Abstract
Objective
Synovial fluid (SF) plays an important role in the maintenance of articular cartilage. SF is a dynamic reservoir of proteins derived from cartilage and synovial tissue; thus, its composition may serve as a biomarker that reflects the health and pathophysiological condition of the joint. The purpose of the current study was to evaluate the osteoarthritic synovial fluid (OASF) and transforming growth factor-β1 (TGF-β1) activity in articular chondrocytes catabolic and inflammatory responses.
Design
Chondrocytes were seeded at passage 2 and cultured for 72 hours under different conditions. Human chondrocytes were subjected to OASF while rat chondrocytes were subjected to either healthy synovial fluid (rSF) or TGF-β1 and then assigned for cell viability analysis. In addition, the effects of OASF and TGF-β1 on chondrocytes metalloprotease (MMP)-3 and MMP-13 and interleukin-18 (IL-18) expression were evaluated by immunocytochemistry, ELISA, and reverse transcriptase-polymerase chain reaction.
Results
SF from osteoarthritic patients significantly induced MMP-3, MMP-13, and IL-18 receptor expression in chondrocytes. To put in evidence the inflammatory activity of OASF, healthy chondrocytes from rat were cultured with TGF-β1. In the presence of TGF-β1 these cells started to express MMP-3, MMP-13, and IL-18 genes and attached to each other forming a chondrocyte aggregated structure. Healthy SF was able to maintain a typical monolayer of rounded chondrocytes with no inflammatory response.
Conclusion
In summary, these observations demonstrated that TGF-β1, one of the components of OASF, has a dual effect, acting in chondrocyte maintenance and also inducing inflammatory and catabolic properties of these cells.
Keywords: chondrocytes, synovial fluid, TGF-β1, interleukin-18, osteoarthritis
Introduction
Articular cartilage is a tissue that is nourished by synovial fluid (SF), resistant to mechanical stress but undergoes atrophy with loading deprivation1,2 and whose remodeling is slower and decreases with aging. SF has been implicated in the maintenance and health of the articular cartilage in vivo. It has been shown that SF derived from healthy joints could induce glycosaminoglycan synthesis through release of insulin-like growth factor (IGF-I) and chondrocyte differentiation from fibrocytes derived from the perichondrium.3,4 However, the biological effect of SF in vivo on chondrogenic processes of hyaline cartilage is still unclear. Physiologically, cartilage development is regulated by an array of growth factors, especially IGF-1, transforming growth factor-β1 (TGF-β1), basic fibroblast growth factor, Sonic Hedgehog, and Indian Hedgehog,5-7 and it has been reported that these growth factors when added exogenously to chondrocytes encapsulated in hydrogels in vitro also improve the quality of engineered cartilage.8 Throughout the inflammatory process, the SF molecular and cellular composition changes drastically and shows high levels of inflammatory cytokines as TGF-β1, tumor necrosis factor-α, interleukin-18 (IL-18), and metalloproteases (MMPs), which are related to cartilage degeneration.9-14 IL-18 has been involved in osteoarthritis development such as rheumatoid arthritis.15-17 Moreover, in vitro, IL-18 was able to induce inflammatory responses in synoviocyte and chondrocyte primary cultures18 and also was produced by chondrocytes when treated with IL-1β.
In contrast to in vivo conditions, there is extensive in vitro data regarding the chondrogenic effect of SF on cultures of chondrocytes. Chondrocyte cultures supplemented with 20% to 100% of healthy SF for 21 days induced the synthesis of glycosaminoglycan and hyaluronic acid, as well as Collagen II and Sox9 expression.19-21 Furthermore, SF and hyaluronic acid were able to induce chondrocyte differentiation from equine mesenchymal stem cells.22 Different chondrocytes culture systems, such as 3D micromass cultures, and chondrogenic media supplemented with TGF-β1 have also been developed, inducing the differentiation of mesenchymal stem cells into chondrocytes.23-25 However, recently it has been demonstrated that high concentrations of TGF-β1 has been involved in osteoarthritis development, whereas inhibition of TGF-β1 attenuates cartilage degeneration in mice.26 This suggested that TGF-β1 could have a paradoxical effect, due to its ability to stimulate chondrocyte growth and chondrogenic differentiation in vitro, while in vivo it is involved in the cartilage degeneration process.
This study investigated the direct role of SF derived from osteoarthritis patients (osteoarthritic synovial fluid [OASF]) as well as SF derived from healthy rat knee joints (rSF) compared with established chondrogenic media supplemented with TGF-β1 in articular chondrocytes cultures. Here, chondrocytes at the second passage were able to retain their chondrogenic characteristics through Sox9, Aggrecan, and CollagenII expression when cultured in all conditions. However, in the presence of TGF-β1 or OASF these cells tend to aggregate and express pro-inflammatory IL-18 or IL-18 receptor, respectively. Therefore, our data showed the inflammatory properties of TGF-β1 and OASF on primary cultures of chondrocytes, even though all conditions were able to maintain chondrocyte cultures in vitro.
Methods
This study was approved by the institutional review board (IRB #: DIMED 030/2011) at Hospital São Vicente de Paulo and our Institutional Animal Care and Use Committee (IACUC #: DAHEICB075) at Federal University of Rio de Janeiro.
Human Samples
Patients under total knee arthroplasty (n = 4) had their cartilage damage identified by direct surgical observation, and the compartment considered “relatively normal” or non-fibrillated were harvested. Prior to the surgical procedure SF samples were aspirated. Cartilage and SF were taken to the laboratory without breaking sterility. Fresh SF samples were then filtered through a 70-µm nylon filter (Becton Dickinson, Franklin Lakes, NJ) to remove debris, centrifuged at 400 × g for 10 minutes, and the supernatant was stored at −80°C until use. For all experiments, SF supernatants were diluted in complete media with a final protein concentration of 3 µg/µL.
Human Chondrocyte Isolation and Primary Culture
Cartilage was sliced in small pieces and human primary chondrocytes were isolated by overnight digestion in 0.5% collagenase II (Gibco, Carlsbad, CA) at 37°C in a shaker. The primary chondrocytes were seeded at 50 × 103 cells/cm2 and cultured in a complete media containing Dulbecco’s Modified Eagle’s Medium (DMEM-F12) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin (100 U mL−1) and streptomycin (U mL−1) (P/S) at 37°C in a humid atmosphere with 5% CO2. The medium was renewed every 2 days, cells were kept in monolayer, and used for experiments at passage 2.
Rat Samples
Knee SF, as well as fragments of cartilage from the femoral head, were obtained from male Wistar rats, 4 months of age and weighing approximately 300 to 330 g. The rats were euthanized by anesthesia (Ketamine 2 mL/kg and 0.5 mL/kg Xylazine—both were from König, MG, Brazil) and the SF was harvested through 2 washes (200 µL each) of complete media into the knee joint cavity using a tuberculin syringe. The final volume average harvested from both knees was 500 µL, the rat synovial fluid (rSF) was centrifuged at 12,000 × g for 1 minute, and the supernatant was stored at −80°C until use.
Rat Chondrocyte Isolation and Primary Culture
Cartilage of the femoral head was dissected, minced into small fragments, and digested overnight in 0.5% collagenase II (Gibco, Carlsbad, CA) at 37°C in a shaker, following the method adapted by Khatib et al.27 Rat primary chondrocytes were seeded at 1.2 × 104 cells/cm2 and cultured in a complete media: DMEM-F12 supplemented with 10% FBS and 1% penicillin (100 U mL−1) and streptomycin (U mL−1) (P/S) at 37°C in a humid atmosphere with 5% CO2. The medium was renewed every 2 days until the cell monolayer was nearly confluent and used for experiments at passage 2.
Chondrocyte Culture Conditions
Chondrocytes were seeded at passage 2 and 24 hours after the medium was removed and the cells were subjected to different culture conditions for 72 hours as follow:
Human: Two different culture conditions: (1) Control—complete media; (2) OASF.
Rat: Three different culture conditions: (1) Control—complete media; (2) rSF; (3) complete media supplemented with 10−6 M insulin (Sigma Aldrich, St. Louis, MO), 8 × 10−8 M apo-transferrin (Gibco, New Zealand), 2.5 × 10−4 M ascorbic acid (Sigma Aldrich, St. Louis, MO) and 10 ng mL−1 TGF-β1.25
Cell Viability
The viabilities of human and rat primary chondrocytes were assessed by using MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide). Briefly, cells were adhered to 96-well plates and exposed to their respective culture conditions described above for 72 hours. Viability was assessed by MTT and cells were disrupted by DMSO (dimethylsulfoxide; Sigma, St. Louis, MO). DMSO light absorption was evaluated at 595 nm.
Immunocytochemistry
Chondrocytes were fixed in 4% paraformaldehyde (PFA) for 3 minutes, blocked with 5% PBS/BSA (phosphate buffered saline/bovine serum albumin) at room temperature, and incubated overnight at 4°C with primary antibodies, as follows: for rat chondrocytes, rabbit anti-rat collagen II (1:200 U mL−1; Abcam, Cambridge, MA), and mouse anti-rat tenascin (1:200; Sigma Aldrich, St. Louis, MO); for human chondrocytes, rabbit anti-human collagen II (1:20, Millipore, Burlington, MA) and mouse anti-human IL-18R, MMP-3, and MMP-13 (all three 1:50, R&D Systems, Minneapolis, MN). After rinsing with PBS, the samples were incubated with secondary antibodies: Alexa fluor 488 conjugated anti-mouse IgG and Alexa fluor 546 conjugate anti-rabbit IgG (both 1:200 U mL−1, Invitrogen, Carlsbad, CA) for 1 hour at room temperature. Nuclei were stained with DAPI (4,6-diamidino-2-phenylindole; Sigma, St. Louis, MA). Negative control sections were prepared by omitting the primary antibody.28 The samples were examined under a confocal fluorescence microscope (Leica TCS SP5, Wetzlar, Germany).
ELISA Assay
Human chondrocytes were cultivated into 96-well plate for 72 hours as described above. Cells were fixed with PFA 4% for 3 minutes following 3 washes with PBS/Tween 0.05%. Nonspecific sites were blocked with 0.1% BSA for 2 hours and then incubated for 2 hours at room temperature (RT) with specific primary antibodies diluted in PBS/Tween 0.05%: Mouse anti-human antibodies IL-18R, MMP-3, and MMP-13 (all 1:100, R&D Systems). After washes with 0.1% BSA/PBS/Tween 0.05%, peroxidase-conjugated secondary antibody diluted in the same buffer was incubated for 1 hour at RT. Colorimetric reaction was determined by citrate buffer 0.1 M pH 4.5 containing OPD 1 mg/mL and 0.01% H2O2, and was stopped with H2SO4 3 M. Absorbance was measured at 490 nm in an ELISA microplate automatic reader (BioRad, Hercules, CA).29
Transforming Growth Factor-β1
The concentration of TGF-β1 on the OASF (n = 12) was measured in triplicate using a commercially available ELISA kit (Biomatik Corporation, Ontario, Canada) in appropriately diluted SFs. OASF aliquots were thawed and subsequently subjected to an acid activation treatment consisting of acidification for 10 minutes (1 M HCl) followed by neutralization with an equal volume of 1.2 M NaOH/0.5 M HEPES, and samples were assay immediately. Samples and controls of known concentration (standards) were added in wells and incubated for 1 hour at 37°C, and additional Reagents A and B as well as TMB substrate and stop solution were added and incubated following the manufacturer’s instructions. Absorbance was measured at 450 nm in an ELISA microplate automatic reader (Tecan Infinite M200 PRO).
Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR) Analysis
Cells were homogenized in TRIZOL reagent (Invitrogen Life Technologies, Carlsbad, CA) and RNA was extracted according to the manufacturer’s instructions. cDNA was synthesized as described previously.30 Briefly, 5 µg RNA was reacted with 1 µL of oligo(dT) primer and 1 µL of SuperScript RNase H-reverse transcriptase for 60 minutes at 37°C. For the PCR, 5 µL cDNA was incubated with 13.65 µL nuclease-free water, 0.5 µL 10 mM dNTP, 2.5 µL 10× PCR buffer, 0.75 µL 25 mM MgCl2, 0.1 µL (5 U) Taq polymerase, and 2.5 µL of each primer pair. Reaction settings had an initial denaturation step of 10 minutes at 94°C, followed by 40 cycles of 45 seconds at 94°C, 45 seconds at annealing temperature for each primer used and with beta-actin as internal control ( Table 1 ), and 1 minute 45 seconds at 72°C. Finally, samples were maintained for 7 minutes at 72°C. Reaction products were resolved by electrophoresis on 1.5% agarose gel and incubated with GelRed (Biotium, Fremont, CA) for visualization under UV light.
Table 1.
Rat Oligonucleotides Used in Reverse Transcriptase-Polymerase Chain Reaction.
| Gene | Forward | Reverse | °C | bp |
|---|---|---|---|---|
| ADAMTS-4 | CCCGCACTGTGCCTTTCTCT | CGGTTGCTGGCAGGACTCTT | 65 | 499 |
| ADAMTS-5 | CCTGCTGACCCTGGCCTCTA | CGTGGCTGAAGTGCATTTGG | 65 | 502 |
| Aggrecan | CAACTGGCAGTGGGGAAACC | TGGCAAAAGGCACACCTGAA | 65 | 504 |
| IL-10 | CAGCAAAGGCCATTCCATCC | TCCAGTAGATGCCGGGTGGT | 69 | 504 |
| IL-13 | ATGGCACTCTGGGTGACTGC | GAGATGTCCAGGGCCGGTCT | 69 | 420 |
| IL-17A | CTGATCAGGACGAGCGACCA | GACGCATGGCGGACAATAGA | 69 | 492 |
| IL-18 | GAAACCCGCCTGTGTTCGAG | CATGCGGCCTCAGGTATTTTG | 65 | 501 |
| MMP-3 | CCCATTGCATGGCAGTGAAG | GGGCATAGGCATGAGCCAAG | 65 | 500 |
| MMP-13 | CGGTCTGGACCACTCCAAGG | AGGGTCTTCCCCGTGTCCTC | 65 | 501 |
| Sox 9 | GTTTGGAGCGGGCAACTGAG | TGTTCTTGCTGGAGCCGTTG | 65 | 498 |
| β-Actin | TGGATCGGTGGCTCCATCCTGG | GCAGCTCAGTAACAGTCCGCCTAGA | 68 | 130 |
Statistical Analysis
We analyzed at least 4 independent experiments, performed in triplicate for each assay. For all statistical analysis, t tests were used to compare mean ± SD. Statistical analyses were performed using GraphPad Prism 5, (GraphPad Software, La Jolla, CA), and significance level was defined as P ⩽ 0.05.
Results
Human Chondrocyte Morphology and Viability under OASF Culture
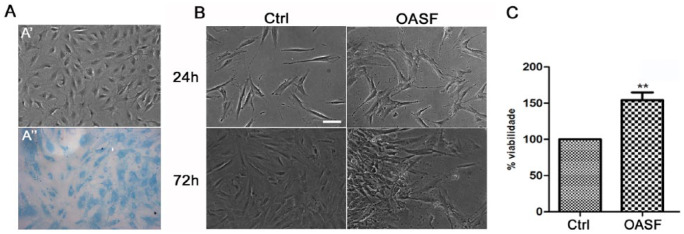
Human chondrocytes harvested from a considered “relatively normal” cartilage compartment were able to keep their rounded morphology in vitro at passage 0, and Alcian blue staining confirmed the presence of sulfated GAGs in the extracellular matrix (ECM) up to second passage ( Fig. 1A ). After 72 hours in culture with 2 different conditions, we observed that chondrocytes kept their morphology as a monolayer when cultured in the presence of complete media (Ctrl), while in the presence of OASF they seem to attach to each other, forming micromass structures ( Fig. 1B ). We also demonstrate that OASF has the ability to significantly increase the viability of these cells (P < 0.01) ( Fig. 1C ).
Figure 1.
Morphology and viability of human chondrocytes after treatment with OASF. Chondrocytes from “relatively normal” cartilage compartment were able to keep their rounded morphology and Alcian blue staining confirmed the presence of sulfated GAGs in the ECM up to second passage (A). Chondrocytes after 72 hours in the presence of complete media (Ctrl) or osteoarthritic synovial fluid (OASF) (B). Significant differences were found between Ctrl and OASF (C). Data were reported as means ± SEM of 4 independent experiments performed in triplicate. Statistical significance indicated by **P < 0.01. Scale bar: 100 µm (For interpretation of the references to colours in this figure legend, refer to the online version of this article).
The Effect of OASF on Chondrocytes IL-18R Expression
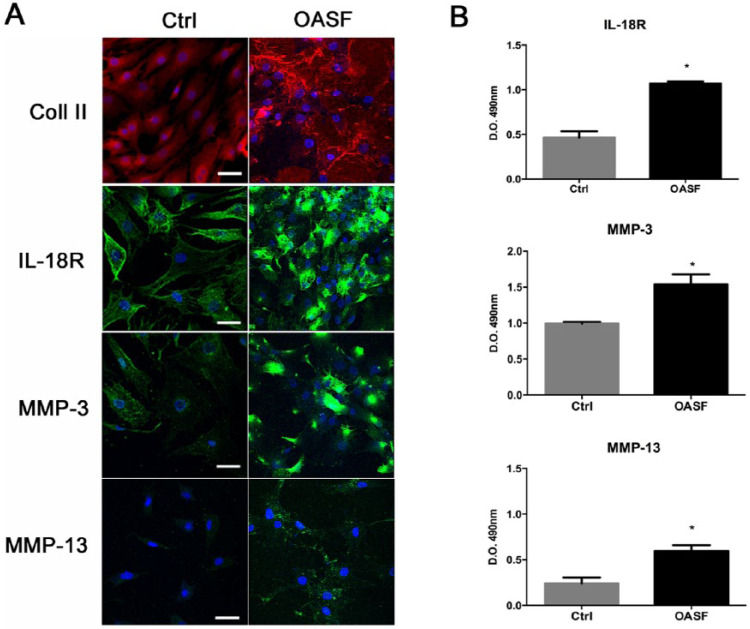
After 72 hours in both culture conditions (Ctrl and OASF), human chondrocytes were able to retain their ECM up to second passage as demonstrated by collagen II staining ( Fig. 2A ). We also demonstrated by immunostaining the ability of OASF to induce the expression of proteinases MMP-3 and MMP-13 and inflammatory interleukin receptor, IL-18R, and which we were able to confirm by ELISA showing that they were significantly expressed in the presence of OASF ( Fig. 2B ).
Figure 2.
Immunocytological analysis of human chondrocytes after OASF treatment. In both culture conditions, chondrocytes after 72 hours were able to retain their ECM as demonstrated by collagen II staining (A). The expression of proteinases MMP-3 and MMP-13 and inflammatory interleukin receptor, IL-18, were significant expressed in the presence of OASF (A and B). In A, the nuclei were stained with DAPI. Data were reported as means ± SEM of 4 independent experiments performed in triplicate. Statistical significance indicated by *P < 0.05. Scale bar: 100 µm.
Rat Chondrocyte Morphology and Viability under rSF and TGF-β1 Culture
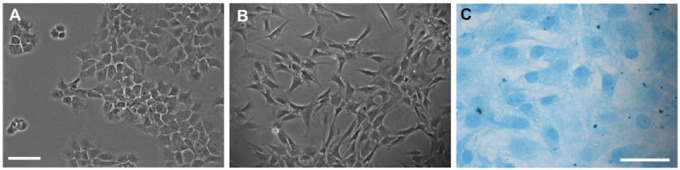
Numerous chondrocytes with their typical rounded morphology were seen by day 7 in culture ( Fig. 3A ). From the first passage to the second passage ( Fig. 3B ), these chondrocytes became elongated, fibroblastoid, or polygonal and reached their confluence of approximately 90% after 28 days in culture. Alcian blue staining showed that the chondrocytes were capable of maintaining the expression of sulfated glycosaminoglycans in their ECM until the second passage ( Fig. 3C ).
Figure 3.
Characterization of rat primary chondrocytes cultured in vitro. (A) After 7 days in culture chondrocytes were able to keep their rounded morphology. (B) From the first passage to the second passage, after 21 days in culture, chondrocytes were more elongated and fibroblastoid. (C) In the second passage, Alcian blue staining confirmed the presence of sulfated GAGs in the ECM. Scale bar: 100 µm (For interpretation of the references to colours in this figure legend, refer to the online version of this article).
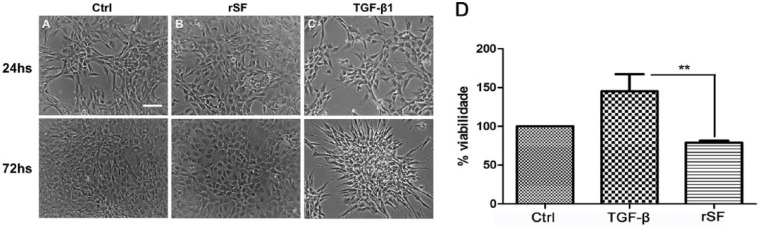
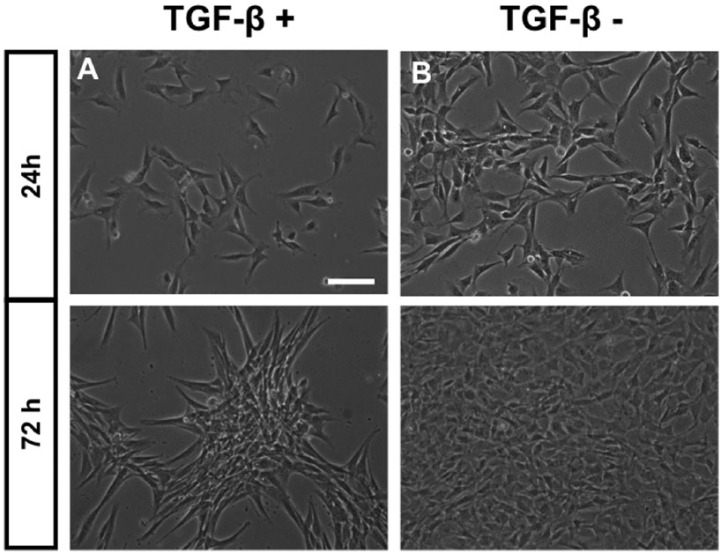
Chondrocytes at passage 2 were cultured for 72 hours under 3 different conditions: (1) complete media (Ctrl), (2) normal synovial fluid (rSF), or (3) media with TGF-β1 ( Fig. 4 ). In the presence of rSF, chondrocytes with a fibroblast-like morphology were able to resume a rounded morphology characteristic of their original chondrogenic phenotype ( Fig. 4B ); and interestingly in the presence of TGF-β1, in addition to increased proliferation, chondrocytes attached to each other forming a micromass structure ( Fig. 4C ). Cell viability was assess by MTT where significant differences were found only between TGF-β1 and the rSF conditions, showing the ability of TGF-β1 to augment the viability of these cells (P < 0.01) ( Fig. 4D ).
Figure 4.
Morphology and viability of rat chondrocytes under 3 different culture conditions. Chondrocytes were cultured for 72 hours in the presence of compete media (Ctrl), normal synovial fluid (rSF), and media with TGF-β1. In the presence of rSF, chondrocytes resumed the rounded shape characteristic of their original chondrogenic phenotype (B); and in the presence of TGF-β1, in addition to increased proliferation, chondrocytes formed an aggregate structure (C). Significant differences were found only between the TGF-β1 and the SF conditions (D). Data were reported as means ± SEM of 3 independent experiments performed in triplicate. Statistical significance indicated by **P < 0.01. Scale bar: 100 µm.
Furthermore, we investigated for 72 hours whether the specific chondrocyte micromass formation was due to the chondrogenic media factors (insulin, apo-transferrin, and ascorbic acid) or due to the presence or absence of TGF-β1. Regardless the other factors presenting in the chondrogenic media, chondrocytes aggregated only when exposed to TGF-β1 ( Fig. 5A ), but not in its absence (Fig. 5B).
Figure 5.
Morphology of rat chondrocytes in the presence of TGF-β1. Independently of the other factors presenting in the chondrogenic media, chondrocytes in the second passage tended to aggregate just in the presence of TGF-β1 (A), but not in its absence (B). Scale bar: 100 µm.
TGF-β1 Induced Chondrocytes to Express IL-18
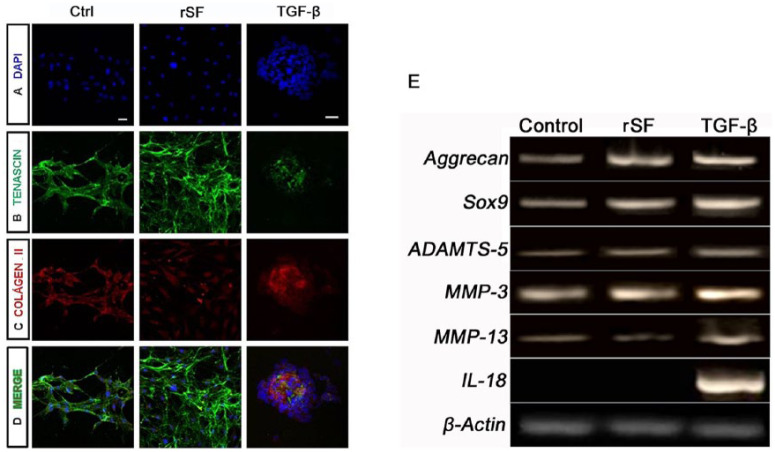
Although morphological differences were observed, immunocytological analyses verified the chondrocytes phenotype by tenascin and collagen II staining ( Fig. 6 ), showing that they were capable of synthesizing an ECM similar to hyaline articular cartilage. Both markers were maintained in the 3 conditions.
Figure 6.
Rat chondrocytes ECM markers and gene expression in 3 different conditions. In all 3 culture conditions, chondrocytes were able to retain the ECM as demonstrated by tenascin (B) and collagen II staining (C). In A, the nuclei were stained with DAPI. D is an overlap of images A, B, and C. Chondrocytes also maintained their chondrogenic characteristics, as confirmed by the expression of Sox9 and aggrecan. Proteinases MMP-3, MMP-13, and ADAMTS-5 were expressed in all 3 conditions. The inflammatory interleukin observed, IL-18, was positive only in the presence of TGF-β1. Amplification of the beta-actin gene was used as the internal control (E). Scale bar: 30 µm.
RT-PCR also confirmed the maintenance of the chondrocyte phenotype in the presence of the 3 different culture conditions through the expression of the master chondrogenic gene, Sox9, and its predominant proteoglycan, aggrecan (Fig. 6E). We also evaluated the expression of the proteinases responsible for collagen cleavage and proteoglycans degradation (MMP-3 and MMP-13 and ADAMTS-5) and an inflammatory interleukin (IL-18). ADAMTS-4 and IL-10, IL-13, and IL-17 were not expressed in any of culture conditions (data not shown). The expression of MMP-13 seems to be stronger and IL-18 was found only in the presence of TGF-β1. Beta-actin was used as the internal control.
OASF and TGF-β1 induced IL-18 expression in human and rat chondrocytes, respectively. To elucidate the inflammatory activity of OASF, TGF-β1 was quantified in 12 SF obtained from osteoarthritic patients, and in all SF analyzed TGF-β1 was detected at a mean concentration of 31.7 ± 47.4 pg/mL ( Table 2 ).
Table 2.
Concentration of TGF-β1 in Osteoarthritic Synovial Fluid (OASF).
| OASF | Mean ± SD |
|---|---|
| TGF-β1 (pg/mL) | 31.7 ± 47.4 |
Discussion
Fifty years have passed since the first demonstration that human articular chondrocytes can be cultured in vitro.31 In the present study, we demonstrated that chondrocytes up to the second passage could maintain their chondrogenic characteristics when cultured with complete media (Ctrl), normal rSF, chondrogenic medium with TGF-β1, and OASF. However, in the presence of OASF or TGF-β1 these cells were induced to express the inflammatory cytokine IL-18. Interestingly, in the presence of normal rSF these cells also showed an ability to return to their original chondrogenic phenotype observed in the primary culture without inflammatory properties.
The isolated chondrocytes from rats healthy cartilage were able to grow in vitro as previously described,27 at passage zero these cells showed their characteristic rounded morphology, and changes in cell behavior was followed by the acquisition of a fibroblast phenotype, in agreement with a previous report where most of monolayer cells tended to dedifferentiate, adopting a fibroblastoid phenotype after the third passage.32 We could also confirm by Alcian blue staining that these cells were capable of secreting an ECM rich in sulfated glycosaminoglycans (Fig. 3C). Moreover, these cells maintained their chondrogenic characteristics, as confirmed by the expression of Sox 9, Aggrecan, and Collagen II expression.
In an attempt to simulate the microenvironment of the joint, different culture systems have been tested,33,34 with the aim of assessing the ability of these cells to maintain their phenotype and to determine their viability rates. We analyzed human and rat chondrocytes at the second passage for 72 hours, in the presence of 2 different conditions (Control and OASF) or 3 different culture conditions (Control, TGF-β1, and rSF), respectively. We observed a statistically significant difference on cell viability between OASF and Ctrl as well as TGF-β1 and rSF conditions.
We also observed morphological changes in our cultures. Human and rat chondrocytes in the second passage had nearly dedifferentiated and adopted a fibroblast phenotype, but in the presence of rSF the rat chondrocytes demonstrated an ability to regain their original (chondrogenic) phenotype. This differs from their behavior in either TGF-β1 or OASF culture, where they showed increased proliferation, as expected,33 and a tendency to form micromasses. For a better evaluation of these cell aggregates in the healthy rat chondrocytes, we analyzed the chondrogenic medium culture with and without TGF-β1, and we were able to confirm that, independently of chondrogenic media factors, the rat chondrocytes were able to aggregate only in the presence of TGF-β1.
The presence of Coll2 indicates a commitment to chondrogenic characteristics. These chondrogenic characteristics of rat chondrocytes were confirmed by RT-PCR using the expression of the chondrogenic gene Sox9 and its predominant proteoglycan, aggrecan, as well as the expression of proteinases, aggrecanases, and interleukins. The chondrocytes maintained their chondrocyte phenotype in all different culture conditions, as confirmed by the expression of Sox9 and aggrecan. The proteinases MMP-3, MMP-13, and ADAMTS-5 were expressed in all 3 conditions. Of the observed interleukins, only IL-18 was positive in the presence of TGF-β1 medium, showing that TGF-β1 induced an inflammatory response in the cultures of chondrocytes.35
IL-18 is present in damaged joints, such as in cases of osteoarthritis and rheumatoid arthritis.17,36 This interleukin has a potential to induce an inflammatory response and contribute to the degradation of cartilage extracellular matrix, mainly through IL-1β,37 identifying IL-18 as a cytokine that regulates chondrocyte responses and contributes to cartilage degradation.12,18 Moreover, it has been demonstrated that IL-18 is capable of inducing angiogenesis in rheumatoid arthritis, the process favoring cartilage degradation.38 Conversely, inhibition of IL-18 ameliorates collagen-induced arthritis in mice.39 These data put in evidence the role of IL-18 in the process of developing arthritis.
The chondrogenic capacity of TGF-β1 is not surprising, since in embryonic cartilage TGF-β1 is expressed abundantly and may be involved in the chondrogenic transformation of primitive mesenchymal condensations. Recently, it has been demonstrated that TGF-β1 was capable of inducing Indian Hedgehog expression in chondrocyte cultures derived from human bone marrow stromal cells.40 Despite the fact that TGF-β1 could induce chondrocyte growth, it was also implicated in the pathological changes of osteoarthritis, and reduction of TGF-β1 could attenuate this process.26 Moreover, it has been shown that the effect of TGF-β on chondrogenesis varies according to cell type, whereas mesenchymal stromal cell culture in the presence of TGF-β differentiate into endochondral chondrocytes, and cultures of human chondrocyte maintain their chondral phenotype.41 These aspects are important because they demonstrate the ability of TGF-β1 to induce different responses within the chondrogenic process, suggesting a dual role of TGF-β1 on cartilage health and disease. This study’s data point out the need to identify and further understand the entire biological activity of TGF-β1 and how its controls the fate of chondrogenesis in vitro and in vivo during osteoarthritis development.
An important limitation of this study is the small amount of SF harvested from the rat knee joint, which would have precluded any comparison between the groups, but nevertheless, we were able to have a significant amount to perform the experiments proposed, analyze, and report an interesting preliminary finding. Although the human articular chondrocytes were harvested from a “healthy” area from an osteoarthritis cartilage and we had a robust immunocytological results with all markers’ activity analysis, we understand the entire osteoarthritic biological environment of what this cartilage has been previously exposed to and the need to evaluate a healthy articular cartilage sample from a donor. Last, we were not able to compare the effects of healthy human SF and rat OASF due to the challenge and difficulty in having a healthy SF donor sample and harvest SF from rat osteoarthritic knee.
In this study, we sought to improve the understanding of SF chondrogenic properties inducing or not inducing chondrocytes inflammatory response. Our results provide preliminary data for the exploration of the roles of OASF and TGF-β1 in joint homeostasis and possible investigations of its potential therapeutic characteristics, in order to develop strategies for maintenance of cartilage homeostasis and ultimately for cartilage regeneration.
Footnotes
Authors’ Note: Institution in which the work was performed: Universidade Federal do Rio de Janeiro, Rio de Janeiro, Brazil.
Acknowledgments and Funding: Thanks to Dr. Maria Eugênia Duarte from National Institute of Traumatology and Orthopedics and to Dr. Scott Rodeo from Hospital for Special Surgery, especially for their critical comments. Also thanks to Drs. Vera Lucia Pannain, Fernando Rosman, Thais Accorsi, and Mariana Lima Vale for their help and discussion of this project. This work was funded by Conselho Nacional de Pesquisa de Desenvolvimento Científico e Tecnológico (CNPq) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Brazil. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the article.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Approval: This study was approved by the institutional review board (IRB #: DIMED 030/2011) at Hospital São Vicente de Paulo and our Institutional Animal Care and Use Committee (IACUC #: DAHEICB075) at Federal University of Rio de Janeiro.
Informed Consent: All patients provided written informed consent for the use of chondrocytes and synovial fluid.
Animal Welfare: The present study followed international, national, and/or institutional guidelines for humane animal treatment and complied with relevant legislation.
References
- 1. Hui AY, McCarty WJ, Masuda K, Firestein GS, Sah RL. A systems biology approach to synovial joint lubrication in health, injury, and disease. Wiley Interdiscip Rev Syst Biol Med. 2012;4(1):15-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Vanwanseele B, Eckstein F, Knecht H, Stüssi E, Spaepen A. Knee cartilage of spinal cord-injured patients displays progressive thinning in the absence of normal joint loading and movement. Arthritis Rheum. 2002;46(8):2073-8. [DOI] [PubMed] [Google Scholar]
- 3. Skoog V, Widenfalk B, Ohlsén L, Wasteson A. The effect of growth factors and synovial fluid on chondrogenesis in perichondrium. Scand J Plast Reconstr Surg Hand Surg. 1990;24(2):89-95. [DOI] [PubMed] [Google Scholar]
- 4. Van den Hoogen BM, van de Lest CH, van Weeren PR, Lafeber FP, Lopes-Cardozo M, van Golde LM, et al. Loading-induced changes in synovial fluid affect cartilage metabolism. Br J Rheumatol. 1998;37(6):671-6. [DOI] [PubMed] [Google Scholar]
- 5. Chiang C, Litingtung Y, Harris MP, Simandl BK, Li Y, Beachy PA, et al. Manifestation of the limb prepattern: limb development in the absence of sonic hedgehog function. Dev Biol. 2001;236(2):421-35. [DOI] [PubMed] [Google Scholar]
- 6. Spiller KL, Liu Y, Holloway JL, Maher SA, Cao Y, Liu W, et al. A novel method for the direct fabrication of growth factor-loaded microspheres within porous nondegradable hydrogels: controlled release for cartilage tissue engineering. J Control Release. 2012;157(1):39-45. [DOI] [PubMed] [Google Scholar]
- 7. Steinert AF, Weissenberger M, Kunz M, Gilbert F, Ghivizzani SC, Göbel S, et al. Indian hedgehog gene transfer is a chondrogenic inducer of human mesenchymal stem cells. Arthritis Res Ther. 2012;14(4):R168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Spiller KL, Maher SA, Lowman AM. Hydrogels for the repair of articular cartilage defects. Tissue Eng Part B Rev. 2011;17(4):281-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Albro MB, Cigan AD, Nims RJ, Yeroushalmi KJ, Oungoulian SR, Hung CT, et al. Shearing of synovial fluid activates latent TGF-β. Osteoarthritis Cartilage. 2012;20(11):1374-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dai SM, Shan ZZ, Xu H, Nishioka K. Cellular targets of interleukin-18 in rheumatoid arthritis. Ann Rheum Dis. 2007;66(11):1411-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Futani H, Okayama A, Matsui K, Kashiwamura S, Sasaki T, Hada T, et al. Relation between interleukin-18 and PGE2 in synovial fluid of osteoarthritis: a potential therapeutic target of cartilage degradation. J Immunother. 2002;25(Suppl. 1):S61-S64. [DOI] [PubMed] [Google Scholar]
- 12. Olee T, Hashimoto S, Quach J, Lotz M. IL-18 is produced by articular chondrocytes and induces proinflammatory and catabolic responses. J Immunol. 1999;162(2):1096-100. [PubMed] [Google Scholar]
- 13. Sandy JD, Verscharen C. Analysis of aggrecan in human knee cartilage and synovial fluid indicates that aggrecanase (ADAMTS) activity is responsible for the catabolic turnover and loss of whole aggrecan whereas other protease activity is required for C-terminal processing in vivo. Biochem J. 2001;358(Pt 3):615-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Schlaak JF, Pfers I, Büschenfelde KHMZ, Märker-Hermann E. Different cytokine profiles in the synovial fluid of patients with osteoarthritis, rheumatoid arthritis and seronegative spondylarthropathies. Clin Exp Rheumatol. 1996;14(2):155-62. [PubMed] [Google Scholar]
- 15. Inoue H, Hiraoka K, Hoshino T, Okamoto M, Iwanaga T, Zenmyo M, et al. High levels of serum IL-18 promote cartilage loss through suppression of aggrecan synthesis. Bone. 2008;42(6):1102-10. [DOI] [PubMed] [Google Scholar]
- 16. Takei S, Hoshino T, Matsunaga K, Sakazaki Y, Sawada M, Oda H, et al. Soluble interleukin-18 receptor complex is a novel biomarker in rheumatoid arthritis. Arthritis Res Ther. 2011;13(2):R52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wang Y, Xu D, Long L, Deng X, Tao R, Huang G. Correlation between plasma, synovial fluid and articular cartilage interleukin-18 with radiographic severity in 33 patients with osteoarthritis of the knee. Clin Exp Med. 2014;14(3):297-304. [DOI] [PubMed] [Google Scholar]
- 18. Fu Z, Liu P, Yang D, Wang F, Yuan L, Lin Z, et al. Interleukin-18-induced inflammatory responses in synoviocytes and chondrocytes from osteoarthritic patients. Int J Mol Med. 2012;30(4):805-10. [DOI] [PubMed] [Google Scholar]
- 19. Bertram KL, Krawetz RJ. Osmolarity regulates chondrogenic differentiation potential of synovial fluid derived mesenchymal progenitor cells. Biochem Biophys Res Commun. 2012;422(3):455-61. [DOI] [PubMed] [Google Scholar]
- 20. Lee DA, Salih V, Stockton EF, Stanton JS, Bentley G. Effect of normal synovial fluid on the metabolism of articular chondrocytes in vitro. Clin Orthop Relat Res. 1997;(342):228-38. [PubMed] [Google Scholar]
- 21. van de, Lest CH, van den Hoogen BM, van Weeren PR. Loading-induced changes in synovial fluid affect cartilage metabolism. Biorheology. 2000;37(1-2):45-55. [PubMed] [Google Scholar]
- 22. Hegewald AA, Ringe J, Bartel J, Krüger I, Notter M, Barnewitz D, et al. Hyaluronic acid and autologous synovial fluid induce chondrogenic differentiation of equine mesenchymal stem cells: a preliminary study. Tissue Cell. 2004;36(6):431-8. [DOI] [PubMed] [Google Scholar]
- 23. Brand JA, McAlindon TE, Zeng L. A 3D system for culturing human articular chondrocytes in synovial fluid. J Vis Exp. 2012(59):e3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Johnstone B, Hering TM, Caplan AI, Goldberg VM, Yoo JU. In vitro chondrogenesis of bone marrow-derived mesenchymal progenitor cells. Exp Cell Res. 1998;238(1):265-72. [DOI] [PubMed] [Google Scholar]
- 25. Muraglia A, Corsi A, Riminucci M, Mastrogiacomo M, Cancedda R, Bianco P, et al. Formation of a chondro-osseous rudiment in micromass cultures of human bone-marrow stromal cells. J Cell Sci. 2003;116(Pt 14):2949-55. [DOI] [PubMed] [Google Scholar]
- 26. Zhen G, Wen C, Jia X, Li Y, Crane JL, Mears SC, et al. Inhibition of TGF-β signaling in mesenchymal stem cells of subchondral bone attenuates osteoarthritis. Nat Med. 2013;19(6):704-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Khatib AM, Lomri A, Moldovan F, Fiet J, Mitrovic DR. Constitutive and inducible expression of endothelin-1 in primary rat articular chondrocyte culture. Cytokine. 1997;9(8):556-62. [DOI] [PubMed] [Google Scholar]
- 28. Faria J, Romão L, Martins S, Alves T, Mendes FA, de Faria GP, et al. Interactive properties of human glioblastoma cells with brain neurons in culture and neuronal modulation of glial laminin organization. Differentiation. 2006;74(9-10):562-72. [DOI] [PubMed] [Google Scholar]
- 29. Alves TR, da Fonseca AC, Nunes SS, da Silva AO, Dubois LG, Faria J, et al. Tenascin-C in the extracellular matrix promotes the selection of highly proliferative and tubulogenesis-defective endothelial cells. Exp Cell Res. 2011;317(15):2073-85. [DOI] [PubMed] [Google Scholar]
- 30. Ehlers S, Smith KA. Differentiation of T cell lymphokine gene expression: the in vitro acquisition of T cell memory. J Exp Med. 1991;173(1):25-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Manning WK, Bonner WM., Jr. Isolation and culture of chondrocytes from human adult articular cartilage. Arthritis Rheum. 1967;10(3):235-9. [DOI] [PubMed] [Google Scholar]
- 32. Surrao DC, Khan AA, McGregor AJ, Amsden BG, Waldman SD. Can microcarrier-expanded chondrocytes synthesize cartilaginous tissue in vitro? Tissue Eng Part A. 2011;17(15-16):1959-67. [DOI] [PubMed] [Google Scholar]
- 33. Gomez-Camarillo MA, Almonte-Becerril M, Tort MV, Tapia-Ramirez J, Flores JBK. Chondrocyte proliferation in a new culture system. Cell Prolif. 2009;42(2):207-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lemare F, Steimberg N, Le Griel C, Demignot S, Adolphe M. Dedifferentiated chondrocytes cultured in alginate beads: restoration of the differentiated phenotype and of the metabolic responses to interleukin-1beta. J Cell Physiol. 1998;176(2):303-13. [DOI] [PubMed] [Google Scholar]
- 35. Otero M, Goldring MB. Cells of the synovium in rheumatoid arthritis. Chondrocytes. Arthritis Res Ther. 2007;9(5):220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tetlow LC, Adlam DJ, Woolley DE. Matrix metalloproteinase and proinflammatory cytokine production by chondrocytes of human osteoarthritic cartilage: associations with degenerative changes. Arthritis Rheum. 2001;44(3):585-94. [DOI] [PubMed] [Google Scholar]
- 37. Joosten LA, Smeets RL, Koenders MI, van den Bersselaar LA, Helsen MM, Oppers-Walgreen B, et al. Interleukin-18 promotes joint inflammation and induces interleukin-1-driven cartilage destruction. Am J Pathol. 2004;165(3):959-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Volin MV, Koch AE. Interleukin-18: a mediator of inflammation and angiogenesis in rheumatoid arthritis. J Interferon Cytokine Res. 2011;31(10):745-51. [DOI] [PubMed] [Google Scholar]
- 39. Smeets RL, van de, Loo FA, Arntz OJ, Bennink MB, Joosten LA, van den Berg WB. Adenoviral delivery of IL-18 binding protein C ameliorates collagen-induced arthritis in mice. Gene Ther. 2003;10(12):1004-11. [DOI] [PubMed] [Google Scholar]
- 40. Handorf AM, Chamberlain CS, Li WJ. Endogenously produced Indian Hedgehog regulates TGFbeta-driven chondrogenesis of human bone marrow stromal/stem cells. Stem Cells Dev. 2015;24(8):995-1007. [DOI] [PubMed] [Google Scholar]
- 41. Dexheimer V, Gabler J, Bomans K, Sims T, Omlor G, Richter W. Differential expression of TGF-β superfamily members and role of Smad1/5/9-signalling in chondral versus endochondral chondrocyte differentiation. Sci Rep. 2016;6:36655. [DOI] [PMC free article] [PubMed] [Google Scholar]