Abstract
Dengue fever, transmitted by Aedes aegypti mosquitoes, is one of the most common vector-borne disease. Its incidence is increasing steadily worldwide, becoming a major public health problem in the tropical and subtropical zone. Neurological manifestations after dengue are not very common and acute disseminated encephalomyelitis (ADEM) following dengue infections is rare with a few cases documented in literature. Clinical characteristics and typical lesions of ADEM on magnetic resonance imaging (MRI) of brain along with serologic positivity for dengue usually confirm the diagnosis. We report a case of ADEM which developed as a neurological complication of dengue during an epidemic in a 39-year-old woman.
Abbreviations: ADEM, Acute Disseminated Encephalomyelitis; CSF, Cerebrospinal Fluid; EEG, Electroencephalography; MRI, Magnetic Resonance Imaging; PCR, Polymerase Chain Reaction
Keywords: Dengue infection, Acute disseminated encephalomyelitis, Mayotte
Introduction
Dengue fever has become one of the most common tropical infections in the past decade and is endemic southwest of the Indian Ocean, including Mayotte island. The disease can be caused by one of the 4 serotypes of the virus, which are immunologically linked, but without cross protection [1]. The involvement of the central nervous system, leading to acute disseminated encephalomyelitis (ADEM), is observed, mainly with serotypes 2 and 3. We present here a case of severe dengue fever with an atypical presentation of acute encephalomyelitis requiring mechanical ventilation, intensive care, and steroids treatment. Despite all efforts, the patient did not survive.
Case report
A 39-year-old female patient with no antecedent comorbidities presented to the emergency department of Mayotte hospital with fever at 38.7 °C associated with chills, headache, cervical pain, and asthenia of 4 days’ duration. She received symptomatic treatment and was discharged.
Five days later, she returned to the emergency unit for acute urine retention in a context of an altered mental state. Clinical examination revealed that she had altered consciousness without fever or neck stiffness. CBC revealed WBC 7500/μL, platelets 356,000/μL and hemoglobin 7.4 g/dL and CRP was 15 mg/L. The results of other biological analysis showed that C-Reactive Protein level was 15 mg/L. Cerebral angiogram did not show any abnormality. A lumbar puncture was performed, the direct examination and culture were negative. However, cerebrospinal fluid (CSF) analysis revealed total leukocyte count 132 cells/μL with a lymphocyte predominance (lymphocytes: 83 %), and elevated level of CSF protein at 79 mg/dL. CSF glucose was within normal limits. The serum PCR dengue serotype 1 performed during her first admission to the emergency department was positive.
She started IV ceftriaxone 4 g per day and amoxicillin 100 mg/kg per day and aciclovir 10 mg/kg/8 h. Due to a deterioration of her neurological state, she was admitted to the intensive care unit. A second lumbar puncture was performed which found an increased number of WBC 7500/μL with a neutrophils predominance (neutrophils: 92 %), red blood cells (2350 cells/μL), the CSF glucose level was 121 mg/dL and CSF protein level was 494 mg/L. Direct examination and the culture of the CSF were again negative.
The injected brain scan showed no visible abnormality or suspect contrast at the meningeal or parenchymal level
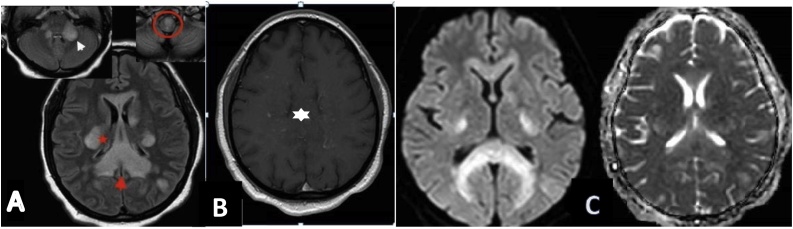
A cranial transdoppler and another repeated after five days showed normocapnia. The electroencephalography (EEG) did not show any signs leading to metabolic or toxic encephalopathy diagnosis. A magnetic resonance imaging (MRI) of her brain was performed and showed lesions of the anterior part of the soft bulb, the left cerebellar peduncles and of the midbrain (bilaterally) in the posterior cerebral fossa which appear as hyperintense signals. After injection, there is a nodular contrast enhancement of the anterior bulbar lesion. Hyperintense signal on T2 fluid attenuated inversion recovery sequence (FLAIR) on the supra-tentorial level (Fig. 1).
Fig. 1.
A. FLAIR axial section showing the diffuse involvement of the corpus callosum (red arrowhead), the corona radiata (red star), left subcortical cortical white matter and middle cerebellar peduncles with predominant involvement on the left (white arrowhead) associated with a hypersignal of the anterior part of the medullary bulb (red circle).B. Axial T1 SE GADO showing nodular contrast enhancement of the corpus callosum (white star). C. Diffusion b 1000 with adc mapping showing bilateral and symmetrical capsulo-lenticular hypersignal and unrestricted corpus callosplenum splenium on adc mapping (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.).
Analysis of the CSF in search of different germs such as Dengue Virus, Zoster Varicella Virus, Herpes Simplex Virus using PCR. Detection of bacterial genes using 16 s RNA of Streptococcus pneumoniae, Neisseria meningitidis, Haemophilus influenzae, Strepto B, Listeria monocytogenes, or Mycobacterium tuberculosis using culture were performed and negative. In the blood, serology of Cytomegalovirus, Epstein-Barr Virus, Human Immunodeficiency Virus, hepatitis C virus, hepatitis B virus, Treponema Palladium and Human Herpesvirus 6 and several blood cultures were negative. Electrophoresis of plasma proteins was performed and showed polyclonal hyper-gamma globulin without a monoclonal peak. A transthoracic echocardiography did not show any valvular vegetation.
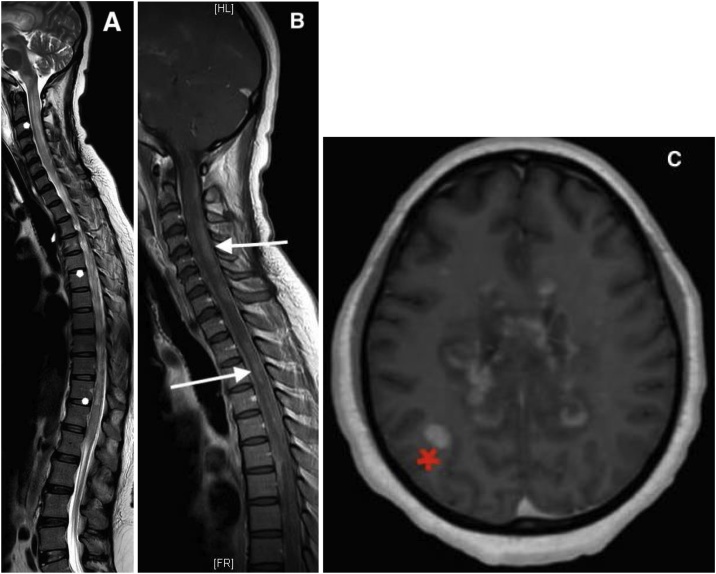
Following a multidisciplinary meeting with the participation of an infectious disease specialist, neuroradiologist, neurologist, neurosurgeon and resuscitator, we concluded that the diagnosis of acute multifocal encephalomyelitis (ADEM) post dengue was the most plausible considering the anamnesis and the imaging (MRI). Therefore, she started an additional treatment using prednisone 2 mg/kg/day for 5 days, then at 1 mg/kg/day. A repeated cerebro-medullary MRI was performed and found an increase number of contrasts enhancing lesions at the level of the brain and spinal cord (Fig. 2). On day 3 of treatment, the patient presented persistent episodes of dysautonomia due to bulbar impairment. A transcranial doppler showed a non-reactive bilateral mydriasis without flux, associated with loss of brain stem reflex. The diagnosis of brain death was confirmed by brain scanner with injection. She was declared died.
Fig. 2.
A- T2 TSE sagittal section showing diffuse myelitis lesions in hypersignal T2 TSE (white star).B. T1 TSE sagittal section after injection of gadolinium showing multifocal centromedullary and posterior cervical and dorsal stage contrast enhancement (white arrowhead).C. FFE axial 3D T1-weighted image after injection showing an increase in contrast enhancement of the corpus callosum and subcortical right parietal white matter (red star) (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.).
Discussion
We reported a case of ADEM during a dengue epidemic in a 39-year-old woman. Dengue fever with atypical neurological complications poses serious diagnosis and clinical challenge to clinicians. The neurological complications in dengue have various pathogenic attributes: a) direct viral invasion, b) systemic metabolic complications and c) post infectious immune-mediated acute disseminated encephalomyelitis [4]. ADEM is a monophasic, immune mediated acute demyelinating disorder of the central nervous system following recent infection or vaccination [5] and characterized by multifocal white matter involvement [6]. The ADEM incidence rate is about 8 per 1,000,000 people per year with a mortality rate estimated at 5% [7]. The pathophysiology involves transient autoimmune response directed towards myelin or other self-antigens, possibly by non-specific activation of auto-reactive T-cell clones or by molecular mimicry [3]. As in other viral infection, the pathogenesis underlying dengue associated ADEM results from an immune system mediated process. Several viral infections (influenza virus, enterovirus, measles, mumps, rubella, varicella-zoster, coxsackievirus, Epstein–Barr, cytomegalovirus, herpes simplex virus, hepatitis A, and rarely dengue); and bacterial infections (Borrelia burgdorferi, Mycoplasma pneumoniae, Leptospira pneumophilia) are known to induce ADEM [8]. Diagnosis of ADEM is very challenging and vigilant suspicion with early imaging are essential and lifesaving modalities.
The neurological symptoms of ADEM begin after the febrile phase, frequently reported in 1–3 weeks after the precipitating event—infection or vaccination. In our patient, ADEM occurred on the 10th day after the onset of clinical manifestations of dengue. The dengue PCR in the negative CSF does not exclude possible neurological complications associated with dengue and moreover before diagnosing ADEM. Brain imaging through MRI has become the most important test in the early diagnosis of ADEM. Three distinct categories of lesions can be classified using MRI criteria: (a) multifocal lesions in the white matter with or without basal ganglia involvement, (b) single or multifocal lesions only in the grey matter and (c) localized lesions in the brain stem, basal ganglia, or cerebellum [9]. Cranial MRI results in our patient matched with the first category involving the white matter and left basal ganglia. ADEM is rarely associated with dengue virus infection. The early diagnosis and administration of glucocorticoid seem to be essential for the functional prognosis of patients with ADEM following dengue fever. A few cases have been reported in the literature [[10], [11], [12]]. Demyelinating lesions with or without foci of hemorrhage on MRI are probably pathognomonic of ADEM following dengue infection [13]. To the best of our knowledge, this is the first reported case of acute disseminated encephalomyelitis due to dengue with serotype-1 without viral presence in the cerebrospinal fluid and which was associated with death on our island. Dengue infection is endemic in Mayotte with 1298 cases recorded in 2019. On a small island in the southwest Indian Ocean such as Mayotte, it would be wise to screen dengue infection in patients with fever and acute neurological manifestations. This could be helpful in early diagnosis, treatment, and better understanding of the physiopathology of the disease.
Conclusion
In a department as Mayotte where dengue infection is endemic, ADEM as one of its complication must be considered in appropriate clinical setting. Early diagnosis and effective treatment with steroids can reverse this potentially fatal disease as it occurred to our patient.
Author contributions
AD, IY conceived, designed the study, collected case data and contributed to manuscript drafting; YD, CM, MJ, MN, PM, BMB, SP revised the manuscript. All authors read and approved the manuscript for publication.
Ethics approval and consent to publish
Patients were contacted after their hospital stay and they agreed to participate in the study and verbal informed and written consent was obtained from them for publication of this case report.
Funding
NA.
Declaration of Competing Interest
The authors declare that they have no conflict of interest.
References
- 1.Sinniah M., Igarashi A. Dengue haemorrhagic fever. Rev Med Virol. 1995;5:193–203. [Google Scholar]
- 3.Murthy J.M. Neurological complication of dengue infection. Neurol India. 2010;58:581–584. doi: 10.4103/0028-3886.68654. [DOI] [PubMed] [Google Scholar]
- 4.Sanjeev Kumar B., Naik S., Jayantee K., Misra U.K. Acute disseminated encephalomyelitis following dengue virus infection. J Neuroinfect Dis. 2014;5:139. doi: 10.4172/2314-7326.1000139. [DOI] [Google Scholar]
- 5.Gera C., George U. Acute disseminating encephalomyelitis with haemorrhage following dengue. Neurol India. 2010;58:595–596. doi: 10.4103/0028-3886.68661. [DOI] [PubMed] [Google Scholar]
- 6.Puccioni-Sohler M., Rosadas C., Cabral-Castro M.J. Neurological complications in dengue infection: a review for clinical practice. Arq Neuropsiquiatr. 2013;71(9b):667–671. doi: 10.1590/0004-282x20130147. Epub 2013/10/22. [DOI] [PubMed] [Google Scholar]
- 7.Leake J.A., Albani S., Kao A.S. Acute disseminated encephalomyelitis in childhood: epidemiologic, clinical and laboratory features. Pediatr Infect Dis J. 2004;23(8):756–764. doi: 10.1097/01.inf.0000133048.75452.dd. [DOI] [PubMed] [Google Scholar]
- 8.(a) Wingerchuk D.M. Post infectious encephalomyelitis. Curr Neurol Neurosci Rep. 2003;3:256–264. doi: 10.1007/s11910-003-0086-x. [DOI] [PubMed] [Google Scholar]; (b) Pal S., Sen K., Biswas N.M., Ghosal A., Jaman S.R., Kumar K.Y. Clinico-radiological profile and outcome of dengue patients with central nervous system manifestations: a case series in an Eastern India tertiary care hospital. J Neurosci Rural Pract. 2016;7:114. doi: 10.4103/0976-3147.165410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pal S., Sen K., Biswas N.M., Ghosal A., Rousan Jaman S.K., Yashavantha Kumar K.Y. Clinico-radiological profile and outcome of dengue patients with central nervous system manifestations: a case series in an Eastern India tertiary care hospital. J Neurosci Rural Pract. 2016;7(January-March (1)):114–124. doi: 10.4103/0976-3147.165410. PMID: 26933357; PMCID: PMC4750307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rastogi N., Nayan A., Sethi P., Nischal N., Brijwal M., Kumar A., Wig N. Dengue fever with acute disseminated encephalomyelitis: sensorium imbroglio. J Assoc Physicians India. 2019;67(10):80–82. [PubMed] [Google Scholar]
- 11.Viswanathan S., Botross N., Rusli B.N., Riad A. Acute disseminated encephalomyelitis complicating dengue infection with neuroimaging mimicking multiple sclerosis: a report of two cases. Mult Scler Relat Disord. 2016;10:112–115. doi: 10.1016/j.msard.2016.10.001. [DOI] [PubMed] [Google Scholar]
- 12.Chakrabarti S. A case of acute disseminated encephalomyelitis following dengue infection. CHRISMED J Health Res. 2015;2:169–171. [Google Scholar]
- 13.Murthy J.M. Acute disseminated encephalomyelitis. Neurol India. 2002;50:238–243. [PubMed] [Google Scholar]