Abstract
Objective
Heat shock proteins are molecules rapidly produced under conditions of environmental stress, and involve in protecting the cells structural integrity and function. Osteoarthritis (OA) is a chronic destructive disorder of the joints manifested by the ongoing deterioration and loss of articular cartilage. The present study aimed to analyze circulating and synovial heat shock protein (Hsp70) values in knee osteoarthritis patients and healthy controls and to determine their relationship with the radiographic grading of the severity of knee OA.
Design
Seventy-two subjects with knee OA and 30 control participants were recruited. Circulating and joint fluid Hsp70 values were quantified by commercially available enzyme-linked immunosorbent assay.
Results
Circulating Hsp70 was markedly higher in knee OA patients compared with that of healthy volunteers (P = 0.01). Correspondingly, synovial fluid Hsp70 was 3-fold greater than paired circulating Hsp70 samples (P < 0.001). Further analysis revealed that circulating and joint fluid Hsp70 values were significantly related with the radiographic severity of knee OA (r = 0.413, P < 0.001 and r = 0.658, P < 0.001, respectively). Subsequently, circulating Hsp70 value was directly associated with joint fluid Hsp70 value (r = 0.704, P < 0.001).
Conclusions
Circulating and synovial Hsp70 levels were positively correlated with the radiographic severity of knee OA. Hsp70 could represent a potential biochemical marker for predicting the severity and may play a fundamental part in the pathogenic mechanism of knee OA.
Keywords: osteoarthritis, knee, Hsp70, circulating, synovial fluid
Introduction
Osteoarthritis (OA) is a prevalent, debilitating, destructive disorder of the joints manifested by the progressive deterioration and loss of articular cartilage.1 The ongoing degenerative changes of articular cartilage often arise from the fibrillation and erosion of hyaline cartilage, subchondral bone sclerosis as well as the formation of osteophytes.2 Degradation of the extracellular matrix (ECM) via excess chondrocyte cell death; caused by an increase in the levels of cellular apoptosis has also been linked to the disorder.3 OA forms one of the most common musculoskeletal disorder seen in the elderly,4,5 with approximately 30% of people older than 65 years in any given population being affected in either the hip or knee joint.6 Currently, treatment is based on a symptomatic method, targeting the pain and limited mobility implicated with a decrease in joint function, thus attempting to improve the patient’s quality of life. The exact causes of the changes seen in OA are mostly unknown; however the limited ability for cartilage self-repair suggests that any lesion to this region will lead to the development of OA.7 In addition to this, OA development and progression has been associated with recurrent trauma.2 Moreover, mechanical, biochemical, and chemical stresses from various sources are widely considered to be contributing factors for the gradual destruction of articular cartilage.8,9
Heat shock proteins (Hsp) or “stress proteins” are molecules rapidly produced under conditions of environmental stress, and function to protect the cells structural integrity and function.10,11 The function of these proteins has been highly conserved throughout evolution—from bacteria to humans. The stress proteins are separated into different families, based on their relative molecular weights: 20 to 30, 40, 60, 70, 90, and 100 kDa. Of these, the 70 kDa family is the most studied.12,13 In normal cells, Hsp70 forms the major heat shock protein, the expression of which is induced by varying stresses (e.g., cytotoxic conditions, hydrostatic pressure, temperature change). It functions to protect the cell from the exerting stress as well as promoting recovery. Hsp70 is thought to act as a molecular chaperone, assisting in the folding, transport and disassembly of intracellular proteins.14,15 Moreover, it is also able to protect the cell from oxidative damage, inhibiting the cell entering a state of apoptosis or necrosis.2 In the recent years, extracellular Hsp70 has been suggested to possess a cytokine-like activity, inducing inflammation in areas of high expression.16
Kubo et al.17 reported that these stress proteins were synthesized in human chondrocytes at both elevated and physiological temperatures on OA cartilage, suggesting that the synthesized stress proteins could make a contribution to the development of osteoarthritis. Following this, it has been reported that chondrocytes present in knee OA cases overexpress the 70 kDa heat shock protein, the levels of which directly correlate to the severity of the disease.1 Additionally, gene transduction of Hsp70 has been shown to cause an increase in cartilage matrix metabolism, protecting against cytotoxic stresses and inhibit apoptosis in vitro.16 Therefore it is sensible to assume, as with previous studies, that the heat shock protein of weight 70 kDa plays an vital functional part in the pathogenic mechanism of autoimmune disorders, including OA.12,13
As far as we know, no preceding investigation has evaluated Hsp70 expression in both circulating and synovial fluid taken from subjects with OA. The present study determined circulating Hsp70 levels between subjects with knee OA and control volunteers, and explored the relationship of circulating and synovial fluid Hsp70 and knee OA severity.
Methods
Study Population
The experimental protocols were approved by the Ethical Committee on Human Research of our institution. The present study was conducted in accordance with the guidelines of the Declaration of Helsinki, and all patients and healthy volunteers signed an informed consent prior to their participation in the study.
Seventy-two subjects with knee OA aged 53 to 84 years (59 women and 13 men, average age 69.9 ± 0.9 years) in accordance with the criteria of the American College of Rheumatology were recruited in the current work. Thirty gender- and age-matched control volunteers with no medical or radiographic evidence of OA (22 women and 8 men, average age 68.9 ± 1.1 years) were enrolled in the current study. Participants with overarching disorders, including diabetes mellitus, reports of long-term steroid treatment, any conditions of arthritis, previous knee injury and/or infection, malignancy, or other chronic inflammatory disorders were excluded from this investigation.
Severity Assessment
Standard weightbearing anteroposterior radiography of the affected knees was performed when each subject maintained full knee extension. Evaluation of radiologic severity was carried out by 2 examiners who were blinded to the findings with regard to the Kellgren and Lawrence (KL) classification.18 The radiographic change of knee OA was categorized into 5 grades (0 to 4): grade 0 (normal findings with no X-ray changes), grade 1 (possibility of osteophytic lipping), grade 2 (clear osteophytes and plausible narrowing of joint space), grade 3 (medium multiple osteophytes, clear narrowing of joints space and some sclerosis), and grade 4 (large osteophytes, severe narrowing of joint space, bone sclerosis, and clear deformity of bone contour). The presence of radiographic knee OA was delineated as KL grade ≥2. Control group was delineated as acquiring none of radiographic hip and knee OA.
Laboratory Methods
Peripheral venous blood specimens were obtained from every subject, centrifuged, and kept instantly at −20°C till use. Synovial fluid samples were collected from the most severe knee during diagnostic or therapeutic arthroscopy or total knee replacement. The specimen was then centrifuged to exclude cells and joint debris and then kept at −20°C for further analysis.
Double-blinded quantitative measurement of circulating and synovial fluid Hsp70 levels were assessed using a commercially available sandwich enzyme-linked immunosorbent assay kit (R&D Systems, Minneapolis, MN, USA). Corresponding to the manufacturer’s instruction, recombinant human Hsp70 standards, plasma, and synovial fluid samples were pipetted into every well of a microplate, which was precoated with specific antibody to Hsp70. After incubating for 2 hours at room temperature, all wells were washed completely 3 times with washing reagent. Subsequently, Hsp70 conjugate was pipetted into every well and incubated for 2 hours at room temperature. After 3 washes, substrate solution was added into each well and then the microplate was incubated for 20 minutes at room temperature without light. Ultimately, all reactions were terminated by the stop reagent and the optical density was evaluated with automated microplate reader at 450 nm. The intensity of color derived is clearly proportionate to the quantity of Hsp70 in the samples. A standard optical density–concentration curve was depicted for the determination of Hsp70 value (range 125-8,000 pg/mL).
Statistical Analysis
Statistical analyses were accomplished with the Statistical Package for Social Sciences (v. 22.0, IBM Corp., Armonk, NY, USA). Comparison among each group was executed by one-way analysis of variance (ANOVA). Pearson’s correlation coefficient was used to examine the relationship between circulating and synovial fluid Hsp70 levels and the radiologic severity. All data were shown as a mean ± standard error of the mean (SEM). Statistical significance for differences and correlations was set at P values <0.05.
Results
Baseline Clinical Characteristics
The detailed demographic features of the participants are presented in Table 1 . No statistical difference in age or gender ratio between subjects with knee OA and control volunteers was observed. As demonstrated in Figure 1 , mean plasma Hsp70 value was notably greater in subjects with OA than in plasma controls (P = 0.01). Mean Hsp70 value in joint fluid of patients with knee OA was significantly (3-fold) greater than that in corresponding blood samples (P < 0.001).
Table 1.
Baseline Clinical Characteristics of Patients with Knee Osteoarthritis (OA) and Controls.
| Patients with Knee OA | Controls | P | |
|---|---|---|---|
| Number | 72 | 30 | |
| Age (years) | 69.9 ± 0.9 | 68.9 ± 1.1 | 0.5 |
| Gender (female/male) | 59/13 | 22/8 | 0.4 |
Figure 1.
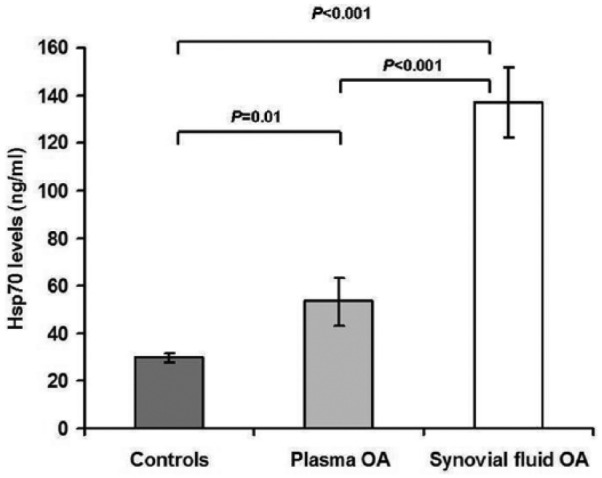
Plasma and synovial fluid Hsp70 levels of patients with knee osteoarthritis (OA) and healthy controls.
Correlation Between Circulating and Synovial Fluid Hsp70 and OA Severity
To explore the association between circulating and joint fluid Hsp70 concentrations and OA severity, the cohort was segregated into 3 groups corresponding to KL grading ( Table 2 ). Of the 72 knee OA subjects included in the study, 26 exhibited a KL grade of 2, 23 had a KL grade of 3, and 23 had a KL grade of 4. The groups were analyzed with regard to both the plasma OA, and joint fluid OA Hsp70 levels ( Fig. 2 ). Both the circulating and joint fluid Hsp70 concentrations presented significant increases with the KL grading of the patient groups (r = 0.413, P <0.001 and r = 0.658 P < 0.01, respectively, Figs. 2 and 3 ). Further analysis revealed a positive relationship between plasma Hsp70 levels and synovial fluid hsp70 levels in patients with knee OA (r = 0.704, P <0.001) ( Fig. 4 ).
Table 2.
Plasma and Synovial Fluid (SF) Hsp70 in Patients with Osteoarthritis and P Values for Differences between Kellgren and Lawrence (KL) Subgroups.
| Total | KL Grade 2 | KL Grade 3 | KL Grade 4 | P | |
|---|---|---|---|---|---|
| n | 72 | 26 | 23 | 23 | |
| SF Hsp70 (ng/mL) | 53.3 ± 9.9 | 15.2 ± 1.4 | 50.9 ± 4.2 | 98.8 ± 28.2 | <0.001 |
| Plasma Hsp70 (ng/ml) | 137.0±14.8 | 61.3±5.8 | 96.3±8.7 | 263.3±31.8 | <0.001 |
Figure 2.
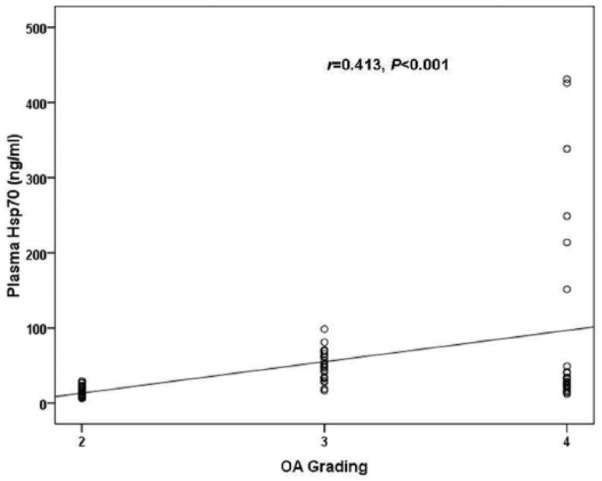
Scattergram showing the positive correlation between plasma Hsp70 and the severity in patients with knee osteoarthritis (OA).
Figure 3.
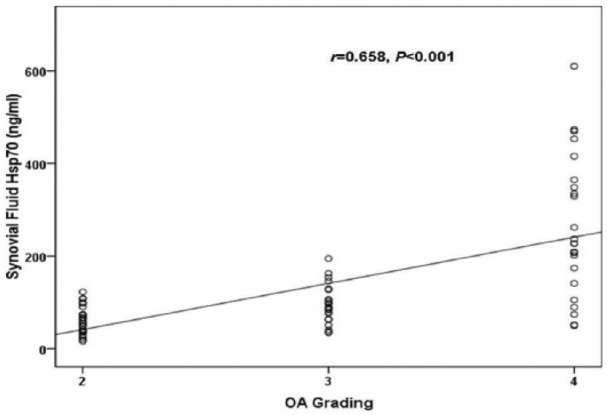
Scattergram showing the positive correlation between synovial fluid Hsp70 and the severity in patients with knee osteoarthritis (OA).
Figure 4.
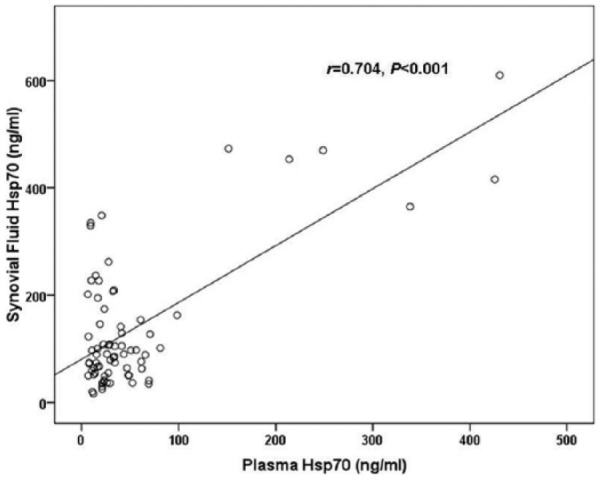
Scattergram showing the positive correlation between plasma and synovial fluid Hsp70 in patients with knee osteoarthritis (OA).
Discussion
Heat shock proteins or stress proteins have been shown to play functional parts in the pathogenic mechanism of autoimmune diseases as well as many human cancers. The most studied, Hsp70, contains both inducible and constitutively expressed proteins generally considered to be intracellular in nature.19 Hsp70 is 1 of 5 families of stress proteins able to act as molecular chaperones in a mechanism reliant on ATP and cofactors.20 This provides the means by which mammalian cells are able to bring about the regulation of proteolysis, in addition to protecting from varying environmental stresses.21,22 Hsp70 has been previously associated with OA development and progression, suggested by differing expression levels and distribution.23 The present study used enzyme immunoassay to quantify Hsp70 protein levels in circulating and joint fluid of OA subjects compared with control plasma.
Our findings demonstrated that circulating Hsp70 levels were significantly increased in subjects with primary knee OA. Additionally, it was shown that the plasma and synovial fluid levels were directly correlated with the radiographic severity of knee OA, agreeing with the conclusions drawn by Takahashi et al.1 Interestingly, the increase in protein level seen in synovial fluid was 3-fold higher than that of circulating plasma. The explanation for this finding could be due to local inflammation and/or oxidative stress in the knee joints of osteoarthritis patients.
Moreover, the potency of the increase in expression appears to be condition dependent. Najafizadeh et al.13 reported significant differences in Hsp70 protein concentrations in subjects with high activity rheumatoid arthritis (RA), or patients in remission. Whereas patients with low activity RA showed no observable concentration difference compared with healthy controls. Likewise, Schett et al.23 found increased levels of Hsp70 in synovial tissues of patients with RA, however this change was not observed in patients with OA. It is not known why plasma and synovial Hsp70 levels were seen to increase in osteoarthritis, while previously reported synovial tissue levels remain constant. Furthermore, Suzuki et al.12 reported elevated Hsp70 levels in synovial fluid samples extracted from patients with internal derangement of the temporomandibular joint; however no significant differences were observed. The differing levels of Hsp70 presented in the study, in particular the relationship existing between synovial fluid and plasma Hsp70 levels may suggest that Hsp70 might serve as a parameter reflecting OA severity. This proposal concurs with the data reported by Sedlackova et al.24 found the Hsp gene expression profiles to differ between inflammatory and noninflammatory joint disorders, suggesting a gene profile, involving Hsp70 to be implemented as a diagnostic tool to differentiate between RA, OA, and healthy individuals.
The Hsp70 protein concentration increase corresponds with mRNA expression changes, demonstrated by Takahashi et al,1 showing gene expression of Hsp47, Hsp70, and Hsp86 to be upregulated in early-stage OA. Additionally, Hsp70 was found to be associated to 2 highly expressed cytokines important in chondrocyte metabolism, suggesting that Hsp70 has a potential role as a diagnostic tool in OA,1 agreeing with the conclusions drawn by the present study.
The exact nature of Hsp70’s involvement in the development and progression of OA is unknown; however it may be linked to a change of localization within the cell. Takahashi et al.4 reported that, as OA progresses, Hsp70 was found in chondrocytes in the deeper layers of knee cartilage. Grossin et al.2 also found the expression of Hsp70 in deep-level chondrocytes in both experimental and in vitro conditions, linking the overexpression to the inhibition of endochondral ossification, reducing chondrocyte distribution. The change in distribution of Hsp70 may explain the differing levels seen between plasma, and synovial fluid. However, further investigations are warranted to explore this possibility.
In conclusion, the present study has found Hsp70 protein levels to be elevated in subjects with primary knee OA, and that the Hsp70 values can be directly correlated with the disease severity. Additionally, a positive relationship between circulating and joint fluid Hsp70 concentration was evident. We are aware that the cohort used for the study was small, and that a repeat would be needed, with a larger sample size in order to confirm the findings. Nonetheless, the findings presented, in combination with previous data suggest Hsp70 possessing a potential role as a prognostic biomarker for both tracking osteoarthritis, and acting as a clinical diagnostic tool; differentiating between the different joint-associated inflammatory conditions.
Footnotes
Acknowledgments and Funding: The authors thank Osteoarthritis and Musculoskeleton Research Unit, the Ratchadapiseksompotch Fund (Faculty of Medicine, Chulalongkorn University) and the Research Chair from National Science and Technology Development Agency (NSTDA). We are grateful to Dr. Wanvisa Udomsinprasert, Research facility of Department of Biochemistry and ChulaMRC for kindly providing technical assistance.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Approval: The experimental protocols were approved by the Ethical Committee on Human Research of our institution (533/54).
Informed Consent: All patients and healthy volunteers signed an informed consent prior to their participation in the study.
Trial Registration: Not applicable.
References
- 1. Takahashi K, Kubo T, Goomer RS, Amiel D, Kobayashi K, Imanishi J, et al. Analysis of heat shock proteins and cytokines expressed during the early stages of osteoarthritis in a mouse model. Osteoarthritis Cartilage. 1997;5:321-9. [DOI] [PubMed] [Google Scholar]
- 2. Grossin L, Cournil-Henrionnet C, Pinzano A, Gaborit N, Dumas D, Etienne S, et al. Gene transfer with HSP 70 in rat chondrocytes confers cytoprotection in vitro and during experimental osteoarthritis. FASEB J. 2006;20:65-75. [DOI] [PubMed] [Google Scholar]
- 3. Etienne S, Gaborit N, Henrionnet C, Pinzano A, Galois L, Netter P, et al. Local induction of heat shock protein 70 (Hsp70) by proteasome inhibition confers condroprotection during surgically induced osteoarthritis in the rat knee. Biomed Mater Eng. 2008;18:253-60. [PubMed] [Google Scholar]
- 4. Takahashi KA, Tonomura H, Arai Y, Terauchi R, Honjo K, Hiraoka N, et al. Hyperthermia for the treatment of articular cartilage with osteoarthritis. Int J Hyperthermia. 2009;25:661-7. [DOI] [PubMed] [Google Scholar]
- 5. Zhao L, Shen XY, Cao YL, Wang LZ, Deng HP, Zhang HM. Effects of laser irradiation on arthritic histopathology and heat shock protein 70 expression in C57 black mice with osteoarthritis. Zhong Xi Yi Jie He Xue Bao. 2011;9:761-7. [DOI] [PubMed] [Google Scholar]
- 6. Siebelt M, Jahr H, Groen HC, Sandker M, Waarsing JH, Kops N, et al. Hsp90 inhibition protects against biomechanically induced osteoarthritis in rats. Arthritis Rheum. 2013;65:2102-12. [DOI] [PubMed] [Google Scholar]
- 7. Sawatzky DA, Foster R, Seed MP, Willoughby DA. Heat-shock proteins and their role in chondrocyte protection, an application for autologous transplantation. Immunopharmacology. 2005;12:569-89. [DOI] [PubMed] [Google Scholar]
- 8. Fujita S, Arai Y, Nakagawa S, Takahashi KA, Terauchi R, Inoue A, et al. Combined microwave irradiation and intraarticular glutamine administration–induced HSP70 expression therapy prevents cartilage degradation in a rat osteoarthritis model. J Orthop Res. 2012;30:401-7. [DOI] [PubMed] [Google Scholar]
- 9. Takahashi K, Kubo T, Arai Y, Imanishi M, Kawata M, Hirasawa Y. Localization of heat shock protein in osteoarthritic cartilage. Scand J Rheumatol. 1997;26:368-75. [DOI] [PubMed] [Google Scholar]
- 10. Tonomura H, Takahashi KA, Mazda O, Arai Y, Shin-Ya M, Inoue A, et al. Effects of heat stimulation via microwave applicator on cartilage matrix gene and HSP70 expression in the rabbit knee joint. J Orthop Res. 2008;26:34-41. [DOI] [PubMed] [Google Scholar]
- 11. Kang EH, Kim DJ, Lee EY, Lee YJ, Lee EB, Song YW. Downregulation of heat shock protein 70 protects rheumatoid arthritis fibroblast-like synoviocytes from nitric oxide-induced apoptosis. Arthritis Res Ther. 2009;11:R130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Suzuki T, Segami N, Nishimura H, Hattori T, Nojima Analysis of 70Kd heat shock protein expression in patients with internal derangement of the temporomandibular joint. Int J Oral Maxillofac Surg. 2000;29:301-4. [PubMed] [Google Scholar]
- 13. Najafizadeh SR, Ghaizadeh Z, Nargesi AA, Mahdavi M, Abtahi S, Mirmiranpour H, et al. Analysis of serum heat shock protein 70 (HSPA1A) concentrations for diagnosis and disease activity monitoring in patients with rheumatoid arthritis. Cell Stress Chaperones. 2015;20:537-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dakappagari N, Neely L, Tangri S, Lundgren K, Hipolito L, Estrellado A, et al. An investigation into the potential use of serum Hsp90 as a novel tumour biomarker for Hsp90 inhibitors. Biomarkers. 2010;15:31-8. [DOI] [PubMed] [Google Scholar]
- 15. Hiraoka N, Arai Y, Takahashi KA, Mazda O, Kishida T, Honjo K, et al. Mild electrical stimulation with heat stimulation increase heat shock protein 70 in articular chondrocyte. J Orthop Res. 2013;31:894-900. [DOI] [PubMed] [Google Scholar]
- 16. Luo X, Zuo X, Zhou Y, Zhang B, Shi Y, Liu M, et al. Extracellular heat shock protein inhibits tumour necrosis factor-alpha induced proinflammatory mediator production in fibroblast-like synoviocytes. Arthritis Res Ther. 2008;10:R41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kubo T, Towle CA, Mankin HJ, Treadwell BV. Stress-induced proteins in chondrocytes from patients with osteoarthritis. Arthritis Rheum. 1985;28:1140-45. [DOI] [PubMed] [Google Scholar]
- 18. Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16:494-502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nguyen TT, Gehrmann M, Zlacka D, Sosna A, Vavrincova P, Multhoff G, et al. Heat shock protein 70 membrane expression on fibroblast-like synovial cells derived from synovial tissue of patients with rheumatoid and juvenile idiopathic arthritis. Scand J Rheumatol. 2006;35:447-53. [DOI] [PubMed] [Google Scholar]
- 20. Kumar S, Stokes J, 3rd, Singh UP, Gunn KS, Acharya A, Manne U, et al. Targetting Hsp70: a possible therapy for cancer. Cancer Lett. 2016;374:156-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Guzhova IV, Shevtsov MA, Abkin SV, Pankratova KM, Margulis BA. Intracellular and extracellular Hsp70 chaperone as a target for cancer therapy. Int J Hyperthermia. 2013;29:399-408. [DOI] [PubMed] [Google Scholar]
- 22. Ito A, Aoyama T, Tajino J, Nagai M, Yamaguchi S, Iijima H, et al. Effects of the thermal environment on articular chondrocyte metabolism: a fundamental study to facilitate establishment of an effective thermotherapy for osteoarthritis. J Jpn Phys Ther Assoc. 2014;17:14-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Schett G, Redlich K, Xu Q, Bizan P, Gröger M, Tohidast-Akrad M, et al. Enhanced expression of heat shock protein 70 (hsp70) and heat shock factor 1 (HSF1) activation in rheumatoid arthritis synovial tissue. Differential regulation of hsp70 expression and hsf1 activation in synovial fibroblasts by proinflammatory cytokines, shear stress, and antiinflammatory drugs. J Clin Invest. 1998;102:302-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sedlackova L, Sosna A, Vavrincova P, Frýdl J, Guerriero V, Raynes DA, et al. Heat shock protein gene expression profile may differentiate between rheumatoid arthritis, osteoarthritis, and healthy controls. Scand J Rheumatol. 2011;40:354-7. [DOI] [PubMed] [Google Scholar]


