Abstract
BACKGROUND
Hypotension is a frequent complication in the intensive care unit (ICU) after adult cardiac surgery.
AIM
To describe frequency of hypotension in the ICU following adult cardiac surgery and its relation to the hospital outcomes.
METHODS
A retrospective study of post-cardiac adult surgical patients at a tertiary academic medical center in a two-year period. We abstracted baseline demographics, comorbidities, and all pertinent clinical variables. The primary predictor variable was the development of hypotension within the first 30 min upon arrival to the ICU from the operating room (OR). The primary outcome was hospital mortality, and other outcomes included duration of mechanical ventilation (MV) in hours, and ICU and hospital length of stay in days.
RESULTS
Of 417 patients, more than half (54%) experienced hypotension within 30 min upon arrival to the ICU. Presence of OR hypotension immediately prior to ICU transfer was significantly associated with ICU hypotension (odds ratio = 1.9; 95% confidence interval: 1.21-2.98; P < 0.006). ICU hypotensive patients had longer MV, 5 (interquartile ranges 3, 15) vs 4 h (interquartile ranges 3, 6), P = 0.012. The patients who received vasopressor boluses (n = 212) were more likely to experience ICU drop-off hypotension (odds ratio = 1.45, 95% confidence interval: 0.98-2.13; P = 0.062), and they experienced longer MV, ICU and hospital length of stay (P < 0.001, for all).
CONCLUSION
Hypotension upon anesthesia-to-ICU drop-off is more frequent than previously reported and may be associated with adverse clinical outcomes.
Keywords: Hypotension, Cardiac surgery, Intensive care, Postoperative care, Care transfer, Drop-off
Core tip: Hypotension is a frequent complication in adult cardiac surgery patients upon intensive care unit admission. This complication has been anecdotally called “anesthesia drop-off syndrome” and we decided to study this retrospectively. Our results suggest that this complication is more frequent than previously reported and that it may be associated with adverse outcomes.
INTRODUCTION
Perioperative hypotension is one of the most common complications after cardiac surgery and this may adversely affect clinical outcomes[1-5]. It is frequently encountered upon intensive care unit (ICU) admission, where patients become hypotensive in the immediate post-operative period, shortly after the arrival from the operating room (OR). This has been anecdotally termed “anesthesia drop-off syndrome”. However, data is limited in the literature regarding the actual prevalence of hypotension that develops shortly after the transfer of patients to the ICU after cardiac surgery. One study evaluated the occurrence of hemodynamic instability in the first 2 h post cardiac surgery and the most common complication was found to be hypotension, occurring in 34% of the patients after admission to the ICU[6]. Hypotensive patients usually require administration of vasopressor boluses prior to or during the transfer from the OR to the ICU as a temporizing measure. The hypotension and necessity for use of vasopressors have been previously associated with increased hospital length of stay (LOS) as well as mortality, relative to the patients who maintained hemodynamic stability[7-9].
Given the proposed discrepancy between the clinical occurrence and limited data on rate of hypotension starting shortly after the anesthesia to ICU transfer, we aimed to evaluate its prevalence and also how this may relate to the pertinent clinical outcomes. We hypothesized that the occurrence of initial hypotension in the ICU is more frequent complication among post-cardiac surgery ICU patients than previously reported and that patients who experience this complication will have more adverse clinical outcomes. We also aimed to better assess the association between the occurrence of initial hypotension in the ICU and the use of vasopressor bolus administered immediately prior to or during the transfer from the OR to the ICU.
MATERIALS AND METHODS
We conducted a retrospective study of adult patients undergoing cardiac surgery at a tertiary academic medical center in the United States in the 2-year period (January 1, 2015 to December 31, 2016). We excluded patients who underwent cardiac transplantation or a combination of other solid organ transplantation and the cardiac surgery. The study protocol was approved by the Mayo Clinic Institutional Review Board as a minimal risk study, therefore the need for informed consent had been waived.
The primary independent variable was the development of hypotension within the first 30 min upon transfer from the OR (“ICU hypotension”). As there is no single, generally accepted, definition of hypotension[10] we used one of the common definitions used in biomedical research: A systolic blood pressure < 90 mmHg or mean arterial pressure < 65 mmHg per arterial catheter tracing. We abstracted demographic and baseline characteristics, comorbidities, including coronary artery disease (CAD), atrial fibrillation, diabetes mellitus (DM), pulmonary hypertension, liver disease, kidney disease, infective endocarditis, immunosuppression; and all pertinent clinical variables including: Vitals, laboratories, type and urgency of surgery, bypass and cross-clamp time (CCT), medications and blood products delivered during the surgery and immediately prior to transfer to ICU, as well as presence of hypotension in the OR (“OR hypotension”). A vasopressor bolus use was abstracted from the electronic chart documentation by the provider. Although the exact doses of vasopressors given were not abstracted, our anesthesiologists mostly use norepinephrine (100 µg) and/or vasopressin (1 unit), and much less frequently epinephrine (10 µg). The primary outcome was hospital mortality and secondary outcomes were duration of mechanical ventilation (MV) in hours, and ICU and LOS in days. All data were manually extracted from an electronic medical record. The anesthesia notes during the surgery were extracted partially from plotted diagrams and partially from nominal data.
Statistical analysis
The continuous variables were reported as median values with interquartile ranges (IQR) and the categorical variables were reported as counts and proportions. We used nonparametric statistical tests; Fisher’s exact and Wilcoxon Rank-Sum tests, as applicable. The predictor variables in univariate analyses with a P value of less than 0.1 were included in the subsequent multivariate analyses. We used nominal logistic and linear regressions, as appropriate. Statistical significance was considered at P value of < 0.05. As we performed analysis mainly for the exploratory purpose, no corrections for multiple comparisons were done. We used JMP 10 Pro statistical software for analysis from SAS (Cary, NC, United States).
RESULTS
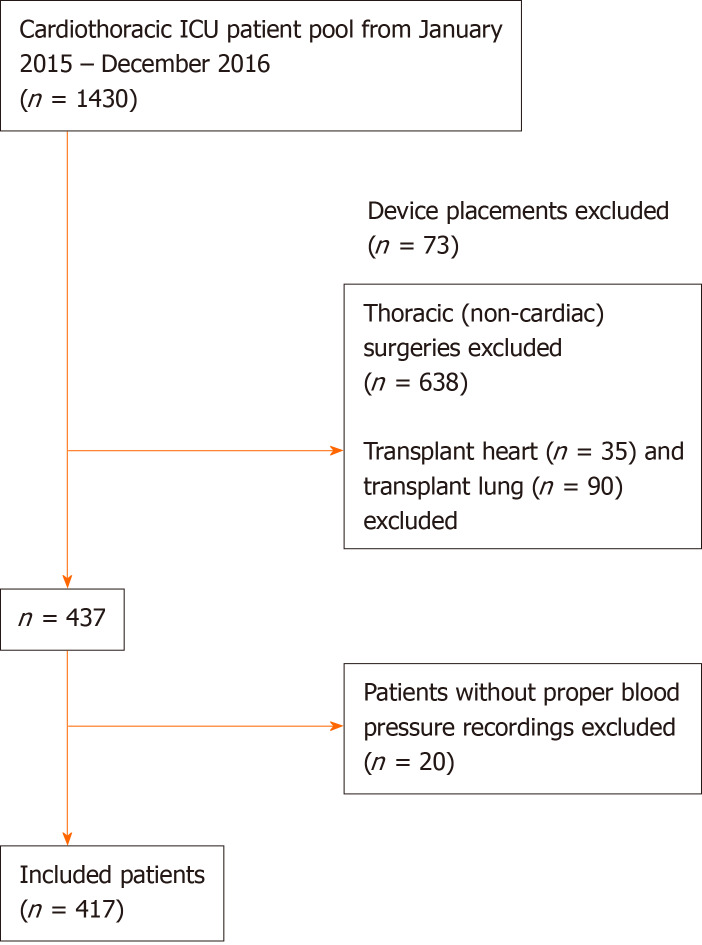
Out of 1273 cardiothoracic surgeries performed within the study period, 437 patients underwent non-transplant cardiac surgery and were eligible for our study. Twenty patients were excluded subsequently as they lacked detailed blood pressure recordings, leaving 417 patients for the study analyses (Figure 1). The majority of patients were white (85%), males (73%), of median age 67 years (IQR 59, 73), and with median body mass index (BMI) of 28 (IQR 25, 32). The two most commonly performed surgeries were coronary artery bypass grafting (46%) and valvular surgery (29%). The detailed baseline characteristics are listed in Table 1. The median bypass time (BT) was 116 min (IQR 90, 150) and the median CCT was 80 min (IQR 55, 105). While 76% of all surgeries were elective (pre-scheduled), 24% were either emergent (within 24 h of admission) or urgent (24-72 h after hospital admission). The overall postoperative mortality was 3%. The median MV duration was 4 h (IQR 3, 9), and the median ICU and hospital LOS were 2 (IQR 1, 3) and 7 days (IQR 5, 10), respectively.
Figure 1.
Schematic representation of the study population. ICU: Intensive care unit.
Table 1.
Basic demographics of the study population, n (%)
| Basic demographics | Overall | ICU hypotension |
| Total | 417 | 227 |
| Sex | ||
| Male | 305 (73) | 172 (76) |
| Female | 112 (27) | 55 (24) |
| Median age (IQR) | 67 (59, 73) | 67 (58, 74) |
| Race | ||
| Not disclosed | 9 (2) | 5 (2) |
| White | 356 (85) | 197 (87) |
| Other | 52 (13) | 25 (11) |
| Median BMI (IQR) | 28 (25, 32) | 29 (26, 33) |
| Mortality | ||
| Alive | 405 (97) | 217 (96) |
| Dead | 12 (3) | 10 (4) |
| Type of surgery | ||
| Aortic graft | 21 (5) | 11 (5) |
| CABG | 193 (46) | 113 (50) |
| Ventriculomyotomy | 26 (6) | 9 (4) |
| Valve | 122 (29) | 63 (28) |
| Aortic graft + CABG | 3 (0.7) | 1 (0.4) |
| Valve + CABG | 30 (7) | 23 (10) |
| ASD repair | 7 (2) | 4 (2) |
| Aortic graft + valve | 12 (3) | 2 (1) |
| ASD repair + valve | 3 (0.7) | 1 (0.4) |
| Need or surgery | ||
| Elective | 318 (76) | 175 (77) |
| Urgent/emergent | 99 (24) | 52 (23) |
| Comorbidities | ||
| CAD | 278 (67) | 164 (72) |
| Afib | 81 (19) | 42 (19) |
| AICD/PM | 21 (5) | 11 (5) |
| DM | 137 (33) | 89 (39) |
| PHTN | 21 (5) | 13 (6) |
| LD | 27 (7) | 18 (8) |
| KI | 88 (21) | 53 (23) |
| Active IE | 6 (1) | 2 (1) |
| IS | 16 (4) | 10 (4) |
ICU: Intensive care unit; IQR: Interquartile range; BMI: Body mass index; CABG: Coronary artery bypass graft; ASD: Atrial septum defect; CAD: Coronary artery disease; Afib: Atrial fibrillation; AICD/PM: Automatic implantable cardioverter defibrillator/pacemaker; DM: Diabetes mellitus; PHTN: Pulmonary hypertension; LD: Liver disease; KI: Kidney injury; IE: Infective endocarditis; IS: Immunosuppressed.
ICU hypotension
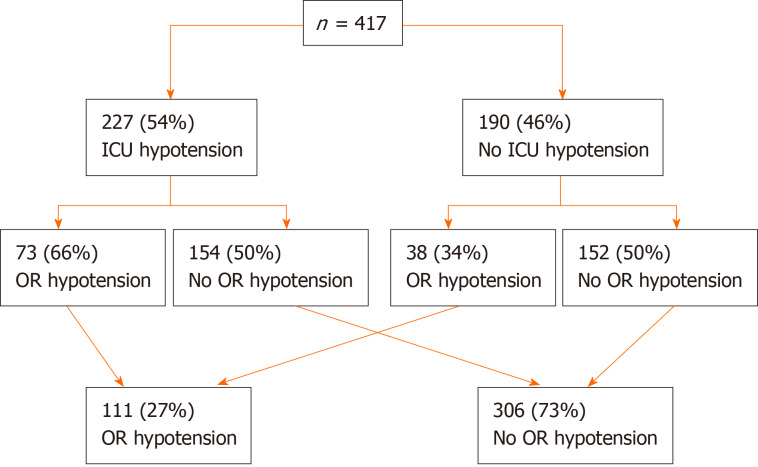
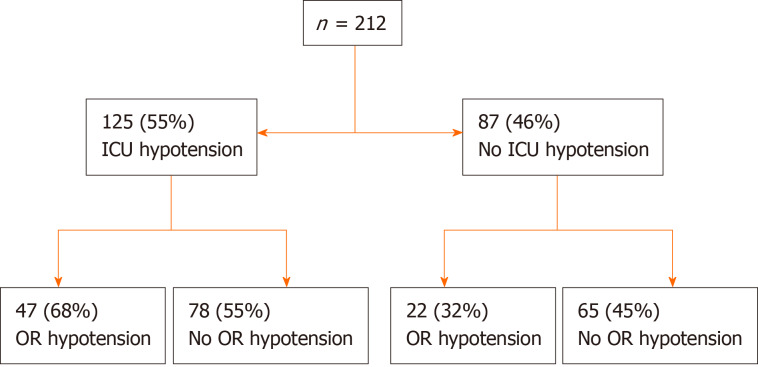
Total of 227 patients (54%) were found to be hypotensive within 30 min upon transfer to the ICU. Nearly three quarters of the whole cohort did not have OR hypotension immediately prior to transfer to the ICU (Figure 2). Presence of OR hypotension immediately prior to ICU transfer was expectedly associated with ICU hypotension [OR = 1.9; 95% confidence interval (CI): 1.21-2.98; P < 0.006]. About two-thirds of patients with preceding OR hypotension continued with ICU hypotension and half of those without preceding OR hypotension developed ICU hypotension upon ICU transfer. Higher BMI, history of DM and CAD were associated with significantly higher unadjusted risk of developing ICU hypotension (Table 2). ICU hypotension was associated with the longer duration of MV in hours: 5 (IQR 3, 15) vs 4 (IQR 3, 6), P = 0.012. Although statistically significant, the clinical significance appeared to be limited only to the patients in the upper quartile (Table 3). Based on the chart documentation, 212 patients received vasopressor boluses around (immediately prior or during) the transfer to the ICU (Figure 3). The patients who received vasopressor bolus on transfer were somewhat more likely to experience ICU drop-off hypotension (OR = 1.45, 95%CI: 0.98-2.13; P = 0.062), although this did not quite reach statistical significance. Of the 212 patients who received bolus, 125 (55%) experienced immediate ICU hypotension. Of these 125 patients with ICU hypotension, 78 did not have preceding OR hypotension and 47 did and continued with ICU hypotension from the OR (OR = 1.78; 95%CI: 0.97-3.26; P = 0.074). Of 12 patients who died, 9 received the bolus during the transfer and 3 did not (OR = 2.99; 95%CI: 0.8-11.2; P = 0.14). Receipt of vasopressor bolus during the transfer was significantly associated with longer MV duration, ICU and hospital LOS (P < 0.001, for all). All variables with α ≤ 0.1 in univariate analysis were included in multivariate analysis. When adjusted in the multivariate analysis, CAD, DM and longer BT were significantly associated with the development of ICU hypotension (Table 4).
Figure 2.
Intensive care unit and operating room hypotension frequency. ICU: Intensive care unit; OR: Operating room.
Table 2.
Association of baseline characteristics with intensive care unit hypotension and mortality
| Baseline characteristic | ICU hypotension, n = 227 | No ICU hypotension, n = 190 | P value | Alive, n = 405 | Dead, n = 12 | P value |
| Age, median (IQR) | 67 (58, 74) | 68 (59, 73) | 0.73 | 67 (59, 73) | 64 (59, 67) | 0.42 |
| Male sex, n (%) | 172 (76) | 133 (70) | 0.22 | 299 (74) | 6 (50) | 0.0935 |
| BMI, median (IQR) | 29 (26, 33) | 27 (25, 31) | 0.01 | 28 (25, 32) | 32 (27, 38) | 0.039 |
| CAD, n (%) | 164 (72.2) | 114 (60.0) | 0.009 | 272 (67) | 6 (50) | 0.23 |
| DM, n (%) | 89 (39.2) | 48 (25.3) | 0.003 | 133 (33) | 4 (33) | 1.0 |
| Afib, n (%) | 42 (19) | 39 (21) | 0.62 | 78 (19) | 3 (25) | 0.71 |
| AICD/PM, n (%) | 11 (5) | 10 (5) | 1.00 | 20 (5) | 1 (8) | 0.47 |
| PHTN, n (%) | 13 (6) | 8 (4) | 0.51 | 18 (12) | 3 (25) | 0.018 |
| IE, n (%) | 2 (1) | 4 (2) | 0.42 | 6 (1) | 0 (0) | 1.0 |
| LD, n (%) | 18 (8) | 9 (5) | 0.23 | 27 (7) | 0 (0) | 1.0 |
| KD, n (%) | 53 (23) | 35 (18) | 0.23 | 86 (21) | 2 (17) | 0.78 |
| IS, n (%) | 10 (4) | 6 (3) | 0.61 | 16 (4) | 0 (0) | 1.0 |
| Elective surgery, n (%) | 175 (77) | 143 (75) | 0.73 | 309 (76) | 9 (75) | 1.0 |
| ASA, n (%) | 0.42 | 0.02 | ||||
| 2 | 1 (0.5) | 1 (0.4) | 2 (0.5) | 0 (0) | ||
| 3 | 94 (49) | 94 (41) | 186 (46) | 2 (17) | ||
| 4 | 94 (49) | 130 (57) | 215 (53) | 9 (75) | ||
| 5 | 1 (0.5) | 2 (0.9) | 2 (0.5) | 1 (8) | ||
| EF%, median (IQR) | 60 (51, 64) | 62 (54, 66) | 0.29 | 60 (53, 65) | 62 (53, 67) | 0.62 |
| BT, median (IQR) | 117 (90, 150) | 114 (85, 148) | 0.10 | 115 (89, 148) | 152 (108, 240) | 0.0008 |
| CCT, median (IQR) | 81 (60, 105) | 77 (53, 108) | 0.10 | 80 (55, 105) | 111 (59, 160) | 0.018 |
| Transfusion, n (%) | 138 (61) | 124 (65) | 0.36 | 252 (62) | 10 (83) | 0.22 |
| Pressors, n (%) | 178 (78) | 138 (73) | 0.21 | 305 (75) | 11 (92) | 0.31 |
| Bolus given, n (%) | 125 (59.0) | 87 (41.0) | 0.06 | 203 (50) | 9 (75) | 0.14 |
| Hb, median (IQR) | 13 (12,14) | 13 (12, 14) | 0.79 | 13 (12, 14) | 13 (10, 14) | 0.48 |
| Hct, median (IQR) | 40 (35, 42) | 40 (36, 42) | 0.86 | 40 (36, 42) | 40 (33, 44) | 0.73 |
| PLT, median (IQR) | 203 (163, 248) | 198 (159, 233) | 0.18 | 201 (161, 243) | 186 (155, 236) | 0.69 |
| Cre, median (IQR) | 1.1 (0.9, 1.4) | 1 (0.9, 1.2) | 0.13 | 1 (0.9, 1.3) | 1.1 (0.9, 1.9) | 0.80 |
| Ca, median (IQR) | 9.3 (8.9, 9.6) | 9.3 (8.9, 9.6) | 0.78 | 9.3 (8.9, 9.6) | 9.2 (8.6, 9.4) | 0.49 |
| Pre-op SBP, median (IQR) | 125 (110, 139) | 126 (110, 139) | 0.69 | 126 (110, 139) | 114 (98, 144) | 0.18 |
| Pre-op MAP, median (IQR) | 84 (73, 94) | 83 (74, 96) | 0.89 | 84 (74, 95) | 80 (49, 91) | 0.066 |
ICU: Intensive care unit; IQR: Interquartile range; n: Number of patients; BMI: Body mass index; CAD: Coronary artery disease; DM: Diabetes mellitus; Afib: Arterial fibrillation; AICD/PM: Automatic implantable cardioverter defibrillator/pacemaker; PHTN: Pulmonary hypertension; IE: Infective endocarditis; LD: Liver disease; KD: Kidney disease; IS: Immunosuppressed; ASA: American Society of Anesthesiologists; EF: Ejection fraction; BT: Bypass time; CCT: Cross-clamp time; Hb: Hemoglobin; Hct: Hematocrit; PLT: Platelet; Cre: Creatinine; Ca: Calcium; pre-op: Pre-operation; SBP: Systolic blood pressure; MAP: Mean arterial pressure.
Table 3.
Unadjusted association of intensive care unit hypotension with clinical outcomes
| Item | ICU hypotension | No ICU hypotension | P value |
| Hosp. LOS, median (IQR) | 7 (5, 10) | 7 (5, 9) | 0.49 |
| ICU LOS, median (IQR) | 2 (1, 4) | 2 (1, 3) | 0.21 |
| MV hours, median (IQR) | 5 (3, 15) | 4 (3, 6) | <0.001 |
| Hosp. mortality, n (%) | 10 (4.4) | 2 (1.1) | 0.07 |
ICU: Intensive care unit; Hosp: Hospital; LOS: Length of stay; IQR: Interquartile range; MV: Mechanical ventilation.
Figure 3.
Number of patients receiving the vasopressor bolus on transfer. ICU: Intensive care unit; OR: Operating room.
Table 4.
Multivariate analysis for intensive care unit hypotension
| Item |
ICU hypotension |
|
| OR; 95%CI | P value | |
| BMI | 1.02; 0.99-1.07 | 0.13 |
| CAD | 1.69; 1.09-2.62 | 0.018 |
| DM | 1.66; 1.06-2.61 | 0.025 |
| BT | 1.004; 1.0002-1.008 | 0.034 |
| Bolus | 1.2; 0.79-1.82 | 0.38 |
Cross clamp time excluded because of linear correlation with bypass time. ICU: Intensive care unit; OR: Odds ratio; CI: Confidence interval; BMI: Body mass index; CAD: Coronary artery disease; DM: Diabetes mellitus; BT: Bypass time.
Mortality and secondary outcomes
Overall hospital mortality was not significantly associated with ICU hypotension (OR = 4.33; 95%CI: 0.94-20.02; P = 0.073); likely given relatively low overall mortality of 3% (Table 5). The female sex was significantly associated with longer ICU and hospital LOS, while longer BT and higher American Society of Anesthesiologists (ASA) physical status score were significantly associated with longer MV, ICU and hospital LOS. When adjusted for multiple covariates, no single variable was significantly associated with the mortality. In order to avoid overfitting of the model, variables such as CCT (collinear with BT) and pulmonary hypertension (low frequency), were excluded.
Table 5.
Multivariate analysis for mortality
| Item |
Mortality |
|
| OR; 95%CI | P value | |
| Sex | 0.74; 0.06-7.99 | 0.80 |
| BMI | 0.93; 0.76-1.14 | 0.51 |
| ICU hypotension | 0.27; 0.03-2.74 | 0.27 |
| Lowest MAP (pre-op) | 0.96; 0.89-1.02 | 0.19 |
| BT | 1.01; 0.43-23.8 | 0.33 |
| ASA | 3.19; 0.79-1.82 | 0.26 |
Cross clamp time excluded because of linear correlation with bypass time; Pulmonary hypertension (low frequency) excluded to prevent overfitting). OR: Odds ratio; CI: Confidence interval; BMI: Body mass index; ICU: Intensive care unit; MAP: Mean artery pressure; pre-op: Pre-operative; BT: Bypass time; ASA: American Society of Anesthesiologists.
DISCUSSION
In this retrospective study from a single academic center, we have demonstrated that hypotension in the initial 30 min upon ICU admission after cardiac surgery occurs more frequently than previously reported and this may be associated with adverse clinical outcomes. More than half of the patients received vasopressor boluses during the OR to ICU transfer, which has also been associated with adverse outcomes.
The results of our study have important implications for anesthesia and ICU practitioners. The frequency of hypotension in the first 30 min upon ICU arrival in our study was substantially higher (54%), relative to a European study which examined the hemodynamic status of cardiac surgical patients in the initial two-hour post-operative period (34%)[6]. It is likely that the frequency of hypotension could have been even higher in our study had we prolonged the observation period to two-hour period similar to the aforementioned study. Given that the patients with ICU hypotension may experience worse clinical outcomes, it is necessary to address potentially modifiable factors. In our cohort, significant unadjusted predictors for hypotension upon arrival to the ICU were elevated BMI, history of DM and CAD, all well-established risk factors for cardiovascular morbidity. After adjustments in the multivariate regression analysis, DM and longer cardiopulmonary bypass remained significantly associated with the development of ICU hypotension. Presence of DM has been previously associated with the higher cardiovascular morbidity, higher rates of pneumonia and sepsis, which may contribute to increased mortality, relative to non-diabetic patients[11-15]. It is important that both preoperative as well perioperative blood sugar control are maximized in order to reduce the hyperglycemia-related adverse outcomes[16-18]. Despite the fact that the significance of longer BT has been well documented to negatively affect post-operative rate of complications and mortality[19], our analysis (Table 5) does not show any significant difference between longer BT and mortality. It is plausible to expect that the future improvements in operative techniques and avoidance of cardiopulmonary bypass altogether would likely further reduce postoperative complications thus improving morbidity and mortality. During the time period of data collection, off-pump surgery was very infrequently done at our institution and this would not affect the results.
Previously, female sex was reported to be significantly associated with adverse postoperative outcomes[20]. Females with the acute coronary syndrome resulting in cardiogenic shock, those with acute aortic dissection, ruptured abdominal aortic aneurysms, or those undergoing non-cardiac surgery, have been shown to have higher mortality rates compared to men[21-25]. Also, females with cerebral complications after cardiac surgery have shown to have a higher mortality than males[24,26]. In our study, females experienced significantly longer unadjusted ICU and hospital lengths of stay. Although the female sex was previously associated with the use of higher tidal volumes (relative to the height measurement) and more ventilator induced lung injury[27], there was no observed difference in duration of MV relative to the males in our cohort. There is a strong impetus for extubation of patients within 6 h of the cardiac surgery[28]. When adjusted for pertinent clinical variables and compared to men, females in our cohort were not more likely to die during the hospital stay.
The ASA physical status score subjectively assesses the patients’ overall health prior to surgery. It has been shown that ASA score is associated with longer ICU and LOS, longer MV, and increased mortality[2,29,30]. In our study, ICU and LOS, as well as MV duration were significantly associated with ASA score, and there was a trend for higher hospital mortality with the rising ASA score, accordingly.
Based on the chart documentation, more than half of patients received boluses of short-acting vasopressors during the transfer from the OR to the ICU. The anesthesiology transport teams routinely carry syringes of resuscitative medications for any unanticipated needs that may occur during the transfer. It is possible that even more patients had received the bolus dosing without the subsequent chart documentation, although this is speculative. Why this may be important? Frequently, ICU receiving team may not be aware of use of vasopressor boluses during the transfer and the development of hypotension soon after the anesthesia drop-off is not anticipated, which leads to delayed and reactive treatment strategy that may be suboptimal. This is anecdotally termed “anesthesia drop-off syndrome” in the ICU, where soon after the transfer from the OR, the patients tend to develop hypotension that was not present at the arrival of the patient into the ICU and during the actual transfer of care from anesthesia to ICU team. As it has been previously suggested that hypotension is associated with adverse outcomes[11,13,31], it is important that any use of vasopressor bolus on transfer is readily communicated to the receiving ICU team, to enable anticipatory rather than reactive management of hypotension. For the same reasons, it may be more appropriate to up titrate the dose of ongoing vasopressor drip rather than to push additional IV bolus, as such bolus dosing may not be obvious to the receiving team. This is currently subject of qualitative improvement and patient safety initiatives spanning both anesthesiology and ICU providers at our institution, as the current process of care needs to be improved.
We have abstracted a vast amount of clinical information on all patients, including vital signs, complete blood counts, pertinent hemodynamic variables such as preoperative and intraoperative echocardiography (systolic and diastolic function, valvular function), transfusion of blood products and cell saver, administration of crystalloid and colloid solutions, CCT, estimated blood loss and development of other OR complications, among others. It is interesting that none of these variables were significant adjusted risk predictors by itself for developing hypotension upon ICU arrival. This implies that the perioperative management of cardiac surgery patients is complex and of a very dynamic nature where the multitude of factors play pertinent roles.
The main study limitation lies in its retrospective design. We relied on abstraction of data from the electronic medical records and at best our data is as good as the chart documentation itself. Relative to this, there might have been time delays between the exact occurrence of the event and the time it was documented in the chart. While this delay may have not been substantial during the intraoperative period, the retrospective charting of the medications administered during the actual patient transfer to the ICU may have been affected, including possibility for the lack of documentation, altogether. This may possibly in part explain why the substantial number of patients, who were not recorded to be hypotensive in the OR immediately prior to the transfer to the ICU, were documented to have received boluses of short-acting vasopressor medications during the transfer.
The study was done at the single academic medical center and since we excluded the patients who underwent transplantation surgery, these factors limit the generalizability of our findings to the certain extent. There was a relatively low mortality and therefore small number of patients who died predisposed multivariate model to overfitting and may not be completely reliable. At our institution, there are no established or preferred teams of certain surgeons and anesthesiologist. All surgeons work with all anesthesiologists depending only on the scheduling. Therefore, it is unlikely that individual surgeons or anesthesiologists could affect the results.
Nevertheless, despite the above limitations, the high proportion of patients who were hypotensive immediately upon transfer from the OR to the ICU dictates the need for novel strategies and protocol implementations to assure the safest transition of care between the anesthesiology and ICU teams, which in turn may improve overall patient outcomes.
In summary, we have demonstrated that the occurrence of hypotension in the initial 30 min upon OR to ICU transfer is frequent and substantially more so than previously reported. Our findings have important implications for the anesthesia and ICU care teams as the occurrence of hypotension have been associated with adverse clinical outcomes. Administration of any medications during the actual transfer of the patient from the OR to the ICU should be readily communicated to the receiving ICU team. It is suggested that there is a room for improvement in the OR to ICU hand off process and renewed strategies that assure smooth transition of care and patient’s safety are needed.
ARTICLE HIGHLIGHTS
Research background
Perioperative hypotension is one of the most common complications after cardiac surgery and this may adversely affect clinical outcomes. However, data is limited in the literature regarding the actual prevalence of hypotension that develops shortly after the transfer of patients to the intensive care unit (ICU) after cardiac surgery. Hypotensive patients usually require administration of vasopressor boluses prior to or during the transfer from the operating room (OR) to the ICU as a temporizing measure. The hypotension and necessity for use of vasopressors have been previously associated with increased hospital length of stay as well as mortality, relative to the patients who maintained hemodynamic stability.
Research motivation
Given the proposed discrepancy between the clinical occurrence and limited data on rate of hypotension starting shortly after the anesthesia to ICU transfer, we aimed to evaluate its prevalence and also how this may relate to the pertinent clinical outcomes.
Research objectives
We hypothesized that the occurrence of initial hypotension in the ICU is more frequent complication among post-cardiac surgery ICU patients than previously reported and that patients who experience this complication would have adverse clinical outcomes. We also aimed to better assess the association between the occurrence of initial hypotension in the ICU and the use of vasopressor bolus administered immediately prior to or during the transfer from the OR to the ICU.
Research methods
We conducted a retrospective study of adult patients undergoing cardiac surgery in a 2-year period. The primary independent variable was the development of hypotension within the first 30 min upon transfer from the OR (“ICU hypotension”). We abstracted demographic and baseline characteristics, comorbidities, and all pertinent clinical variables, as well as presence of hypotension in the OR (“OR hypotension”). A vasopressor bolus use was abstracted from the electronic chart documentation by the provider. All data were manually extracted from an electronic medical record. The anesthesia notes during the surgery were extracted partially from plotted diagrams and partially from nominal data.
Research results
We have demonstrated that hypotension in the initial 30 min upon ICU admission after adult cardiac surgery occurs more frequently than previously reported and this may be associated with adverse clinical outcomes. The results of our study have important implications for anesthesia and ICU practitioners. Given that the patients with ICU hypotension may experience worse clinical outcomes, it is necessary to address potentially modifiable factors. More than half of patients received boluses of short-acting vasopressors during the transfer from the OR to the ICU. Why this may be important? Frequently, ICU receiving team may not be aware of use of vasopressor boluses during the transfer and the development of hypotension soon after the anesthesia drop-off is not anticipated, which leads to delayed and reactive treatment strategy that may be suboptimal. This is currently subject of qualitative improvement and patient safety initiatives spanning both anesthesiology and ICU providers at our institution, as the current process of care needs to be improved. The main study limitation lies in its retrospective design. We relied on abstraction of data from the electronic medical records. The study was done at the single academic medical center and since we excluded the patients who underwent transplantation surgery, these factors limit the generalizability of our findings to the certain extent. Nevertheless, despite the above limitations, the high proportion of patients who were hypotensive immediately upon transfer from the OR to the ICU dictates the need for novel strategies and protocol implementations to assure the safest transition of care between the anesthesiology and ICU teams, which in turn may improve overall patient outcomes.
Research conclusions
We have demonstrated that the occurrence of hypotension in the initial 30 min upon OR to ICU transfer is frequent and substantially more so than previously reported. Our findings have important implications for the anesthesia and ICU care teams as the occurrence of hypotension have been associated with adverse clinical outcomes. Administration of any medications during the actual transfer of the patient from the OR to the ICU should be readily communicated to the receiving ICU team.
Research perspectives
It is suggested that there is a room for improvement in the OR to ICU hand off process and renewed strategies that assure smooth transition of care and patient’s safety are needed.
Footnotes
Institutional review board statement: This study was reviewed and approved by the Mayo Clinic Institution Review Board.
Informed consent statement: Patients were not required to give informed consent to the study because the analysis used anonymous data that were obtained after each patient agreed to treatment by written consent.
Conflict-of-interest statement: The Authors declare that there is no conflict of interest.
Manuscript source: Unsolicited manuscript
Peer-review started: December 26, 2019
First decision: April 9, 2020
Article in press: May 14, 2020
Specialty type: Critical care medicine
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Liu Y S-Editor: Tang JZ L-Editor: A E-Editor: Xing YX
Contributor Information
Sabina Cengic, Department of Critical Care Medicine, Mayo Clinic, Jacksonville, FL 32224, United States; Department of General Surgery, Stadtspital Triemli, Zurich 8063, Switzerland.
Muhammad Zuberi, Department of Critical Care Medicine, Mayo Clinic, Jacksonville, FL 32224, United States.
Vikas Bansal, Department of Critical Care Medicine, Mayo Clinic, Jacksonville, FL 32224, United States.
Robert Ratzlaff, Department of Critical Care Medicine, Mayo Clinic, Jacksonville, FL 32224, United States; Department of Anesthesiology and Perioperative Medicine, Mayo Clinic, Jacksonville, FL 32224, United States.
Eduardo Rodrigues, Department of Anesthesiology and Perioperative Medicine, Mayo Clinic, Jacksonville, FL 32224, United States.
Emir Festic, Department of Critical Care Medicine, Mayo Clinic, Jacksonville, FL 32224, United States. festic.emir@mayo.edu.
Data sharing statement
No additional data are available.
References
- 1.Barbour CM, Little DM., Jr Postoperative hypotension. J Am Med Assoc. 1957;165:1529–1532. doi: 10.1001/jama.1957.02980300009003. [DOI] [PubMed] [Google Scholar]
- 2.Brovman EY, Gabriel RA, Lekowski RW, Dutton RP, Urman RD. Rate of Major Anesthetic-Related Outcomes in the Intraoperative and Immediate Postoperative Period After Cardiac Surgery. J Cardiothorac Vasc Anesth. 2016;30:338–344. doi: 10.1053/j.jvca.2015.08.006. [DOI] [PubMed] [Google Scholar]
- 3.Hori D, Ono M, Rappold TE, Conte JV, Shah AS, Cameron DE, Adachi H, Everett AD, Hogue CW. Hypotension After Cardiac Operations Based on Autoregulation Monitoring Leads to Brain Cellular Injury. Ann Thorac Surg. 2015;100:487–493. doi: 10.1016/j.athoracsur.2015.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roock SD, Mesana TG, and Sun L. “Abstract 13021: Impact of Preoperative Risk on the Association Between Hypotension and Mortality After Cardiac Surgery”. Circulation. 2019;140:A13021–A13021. [Google Scholar]
- 5.Sun LY, Chung AM, Farkouh ME, van Diepen S, Weinberger J, Bourke M, Ruel M. Defining an Intraoperative Hypotension Threshold in Association with Stroke in Cardiac Surgery. Anesthesiology. 2018;129:440–447. doi: 10.1097/ALN.0000000000002298. [DOI] [PubMed] [Google Scholar]
- 6.Currey J, Botti M. The haemodynamic status of cardiac surgical patients in the initial 2-h recovery period. Eur J Cardiovasc Nurs. 2005;4:207–214. doi: 10.1016/j.ejcnurse.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 7.Magruder JT, Dungan SP, Grimm JC, Harness HL, Wierschke C, Castillejo S, Barodka V, Katz N, Shah AS, Whitman GJ. Nadir Oxygen Delivery on Bypass and Hypotension Increase Acute Kidney Injury Risk After Cardiac Operations. Ann Thorac Surg. 2015;100:1697–1703. doi: 10.1016/j.athoracsur.2015.05.059. [DOI] [PubMed] [Google Scholar]
- 8.Rady MY, Ryan T, Starr NJ. Perioperative determinants of morbidity and mortality in elderly patients undergoing cardiac surgery. Crit Care Med. 1998;26:225–235. doi: 10.1097/00003246-199802000-00016. [DOI] [PubMed] [Google Scholar]
- 9.Weis F, Kilger E, Beiras-Fernandez A, Nassau K, Reuter D, Goetz A, Lamm P, Reindl L, Briegel J. Association between vasopressor dependence and early outcome in patients after cardiac surgery. Anaesthesia. 2006;61:938–942. doi: 10.1111/j.1365-2044.2006.04779.x. [DOI] [PubMed] [Google Scholar]
- 10.Bijker JB, van Klei WA, Kappen TH, van Wolfswinkel L, Moons KG, Kalkman CJ. Incidence of intraoperative hypotension as a function of the chosen definition: literature definitions applied to a retrospective cohort using automated data collection. Anesthesiology. 2007;107:213–220. doi: 10.1097/01.anes.0000270724.40897.8e. [DOI] [PubMed] [Google Scholar]
- 11.Bannier K, Lichtenauer M, Franz M, Fritzenwanger M, Kabisch B, Figulla HR, Pfeifer R, Jung C. Impact of diabetes mellitus and its complications: survival and quality-of-life in critically ill patients. J Diabetes Complications. 2015;29:1130–1135. doi: 10.1016/j.jdiacomp.2015.08.010. [DOI] [PubMed] [Google Scholar]
- 12.Gadboys HL, Wisoff G, Litwak RS. Surgical treatment of complete heart block. An analysis of 36 cases. JAMA. 1964;189:97–102. doi: 10.1001/jama.1964.03070020025005. [DOI] [PubMed] [Google Scholar]
- 13.Kannel WB, McGee DL. Diabetes and glucose tolerance as risk factors for cardiovascular disease: the Framingham study. Diabetes Care. 1979;2:120–126. doi: 10.2337/diacare.2.2.120. [DOI] [PubMed] [Google Scholar]
- 14.Ouattara A, Lecomte P, Le Manach Y, Landi M, Jacqueminet S, Platonov I, Bonnet N, Riou B, Coriat P. Poor intraoperative blood glucose control is associated with a worsened hospital outcome after cardiac surgery in diabetic patients. Anesthesiology. 2005;103:687–694. doi: 10.1097/00000542-200510000-00006. [DOI] [PubMed] [Google Scholar]
- 15.Haines D, Miranda HG, Flynn BC. The Role of Hemoglobin A1c as a Biomarker and Risk Assessment Tool in Patients Undergoing Non-cardiac and Cardiac Surgical Procedures. J Cardiothorac Vasc Anesth. 2018;32:488–494. doi: 10.1053/j.jvca.2017.05.047. [DOI] [PubMed] [Google Scholar]
- 16.Doenst T, Wijeysundera D, Karkouti K, Zechner C, Maganti M, Rao V, Borger MA. Hyperglycemia during cardiopulmonary bypass is an independent risk factor for mortality in patients undergoing cardiac surgery. J Thorac Cardiovasc Surg. 2005;130:1144. doi: 10.1016/j.jtcvs.2005.05.049. [DOI] [PubMed] [Google Scholar]
- 17.Kotagal M, Symons RG, Hirsch IB, Umpierrez GE, Dellinger EP, Farrokhi ET, Flum DR SCOAP-CERTAIN Collaborative. Perioperative hyperglycemia and risk of adverse events among patients with and without diabetes. Ann Surg. 2015;261:97–103. doi: 10.1097/SLA.0000000000000688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Robich MP, Iribarne A, Leavitt BJ, Malenka DJ, Quinn RD, Olmstead EM, Ross CS, Sawyer DB, Klemperer JD, Clough RA, Kramer RS, Baribeau YR, Sardella GL, DiScipio AW Northern New England Cardiovascular Disease Study Group. Intensity of Glycemic Control Affects Long-Term Survival After Coronary Artery Bypass Graft Surgery. Ann Thorac Surg. 2019;107:477–484. doi: 10.1016/j.athoracsur.2018.07.078. [DOI] [PubMed] [Google Scholar]
- 19.Shultz B, Timek T, Davis AT, Heiser J, Murphy E, Willekes C, Hooker R. Outcomes in patients undergoing complex cardiac repairs with cross clamp times over 300 minutes. J Cardiothorac Surg. 2016;11:105. doi: 10.1186/s13019-016-0501-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hassan A, Chiasson M, Buth K, Hirsch G. Women have worse long-term outcomes after coronary artery bypass grafting than men. Can J Cardiol. 2005;21:757–762. [PubMed] [Google Scholar]
- 21.Alonso-Pérez M, Segura RJ, Sánchez J, Sicard G, Barreiro A, García M, Díaz P, Barral X, Cairols MA, Hernández E, Moreira A, Bonamigo TP, Llagostera S, Matas M, Allegue N, Krämer AH, Mertens R, Coruña A. Factors increasing the mortality rate for patients with ruptured abdominal aortic aneurysms. Ann Vasc Surg. 2001;15:601–607. doi: 10.1007/s100160010115. [DOI] [PubMed] [Google Scholar]
- 22.Harris LM, Faggioli GL, Fiedler R, Curl GR, Ricotta JJ. Ruptured abdominal aortic aneurysms: factors affecting mortality rates. J Vasc Surg. 1991;14:812–818; discussion 819-820. doi: 10.1067/mva.1991.33494. [DOI] [PubMed] [Google Scholar]
- 23.Kamiński KA, Tycińska AM, Stepek T, Szpakowicz A, Olędzka E, Dobrzycki S, Musiał WJ. Natural history and risk factors of long-term mortality in acute coronary syndrome patients with cardiogenic shock. Adv Med Sci. 2014;59:156–160. doi: 10.1016/j.advms.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 24.Nienaber CA, Fattori R, Mehta RH, Richartz BM, Evangelista A, Petzsch M, Cooper JV, Januzzi JL, Ince H, Sechtem U, Bossone E, Fang J, Smith DE, Isselbacher EM, Pape LA, Eagle KA International Registry of Acute Aortic Dissection. Gender-related differences in acute aortic dissection. Circulation. 2004;109:3014–3021. doi: 10.1161/01.CIR.0000130644.78677.2C. [DOI] [PubMed] [Google Scholar]
- 25.Xu L, Yu C, Jiang J, Zheng H, Yao S, Pei L, Sun L, Xue F, Huang Y. Major adverse cardiac events in elderly patients with coronary artery disease undergoing noncardiac surgery: A multicenter prospective study in China. Arch Gerontol Geriatr. 2015;61:503–509. doi: 10.1016/j.archger.2015.07.006. [DOI] [PubMed] [Google Scholar]
- 26.Ridderstolpe L, Ahlgren E, Gill H, Rutberg H. Risk factor analysis of early and delayed cerebral complications after cardiac surgery. J Cardiothorac Vasc Anesth. 2002;16:278–285. doi: 10.1053/jcan.2002.124133. [DOI] [PubMed] [Google Scholar]
- 27.Gajic O, Dara SI, Mendez JL, Adesanya AO, Festic E, Caples SM, Rana R, St Sauver JL, Lymp JF, Afessa B, Hubmayr RD. Ventilator-associated lung injury in patients without acute lung injury at the onset of mechanical ventilation. Crit Care Med. 2004;32:1817–1824. doi: 10.1097/01.ccm.0000133019.52531.30. [DOI] [PubMed] [Google Scholar]
- 28.Kotfis K, Szylińska A, Listewnik M, Lechowicz K, Kosiorowska M, Drożdżal S, Brykczyński M, Rotter I, Żukowski M. Balancing intubation time with postoperative risk in cardiac surgery patients - a retrospective cohort analysis. Ther Clin Risk Manag. 2018;14:2203–2212. doi: 10.2147/TCRM.S182333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lupei MI, Chipman JG, Beilman GJ, Oancea SC, Konia MR. The association between ASA status and other risk stratification models on postoperative intensive care unit outcomes. Anesth Analg. 2014;118:989–994. doi: 10.1213/ANE.0000000000000187. [DOI] [PubMed] [Google Scholar]
- 30.Collins TC, Daley J, Henderson WH, Khuri SF. Risk factors for prolonged length of stay after major elective surgery. Ann Surg. 1999;230:251–259. doi: 10.1097/00000658-199908000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brown DV, O'Connor CJ. Hypotension after coronary artery bypass surgery. J Cardiothorac Vasc Anesth. 2000;14:97–99. doi: 10.1016/s1053-0770(00)90067-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No additional data are available.