Abstract
Breast implant–associated anaplastic large-cell lymphoma (BIA-ALCL) is a CD30-positive, anaplastic lymphoma kinase–negative T-cell lymphoma. Nearly all cases have been associated with textured implants. Most cases are of effusion-limited, indolent disease, with an excellent prognosis after implant and capsule removal. However, capsular invasion and tumor mass have a more aggressive course and a fatal outcome risk. This review summarizes the current knowledge on BIA-ALCL cell of origin and immunologic factors underlying its pathogenesis. Cytokine expression profiling of BIA-ALCL cell lines and clinical specimens reveals a predominantly type 17 helper T-cell (Th17)/Th1 signature, implicating this as its cell of origin. However, a Th2 allergic inflammatory response is suggested by the presence of IL-13, with infiltration of eosinophils and IgE-coated mast cells in clinical specimens of BIA-ALCL. The microenvironment-induced T-cell plasticity, a factor increasingly appreciated, may partially explain these divergent results. Mutations resulting in constitutive Janus kinase (JAK)–STAT activation have been detected and associated with BIA-ALCL pathogenesis in a small number of cases. One possible scenario is that an inflammatory microenvironment stimulates an immune response, followed by polyclonal expansion of Th17/Th1 cell subsets with release of inflammatory cytokines and chemokines and accumulation of seroma. JAK-STAT3 gain-of-function mutations within this pathway and others may subsequently lead to monoclonal T-cell proliferation and clinical BIA-ALCL. Current research suggests that therapies targeting JAK proteins warrant investigation in BIA-ALCL.
In 1997, a case of CD30-positive, anaplastic lymphoma kinase (ALK)–negative T-cell lymphoma in proximity to a breast implant was first reported.1 Other cases of this uncommon malignancy (estimated US incidence, 2.03 per million person-years for textured implants)2 were subsequently reported, with nearly all confirmed cases either associated with textured (versus smooth) implants or occurring in patients who had previously had textured implants.2, 3, 4 In 2016, the World Health Organization recognized breast implant–associated anaplastic large-cell lymphoma (BIA-ALCL) as a provisional entity distinguished from other ALK-negative ALCLs.5 Similar numbers of BIA-ALCL cases have been reported among patients with implants for breast reconstruction for breast cancer or prophylaxis and for cosmetic purposes.2, 3, 4,6 Most patients with BIA-ALCL present with seroma without capsular invasion.3,6,7 Time of seroma occurrence after initial implantation or reimplantation ranges from 0.2 to 27 years in retrospective studies.6,8 In these cases, BIA-ALCL follows an indolent course, with patients having a favorable prognosis after complete surgical excision.9 However, patients with tumor mass infiltration of capsule and adjoining tissue face a more aggressive disease.9, 10, 11 The spectrum of cytologic and histologic changes encountered in BIA-ALCL is illustrated in Figure 1. The purpose of this review is to summarize what is known about the BIA-ALCL cell of origin and immunologic factors underlying the pathogenesis of BIA-ALCL and to identify key areas where we believe future research is needed.
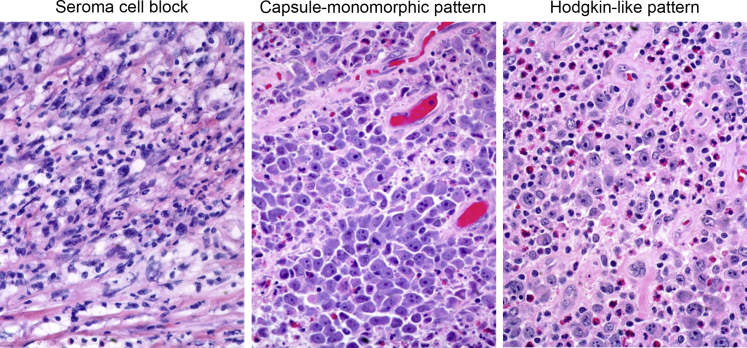
Figure 1.
Spectrum of cytologic and histologic changes in breast implant–associated anaplastic large-cell lymphoma (BIA-ALCL). Left panel: The seroma cell block contains large anaplastic cells with an admixture of inflammatory cells and fibrin. Seroma cell blocks are helpful for the diagnosis of BIA-ALCL. Middle and right panels: The spectrum of morphologic changes that can be encountered when BIA-ALCL infiltrates the capsule and surrounding breast tissue. Original magnification, ×400.
Triggering Event
Much of the underlying etiology of BIA-ALCL remains to be elucidated, but multiple factors appear to be involved in its development, including host genetic factors.7,12 Various triggers, with varying degrees of evidence, have been proposed for the development of BIA-ALCL, including mechanical friction, implant shell particulates, implant components leaching into surrounding tissue, and bacterial biofilm.13,14 The evidence supporting the association of bacterial biofilm with a greater risk of BIA-ALCL is that the uneven surfaces of textured breast implants provide a sheltered environment for bacterial proliferation, supporting a higher biofilm load than is possible for smooth implants and explaining the higher rates of BIA-ALCL with textured implants.6,15 Another possibility for triggering a pathophysiological cascade is that a superantigen may be involved, as is the case in cutaneous T-cell lymphoma,16 although this remains speculative for BIA-ALCL at present. The discovery of ribosomal protein S10 in BIA-ALCL samples suggests that viral etiology is another possibility because ribosomal protein S10 contributes to formation of the internal ribosome entry site by which viral transcripts gain entry to the ribosome.17,18
Innate Immune Response
Cellular and cytokine studies suggest that an inflammatory milieu may be a necessary component of the pathobiology of BIA-ALCL.19, 20, 21 In response to bacteria or other yet undefined antigens, acute inflammation is initiated by innate immune cells (eg, mast cells, neutrophils, and macrophages).22 Through antigen presentation, these cells may activate adaptive immune cell responses via the release of cytokines and chemokines.23
The lymphoma cells of most cases of BIA-ALCL lack T-cell receptors α/β or γ/δ or have gene mutations that may contribute to a defective T-cell receptor phenotype.11,21,24, 25, 26, 27 Lack of adaptive T-cell functionality suggests that an innate immune response may significantly contribute to BIA-ALCL immunopathophysiology. This may be further supported by a study using human peripheral blood mononuclear cell cultures, which found that exposure to silicone breast implant surfaces did not induce T-cell activation. However, some implant surfaces resulted in increased expression of the proinflammatory cytokines IL-1β, IL-6, and tumor necrosis factor-α, which play a role in macrophage activation.28 Furthermore, eosinophils and mast cells with strongly bound cell-surface IgE, which are characteristic of allergic inflammation, have been found in BIA-ALCL tissue20 (Figure 2).
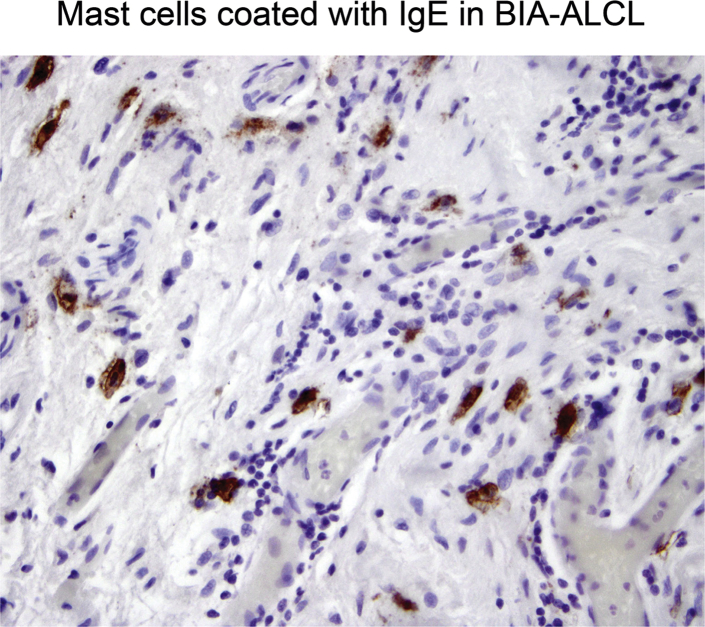
Figure 2.
Mast cells in breast implant–associated anaplastic large-cell lymphoma (BIA-ALCL) show strong IgE surface expression (brown stain). Rabbit antibody (catalog number 75673; Abcam, Cambridge, UK) was used neat. Second-stage antibody was from R&D Systems (Minneapolis, MN; catalog number CTS005). Original magnification, ×400.
IL-22, produced by group 3 innate lymphoid cells (ILC3), has been associated with malignancies, including T-cell lymphomas.29,30 Up-regulation of ILC3 genes and secretion of IL-22 have been observed in ALCL cell lines, pointing to ILC3 cells as a potential cell of origin for ALK-positive ALCLs.31
Cell of Origin
Thymic Progenitor Cells in ALCL
Because of the relatively recent recognition of BIA-ALCL as a clinical entity and its relative rarity, knowledge of its pathobiology is more limited than for the other types of ALCL, such as systemic ALK-positive ALCL (ALK-positive sALCL), systemic ALK-negative ALCL (ALK-negative sALCL), and primary cutaneous ALCL (pcALCL).32 Although ALCL subtypes are associated with divergent clinical presentations and prognoses, some aspects of their pathogenesis are shared.33 All ALCL subtypes have common features, including variable expression of surface markers that support a T-cell origin. All are positive for CD30, which is a cell membrane protein of the tumor necrosis factor receptor family normally found on the surface of activated B and T cells.11,26,34, 35, 36 In addition, T-cell receptor gene rearrangements are seen and there is rare cell surface T-cell receptor expression and the frequent loss of CD3.11,21,26,27,35,37, 38, 39 All studied subtypes have anaplastic morphology40 and strongly express antigen presentation–associated proteins (human leukocyte antigen-DR+CD80+CD86+).21,35,41 Malignant cells are commonly CD4 positive, less frequently CD8 positive, and rarely CD4/CD8 double positive.11,26,27,35,42,43 Some cases are CD4-/CD8-/CD3- (triple negative; null cell phenotype), but are still T-cell derived, as evidenced by molecular T-cell receptor rearrangements.11,26,27,35,42,44 Nonetheless, ALCL often expresses perforin and/or granzyme B, irrespective of CD4/CD8 phenotype.11,26,42,43,45, 46, 47 BIA-ALCL has similarities with pcALCL in terms of morphology, biomarkers, a typically localized disease with frequent absence of systemic symptoms, and a generally favorable prognosis.9,48
Assigning a cell of origin to any ALCL has proved challenging, although all ALCLs fall under the umbrella of peripheral T-cell lymphomas and, thus, by definition, are of post-thymic origin. In one study, gene expression profiling (GEP) of a subpopulation of ALK-positive and ALK-negative ALCL cells reflected an early thymic progenitor origin.49 Yet, in another study of ALK-positive ALCL, ALK-negative ALCL, and pcALCL, GEP was unable to clearly assign a pattern of CD4+ helper T cell, CD8+ cytotoxic T cell, or CD30+ activated T cell of origin to ALCLs.50 However, in this study, the GEP of subsets of helper and cytotoxic T cells was not considered. In the case of ALK-positive sALCL, in vivo studies have provided evidence of an early thymocyte origin with T-cell receptor expression, which is necessary for thymic egress.39 In addition, DNA methylation fingerprints in both ALK-positive and ALK-negative ALCL samples were found to be consistent with a thymic origin, although epigenomic reprogramming at a later stage could not be ruled out.51
Th17/Th1 Phenotype
A different cell of origin was recently suggested by a GEP study of several ALK-positive and ALK-negative cell lines.31 These cells demonstrated a GEP characteristic of type 17 helper T cells (Th17) and, in some cases, ILC3 cells, with AP-1-BATF and AP-1-BATF3 playing a crucial role in Th17/ILC3 skewing.31,52 Further supporting a Th17 phenotype, the IL-17A and IL-17F cytokines were secreted by these cells and were detectable in patients with ALCL. These findings were consistent with a previous study in which IL-17A and IL-17F were shown to be associated with ALCL.52 Although these studies did not include BIA-ALCL, the origin of these ALCL subtypes is of more than academic interest because eradication of clinical disease depends on elimination of any reservoirs that might cause relapse.53
In considering cellular origin and phenotype, CD4+ helper T-cell plasticity must be kept in mind.54 Differentiation of naïve CD4+ T cells into discrete Th1, Th2, Th17, regulatory T cells, and other subsets is not only context dependent, but the polarization states reached in some cases maintain context-dependent late plasticity governed by epigenetic regulation that is, in turn, influenced by secondary stimulation.55 Specifically, Th17 cells are known to be capable of acquiring functional characteristics associated with Th1 cells, although the reverse does not seem to occur,56 and cells producing both IL-17A and interferon-γ have been reported. Thus, Th17 cells recruited in response to extracellular antigens may initiate an inflammatory response via IL-17A and IL-17F before transitioning into Th1-like cells, thereby providing characteristics of both cell types.55
A study of BIA-ALCL GEP was performed on cell lines derived from patient samples as well as pcALCL cell lines.48 BIA-ALCL cells displayed moderate to high expression levels of the Th1 signature cytokine interferon-γ as well as IL-17A and IL-17F. Interestingly, high expression of suppressor of cytokine signaling 3, which has been proposed to play a role in ALCL pathogenesis via the Janus kinase 3–signal transducer and activator of transcription 3 (JAK3-STAT3) pathway,57 was also characteristic of BIA-ALCL cells. BIA-ALCL cells and pcALCL cells had similar cytokine and transcription factor profiles, including high levels of suppressor of cytokine signaling 3, SATB1, and JunB, which may promote lymphoma development through transcriptional regulation of platelet-derived growth factor receptor-β.48,58,59 Expression of IL-17F and interferon-γ was confirmed in BIA-ALCL tumor cells and surrounding capsule from clinical samples, with stronger IL-17F expression in capsular infiltrates.48
Collectively, these results suggest that breast implants may, by still undefined mechanisms, trigger a local Th17/Th1 immune response, leading to subsequent cytokine-promoted fibrosis and the formation of the peri-implant capsule.48 Given the extremely low incidence rate of BIA-ALCL, in the vast majority of cases, this must not have downstream malignant sequelae, although it may be a factor in the capsular contracture sometimes encountered. Accepting the hypothesis of a predominantly Th17/Th1 phenotype, what then remains to be elucidated are the additional hits and downstream transformational processes necessary for the development of BIA-ALCL in this milieu.
Immunologic Pathways in BIA-ALCL Pathogenesis
The Role of Inflammation
Several lines of evidence point to the role of an inflammatory environment with chronic T-cell stimulation in BIA-ALCL pathogenesis. In clinical specimens from patients with BIA-ALCL that was invasive through the peri-implant capsule, malignant cells were associated with an inflammatory background with a large number of eosinophils (Figure 1).11 BIA-ALCL cell lines and 14 of 14 clinical BIA-ALCL capsule specimens were found to secrete or express IL-13, the Th2-associated signature cytokine of allergic inflammation, which was only infrequently expressed in ALK-negative sALCL, ALK-positive sALCL,60 or benign capsular tissue specimens.20 In this study, BIA-ALCL tissue samples contained numerous eosinophils and mast cells with surface-expressed IgE, also characteristic of allergic inflammation. In contrast, few ALK-negative or ALK-positive sALCL samples contained eosinophils, and ALK-positive ALCL has been associated with predominantly Th17 cytokines.61 These results suggest that IL-13 is a functional biomarker of BIA-ALCL and imply that allergic inflammation may be a unique component of its pathogenesis. Furthermore, allergen-bound IgE causes mast cell activation, resulting in the release of cytokines, such as IL-9, IL-13, and the inflammatory mediator histamine, and chemokines, including prostaglandin D2.62, 63, 64 Histamine, in turn, promotes microvascular permeability; its release from mast cells may contribute to the pathogenesis of BIA-ALCL.65
The presence of numerous eosinophils is also characteristic of most Hodgkin lymphomas.66 It was found that these express functionally active CD30 ligand on their surfaces, which acts to transduce proliferative signaling in CD30+ cells, including Reed-Sternberg cells, thereby representing an important element in Hodgkin lymphoma pathology.66 In a subsequent investigation, multivariate analysis revealed that eosinophilia was the strongest prognostic factor in nodular sclerosing Hodgkin lymphoma, predicting poor outcomes for freedom from treatment failure (P < 0.001) and overall survival (P < 0.001) in a stage-stratified model.67 Although CD30 ligand is known to be expressed in several B- and T-cell malignancies,68 its expression and potential role in BIA-ALCL, although intriguing, remain to be elucidated.
The apparent divergence with the IL-17F– and interferon-γ–producing Th17/Th1 phenotype that evidence suggests comprises the cell of origin may be explained, in part, by several factors. In a chronically inflamed milieu, subsets of Th2 cells would be expected to be present. The known plasticity of helper T-cell subtypes could also factor into this difference. Multiple subtypes, including regulatory T cells, appear to be capable of differentiation into Th17-producing cells.69 In addition, CD30-mediated IL-13 production from CD4+ T cells that is entirely independent of T-cell receptor signaling has been reported in a murine model.70 Thus, it is conceivable that neoplastic BIA-ALCL cells, which, as previously noted (Innate Immune Response), generally lack surface T-cell receptor expression, may still serve as a source of this inflammatory cytokine.
JAK-STAT Pathway Centrality
Cytokine signaling predominantly uses the JAK-STAT pathway, which is, therefore, central in converting extracellular signals into changes in cellular protein expression.71 Acquired mutations in JAK-STAT signaling have been associated with oncogenesis in T-cell lymphomas.72,73 JAK1 and/or STAT3 mutations were found in 37.5% of ALK-negative sALCL specimens; fusion proteins involving the kinases TYK2 and ROS1, leading to constitutive STAT activation, were also identified, implicating STAT3 activation as a key oncogenic driver in this cancer type.12,74,75
A study of whole exome sequencing on DNA extracted from effusion cytology fluid and germline DNA of two patients with effusion-limited BIA-ALCL revealed somatic, activating mutations in JAK1 and STAT3 as well as a germline JAK3 mutation, the latter suggesting a possible genetic risk factor for the development of this lymphoma.12 Clinical specimens from 12 of 12 patients with BIA-ALCL tested positive for phospho-STAT3 (pSTAT3) by immunohistochemistry.11 Examination of capsular tissue from patients with BIA-ALCL by immunohistochemistry found that all tested cases (n = 27) were positive for the presence of activated STAT3, which is found in only 38% to 47% of ALK-negative ALCLs76 (Figure 3). BIA-ALCL cell lines expressed high levels of p-STAT3, which correlated with aggressiveness in xenografted mice.21 These cell lines also produce high levels of the cytokines IL-6 and IL-10, both of which transduce signals via STAT3; and investigators have proposed that autocrine signaling by IL-6 is necessary for survival of these cells.21,77,78 Two of the three BIA-ALCL cell lines tested (both having activating STAT3 mutations) and most of other ALK-negative cell lines exhibited absolute JAK1-STAT3 dependence; in these cell lines, the JAK inhibitors ruxolitinib, tofacitinib, and AZ-3 as well as JAK1 and STAT3 knockdown by shRNA inhibited proliferation.79 Cells that were p-STAT3 positive were susceptible to JAK inhibition regardless of the presence of JAK-STAT mutations, which implies that other mechanisms resulting in constitutive p-STAT3 expression are operative but that these cells still depend on JAK1-STAT3 signaling.79
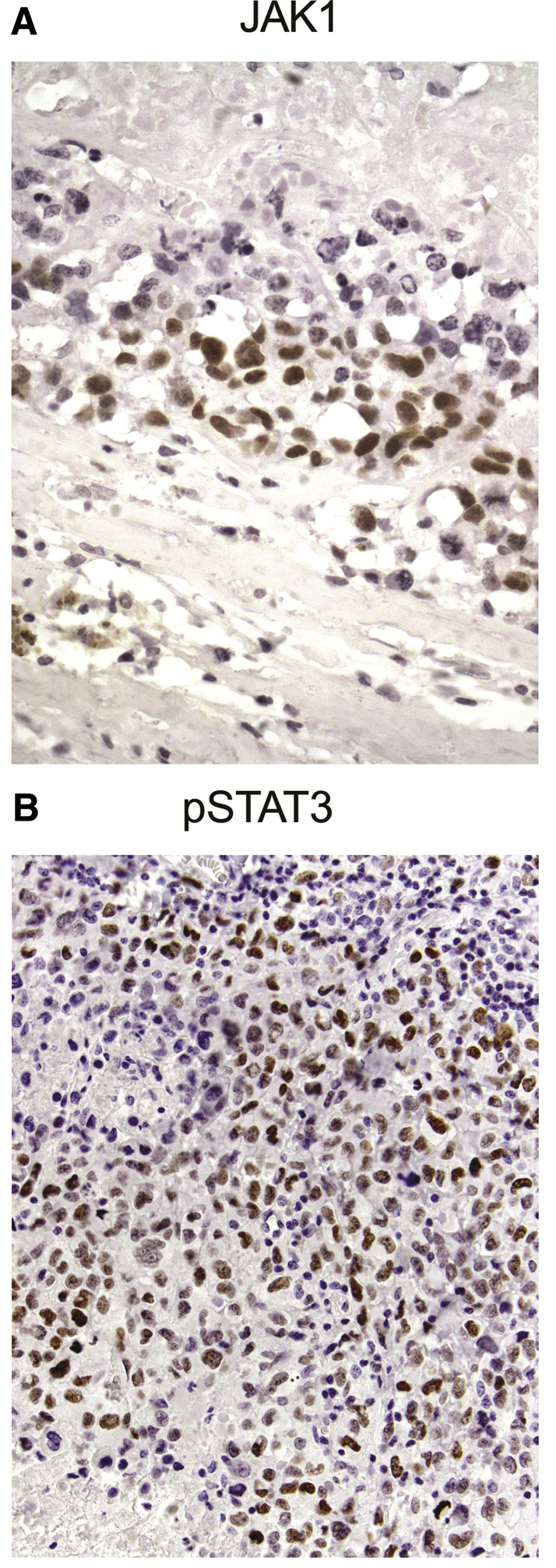
Figure 3.
A: Anaplastic cells in breast implant–associated anaplastic large-cell lymphoma (BIA-ALCL) express Janus kinase 1 (JAK1; nucleus, brown stain). Rabbit monoclonal antibody (mAb) IgG number 3344 (Cell Signaling Technology, Danvers, MA) was used at 1:100 dilution. B: Anaplastic BIA-ALCL cells express phospho-STAT3 (pSTAT3) in the nucleus (brown stain). (Tyr705)(D3A7) rabbit mAb IgG number 9145 (Cell Signaling Technology) was used at 1:200 dilution. Original magnification: ×600 (A); ×400 (B).
These findings have several noteworthy implications. Dysregulation of JAK-STAT signaling appears to play a central role in the development of BIA-ALCL. From a therapeutic standpoint, JAK-STAT dependence may, therefore, constitute an attractive target in BIA-ALCL, and could represent an additional targeted approach, with case studies currently reporting efficacy for the anti-CD30 antibody-drug conjugate brentuximab vedotin.80,81
Conclusions
The existing data suggest that BIA-ALCL is a complex disease resulting from the interplay of several pathophysiological processes. It is likely that a triggering event stimulates an immune response, leading to the recruitment of lymphocytes and other inflammatory cells (Figure 4). However, much remains to be understood regarding its etiology and the mechanisms driving its progression, necessitating further investigation. The proposed role of bacterial biofilms and other possible triggers of the inflammatory immune cascade requires further studies. The presence of both a distinctly inflammatory phenotype with a Th17/Th1 cell of origin and a distinct Th2 phenotype characteristic of allergic inflammation must be reconciled, and it will need to be determined if this duality reflects T-cell plasticity and/or a downstream effect of Th17/Th1 inflammation. In addition, a thorough assessment of the impact of JAK-STAT pathway mutations may give additional insight into the mechanisms of disease, as well as direct therapeutic strategies for its management. Ongoing and future research on the molecular mechanisms underlying BIA-ALCL may provide still better options for its prevention and treatment in all patients.
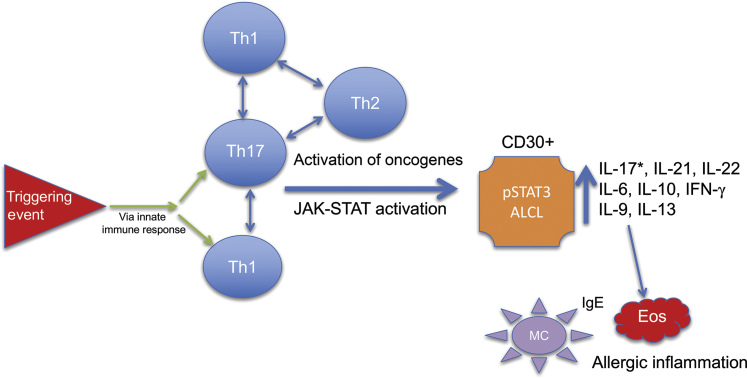
Figure 4.
Proposed hypothesis for progression of immune T-cell infiltrates to breast implant–associated anaplastic large-cell lymphoma (BIA-ALCL). In this proposed model, a triggering event elicits both innate and adaptive [type 17 helper T-cell (Th17)/Th1/Th2] immune responses. Oncogenic events, including critical mutations leading to constitutive Janus kinase (JAK)–STAT activation and/or other oncogenic drivers, result in the emergence of monoclonal, CD30-positive BIA-ALCL cells capable of amplifying the inflammatory environment through plasticity and secretion of cytokines, leading to infiltration of eosinophils (Eos) and IgE-coated mast cells (MC). *Variable reports of IL-17 expression in the literature. IFN-γ, interferon-γ; pSTAT3, phospho-STAT3.
Acknowledgments
We thank Robert Rydzewski, M.S., C.M.P.P. (Peloton Advantage, LLC, Parsippany, NJ) for providing writing and editorial assistance (funded by Allergan plc, Dublin, Ireland).
Footnotes
Supported by Allergan plc, Dublin, Ireland.
Disclosures: M.E.K., G.I., and R.N.M. have served on scientific advisory boards on breast implant–associated anaplastic large-cell lymphoma (BIA-ALCL) for Allergan; S.D.T. has served on scientific advisory boards on BIA-ALCL for Allergan without remuneration.
Author Contributions
M.E.K. co-authored the manuscript, provided illustrations, and introduced concepts of pathogenesis of breast implant–associated anaplastic large-cell lymphoma; S.D.T. developed concepts discussed in the manuscript and coauthored and edited the manuscript; all authors approved the manuscript.
References
- 1.Keech J.A., Jr., Creech B.J. Anaplastic T-cell lymphoma in proximity to a saline-filled breast implant. Plast Reconstr Surg. 1997;100:554–555. doi: 10.1097/00006534-199708000-00065. [DOI] [PubMed] [Google Scholar]
- 2.Doren E.L., Miranda R.N., Selber J.C., Garvey P.B., Liu J., Medeiros L.J., Butler C.E., Clemens M.W. United States epidemiology of breast implant-associated anaplastic large cell lymphoma. Plast Reconstr Surg. 2017;139:1042–1050. doi: 10.1097/PRS.0000000000003282. [DOI] [PubMed] [Google Scholar]
- 3.Campanale A., Boldrini R., Marletta M. 22 Cases of breast implant-associated ALCL: awareness and outcome tracking from the Italian Ministry of Health. Plast Reconstr Surg. 2018;141:11e–19e. doi: 10.1097/PRS.0000000000003916. [DOI] [PubMed] [Google Scholar]
- 4.de Boer M., van Leeuwen F.E., Hauptmann M., Overbeek L.I.H., de Boer J.P., Hijmering N.J., Sernee A., Klazen C.A.H., Lobbes M.B.I., van der Hulst R., Rakhorst H.A., de Jong D. Breast implants and the risk of anaplastic large-cell lymphoma in the breast. JAMA Oncol. 2018;4:335–341. doi: 10.1001/jamaoncol.2017.4510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Swerdlow S.H., Campo E., Pileri S.A., Harris N.L., Stein H., Siebert R., Advani R., Ghielmini M., Salles G.A., Zelenetz A.D., Jaffe E.S. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127:2375–2390. doi: 10.1182/blood-2016-01-643569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Loch-Wilkinson A., Beath K., Knight R.J.W., Wessels W.L.F., Magnusson M., Papadopoulos T., Connell T., Lofts J., Locke M., Hopper I., Cooter R., Vickery K., Joshi P.A., Prince H.M., Deva A.K. Breast implant associated anaplastic large cell lymphoma in Australia and New Zealand: high surface area textured implants are associated with increased risk. Plast Reconstr Surg. 2017;140:645–654. doi: 10.1097/PRS.0000000000003654. [DOI] [PubMed] [Google Scholar]
- 7.Leberfinger A.N., Behar B.J., Williams N.C., Rakszawski K.L., Potochny J.D., Mackay D.R., Ravnic D.J. Breast implant-associated anaplastic large cell lymphoma: a systematic review. JAMA Surg. 2017;152:1161–1168. doi: 10.1001/jamasurg.2017.4026. [DOI] [PubMed] [Google Scholar]
- 8.Srinivasa D.R., Miranda R.N., Kaura A., Francis A.M., Campanale A., Boldrini R., Alexander J., Deva A., Gravina P., Medeiros L.J., Nast K., Butler C.E., Clemens M.W. Global adverse event reports of breast implant-associated ALCL: an international review of 40 government authority databases. Plast Reconstr Surg. 2017;139:1029–1039. doi: 10.1097/PRS.0000000000003233. [DOI] [PubMed] [Google Scholar]
- 9.Clemens M.W., Medeiros L.J., Butler C.E., Hunt K.K., Fanale M.A., Horwitz S., Weisenburger D.D., Liu J., Morgan E.A., Kanagal-Shamanna R., Parkash V., Ning J., Sohani A.R., Ferry J.A., Mehta-Shah N., Dogan A., Liu H., Thormann N., Di Napoli A., Lade S., Piccolini J., Reyes R., Williams T., McCarthy C.M., Hanson S.E., Nastoupil L.J., Gaur R., Oki Y., Young K.H., Miranda R.N. Complete surgical excision is essential for the management of patients with breast implant-associated anaplastic large-cell lymphoma. J Clin Oncol. 2016;34:160–168. doi: 10.1200/JCO.2015.63.3412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miranda R.N., Aladily T.N., Prince H.M., Kanagal-Shamanna R., de Jong D., Fayad L.E., Amin M.B., Haideri N., Bhagat G., Brooks G.S., Shifrin D.A., O'Malley D.P., Cheah C.Y., Bacchi C.E., Gualco G., Li S., Keech J.A., Jr., Hochberg E.P., Carty M.J., Hanson S.E., Mustafa E., Sanchez S., Manning J.T., Jr., Xu-Monette Z.Y., Miranda A.R., Fox P., Bassett R.L., Castillo J.J., Beltran B.E., de Boer J.P., Chakhachiro Z., Ye D., Clark D., Young K.H., Medeiros L.J. Breast implant-associated anaplastic large-cell lymphoma: long-term follow-up of 60 patients. J Clin Oncol. 2014;32:114–120. doi: 10.1200/JCO.2013.52.7911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Laurent C., Delas A., Gaulard P., Haioun C., Moreau A., Xerri L., Traverse-Glehen A., Rousset T., Quintin-Roue I., Petrella T., Emile J.F., Amara N., Rochaix P., Chenard-Neu M.P., Tasei A.M., Menet E., Chomarat H., Costes V., Andrac-Meyer L., Michiels J.F., Chassagne-Clement C., de L.L., Brousset P., Delsol G., Lamant L. Breast implant-associated anaplastic large cell lymphoma: two distinct clinicopathological variants with different outcomes. Ann Oncol. 2016;27:306–314. doi: 10.1093/annonc/mdv575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blombery P., Thompson E.R., Jones K., Arnau G.M., Lade S., Markham J.F., Li J., Deva A., Johnstone R.W., Khot A., Prince H.M., Westerman D. Whole exome sequencing reveals activating JAK1 and STAT3 mutations in breast implant-associated anaplastic large cell lymphoma anaplastic large cell lymphoma. Haematologica. 2016;101:e387–e390. doi: 10.3324/haematol.2016.146118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Santanelli di Pompeo F., Sorotos M. EURAPS editorial: BIA-ALCL, a brief overview. J Plast Reconstr Aesthet Surg. 2018;71:785–787. doi: 10.1016/j.bjps.2018.04.012. [DOI] [PubMed] [Google Scholar]
- 14.Flassbeck D., Pfleiderer B., Klemens P., Heumann K.G., Eltze E., Hirner A.V. Determination of siloxanes, silicon, and platinum in tissues of women with silicone gel-filled implants. Anal Bioanal Chem. 2003;375:356–362. doi: 10.1007/s00216-002-1694-z. [DOI] [PubMed] [Google Scholar]
- 15.Jacombs A., Tahir S., Hu H., Deva A.K., Almatroudi A., Wessels W.L., Bradshaw D.A., Vickery K. In vitro and in vivo investigation of the influence of implant surface on the formation of bacterial biofilm in mammary implants. Plast Reconstr Surg. 2014;133:471e–480e. doi: 10.1097/PRS.0000000000000020. [DOI] [PubMed] [Google Scholar]
- 16.Linnemann T., Gellrich S., Lukowsky A., Mielke A., Audring H., Sterry W., Walden P. Polyclonal expansion of T cells with the TCR V beta type of the tumour cell in lesions of cutaneous T-cell lymphoma: evidence for possible superantigen involvement. Br J Dermatol. 2004;150:1013–1017. doi: 10.1111/j.1365-2133.2004.05970.x. [DOI] [PubMed] [Google Scholar]
- 17.Di Napoli A., De Cecco L., Piccaluga P.P., Navari M., Cancila V., Cippitelli C., Pepe G., Lopez G., Monardo F., Bianchi A., D'Amore E.S.G., Gianelli U., Facchetti F., Berti E., Bhagat G. Transcriptional analysis distinguishes breast implant-associated anaplastic large cell lymphoma from other peripheral T-cell lymphomas. Mod Pathol. 2019;32:216–230. doi: 10.1038/s41379-018-0130-7. [DOI] [PubMed] [Google Scholar]
- 18.Kang S.T., Wang H.C., Yang Y.T., Kou G.H., Lo C.F. The DNA virus white spot syndrome virus uses an internal ribosome entry site for translation of the highly expressed nonstructural protein ICP35. J Virol. 2013;87:13263–13278. doi: 10.1128/JVI.01732-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fitzal F., Turner S.D., Kenner L. Is breast implant-associated anaplastic large cell lymphoma a hazard of breast implant surgery? Open Biol. 2019;9:190006. doi: 10.1098/rsob.190006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kadin M.E., Morgan J., Xu H., Epstein A.L., Sieber D., Hubbard B.A., Adams W.P., Jr., Bacchi C.E., Goes J.C.S., Clemens M.W., Medeiros L.J., Miranda R.N. IL-13 is produced by tumor cells in breast implant associated anaplastic large cell lymphoma: implications for pathogenesis. Hum Pathol. 2018;78:54–62. doi: 10.1016/j.humpath.2018.04.007. [DOI] [PubMed] [Google Scholar]
- 21.Lechner M.G., Megiel C., Church C.H., Angell T.E., Russell S.M., Sevell R.B., Jang J.K., Brody G.S., Epstein A.L. Survival signals and targets for therapy in breast implant-associated ALK--anaplastic large cell lymphoma. Clin Cancer Res. 2012;18:4549–4559. doi: 10.1158/1078-0432.CCR-12-0101. [DOI] [PubMed] [Google Scholar]
- 22.Weiser J.N., Nahm M.H. Immunity to extracellular bacteria. Fundamental Immunology, ed 6. In: Paul W.E., editor. Lippincott Williams & Wilkins; Philadelphia, PA: 2008. pp. 1182–1203. [Google Scholar]
- 23.Medzhitov R. The innate immune system. Fundamental Immunology, ed 6. In: Paul W.E., editor. Lippincott Williams & Wilkins; Philadelphia, PA: 2008. pp. 427–450. [Google Scholar]
- 24.Aladily T.N., Medeiros L.J., Amin M.B., Haideri N., Ye D., Azevedo S.J., Jorgensen J.L., de Peralta-Venturina M., Mustafa E.B., Young K.H., You M.J., Fayad L.E., Blenc A.M., Miranda R.N. Anaplastic large cell lymphoma associated with breast implants: a report of 13 cases. Am J Surg Pathol. 2012;36:1000–1008. doi: 10.1097/PAS.0b013e31825749b1. [DOI] [PubMed] [Google Scholar]
- 25.Quesada A.E., Medeiros L.J., Clemens M.W., Ferrufino-Schmidt M.C., Pina-Oviedo S., Miranda R.N. Breast implant-associated anaplastic large cell lymphoma: a review. Mod Pathol. 2019;32:166–168. doi: 10.1038/s41379-018-0134-3. [DOI] [PubMed] [Google Scholar]
- 26.Taylor C.R., Siddiqi I.N., Brody G.S. Anaplastic large cell lymphoma occurring in association with breast implants: review of pathologic and immunohistochemical features in 103 cases. Appl Immunohistochem Mol Morphol. 2013;21:13–20. doi: 10.1097/PAI.0b013e318266476c. [DOI] [PubMed] [Google Scholar]
- 27.Di Napoli A., Pepe G., Giarnieri E., Cippitelli C., Bonifacino A., Mattei M., Martelli M., Falasca C., Cox M.C., Santino I., Giovagnoli M.R. Cytological diagnostic features of late breast implant seromas: from reactive to anaplastic large cell lymphoma. PLoS One. 2017;12:e0181097. doi: 10.1371/journal.pone.0181097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cappellano G., Ploner C., Lobenwein S., Sopper S., Hoertnagl P., Mayerl C., Wick N., Pierer G., Wick G., Wolfram D. Immunophenotypic characterization of human T cells after in vitro exposure to different silicone breast implant surfaces. PLoS One. 2018;13:e0192108. doi: 10.1371/journal.pone.0192108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hazenberg M.D., Spits H. Human innate lymphoid cells. Blood. 2014;124:700–709. doi: 10.1182/blood-2013-11-427781. [DOI] [PubMed] [Google Scholar]
- 30.Miyagaki T., Sugaya M., Suga H., Kamata M., Ohmatsu H., Fujita H., Asano Y., Tada Y., Kadono T., Sato S. IL-22, but not IL-17, dominant environment in cutaneous T-cell lymphoma. Clin Cancer Res. 2011;17:7529–7538. doi: 10.1158/1078-0432.CCR-11-1192. [DOI] [PubMed] [Google Scholar]
- 31.Schleussner N., Merkel O., Costanza M., Liang H.C., Hummel F., Romagnani C., Durek P., Anagnostopoulos I., Hummel M., Johrens K., Niedobitek A., Griffin P.R., Piva R., Sczakiel H.L., Woessmann W., Damm-Welk C., Hinze C., Stoiber D., Gillissen B., Turner S.D., Kaergel E., von Hoff L., Grau M., Lenz G., Dorken B., Scheidereit C., Kenner L., Janz M., Mathas S. The AP-1-BATF and -BATF3 module is essential for growth, survival and TH17/ILC3 skewing of anaplastic large cell lymphoma. Leukemia. 2018;32:1994–2007. doi: 10.1038/s41375-018-0045-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Montes-Mojarro I.A., Steinhilber J., Bonzheim I., Quintanilla-Martinez L., Fend F. The pathological spectrum of systemic anaplastic large cell lymphoma (ALCL) Cancers (Basel) 2018;10:E107. doi: 10.3390/cancers10040107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Turner S.D. An exploration into the origins and pathogenesis of anaplastic large cell lymphoma, anaplastic lymphoma kinase (ALK)-positive. Cancers (Basel) 2017;9:141. [Google Scholar]
- 34.Stein H., Foss H.D., Durkop H., Marafioti T., Delsol G., Pulford K., Pileri S., Falini B. CD30(+) anaplastic large cell lymphoma: a review of its histopathologic, genetic, and clinical features. Blood. 2000;96:3681–3695. [PubMed] [Google Scholar]
- 35.Juco J., Holden J.T., Mann K.P., Kelley L.G., Li S. Immunophenotypic analysis of anaplastic large cell lymphoma by flow cytometry. Am J Clin Pathol. 2003;119:205–212. doi: 10.1309/HEFL-7KC4-35KF-WEX8. [DOI] [PubMed] [Google Scholar]
- 36.van der Weyden C.A., Pileri S.A., Feldman A.L., Whisstock J., Prince H.M. Understanding CD30 biology and therapeutic targeting: a historical perspective providing insight into future directions. Blood Cancer J. 2017;7:e603. doi: 10.1038/bcj.2017.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bonzheim I., Geissinger E., Roth S., Zettl A., Marx A., Rosenwald A., Muller-Hermelink H.K., Rudiger T. Anaplastic large cell lymphomas lack the expression of T-cell receptor molecules or molecules of proximal T-cell receptor signaling. Blood. 2004;104:3358–3360. doi: 10.1182/blood-2004-03-1037. [DOI] [PubMed] [Google Scholar]
- 38.Geissinger E., Sadler P., Roth S., Grieb T., Puppe B., Muller N., Reimer P., Vetter-Kauczok C.S., Wenzel J., Bonzheim I., Rudiger T., Muller-Hermelink H.K., Rosenwald A. Disturbed expression of the T-cell receptor/CD3 complex and associated signaling molecules in CD30+ T-cell lymphoproliferations. Haematologica. 2010;95:1697–1704. doi: 10.3324/haematol.2009.021428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Malcolm T.I., Villarese P., Fairbairn C.J., Lamant L., Trinquand A., Hook C.E., Burke G.A., Brugieres L., Hughes K., Payet D., Merkel O., Schiefer A.I., Ashankyty I., Mian S., Wasik M., Turner M., Kenner L., Asnafi V., Macintyre E., Turner S.D. Anaplastic large cell lymphoma arises in thymocytes and requires transient TCR expression for thymic egress. Nat Commun. 2016;7:10087. doi: 10.1038/ncomms10087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stein H., Mason D.Y., Gerdes J., O'Connor N., Wainscoat J., Pallesen G., Gatter K., Falini B., Delsol G., Lemke H., Schwarting R., Lennert K. The expression of the Hodgkin's disease associated antigen Ki-1 in reactive and neoplastic lymphoid tissue: evidence that Reed-Sternberg cells and histiocytic malignancies are derived from activated lymphoid cells. Blood. 1985;66:848–858. [PubMed] [Google Scholar]
- 41.Kesler M.V., Paranjape G.S., Asplund S.L., McKenna R.W., Jamal S., Kroft S.H. Anaplastic large cell lymphoma: a flow cytometric analysis of 29 cases. Am J Clin Pathol. 2007;128:314–322. doi: 10.1309/GUHKGAJEJ72CEAL7. [DOI] [PubMed] [Google Scholar]
- 42.Massone C., Cerroni L. Phenotypic variability in primary cutaneous anaplastic large T-cell lymphoma: a study on 35 patients. Am J Dermatopathol. 2014;36:153–157. doi: 10.1097/DAD.0b013e3182a5683a. [DOI] [PubMed] [Google Scholar]
- 43.Krenacs L., Wellmann A., Sorbara L., Himmelmann A.W., Bagdi E., Jaffe E.S., Raffeld M. Cytotoxic cell antigen expression in anaplastic large cell lymphomas of T- and null-cell type and Hodgkin's disease: evidence for distinct cellular origin. Blood. 1997;89:980–989. [PubMed] [Google Scholar]
- 44.Kumura T., Hino M., Yamane T., Ohta K., Nakao T., Wakasa K., Tatsumi N. Triple-negative (CD3-/CD4-/CD8-) adult T cell leukemia/lymphoma, histologically presenting as CD30 (Ki-1)-positive anaplastic large cell lymphoma with clonal Epstein-Barr virus genome. Leukemia. 2001;15:994–995. doi: 10.1038/sj.leu.2402126. [DOI] [PubMed] [Google Scholar]
- 45.Foss H.D., Anagnostopoulos I., Araujo I., Assaf C., Demel G., Kummer J.A., Hummel M., Stein H. Anaplastic large-cell lymphomas of T-cell and null-cell phenotype express cytotoxic molecules. Blood. 1996;88:4005–4011. [PubMed] [Google Scholar]
- 46.de Jong D., Vasmel W.L., de Boer J.P., Verhave G., Barbe E., Casparie M.K., van Leeuwen F.E. Anaplastic large-cell lymphoma in women with breast implants. JAMA. 2008;300:2030–2035. doi: 10.1001/jama.2008.585. [DOI] [PubMed] [Google Scholar]
- 47.Plaza J.A., Feldman A.L., Magro C. Cutaneous CD30-positive lymphoproliferative disorders with CD8 expression: a clinicopathologic study of 21 cases. J Cutan Pathol. 2013;40:236–247. doi: 10.1111/cup.12047. [DOI] [PubMed] [Google Scholar]
- 48.Kadin M.E., Deva A., Xu H., Morgan J., Khare P., MacLeod R.A., Van Natta B.W., Adams W.P., Jr., Brody G.S., Epstein A.L. Biomarkers provide clues to early events in the pathogenesis of breast implant-associated anaplastic large cell lymphoma. Aesthet Surg J. 2016;36:773–781. doi: 10.1093/asj/sjw023. [DOI] [PubMed] [Google Scholar]
- 49.Moti N., Malcolm T., Hamoudi R., Mian S., Garland G., Hook C.E., Burke G.A., Wasik M.A., Merkel O., Kenner L., Laurenti E., Dick J.E., Turner S.D. Anaplastic large cell lymphoma-propagating cells are detectable by side population analysis and possess an expression profile reflective of a primitive origin. Oncogene. 2015;34:1843–1852. doi: 10.1038/onc.2014.112. [DOI] [PubMed] [Google Scholar]
- 50.Eckerle S., Brune V., Doring C., Tiacci E., Bohle V., Sundstrom C., Kodet R., Paulli M., Falini B., Klapper W., Chaubert A.B., Willenbrock K., Metzler D., Brauninger A., Kuppers R., Hansmann M.L. Gene expression profiling of isolated tumour cells from anaplastic large cell lymphomas: insights into its cellular origin, pathogenesis and relation to Hodgkin lymphoma. Leukemia. 2009;23:2129–2138. doi: 10.1038/leu.2009.161. [DOI] [PubMed] [Google Scholar]
- 51.Hassler M.R., Pulverer W., Lakshminarasimhan R., Redl E., Hacker J., Garland G.D., Merkel O., Schiefer A.I., Simonitsch-Klupp I., Kenner L., Weisenberger D.J., Weinhaeusel A., Turner S.D., Egger G. Insights into the pathogenesis of anaplastic large-cell lymphoma through genome-wide DNA methylation profiling. Cell Rep. 2016;17:596–608. doi: 10.1016/j.celrep.2016.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Iqbal J., Weisenburger D.D., Greiner T.C., Vose J.M., McKeithan T., Kucuk C., Geng H., Deffenbacher K., Smith L., Dybkaer K., Nakamura S., Seto M., Delabie J., Berger F., Loong F., Au W.Y., Ko Y.H., Sng I., Armitage J.O., Chan W.C. Molecular signatures to improve diagnosis in peripheral T-cell lymphoma and prognostication in angioimmunoblastic T-cell lymphoma. Blood. 2010;115:1026–1036. doi: 10.1182/blood-2009-06-227579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Malcolm T.I., Hodson D.J., Macintyre E.A., Turner S.D. Challenging perspectives on the cellular origins of lymphoma. Open Biol. 2016;6:160232. doi: 10.1098/rsob.160232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.O'Shea J.J., Paul W.E. Mechanisms underlying lineage commitment and plasticity of helper CD4+ T cells. Science. 2010;327:1098–1102. doi: 10.1126/science.1178334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Muranski P., Restifo N.P. Essentials of Th17 cell commitment and plasticity. Blood. 2013;121:2402–2414. doi: 10.1182/blood-2012-09-378653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Guery L., Hugues S. Th17 cell plasticity and functions in cancer immunity. Biomed Res Int. 2015;2015:314620. doi: 10.1155/2015/314620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cho-Vega J.H., Rassidakis G.Z., Amin H.M., Tsioli P., Spurgers K., Remache Y.K., Vega F., Goy A.H., Gilles F., Medeiros L.J. Suppressor of cytokine signaling 3 expression in anaplastic large cell lymphoma. Leukemia. 2004;18:1872–1878. doi: 10.1038/sj.leu.2403495. [DOI] [PubMed] [Google Scholar]
- 58.Laimer D., Dolznig H., Kollmann K., Vesely P.W., Schlederer M., Merkel O. PDGFR blockade is a rational and effective therapy for NPM-ALK-driven lymphomas. Nat Med. 2012;18:1699–1704. doi: 10.1038/nm.2966. [DOI] [PubMed] [Google Scholar]
- 59.Schiefer A.I., Vesely P., Hassler M.R., Egger G., Kenner L. The role of AP-1 and epigenetics in ALCL. Front Biosci (Schol Ed) 2015;7:226–235. doi: 10.2741/S436. [DOI] [PubMed] [Google Scholar]
- 60.Vassallo J., Lamant L., Brugieres L., Gaillard F., Campo E., Brousset P., Delsol G. ALK-positive anaplastic large cell lymphoma mimicking nodular sclerosis Hodgkin's lymphoma: report of 10 cases. Am J Surg Pathol. 2006;30:223–229. doi: 10.1097/01.pas.0000179123.66748.c2. [DOI] [PubMed] [Google Scholar]
- 61.Savan R., McFarland A.P., Reynolds D.A., Feigenbaum L., Ramakrishnan K., Karwan M., Shirota H., Klinman D.M., Dunleavy K., Pittaluga S., Anderson S.K., Donnelly R.P., Wilson W.H., Young H.A. A novel role for IL-22R1 as a driver of inflammation. Blood. 2011;117:575–584. doi: 10.1182/blood-2010-05-285908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen C.Y., Lee J.B., Liu B., Ohta S., Wang P.Y., Kartashov A.V., Mugge L., Abonia J.P., Barski A., Izuhara K., Rothenberg M.E., Finkelman F.D., Hogan S.P., Wang Y.H. Induction of interleukin-9-producing mucosal mast cells promotes susceptibility to IgE-mediated experimental food allergy. Immunity. 2015;43:788–802. doi: 10.1016/j.immuni.2015.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Burd P.R., Thompson W.C., Max E.E., Mills F.C. Activated mast cells produce interleukin 13. J Exp Med. 1995;181:1373–1380. doi: 10.1084/jem.181.4.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Galli S.J., Tsai M. IgE and mast cells in allergic disease. Nat Med. 2012;18:693–704. doi: 10.1038/nm.2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kadin M.E., Morgan J., Xu H., Epstein A., Miranda R.N., Sieber D., Clemens M.W. American Association of Plastic Surgeons; Arlington Heights, IL: 2018. Breast implant associated ALCL tumor expresses prostaglandin D2 receptor: an allergic inflammation pathogenesis. Annual Meeting of the American Association of Plastic Surgeons [abstract 34], April 7–10, 2018, Seattle, WA. [Google Scholar]
- 66.Pinto A., Aldinucci D., Gloghini A., Zagonel V., Degan M., Improta S., Juzbasic S., Todesco M., Perin V., Gattei V., Herrmann F., Gruss H.J., Carbone A. Human eosinophils express functional CD30 ligand and stimulate proliferation of a Hodgkin's disease cell line. Blood. 1996;88:3299–3305. [PubMed] [Google Scholar]
- 67.von Wasielewski R., Seth S., Franklin J., Fischer R., Hubner K., Hansmann M.L., Diehl V., Georgii A. Tissue eosinophilia correlates strongly with poor prognosis in nodular sclerosing Hodgkin's disease, allowing for known prognostic factors. Blood. 2000;95:1207–1213. [PubMed] [Google Scholar]
- 68.Gattei V., Degan M., Gloghini A., De Iuliis A., Improta S., Rossi F.M., Aldinucci D., Perin V., Serraino D., Babare R., Zagonel V., Gruss H.J., Carbone A., Pinto A. CD30 ligand is frequently expressed in human hematopoietic malignancies of myeloid and lymphoid origin. Blood. 1997;89:2048–2059. [PubMed] [Google Scholar]
- 69.Xu L., Kitani A., Fuss I., Strober W. Cutting edge: regulatory T cells induce CD4+CD25-Foxp3- T cells or are self-induced to become Th17 cells in the absence of exogenous TGF-beta. J Immunol. 2007;178:6725–6729. doi: 10.4049/jimmunol.178.11.6725. [DOI] [PubMed] [Google Scholar]
- 70.Harlin H., Podack E., Boothby M., Alegre M.L. TCR-independent CD30 signaling selectively induces IL-13 production via a TNF receptor-associated factor/p38 mitogen-activated protein kinase-dependent mechanism. J Immunol. 2002;169:2451–2459. doi: 10.4049/jimmunol.169.5.2451. [DOI] [PubMed] [Google Scholar]
- 71.Jatiani S.S., Baker S.J., Silverman L.R., Reddy E.P. Jak/STAT pathways in cytokine signaling and myeloproliferative disorders: approaches for targeted therapies. Genes Cancer. 2010;1:979–993. doi: 10.1177/1947601910397187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Elenitoba-Johnson K.S.J., Lim M.S. New insights into lymphoma pathogenesis. Annu Rev Pathol. 2018;13:193–217. doi: 10.1146/annurev-pathol-020117-043803. [DOI] [PubMed] [Google Scholar]
- 73.Pizzi M., Margolskee E., Inghirami G. Pathogenesis of peripheral T cell lymphoma. Annu Rev Pathol. 2018;13:293–320. doi: 10.1146/annurev-pathol-020117-043821. [DOI] [PubMed] [Google Scholar]
- 74.Prutsch N., Gurnhofer E., Suske T., Liang H.C., Schlederer M., Roos S., Wu L.C., Simonitsch-Klupp I., Alvarez-Hernandez A., Kornauth C., Leone D.A., Svinka J., Eferl R., Limberger T., Aufinger A., Shirsath N., Wolf P., Hielscher T., Aberger F., Schmoellerl J., Stoiber D., Strobl B., Jager U., Staber P.B., Grebien F., Moriggl R., Muller M., Inghirami G.G., Sanda T., Look A.T., Turner S.D., Kenner L., Merkel O. Dependency on the TYK2/STAT1/MCL1 axis in anaplastic large cell lymphoma. Leukemia. 2019;33:696–709. doi: 10.1038/s41375-018-0239-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Crescenzo R., Abate F., Lasorsa E., Tabbo F., Gaudiano M., Chiesa N. Convergent mutations and kinase fusions lead to oncogenic STAT3 activation in anaplastic large cell lymphoma. Cancer Cell. 2015;27:516–532. doi: 10.1016/j.ccell.2015.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Oishi N., Brody G.S., Ketterling R.P., Viswanatha D.S., He R., Dasari S., Mai M., Benson H.K., Sattler C.A., Boddicker R.L., McPhail E.D., Bennani N.N., Harless C.A., Singh K., Clemens M.W., Medeiros L.J., Miranda R.N., Feldman A.L. Genetic subtyping of breast implant-associated anaplastic large cell lymphoma. Blood. 2018;132:544–547. doi: 10.1182/blood-2017-12-821868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hodge D.R., Hurt E.M., Farrar W.L. The role of IL-6 and STAT3 in inflammation and cancer. Eur J Cancer. 2005;41:2502–2512. doi: 10.1016/j.ejca.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 78.Hutchins A.P., Diez D., Miranda-Saavedra D. The IL-10/STAT3-mediated anti-inflammatory response: recent developments and future challenges. Brief Funct Genomics. 2013;12:489–498. doi: 10.1093/bfgp/elt028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chen J., Zhang Y., Petrus M.N., Xiao W., Nicolae A., Raffeld M., Pittaluga S., Bamford R.N., Nakagawa M., Ouyang S.T., Epstein A.L., Kadin M.E., Del Mistro A., Woessner R., Jaffe E.S., Waldmann T.A. Cytokine receptor signaling is required for the survival of ALK- anaplastic large cell lymphoma, even in the presence of JAK1/STAT3 mutations. Proc Natl Acad Sci U S A. 2017;114:3975–3980. doi: 10.1073/pnas.1700682114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Alderuccio J.P., Desai A., Yepes M.M., Chapman J.R., Vega F., Lossos I.S. Frontline brentuximab vedotin in breast implant-associated anaplastic large-cell lymphoma. Clin Case Rep. 2018;6:634–637. doi: 10.1002/ccr3.1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Johnson L., O'Donoghue J.M., McLean N., Turton P., Khan A.A., Turner S.D., Lennard A., Collis N., Butterworth M., Gui G., Bristol J., Hurren J., Smith S., Grover K., Spyrou G., Krupa K., Azmy I.A., Young I.E., Staiano J.J., Khalil H., MacNeill F.A. Breast implant associated anaplastic large cell lymphoma: the UK experience: recommendations on its management and implications for informed consent. Eur J Surg Oncol. 2017;43:1393–1401. doi: 10.1016/j.ejso.2017.05.004. [DOI] [PubMed] [Google Scholar]