Abstract
Objective
Osteochondritis dissecans (OCD) is a knee disorder of predominately pediatric populations. Because of low incidence, it has traditionally been difficult to study OCD. The purpose of this study was to report long-term outcomes of skeletally immature OCD lesions and determine risk factors for persistent knee pain at final follow-up.
Design
A geographic database of more than 500,000 patients was reviewed to identify patients with knee OCD. Clinical course including operative management, persistent knee pain, and total knee arthroplasty (TKA) were analyzed through review of radiographs, magnetic resonance images, and physician notes.
Results
A total of 95 skeletally immature patients (70 male, 25 female, mean age 12.5 ± 2.0 years) were followed for a mean of 14 years (range, 2-40 years). Fifty-three patients were treated operatively and 42 were treated nonoperatively. At final follow-up, 13 patients noted persistent knee pain, 8 treated operatively versus 5 treated nonoperatively. Risk factors for knee pain were female gender, patellar lesions, and unstable lesions. Four patients (8%) treated operatively and 2 patients (5%) treated nonoperatively developed symptomatic osteoarthritis at a mean of 28.6 years following diagnosis. Three patients underwent TKA at a mean age of 52 years, significantly younger than that observed for primary TKA at our institution (P = 0.004).
Conclusions
Skeletally immature OCD patients have promising histories, with an estimated 14% risk of persistent knee pain, 6% symptomatic osteoarthritis, and 3% conversion to TKA at 14 years’ mean follow-up. Females, patellar lesions, and unstable lesions demonstrated increased persistent knee pain risk. Patients with OCD undergo TKA at a significantly younger age than the general population.
Keywords: knee, articular cartilage, procedures, nonsurgical therapy, osteoarthritis
Introduction
Osteochondritis dissecans (OCD) is a disorder commonly affecting the knee of pediatric populations, with particular predilection for the medial femoral condyle.1,2 OCD has an incidence of approximately 0.03% in males and 0.02% in females aged 10 to 20 years old.3,4 Although the underlying pathogenesis is not completely understood, lesions are characterized by focal osseous necrosis, collapse, and subsequent destabilization of overlying articular cartilage.2,5,6 Etiologies including vascular disruption,2,6 microtrauma,7 and hereditary factors8 have been proposed as lesions can histologically resemble avascular necrosis (AVN), demonstrate scintographic findings similar to traumatic fractures, and 14% of OCD patients have a family history of OCD.
Management of OCD lesions remains controversial, and while the general treatment algorithm for clinical decisions regarding operative and nonoperative management is fairly well accepted, significant room exists for interpretation on a case-by-case basis. Current studies recommend complex decision making taking into account history, physical exam findings, and magnetic resonance imaging (MRI).1,9 To further complicate management, the reliability and accuracy of MRI in identifying lesions as stable or unstable, especially in the skeletally immature population, has recently been brought into question in studies correlating imaging and arthroscopic findings.10
If left untreated, focal OCD can progress to early arthritis, pain, and disability.9 It has been previously estimated that four percent of primary knee osteoarthritis in men is caused by previously undiagnosed OCD lesions.3 However, few studies have reported the rate of OCD progression to arthritis or compared the long-term effects of operative versus nonoperative management. Additionally, most studies have been limited by small sample size and short duration of follow-up due to the relative rarity of the condition.11-14 Considering the risk for knee pain and progression to early total knee arthroplasty, better understanding of the long-term outcomes of operative and nonoperative management of OCD lesions is necessary.
The purpose of this study, therefore, was to (1) to evaluate the rate of persistent knee pain, symptomatic osteoarthritis and conversion to total knee arthroplasty (TKA) in a skeletally immature population with OCD and (2) to determine factors associated with persistent knee pain at time of final follow-up. Our hypotheses were that (1) patients with OCD of the knee would have a clinically significant risk of symptomatic knee pain at final follow-up and undergo TKA at an earlier age than the general population and (2) previously established short-term risk factors such as unstable lesions and patellar location would predict worse outcomes.
Methods
Patients with OCD lesions were identified using the Rochester Epidemiology Project (REP), a collaborative database providing access to the complete medical records for all residents of Olmsted County, Minnesota, and neighboring areas. Patients authorize and can choose not to participate in health record disclosure for external use through Minnesota Statute 144.295. This database has previously been described in detail and allows for the determination of the true geographic demographics and incidence of diseases as well as provides records for associated surgical procedures through the use of physician-determined diagnostic codes and health record information.15 The study was conducted after approval from the institutional review board (15-005636).
Patients with OCD lesions were identified by using the International Classification of Diseases, Ninth Revision (ICD-9) diagnosis code for OCD (732.7) in a period ranging from 1976 to 2015. Following identification of n = 95 potential patients, the record for each subject was manually reviewed to verify the accuracy of the diagnosis, note demographics including age, body mass index, and lesion laterality, as well as to capture details regarding treatment course on a standardized documentation form.
Inclusion criteria consisted of (1) symptomatic OCD lesions of the femoral condyle (medial or lateral), trochlea, or patella and (2) skeletally immature patients with open distal femoral and proximal tibial physes on plain radiographs. To be considered an OCD lesion, knee radiographs must have demonstrated a radiolucent line separating an osteochondral segment in the femur or patella from the underlying bone or MRI showing high-signal intensity between an osteochondral segment and the underlying bone. Patients that had closed physes at the time of diagnosis were excluded from analysis. On manual review, all 95 patients were confirmed to have met criteria for inclusion in this study.
Plain radiographs at time of diagnosis were reviewed to describe lesions according to guideline established by the Research in Osteochondritis of the Knee (ROCK) group16 and MRI scans at the time of presentation were evaluated by a musculoskeletal-trained radiologist and verified by a senior orthopedic resident to evaluate lesion size and factors associated with lesion stability (edema, cystic changes, breach in articular cartilage, and lesion detachment) according to the De Smet classification.17 Lesions were defined as radiographically unstable if plain films or MRI images showed evidence of lesion detachment, cystic changes under the lesion, high signal intensity under the lesion on MRI, or breach of the articular surface with high signal intensity traversing the cartilage layer on MRI.
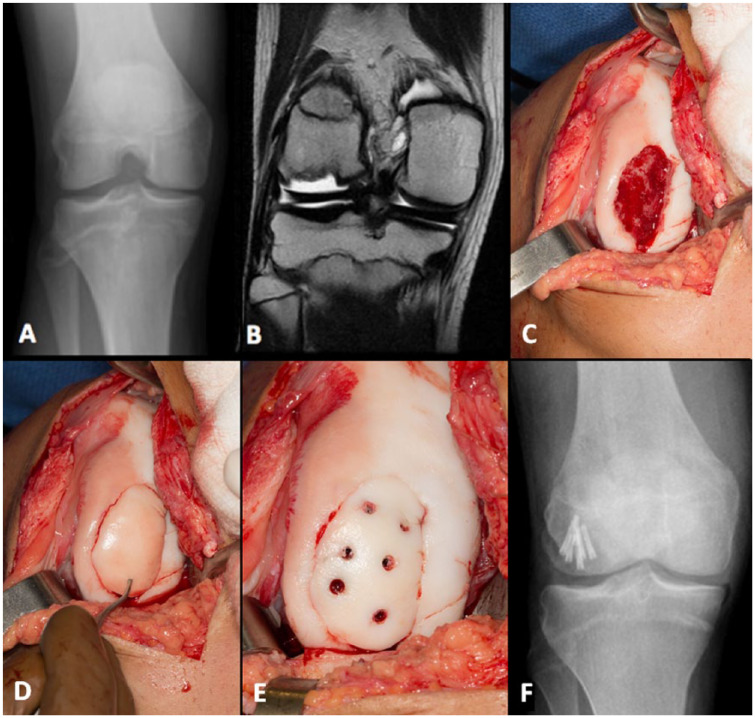
Surgical procedures captured included fragment excision, fragment preservation (lesion drilling and/or fragment fixation), and chondral defect grafting (osteochondral allograft or autograft). When employed, OCD lesion drilling was performed using multiple trans-articular drill holes. Fragment preservation was attempted when intraoperative tissue quality and subchondral bone stock was deemed to be sufficient for headless compression screw fixation with viable articular cartilage ( Fig. 1 ). Treatment decisions were made on an individualized basis by the treating surgeon.
Figure 1.
Preoperative (A) radiograph and (B) magnetic resonance image of a large lateral femoral condyle lesion with subsequent (C) intraoperative lesion bed visualization and preparation, (D) fragment reduction, and (E) fixation. (F) Postoperative radiograph demonstrating fragment fixation. Patient subsequently returned to competitive basketball with no pain.
Following initial operative or nonoperative management, medical records were reviewed to determine whether patients developed persistent knee pain, symptomatic osteoarthritis, or went on to TKA. Persistent knee pain was defined as pain or soreness, with or without catching, locking, or swelling, that caused limitations in activities of daily living or prevented the patient from participating in sports or recreational activities. Symptomatic osteoarthritis was defined as physician-diagnosed osteoarthritis with supportive radiographs demonstrating degenerative Grade 2+ changes in the tibiofemoral or patellofemoral joint as reported by the Kellgren-Lawrence grading system.
Statistical Analysis
Demographic variables and their associated standard deviations and percentages were reported for descriptive representation of the study cohorts. Operative and nonoperative groups were compared to evaluate for potential differences using Fisher’s exact test for proportions and the Kruskall-Wallis test for continuously distributed and ordinal variables. Cox proportional hazards regression was performed to determine risk factors for symptomatic knee pain at the time of final follow-up. P values <0.05 were considered significant. Analyses were conducted in R 3.4.1 (R Core Team, Vienna, Austria).
Results
Ninety-five skeletally immature patients with confirmed OCD lesions, 70 males and 25 females, were included in the study. Mean age at the time of diagnosis was 12.5 ± 2.0 years (range, 7-16 years, Table 1 ). Patients were followed for a mean of 13.8 years (range, 2-40 years). Lesions occurred most commonly on the medial femoral condyle (MFC, 82%) followed by the lateral femoral condyle (LFC, 12%), patella (4%), and then trochlea (2%) ( Table 2 ). Eighty patients (84%) were playing sports at the time of diagnosis. Overall, 53 patients (56%) were treated operatively and 42 patients (44%) were treated nonoperatively. 49.1% of lesions in the operative group were unstable as determined by the De Smet classification, while 38.1 % of lesions in the nonoperative group were unstable (P = 0.39, Table 3 ).
Table 1.
Demographics of Skeletally Immature Study Patients with Osteochondritis Dissecans of the Knee.a
| Variable | Operative (n = 53) | Nonoperative (n = 42) | P |
|---|---|---|---|
| Male | 38 (71.7) | 31 (73.8) | 1.00 |
| Age (years) | 12.8 ± 1.9 | 12.0 ± 2.0 | 0.05 |
| Body mass index (kg/m2) | 21.7 ± 4.4 | 23.0 ± 5.6 | 0.32 |
| Laterality | |||
| Left | 30 (56.6) | 18 (42.9) | |
| Right | 23 (43.4) | 24 (57.1) | 0.26 |
| Race | |||
| White | 46 (86.8) | 36 (85.7) | |
| Black | 1 (1.9) | 2 (4.8) | |
| Unknown | 6 (11.3) | 4 (9.5) | 0.71 |
| Playing sports at diagnosis | 45 (84.9) | 35 (83.3) | 1.00 |
| Mechanism of injury | |||
| Sports | 42 (79.2) | 33 (78.6) | |
| Trauma | 3 (5.7) | 4 (9.5) | |
| Other | 8 (15.1) | 5 (11.9) | 0.72 |
Values are presented as mean ± standard deviation or n (%).
Table 2.
X-Ray and Research in Osteochondritis of the Knee (ROCK) Lesion Characteristics for Operatively and Nonoperatively Treated Osteochondritis Dissecans Lesions.a
| Variable | Operative | Nonoperative | P b |
|---|---|---|---|
| Lesion location | |||
| MFC | 69.2 | 97.6 | |
| LFC | 19.2 | 2.4 | |
| Patella | 7.7 | 0.0 | |
| Trochlea | 3.8 | 0.0 | <0.01 |
| AP radiograph measurements | |||
| Width (mm) | 15.3 ± 4.0 | 13.0 ± 4.9 | 0.05 |
| Depth (mm) | 4.4 ± 1.6 | 4.1 ± 2.0 | 0.46 |
| Lateral radiograph measurements | |||
| Width (mm) | 18.6 ± 4.6 | 14.6 ± 6.4 | <0.01 |
| Depth (mm) | 4.9 ± 2.2 | 4.2 ± 2.3 | 0.21 |
| Location | |||
| Anterior | 4.3 | 17.4 | |
| Posterior | 95.7 | 82.6 | 0.34 |
| Parent bone radiodensity | |||
| Same | 100.0 | 100.0 | |
| Different | 0.0 | 0.0 | 1.00 |
| Progeny bone | |||
| Fragmented | 16.7 | 0.0 | |
| Displaced | 8.3 | 0.0 | |
| Distinct from lesion | 54.2 | 54.2 | 0.08 |
| Lesion contour | |||
| Convex | 58.3 | 91.7 | |
| Concave | 41.7 | 8.3 | <0.01 |
AP = anterior-posterior; LFC = lateral femoral condyle; MFC = medial femoral condyle.
Values provided as percentage of patients in whom corresponding x-ray views were available or as mean ± standard deviation.
Comparisons made using Fisher’s exact test to simultaneously evaluate proportions (i.e., location) between operative and nonoperative management. Continuous variables (AP and lateral measurements) evaluated using the Kruskall-Wallis test. P values in boldface indicate statistical significance.
Table 3.
MRI Characteristics of Operatively and Nonoperatively Treated OCD Lesions.a
| MRI Characteristic | Operative | Nonoperative | P b |
|---|---|---|---|
| Increased T2 signal intensity | 100.0 | 100.0 | 1.00 |
| High signal at fragment/femur interface | 88.0 | 92.9 | 1.00 |
| Disruption of subchondral bone plate | 56.0 | 14.3 | 0.03 |
| Adjacent focal cystic areas | 28.0 | 42.9 | 0.56 |
| Intra-articular fragment displacement | 32.0 | 0.0 | 0.0499 |
| Focal articular cartilage defects adjacent to lesion (≥5 mm in width) | 32.0 | 0.0 | 0.0499 |
| Line of high signal (≥5 mm) between OCD lesion and underlying bone | 64.0 | 64.3 | 1.00 |
| Discrete area of high signal (≥5 mm) beneath OCD lesion | 20.0 | 14.3 | 0.99 |
| High signal line traversing cartilage and subchondral bone into lesion | 40.0 | 7.1 | 0.07 |
| Lesion stability | |||
| Unstable | 49.1 | 38.1 | 0.39 |
| Stable | 50.9 | 61.9 |
MRI = magnetic resonance imaging; OCD = osteochondritis dissecans.
Values provided as percentage of patients in whom corresponding MRI imaging was available.
P values in boldface indicate statistical significance.
Operative Group
Thirty-nine males (74%) and 14 females (26%) with a mean age of 12.8 years (range, 7-16 years) were treated operatively and followed for a mean of 14.4 years (range, 2-40 years). Twenty-five patients (40%) underwent repair procedures (drilling, fixation), 1 patient underwent osteochondral allograft (OCA) transplantation for a 5.6 cm2 defect, and 27 patients (51%) underwent palliative procedures. Of those undergoing palliative procedures, 11 patients underwent loose body removal, 8 underwent fragment excision, and 8 underwent isolated debridement / chondroplasty. At the time of final follow-up, a total of 8 patients had symptomatic knee pain, 5 (16%) in the palliative group and 3 patients (13.6%) in the restorative group. During the course of follow-up, 13 patients (25%) had repeat interventions. Of these, 5 were removals of hardware, 2 received fragment fixation, 1 patient was salvaged with a combined OCA and valgus producing osteotomy, 4 were removals of newly formed loose bodies, and 1 underwent isolated debridement. At a mean of 25.1 years postoperatively, 4 patients developed symptomatic osteoarthritis, 2 from the palliative group (mean age, 49.3 years) and 2 from the restorative group (mean age, 24.4 years). Of these, 1 patient from the palliative group converted to TKA at 39.6 years following the time of initial diagnosis at an age of 52.7 years. The 10-, 20-, and 30-year survival free from osteoarthritis was 100%, 91.4%, and 91.4%, respectively. The 10-, 20-, and 30-year survival free from TKA was 100%, 100%, and 100%, respectively.
Nonoperative Group
Thirty-one males (74%) and 11 females (26%) with a mean age of 12.0 years (range, 7-15) were treated nonoperatively and followed for a mean of 13.0 years (range, 2-38 years). Seven patients were immobilized and made nonweightbearing, 32 patients were given a brace, and 13 patients were given an assistance device to walk. At the time of final follow-up, a total of 4 patients had symptomatic knee pain. At a mean of 33.5 years postoperatively, 2 patients had developed symptomatic osteoarthritis. Of these, both converted to TKA, 1 at 35.0 years of follow-up, and the other at 38.0 years. Thus, the 10-, 20-, and 30-year survival free from both osteoarthritis and TKA was 100%, 100%, and 100%, respectively.
In total, 3 patients managed operatively or nonoperatively converted to total knee arthroplasty at a mean age of 51.5 ± 2.6 years, which is statistically below (P = 0.004) our latest available institutional average age of 68.0 ± 10.0 years for primary TKA in the 2002-2005 period.18
Risk Factors for Symptomatic Knee Pain at Final Follow-Up
Demographic variables and factors, which differed between the operative and nonoperative groups, such as lesion location were assessed for predictive value for symptomatic knee pain at the time of final follow-up using Cox proportional hazards modeling. It was determined that males were at a decreased risk of symptomatic knee pain (hazard ratio [HR] = 0.24, 95% confidence interval [CI] = 0.07-0.81, P = 0.02) whereas patellar lesion location (HR = 5.30, 95% CI = 1.37-20.48, P = 0.02) and unstable lesions according to the De Smet criteria (HR = 10.58, 95% CI = 1.26-88.63, P = 0.03) were associated with an increased risk of symptomatic knee pain at final follow-up ( Table 4 ). Operative versus nonoperative treatment, patient age, lesion width, depth, and contour, subchondral bone disruption, intra-articular fragment displacement, and adjacent cartilage defects did not predict symptomatic knee pain. In a subanalysis of the individual operative and nonoperative management groups, unstable lesions trended toward predicting knee pain at final follow-up in the operative cohort (HR = 4.73, 95% CI = 0.46-48.05, P = 0.19) whereas unstable lesions remained highly significant in predicting knee pain in the nonoperative cohort (HR > 1000, P < 0.001).
Table 4.
Risk Factors for Persistent Knee Pain at the Time of Final Clinical Follow-Up.
| Variable | HR (95% CI) | P a |
|---|---|---|
| Treatment | ||
| Nonoperative | Reference | |
| Operative | 1.16 (0.35-3.83) | 0.81 |
| Age at diagnosis, years | ||
| <10 | Reference | |
| ≥10 | 10.3 (0.11-9.63) | 0.98 |
| Gender | ||
| Female | Reference | |
| Male | 0.24 (0.07-0.81) | 0.02 |
| Location | ||
| MFC | Reference | |
| LFC | 2.91 (0.57-14.97) | 0.20 |
| Patella | 5.30 (1.37-20.48) | 0.02 |
| Trochlea | 0.00 (0.00-0.00)b | <0.01 b |
| AP radiograph lesion width, mm | ||
| <20 | Reference | |
| ≥20 | 1.74 (0.23-12.97) | 0.59 |
| AP radiograph lesion depth, mm | ||
| <5 | Reference | |
| ≥5 | 0.68 (0.08-6.00) | 0.73 |
| Lateral radiograph lesion width, mm | ||
| <20 | Reference | |
| ≥20 | 1.75 (0.31-10.01) | 0.53 |
| Lateral radiograph lesion depth, mm | ||
| <5 | Reference | |
| ≥5 | 2.14 (0.38-11.95) | 0.38 |
| Lesion contour | ||
| Concave | Reference | |
| Convex | 0.45 (0.08-2.54) | 0.37 |
| Disruption of subchondral bone | ||
| No | Reference | |
| Yes | 0.73 (0.15-3.54) | 0.70 |
| Intra-articular displaced fragment | ||
| No | Reference | |
| Yes | 0.85 (0.10-7.48) | 0.88 |
| Adjacent focal articular cartilage defects | ||
| No | Reference | |
| Yes | 0.85 (0.10-7.48) | 0.88 |
| Stability | ||
| Stable | Reference | |
| Unstable | 10.58 (1.26-88.63) | 0.03 |
AP = anterior-posterior; CI = confidence interval; HR = hazard ratio; LFC = lateral femoral condyle; MFC = medial femoral condyle.
P values in boldface indicate statistical significnace.
Of the n = 2 lesions present on the trochlea, none developed symptomatic knee pain. The estimated hazard ratio for trochlear location should be interpreted with caution given the limited number of trochlear lesions.
Discussion
OCD is a disorder that affects pediatric populations and can lead to long-term consequences including persistent pain, early arthritis, and TKA. The purpose of this study was to evaluate the rates of symptomatic knee pain, clinical osteoarthritis, and subsequent surgical interventions in a skeletally immature population with OCD and to determine factors associated with symptomatic knee pain at the time of final follow-up. Our hypothesis was supported in that patients with OCD demonstrated overall positive clinical outcomes while demonstrating low but clinically significant rates of knee pain at final follow-up as well as undergo TKA at a younger age than observed for the primary TKA population at our institution. Additionally, unstable and patellar lesions as well as female gender were found to predict symptomatic knee pain at the time of final follow-up.
Because of the relative rarity of OCD, little is documented regarding the long-term expected clinical course of lesions managed operatively and nonoperatively. The rates of radiographic arthritis and conversion to TKA observed at a mean of 14 years of follow-up in this study are reassuring and fall at or below previous estimates for skeletally mature and mixed maturity populations, with previously available studies trending toward lower rates of arthritis with younger patient age at presentation.13,19,20 The causes for this are likely 2-fold, namely skeletal immaturity plays a protective role in natural disease history and additionally, any effects that might hasten the rate at which patients undergo total knee arthroplasty are better captured in older patients. The longest currently available mean follow-up in the OCD literature is 33.6 years for a series of 22 knees.21 However, these results outline the clinical history of initial presentations between 1953 and 1971 and are the product of now antiquated methods of OCD treatment including patellectomy.20 Continued surveillance of our reported group, which we believe to be the largest and longest followed contemporary cohort of skeletally immature patients, will further outline the long-term history of OCD lesions in the knee in the setting of modern techniques.
In this study, unstable lesions were found to predict persistent pain at the time of final follow-up. It is important to note that unstable lesions likely represent more severe forms of pathology, with larger fragments, less intact subchondral bone, and higher contact forces, making them unlikely to undergo spontaneous healing. The presence of unstable lesions as an indication for surgery and potential for poor outcomes if left untreated has been previously described.11,17 This is supported in our data, which suggest that the risk of persistent symptomatic pain in patients with nonoperative management of unstable lesions is significantly higher than that of unstable lesions undergoing operative management. It is important to note that there is likely a degree of confounding present, with operative lesions implying worse pathology than can be captured by stable versus unstable categorization. The fact that 38% of the nonoperative group had unstable lesions highlights the room for interpretation present from provider to provider with regard to OCD management strategies as well as the presence of shared decision making with patients with regard to preference in pursuing operative versus nonoperative management. The observed trend of unstable lesions predicting persistent pain despite of operative management (HR = 4.73, P = 0.19) is also in accordance with literature that suggests that even though 92% of patients with unstable lesions undergoing open reduction internal fixation go on to stable union, knee function and subjective outcome scores do not return to normal, even at a mean of 9.2 years of follow-up.11
Patellar lesions as a risk factor for continued symptomatology is a relatively novel finding of this study. Previous literature has suggested that medial condyle lesions demonstrate favorable natural histories whereas lateral condyle lesions fare worse than medial condyle and patellofemoral lesions.21,22 In contrast, Kramer et al.23 suggested that patients with patellofemoral OCD lesions demonstrate poor clinical outcomes due to the high forces present at the joint and that lesions in this area may heal more poorly than those on the femoral condyles. Biomechanical analyses have certainly demonstrated patellofemoral forces parallel and exceed those found at the tibiofemoral articulation, supporting that the patella is a high-stress location for healing to occur.24,25 Of note, the authors believe that a key component to successful management of patellar lesions is addressing any underlying malalignment and instability. While patellar cartilage lesions have demonstrated historically poor outcomes, positive results have been published in contemporary series employing concomitant biomechanical normalization procedures such as tibial tubercle osteotomy.26,27
The finding that female gender was a risk factor for pain at final follow-up has been previously documented. In a series of 26 patients with a mean of 3.8 years of follow-up, female patients demonstrated a 9-fold increased risk of persistent pain following surgical management.23 Additionally, a nonoperative MRI study trended toward increased healing rates in males following both 6 and 12 months of nonoperative management.28 Further research regarding the role of gender in determining outcomes is needed, however, there is likely some degree of interplay with skeletal immaturity, which is prolonged in males and has been consistently demonstrated to be an independent predictor of positive outcomes.29-31 It is also noteworthy that the observed 2.8-fold predominance of males in this study matches previous literature estimating that males are 2.0 to 3.8-fold more common than females in the OCD population.3,32
The current study has important limitations. Because of the rarity of OCD and young age of patients at diagnosis, outcomes measuring progression of radiographic arthritis, the effect of operative management, and conversion to total knee arthroplasty require extended periods of follow-up and are limited by patient volumes. The current series followed 95 patients for a mean of 14 years and maximum of 40 years, resulting in 3 patients converting to TKA. Because of this, we elected to undertake univariate proportional hazards modeling for symptomatic knee pain, which was present in 8 of the operative patients and 4 of the nonoperative patients at the time of final follow-up for a total of 12 patients. Previous literature has suggested that approximately 5 to 10 events are required per variable modeled for a reasonably well-powered analysis.33,34 Additionally, because of the nature of the epidemiological project from which our data were obtained, the accuracy of diagnosis is dependent on accurate record keeping, as is the case in any retrospective review. By employing ICD-9 codes to identify OCD cases, there may be a selection bias for operative cases as clinical coding is often more specific following operative management than for nonoperative clinic visits. Additionally, patients with symptomatology not warranting clinical presentation and subsequent documentation are not captured in the database, leading to some degree of underrepresentation of clinically mild OCD.
Conclusion
Skeletally immature patients with OCD of the knee demonstrate clinically promising outcomes at a mean of 14 years’ follow-up, with persistent knee pain rates at or less than 15%, symptomatic osteoarthritis rates at or less than 8% and conversion to total knee surgery less than 5%, regardless of initial operative or nonoperative management. However, unstable lesions, patellar lesion location, and female gender are risk factors for persistent knee pain at the time of final follow-up. While operative intervention appears to decrease the risk of persistent knee pain, patients presenting with unstable pathology tend to have worse outcomes than those presenting with stable lesions. As such, we recommend clinical counseling for patients in higher-risk categories and continued long-term orthopedic follow-up for patients with symptomatic OCD lesions.
Footnotes
Authors’ Note: The findings and conclusions of this report are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
Acknowledgments and Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was made possible by the Rochester Epidemiology Project, which is supported by the National Institute on Aging of the National Institutes of Health under award number R01AG034676.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Todd A. Milbrandt is a board or committee member of the American Academy of Orthopedic Surgeons, Pediatric Orthopaedic Society of North America, and the Scoliosis Research Society. He is a paid consultant for Orthopediatrics, receives stock or stock options from Viking Scientific, and receives other financial support from Broadwater. Bruce A. Levy receives research support from Arthrex, Biomet, Smith & Nephew, and Stryker. He serves on the editorial or governing board of the Journal of Arthroscopy, Clinical Orthopedics and Related Research, Journal of Knee Surgery, and Knee Surgery, Sports Traumatology, Arthroscopy. He is a paid consultant or receives royalties from Arthrex and Smith & Nephew. Michael J. Stuart receives research support from Stryker. He serves on the editorial or governing board for American Journal of Sports Medicine and is a paid consultant for Arthrex. Daniel B. F. Saris receives research support from Arthrex, Ivy Sports, and Smith & Nephew. He serves on the editorial or governing board for Cartilage. He is a paid consultant for Cartiheal, Smith & Nephew, and Vericel. Aaron J. Krych receives research support from Aesculap/B. Braun, the Arthritis Foundation, Ceterix, and Histogenics. He serves on the editorial or governing board for American Journal of Sports Medicine and is a board or committee member of the International Cartilage Repair Society, International Society of Arthroscopy, Knee Surgery, and Orthopedic Sports Medicine, Minnesota Orthopedic Society, and Musculoskeletal Transplantation Foundation. He receives royalties or is a consultant of Arthrex and Vericel. Mario Hevesi, Thomas L. Sanders, and Ayoosh Pareek have no relevant disclosures.
Ethical Approval: This study was conducted after approval from the institutional review board (15-005636).
Informed Consent: Informed consent was not sought for the present study because it is not required for data from the Rochester Epidemiology Project and is rather governed by Minnesota Research Authorization law.
Trial Registration: Not applicable.
ORCID iD: Todd A. Milbrandt  https://orcid.org/0000-0001-9950-3668
https://orcid.org/0000-0001-9950-3668
Aaron J. Krych  https://orcid.org/0000-0003-3248-8007
https://orcid.org/0000-0003-3248-8007
References
- 1. Erickson BJ, Chalmers PN, Yanke AB, Cole BJ. Surgical management of osteochondritis dissecans of the knee. Curr Rev Musculoskelet Med. 2013;6(2):102-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Grimm NL, Weiss JM, Kessler JI, Aoki SK. Osteochondritis dissecans of the knee: pathoanatomy, epidemiology, and diagnosis. Clin Sports Med. 2014;33(2):181-8. [DOI] [PubMed] [Google Scholar]
- 3. Linden B. The incidence of osteochondritis dissecans in the condyles of the femur. Acta Orthop Scand. 1976;47(6):664-7. [DOI] [PubMed] [Google Scholar]
- 4. Pareek A, Sanders TL, Wu IT, Larson DR, Saris DBF, Krych AJ. Incidence of symptomatic osteochondritis dissecans lesions of the knee: a population-based study in Olmsted County. Osteoarthritis Cartilage. 2017;25(10):1663-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Edmonds EW, Polousky J. A review of knowledge in osteochondritis dissecans: 123 years of minimal evolution from König to the ROCK study group. Clin Orthop Relat Res. 2013;471(4):1118-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shea KG, Jacobs JC, Jr, Carey JL, Anderson AF, Oxford JT. Osteochondritis dissecans knee histology studies have variable findings and theories of etiology. Clin Orthop Relat Res. 2013;471(4):1127-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cahill BR, Berg BC. 99m-Technetium phosphate compound joint scintigraphy in the management of juvenile osteochondritis dissecans of the femoral condyles. Am J Sports Med. 1983;11(5):329-35. [DOI] [PubMed] [Google Scholar]
- 8. Gornitzky AL, Mistovich RJ, Atuahuene B, Storey EP, Ganley TJ. Osteochondritis dissecans lesions in family members: does a positive family history impact phenotypic potency? Clin Orthop Relat Res. 2017;475(6):1573-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pascual-Garrido C, McNickle AG, Cole BJ. Surgical treatment options for osteochondritis dissecans of the knee. Sports Health. 2009;1(4):326-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ellermann JM, Donald B, Rohr S, Takahashi T, Tompkins M, Nelson B, et al. Magnetic resonance imaging of osteochondritis dissecans: validation study for the ICRS classification system. Acad Radiol. 2016;23(6):724-9. [DOI] [PubMed] [Google Scholar]
- 11. Magnussen RA, Carey JL, Spindler KP. Does operative fixation of an osteochondritis dissecans loose body result in healing and long-term maintenance of knee function? Am J Sports Med. 2009;37(4):754-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wright RW, McLean M, Matava MJ, Shively RA. Osteochondritis dissecans of the knee: long-term results of excision of the fragment. Clinl Orthop Relat Res. 2004;(424):239-43. [PubMed] [Google Scholar]
- 13. Aglietti P, Ciardullo A, Giron F, Ponteggia F. Results of arthroscopic excision of the fragment in the treatment of osteochondritis dissecans of the knee. Arthroscopy. 2001;17(7):741-6. [DOI] [PubMed] [Google Scholar]
- 14. Baltzer AW, Ostapczuk MS, Terheiden HP, Merk HR. Good short- to medium-term results after osteochondral autograft transplantation (OAT) in middle-aged patients with focal, non-traumatic osteochondral lesions of the knee. Orthop Traumatol Surg Res. 2016;102(7):879-84. [DOI] [PubMed] [Google Scholar]
- 15. St Sauver JL, Grossardt BR, Leibson CL, Yawn BP, Melton LJ, 3rd, Rocca WA. Generalizability of epidemiological findings and public health decisions: an illustration from the Rochester Epidemiology Project. Mayo Clin Proc. 2012;87(2):151-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wall EJ, Polousky JD, Shea KG, Carey JL, Ganley TJ, Grimm NL, et al. Novel radiographic feature classification of knee osteochondritis dissecans: a multicenter reliability study. Am J Sports Med. 2015;43(2):303-9. [DOI] [PubMed] [Google Scholar]
- 17. De Smet AA, Fisher DR, Graf BK, Lange RH. Osteochondritis dissecans of the knee: value of MR imaging in determining lesion stability and the presence of articular cartilage defects. AJR Am J Roentgenol. 1990;155(3):549-53. [DOI] [PubMed] [Google Scholar]
- 18. Singh JA, Lewallen DG. Time trends in the characteristics of patients undergoing primary total knee arthroplasty. Arthritis Care Res (Hoboken). 2014;66(6):897-906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sanders TL, Pareek A, Obey MR, Johnson NR, Carey JL, Stuart MJ, et al. High rate of osteoarthritis after osteochondritis dissecans fragment excision compared with surgical restoration at a mean 16-year follow-up. Am J Sports Med. 2017;45(8):1799-1805. [DOI] [PubMed] [Google Scholar]
- 20. Aichroth P. Osteochondritis dissecans of the knee. A clinical survey. J Bone Joint Surg Br. 1971;53(3):440-7. [PubMed] [Google Scholar]
- 21. Twyman RS, Desai K, Aichroth PM. Osteochondritis dissecans of the knee. A long-term study. J Bone Joint Surg Br. 1991;73(3):461-4. [DOI] [PubMed] [Google Scholar]
- 22. Backes JR, Durbin TC, Bentley JC, Klingele KE. Multifocal juvenile osteochondritis dissecans of the knee: a case series. J Pediatr Orthop. 2014;34(4):453-8. [DOI] [PubMed] [Google Scholar]
- 23. Kramer DE, Yen YM, Simoni MK, Miller PE, Micheli LJ, Kocher MS, et al. Surgical management of osteochondritis dissecans lesions of the patella and trochlea in the pediatric and adolescent population. Am J Sports Med. 2015;43(3):654-62. [DOI] [PubMed] [Google Scholar]
- 24. Kaufman KR, An KN, Litchy WJ, Morrey BF, Chao EY. Dynamic joint forces during knee isokinetic exercise. Am J Sports Med. 1991;19(3):305-16. [DOI] [PubMed] [Google Scholar]
- 25. Cleather DJ, Goodwin JE, Bull AM. Hip and knee joint loading during vertical jumping and push jerking. Clin Biomech (Bristol, Avon). 2013;28(1):98-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Brittberg M, Lindahl A, Nilsson A, Ohlsson C, Isaksson O, Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994;331(14):889-95. [DOI] [PubMed] [Google Scholar]
- 27. Gomoll AH, Gillogly SD, Cole BJ, Farr J, Arnold R, Hussey K, et al. Autologous chondrocyte implantation in the patella: a multicenter experience. Am J Sports Med. 2014;42(5):1074-81. [DOI] [PubMed] [Google Scholar]
- 28. Krause M, Hapfelmeier A, Möller M, Amling M, Bohndorf K, Meenen NM. Healing predictors of stable juvenile osteochondritis dissecans knee lesions after 6 and 12 months of nonoperative treatment. Am J Sports Med. 2013;41(10):2384-91. [DOI] [PubMed] [Google Scholar]
- 29. De Smet AA, Ilahi OA, Graf BK. Untreated osteochondritis dissecans of the femoral condyles: prediction of patient outcome using radiographic and MR findings. Skeletal Radiol. 1997;26(8):463-7. [DOI] [PubMed] [Google Scholar]
- 30. Pill SG, Ganley TJ, Flynn JM, Milam RA, King PJ, Gregg JR. Osteochondritis dissecans of the knee: experiences at The Children’s Hospital of Philadelphia and a review of literature. Univ Penn Orthop J. 2001;14:25-33. [Google Scholar]
- 31. Linden B. Osteochondritis dissecans of the femoral condyles: a long-term follow-up study. J Bone Joint Surg Am. 1977;59(6):769-76. [PubMed] [Google Scholar]
- 32. Kessler JI, Nikizad H, Shea KG, Jacobs JC, Jr, Bebchuk JD, Weiss JM. The demographics and epidemiology of osteochondritis dissecans of the knee in children and adolescents. Am J Sports Med. 2014;42(2):320-6. [DOI] [PubMed] [Google Scholar]
- 33. Vittinghoff E, McCulloch CE. Relaxing the rule of ten events per variable in logistic and Cox regression. Am J Epidemiol. 2007;165(6):710-8. [DOI] [PubMed] [Google Scholar]
- 34. Peduzzi P, Concato J, Kemper E, Holford TR, Feinstein AR. A simulation study of the number of events per variable in logistic regression analysis. J Clin Epidemiol. 1996;49(12):1373-9. [DOI] [PubMed] [Google Scholar]