Abstract
Objective
The purpose of this study was to evaluate changes in the synovial fluid proteome following acute anterior cruciate ligament (ACL) injury.
Design
This study represents a secondary analysis of synovial fluid samples collected from the placebo group of a previous randomized trial. Arthrocentesis was performed twice on 6 patients with an isolated acute ACL tear at a mean of 6 and 14 days postinjury. Synovial fluid was analyzed by a highly multiplexed assay of 1129 proteins (SOMAscan version 3, SomaLogic, Inc., Boulder, CO). Pathway analysis using DAVID was performed; genes included met 3 criteria: significant change between the 2 study time points using a paired t test, significant change between the 2 study time points using a Mann-Whitney nonparametric test, and significant Benjamini post hoc analysis.
Results
Fifteen analytes demonstrated significant increases between time points. Five of the 15 have been previously associated with the onset and/or severity of rheumatoid arthritis, including apoliopoprotein E and isoform E3, vascular cell adhesion protein 1, interleukin-34, and cell surface glycoprotein CD200 receptor 1. Chondrodegenerative enzymes and products of cartilage degeneration all increased over time following injury: MMP-1 (P = 0.08, standardized response mean [SRM] = 1.00), MMP-3 (P = 0.05, SRM = 0.90), ADAM12 (P = 0.03, SRM = 1.31), aggrecan (P = 0.08, SRM = 1.13), and CTX-II (P = 0.07, SRM = 0.56). Notable pathways that were differentially expressed following injury were the cytokine-cytokine receptor interaction and osteoclast differentiation pathways.
Conclusions
The proteomic results and pathway analysis demonstrated a pattern of cartilage degeneration, not only consistent with previous findings but also changes consistent with an inflammatory arthritogenic process post-ACL injury.
Keywords: anterior cruciate ligament, osteoarthritis, biomarker, proteome, pathway analysis
Introduction
Posttraumatic osteoarthritis (OA) is considered to be the consequence of an initial mechanical disruption or injury. The initial acute damage to the joint includes mechanical damage to the tissue itself with elevated expression of catabolic enzymes1 and inflammatory cytokines and ultimately death of chondrocytes.2-5 The hemarthrosis following anterior cruciate ligament (ACL) injury6 can further affect cartilage stability and viability. Recently, there has been a renewed focus on the inflammatory milieu and its effects on the ability to repair or restore cartilage after acute traumatic injury. Increased markers of type II collagen turnover and inflammatory cytokines have been detected in the acute phase after injury.7-13
Historically, the principal evidence for the onset of posttraumatic OA has been radiographic joint space narrowing. However, joint space narrowing often takes years to manifest.14 To better understand the etiology and evolution of posttraumatic OA, a new emphasis has been placed on identifying biomarkers that could be used as objective indicators of the key pathogenic processes or responses to therapeutic interventions.15 Lattermann et al.13 recently evaluated the potential to alter chondral breakdown following acute ACL injury through the use of a corticosteroid injection within the first week after injury. Monitoring of the progression of soluble analytes associated with chondral breakdown demonstrated early potentially irreversible cartilage changes after injury (as demonstrated by loss of type II collagen fragments from cartilage within the first 2 weeks after injury). However, as observed for established OA,16 Lattermann et al.13 also observed variability in responses to anti-inflammatory treatment after ACL injury. This experience underscores the need to continue to explore potential therapeutic agents to lessen chondrodegeneration following ACL injury and the need to define patient phenotypes that may or may not respond to specific interventions.15 With this in mind, our long-term goal is to identify potential ACL patient phenotypes that either do or do not respond to early anti-inflammatory treatment. However, to both determine if different patient phenotypes exist and to potentially identify additional therapeutic targets, the proteomic response to acute ACL injury must first be quantified. Proteomic analyses of synovial fluid from patients with idiopathic knee OA have been performed17; however, to our knowledge, the proteomic response after acute ACL injury has not been performed. As such, the purpose of this study was to explore synovial fluid proteome changes to potentially provide insights into mediators of the cartilage degradative process following acute joint injury. We hypothesized that analytes previously associated with idiopathic OA would be differentially expressed following acute ACL injury.
Methods
Study Design
This study represents a secondary analysis of patients with acute ACL tears who participated in an institutional review board (IRB)–approved randomized clinical trial (clinicaltrials.gov: NCT01692756) to assess the efficacy of early anti-inflammatory treatment following ACL injury.13 The procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000.
While this IRB-approved randomized controlled trial did evaluate the effect of intra-articular triamcinolone acetonide, none of the patients from the treatment group were analyzed for this study. Samples for the current proteomic analysis were selected from among the placebo-treated group who underwent direct joint aspirations and subsequent small volume (10 mL) intra-articular saline injections. Arthrocentesis to dryness was performed on the day of initial presentation (mean = 6 days postinjury, range = 2-8 days) and at a second time point between 6 and 12 days after the initial visit (mean = 14 days postinjury, range = 8-20 days). After arthrocentesis, patients administered 10 mL of normal saline (placebo) at both of the 2 study visits were included in this analysis.
Patient Characteristics
This study involved a subset of 6 patients (3 females, 3 males; mean age 17.5 years, range 16.4-20.4 years; mean body mass index 22.8 kg/m2, range 20-24.4 kg/m2) with acute ACL tears. Study inclusion criteria included the following: isolated ACL injury as determined via clinical exam (positive Lachman test with intra-articular effusion) and validated using magnetic resonance imaging (MRI) examination, age 14 years or older with closed growth plates as visualized on plain knee radiographs, no history of previous traumatic ipsilateral knee injury, occurrence of the ACL injury during sports activity, no clinical evidence of posterior cruciate ligament injury, and no more than grade 1 medial or lateral collateral ligament injury. Exclusion criteria included: onset of injury more than 8 days prior to enrollment, previous ipsilateral knee surgery, intra-articular cortisone injection into either knee within 3 months of injury, or a history of any inflammatory disease.
Preoperative MRI of the knee was used to confirm the absence of concomitant injury as suggested by the clinical exam, and an ACL injury was confirmed intraoperatively at the time of ACL reconstruction. Patients were instructed to avoid any prescription or over-the-counter nonsteroidal anti-inflammatory medications but were encouraged to rest, ice, and elevate the involved knee to control inflammation. All patients had similar self-directed, home-based preoperative rehabilitation, which included quadriceps initiation exercises and passive range of motion.
Proteomic Analyses
Proteomic analysis of the synovial fluid biospecimens was performed using a high throughput and highly multiplexed assay (SOMAscan version 3, SomaLogic, Inc., Boulder, CO).18 SOMAscan uses aptamers, chemically modified oligonucleotides that recognize the 3 dimensional structure of proteins with high specificity and high sensitivity. Each aptamer, recognizing a specific protein, was tagged with a short DNA sequence enabling quantification using a custom hybridization array. The assay transformed the measurement of proteins into the measurement of the corresponding DNA via hybridization to an antisense probe array using a hybridization gasket slide with 8 microarrays per slide. Data were recorded for each protein as relative fluorescent units (RFU). The median coefficient of variation for the SOMAscan Assay is 5% (somalogic.com/somascan-assay-faqs).
We also assessed changes in specific analytes, measured by the SOMAscan Assay, that have been previously linked to OA: aggrecan, disintegrin and metalloproteinase domain-containing protein 12 (ADAM12), metalloproteinase inhibitor-1 (TIMP-1), and matrix metalloproteinases 1, 3, and 9 (MMP-1, MMP-3, and MMP-9, respectively).19-23 With the exception of TIMP-1 for which decreasing values have been associated with OA changes, increasing values of these proteins would be indicative of greater cartilage breakdown. Lower values of TIMP-1 would also be consistent with a chondrodegenerative process as it has been identified as the primary inhibitor of MMP-1 and -3.
Statistical Analyses
The chondrodegenerative process following acute ACL injury was quantified by comparing biomarker values at the 2 study time points using paired t tests. However, because of the small sample size, we also calculated standardized response means (SRMs) to determine if potentially meaningful trends could be identified. SRM is the pre- to postoperative change divided by the standard deviation of the change scores for that particular variable, with values >0.8 considered large.24-26
Pathway analysis was performed using the Database for Annotation, Visualization, and Integrated Discovery (DAVID Bioinformatics Resources 6.8, National Institute of Allergy and Infectious Diseases, NIH). Genes included in the pathway analyses were those that met all 3 of the following criteria: significant change between the 2 study time points using a paired t test, significant change between the 2 study time points using a Mann-Whitney nonparametric test, and significant Benjamini post hoc analysis. The Gene Functional Analysis Tool was used to identify pathways that were differentially expressed, with Benjamini post hoc analyses performed to account for multiple comparisons.27,28
Results
Analytes Demonstrating Greatest Increases
From among the 1,129 analytes measured (Supplemental Table S1, available in the online version of the article), we identified the 30 proteins that demonstrated the largest change during the first 2 weeks after ACL injury ( Table 1 ). While likely due to the small sample size of this pilot work, large variation in analyte concentrations were observed during the first 2 weeks following ACL injury (SRMs ranged from 1.22 to 4.22); 15 proteins from among these 30 were statistically significantly changed during the first 2 weeks after ACL injury (based on P < 0.05). Interestingly, 5 of the 15 analytes (33%) that significantly increased after ACL injury have been previously associated with the onset and/or severity of rheumatoid arthritis (RA), including apoliopoprotein E and isoform E3, vascular cell adhesion protein 1, interleukin-34, and cell surface glycoprotein CD200 receptor 1 ( Table 1 ). In contrast, only 1 of the 15 significant analytes (ADAM12) has been previously associated with idiopathic OA ( Table 1 ).29
Table 1.
Thirty Proteins That Demonstrated the Largest SRMs between the 2 Study Time Points Following Acute Anterior Cruciate Ligament Injury.a
| Protein | Time 1 | Time 2 | P | SRM | OA- or RA-Related? |
|---|---|---|---|---|---|
| Apoliopoprotein E (isoform E3) | 3157.6 ± 1761.5 | 7666.4 ± 2715.3 | 0.008 | 4.22 | RA |
| Vascular cell adhesion protein 1 | 2497.3 ± 292.2 | 3090.1 ± 253.4 | 0.004 | 3.21 | RA |
| α-l-iduronidase | 1362.1 ± 251.7 | 2052.0 ± 379.8 | 0.005 | 1.89 | |
| Cell adhesion molecule–related/downregulated by oncogenes | 11526.1 ± 5924.8 | 16915.3 ± 5009.8 | 0.121 | 1.87 | |
| Extracellular matrix protein 1 | 5144.7 ± 2491.7 | 8492.1 ± 2147.3 | 0.032 | 1.85 | |
| Layilin | 1166.9 ± 143.0 | 1491.1 ± 307.5 | 0.051 | 1.79 | OA |
| Apoliopoprotein E | 351.2 ± 124.3 | 601.2 ± 229.3 | 0.048 | 1.70 | RA |
| 1-phosphatidylinositol 4,5-bisphosphate phosphodiesterase γ-1 | 359.4 ± 97.4 | 560.6 ± 162.1 | 0.031 | 1.66 | |
| Interleukin-17 receptor A | 741.3 ± 328.9 | 955.3 ± 216.5 | 0.217 | 1.59 | RA |
| Tumor necrosis factor receptor superfamily member 25 | 2105.4 ± 1136.5 | 2405.6 ± 1164.0 | 0.661 | 1.58 | RA |
| Desmoglein-2 | 9852.9 ± 2731.3 | 11791.2 ± 2636.1 | 0.240 | 1.53 | |
| Bone morphogenetic protein 1 | 713.7 ± 340.0 | 960.0 ± 300.2 | 0.213 | 1.51 | OA |
| Plexin-C1 | 460.6 ± 58.9 | 717.1 ± 175.1 | 0.014 | 1.51 | |
| Interleukin-6 receptor subunit alpha | 4345.4 ± 1136.3 | 5501.6 ± 1382.7 | 0.146 | 1.47 | |
| Tyrosine-protein kinase receptor Tie-1, soluble | 1902.8 ± 964.6 | 3006.8 ± 1153.5 | 0.103 | 1.45 | |
| Interleukin-17F | 128.6 ± 20.8 | 161.7 ± 28.1 | 0.045 | 1.42 | |
| Nidogen-2 | 1792.6 ± 1037.2 | 2607.6 ± 1503.4 | 0.303 | 1.33 | OA |
| Fibronectin | 24540.4 ± 10592.9 | 31566.9 ± 6142.6 | 0.197 | 1.32 | |
| Growth hormone receptor | 390.2 ± 81.1 | 459.2 ± 100.1 | 0.221 | 1.30 | |
| Tumor necrosis factor receptor superfamily member 3 | 1617.3 ± 355.3 | 2006.3 ± 386.3 | 0.100 | 1.29 | |
| Carbohydrate sulfotransferase 15 | 4679.3 ± 7382.8 | 8429.7 ± 3698.6 | 0.141 | 1.29 | |
| Interleukin-34 | 325.7 ± 60.1 | 454.4 ± 105.8 | 0.032 | 1.28 | RA |
| ADAM12 | 1242.4 ± 717.7 | 3198.5 ± 1442.4 | 0.020 | 1.27 | OA |
| Fibroblast growth factor receptor 1 | 7383.7 ± 1938.5 | 12320.0 ± 3494.8 | 0.017 | 1.27 | |
| Scavenger receptor cysteine-rich type 1 protein M130 | 6550.4 ± 3777.5 | 10863.1 ± 2591.3 | 0.047 | 1.25 | |
| Cell surface glycoprotein CD200 receptor 1 | 554.5 ± 216.0 | 921.6 ± 244.4 | 0.021 | 1.25 | RA |
| Inducible T-cell costimulator | 2028.6 1460.3 | 3251.2 ± 1874.78 | 0.238 | 1.24 | |
| Neurexophilin-1 | 856.4 ± 87.2 | 1050.9 ± 154.0 | 0.028 | 1.23 | |
| Fibroblast growth factor 10 | 133.9 ± 51.5 | 177.0 ± 60.1 | 0.213 | 1.23 | |
| Roundabout homolog 2 | 2152.1 ± 483.6 | 3428.2 ± 945.7 | 0.020 | 1.22 |
OA = osteoarthritis; RA = rheumatoid arthritis; SRM = standardized response mean.
All values are expressed in relative florescent units (RFU; mean ± standard deviation).
Pathway Analysis
Of the full list of 1,129 protein analytes that were assessed, 83 met the criteria for inclusion in the pathway analysis. The pathway analysis identified the following 8 pathways that were differentially expressed: cytokine-cytokine receptor interaction pathway (P = 0.005, 7 analytes identified in this pathway), phagosome pathway (P = 0.004; 6 analytes identified), hematopoietic cell lineage pathway (P = 0.003; 5 analytes identified), extracellular matrix receptor interaction pathway (P = 0.003; 5 analytes identified), osteoclast differentiation pathway (P = 0.01; 5 analytes identified), natural killer cell–mediated toxicity pathway (P = 0.01; 5 analytes identified), hypoxia-inducible factor 1 (HIF-1) signaling pathway (P = 0.03; 4 analytes identified), and T cell receptor signaling pathway (P = 0.03; 4 analytes identified). Specific analytes associated with each pathway are presented in Table 2 .
Table 2.
Analytes Identified for Each of the Significant Pathways.
| Pathway | Analytes |
|---|---|
| Cytokine-cytokine receptor interaction | TNF (tumor necrosis factor) receptor superfamily members 3 (TNFRSF3)a
TNF receptor superfamily members 4 (TNFRSF4) TNF receptor superfamily members 9 (TNFRSF9) TNF receptor superfamily members 25 (TNFRSF25) Interleukin 17 receptor A (IL17RA) Interleukin 6 receptor (IL6R) Colony-stimulating factor 1 receptor (CSF1R) |
| Phagosome | Fc fragment of IgA receptor (FCAR) Fc fragment of IgG receptor IIIb (FCGR3B) CD36 molecule (CD36) Integrin subunit beta 3 (ITGB3) Cathepsin S (CTSS) Myeloperoxidase (MPO) |
| Hematopoietic cell lineage | CD36 molecule (CD36) Integrin subunit beta 3 (ITGB3) Integrin subunit alpha 2b (ITGA2B) Interleukin 6 receptor (IL6R) Colony-stimulating factor 1 receptor (CSF1R) |
| Extracellular matrix receptor interaction | CD36 molecule (CD36) Fibronectin 1 (FN1) Glycoprotein VI platelet (GP6) Integrin subunit alpha 2b (ITGA2B) Integrin subunit beta 3 (ITGB3) |
| Osteoclast differentiation | Proto-oncogene, src family tyrosine kinase (FYN) Fc fragment of IgG receptor IIIb (FCGR3B) Colony-stimulating factor 1 receptor (CSF1R) Integrin subunit beta 3 (ITGB3) Leukocyte immunoglobin like receptor B1 (LILRB1) |
| Natural killer cell mediated toxicity | Proto-oncogene, src family tyrosine kinase (FYN) Fc fragment of IgG receptor IIIb (FCGR3B) SH2 domain containing 1A (SH2D1A) Phospholipase C gamma 1 (PLCG1) Protein tyrosine phosphatase, non-receptor type 6 (PTPN6) |
| Hypoxia-inducible factor 1 (HIF-1) signaling | Interleukin 6 receptor (IL6R) TNF receptor superfamily members 3 (TNFRSF3)a Phospholipase C gamma (PLCG1) Serpin family E member 1 (SERPINE1) |
| T cell receptor signaling | T cell co-stimulator (ICOS) hematopoiesis factor PTPN6 (PTPN6) phospholipase C gamma 1 (PLCG1) proto-oncogene, src family tyrosine kinase (FYN) |
TNFRSF3 also known as lymphotoxin B receptor (LTBR).
Analytes Previously Linked to Osteoarthritis
In addition to the 30 proteins that demonstrated the largest change during the first 2 weeks after ACL injury, we also evaluated 6 biomarkers previously linked to OA ( Table 2 ). TIMP-1, the primary inhibitor of MMP-1 and MMP-3, tended to be downregulated in the first 2 weeks after ACL injury (P = 0.09, SRM = −0.89, Table 3 ). In contrast, chondrodegenerative enzymes and products of cartilage degeneration all tended to increase: MMP-1 (P = 0.08, SRM = 0.88), MMP-3 (P = 0.12, SRM = 1.04), ADAM12 (P = 0.02, SRM = 1.27), and aggrecan (P = 0.06, SRM = 0.89). Interestingly, MMP-9, previously shown to decrease with OA, tended to decrease over the first 2 weeks after ACL injury (P = 0.24, SRM = −0.84).
Table 3.
Osteoarthritis-Related Proteomic Changes Occurring between 1 and 2 Weeks Following Acute Anterior Cruciate Ligament Injury.
| Protein | Time 1 | Time 2 | P | SRM |
|---|---|---|---|---|
| Aggrecan | 6219.0 ± 3631.2 | 15680.5 ± 9367.2 | 0.057 | 0.89 |
| ADAM12 | 1242.4 ± 717.7 | 3198.5 ± 1442.4 | 0.020 | 1.27 |
| TIMP-1 | 333.7 ± 1020.4 | 2428.6 ± 490.0 | 0.090 | −0.89a |
| MMP-1 | 62479.7 ± 44114.5 | 122818.6 ± 61805.1 | 0.083 | 0.88 |
| MMP-3 | 39576.1 ± 33816.1 | 77540.1 ± 43491.1 | 0.124 | 1.04 |
| MMP-9 | 4192.9 ± 5527.6 | 1135.9 ± 938.3 | 0.236 | −0.84a |
SRM = standardized response mean.
Negative SRMs are indicative of a decrease between the 2 time points.
Discussion
An acute ACL tear has been established as a risk factor for the development of posttraumatic knee OA. Greater than 50% of patients will develop radiographic evidence of posttraumatic OA within 10 to 20 years following ACL injury.30,31 While surgical stabilization of the knee can normalize biomechanics, it does not alone reduce the risk of progressive cartilage degeneration. A critical response to joint injury involves activation of inflammatory pathways; however, prolonged activation of these pathways results in increased biomarkers associated with progressive cartilage breakdown and matrix catabolism.32-34 Elevated inflammatory markers and cytokine concentrations at the time of ACL reconstruction have been reported to be predictive of cartilage matrix composition 3 years after surgery,35 and biomarker concentrations on the day of surgery may be predictive of patient-reported outcomes 2 years following surgery for female patients.36
The changes we observed in markers previously associated with cartilage degradation and OA were consistent with prior studies, namely increased synovial fluid aggrecan fragments, MMP-1, MMP-3, and ADAM12 following knee injuries and in early stage OA.32,37 In addition to these results, we were intrigued by the number of RA-related markers that were upregulated following acute ACL injury suggesting that the initial response to injury may have distinct biological pathways in common with RA. Proteins upregulated acutely after ACL injury included apoliopoprotein E and isoform E3, vascular cell adhesion protein 1, interleukin-34, and cell surface glycoprotein CD200 receptor 1. This is perhaps suggestive of chondral breakdown driven by an inflammatory process, not unlike what is seen in the early stages of RA. Others have noted the similarity of early stages of posttraumatic OA and early stages of RA.38 Both are associated with synovial membrane inflammation with increases in inflammatory cytokines with no radiographic evidence of OA. In RA, this initial response ultimately leads to T and B cell proliferation in the synovium that is not present in OA.
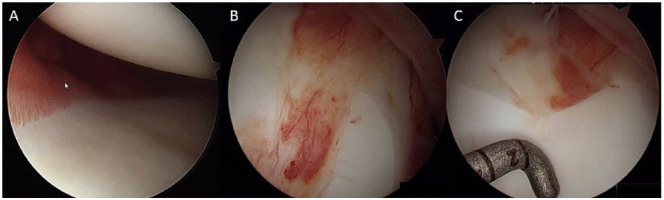
The similarities of RA and posttraumatic OA processes do not extend to the features of stage III RA that include development of a pannus. However, while the B and T cell response and subsequent pannus development are clearly not a part of the posttraumatic OA progression, we have anecdotally noted the presence of an intra-articular film-like membrane during second-look arthroscopy of knees with persistent pain and synovitis that may be suggestive of a persistent inflammatory response following acute ACL injury consistent with a central role of inflammation long after the initial injury ( Fig. 1 ). Taken together, the results of the current study lend further support to the concept of controlling the early inflammatory response to joint injury as a potential chondroprotective strategy.
Figure 1.
Second look arthroscopy of a patient not involved in the current study performed 4 weeks after anterior cruciate ligament (ACL) reconstruction. After ACL injury, the patient showed a subacute inflammatory response in the surrounding synovium that can be strictly localized as shown in (A). The synovial inflammation is sharply demarcated along the base of the medial meniscus in a patient 4 weeks after ACL injury. Some patients develop more severe synovitis that is often accompanied by pain and stiffness. In those patients, the chronic inflammatory state may result in a pannus-like synovial response that can cover the condyle like a vascularized membrane (B). The membrane covers normal articular cartilage and can easily be stripped with an arthroscopic hook. The underlying cartilage, while appearing normal, appears softer and less light reflective than normal articular cartilage during arthroscopy (C).
Exploratory Pathway Analyses
Eight pathways were noted to be differentially upregulated. Several pathways, such as the phagosome, hematopoietic cell lineage, extracellular matrix receptor interaction, natural killer cell–mediated toxicity, hypoxia-inducible factor 1 signaling, and T cell receptor signaling pathways, are to be expected as a result of the acute inflammatory response after ACL injury. However, 2 pathways, the cytokine-cytokine receptor interaction and osteoclast differentiation pathways, may have implications for the observed RA-like response and progressive cartilage degradation following ACL injury.
Cytokine-Cytokine Receptor Interaction Pathway
It is not surprising that the cytokine-cytokine receptor interaction pathway is upregulated following acute ACL injury as cytokines play a crucial role in both innate and adaptive inflammatory host defenses, and development and repair processes aimed at the restoration of homeostasis. However, this pathway has been previously linked to many diseases, including juvenile idiopathic arthritis suggesting a role in cartilage degradation and not solely a response to acute injury.29 Four of the seven analytes belong to the tumor necrosis factor (TNF) family and include TNF receptor superfamily members 3, (TNFRSF3), 4 (TNFRSF4), 9 (TNFRSF9), and 25 (TNFRSF25). Interleukin 17 receptor A (IL17RA) was also upregulated and is involved in the pathogenesis of inflammatory and autoimmune diseases including RA.29
Cytokine concentrations in the synovial fluid early after ACL injury have been associated with degenerative cartilage changes over the ensuing three years.35 OA is a chronic, inflammatory condition with monocytes and macrophages acting as mediators in the cycle of cartilage degradation in knee OA.39,40 Synovial macrophages activated by proinflammatory cytokines as a result of cartilage breakdown produce proinflammatory cytokines, which then activate chondrocytes and production of MMPs, thereby creating a cyclical process of cartilage degradation.39
As such, it is particularly noteworthy that four protein analytes in the TNF family were upregulated following acute ACL injury. Genes in the TNF family have been previously associated with both RA and OA,41,42 and this may be a potential therapeutic target. Corticosteroids have been demonstrated to reduce markers of type II collagen breakdown following acute ACL injury13; and it may be the underlying ability of corticosteroids to modulate macrophage function that is causing the reduction in collagen breakdown. Acute injury initiates a cascade of activity, including activation of synovial macrophages and increased concentrations of TNF-α and other proinflammatory cytokines, which thereby results in increased markers of type II collagen turnover in the acute phase after injury.7-13 Intra-articular corticosteroids have demonstrated the ability to disrupt this cycle by lessening TNF and cytokine concentrations in chronic arthritis,43 albeit transiently.
Osteoclast Differentiation Pathway
While OA progression has been linked to the innate immunologic response, OA progression is also potentially affected by cross-talk between the cartilage (chondrocytes) and subchondral bone (osteoclasts and osteoblasts).44 Not only does ACL injury result in an intraarticular inflammatory response, there is also substantial injury to the subchondral bone. So-called “bone bruises” in the proximal tibia and distal femur are commonly found in combination with ACL injury.45-47 There are conflicting reports as to whether these bone bruises resolve over time,46,47 but other characteristics, including bone mineral density, bone mass, and bone content, are all altered after ACL injury and do not return to preinjury status even years after injury.48,49 Furthermore, bone bruise volume in the lateral compartment shortly after ACL injury has been associated with cartilage degradation during the first 2 to 3 years after injury.46
In our study, colony-stimulating factor 1 receptor (CSF1R) was upregulated after ACL injury (P = 0.04, SRM = 1.16). Bone bruises were present on preoperative MRIs for all 6 patients and, while not statistically significant because of the small sample size (P = 0.16), there was a positive correlation between the volume of preoperative bone bruises in the lateral compartment and the change in CSF1R between the 2 study time points (Spearman’s ρ = 0.66). CSF1R is involved with both the cytokine-cytokine receptor interaction and osteoclast differentiation pathways. CSF1R is a cytokine that controls the production, differentiation and function of macrophages and osteoclasts.35 Importantly, CSF1R has been known to be involved in transmembrane signaling in both bone marrow and blood mononuclear cells and as such, could potentially play a role in both innate immunologic and subchondral bone mechanisms of OA progression.35 Synovial CSF1 expression has been demonstrated to be significantly greater for both RA and OA patients that controls.50 CSF1R is expressed by synovial macrophages activated by TNF; CSF1R also differentially regulates MMP expression, osteoclast differentiation, and TNF-induced osteolysis, and thereby appears to have an essential role in cartilage degradation.50-52 Recent animal model results suggest that blockade of CSF1R may suppress the synovial inflammatory response50; this highlights the need for future studies to aid in determining optimal early interventions for minimizing cartilage degeneration, and hopefully the incidence of PTOA following acute knee injuries.
This preliminary study was exploratory in design and thus, not without limitations. An a priori power analysis was not used to justify the sample size. The sample size was small consisting of only 6 relatively young patients (mean age of 17.5 years) thereby limiting the conclusions that can be drawn and whether the results can be generalized to older patient populations. The development of OA and the progression of articular cartilage damage after ACL injury and reconstruction are undoubtedly multifactorial in nature. Several factors, including patient age, body mass index, and treatment of the menisci, have been associated with progressive articular cartilage changes.53 While the onset and progression of posttraumatic OA after ACL injury are multifactorial, elevated inflammatory markers and cytokine concentrations at the time of ACL reconstruction have been reported to be predictive of cartilage matrix composition 3 years after surgery,35 and biomarker concentrations on the day of surgery are predictive of patient-reported outcomes 2 years following surgery for female patients.36 The current analyses address the biologic factors potentially associated with posttraumatic OA progression. The samples subjected to proteomic analysis were limited to 2 very early time points, but nevertheless demonstrate the feasibility and utility of proteomic analyses of synovial fluid. The combination of our current proteomic results and previously reported synovial fluid biomarker results highlight the need for additional studies to determine if progression of posttraumatic OA after ACL injury is driven by an inflammatory process represented by the autoimmune arthropathy as in rheumatoid arthritis, or switches to a subacute innate immune inflammatory arthropathy represented by idiopathic OA. A better understanding of the underlying mechanisms of posttraumatic OA progression may then yield novel treatments to lessen the burden of posttraumatic OA after acute ACL injury. These data may also contribute to identification of pharmacodynamic biomarkers to quantify treatment efficacy, and data with which to estimate adequate power in future studies.
Conclusions
In conclusion, the proteomic results demonstrate a pattern of biomarker expression following ACL injury that was indicative of articular cartilage degeneration. To our knowledge, this is the first clinical study to assess proteomic changes following acute ACL injury. We found that the protein responses postinjury were similar in direction to those previously reported with knee OA. The proteomic analysis also revealed large post-injury increases in RA associated markers, and differential upregulation of the cytokine-cytokine receptor interaction pathway that is closely associated with inflammation. These results not only concur with other recent studies that have reported that the chondrodegenerative process begins early after acute ACL injury12,13,54 but also provide unique insights into potential therapeutic targets.
Supplemental Material
Supplemental material, Additional_File_1 for Joint Fluid Proteome after Anterior Cruciate Ligament Rupture Reflects an Acute Posttraumatic Inflammatory and Chondrodegenerative State by John D. King, Grant Rowland, Alejandro G. Villasante Tezanos, James Warwick, Virginia B. Kraus, Christian Lattermann and Cale A. Jacobs in CARTILAGE
Footnotes
Acknowledgments and Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study received funding from The Arthritis Foundation of America. Research reported in this publication was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Number 5K23AR060275 and was supported in part by pilot awards from NIH NICHD National Center for Medical Rehabilitation Research (R24HD050846), and the Clark Charitable Foundation. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Data collection and study administration was supported by the University of Kentucky CTSA award (UL1TR000117).
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Approval: This study was approved by the University of Kentucky Institutional Review Board (Protocol #12-0706).
Informed Consent: all subjects consented prior to study participation.
Trial Registration: ClinicalTrials.gov Identifier: NCT01692756.
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Brandt KD, Dieppe P, Radin E. Etiopathogenesis of osteoarthritis. Med Clin North Am. 2009;93(1):1-24. [DOI] [PubMed] [Google Scholar]
- 2. DiMicco MA, Patwari P, Siparsky PN, Kumar S, Pratta MA, Lark MW, et al. Mechanisms and kinetics of glycosaminoglycan release following in vitro cartilage injury. Arthritis Rheum. 2004;50(3):840-8. [DOI] [PubMed] [Google Scholar]
- 3. Patwari P, Kurz B, Sandy JD, Grodzinsky AJ. Mannosamine inhibits aggrecanase-mediated changes in the physical properties and biochemical composition of articular cartilage. Arch Biochem Biophys. 2000;374(1):79-85. [DOI] [PubMed] [Google Scholar]
- 4. Lee JH, Fitzgerald JB, DiMicco MA, Grodzinsky AJ. Mechanical injury of cartilage explants causes specific time-dependent changes in chondrocyte gene expression. Arthritis Rheum. 2005;52(8):2386-95. [DOI] [PubMed] [Google Scholar]
- 5. Hembree W, Ward B, Furman B, Zura R, Nichols L, Guilak F, et al. Viability and apoptosis of human chondrocytes in osteochondral fragments following joint trauma. J Bone Joint Surg Br. 2007;89(10):1388-95. [DOI] [PubMed] [Google Scholar]
- 6. Olsson O, Isacsson A, Englund M, Frobell RB. Epidemiology of intra-and peri-articular structural injuries in traumatic knee joint hemarthrosis—data from 1145 consecutive knees with subacute MRI. Osteoarthritis Cartilage. 2016;24(11):1890-7. [DOI] [PubMed] [Google Scholar]
- 7. Lohmander LS, Atley LM, Pietka TA, Eyre DR. The release of crosslinked peptides from type II collagen into human synovial fluid is increased soon after joint injury and in osteoarthritis. Arthritis Rheum. 2003;48(11):3130-9. [DOI] [PubMed] [Google Scholar]
- 8. Irie K, Uchiyama E, Iwaso H. Intraarticular inflammatory cytokines in acute anterior cruciate ligament injured knee. Knee. 2003;10(1):93-6. [DOI] [PubMed] [Google Scholar]
- 9. Dare D, Rodeo S. Mechanisms of post-traumatic osteoarthritis after ACL injury. Curr Rheumatol Rep. 2014;16(10):448. [DOI] [PubMed] [Google Scholar]
- 10. Swärd P, Frobell R, Englund M, Roos H, Struglics A. Cartilage and bone markers and inflammatory cytokines are increased in synovial fluid in the acute phase of knee injury (hemarthrosis)—a cross-sectional analysis. Osteoarthritis Cartilage. 2012;20(11):1302-8. [DOI] [PubMed] [Google Scholar]
- 11. Bigoni M, Sacerdote P, Turati M, Franchi S, Gandolla M, Gaddi D, et al. Acute and late changes in intraarticular cytokine levels following anterior cruciate ligament injury. J Orthop Res. 2013;31(2):315-21. [DOI] [PubMed] [Google Scholar]
- 12. Lattermann C, Jacobs CA, Bunnell MP, Jochimsen KN, Abt JP, Reinke EK, et al. Logistical challenges and design considerations for studies using acute anterior cruciate ligament injury as a potential model for early posttraumatic osteoarthritis. J Orthop Res. 2017;35(3):641-50. [DOI] [PubMed] [Google Scholar]
- 13. Lattermann C, Jacobs CA, Bunnell MP, Huston LJ, Gammon LG, Johnson DL, et al. A multicenter study of early anti-inflammatory treatment in patients with acute anterior cruciate ligament tear. Am J Sports Med. 2017;45(2):325-33. [DOI] [PubMed] [Google Scholar]
- 14. Hunter DJ, Nevitt M, Losina E, Kraus V. Biomarkers for osteoarthritis: current position and steps toward further validation. Best Pract Res Clin Rheumatol. 2014;28(1):61-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kraus VB, Blanco FJ, Englund M, Henrotin Y, Lohmander LS, Losina E, et al. OARSI Clinical Trials Recommendations: soluble biomarker assessments clinical trials in osteoarthritis. Osteoarthritis Cartilage. 2015;23(5):686-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hirsch G, Kitas G, Klocke R. Intra-articular corticosteroid injection in osteoarthritis of the knee and hip: factors predicting pain relief—a systematic review. Sem Arthritis Rheum. 2013;42(5):451-73. [DOI] [PubMed] [Google Scholar]
- 17. Hsueh MF, Önnerfjord P, Kraus VB. Biomarkers and proteomic analysis of osteoarthritis. Matrix Biol. 2014;39:56-66. [DOI] [PubMed] [Google Scholar]
- 18. Hathout Y, Conklin LS, Seol H, Gordish-Dressman H, Brown KJ, Morgenroth LP, et al. Serum pharmacodynamic biomarkers for chronic corticosteroid treatment of children. Sci Rep. 2016;6:31727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Struglics A, Larsson S, Lumahashi N, Frobell R, Lohmander LS. Changes in cytokines and aggrecan ARGS neoepitope in synovial fluid and serum and in C-terminal crosslinking telopeptide of type II collagen and N-terminal crosslinking telopeptide of type I collagen in urine over five years after anterior cruciate ligament rupture: an exploratory analysis in the Knee Anterior Cruciate Ligament, Nonsurgical Versus Surgical Treatment Trial. Arthritis Rheumatol. 2015;67(7):1816-25. [DOI] [PubMed] [Google Scholar]
- 20. Martel-Pelletier J, McCollum R, Fujimoto N, Obata K, Cloutier JM, Pelletier JP. Excess of metalloproteases over tissue inhibitor of metalloprotease may contribute to cartilage degradation in osteoarthritis and rheumatoid arthritis. Lab Invest. 1994;70(6):807-15. [PubMed] [Google Scholar]
- 21. Kerna I, Kisand K, Suutre S, Murde M, Tamm A, Kumm J, et al. The ADAM12 is upregulated in synovitis and postinflammatory fibrosis of the synovial membrane in patients with early radiographic osteoarthritis. Joint Bone Spine. 2014;81(1):51-6. [DOI] [PubMed] [Google Scholar]
- 22. Naito K, Takahashi M, Kushida K, Suzuki T, Ohishi T, Miura M, et al. Measurement of matrix metalloproteinases (MMPs) and tissue inhibitor of metalloproteinase-1 (TIMP-1) in patients with knee osteoarthritis: comparison with generalized osteoarthritis. Rheumatology (Oxford). 1999;38(6):510-5. [DOI] [PubMed] [Google Scholar]
- 23. Brew K, Nagase H. The tissue inhibitors of metalloproteinases (TIMPs): an ancient family with structural and functional diversity. Biochim Biophys Acta. 2010;1803(1):55-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Liang MH. Longitudinal construct validity: establishment of clinical meaning in patient evaluative instruments. Med Care. 2000;38(9 Suppl):II84-II90. [PubMed] [Google Scholar]
- 25. Liang MH, Larson MG, Cullen KE, Schwartz JA. Comparative measurement efficiency and sensitivity of five health status instruments for arthritis research. Arthritis Rheum. 1985;28(5):542-7. [DOI] [PubMed] [Google Scholar]
- 26. Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. Hillsdale, NJ: Lawrence Earlbaum; 1988. [Google Scholar]
- 27. Huang da W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4(1):44-57. [DOI] [PubMed] [Google Scholar]
- 28. Huang da W, Sherman BT, Lempicki RA. Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 2009;37(1):1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. National Center for Biotechnology Information U.S. National Library of Medicine. NCBI Gene database. Available from: https://www.ncbi.nlm.nih.gov/guide/
- 30. Lohmander LS, Englund PM, Dahl LL, Roos EM. The long-term consequence of anterior cruciate ligament and meniscus injuries: osteoarthritis. Am J Sports Med. 2007;35(10):1756-69. [DOI] [PubMed] [Google Scholar]
- 31. Oiestad BE, Engebretsen L, Storheim K, Risberg MA. Knee osteoarthritis after anterior cruciate ligament injury: a systematic review. Am J Sports Med. 2009;37(7):1434-43. [DOI] [PubMed] [Google Scholar]
- 32. Neuman P, Dahlberg LE, Englund M, Struglics A. Concentrations of synovial fluid biomarkers and the prediction of knee osteoarthritis 16 years after anterior cruciate ligament injury. Osteoarthritis Cartilage. 2017;25(4):492-8. doi: 10.1016/j.joca.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 33. Lieberthal J, Sambamurthy N, Scanzello CR. Inflammation in joint injury and post-traumatic osteoarthritis. Osteoarthritis Cartilage. 2015;23(11):1825-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Watt FE, Paterson E, Freidin A, Kenny M, Judge A, Saklavala J, et al. Acute molecular changes in synovial fluid following human knee injury: association with early clinical outcomes. Arthritis Rheumatol. 2016;68(9):2129-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Amano K, Huebner JL, Stabler TV, Tanaka M, McCulloch CE, Lobach I, et al. Synovial fluid profile at the time of anterior cruciate ligament reconstruction and its association with cartilage matrix composition 3 years after surgery. Am J Sports Med. 2018;46(4):890-9. doi: 10.1177/0363546517749834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lattermann C, Jacobs CA, Conley CW, Jochimsen KN, Johnson DJ, Reinke EK, et al. Biomarkers on the day of ACL reconstruction and sex predictive of knee-related quality of life at 2-year follow-up. Orthop J Sports Med. 2017;5(3 Suppl 3):2325967117S2325900127. [Google Scholar]
- 37. Tchetverikov I, Lohmander LS, Verzijl N, Huizinga TW, TeKoppele JM, Hanemaaijer R, et al. MMP protein and activity levels in synovial fluid from patients with joint injury, inflammatory arthritis, and osteoarthritis. Ann Rheum Dis. 2005;64(5):694-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wheeless CR. Stages of rheumatoid arthritis. In: Wheeless CR, Nunley JA, Urbaniak JR, editors. Wheeless’ text of orthopaedics. Towson, MD: Data Trace Internet Publishing; 2012. Available from: http://www.wheelessonline.com/ortho/stages_of_rheumatoid_arthritis [Google Scholar]
- 39. Berenbaum F. Osteoarthritis as an inflammatory disease (osteoarthritis is not osteoarthrosis!). Osteoarthritis Cartilage. 2013;21(1):16-21. [DOI] [PubMed] [Google Scholar]
- 40. Issa RI, Griffin TM. Pathobiology of obesity and osteoarthritis: integrating biomechanics and inflammation. Pathobiol Aging Age Relat Dis. 2012;2(2012):17470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Noack M, Miossec P. Selected cytokine pathways in rheumatoid arthritis. Semin Immunopathol. 2017;39(4):365-83. [DOI] [PubMed] [Google Scholar]
- 42. Feng Z, Lian KJ. Identification of genes and pathways associated with osteoarthritis by bioinformatics analyses. Eur Rev Med Pharmacol Sci. 2015;19(5):736-44. [PubMed] [Google Scholar]
- 43. af Klint E, Grundtman C, Engström M, Catrina AI, Makrygiannakis D, Klareskog L, et al. Intraarticular glucocorticoid treatment reduces inflammation in synovial cell infiltrations more efficiently than in synovial blood vessels. Arthritis Rheum. 2005;52(12):3880-9. [DOI] [PubMed] [Google Scholar]
- 44. Findlay DM, Atkins GJ. Osteoblast-chondrocyte interactions in osteoarthritis. Curr Osteoporos Rep. 2014;12(1):127-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lattermann C, Jacobs CA, Reinke EK, Scaramuzza EA, Huston LJ, Dunn WR, et al. Are bone bruise characteristics and articular cartilage pathology associated with inferior outcomes two and six years after anterior cruciate ligament reconstruction? Cartilage. 2017;8(2):139-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Potter HG, Jain SK, Ma Y, Black BR, Fung S, Lyman S. Cartilage injury after acute, isolated anterior cruciate ligament tear: immediate and longitudinal effect with clinical/MRI follow-up. Am J Sports Med. 2012;40(2):276-85. [DOI] [PubMed] [Google Scholar]
- 47. Costa-Paz M, Muscolo DL, Ayerza M, Makino A, Aponte-Tinao L. Magnetic resonance imaging follow-up sudy of bone bruises associated with anterior cruciate ligament ruptures. Arthroscopy. 2001;17(5):445-9. [DOI] [PubMed] [Google Scholar]
- 48. Nyland J, Fisher B, Brand E, Krupp R, Caborn DNM. Osseous deficits after anterior cruciate ligament injury and reconstruction: a systematic literature review with suggestions to improve osseous homeostasis. Arthroscopy. 2010;26(9):1248-57. [DOI] [PubMed] [Google Scholar]
- 49. van Meer BL, Waarsing JH, van Eijsden WA, Meuffles DE, van Arkel ERA, Verhaar JAN, et al. Bone mineral density changes in the knee following anterior cruciate ligament rupture. Osteoarthritis Cartilage. 2014;22(1):154-61. [DOI] [PubMed] [Google Scholar]
- 50. Garcia S, Hartkamp LM, Malvar-Fernandez B, van Es IE, Lin H, Wong J, et al. Colony-stimulating factor (CSF) 1 receptor blockade reduces inflammation in human and murine models of rheumatoid arthritis. Arthritis Res Ther. 2016;18:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kitaura H, Zhou P, Kim HJ, Novack DV, Ross FP, Teitelbaum SL. M-CSF mediates TNF-induced inflammatory osteolysis. J Clin Invest. 2005;115(12):3418-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ota T, Urakawa H, Kozawa E, Ikuta K, Hamada S, Tsukushi S, et al. Expression of colony-stimulating factor 1 is associated with occurence of osteochondral change in pigmented villonodular synovitis. Tumor Biol. 2015;36(7):5361-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. MARS Group;Magnussen RA, Borchers JR, Pedroza AD, Huston LJ, Haas AK, et al. Risk factors and predictors of significant chondral surface change from primary to revision anterior cruciate ligament reconstruction: a MOON and MARS cohort study. Am J Sports Med. 2018;46(3):557-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Catterall JB, Stabler TV, Flannery CR, Kraus VB. Changes in serum and synovial fluid biomarkers after acute injury (NCT00332254). Arthritis Res Ther. 2010;12(6):R229. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Additional_File_1 for Joint Fluid Proteome after Anterior Cruciate Ligament Rupture Reflects an Acute Posttraumatic Inflammatory and Chondrodegenerative State by John D. King, Grant Rowland, Alejandro G. Villasante Tezanos, James Warwick, Virginia B. Kraus, Christian Lattermann and Cale A. Jacobs in CARTILAGE