Abstract
Clinical evidence suggests that patients with subcortical ischemic vascular dementia (SIVD) perform better at cognitive tests after exercise. However, the underlying mechanism for this effect is largely unknown. Here, we examined how treadmill exercise changes the cognitive function and white matter cellular pathology in a mouse model of SIVD. Prolonged cerebral hypoperfusion was induced in 2-month-old male C57BL/6J mice by bilateral common carotid artery stenosis. A week later, the mice were randomly divided into a group that received 6-week treadmill exercise and a sedentary group for observation. In multiple behavioral tests (Y-maze, novel object recognition, and Morris water maze tests), the treadmill exercise training was shown to ameliorate cognitive decline in the hypoperfused SIVD mice. In addition, immunohistological analyses confirmed that there was a larger population of oligodendrocyte precursor cells in the subventricular zone of exercised versus sedentary mice. Although further investigations are needed to confirm a causal link between these findings, our study establishes a model and cellular foundation for investigating the mechanisms through which exercise preserves cognitive function in SIVD.
Keywords: Treadmill exercise, Subcortical ischemic vascular dementia, White matter, Oligodendrocyte precursor cell, Subventricular zone
Introduction:
Subcortical ischemic vascular dementia (SIVD) is the most common form of vascular cognitive impairment and dementia (VCID) [1, 2]. Patients typically suffer from peri-ventricular white matter degeneration that leads to stepwise development of neurological deficits and loss of executive function such as difficulties with working memory [3–6]. The prevalence of SIVD is expected to increase as the population ages. To date, there are no clinically effective drugs. Taken together with the fact that polypharmacy among elderly patients has become a serious social issue around the world [7], it is essential to seek non-pharmacological therapies for this disease.
Recent clinical data have shown that physical activity is inversely associated with the progression of Alzheimer’s disease (AD) [8]. These results are supported by studies in experimental studies that verified the exercise-induced attenuation of disease-related pathology in rodent models [9–11]. Likewise, there is also an emerging body of evidence that patients with mild VCID perform better at cognitive functioning tests after aerobic exercise [12]. However, unlike AD, there is a lack of basic data in experimental models that supports the efficacy of exercise for preventing SIVD progression. In this study, we asked whether exercise-induced improvements in cognitive function are accompanied by changes in oligodendrocyte precursor cells (OPCs) in the white matter of mice after bilateral common carotid artery stenosis (BCAS), a model of prolonged cerebral hypoperfusion that is known to mimic the pathophysiology of SIVD [13, 14].
Materials and methods:
Overall Experimental Design
In this mouse model of SIVD, working memory is known to decline in 4 weeks presumably due to rarefaction of the white matter [15]. Our intervention was a 6-week treadmill training which, according to our hypothesis, would slow cognitive decline and ameliorate the deterioration in performance over time. The overall experimental protocol consisted of memory assessment using Y-maze (day 13 and day 48), novel object recognition (NORT; day 14 and day 49), and the Morris water maze (day 50–56) tests with treadmill training in intervening time period for the training group and the lack of any specific exercise for the sedentary group. After the BCAS surgery on day 1, treadmill training occurred from day 8 to day 47 in the training group (n=31) while mice in the sedentary group (n=31) were placed on the treadmill for an equivalent amount of time, but without running (Figure 1A). In addition to the memory assessment, we also conducted immunohistological analysis using mouse brain sections. Please see our supplementary methods for detailed procedures of preparation of hypoperfused BCAS mice, treadmill exercise, behavior tests, assessment of cerebral blood flow, and immunohistochemistry. In this study, total 88 mice were used, and the detailed information of animal numbers for each experiment were also presented in our supplementary information.
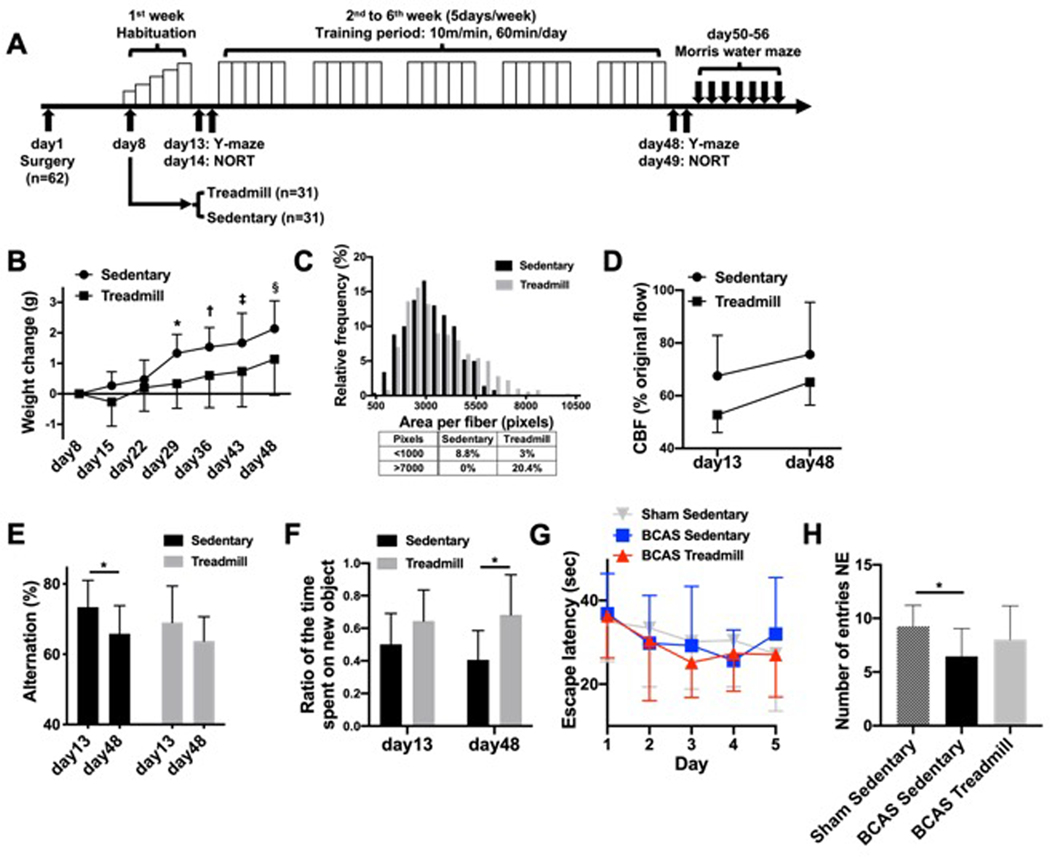
Figure 1.
(A) Schematic overview of the treadmill protocol. Sixty-two BCAS mice were separated into 2 groups (Sedentary: n=31, Treadmill: n=31) 7 days after the surgery. Following a week of habituation period, mice in the treadmill group were placed on a treadmill by which they were obligated to run for 60 minutes/day at the maximum speed of 10 m/min for 5 weeks on weekdays. Y-maze was conducted 2 times, on day 13 and day 48 (n=15/group). NORT was conducted 2 times, on day 14 and day 49 (n=12/group). Water maze was conducted between days 50–56 (n=11–12/group). Please see our supplementary information for the detailed animal numbers for each experiment. (B) Temporal weight change of mice. A significant difference was observed between the 2 groups after day 29 (*, †, ‡, §P<0.05, Sidak’s multiple comparisons test). Data are expressed as mean±SD, with 15 mice for each group. (C) Distribution of the area of muscle fibers showed significant difference between the 2 groups (P<0.05, Mann-Whitney U test). Data are expressed as mean±SD, with 5 mice (100 fibers per mouse) for each group. Representative cross-sectional image of the gastrocnemius muscle was presented in Supplementary Figure S1D. (D) Laser-doppler flowmetry comparing CBF of sedentary BCAS mice and treadmill BCAS mice at day 13 and day 48. No statistical difference was observed between the 2 groups at both time points. Data are expressed as mean±SD, with 4 mice for each group. (E) Change in alternation in Y-maze test before and after the training period. Significant decline was observed only in the sedentary group (*P<0.05, Sidak’s multiple comparisons test). Data are mean±SD from 15 mice for each group. Data for arm entry number were presented in Supplementary Figure S2A. (F) While there was no difference in recognition memory between the 2 groups on day 14, statistical significance was observed on day 49 in NORT (*P<0.05, Sidak multiple comparisons test). Data are expressed as mean±SD, with 11 mice for each group. (G) During the acquisition phase of Morris water maze, no difference was observed in latencies to locate the hidden platform between the sham-operated sedentary group, the BCAS sedentary group, and the BCAS treadmill group. Data are expressed as mean±SD, with 11–12 mice for each group. (H) Result of the probe trial in the Morris water maze test. In comparison to the sham sedentary group, number of entries into the northeast (NE) quadrant which formerly contained a platform in the acquisition phase, was significantly decreased in BCAS sedentary group, but not in BCAS treadmill group (*P<0.05, Dunnett’s T3 multiple comparisons test). Data are expressed as mean±SD, with 11–12 mice for each group.
Statistics
Statistical analysis was performed with R version 3.4.0 (https://www.r-project.org/) and PRISM® 7. For body weight, cerebral blood flow (CBF), Y-maze, and NORT, data were first tested with two-way analysis of variance (ANOVA) followed by post hoc Sidak’s multiple comparisons test. For the acquisition phase of Morris water maze test, data were first tested with two-way ANOVA followed by post hoc Tukey’s multiple comparisons test. Data from probe test were first analyzed with Brown-Forsythe ANOVA followed by post hoc Dunnett’s T3 multiple comparisons test. Mann-Whitney U test was used to analyze the area distribution of muscle fibers. Welch’s t-test was used for other analyses. All values were expressed as mean ± SD. P-values less than 0.05 were considered as statistically significant.
Results:
Cerebral hypoperfusion and demyelination in BCAS mice
To determine whether the operated mice showed an expected degree of CBF decline by the time they started to exercise, laser-doppler flowmetry was performed to check the temporal patterns of CBF after BCAS surgery. CBF dropped to 50–60% of the pre-surgery normal baselines, but gradually stabilized around 70–80% by the 7th day after surgery, consistent with previous reports [16] (Supplementary Figure S1A). To check on white matter degeneration, segments of the corpus callosum were dissected (Supplementary Figure S1B). By 6 weeks post-surgery, western blots showed that myelin basic protein (MBP) was significantly decreased in BCAS mice, indicating degeneration of the white matter as expected (Supplementary Figure S1C).
Physical changes in BCAS mice after exercise
Over time during the treadmill exercise training, all mice gained weight, as expected, but the sedentary group gained more weight compared to the treadmill-exercised group, with a significant difference between the 2 groups (Figure 1B, day 29: P=0.005, day36: P=0.01, day 43: P=0.01, day 48: P=0.005). Evidence of the physical changes resulting from exercise was also observed in biopsies of the right gastrocnemius muscles from randomly selected mice in each group. Histological analysis demonstrated a significant difference in the distribution of muscle fiber thickness between the 2 groups (Figure 1C, P=0.006). Mice with treadmill exercise tended to have a lower number of small fibers (<1500 pixels) and higher number of large fibers (>7000 pixels), confirming that the treadmill exercise did change the physical parameters in BCAS mice. When CBF of sedentary and treadmill BCAS mice was measured on day 13 (after the 1-week habituation for treadmill) and on day 48 (after the full-length treadmill program) to evaluate the effect of exercise on the blood flow, both BCAS/sedentary and BCAS/treadmill groups showed the mild CBF recovery as expected [16] and there was no significant difference between those two groups at either time point (Figure 1D).
Amelioration of cognitive decline in BCAS mice after exercise
At 13 and 48 days after BCAS, the standard Spontaneous Alternation Y-maze was used to track working memory in sedentary versus treadmill-exercised mice. In sedentary mice, the number of spontaneous alternation significantly decreased over time, indicating a deterioration in working memory (Figure 1E, P=0.04), as expected [15]. In contrast, there was no significant decline in alternation in exercised mice (Figure 1E, P=0.2), indicating that the progressive deterioration in memory was ameliorated by treadmill exercise. No difference was observed in spontaneous activity (Supplementary Figure S2A, P=0.8 (Sedentary), P=0.8 (Treadmill)). To further determine the effect of exercise on recognition memory, the ratio of the time spent on new object to the total time spent on familiar and new objects was measured by NORT. While there was no difference at day 14, the value was significantly higher for mice in treadmill group after 5 weeks (day 49) of full-length exercise (Figure 1F, P=0.006), supporting our hypothesis that exercise is effective in preserving cognitive function in the hypoperfused BCAS mice. In addition, when spatial learning and memory were assessed in the Morris water maze test, there was no difference between the groups during acquisition (Figure 1G, P=0.8). However, number of entries into the target quadrant in the probe trial (conducted 4 hours after the last acquisition trial) was significantly decreased in the BCAS sedentary group compared to sham-operated sedentary group (Figure 1H, P=0.03) while no significant decline was observed in BCAS treadmill group (Figure 1H, P=0.6), suggesting a preventive effect of exercise towards memory loss caused by cerebral hypoperfusion.
Cellular effects of exercise in white matter after BCAS
Within the sensitivity limits of corpus callosum whole tissue homogenates, western blots suggested that there were no overall differences in MBP in exercised versus sedentary mice after BCAS (Figure 2A and C, P=0.4). However, probing for platelet derived growth factor receptor alpha (PDGFRα) suggested that exercise increases this protein marker of OPCs (Figure 2B–C, P=0.02). To further assess the potential cellular effects of exercise, we used quantitative immunostaining to examine two known pockets of mitogenic activity in the adult CNS, i.e. the subventricular zone (SVZ) and the subgranular zone (SGZ). In a discrete area of corpus callosum adjacent to the lateral ventricle and next to SVZ, immunostaining demonstrated a population of OPCs (Figure 2D). Within this region, the number of OPCs was significantly increased in exercised mice compared to sedentary mice (Figure 2E–F, P=0.03). In contrast, there were no significant differences in nestin-positive neural progenitor cells (Figure 2F, P=0.5), indicating that the increase in OPCs was not due to a non-specific change to immature progenitor cells. Consistent with this observation, there were also no significant differences in nestin-positive or Ki-67-positive cells in the SGZ of the hippocampus (Figure 2G–I, P=0.9 (Nestin), P=0.5 (Ki-67)).
Figure 2.
(A), (B) Western blot image of MBP and PDGFRα. S: Sedentary, T: Treadmill. (C) Semi-quantification revealed that PDGFRα was significantly increased in the treadmill group (*P<0.05, Welch’s t-test). Data represents the observations from 5 mice for each group. (D) Schematic view of the ROI predetermined for the evaluation of SVZ. SVZ was defined as an area surrounded by white dashed lines. (E) Immunohistochemistry showed that the number of PDGFRα-positive cells (colored with red) is increased within the SVZ. Bar = 100 μm. (F) Intensity of PDGFRα-positive cells is increased in the treadmill group (*P<0.05, Welch’s t-test. Four ROIs examined per mouse). Data from 5 mice for each group. (G) Schematic view of the ROI predetermined for the evaluation of dentate gyrus in the hippocampus. Area for immunofluorescence measurement was defined as an area surrounded by white-dashed lines. (H), (I) Immunohistochemistry showed that neither nestin-positive nor Ki-67-positive cells were increased within the area of interest. Bar = 100 μm. (Four ROIs examined per mouse). Data from 5 mice for each group. CC: corpus callosum, CP: caudate putamen, DG: dentate gyrus, GCL: granule cell layer, LV: lateral ventricle, ML: molecular layer, Pα: PDGFRα.
Discussion:
Our current study demonstrated that treadmill exercise ameliorates the decline of working memory in mice with prolonged cerebral hypoperfusion. We also showed that exercise induces an increase of OPCs in the white matter, especially within white matter regions adjacent to the SVZ. A number of basic studies investigating the efficacy of exercise in preventing cognitive decline have attributed its positive effect to a reversal of pathological alterations in the cerebral limbic system [9–11, 17, 18]. However, despite recent data showing that structural abnormalities of the white matter is associated with a high risk of disease progression in AD patients [19–21] and that preservation of myelin may be beneficial in the treatment of dementia, there are only a few basic studies investigating the effect of exercise on white matter in dementia models [22–25]. To the best of our knowledge, there are only two previous studies that evaluate the effectiveness of exercise in preventing white matter degeneration caused by chronic cerebral hypoperfusion [24, 25]. In these studies using a rat model of SIVD, trained rats had a greater number of proliferating OPCs within their corpus callosum, and performed better in behavioral tests. Our findings further add to this existing literature by localizing the area in which the number of OPCs increases. However, our results differ from the previous report in that we did not find a significant difference in myelin density between the sedentary and exercise group. This discrepancy may be explained by the fact that CBF levels after hypoperfusion may differ between the rat versus mouse model, as the dramatic decline of CBF in the rat model during the perioperative period could lead to more severe demyelination compared to our mouse model [26].
An important aspect of our current study is the novel finding that OPCs in the SVZ region may be sensitive to the effect of treadmill exercise. OPCs are known to be active during the developmental phase and generate mature oligodendrocytes that would eventually form myelin sheaths. Although formation of myelinated tracts occurs at an early stage of life, their renewal lasts throughout life due to the presence of residual OPCs and neural stem cells (NSCs) that can transform into OPCs when needed [27–30]. In adult brains, residual OPCs outside the SVZ and NSCs inside the SVZ both proliferate and differentiate to conduct endogenous repairs in response to white matter injury [31–33]. NSC-derived Olig2-expressing cells, located in SVZ, differentiate into highly migratory OPCs which are situated in close proximity to blood vessels. The vessels serve as a scaffold for the migration to the area of injury by releasing chemoattractants such as brain-derived neurotropic factor (BDNF) [32–35]. Since exercise is known to upregulate systemic growth factors such as BDNF [36], it is possible that exercise-induced increases in trophic factors and their associated molecular signals underlie the effects of exercise on OPCs.
Although we have demonstrated the treadmill exercise ameliorates white matter damage caused by cerebral hypoperfusion, there are some limitations and caveats to this study. First, we have not yet confirmed how the histological changes documented in the exercised mice contribute to the alleviation of cognitive decline. Additional time-course studies would be necessary to capture cell type transformation induced by prolonged cerebral hypoperfusion, from NSPC to OPC within the SVZ, and to trace the fate of those cells after they migrate from the SVZ to the site of injury. Another next goal would be an application of BCAS to genetically or pharmacologically manipulated mice fated to lose functional activity of the OPCs. Investigations of their post-surgical behavior could consolidate the interconnection between the OPCs and cognitive function. Second, we lack values for blood gas and white matter CBF data during exercise. Arterial blood gas measurements were initially considered for screening hypercapnia but was not executed in order to avoid giving additional stress to the mice. Although none of the mice were excluded from the study by pre- and post-training neurological examination conducted to assess possible exercise-induced stroke (either ischemic or hemorrhagic), there could have been a change in CBF during exercise, which would have affected our results. Our result of laser-doppler flowmetry on days 13 and 48 only indicates that treadmill exercise may not affect the blood flow of the superficial arteries. Therefore, we cannot rule out the possibility that exercise may affect the white matter CBF, and utilization of imaging technology such as optical coherence tomography should be considered to capture the effect of exercise towards capillary blood flow of the white matter. Third, systemic factors released into circulation during exercise are still undetermined. Comprehensive screening of candidate factors using blood samples and an immunohistological approach to confirm how those factors are distributed within the brain are essential in future experiments. The final caveat of our study is that we used only young male mice for our experiment. Since differences in age and gender may alter the dynamics of systemic humoral factors as mentioned in published literature [37–39], it will be important to determine if our results could be replicated in old male mice and in female mice.
In conclusion, we have demonstrated that treadmill exercise suppresses cognitive decline in mice with prolonged cerebral hypoperfusion that mimics the pathophysiology of SIVD. This beneficial effect of exercise may be associated partly with an increase of OPCs in white matter adjacent to the SVZ.
Supplementary Material
Acknowledgements:
Supported in part by National Institutes of Health (R01 NS065089, P01 NS055104, R01 AG055559, R01 NS113556), JSPS KAKENHI (16H05319). The authors thank Nobuhiro Okagaki and Shuntaro Oribe for technical assistance.
Funding: This study was funded by National Institutes of Health (R01 NS065089, P01 NS055104, R01 AG055559, R01 NS113556) and JSPS KAKENHI (16H05319).
Footnotes
Disclosures: None.
Compliance with Ethical Standards:
Ethical approval: All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. This article does not contain any studies with human participants performed by any of the authors.
Conflict of Interest: All authors declare that they have no conflict of interest.
References
- 1.Erkinjuntti T, et al. , Research criteria for subcortical vascular dementia in clinical trials. J Neural Transm Suppl, 2000. 59: p. 23–30. [DOI] [PubMed] [Google Scholar]
- 2.Erkinjuntti T, et al. , Limitations of clinical criteria for the diagnosis of vascular dementia in clinical trials. Is a focus on subcortical vascular dementia a solution? Ann N Y Acad Sci, 2000. 903: p. 262–72. [DOI] [PubMed] [Google Scholar]
- 3.Roman GC, et al. , Subcortical ischaemic vascular dementia. Lancet Neurol, 2002. 1(7): p. 426–36. [DOI] [PubMed] [Google Scholar]
- 4.Prins ND and Scheltens P, White matter hyperintensities, cognitive impairment and dementia: an update. Nat Rev Neurol, 2015. 11(3): p. 157–65. [DOI] [PubMed] [Google Scholar]
- 5.Roh JH and Lee JH, Recent updates on subcortical ischemic vascular dementia. J Stroke, 2014. 16(1): p. 18–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wallin A, et al. , Update on Vascular Cognitive Impairment Associated with Subcortical Small-Vessel Disease. J Alzheimers Dis, 2018. 62(3): p. 1417–1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fried TR, et al. , Health outcomes associated with polypharmacy in community-dwelling older adults: a systematic review. J Am Geriatr Soc, 2014. 62(12): p. 2261–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stephen R, et al. , Physical Activity and Alzheimer’s Disease: A Systematic Review. J Gerontol A Biol Sci Med Sci, 2017. 72(6): p. 733–739. [DOI] [PubMed] [Google Scholar]
- 9.Nigam SM, et al. , Exercise and BDNF reduce Abeta production by enhancing alpha-secretase processing of APP. J Neurochem, 2017. 142(2): p. 286–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gratuze M, et al. , Differential effects of voluntary treadmill exercise and caloric restriction on tau pathogenesis in a mouse model of Alzheimer’s disease-like tau pathology fed with Western diet. Prog Neuropsychopharmacol Biol Psychiatry, 2017. 79(Pt B): p. 452–461. [DOI] [PubMed] [Google Scholar]
- 11.Baek SS and Kim SH, Treadmill exercise ameliorates symptoms of Alzheimer disease through suppressing microglial activation-induced apoptosis in rats. J Exerc Rehabil, 2016. 12(6): p. 526–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu-Ambrose T, et al. , Aerobic exercise and vascular cognitive impairment: A randomized controlled trial. Neurology, 2016. 87(20): p. 2082–2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shibata M, et al. , White matter lesions and glial activation in a novel mouse model of chronic cerebral hypoperfusion. Stroke, 2004. 35(11): p. 2598–603. [DOI] [PubMed] [Google Scholar]
- 14.Yang Y, et al. , Rodent Models of Vascular Cognitive Impairment. Transl Stroke Res, 2016. 7(5): p. 407–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shibata M, et al. , Selective impairment of working memory in a mouse model of chronic cerebral hypoperfusion. Stroke, 2007. 38(10): p. 2826–32. [DOI] [PubMed] [Google Scholar]
- 16.Ohtomo R, et al. , Cilostazol alleviates white matter degeneration caused by chronic cerebral hypoperfusion in mice: Implication of its mechanism from gene expression analysis. Neurosci Lett, 2018. 662: p. 247–252. [DOI] [PubMed] [Google Scholar]
- 17.Maejima H, et al. , Exercise enhances cognitive function and neurotrophin expression in the hippocampus accompanied by changes in epigenetic programming in senescence-accelerated mice. Neurosci Lett, 2018. 665: p. 67–73. [DOI] [PubMed] [Google Scholar]
- 18.Parrini M, et al. , Aerobic exercise and a BDNF-mimetic therapy rescue learning and memory in a mouse model of Down syndrome. Sci Rep, 2017. 7(1): p. 16825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Giulietti G, et al. , Whole brain white matter histogram analysis of diffusion tensor imaging data detects microstructural damage in mild cognitive impairment and alzheimer’s disease patients. J Magn Reson Imaging, 2018. [DOI] [PubMed] [Google Scholar]
- 20.Zhuang L, et al. , Microstructural white matter changes in cognitively normal individuals at risk of amnestic MCI. Neurology, 2012. 79(8): p. 748–54. [DOI] [PubMed] [Google Scholar]
- 21.Zhuang L, et al. , Microstructural white matter changes, not hippocampal atrophy, detect early amnestic mild cognitive impairment. PLoS One, 2013. 8(3): p. e58887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang L, et al. , Exercise Prevents Cognitive Function Decline and Demyelination in the White Matter of APP/PS1 Transgenic AD Mice. Curr Alzheimer Res, 2017. 14(6): p. 645–655. [DOI] [PubMed] [Google Scholar]
- 23.Zhang Y, et al. , Effects of exercise on capillaries in the white matter of transgenic AD mice. Oncotarget, 2017. 8(39): p. 65860–65875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jiang T, et al. , Physical Exercise Improves Cognitive Function Together with Microglia Phenotype Modulation and Remyelination in Chronic Cerebral Hypoperfusion. Front Cell Neurosci, 2017. 11: p. 404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee JM, et al. , The ameliorative effects of exercise on cognitive impairment and white matter injury from blood-brain barrier disruption induced by chronic cerebral hypoperfusion in adolescent rats. Neurosci Lett, 2017. 638: p. 83–89. [DOI] [PubMed] [Google Scholar]
- 26.Farkas E, Luiten PG, and Bari F, Permanent, bilateral common carotid artery occlusion in the rat: a model for chronic cerebral hypoperfusion-related neurodegenerative diseases. Brain Res Rev, 2007. 54(1): p. 162–80. [DOI] [PubMed] [Google Scholar]
- 27.Dimou L, et al. , Progeny of Olig2-expressing progenitors in the gray and white matter of the adult mouse cerebral cortex. J Neurosci, 2008. 28(41): p. 10434–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Young KM, et al. , Oligodendrocyte dynamics in the healthy adult CNS: evidence for myelin remodeling. Neuron, 2013. 77(5): p. 873–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maki T, et al. , Mechanisms of oligodendrocyte regeneration from ventricular-subventricular zone-derived progenitor cells in white matter diseases. Front Cell Neurosci, 2013. 7: p. 275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Takagi T, et al. , Novel Regenerative Therapies Based on Regionally Induced Multipotent Stem Cells in Post-Stroke Brains: Their Origin, Characterization, and Perspective. Transl Stroke Res, 2017. 8(6): p. 515–528. [DOI] [PubMed] [Google Scholar]
- 31.Menn B, et al. , Origin of oligodendrocytes in the subventricular zone of the adult brain. J Neurosci, 2006. 26(30): p. 7907–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gonzalez-Perez O and Alvarez-Buylla A, Oligodendrogenesis in the subventricular zone and the role of epidermal growth factor. Brain Res Rev, 2011. 67(1–2): p. 147–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gonzalez-Perez O, et al. , Epidermal growth factor induces the progeny of subventricular zone type B cells to migrate and differentiate into oligodendrocytes. Stem Cells, 2009. 27(8): p. 2032–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shen Q, et al. , Endothelial cells stimulate self-renewal and expand neurogenesis of neural stem cells. Science, 2004. 304(5675): p. 1338–40. [DOI] [PubMed] [Google Scholar]
- 35.Snapyan M, et al. , Vasculature guides migrating neuronal precursors in the adult mammalian forebrain via brain-derived neurotrophic factor signaling. J Neurosci, 2009. 29(13): p. 4172–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cotman CW, Berchtold NC, and Christie LA, Exercise builds brain health: key roles of growth factor cascades and inflammation. Trends Neurosci, 2007. 30(9): p. 464–72. [DOI] [PubMed] [Google Scholar]
- 37.Stanford KI, et al. , Maternal Exercise Improves Glucose Tolerance in Female Offspring. Diabetes, 2017. 66(8): p. 2124–2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McMullan RC, et al. , Long-term exercise in mice has sex-dependent benefits on body composition and metabolism during aging. Physiol Rep, 2016. 4(21). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Herson PS, Palmateer J, and Hurn PD, Biological sex and mechanisms of ischemic brain injury. Transl Stroke Res, 2013. 4(4): p. 413–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.