Abstract
Head and neck squamous cell carcinoma (HNSCC) has a poor 5-year survival rate of 50%. One potential reason for treatment failure is the presence of cancer stem cells (CSCs). Several cell markers, particularly CD44, have been used to isolate CSCs. However, isolating a pure population of CSC in HNSCC still remains a challenging task. Recent findings show that normal oral stem cells were isolated using CD271 as a marker. Thus, we investigated the combined use of CD271 and CD44 to isolate an enriched subpopulation of CSCs, followed by their characterization in vitro, in vivo, and in patients’ tissue samples. Fluorescent-activated cell sorting was used to isolate CD44+/CD271+ and CD44+/CD271− from two human HNSCC cell lines. Cell growth and self-renewal were measured with MTT and sphere/colony formation assays. Treatment-resistance was tested against chemotherapy (cisplatin and 5-fluorouracil) and ionizing radiation. Self-renewal, resistance, and stemness-related genes expression were measured with qRT-PCR. In vivo tumorigenicity was tested with an orthotopic immunodeficient mouse model of oral cancer. Finally, we examined the co-localization of CD44+/CD271+ in patients’ tissue samples. We found that CD271+ cells were a subpopulation of CD44+ cells in human HNSCC cell lines and tissues. CD44+/CD271+ cells exhibited higher cell proliferation, sphere/colony formation, chemo- and radio-resistance, upregulation of CSCs-related genes, and in vivo tumorigenicity when compared to CD44+/CD271− or the parental cell line. These cell markers showed increased expression in patients with the increase of the tumor stage. In conclusion, using both CD44 and CD271 allowed the isolation of CSCs from HNSCC. These enriched CSCs will be more relevant in future treatment and HNSCC progression studies.
Introduction
Cancer is the second leading cause of death in the USA and the first cause in Canada, as it is responsible for over 30% of all deaths annually (1,2). Head and neck squamous cell carcinoma (HNSCC) is the seventh most common cancer worldwide, as it accounts for over 580,000 new diagnosed cases in 2018 (3). In Canada, 5850 new cancer patients were diagnosed with HNSCC and it was responsible for 1690 deaths in 2017 (1). Despite recent advances for diagnosis and cancer treatment, the current prognosis for HNSCC is poor due to relapse in the form of local recurrence or metastasis. The 5-year survival rate has remained approximately 50% for the last three decades (4).
One reason for cancer treatment failure is considered to be related to the presence of a subpopulation of cells in the tumor called “cancer stem cells” (CSCs), which are suggested to have tumor-initiating potential combined with the ability of self-renewal and multilineage differentiation (5). Acute myeloid leukemia was the first malignancy that was discovered to contain CSCs; this was followed by the discovery in multiple tumor types, including lung, breast, brain, liver, pancreas and colon cancers (6,7). CSCs share some of the characteristics of normal stem cells such as the ability to undergo self-renewal, maintain quiescence, show multipotentiality, and exert survival/anti-apoptosis proteins (7). In some tumors, CSCs were linked to chemo-resistance (8), radio-resistance (9), recurrence (10), and metastasis (11). In HNSCC, cancer stem cells have been suggested to be responsible for tumor recurrence, metastasis initiation because of high migration capacity, and radio/chemotherapy treatment resistance (12–14). Several studies reported a prognostic value for CSCs in HNSCC (15).
Cluster of differentiation 44 (CD44) is a transmembrane glycoprotein and a receptor for hyaluronic acid, an important component of the extracellular matrix, and a coreceptor for many growth factors and cytokines. The CD44+ cell population in cancer was shown to be CSC, as these purified CD44+ cells from primary tumors gave rise to tumors more rapidly despite a lower cell-seeding number in a xenograft model when compared to CD44− cells; these xenograft tumors subsequently reproduced the original tumor heterogeneity observed in the primary tumor. CD44+ cells can resist oxidative stress and thus, are more radio-resistant (16). CD44+ cells also have a greater ability to metastasize to regional lymph nodes in animal models (17). Patients whose tumors had greater percentages of CD44+ cells had a significantly poorer clinical outcome (18). Recent studies used CD44 surface marker expression as a sole marker for HNSCC-CSCs (19–21). However, some studies demonstrated that CD44− cells can also initiate tumor growth in vivo, form tumor-spheres, and express treatment resistance similar to CD44+ cells (22,23). With different microenvironments, there will be some heterogeneity in the CSCs (24), and the CD44+ cells may not represent pure HNSCC-CSCs.
In normal human oral epithelium, we can find a subpopulation of cells with stem cell–like properties. These cells express a cell surface molecule, designated as CD271+ cells (25,26). Recently, this molecule was identified as a marker of CSCs in many tumors, such as human melanoma (27), esophageal carcinoma (28,29), and hypopharyngeal carcinoma (30). Besides being expressed in discrete cells within the basal layer of normal oral epithelium, CD271 is also found in oral epithelial dysplasia and oral squamous cell carcinoma (31).
In the present study, we have used head and neck cancer cell lines to analyze the expression of CD44 and CD271 followed by isolation of CD44+/CD271+ and CD44+/CD271− subpopulations for further experiments. These populations were subjected to various molecular and cellular assays to determine whether CD271 has any significant contribution in the context of CD44 population towards defining CSC marker. We have clearly demonstrated that CD271+ cells comprised a purer CSCs subpopulation within the CD44+ cells, especially with regard to self-renewal, proliferation, treatment resistance, and strong in vivo tumorigenic capacity.
Materials and methods
Human squamous cell carcinoma cell lines and patient tissue samples
SCC12 (laryngeal SCC, RRID: CVCL_7717) and SCC38 (tonsillar SCC, RRID: CVCL_7749) cell lines were purchased from the University of Michigan in 2014 and were used as models for HNSCC (32). The cell lines have been tested and authenticated using STR analysis in 2019 at Genome Quebec. They were cultured in Dulbecco’s modified Eagle medium (DMEM) supplemented with 1% nonessential amino acids, 10% fetal bovine serum and 2% antibiotic–antimycotic (Thermo Fisher). All experiments were performed with mycoplasma-free cells.
A total of 10 oral squamous cell carcinomas were obtained from the Pathology Department of the McGill University Hospital Center. The sections were obtained from formalin-fixed paraffin-embedded (FFPE) tissue blocks. All tumor samples were intraoral and either located in the tongue, floor of the mouth, or gingiva. Six samples were from male patients and four from female patients. This study was performed with approval from the McGill Faculty of Medicine Institutional Review Board (IRB study number A05-M62-05B). The specimens were obtained as an incisional biopsy or surgical resection for oral squamous cell carcinoma. Normal mucosa was obtained from blocks of the same patient but were free of dysplasia and distant from the primary tumor location (taken from the farthest margin of the surgical resection).
Flow cytometry and fluorescence-activated cell sorting (FACS)
Alexa Fluor® 700 Mouse Anti-Human CD44 (Clone G44-26) and PerCP-Cy™5.5 Mouse Anti-Human CD271 (Clone C40-1457) monoclonal antibodies for flow cytometry and sorting were obtained from BD Pharmingen. Cells from the two tumor cell lines were harvested using Accutase™ Cell Detachment Solution (BD Bioscience) and resuspended as a single-cell suspension in the staining buffer (1% FBS in ice-cold PBS) with a final concentration 1 × 106 cells/100 µl. Cells were then incubated with the Fixable Viability Stain 450 (BD Bioscience) for 15 min on ice protected from light and washed twice in staining buffer to allow the exclusion of non-viable cells. Cells were blocked by incubation with Human TruStain FcX™ (Fc Receptor Blocking Solution, Biolegend) for 10 min then washed once with the staining buffer to block nonspecific staining. Cells were then stained by the antibodies for CD44 and CD271 at a dilution of 1:20 for 30 min on ice protected from light and washed twice with the staining buffer. The samples were analyzed using LSR Fortessa (BD Biosciences). Data analysis was performed using FlowJo vX (FlowJo LCC). FACS of CD44+/CD271− and CD44+/CD271+ cells were performed using a BD FACSARIA III cells sorter (BD Bioscience). Only the highly positive stained cells were isolated as CD271+ cells. Cells incubated with the viability stain, blocking agent, and the monoclonal antibodies, and passed through the BD FACSARIA III cells sorter without sorting were used as the parent cell population. UltraComp eBeads™ Compensation Beads (Thermo Fisher) was used as control.
Immunofluorescent staining
For immunofluorescent staining, 5-μm thick sections were cut on coated slides from FFPE tissue samples blocks. Slides were dewaxed with CitriSolv and rehydrated through graded alcohol. For antigen retrieval, they were immersed in 10% citrate buffer and treated in a water bath at 98°C for 15 min. The slides were then blocked with Power Block Universal Blocking Reagent (Biogenex) for 10 min followed by goat and donkey serum 5% for 1 h to inhibit any potential nonspecific binding. The slides were reacted with various prediluted primary antibodies overnight at 4°C in a humid chamber. The primary antibodies used in this study were: Mouse monoclonal anti CD44 (1:150, ab6124) and Rabbit monoclonal anti-CD271 (1:200, ab52987) from Abcam. After three washes in PBS, slides were incubated with secondary antibodies (1:100) in the dark for 1 h at room temperature. Secondary antibodies were Fluorescein (FITC) AffiniPure Donkey Anti-Mouse IgG (H+L) and Rhodamine Red™-X (RRX) AffiniPure Donkey Anti-Rabbit IgG (H+L) (Jackson ImmunoReserach). Then 4,6-diamidino-2-phenylindole, dihydrochloride (DAPI, Invitrogen) was added for 3 min to label cell nuclei. The same tissue sections treated without the primary antibodies were used as negative controls and human skin tissue slides were used as positive control (photos not shown). Fluorescence pictures were taken using theLeica DM4000 fluorescent microscope and the corrected total cell fluorescence (CTCF) using ImageJ software (NIH).
MTT assay
Cell growth was assayed with MTT assay. 1.5 × 103 cells from CD44+/CD271−, CD44+/CD271+, and unsorted parental cells were seeded in 96-well plates. Each day—for 7 consecutive days—the cell medium was removed and 10% solution of 5 mg/ml MTT in medium (Sigma Aldrich (3-(4,5-dimethylthoiazol-2-yl)-2,5-diphenyltetrazolium bromide) was added to each well and incubated at 37°C for 2 h. The medium was removed, and formazan was dissolved by adding 100 µl DMSO to each well. The optical density was measured at 562/540 nm in EL800 Microplate Reader (BIO-TEK Instruments). The assay was done in triplicates and three independent experiments were carried out.
Colony-forming assay
CD44+/CD271−, CD44+/CD271+, and the unsorted parental cells were prepared as single cell suspensions and 400 cells/well were plated into a six-well plate. Cells were allowed 2 weeks to form colonies under standard conditions, and the rate at which this occurred was recorded. To determine colony formation, culture medium was removed, and colonies were fixed and stained with 1% crystal violet, 50% methanol in DDH2O for 1 h. The number of colonies with >50 cells were counted under an inverted microscope. The assay was done in triplicates and three independent experiments were carried out.
Sphere-forming assay
CD44+/CD271−, CD44+/CD271+, and the unsorted parental cells were cultured overnight to eliminate dead cells. The next day, 5000 cells/well were seeded in 24 Ultra-Low Attachment Multiple Well Plate (Millipore Sigma) in 500 µl DMEM-F-12 serum-free media (Gibco) reconstituted with 20 ng/ml of Epidermal Growth factor, 20 ng/ml of Basic Fibroblast Growth Factor, 0.5% N2 supplement (STEMCELL Technologies), 1% B27 supplement and 2% antibiotic–antimycotic (Thermo Fisher). The medium was added every 2–3 days. Formation of sphere-like structures was visible at 4–7 days and the photographs of experiment and control groups were captured under Leica DM IL phase-contrast microscope (Leica Microsystems) using QICAM (QImaging) at ×5 and ×40 magnification at 14 days. All experiments were done in triplicate. Spheres were then collected by centrifugation and dissociated by trypsin (Thermo Fisher) to single cells. Cells were counted for each group using a hemocytometer with Trypan blue staining to exclude the dead cells.
Drug resistance assay
Cisplatin (Cayman Chemical) was prepared in phosphate-buffered saline to a 0.3 mg/ml stock and kept at 4°C protected from light. 5-Fluorouracil (Sigma–Aldrich) was prepared in dimethyl sulfoxide (DMSO) to a 50 mg/ml stock. Final concentrations of the solvents in the working solution medium were 0.1% or less. CD44+/CD271−, CD44+/CD271+, and the unsorted parental cells were seeded in 96-well plate at a density of 1500 cells/well and allowed to grow in normal medium. After 24 h, the medium was replaced with 100 µl fresh medium containing Cisplatin (Cayman Chemical) at concentration of 0, 0.125, 0.25, 0.5, 1, or 2 µg/ml or 5-fluorouracil (Sigma–Aldrich) at concentration of 0, 0.125, 0.5, 2, 8, or 32 µg/ml in triplicates and kept under standard culture conditions for another 72 h. Afterward, MTT assay was performed as mentioned above. Four independent experiments were carried out.
Radiation resistance assay
CD44+/CD271−, CD44+/CD271+, and the unsorted parental cells were subjected to ionizing radiation of 0, 2, or 4 Gray using RS200 X-ray biological irradiator (Rad source technologies). After that, 400 single live cells were seeded in 6-well plates and colony forming assay was continued as mentioned above. The assay was done in triplicates and three independent experiments were carried out.
Real-time qRT-PCR
Total RNA was extracted from CD44+/CD271−, CD44+/CD271+ and the unsorted parental cells using TRIzol (Thermo Fisher Scientific). The first-strand cDNA was synthesized from 1 μg total RNA using High-Capacity cDNA Reverse Transcription kit (Thermo Fisher Scientific). For the quantification of gene amplification, QPCR was performed using StepOnePlus™ Real-Time PCR System (Thermo Fisher Scientific) in the presence of PowerUp SYBR Green Master Mix (Thermo Fisher Scientific). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as the endogenous expression standard. Target sequences were amplified at 95°C for 10 min, followed by 40 cycles of 95°C for 15 s then 57.5–65°C annealing temperature for each gene for 1 min. The following gene-specific primers were used:
GAPDH: (5′-GAGAAGGCTGGGGCTCATTT-3′, 5′-AGTGATGGCATGGACT GTGG-3′), BMI-1: (5′-TCCTTAACAGTCTCAGGTATCAACC-3′, 5′-CACAGTTTCC TCACATTTCCA-3′), SMO: (5′-TGGTCACTCCCCTTTGTCCTCAC-3′, 5′-GCACGGTATCGGTAGTTCTTGTAGC-3′), GLI1: (5′-TTGGAGAAGCCGAGCCGAGTATC-3′, 5′-GAGTAGACAGAGGTTGGGAGGTAAGG-3′), NOTCH1: (5′-GCAGAGGCGTGGCAGACTAT-3′, 5′-ACTTGTACTCCGTCAGCGTG-3′), SOX2: (5′-ACACCAATCCCATCCACACT-3′, 5′-CAAACTTCCTGCAAAGCTCC-3′) OCT4: (5′-CTCGAGAAGGATGTGGTCCG-3′, 5′-GAAGTGAGGGCTCCCATAGC-3′) and ALDH1A1: (5′-ATCAAAGAAGCTGCCGGGAA-3′, 5′- GCATTGTCCAAGTCGG CATC-3′). All assays were performed in triplicate and the expression was calculated on the basis of ΔΔCt method. The n-fold difference in mRNAs expression was determined according to the method of 2−ΔΔCT.
In vivo tumor formation assay
The animal experiments were approved by the University Animal Care Committee at McGill University (Protocol #5330, www.animalcare.mcgill.ca). The total number of animals used was 50 NU/NU Nude (Crl:NU-Foxn1nu) mice (Charles River). Mice were anesthetized with isoflurane (Isoba VetTM) (Schering Plough) (4% induction and 2% maintenance). Six to ten-week-old male mice were injected with either 1 × 103, 1 × 104 or 1 × 105 viable CD44+/CD271+, CD44+/CD271− or unsorted SCC12 cells suspended in 30 μl of normal saline, into the side of the tongue, using a 1-ml tuberculin syringe with a 30-gauge hypodermic needle. There were 5 mice per experimental group (5 mice × 6 experimental groups) and 5 mice in the control group. The mice were examined for tumor formation on the tongue each week, measured bidirectionally using a caliber under gas anesthesia. Tumor size was calculated using the following formula: Volume = (width2 × length)/2. Animals were sacrificed after 32 days and their tongues were collected, fixed in 10 % neutral buffered formalin, and embedded in paraffin. Tumor formation was confirmed using H&E stained sections. Tumor sizes (in mm3) from the 1 × 105 cells injected group, as recorded every week, were compared between the three animal groups (CD44+/CD271+, CD44+/CD271−, and unsorted parental).
Statistical analysis
Statistical analysis was performed through the software GraphPad Prism 8 (GraphPad Software). Data were presented as the means ± standard deviation (SD) of three independent experiments with comparable results. One-way analysis of variance (ANOVA) followed by post hoc Tukey’s test were used to assess significant differences between three groups or more, while Student’s t-test (Unpaired) was used between two groups. p-values < 0.05 were considered statistically significant and <0.01 were considered extremely statistically significant.
Results
CD271+ cells are a subpopulation of CD44+ cells
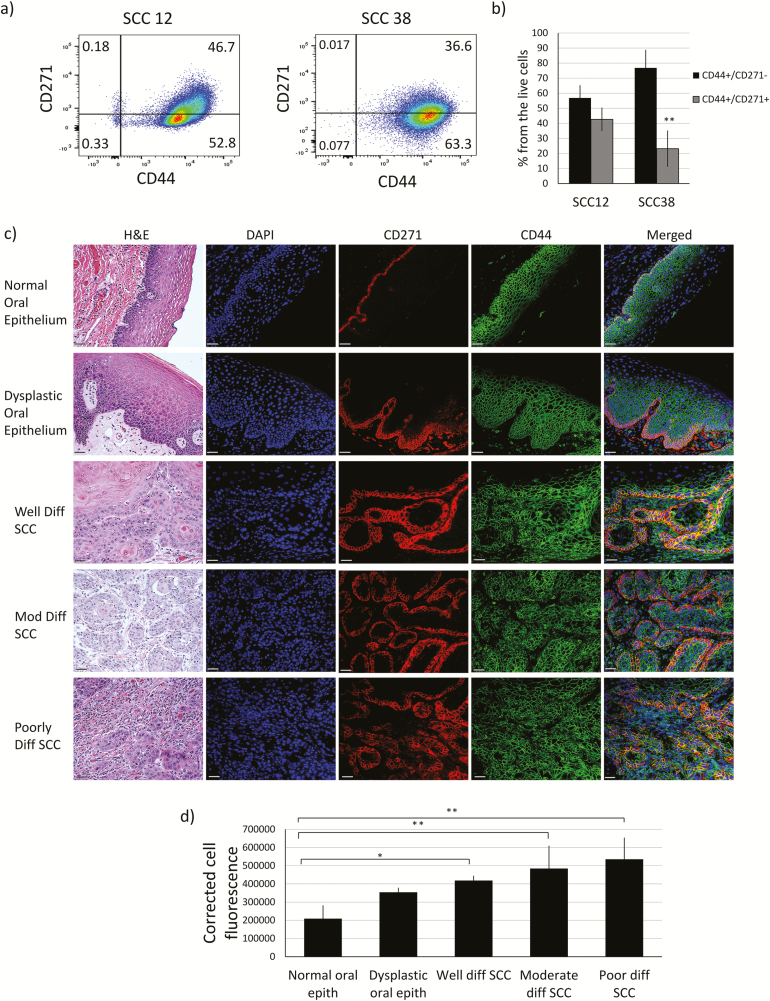
We assessed the prevalence of CD271 and CD44 expressions in the two tested human HNSCC cell lines using immunofluorescence staining and flow cytometry. The cells in the two cell lines expressed CD44 uniformly, with a negligible number of CD44 negative cells (less than 0.4% for SCC12 and 0.1% for SCC38). CD271+ cells were a subpopulation of CD44+ ones as they comprised 42.6% (±7.7) of the CD44+ cells in SCC12, and 23.1% (±11.8) in SCC38 (Figure 1a and b). To examine the colocalization of CD271 and CD44 in HNSCC human tissues, we used immunofluorescent staining of the patient’s oral squamous cell carcinoma tissue samples (Figure 1c). By using double anti CD44/CD271 staining, we detected a discrete expression of CD271 surface receptor on a distinct subpopulation of cells in the normal oral epithelium (Figure 1c). The dysplastic oral epithelium, as well as the well-differentiated oral SCC tumors, showed higher expression of CD271 in the “basal” aspect of the malignant epithelium (invasion front) compared to the normal epithelium while maintaining polarity. In higher grade (less differentiated) tumors, CD271 expression was less organized with an increase in the CD271 expression, as shown by measuring the CTCF of the CD271 staining (Figure 1d). Most importantly, since the basal half of the normal and basal two-thirds of dysplastic oral epithelium and most of the epithelial cell nests in HNSCC are CD44+, there was a colocalization of CD44 and CD271 expression in the normal and cancerous epithelial cells with CD271+ being a part of CD44+ cells.
Figure 1.
CD271+ cells are a subpopulation of CD44+ cells. (a) SCC12 and SCC38 cells were examined for the expression of CD44 and CD271 markers using flow cytometry. (b) Percentage of CD44+/CD271− cells and CD44+/CD271+ cells in SCC12 and SCC38. (c) Human normal and oral SCC samples were stained with H&E and monoclonal immunofluorescence antibodies against CD44 and CD271 for the assessment of the tumor grade and the localization of CD44+ and CD271+ cells (×20 magnification. Scale bar: 37 μm). (d) Quantification of cell fluorescence of CD271+ from stained images in (c) with ImageJ software (n = 3 to 6 per group). Data are presented as mean ± SD (*P < 0.05 and **P < 0.01). ).
CD44+/CD271+ cells have a higher growth rate in 2D and 3D culture conditions
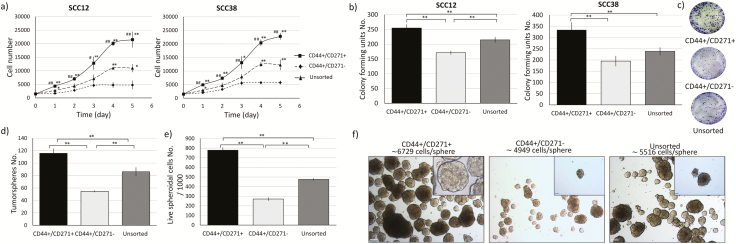
MTT assay was used to assess the proliferation of the isolated subpopulation in SCC12 and SCC38 cells. CD44+/CD271+ cells showed a higher proliferation rate compared to the CD44+/CD271− cells and the parent cell population in both tested cell lines. Furthermore, CD44+/CD271+ cells did not reach the stationary growth phase until up to 5 days while the unsorted parental cells reached it after 4 days, and the CD44+/CD271− cells reached a stationary phase after 3 days (Figure 2a).
Figure 2.
CD44+/CD271+ cells have higher growth rate in 2D and 3D culture conditions. (a) CD44+/CD271+, CD44+/CD271− and the unsorted parental cell population from SCC12 and SCC38 cell lines were seeded in 96-well plates and their cell growths were assessed using MTT assay (in triplicates from three independent experiments). Data are presented as mean ± SD (* p < 0.05 and ** p < 0.01 with CD44+/CD271−, #p < 0.05 and ##p < 0.01 with parental cells). (b) Assessment of self-renewal characteristic in 2D culture with the colony forming assay. Single cells from each of the three cell populations from SCC12 and SCC38 were seeded in 6-well plates and allowed to form colonies for 14 days (400 cells seeded per well). Fixed and stained colonies containing > 50 cells were counted under an inverted light microscope. Data are presented as mean ± SD (** p < 0.01). Sample photographs of the fixed and stained colonies are presented on the (c) panel. (d) To assess self-renewal in 3D culture, tumor-sphere formation assay was used. Single cells from each of the three cell populations from SCC12 were cultured in anchorage-independent and serum-free culture conditions and allowed to form spheres for 14 days. Data are presented as mean ± SD (** p < 0.01). (e) The spheres were dissociated into single cells and counted in the presences of trypan blue stain. Cells numbers are presented as mean ± SD (** p < 0.01). (f) The spheres were counted, and photos were taken under a phase-contrast microscope with ×5 (the main photos) and ×40 (the inserts) magnification. Scale bar = 90 µM and 10 µM, respectively.
CD271+ cells formed more colonies and in a shorter timeframe when compared to CD44+/CD271− and the unsorted parental cells in SCC12 and SCC38 cell lines (Figure 2b and c). The SCC12 cell line was selected for further experiments because it yielded more CD271+ cells.
The ability to grow in 3D conditions was tested by tumor-sphere formation in suspension culture. CD44+/CD271+ cells formed significantly more spheres compared to CD44+/CD271− and the parental cell line (Figure 2d). Because we had different sizes of tumor-spheres, we collected the spheres, dissociated them and counted the living cells using trypan blue staining (Figure 2e). By dividing the cells number by spheres’ number, we obtained an estimated number of cells per sphere. CD271+ cells had ~6729 cells/sphere while the CD271− cells had ~4949 cells/sphere and the parental population had ~5516 cells/sphere (Figure 2f). Because the cell size was comparable, we could imply that the spheres formed by CD271+ cells were bigger in size (Figure 2e and f).
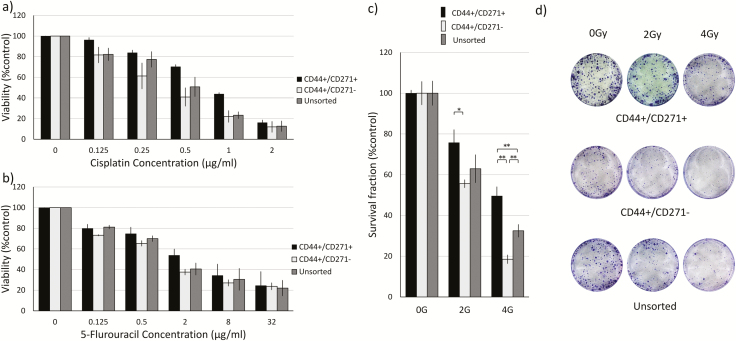
CD44+/CD271+ cells possess higher resistance to chemotherapy and radiotherapy treatments
To assess the chemo-resistance ability of the isolated cell populations, we exposed the cells to different concentrations of Cisplatin (CIS) and 5-Fluorouracil (5-FU) and calculated the inhibitory drug concentration that killed 50% of the cells (IC50). The drug resistance assay showed a statistically significant difference between the IC50 values of CIS in the three cell populations. IC50 of CIS was 0.817 μg/ml for CD44+/CD271+, 0.375 μg/ml for CD44+/CD271−, and 0.496 μg/ml for the unsorted parental cell line (Figure 3a). The same trend of results was observed with 5-FU as the IC50 was 3.644 μg/ml, 0.766 μg/ml and 1.49 μg/ml with CD44+/CD271+, CD44+/CD271− and the unsorted parental cell line, respectively (Figure 3b).
Figure 3.
CD44+/CD271+ cells possess higher onco-treatment resistance. (a) CD44+/CD271+, CD44+/CD271−, and the unsorted parental cell population from SCC12 cells were treated with 0, 0.125, 0.25, 0.5, 1, and 2 μg/ml of CIS for 72 h. Cell viability was evaluated in triplicate by MTT assay. Data are presented as mean ± SD. (b) The three cell population from SCC12 were treated with 0, 0.125, 0.5, 2, 8, and 32 μg/ml of 5-FU for 72 h. Cell viability was evaluated in triplicate by MTT assay. (c) The three cell populations from SCC12 were exposed to 0, 2, or 4 Gy radiation doses then 400 single live cells were seeded in 6-well plate and allowed to form colonies for 14 days. Fixed and stained colonies containing >50 cells were counted under an inverted light microscope. Data are presented as mean ± SD (*p < 0.05, ** p < 0.01). Sample photographs of the fixed and stained colonies are presented on the (d) panel.
CD44+/CD271+ cells showed more resistance to radiotherapy when compared to CD44+/CD271− cells and the unsorted parental cell line. The examined cell populations were exposed to 2 Gy and 4 Gy radiation doses then plated as single cells and allowed to form colonies for 14 days. CD271+ cells formed significantly more colonies compared to the CD271− cells at 2 Gy radiation, and more than both CD271- and the total cells populations at 4 Gy radiation (Figure 3c and d)
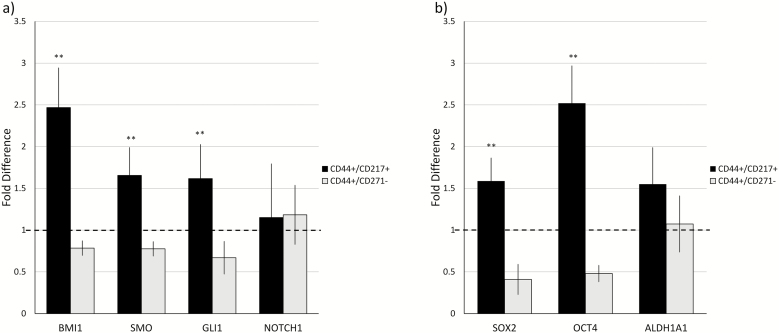
CD44+/CD271+ cells expressed higher levels of stem cell-related markers
qRT-PCR revealed that compared to CD44+/CD271− cells, the CD44+/CD271+ SCC12 cells have significantly higher expression levels of genes (previously reported as self-renewal organizing genes), namely BMI1, SMO, and GLI1. The NOTCH gene expression was found to be expressed at the same level by both CD44+/CD271+ and CD44+/CD271− SCC12 cells (Figure 4a). Additionally, expression of stemness-related genes SOX2 and OCT4 were found to be higher in CD44+/CD271+ SCC12 cells compared to the CD44+/CD271− cells; however even with the higher expression of drug resistance-related gene ALDH1A1 in CD44+/CD271+ SCC12 cells, the difference was not statistically significant (Figure 4b).
Figure 4.
CD44+/CD271+ cells expressed higher levels of stem cell-related markers than CD44+/CD271− cells. Expression levels of CSCs self-renewal related genes: (a) BMI1, SMO, GLI1, and NOTCH1, (b) stemness-related genes; SOX2 and OCT4, and drug resistance-related gene ALDH1A1 were analyzed by quantitative RT-PCR. Y-axis shows the relative expression of the gene compared to GAPDH. The horizontal dashed line represents the relative genes expression level of the unsorted parental cell population. All assays were performed in triplicate in three independent experiments and were calculated on the basis of ΔΔCt method. Data represent mean ± SD (**P < 0.01).
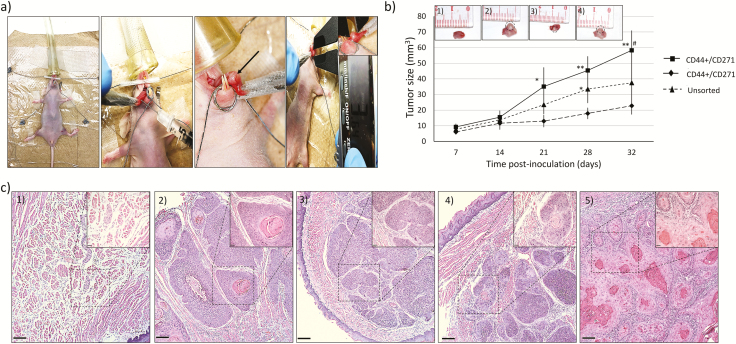
CD44+/CD271+ cells have higher in vivo tumorigenicity in an orthotopic immunodeficient mouse model of oral cancer
FACS-sorted CD44+CD271+ cells, CD44+CD271− cells, and unsorted parental SCC12 cells were implanted into the tongue of NU/NU Nude (Crl:NU-Foxn1nu) mice through a limiting dilution approach (Figure 5a). The results indicated that CD44+CD271+ cells have the greatest capacity to form tumors among these three cell populations (Table 1). CD44+CD271+ cells also generated tumors with the most robust in vivo tumor growth (Figure 5b). H&E staining was used to identify morphological differences between the three groups. Stained sections were observed and digital images were captured with a light microscope. All formed tumors appeared to have a typical SCC tumor morphology, well-differentiated, with keratin pearl formation, cellular and nuclear pleomorphism, and invasion in the surrounding skeletal muscle with no intercellular bridges and abnormal mitosis (Figure 5c).
Figure 5.
CD44+/CD271+ cells have higher tumorigenicity when compared to CD44+/CD271− cells. (a) CD44+/CD271+, CD44+/CD271−, and the parental cell population from SCC12 were injected in the tongue of NU/NU Nude (Crl:NU-Foxn1nu) mice and tumor formation and size were followed weekly. (b) Tumor volume (mm3) was measured weekly after cancer cell inoculation. Data represent mean ± SD (*P < 0.05 and **P < 0.01 compared with CD44+/CD271− and #P < 0.05 compared with parental cells). (b1) Normal mouse tongue as negative control, (b2) tumor formation after 35 days with CD44+/CD271+ injection, (b3) tumor formation with CD44+/CD271− injection, (b4) tumor formation with the unsorted parental cell population injection. (c) Tumor formation following inoculation with the three cellular populations (hematoxylin and eosin staining). (c1) Normal mouse tongue as negative control, (c2) CD44+/CD271+, (c3) CD44+/CD271−, (c4) Unsorted parental cell population, and (c5) Well differentiated human HNSCC, used as a positive control (×5 magnification. Scale bar: 150 μm, inserts ×20 magnification. Scale bar: 37 μm).
Table 1.
Tumorigenicity of CD44+/CD271+, CD44+/CD271−, and unsorted SCC12 cells in a nude mouse orthotopic xenograft model.
| Mice with tumor formed in relation to the number and types of cells injected. | |||
|---|---|---|---|
| 1 × 103 cells | 1 × 104 cells | 1 × 105 cells | |
| CD44+/CD271+ | 3/5 mice | 5/5 mice | 5/5 mice |
| CD44+/CD271− | 0/5 mice | 1/5 mice | 3/5 mice |
| Unsorted parental cells | 0/5 mice | 2/5 mice | 4/5 mice |
Discussion
The inability to eradicate CSCs is among the most supported theories to explain cancer treatment resistance, recurrence, and metastases. Designing treatments that target CSCs should decrease the mortality rates of cancer patients. Accurately identifying CSCs in cancer is a crucial step and requires the fulfillment of three characteristics (33,34): first, CSCs must express specific stem cell-related genes; second, CSCs have a high self-renewal ability; and third, CSCs are tumorigenic and generate tumors in mice, even with a very few number of cells. In this study, we extend the current understanding and characterization of CSCs in HNSCCs by demonstrating that, within the CD44+ cell population, the CD44+/CD271+ cell subpopulation is an enriched CSC population. In addition, we demonstrated that CD44+/CD271+ cell subpopulation may play a major role in the development of treatment resistance of CD44+ cells, making these cells a more suitable target for therapy.
Our data is compatible with the theory that CSCs come from deregulated normal stem cells. In normal human oral epithelium, CD271 was used to identify a subset of cells in the basal layer of the epithelium that possessed stem cell-like characteristics (25). These CD271+ cells showed a higher growth rate, had more colonies in vitro, and formed cellular layers expressing key differentiation markers, comparable to culturing stratified epithelium on amniotic membranes. Because cells in the basal half of the oral epithelium express CD44, the CD271+ cells are a subset of CD44+ cells in normal oral tissues. Our flow cytometry analysis and immunofluorescence staining showed colocalization of CD271 and CD44 in HNSCC cell lines and human tissue samples (Figure 1). Immunofluorescent staining showed that the number of CD271+ cells increased proportionally with increased tumor severity and were more randomly distributed (Figure 1c and d). This suggested an increase in cell proliferation and a loss of normal function, especially at the invasion front. In our study, obtaining normal tissues from subjects without cancer to test the specificity of CD44 and CD271 was not possible, and thus we used normal tissues from cancer patients. The caveat of using normal (noncancerous) tissue from the same cancer patient as a healthy tissue control is controversial because this tissue has been exposed to the same risk factors as the area where the tumor developed. This could explain the detection of CD271 expression in cells of the normal epithelium. Still, previous studies confirmed our findings of CD271 cellular distribution in healthy normal oral (25,26), laryngeal (35), hypopharyngeal (30), and esophageal epithelial tissues (29). Thus, we suggest that CD44+/CD271+ cells in HNSCC possess many similar stemness characteristics as those CD44+/CD271+ cells found in the normal oral epithelium, but their number increased when the epithelium becomes more dysplastic.
Several studies have correlated the percentage of CSCs in cancer with patient prognosis. The higher percentage of CD44+ cells in HNSCC was found to correlate with metastasis and recurrence (18). Our data, along with what was reported in esophageal carcinoma (36), hypopharyngeal carcinoma (30), and oral carcinoma (37) suggest that the percentage of CD44+/CD271+ cells could be an indicator of prognosis (Figure 1c and d). Perineural invasion (PNI) was documented to be an indicator related to poor prognosis in HNSCC, as it was associated with a higher rate of recurrence and metastasis (38). It was suggested that CD271 expression may cause PNI in HNSCC as it may become activated by nerve growth factors (NGF) in Schwann cell surrounding neurons (39). Indeed, CD271 expression was correlated with PNI in melanoma (40), pancreatic cancer (41) and oral cancer (42).
Our results revealed that the growth rate of CD44+/CD271+ cells was much higher and reached a stationary phase much later when compared to CD44+/CD271− cells and the unsorted parental cells (Figure 2a). These results were similar to those reported by studies comparing CD271+ cells to the CD271− negative cells in tongue carcinoma (43), oral carcinoma (44), hypopharyngeal carcinoma (30), gastric cancers (45), prostate cancer (46), and melanoma (27). Self-renewal is a hallmark of CSCs, as these cells can give rise to new cells that will expand and proliferate. The colony-forming ability of a cell is used to measure its self-renewal characteristic and previous studies have used this assay as one of the methods to characterize CSCs in 2D culture (47,48). Analogously in 3D suspension culture, the sphere-forming ability of cells is used to measure the self-renewal ability of different cancer cells (49). Our results demonstrated that CD44+/CD271+ cells not only possessed both a greater colony-forming and a sphere-forming ability when compared to the CD271- cells and their unsorted parental cells but also formed bigger spheres (Figure 2b –f).
Due to the increasing evidence that CSCs from several types of cancers possess more chemotherapeutic- and radiotherapeutic-resistance when compared to the non-CSCs population, cancer treatment strategies are being developed to target CSCs (23). In HNSCC, CD44+ cells showed more resistance to oxidative stress and remained in higher numbers in xenografts following radiotherapy, when compared to CD44− cells (16). In esophageal squamous cell carcinoma, CD271+ cells were found more resistant to oxidative stress (which is a cytotoxic effect of cisplatin) (28). Tolerance to reactive oxygen species was found increased in PC12 cells using neurotrophins in a CD271-dependent manner (50). Also, CD271+ cells were more resistant to DNA-damaging agents in melanoma when compared to CD271− cells (51). These studies support our findings that CD44+CD271+ cells were the subpopulation with the most resistance to oxidative stress in HNSCC. The exact mechanisms remain to be confirmed, but current studies suggest several mechanisms in CD271+ cells, such as the increased expression of ALDH1A1, ALDH1A1-dependent activation of the drug-efflux pump, ATP-binding cassette subfamily B member 1(ABCB1), and survival proteins (AKT and BCL2) (52).
CD44+/CD271+ cells showed higher expression of self-renewal–related genes, namely BMI1, SMO, and GLI1, when compared to CD44+/CD271- cells and the unsorted parental cell population (Figure 4). These findings suggest higher activation of the Hedgehog signaling pathway of self-renewal. There was also a higher expression of stemness-related genes, SOX2 and OCT4, which is responsible for maintaining pluripotency (53). Our results are in line with previous studies reporting that CD271+ cells expanded and possessed stem-like characteristics of the oral mucosa (25), esophagus (29), and esophageal cancer (36).
In addition to in vitro assays using human HNSCC derived cell lines, we performed in vivo tumorigenic assays by injecting these cells using a limiting dilution approach (Table 1). Compared to CD44+/CD271− cells, CD44+/CD271+ subpopulation was found to have a higher tumor formation incidence and with a faster tumor growth rate when implanted at the same low injection cell number into immune-deficient mice. Other studies, which tested CD271 expression only, reported high tumorigenicity of CD271+ cells when compared to the negative subpopulation of other solid tumors such as hypopharyngeal carcinoma and oral cancer (54,55).
In conclusion, we demonstrated that combining two cell markers CD44+/CD271+ was better than using CD44 alone. This was because CD271+ cells represented a subpopulation of CD44+ cells with increased tumorigenicity and treatment resistance in HNSCC. In the future, it should be possible to target CD271+ cells to eradicate HNSCC resistance (44). Our findings support the idea of CSCs role in the development of treatment resistance and tumor progression. Further studies should be conducted in a larger patient cohort to better understand the pathogenesis, development of targeted cancer treatments, clinical and prognostic impact on HNSCC.
Acknowledgements
We would like to thank Camille Stegen for her technical assistant with the FACS, Li-Chieh for his help in the cell lines authentication and Hieu Pham for proofreading and editing.
Glossary
Abbreviations
- CD44
cluster of differentiation 44
- CSC
cancer stem cell
- CTCF
corrected total cell fluorescence
- DMEM
Dulbecco’s modified Eagle medium
- FACS
fluorescence-activated cell sorting
- FFPE
formalin-fixed paraffin-embedded
- HNSCC
head and neck squamous cell carcinoma
Funding
This work was partly funded by: Canadian Institutes of Health Research (CIHR grant 119585), Natural Sciences and Engineering Research Council of Canada (NSERC grant 05247), MJW Kim research fund and the Ministry of Higher Education in Egypt (MOHE post graduate studies funding).
Conflict of interest statement: None declared.
Authors’ contributions
Osama A. Elkashty and Ghada Abu Elghanam conceived and carried out experiments and analyzed data, Xinyun Su, Peter J. Chauvin, and Simon D. Tran conceived experiments and analyzed data. Younan Liu carried out experiments. All authors were involved in writing the paper and had final approval of the submitted and published versions.
References
- 1. Canadian-Cancer-Statistics-Advisory-Committee. (2018) Canadian Cancer Statistics 2018. Toronto, ON: Canadian Cancer Society; 2018. Available at: cancer.ca/Canadian-Cancer-Statistics-2018-EN (accessed 21-11-2018). http://www.cancer.ca/en/cancer-information/cancer-101/canadian-cancer-statistics-publication/?region=on#ixzz5XXikeMEh. [Google Scholar]
- 2. Heron M. (2018) Deaths: leading causes for 2016. Natl. Vital Stat. Rep., 67, 1–77. [PubMed] [Google Scholar]
- 3. Bray F. et al. (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA. Cancer J. Clin., 68, 394–424. [DOI] [PubMed] [Google Scholar]
- 4. Machiels J.P. et al. (2014) Advances in the management of squamous cell carcinoma of the head and neck. F1000Prime Rep., 6, 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Finlan L.E. et al. (2006) Epidermal stem cells and cancer stem cells: insights into cancer and potential therapeutic strategies. Eur. J. Cancer, 42, 1283–1292. [DOI] [PubMed] [Google Scholar]
- 6. Bonnet D. et al. (1997) Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat. Med., 3, 730–737. [DOI] [PubMed] [Google Scholar]
- 7. Visvader J.E. et al. (2008) Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. Nat. Rev. Cancer, 8, 755–768. [DOI] [PubMed] [Google Scholar]
- 8. Donnenberg V.S. et al. (2005) Multiple drug resistance in cancer revisited: the cancer stem cell hypothesis. J. Clin. Pharmacol., 45, 872–877. [DOI] [PubMed] [Google Scholar]
- 9. Baumann M. et al. (2008) Exploring the role of cancer stem cells in radioresistance. Nat. Rev. Cancer, 8, 545–554. [DOI] [PubMed] [Google Scholar]
- 10. Jiang X. et al. (2007) Chronic myeloid leukemia stem cells possess multiple unique features of resistance to BCR-ABL targeted therapies. Leukemia, 21, 926–935. [DOI] [PubMed] [Google Scholar]
- 11. Brabletz T. et al. (2005) Opinion: migrating cancer stem cells—an integrated concept of malignant tumour progression. Nat. Rev. Cancer, 5, 744–749. [DOI] [PubMed] [Google Scholar]
- 12. Krishnamurthy S. et al. (2012) Head and neck cancer stem cells. J. Dent. Res., 91, 334–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Satpute P.S. et al. (2013) Cancer stem cells in head and neck squamous cell carcinoma: a review. Asian Pac. J. Cancer Prev., 14, 5579–5587. [DOI] [PubMed] [Google Scholar]
- 14. Elkashty O.A. et al. (2019) Head and neck cancer management and cancer stem cells implication. Saudi Dental J., 31, 395–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fan Z. et al. (2017) Prognostic value of cancer stem cell markers in head and neck squamous cell carcinoma: a meta-analysis. Sci. Rep., 7, 43008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Diehn M. et al. (2009) Association of reactive oxygen species levels and radioresistance in cancer stem cells. Nature, 458, 780–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chinn S.B. et al. (2015) Cancer stem cells: mediators of tumorigenesis and metastasis in head and neck squamous cell carcinoma. Head Neck, 37, 317–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Joshua B. et al. (2012) Frequency of cells expressing CD44, a head and neck cancer stem cell marker: correlation with tumor aggressiveness. Head Neck, 34, 42–49. [DOI] [PubMed] [Google Scholar]
- 19. Irani S. et al. (2018) Expression of vimentin and CD44 in mucoepidermoid carcinoma: a role in tumor growth. Indian J. Dent. Res., 29, 333–340. [DOI] [PubMed] [Google Scholar]
- 20. Alsheddi M. et al. (2018) Expression of stem cell marker CD44 in selected benign and malignant salivary gland tumors. Saudi J. Oral Sci., 5, 80–83. [Google Scholar]
- 21. Mishra A. et al. (2018) Decreased expression of cell adhesion genes in cancer stem-like cells isolated from primary oral squamous cell carcinomas. Tumour Biol., 40, 1010428318780859. [DOI] [PubMed] [Google Scholar]
- 22. Oh S.Y. et al. (2013) CD44-negative cells in head and neck squamous carcinoma also have stem-cell like traits. Eur. J. Cancer, 49, 272–280. [DOI] [PubMed] [Google Scholar]
- 23. Eyler C.E. et al. (2008) Survival of the fittest: cancer stem cells in therapeutic resistance and angiogenesis. J. Clin. Oncol., 26, 2839–2845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Coulon A. et al. (2011) Functional sphere profiling reveals the complexity of neuroblastoma tumor-initiating cell model. Neoplasia, 13, 991–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nakamura T. et al. (2007) Identification of human oral keratinocyte stem/progenitor cells by neurotrophin receptor p75 and the role of neurotrophin/p75 signaling. Stem Cells, 25, 628–638. [DOI] [PubMed] [Google Scholar]
- 26. Thompson S.J. et al. (1989) A monoclonal antibody against nerve growth factor receptor. Immunohistochemical analysis of normal and neoplastic human tissue. Am. J. Clin. Pathol., 92, 415–423. [DOI] [PubMed] [Google Scholar]
- 27. Civenni G. et al. (2011) Human CD271-positive melanoma stem cells associated with metastasis establish tumor heterogeneity and long-term growth. Cancer Res., 71, 3098–3109. [DOI] [PubMed] [Google Scholar]
- 28. Huang S.D. et al. (2009) Self-renewal and chemotherapy resistance of p75NTR positive cells in esophageal squamous cell carcinomas. BMC Cancer, 9, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Okumura T. et al. (2003) Neurotrophin receptor p75(NTR) characterizes human esophageal keratinocyte stem cells in vitro. Oncogene, 22, 4017–4026. [DOI] [PubMed] [Google Scholar]
- 30. Imai T. et al. (2013) CD271 defines a stem cell-like population in hypopharyngeal cancer. PLoS One, 8, e62002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kiyosue T. et al. (2013) Immunohistochemical location of the p75 neurotrophin receptor (p75NTR) in oral leukoplakia and oral squamous cell carcinoma. Int. J. Clin. Oncol., 18, 154–163. [DOI] [PubMed] [Google Scholar]
- 32. Elkashty O.A. et al. (2018) Broccoli extract improves chemotherapeutic drug efficacy against head-neck squamous cell carcinomas. Med. Oncol., 35, 124. [DOI] [PubMed] [Google Scholar]
- 33. Bhaijee F. et al. (2012) Cancer stem cells in head and neck squamous cell carcinoma: a review of current knowledge and future applications. Head Neck, 34, 894–899. [DOI] [PubMed] [Google Scholar]
- 34. Sheng X. et al. (2013) Isolation and enrichment of PC-3 prostate cancer stem-like cells using MACS and serum-free medium. Oncol. Lett., 5, 787–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Li X. et al. (2012) Biological and clinical significance of p75NTR expression in laryngeal squamous epithelia and laryngocarcinoma. Acta Otolaryngol., 132, 314–324. [DOI] [PubMed] [Google Scholar]
- 36. Okumura T. et al. (2006) The biological role of the low-affinity p75 neurotrophin receptor in esophageal squamous cell carcinoma. Clin. Cancer Res., 12, 5096–5103. [DOI] [PubMed] [Google Scholar]
- 37. Søland T.M. et al. (2008) Nerve growth factor receptor (p75 NTR) and pattern of invasion predict poor prognosis in oral squamous cell carcinoma. Histopathology, 53, 62–72. [DOI] [PubMed] [Google Scholar]
- 38. Fagan J.J. et al. (1998) Perineural invasion in squamous cell carcinoma of the head and neck. Arch. Otolaryngol. Head. Neck Surg., 124, 637–640. [DOI] [PubMed] [Google Scholar]
- 39. Tosaki T. et al. (2008) Reduced NGF secretion by Schwann cells under the high glucose condition decreases neurite outgrowth of DRG neurons. Exp. Neurol., 213, 381–387. [DOI] [PubMed] [Google Scholar]
- 40. Chan M.M. et al. (2010) Low-affinity nerve growth factor receptor (P75 NGFR) as a marker of perineural invasion in malignant melanomas. J. Cutan. Pathol., 37, 336–343. [DOI] [PubMed] [Google Scholar]
- 41. Zhu Z. et al. (1999) Nerve growth factor expression correlates with perineural invasion and pain in human pancreatic cancer. J. Clin. Oncol., 17, 2419–2428. [DOI] [PubMed] [Google Scholar]
- 42. Kolokythas A. et al. (2010) Nerve growth factor and tyrosine kinase A receptor in oral squamous cell carcinoma: is there an association with perineural invasion? J. Oral Maxillofac. Surg., 68, 1290–1295. [DOI] [PubMed] [Google Scholar]
- 43. Tong D. et al. (2017) p75 neurotrophin receptor: a potential surface marker of tongue squamous cell carcinoma stem cells. Mol. Med. Rep., 15, 2521–2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Murillo-Sauca O. et al. (2014) CD271 is a functional and targetable marker of tumor-initiating cells in head and neck squamous cell carcinoma. Oncotarget, 5, 6854–6866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Jin H. et al. (2007) p75 neurotrophin receptor suppresses the proliferation of human gastric cancer cells. Neoplasia, 9, 471–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Khwaja F. et al. (2006) The p75(NTR) tumor suppressor induces cell cycle arrest facilitating caspase mediated apoptosis in prostate tumor cells. Biochem. Biophys. Res. Commun., 341, 1184–1192. [DOI] [PubMed] [Google Scholar]
- 47. Liu S. et al. (2006) Hedgehog signaling and Bmi-1 regulate self-renewal of normal and malignant human mammary stem cells. Cancer Res., 66, 6063–6071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Diamandis P. et al. (2007) Chemical genetics reveals a complex functional ground state of neural stem cells. Nat. Chem. Biol., 3, 268–273. [DOI] [PubMed] [Google Scholar]
- 49. Krishnamurthy S. et al. (2010) Endothelial cell-initiated signaling promotes the survival and self-renewal of cancer stem cells. Cancer Res., 70, 9969–9978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Mi Z. et al. (2009) p75NTR-dependent modulation of cellular handling of reactive oxygen species. J. Neurochem., 110, 295–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Redmer T. et al. (2017) The role of the cancer stem cell marker CD271 in DNA damage response and drug resistance of melanoma cells. Oncogenesis, 6, e291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Yang Y. et al. (2014) NEK2 mediates ALDH1A1-dependent drug resistance in multiple myeloma. Oncotarget, 5, 11986–11997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Shi G. et al. (2010) Role of Oct4 in maintaining and regaining stem cell pluripotency. Stem Cell Res. Ther., 1, 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Osman T.A. et al. (2015) The low-affinity nerve growth factor receptor p75NTR identifies a transient stem cell-like state in oral squamous cell carcinoma cells. J. Oral Pathol. Med., 44, 410–419. [DOI] [PubMed] [Google Scholar]
- 55. Mochizuki M. et al. (2016) CD271 regulates the proliferation and motility of hypopharyngeal cancer cells. Sci. Rep., 6, 30707. [DOI] [PMC free article] [PubMed] [Google Scholar]