1. Introduction
Effective aids to smoking cessation are available,[1] however, the majority of smokers (between 70% and 90%) are not actively preparing to quit at any given time.[2] Tools and experiences designed specifically for smokers who are not trying to quit can help direct them towards successful quit attempts earlier and more effectively. In the Phased Framework of tobacco cessation interventions,[3] smokers are categorized into four phases: 1) motivation, 2) pre-cessation, 3) cessation, and 4) maintenance. Those not actively preparing to quit are ‘motivation phase’ smokers. Although some inveterate motivation phase smokers are unwilling to quit (estimated between 29% and 31%),[4, 5] many may be thinking of quitting in the future. Health education and motivational interventions can induce cessation behaviors among lower-motivated smokers at an accelerated rate.[2, 6] Trials to motivate smokers not ready to quit are termed “cessation induction trials”, and are far less frequent than the common “aid to cessation” trials that recruit pre-cessation and cessation phase smokers.
While most smoking interventions target smokers actively attempting to quit,[7] prior cessation-induction interventions focused on motivation phase smokers have successfully engaged up to half of the smokers in brief, non-cessation experiences.[2, 6] These experiences frequently consist of Nicotine Replacement Therapy (NRT) sampling during a brief abstinence period.[8, 9] NRT sampling refers to a brief course of NRT provided to smokers who are not currently interested in quitting smoking. Short-term, pre-cessation NRT sampling can help induce cessation and is safe for active smokers.[10–12] Non-cessation experiences designed to teach new skills and increase self-efficacy[13, 14] for future quit attempts could add benefits to NRT sampling for motivation phase smokers. However, few rigorous trials of behavioral interventions specifically targeting motivation phase smokers have been reported.
A State of the Science conference on tobacco research emphasized that interventions need to be palatable and engaging for all smokers so that they are supported, desired, and asked-for.[15] Framing smoking interventions as brief games may increase motivation phase smokers’ willingness to engage. Drawing from behavioral theory, game mechanics can provide immediate rewards while combining entertainment with motivation.[16–18] Effective games reinforce desired behaviors by rewarding participants for achievements while providing helpful feedback and support.[18–20] This gaming framework can guide the delivery and content of smoking intervention components to enhance smoker motivation and engagement in pre-cessation interventions.
Below we describe the protocol of our multi-site randomized controlled trial to test a pre-cessation, gamified intervention for motivation phase smokers. The intervention, called Take a Break, is a 3-week game experience, and includes components designed to maintain participation, enhance self-efficacy, and obtain new skills to support future long-term quit attempts. Smokers are encouraged to engage with the intervention components described below and set abstinence goals at specified time points during the intervention period. Along the way, smokers receive participation points and compete with others, and themselves, during the Take a Break intervention experience.
2. Methods
2.1. Study Design
Study participants are recruited and randomized to one of two groups (see Figure 1) and blinded to study group assignment (Take a Break intervention group versus NRT-only comparison group). Participants are requested to complete follow-up assessments three weeks and six months after enrollment. We hypothesize that compared with smokers in the comparison group, those in the Take a Break intervention group will have: Hypothesis 1) greater mean number of days abstinent during the first three weeks; Hypothesis 2) greater increase in self-efficacy comparing baseline and at the end of the first three weeks; Hypothesis 3) lower time to first quit attempt during the 6-month study period; and Hypothesis 4) higher rates of 7-day point prevalent carbon-monoxide verified abstinence measured at the 6-month final visit. Hypotheses 1 and 2 will test the process of the Take a Break experience and Hypotheses 3 and 4 will be used to test the effect of the Take a Break intervention on outcome measures. As this is a motivational study, Hypothesis 3 will be considered the primary hypothesis. This study is approved by the Institutional Review Boards (IRBs) at each participating study site.
Figure 1:
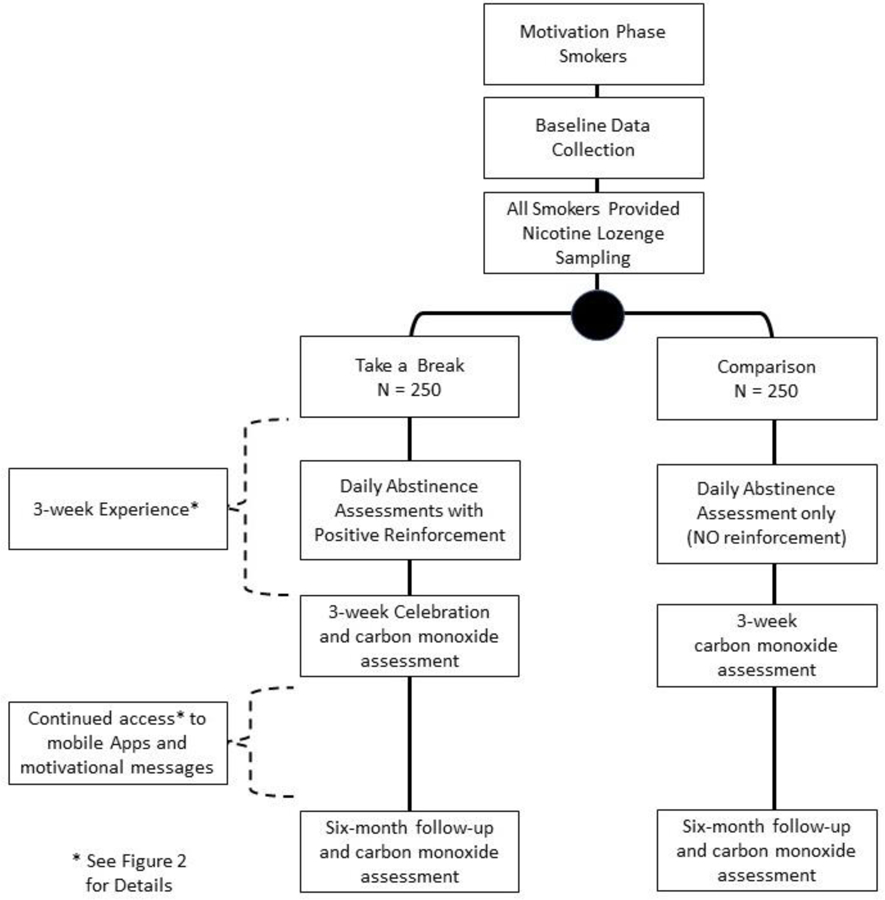
Study Workflow Diagram
2.2. Study Participants
This study targets motivation phase smokers, those who are not preparing to quit, or already actively trying to quit. Inclusion criteria specifies that smokers are over 18 years of age, English speaking, actively smoking cigarettes, and not trying to quit smoking at the time of recruitment. Pregnant women, prisoners and smokers with active depression symptoms at time of recruitment are excluded from the study. Depression symptoms are assessed using the Patient Health Questionnaire-2 (PHQ-2) tool.[21] Those with a PHQ-2 cut-off score of ≥3, the score recommended for depression diagnosis, [18] are excluded.
2.3. Take a Break Intervention Overview
The Take a Break intervention was developed with a user-centered design approach[22] for smokers not ready to quit.[23] The intervention components were specifically conceptualized to increase self-efficacy (perceived capacity to quit).[13, 14] Tailored for lower-motivated smokers, Take a Break acknowledges that quitting is a personal choice and allows for variability in participation. Smokers can engage with as little or as much of the Take a Break experience as they choose. To increase engagement (defined as active participation in the Take a Break components) and enhance motivation, Take a Break uses game mechanics, as described in more detail in our formative research,[24] and motivation messaging adapted from prior interventions shown to successfully assist smokers in quitting.[25] As part of the user-centered design of the intervention, usability testing of each intervention component by smokers (n=7) with think aloud protocol[24, 26] and feasibility pilot testing to assess engagement with smokers (n=41) were conducted.[26] In the following paragraphs, we first describe the 3-week Take a Break experience, followed by a description of each of the intervention components.
The 3-week Take a Break experience includes a training week followed by a 2-week game challenge. The week 1 training is designed to help prepare the smoker to participate in the following 2-week challenge. At the end of week 1, Take a Break participants are contacted by phone by a Tobacco Treatment Specialist (TTS). During this brief 20–30-minute call, the week 1 training experience is reviewed, and smokers are asked if they wish to set an abstinence goal for the challenge. The goal is self-determined and can range from 0 to the total 14 days. The goal establishes their personal challenge - the number of days they want to try “taking a break” from smoking during the 2-week challenge. This ‘abstinence challenge’ is the core of the game experience. Take a Break intervention components are designed to help the smokers prepare and meet the challenge, as well as learn skills during the attempt (Figure 2). Across the 3-week experience, smokers accumulate recognition points and rewards for participation. In the following paragraphs, additional details on the key components of the Take a Break intervention and the rational for each are provided.
Figure 2: The Take a Break Three-Week Experience with Five Behavior Support Components.
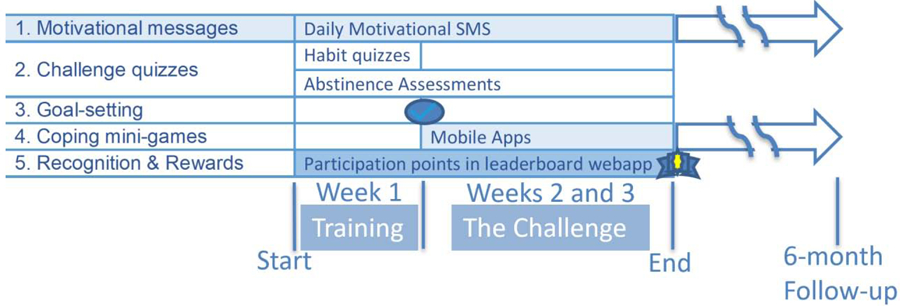
Week 1 is the training week. In this week, the experience focuses on:
1. Motivational Messages: tailored for smokers not ready to quit
2. Challenge Quizzes: to assess habits (cravings and urges) and daily abstinence assessments (number of cigarettes smoked in past 24 hours) with positive feedback.
At the end of Week 1:
3. Goal-setting call: a brief 20–30-minute call with the week 1 training experience reviewed and smokers asked if they wish to set an abstinence goal.
Weeks 2 and 3 are the “The Challenge” where smokers work toward their abstinence goal:
During The Challenge, Motivational Messages and daily abstinence assessments with positive feedback continue. The challenge focuses on achieving the abstinence goal and includes:
4. Coping mini-games: apps encouraged to use for distraction and/or relaxation to cope with cravings - see also Figure 3.
5. Recognition and Rewards: throughout the three weeks smokers receive points for responding to Challenge quizzes and medals/rewards at the 3-week follow-up visit.
*Note that all participants receive NRT sampling (Seventy-two Nicorette lozenges provided so smokers may use them during the challenge).
Motivational Messages
Motivational text messages are provided daily throughout the 3-week experience. These messages were adopted from an effective computer-tailored motivational messaging system evaluated in a prior trial.[25, 27, 28] The motivational message system includes expert messages (written by smoking cessation providers and researchers) and peer messages (written by smokers to motivate other smokers).[28] All messages in the system have been previously rated by former and current smokers and message phrasing is tailored to smoker’s stage of readiness to quit.[29, 30] In the Take a Break intervention, the highest rated messages tailored for smokers who are not ready to quit are sent out daily during the three weeks (1-week training and 2-week challenge) of the intervention. After the 3-week Take a Break experience, the motivational messages continue at a rate of twice per week for six months from registration.
Challenge Quizzes
During the 1-week training, intervention participants receive daily self-assessment text messages and motivational replies. The first daily assessment asks, “Over the past 24 hours, about how many cigarettes have you smoked?” Participants are directed to reply to the text message with a numerical (digit) response “such as 0, 10, or 24”. Intervention participants who reply to the daily question are sent a motivational reply followed by a “Bonus Question” to assess their smoking cravings and urges. Responses to the daily assessment and Bonus Questions are used to inform the 1-week TTS goal-setting phone call and to create an opportunity for participants to reflect on their current smoking behaviors. After the TTS call, the 2-week challenge begins. The daily self-assessment challenge quizzes are continued, along with motivational replies and messages reinforcing participation, as described above.
Goal-Setting Call
After completing the 1-week training, participants have a goal-setting telephone call with a TTS. During this call, data from the baseline questionnaire and week 1 challenge quizzes are reviewed, including the participant’s smoking history, status, cravings, and urges. Motivational interviewing techniques [31] are used to encourage participants to begin thinking about quitting. At the end of the call, participants are asked if they would like to set an abstinence goal for the following 2-week challenge.
Coping Mini-Games
Take a Break intervention participants are encouraged to use apps to help manage smoking cravings by providing relaxation or distraction (Figure 3). After testing a distraction app developed by our team,[32] we discovered that individuals have differing preferences for game complexity and difficulty. Thus, we pilot tested a series of games freely available for common smart phone operating systems with current smokers (n=7).[24] All apps included in the intervention are frequently downloaded, highly rated by users, free to download, and available on both iOS and Android platforms. To accommodate varying individual preferences, intervention participants choose from a menu of three distraction-related and three relaxation-related apps. The distraction apps suggested to choose from are Wordscapes, Piano Tiles, and Flow Free. The relaxation apps suggested to choose from are Calm, Take A Break (unrelated to the Take a Break intervention), and Breathe2Relax (Figure 3). Participants are given a brief trial of each app during enrollment before they select their preferred apps. Note that the Take a Break team did not develop these apps, nor do we have any competing interest associated with their use. Participants are also given the option to use alternative apps that they already use on their phone. In such cases, the alternative apps used are documented at the baseline visit.
Figure 3. The Six Coping Mini Games Provided to Manage Cravings with Distraction and Relaxation during the The Take a Break Challenge.

We pilot tested a series of games freely available for common smart phone operating systems with current smokers. All six apps included in the Take a Break are frequently downloaded, highly rated by users, free to download, and available on both iOS and Android platforms. Note that the Take a Break team did not develop these Apps nor do we have any competing interest associated with their use.
Recognition & Rewards
In all 3 weeks, Take a Break participants receive recognition for participation, and these accumulate into a reward at study end. Participants provide an alias during enrollment to track points earned on a leaderboard. Study staff explains that points are rewarded for participation in the intervention such as replying to daily challenge quizzes and completing the 3-week follow-up visit. Importantly, note that rewards are not given for abstinence, only participation. The rationale was that we did not want to over-incentivize reports of abstinence and create bias in reporting. Thus, participants received points after responding, regardless of the number of cigarettes smoked. All participants receive 100 points to start (at baseline) and for returning for 3-week follow-up visit, and 10 additional points per response to a text question. The leaderboard tracks the number of points accumulated and is used to reward participants at the 3-week follow-up visit, with the top 25% receiving a ‘Gold medal’ and $15 gift card, the middle 50% receiving a ‘Silver medal’ and $10 gift card, and the lowest 25% receiving a ‘Bronze medal’ and $5 gift card. All participants who return for 3-week follow-up appointment therefore receive a reward.
NRT Sampling
NRT sampling, defined as provision of a brief NRT starter kit,[33]were provided to Take a Break intervention participants. Intervention participants were encouraged to consider using the NRT during the two-week abstinence challenge. Based on successful prior trials, smokers were provided Nicorette nicotine lozenge with 2-mg or 4-mg doses, based on individual smoking level (per package instructions). Seventy-two lozenges were provided for the two weeks. Again, smokers could choose whether they used the NRT samples or not.
2.4. Comparison group
To isolate the effect of the Take a Break game experience, we chose an active comparison. As prior trials have demonstrated the effectiveness of short-term NRT sampling, we provided the samples (72 lozenges) to the comparison group. The comparison group does not receive the motivational messages, challenge quizzes with feedback, goal-setting, or recognition and rewards components of the Take a Break intervention.
Further, we also controlled for attention, or the level of interaction between the study staff and participants, in the comparison group. Comparison participants received a 1-week telephone call, and 3-week visit that did not include the active components of the Take a Break intervention (i.e.: no review of week 1 experience, no goal-setting, no recognition and reward at 3-week visit). Similarly, and for assessment purposes, the comparison received the daily text measurement of number of cigarettes smoked but did not receive the motivational reply or reply reward points in the Take a Break intervention.
2.5. Recruitment, randomization, and allocation concealment
Recruitment and Informed Consent
In the study, both in-person and telephone recruitment methods are used to enroll the target sample of motivation phase smokers. The recruitment target was calculated to ensure adequate statistical power to detect a difference in time to first quit attempt (Hypothesis 3) and 7-day point prevalent carbon-monoxide verified abstinence measured after 6 months (Hypothesis 4). We calculated that we would need analyzable data for 162 participants per group to maintain 80% statistical power to detect a difference in days of one third the standard deviation of the expected overall time to first quit attempt (estimated at mean (SD) of 121 (64)). We calculated that we would need analyzable data for 170 participants per group to maintain 80% statistical power to detect a difference of 11% in six-month cessation, considering a cessation rate in comparison group of 10%. Assuming an attrition rate of 15–20%, we targeted a recruited sample of at least 440 smokers. The attrition rate of 15–20% is comparable to attrition rates observed in previous smoking cessation studies.[34]
In-person recruitment is conducted during routine clinical appointments for patients who screened positive for tobacco-use and meet the study inclusion criteria. To facilitate telephone recruitment, a registry of smokers was created to proactively recruit smokers across the study sites. Study sites include University of Massachusetts Medical School (UMMS), Northwell Health, VA Central Western MA (VACWM) and Reliant Medical Group (RMG). HIPAA waivers of authorization were obtained to identify potential participants using each site’s Electronic Health Record (EHR) system. Patients identified as being active smokers in the EHR and having at least one primary care visit in the preceding twelve months are added to the smoker registry. An “opt-out” recruitment strategy is then used to recruit study participants. In this strategy, potential participants are mailed an initial communication which includes a letter from the study’s principal investigator outlining the purpose of the study and a stamped “opt-out” postcard to mail back if they wish to be removed from the recruitment list. Potential participants who do not opt-out after two weeks are phoned to determine interest and screen for eligibility. Smokers who do not meet the inclusion criteria are excluded from the study. Interested and eligible patients schedule an in-person appointment to provide written informed consent, complete a baseline questionnaire, and randomized into a study group.
Randomization and Allocation Concealment
To execute randomization, the study biostatistician generated a randomization table using a sequence of two random blocks of different sizes (four and eight) to assure balance between the study groups. Upon completing the baseline questionnaire at the baseline visit, enrolled participants are randomized by an automated program using the pre-specified randomization table. Study staff then provides training to participants according to their group assignments. Participants are blinded to Take a Break intervention versus comparison group.
2.6. Data Collection and Safety Monitoring
Data are collected throughout the study via text-based assessments, questionnaires, and carbon monoxide (CO) readings. Text-based assessment responses to the daily Challenge Quizzes are collected throughout the training and challenge periods. Data for the mean number of days abstinent from smoking during the challenge period are calculated from text message responses. Questionnaire data are collected at the baseline visit, the 1-week phone call, the 3-week follow-up visit, and the 6-month final visit. Data collection at the follow-up visits is conducted by study staff members blinded to randomized group allocations. Data collected at these visits include patient characteristics and smoking history (see Table 1 for a summary of data collected). Carbon monoxide (CO) readings are collected at the baseline, 3-week follow-up, and 6-month final visits. CO readings are assessed using Covita Micro+ Pro CO meters and are collected with the time of the last cigarette smoked. Questionnaires are completed on paper and include validated scales assessing social and smoking behavior-related factors (See Table 1). All questionnaire and CO data collected are manually entered into a REDCap data collection system by study staff. Study staff are blinded to group allocation when completing follow-up assessments of smoking status.
Table 1:
Key Data Elements
| HER registry | Baseline | Challenge | Follow-up (3-week, 6-month) | |
|---|---|---|---|---|
| Patient demographics | X | X | ||
| Level of Addiction[45] | X | |||
| Medical Comorbidities | X | X | ||
| Depression scale (CES-D)[46–48] | X | |||
| Past Quit History[49] | X | |||
| Readiness to Quit | X | |||
| Self-efficacy (SEQ-12)[50] | X | X | ||
| Cessation Treatment Beliefs[51] | X | X | ||
| Smoking Urges (QSU)[52] | X | X | ||
| Abstinence Reports (daily) | X | |||
| Challenge Quizzes | X | |||
| Use of Mini-Games | X | X | ||
| Quit Attempts | X | |||
| Smoking Cessation* (biochemically verified at 3-week and 6-month follow-up visits) | X |
Prior to the start of recruitment, a Data Safety and Monitoring Board (DSMB), consisting of three external faculty members, was assembled to review the study protocol, suggest revisions, and provide approval to commence recruitment. The external DSMB members specialize in smoking cessation interventions, biostatistics, and clinical research studies. The DSMB meets twice per year to ensure the safety of participants and integrity of the data collected, and to provide recommendations to the study team.
2.7. Primary and Secondary Outcomes
The primary outcome for the study is the time to first quit attempt. Secondary outcomes include mean number of days abstinent from smoking during the challenge, change in patient-reported self-efficacy after the challenge, and 7-day point prevalent abstinence after six months. The date of the first quit attempt after the challenge is collected at the 6-month final visit using the Timeline Follow-back (TLFB) Method Assessment. The TLFB has been shown to be a reliable method of assessing patterns of change in smoking and cessation over extended time frames.[35] Participants are asked if they had a quit attempt in the past six months and if so, to indicate on a calendar the start of their first attempt. Start and end dates of all quit attempts during the follow-up period are collected. Change in self-efficacy is calculated from completion of the Self-Efficacy Questionnaire (SEQ-12) during enrollment and the 3-week follow-up visit.[36] Point prevalent abstinence is measured at the 6-month final visit using the single item question: “Do you currently smoke cigarettes (smoked even 1 puff in the last 7 days)?”
2.8. Analytic Plan
The primary outcomes analysis will be based on the intent-to-treat principle. In process measure analyses, we will test whether the mean number of days abstinent during the challenge period will be greater in the intervention group than in the comparison group (Hypothesis 1) by computing average number of days reported abstinent for each participant over the 2-week challenge and use a t-test to compare the mean number of days abstinent between groups. We will then calculate adjusted effect size using a regression model, including subject characteristics different between groups as covariates. To test whether the intervention group reports a greater increase in self-efficacy than the comparison group (Hypothesis 2), a 2-sample t-test will be used to compare the mean change in scores and then a regression model to adjust for potential confounders (e.g., baseline SEQ score, subject characteristics, and site effects). Unadjusted and adjusted effect size of the intervention on the SEQ change score will then be calculated.
For the primary outcome (Hypothesis 3), we will compare the survival time (time to quit), in the two groups. We will first produce Kaplan-Meier curves for the two study groups and use the log-rank test to test the null hypothesis of no difference in time to first quit attempt between groups. We will then use Cox regression models to estimate the ratio of the probability of any quit attempt for intervention vs. comparison groups adjusting for covariates (e.g., subject characteristic and site effects).[37]
A secondary outcome is six-month carbon-monoxide verified smoking cessation (Hypothesis 4). To test whether point prevalent abstinence measured at six months will be greater in the intervention group than in the comparison group, we will assess differences in percent distributions and means (with standard deviations) with a logistic regression model to provide the odds of cessation in the intervention group compared to the comparison group.
3. Discussion
With the support of policy changes, public health and clinical interventions, and behavioral and pharmacotherapy, we have seen progress in smoking cessation. The prevalence of cigarette smoking declined from 21% in 2005 to 14% in 2017.[38] Those who remain smokers are complex. Many of these individuals who continue to smoke are less motivated and will require creative strategies to capture their attention and engagement. Responding to the need for evaluation of additional cessation-induction innovations designed to support smokers who are not yet ready to quit,[7] we designed Take a Break. As described, Take a Break is a brief, self-directed game experience. By allowing smokers to practice cessation-supporting strategies for quitting over the 3-week experience, we aim to improve smoker self-efficacy. In the Take a Break experience, smokers can gain knowledge, develop skills and experience using tools needed to support a cessation attempt when they are ready to quit. In special situations, some smokers may decide to turn the initial brief abstinence into permanent cessation.
To attract smokers who are not ready to quit, the intervention was designed to be brief, accessible, and engaging. Brief, short-term health behavior change is easier to obtain than long-term change, and more likely to encourage motivation phase smokers to further pursue smoking cessation. During recruitment, we emphasized that the primary goal of Take a Break is not for participants to quit smoking tobacco by the end of the experience, but rather to be better prepared with information, tools, and practice for when they are ready for a real quit attempt. Our assumption is that use of cessation induction techniques in Take a Break will serve as a motivating and learning experience likely to prompt quit attempts among smokers previously uninterested in quitting.
In anticipation of challenges enrolling this population of smokers, recruitment occurs both in-person during clinical appointments and over the telephone using a large registry of smokers. By using the opt-out mailing recruitment method, we provide smokers the opportunity to decline being contacted. As we expect relatively few smokers to return opt-out cards,[39] a large pool of prospective participants is available to recruit from. Once enrolled in the intervention, smokers are provided with options to tailor their experience and are encouraged to set goals that are realistic and relevant for them. Encouraging smokers to set reasonable and achievable goals may increase their self-efficacy and help move them along the motivation-to-quit spectrum so they will be better prepared for a successful quit attempt when they are ready.
NRT sampling has been shown to enhance cessation behaviors,[40–42] with NRT use doubling six-month cessation in motivation phase smokers.[7] NRT sampling reduces withdrawal symptoms and cravings that lead to negative experiences[43] and provides an experience of treatment that prepares smokers for future cessation efforts.[44] Take a Break was designed to build upon NRT sampling by providing an experience that further promotes self-efficacy, skill development, and effective use of increasingly available mHealth-based tools to achieve long-term cessation when ready to quit. Therefore, both intervention and comparison group participants are provided with optional NRT sampling. While providing NRT samples to both the intervention and the comparison groups may limit differences in benefits observed, it is essential that interventions create innovative approaches that build upon effective techniques to capture and engage smokers of all motivation phases to build towards a successful quit attempt. In addition to NRT sampling, the level of interaction between the study staff and participants was controlled for by having comparison group participants receive a 1-week telephone call and a 3-week study visit that did not include the active ingredients of the intervention (e.g.: no review of week 1 experience, no goal-setting, no recognition and rewards at 3-weeks). Similarly, the comparison received the daily measurement of number of cigarettes smoked but did not reactive the automated feedback messages that the Take a Break intervention participants received after replying to the daily texting assessment.
Take a Break’s packaging as an accomplishable challenge and the continuously accessible delivery mechanism of mobile technology create a point-of-need gaming intervention designed specifically for smokers who are not ready to quit. Take a Break uses game mechanics [24] whereby individuals challenge themselves by setting a goal and also have social competition with points and alias names displayed on a leaderboard. Combining knowledge gained from years of smoking cessation research with feedback from current and former smokers, Take a Break is designed to be an engaging intervention that can help smokers who are not ready to quit become better prepared, experienced, and confident for when they are ready to ‘take a break’ from smoking.
ACKNOWLEDGEMENTS
Funding: NCI 1R01CA190866-01A1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fiore MC, Jaen CR, Baker TB, Bailey WC, Benowitz NL, Curry SJ, Dorfman SF et al. , Treating tobacco use and dependence, P.H. Service, Editor. 2008, United States Department of Health and Human Services: Rockville, MD. [Google Scholar]
- 2.Carpenter MJ, et al. , Both smoking reduction with nicotine replacement therapy and motivational advice increase future cessation among smokers unmotivated to quit. J Consult Clin Psychol, 2004. 72(3): p. 371–81. [DOI] [PubMed] [Google Scholar]
- 3.Baker TB, et al. , New methods for tobacco dependence treatment research. Ann Behav Med, 2011. 41(2): p. 192–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lader D, Smoking-related behaviour and attitudes 2008/2009: Office for National Statistics; 2009. London. UK. [Google Scholar]
- 5.Control, C.f.D. and Prevention, Quitting smoking among adults--United States, 2001–2010. MMWR. Morbidity and mortality weekly report, 2011. 60(44): p. 1513. [PubMed] [Google Scholar]
- 6.Catley D, et al. , A randomized trial of motivational interviewing: cessation induction among smokers with low desire to quit. American journal of preventive medicine, 2016. 50(5): p. 573–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.National Institutes of Health State-of-the-Science conference statement: tobacco use: prevention, cessation, and control. Ann Intern Med, 2006. 145(11): p. 839–44. [DOI] [PubMed] [Google Scholar]
- 8.Rose JE, Nicotine preloading: the importance of a pre-cessation reduction in smoking behavior. Psychopharmacology, 2011. 217(3): p. 453–454. [DOI] [PubMed] [Google Scholar]
- 9.Carpenter MJ, et al. , Both smoking reduction with nicotine replacement therapy and motivational advice increase future cessation among smokers unmotivated to quit. Journal of consulting and clinical psychology, 2004. 72(3): p. 371. [DOI] [PubMed] [Google Scholar]
- 10.Rose JE, et al. , Precessation treatment with nicotine skin patch facilitates smoking cessation. Nicotine & Tobacco Research, 2006. 8(1): p. 89–101. [DOI] [PubMed] [Google Scholar]
- 11.Stead LF, et al. , Nicotine replacement therapy for smoking cessation. Cochrane database of systematic reviews, 2012(11). [DOI] [PubMed] [Google Scholar]
- 12.Bullen C, et al. , Pre-cessation nicotine replacement therapy: pragmatic randomized trial. Addiction, 2010. 105(8): p. 1474–1483. [DOI] [PubMed] [Google Scholar]
- 13.Bandura A, Self-efficacy: toward a unifying theory of behavioral change. Psychol Rev, 1977. 84(2): p. 191–215. [DOI] [PubMed] [Google Scholar]
- 14.Bandura A, Self-efficacy: The exercise of control. 1997, New York: W H Freeman. [Google Scholar]
- 15.NIH State-of-the-Science: tobacco use. Ann Intern Med, 2006. 145(11): p. 839–44. [DOI] [PubMed] [Google Scholar]
- 16.Thompson D, et al. , Serious Video Games for Health How Behavioral Science Guided the Development of a Serious Video Game. Simul Gaming, 2010. 41(4): p. 587–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thompson D, et al. , In pursuit of change: youth response to intensive goal setting embedded in a serious video game. J Diabetes Sci Technol, 2007. 1(6): p. 907–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lieberman DA and Donner A Using Electronic Games to Empower Healthy Lifestyles, Prevention and Self-Care: Theory and Research Findings 2008. 24. [Google Scholar]
- 19.Aoki N, et al. , INSULOT: a cellular phone-based edutainment learning tool for children with type 1 diabetes. Diabetes Care, 2005. 28(3): p. 760. [DOI] [PubMed] [Google Scholar]
- 20.Lieberman DA, Management of chronic pediatric diseases with interactive health games: theory and research findings. J Ambul Care Manage, 2001. 24(1): p. 26–38. [DOI] [PubMed] [Google Scholar]
- 21.Löwe B, Kroenke K, and Gräfe K, Detecting and monitoring depression with a two-item questionnaire (PHQ-2). Journal of psychosomatic research, 2005. 58(2): p. 163–171. [DOI] [PubMed] [Google Scholar]
- 22.Lyon AR and Koerner K, User-centered design for psychosocial intervention development and implementation. Clinical Psychology: Science and Practice, 2016. 23(2): p. 180–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McClure JB, et al. , Design considerations for mHealth programs targeting smokers not yet ready to quit: results of a sequentialmixed-methods study. JMIR mHealth and uHealth, 2017. 5(3): p. e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blok A, et al. Gamification of a Digital Health Technology for Unmotivated Smokers: Concept and User-driven Development. in Proceedings of the 52nd Hawaii International Conference on System Sciences 2019. [Google Scholar]
- 25.Houston TK, et al. , Evaluating the QUIT-PRIMO clinical practice ePortal to increase smoker engagement with online cessation interventions: a national hybrid type 2 implementation study. Implementation Science, 2015. 10(1): p. 154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blok AC, et al. , Gamification to Motivate the Unmotivated Smoker: The “Take a Break” Digital Health Intervention. Games for Health Journal, 2019. 8(4): p. 275–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Houston TK, et al. , The QUIT-PRIMO provider-patient Internet-delivered smoking cessation referral intervention: a cluster-randomized comparative effectiveness trial: study protocol. Implementation Science, 2010. 5(1): p. 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Coley HL, et al. , Crowdsourced peer-versus expert-written smoking-cessation messages. American journal of preventive medicine, 2013. 45(5): p. 543–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marlin BM, et al. Towards collaborative filtering recommender systems for tailored health communications. in AMIA annualsymposium proceedings 2013. American Medical Informatics Association. [PMC free article] [PubMed] [Google Scholar]
- 30.Adams RJ, et al. PERSPeCT: collaborative filtering for tailored health communications. in Proceedings of the 8th ACM Conference on Recommender systems 2014. [PMC free article] [PubMed] [Google Scholar]
- 31.Lai DT, et al. , Motivational interviewing for smoking cessation. Cochrane database of systematic reviews, 2010(1). [DOI] [PubMed] [Google Scholar]
- 32.DeLaughter KL, et al. , Crave-Out: a distraction/motivation mobile game to assist in smoking cessation. JMIR serious games, 2016. 4(1): p. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dahne J, et al. , Nicotine replacement therapy sampling via primary care: Methods from a pragmatic cluster randomized clinical trial. Contemporary clinical trials, 2018. 72: p. 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Belita E and Sidani S, Attrition in smoking cessation intervention studies: a systematic review. Canadian Journal of Nursing Research Archive, 2015. 47(4). [DOI] [PubMed] [Google Scholar]
- 35.Brown RA, et al. , Reliability and validity of a smoking timeline follow-back interview. Psychology of Addictive Behaviors, 1998. 12(2): p. 101. [Google Scholar]
- 36.Etter JF, et al. , Development and validation of a scale measuring self-efficacy of current and former smokers. Addiction, 2000. 95(6): p. 901–913. [DOI] [PubMed] [Google Scholar]
- 37.Cox DR, Regression models and life-tables. Journal of the Royal Statistical Society: Series B (Methodological), 1972. 34(2): p. 187–202. [Google Scholar]
- 38.Wang TW, et al. , Tobacco product use among adults—United States, 2017. Morbidity and Mortality Weekly Report, 2018. 67(44): p. 1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Junghans C, et al. , Recruiting patients to medical research: double blind randomised trial of “opt-in” versus “opt-out” strategies. Bmj, 2005. 331(7522): p. 940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jolicoeur DG, et al. , Smoking cessation, smokingreduction, and delayed quitting among smokers given nicotine patches and a self-help pamphlet. Substance Abuse, 2003. 24(2): p. 101–106. [DOI] [PubMed] [Google Scholar]
- 41.Jolicoeur DG, et al. , The use of nicotine patches with minimal intervention. Preventive Medicine, 2000. 30(6): p. 504–512. [DOI] [PubMed] [Google Scholar]
- 42.Alberg AJ, et al. , The influence of offering free transdermal nicotine patches on quit rates in a local health department’s smoking cessation program. Addictive behaviors, 2004. 29(9): p. 1763–1778. [DOI] [PubMed] [Google Scholar]
- 43.Burris JL, et al. , A mechanistic test of nicotine replacement therapy sampling for smoking cessation induction. Psychology of Addictive Behaviors, 2015. 29(2): p. 392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Carpenter MJ, et al. , Nicotine therapy sampling to induce quit attempts among smokers unmotivated to quit: a randomized clinical trial. Archives of internal medicine, 2011. 171(21): p. 1901–1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Heatherton T, Kozlowski L, and Frecker R, The Fagerstrom test for nicotine dependence: a revision of the Fagerstrom tolerance questionnaire. Br J Addict, 1991. 86(9): p. 1119–1127. [DOI] [PubMed] [Google Scholar]
- 46.Covey LS, Glassman AH, and Stetner F, Cigarette smoking and major depression. J Addict Dis, 1998. 17(1): p. 35–46. [DOI] [PubMed] [Google Scholar]
- 47.Killen JD, et al. , Interactive effects of depression symptoms, nicotine dependence, and weight change on late smoking relapse. J Consult Clin Psychol, 1996. 64(5): p. 1060–7. [DOI] [PubMed] [Google Scholar]
- 48.Kinnunen T, et al. , Depression and smoking cessation: characteristics of depressed smokers and effects of nicotine replacement. J Consult Clin Psychol, 1996. 64(4): p. 791–8. [DOI] [PubMed] [Google Scholar]
- 49.Shadel WG and Shiffman S, Behavioral Assessment, in Assessment of addictive behaviors, Donovan DM and Marlatt GA, Editors. 2005, Guilford Press: New York: p. 139–199. [Google Scholar]
- 50.Velicer WF, et al. , Relapse situations and self-efficacy: an integrative model. Addict Behav, 1990. 15(3): p. 271–83. [DOI] [PubMed] [Google Scholar]
- 51.Fucito LM, et al. , Beliefs and attitudes about bupropion: implications for medication adherence and smoking cessation treatment. Psychol Addict Behav, 2009. 23(2): p. 373–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tiffany ST and Drobes DJ, The development and initial validation of a questionnaire on smoking urges. Br J Addict, 1991. 86(11): p. 1467–76. [DOI] [PubMed] [Google Scholar]


