H., Mackey D.W., Esser V., Foster D.W., Burns D.K., Yarnall D.P., Froguel P., McGarry J.D. Fine chromosome mapping of the genes for human liver and muscle carnitine palmitoyltransferase I (CPT1A and CPT1B) Genomics. 1997;40:209–211. doi: 10.1006/geno.1996.4539. [DOI] [PubMed] [Google Scholar]
- Buck-Koehntop B.A., Defossez P.A. On how mammalian transcription factors recognize methylated DNA. Epigenetics. 2013;8:131–137. doi: 10.4161/epi.23632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capraro A., O'Meally D., Waters S.A., Patel H.R., Georges A., Waters P.D. Waking the sleeping dragon: gene expression profiling reveals adaptive strategies of the hibernating reptile Pogona vitticeps. BMC Genomics. 2019;20:460. doi: 10.1186/s12864-019-5750-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey H.V., Andrews M.T., Martin S.L. Mammalian hibernation: cellular and molecular responses to depressed metabolism and low temperature. Physiol. Rev. 2003;83:1153–1181. doi: 10.1152/physrev.00008.2003. [DOI] [PubMed] [Google Scholar]
- Chen B.H., Hua T.M., Wu X.B., Wang C.L. Shanghai Scientific and Technological Education Publishing House; 2003. Research on Chinese Alligator. [Google Scholar]
- Cooper S.T., Sell S.S., Fahrenkrog M., Wilkinson K., Howard D.R., Bergen H., Cruz E., Cash S.E., Andrews M.T., Hampton M. Effects of hibernation on bone marrow transcriptome in thirteen-lined ground squirrels. Physiol. Genomics. 2016;48:513–525. doi: 10.1152/physiolgenomics.00120.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costanzo J.P. The bioenergetics of hibernation in the eastern garter snake Thamnophis sirtalis sirtalis. Physiol. Zool. 1985;58:682–692. [Google Scholar]
- Daniels C.B. The importance of caudal lipid in the gecko Phyllodactylus marmoratus. Herpetologica. 1984;40:337–344. [Google Scholar]
- Dessauer H.C. Hibernation of the lizard, Anolis carolinensis. Proc. Soc. Exp. Biol. Med. 1953;82:351–353. doi: 10.3181/00379727-82-20114. [DOI] [PubMed] [Google Scholar]
- Faherty S.L., Villanueva-Canas J.L., Blanco M.B., Alba M.M., Yoder A.D. Transcriptomics in the wild: hibernation physiology in free-ranging dwarf lemurs. Mol. Ecol. 2018;27:709–722. doi: 10.1111/mec.14483. [DOI] [PubMed] [Google Scholar]
- Faherty S.L., Villanueva-Canas J.L., Klopfer P.H., Alba M.M., Yoder A.D. Gene expression profiling in the hibernating primate, Cheirogaleus medius. Genome Biol. Evol. 2016;8:2413–2426. doi: 10.1093/gbe/evw163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang L.M., Zhai T., Zhao L., Fang S.G. Deep brumation features of Zhejiang Chinese alligators. Chin. J. Wildl. 2015;36:284–287. [Google Scholar]
- Fujii G., Nakamura Y., Tsukamoto D., Ito M., Shiba T., Takamatsu N. CpG methylation at the USF-binding site is important for the liver-specific transcription of the chipmunk HP-27 gene. Biochem. J. 2006;395:203–209. doi: 10.1042/BJ20051802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigg G.C., Kirshner D. Comstock Publishing Associates a division of Cornell University Press; 2015. Biology and Evolution of Crocodylians. [Google Scholar]
- Hampton M., Melvin R.G., Andrews M.T. Transcriptomic analysis of brown adipose tissue across the physiological extremes of natural hibernation. PLoS One. 2013;8:e85157. doi: 10.1371/journal.pone.0085157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert C.V., Jackson D.C. Temperature effects on the responses to prolonged submergence in the turtle Chrysemys Picta Bellii .2. Metabolic-rate, blood acid-base and ionic changes, and cardiovascular function in aerated and anoxic water. Physiol. Zool. 1985;58:670–681. [Google Scholar]
- Humphries M.M., Thomas D.W., Speakman J.R. Climate-mediated energetic constraints on the distribution of hibernating mammals. Nature. 2002;418:313–316. doi: 10.1038/nature00828. [DOI] [PubMed] [Google Scholar]
- Inouye D.W., Barr B., Armitage K.B., Inouye B.D. Climate change is affecting altitudinal migrants and hibernating species. Proc. Natl. Acad. Sci. U S A. 2000;97:1630–1633. doi: 10.1073/pnas.97.4.1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivascu C., Wasserkort R., Lesche R., Dong J., Stein H., Thiel A., Eckhardt F. DNA methylation profiling of transcription factor genes in normal lymphocyte development and lymphomas. Int. J. Biochem. Cell Biol. 2007;39:1523–1538. doi: 10.1016/j.biocel.2007.02.006. [DOI] [PubMed] [Google Scholar]
- Jones P.A. Functions of DNA methylation: islands, start sites, gene bodies and beyond. Nat. Rev. Genet. 2012;13:484–492. doi: 10.1038/nrg3230. [DOI] [PubMed] [Google Scholar]
- Karpovich S.A., Toien O., Buck C.L., Barnes B.M. Energetics of arousal episodes in hibernating arctic ground squirrels. J. Comp. Physiol. B. 2009;179:691–700. doi: 10.1007/s00360-009-0350-8. [DOI] [PubMed] [Google Scholar]
- Kornfeld S.F., Biggar K.K., Storey K.B. Differential expression of mature microRNAs involved in muscle maintenance of hibernating little brown bats, Myotis lucifugus: a model of muscle atrophy resistance. Genomics Proteomics Bioinformatics. 2012;10:295–301. doi: 10.1016/j.gpb.2012.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krivoruchko A., Storey K.B. Regulation of the heat shock response under anoxia in the turtle, Trachemys scripta elegans. J. Comp. Physiol. B. 2010;180:403–414. doi: 10.1007/s00360-009-0414-9. [DOI] [PubMed] [Google Scholar]
- Lei M., Dong D., Mu S., Pan Y.H., Zhang S. Comparison of brain transcriptome of the greater horseshoe bats (Rhinolophus ferrumequinum) in active and torpid episodes. PLoS One. 2014;9:e107746. doi: 10.1371/journal.pone.0107746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luan Y., Ou J., Kunze V.P., Qiao F., Wang Y., Wei L., Li W., Xie Z. Integrated transcriptomic and metabolomic analysis reveals adaptive changes of hibernating retinas. J. Cell Physiol. 2018;233:1434–1445. doi: 10.1002/jcp.26030. [DOI] [PubMed] [Google Scholar]
- Lyons P.J., Lang-Ouellette D., Morin P., Jr. CryomiRs: towards the identification of a cold-associated family of microRNAs. Comp. Biochem. Physiol. Part D Genomics Proteomics. 2013;8:358–364. doi: 10.1016/j.cbd.2013.10.001. [DOI] [PubMed] [Google Scholar]
- Mamady H., Storey K.B. Coping with the stress: expression of ATF4, ATF6, and downstream targets in organs of hibernating ground squirrels. Arch. Biochem. Biophys. 2008;477:77–85. doi: 10.1016/j.abb.2008.05.006. [DOI] [PubMed] [Google Scholar]
- Mateju K., Bendova Z., El-Hennamy R., Sladek M., Sosniyenko S., Sumova A. Development of the light sensitivity of the clock genes Period1 and Period2, and immediate-early gene c-fos within the rat suprachiasmatic nucleus. Eur. J. Neurosci. 2009;29:490–501. doi: 10.1111/j.1460-9568.2009.06609.x. [DOI] [PubMed] [Google Scholar]
- Nespolo R.F., Gaitan-Espitia J.D., Quintero-Galvis J.F., Fernandez F.V., Silva A.X., Molina C., Storey K.B., Bozinovic F. A functional transcriptomic analysis in the relict marsupial Dromiciops gliroides reveals adaptive regulation of protective functions during hibernation. Mol. Ecol. 2018;27:4489–4500. doi: 10.1111/mec.14876. [DOI] [PubMed] [Google Scholar]
- Oaks J.R. A time-calibrated species tree of Crocodylia reveals a recent radiation of the true crocodiles. Evolution. 2011;65:3285–3297. doi: 10.1111/j.1558-5646.2011.01373.x. [DOI] [PubMed] [Google Scholar]
- Ono M., Hosoe Y., Azuma S., Shoji M., Nara K., Kondo N., Shiba T., Takamatsu N. HNF-1 regulates the liver-specific transcription of the chipmunk HP-20 gene. Gene. 2001;277:121–127. doi: 10.1016/s0378-1119(01)00699-0. [DOI] [PubMed] [Google Scholar]
- Pelizzola M., Ecker J.R. The DNA methylome. FEBS Lett. 2011;585:1994–2000. doi: 10.1016/j.febslet.2010.10.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito T., Sugimoto K., Adachi Y., Wu Q., Mori K.J. Cloning and characterization of amphibian cold inducible RNA-binding protein. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2000;125:237–245. doi: 10.1016/s0305-0491(99)00174-1. [DOI] [PubMed] [Google Scholar]
- Sheriff M.J., Fridinger R.W., Toien O., Barnes B.M., Buck C.L. Metabolic rate and prehibernation fattening in free-living arctic ground squirrels. Physiol. Biochem. Zool. 2013;86:515–527. doi: 10.1086/673092. [DOI] [PubMed] [Google Scholar]
- Smith Z.D., Meissner A. DNA methylation: roles in mammalian development. Nat. Rev. Genet. 2013;14:204–220. doi: 10.1038/nrg3354. [DOI] [PubMed] [Google Scholar]
- Staples J.F. Metabolic flexibility: hibernation, torpor, and estivation. Compr. Physiol. 2016;6:737–771. doi: 10.1002/cphy.c140064. [DOI] [PubMed] [Google Scholar]
- Storey K.B. Metabolic adaptations supporting anoxia tolerance in reptiles: recent advances. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 1996;113:23–35. doi: 10.1016/0305-0491(95)02043-8. [DOI] [PubMed] [Google Scholar]
- Storey K.B. Reptile freeze tolerance: metabolism and gene expression. Cryobiology. 2006;52:1–16. doi: 10.1016/j.cryobiol.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Storey K.B., Storey J.M. Tribute to P. L. Lutz: putting life on `pause' – molecular regulation of hypometabolism. J. Exp. Biol. 2007;210:1700–1714. doi: 10.1242/jeb.02716. [DOI] [PubMed] [Google Scholar]
- Storey K.B., Storey J.M. Molecular biology of freezing tolerance. Compr. Physiol. 2013;3:1283–1308. doi: 10.1002/cphy.c130007. [DOI] [PubMed] [Google Scholar]
- Su Z., Han L., Zhao Z. Conservation and divergence of DNA methylation in eukaryotes: new insights from single base-resolution DNA methylomes. Epigenetics. 2011;6:134–140. doi: 10.4161/epi.6.2.13875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimoto K., Jiang H.J. Cold stress and light signals induce the expression of cold-inducible RNA binding protein (cirp) in the brain and eye of the Japanese treefrog (Hyla japonica) Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2008;151:628–636. doi: 10.1016/j.cbpa.2008.07.027. [DOI] [PubMed] [Google Scholar]
- Sun H.J., Zuo X.B., Sun L., Yan P., Zhang F., Xue H., Li E., Zhou Y.K., Wu R., Wu X.B. Insights into the seasonal adaptive mechanisms of Chinese alligators (Alligator sinensis) from transcriptomic analyses. Aust. J. Zool. 2018;66:93–102. [Google Scholar]
- Wan Q.H., Pan S.K., Hu L., Zhu Y., Xu P.W., Xia J.Q., Chen H., He G.Y., He J., Ni X.W. Genome analysis and signature discovery for diving and sensory properties of the endangered Chinese alligator. Cell Res. 2013;23:1091–1105. doi: 10.1038/cr.2013.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C.W., Biggar K.K., Storey K.B. Expression profiling and structural characterization of microRNAs in adipose tissues of hibernating ground squirrels. Genomics Proteomics Bioinformatics. 2014;12:284–291. doi: 10.1016/j.gpb.2014.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia T.S., Zhu J.L., Shao M. Relationship between temperature and diseases occurrence of Chinese alligator during hibernation. Sichuan J. Zool. 2006;25:400–402. [Google Scholar]
- Xiao Y., Wu Y., Sun K., Wang H., Jiang T., Lin A., Huang X., Yue X., Shi L., Feng J. Gene expression and adaptive evolution of ZBED1 in the hibernating greater horseshoe bat (Rhinolophus ferrumequinum) J. Exp. Biol. 2016;219:834–843. doi: 10.1242/jeb.133272. [DOI] [PubMed] [Google Scholar]
- Zani P.A., Irwin J.T., Rollyson M.E., Counihan J.L., Healas S.D., Lloyd E.K., Kojanis L.C., Fried B., Sherma J. Glycogen, not dehydration or lipids, limits winter survival of side-blotched lizards (Uta stansburiana) J. Exp. Biol. 2012;215:3126–3134. doi: 10.1242/jeb.069617. [DOI] [PubMed] [Google Scholar]
- Zhang G.L., Geng Y.J., XIao J.G., Yang S.H. Comparison of two overwintering ways for Chinese alligator in captivity. J. Econ. Anim. 2003;7:57–59. [Google Scholar]
- Zhang Y., Storey K.B. Regulation of gene expression by NFAT transcription factors in hibernating ground squirrels is dependent on the cellular environment. Cell Stress Chaperones. 2016;21:883–894. doi: 10.1007/s12192-016-0713-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H., Wang G.H., Qian J. Transcription factors as readers and effectors of DNA methylation. Nat. Rev. Genet. 2016;17:551–565. doi: 10.1038/nrg.2016.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinger L., Bonin A., Alsos I.G., Balint M., Bik H., Boyer F., Chariton A.A., Creer S., Coissac E., Deagle B.E. DNA metabarcoding-Need for robust experimental designs to draw sound ecological conclusions. Mol. Ecol. 2019;28:1857–1862. doi: 10.1111/mec.15060. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The Chinese alligator reference genome is available in the NCBI with the assembly accession number GCA_000455745.1. The BS-seq, mRNA-seq, and sRNA-seq data generated in this work have been deposited in the SRA database under NCBI BioProjects: PRJNA556094, PRJNA556093, and PRJNA556092, respectively. The iTRAQ and TMT data have been deposited in the ProteomeXchange with identifier PXD019278 and PXD019277, respectively. The DNA methylome data of Petromyzon marinus, Danio rerio, Bos grunniens, Homo sapiens, and Gallus gallus were downloaded from NCBI SRA database: SRR2457525, SRR800080, SRR8834688, SRR3427332, and SRR5003428, respectively.