Abstract
Introduction:
Venous thromboembolism (VTE) in pregnancy and postpartum is a leading cause of maternal morbidity and mortality in developed countries, where obesity is a known risk for this complication. Current guidelines vary in which patients qualify for VTE prophylaxis, precluding a uniform approach for management. We sought to derive a risk prediction model for VTE in obese pregnant women.
Materials and Methods:
We performed a retrospective cohort analysis using the Consortium on Safe Labor (CSL) database. Women ages 16–45 who were pregnant with singletons and had an obese body mass index (>30kg/m2) were included in our study population. Multivariable logistic regression was used in order to identify predictors of venous thromboembolism.
Results:
Of the 83,500 women who met inclusion criteria, on average women were 27.8 years old, 38.6 weeks gestation, with body mass index of 35.8, and cesarean delivery incidence of 35.2%. 109 women (0.13%) experienced a VTE event. Independent predictors of VTE in our final multivariable predictive model included: mode of delivery, body mass index, pregestational diabetes, chronic heart disease, preeclampsia, blood transfusion (intrapartum or postpartum), prenatal history of thromboembolic disorder, and postpartum maternal length of stay. A receiver operating characteristic curve was developed to assess the model; area under the curve was 0.826.
Conclusions:
We developed a strong predictive model using a large, retrospective database to distinguish risk of VTE in obese pregnant women, which may provide the foundation for future protocol development of obstetrical thromboprophylaxis in obese women.
Keywords: Low molecular weight heparin, maternal morbidity, obesity, pregnancy, thromboprophylaxis, venous thromboembolism
Introduction
Venous thromboembolism (VTE), which includes both deep venous thrombosis (DVT) and pulmonary embolism (PE), is a leading cause of maternal morbidity and mortality.1 In developed countries, VTE accounts for 14.9% of maternal deaths, defined as death of a woman while pregnant or within 42 days of termination of pregnancy (or 1 year for late maternal deaths) from any cause related to its management.2 Between 2011 and 2013 in the U.S., VTE accounted for 9.2% of maternal mortality. Although rare, this catastrophic adverse event was among the three most common causes of maternal mortality, alongside cardiomyopathy and other cardiovascular conditions.3 In comparison to nonpregnant women, pregnant or postpartum women have a fourfold to fivefold increased risk of thromboembolism.4 The epidemic of obesity in the US does not exclude women of reproductive age. In fact, almost one in two US women of child bearing age are either overweight or obese.5 Obesity is also a known risk factor for VTE during pregnancy and postpartum.6 Many providers will, therefore, treat a pregnant population with obesity and optimal management for this demographic has yet to be established.
Current guidelines for pharmacologic VTE prophylaxis provided by The American College of Obstetrics and Gynecologists (ACOG), the American College of Chest Physicians (ACCP) and the Royal College of Obstetricians and Gynecologists (RCOG) do not offer consensus for which patients should receive treatment. The considerable difference between the professional opinions of these societies has been demonstrated by a cross-sectional study of patients who were all postoperative day one from cesarean delivery. Applying the three criteria to this population to see who would theoretically qualify for treatment yielded the following: by the least inclusive interpretation of the ACOG guidelines, only 1.0% of patients would receive post-cesarean section pharmacologic prophylaxis, 34.8% under ACCP guidelines, and 85% under RCOG guidelines.7 The disparity in recommendations of these governing organizations poses questions about types of studies these guidelines are based on. Existing evidence in the literature is devoid of large randomized control trials, and case-control combined with observational studies provide a suboptimal base on which to derive clinical decision-making tools. Our objective in this study is to utilize a national database to elucidate the factors that make the obese pregnant population most at risk for VTE. Identifying these individuals through development and validation of a predictive model may facilitate more informed decisions about who should receive pharmacologic prophylaxis in the clinical setting.
Materials and Methods
We conducted a retrospective cohort study of women who delivered from the Consortium on Safe Labor (CSL), a publicly available database of the Eunice Shriver Kennedy National Institute of Child Health and Human Development, National Institutes of Health. The CSL includes detailed information from the electronic medical record for 228,562 deliveries in the United States from 2002 through 2008. This database was designed as an observational study with the ultimate goal of creating contemporary, evidence-based definitions of labor protraction and arrest. The CSL aimed to provide information that could identify the underlying causes of high cesarean rate in the US and determine the appropriate time to perform a cesarean delivery in patients with labor protraction and arrest.8 Within the CSL, all patients delivered greater than or equal to 23 weeks gestation. There were 12 clinical centers with 19 hospitals that ranged across 9 American Congress of Obstetrician and Gynecologist (ACOG) districts.9 The participating institutions included 8 university affiliated teaching hospitals, 9 teaching community hospitals, and 2 nonteaching community hospitals, all of which obtained institutional review board approval. Data from manual chart extraction was compared with the data from the electronic medical record. This analysis found that most variables from the electronic medical record were highly accurate, and that this level of precision could be likely applied to the entire database. The validity of data from electronic medical records comparing to medical charts in selected variables is documented in Appendix I of Zhang et al.10
The outcomes of VTE were confirmed by a combination of electronic medical record extraction and ICD 9 codes. The four variables from CSL that we used to define VTE include: “Postpulembol” – Maternal Postpartum: Pulmonary thromboembolism; “Postthrombosis” – Maternal Postpartum: Thrombosis; “Pulmemb_new” – Derived variable: Pulmonary embolism; and “pulmonary_embolism9” – Maternal ICD-9 collection: Pulmonary Embolism (ICD-9 code 415, 673).
Inclusion criteria were women with singleton gestation between the ages of 16 and 45 with an obese body mass index (BMI > 30 kg/m2). Weight was given in the CSL as weight on admission and only one height per patient was listed. Therefore, we calculated BMI at admission using these 2 variables. These values were reported and we do not know if they were measured or self-reported. According to Zhang et al, in order to avoid intra-person correlation within the CSL, only the first delivery from each subject was selected for analysis.
The primary outcome of interest was VTE, defined as pulmonary embolism or venous thrombosis either intrapartum or postpartum. Predictive model construction was performed by way of the following algorithm for the aforementioned CSL-defined VTE outcome. Cohorts for comparison were split by VTE occurrence and NO VTE occurrence. Demographic and clinical variables were first analyzed to identify associations with VTE at the univariate level. Normally distributed and nonparametric continuous variables were analyzed by way of independent samples t-test and Kruskal-Wallis test, respectively, while adequate cell-count and low cell-count categorical variables were analyzed by way of Chi-square and Fisher’s exact test, respectively. Variables that had corresponding univariate-test p-value<0.1 were considered to have at least a trend-level significant association with VTE occurrence and were then entered into a multivariable logistic regression model for risk-prediction of VTE. Multicollinearity of covariates was assessed using a variance inflation factor (VIF) analysis and the condition index with acceptable VIF < 2. Through stepwise purposeful selection, our final predictive model identifies significant independent predictors of VTE and maximizes the concordant classification ability of our model, which is represented by the Receiver Operating Characteristic (ROC) and area under the curve (AUC). This methodological algorithm results in the exact equation used for predicted probabilities of VTE occurrence.
Validation of our predictive model was performed internally using 250 bootstrapped iterations of 50% randomly selected sub-samples of our overall study population in order to attain the empirical distribution of each parameter in our prediction model. Multivariable odds ratios with 95% confidence intervals, corresponding p-values, AUC with 95% confidence intervals, and overall correlation between validation model and original predictive model will be performed to provide validation diagnostics.
Additionally, observed VTE events by predicted probability of VTE were plotted overall by predicted probability decile, as well as by decile only within the top decile of predicted probabilities in order to visually represent model calibration.
High versus low risk patient groups were also defined using a weighted point system. The weight of points for each predictor variable was achieved by rounding the odds ratio estimate for binary variables to the nearest whole number. Prenatal History of Thromboembolic Disorder was assigned a weight of 5 points after truncating point weights for this variable due to its paramount relationship with increased odds of VTE (adjusted odds ratio estimate = 140). Continuous predictors were assigned point weights between clinically relevant binary unit ranges. The sum of all weighted points was defined as the variable “sumpts”, where sumpts > 3 was defined as the cutoff for high risk patients. All other patients who were not high risk were considered low risk. The decision to use this cutoff was achieved by analyzing the distribution of sumpts by VTE cohort and identifying the inflection point at which the weighted point range changed from being more common in the no-VTE cohort to being more common in the VTE cohort. Tables outlining these methods and results, as well as corresponding diagnostics with 95% confidence intervals are provided for High vs Low risk predictions by true VTE and no-VTE occurrence.
All statistical analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC) with p < 0.05 considered statistically significant. This secondary analysis of the CSL database has exemption status by the George Washington University Hospital Institutional Review Board.
Results
Our study population included 83,500 pregnancies, 109 in the VTE group and 83,391 in the NO VTE group and. Of the 83,500 women who met inclusion criteria, on average women were 27.8 ± 6.0 years old, 38.6 ± 2.21 weeks gestation, with BMI of 35.8 ± 5.45 kg/m2, and cesarean delivery incidence of 35.2%. VTE occurred in 109 women (0.13%).
VTE occurrence was significantly associated with older maternal age, African American race, higher BMI, pregestational diabetes, history of heart disease, prenatal thromboembolic disorder, cesarean delivery (CD), younger gestational age, gestational diabetes, postpartum hypertension, preeclampsia, endometritis, placenta previa, blood transfusion(s), drug use during pregnancy, intrapartum fever, postpartum hemorrhage, preterm birth, and longer postpartum maternal hospital length of stay (Table 1, Table 2).
Table 1.
Maternal Characteristics and Preexisting Conditions N=83,500
| Variable | VTE (n=109) | No VTE (n=83,391) | p-value |
|---|---|---|---|
| Maternal age | 29.4 ± 6.4 | 27.8 ± 6.0 | 0.0043* |
| Maternal race | 0.0036* | ||
| BMI | 38.6 ± 7.7 | 35.8 ± 5.5 | 0.0003* |
| Parity | 1 (0, 2) | 1 (0, 2) | 0.3820 |
| Education | 0.2134 | ||
| Pregestational diabetes | 13 (11.9%) | 2057 (2.5%) | <.0001* |
| History of heart disease | 10 (9.2%) | 1217 (1.5%) | <.0001* |
| Prenatal thromboembolic disorder | 30 (27.5%) | 145 (0.2%) | <.0001* |
VTE: venous thromboembolism;
p<0.05 statistically significant.
Data are reported as n (%), mean ± standard deviation, and/or median (Q1, Q3)
Table 2.
Pregnancy Conditions and Complications N=83,500
| Variable | VTE (n=109) | No VTE (n=83,391) | p-value |
|---|---|---|---|
| Cesarean delivery (delivery mode) | 68 (62.4%) | 29295 (35.1%) | <.0001* |
| Emergency C-section | - | 89 (0.1%) | 0.9999 |
| Gestational age | 36.8 ± 3.6 | 38.6 ± 2.3 | <.0001* |
| Gestational diabetes | 13 (11.9%) | 5827 (7.0%) | 0.0433* |
| Postpartum HTN | 6 (5.5%) | 992 (1.2%) | 0.0021* |
| Gestational HTN | 4 (3.7%) | 3562 (4.3%) | 0.9999 |
| Preeclampsia | 24 (22.0%) | 6369 (7.6%) | <.0001* |
| Eclampsia | 1 (0.9%) | 92 (0.1%) | 0.1145 |
| Chorioamni onitis | 4 (3.7%) | 2858 (3.4%) | 0.7903 |
| Endometritis | 3 (2.8%) | 283 (0.3%) | 0.0064* |
| Placenta previa | 3 (2.8%) | 529 (0.6%) | 0.0329* |
| Placental abruption | 1 (0.9%) | 1251 (1.5%) | 0.9999 |
| Smoked during Pregnancy | 8 (7.3%) | 5742 (6.9%) | 0.8517 |
| Blood transfusion (intra or post) | 13 (11.9%) | 2343 (2.8%) | <.0001* |
| Stillbirth | - | 346 (0.4%) | 0.9999 |
| Drug use during pregnancy | 7 (6.4%) | 1555 (1.9%) | 0.0045* |
| Antepartum UTI | 5 (4.6%) | 3107 (3.7%) | 0.6069 |
| Intrapartum fever | 11 (10.1%) | 4328 (5.2%) | 0.0295* |
| Postpartum hemorrhage | 25 (22.9%) | 5783 (6.9%) | <.0001* |
| Preterm birth | 35 (32.1%) | 9021 (10.8%) | <.0001* |
| Postpartum maternal length of stay (days) | 5 (3, 8) | 2 (2, 3) | <.0001* |
VTE: venous thromboembolism;
p<.05, statistically significant.
Data are reported as n (%), mean ± standard deviation, and/or median (Q1, Q3)
Significant independent predictors of VTE occurrence which constitute our predictive model include mode of delivery, BMI, pregestational diabetes, chronic heart disease, preeclampsia, blood transfusion(s), prenatal history of thromboembolic disorder, and postpartum maternal length of stay (Table 3). While there is information about emergency, planned versus unplanned was not readily available and so we did not separate these diagnoses to examine them further.
Table 3.
Multivariable predictive model for venous thromboembolism
| Predictor Variable | β | SE | Adjusted OR (95% CI) | p-value |
|---|---|---|---|---|
| Mode of delivery (cesarean vs vaginal delivery) | 0.6442 | 0.2234 | 1.90 (1.23 – 2.95) | 0.004* |
| BMI (admission), per 5 unit increase+ | 0.0451 | 0.0143 | 1.25 (1.08 – 1.43) | 0.002* |
| Pre-gestational diabetes | 1.2147 | 0.3337 | 3.37 (1.75 – 6.48) | <0.001* |
| Chronic heart disease | 1.1833 | 0.4154 | 3.27 (1.45 – 7.37) | 0.004* |
| Preeclampsia | 0.6899 | 0.2717 | 2.03 (1.21 – 3.40) | 0.011* |
| Blood transfusion (intra-partum or postpartum) | 1.1912 | 0.3248 | 3.29 (1.74 – 6.22) | <0.001* |
| Prenatal history of thromboembolic disorder | 4.9461 | 0.2556 | 140.00 (85.20 – 232.00) | <0.001* |
| Postpartum maternal length of stay, per 1 day increase | 0.0604 | 0.0123 | 1.06 (1.04 – 1.09) | <0.001* |
p<.05, statistically significant;
adjusted odds ratio and 95% confidence interval reported as binary 5 unit increase, parameter estimate (β) and SE (standard error) reported per 1 unit increase for more accurate long-form calculation.
Independent of other significant predictors/covariates, patients undergoing CD rather than vaginal delivery had 1.9 times higher odds of VTE occurrence (95% CI: 1.23 – 2.95; p=0.004), patients with pre-gestational diabetes had 3.37 times higher odds of VTE occurrence (95% CI: 1.75 – 6.48; p<.001), and patients with a prenatal history of thromboembolic disorder had 140 times higher odds of VTE occurrence (95% CI: 85.20 – 232.00; p<.001).
The exact calculation for a given patient’s predicted probability for VTE can be ascertained by way of the below equation (Figure 1) which corresponds to the prediction model from Table 3.
Figure 1. Equation for Prediction Model Calculator using Table 3.

Where: P=predicted probability of VTE; Csec=Cesarean section (CD), enter 1 if CD, enter 0 if vaginal delivery; BMInew=Maternal BMI on admission, enter continuous BMI value; Prediab=Pre-gestational diabetes, enter 1 if present, 0 if absent; Heartdis=Chronic heart disease, enter 1 if present, 0 if absent; Preeclamp=Preeclampsia, enter 1 if present, 0 if absent; Bloodtrans=Blood transfusion, enter 1 if present, 0 if absent; Antethrombo=Prenatal history of thromboembolic disorder, enter 1 if present, 0 if absent; momLOS=Maternal length of stay, enter continuous LOS value (in days).
We operationalized these findings into a Risk of VTE in Pregnancy calculator which is a prototype tool that providers can use in real time to help guide their clinical decision making for management of high risk patients. This calculator can be found at: http://www.gwdocs.com/mfm/thrombosis-calculator. Using the calculator above, a suspected low risk clinical patient BMI of 35 who delivered vaginally with an appropriate 1 day postpartum stay who has no other risk factors has a 0.04% chance of VTE occurrence. In comparison, a suspected clinically high risk patient with BMI 40, cesarean delivery, pre-gestational diabetes, and preeclampsia has a 51.16% of VTE occurrence.
The predictive strength of our model from Table 3 is visually represented by way of the ROC curve in Figure 2. The AUC (95% CI)/C-statistic associated with our model/ROC curve is 0.826 (0.775 – 0.877) and the Hosmer-Lemeshow test p-value=0.6197. This indicates strong classification and calibration of our predictive model.
Figure 2.
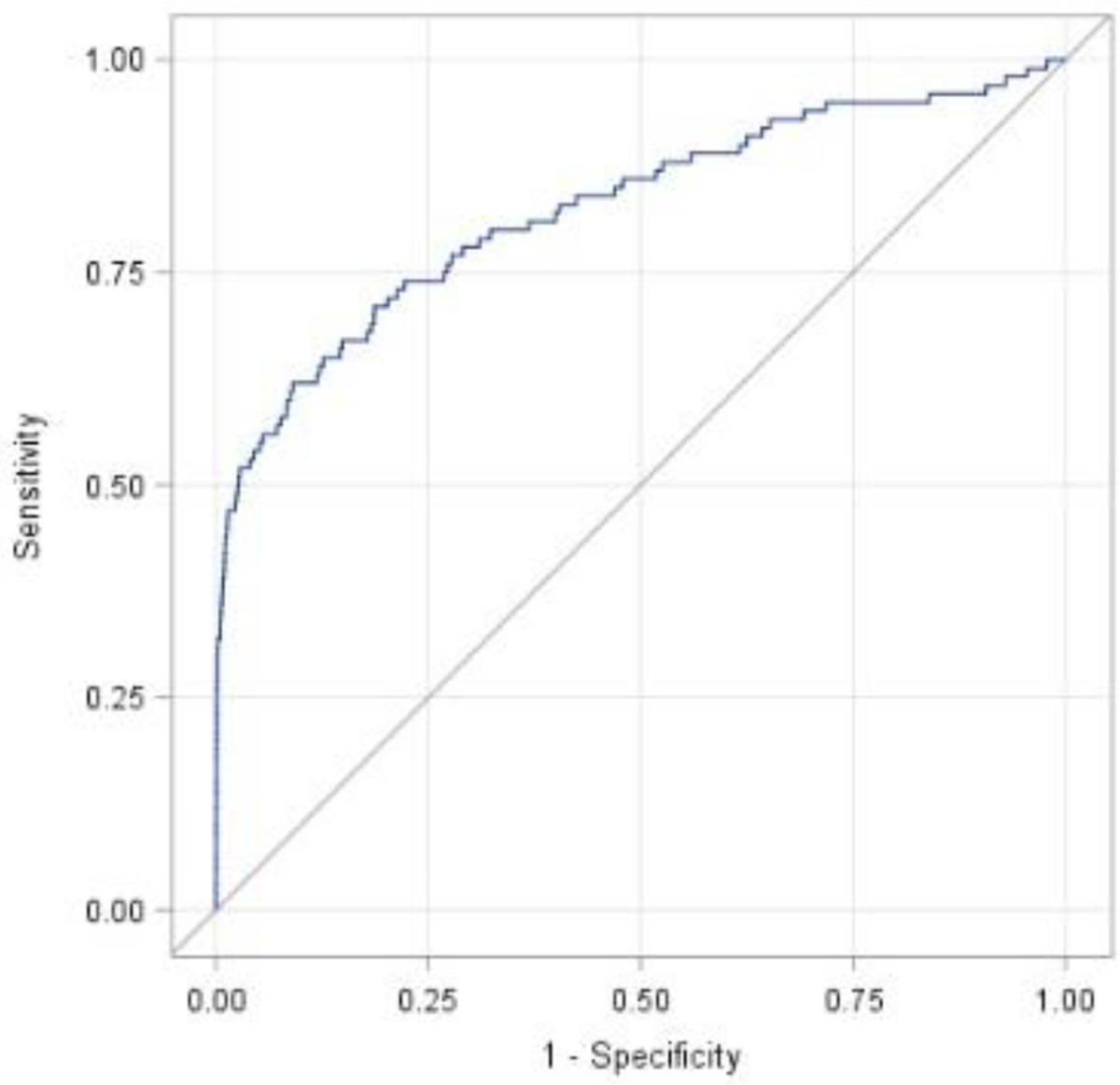
Receiver operating characteristic curve for prediction model of venous thromboembolism in obese pregnant women (Table 3)
Additionally, we created a second predictive model which excludes the most significant predictor variable of prenatal history of thromboembolic disorder. Significant predictor variables which constitute this model include mode of delivery, BMI, gestational age, pre-gestational diabetes, chronic heart disease, preeclampsia, blood transfusion(s), drug use during pregnancy, and postpartum maternal length of stay (Table 4).
Table 4.
Multivariable Predictive Model for VTE, without antepartum thromboembolic disorder
| Predictor Variable | β | SE | Adjusted OR (95% CI) | p-value |
|---|---|---|---|---|
| Mode of delivery (cesarean vs vaginal delivery) | 0.6179 | 0.2182 | 1.86 (1.21 – 2.85) | <0.001* |
| BMI (admission), per 5 unit increase+ | 0.0447 | 0.0135 | 1.25 (1.09 – 1.42) | <0.001* |
| Gestational age, per 1 week increase | −0.0778 | 0.0318 | 0.93 (0.87 – 0.99) | 0.014* |
| Pre-gestational diabetes | 0.8383 | 0.3306 | 2.31 (1.21 – 4.42) | 0.011* |
| Chronic heart disease | 1.3531 | 0.3744 | 3.87 (1.86 – 8.06) | <0.001* |
| Preeclampsia | 0.5368 | 0.2693 | 1.71 (1.01 – 2.90) | 0.046* |
| Blood transfusion (intra-partum or postpartum) | 1.158 | 0.3089 | 3.18 (1.74 – 5.83) | <0.001* |
| Drug use during pregnancy | 1.0557 | 0.4103 | 2.87 (1.29 – 6.42) | 0.010* |
| Postpartum maternal length of stay, per 1 day increase | 0.0655 | 0.0110 | 1.07 (1.05 – 1.09) | <.0001* |
p<.05, statistically significant;
adjusted odds ratio and 95% confidence interval reported as binary 5 unit increase, parameter estimate (β) and SE (standard error) reported per 1 unit increase for more accurate long-form calculation.
The exact calculation for a given patient’s predicted probability for VTE can be ascertained by way of the below equation (Figure 3) which corresponds to the prediction model from Table 4.
Figure 3. Equation for Prediction Model Calculator using Table 4.

Where: P=predicted probability of VTE; Csec=Cesarean section (CD), enter 1 if CD, enter 0 if vaginal delivery; BMInew=Maternal BMI on admission, enter continuous BMI value; GA=Gestational age, enter continuous value in weeks; Prediab=Pre-gestational diabetes, enter 1 if present, 0 if absent; Heartdis=Chronic heart disease, enter 1 if present, 0 if absent;Preeclamp=Preeclampsia, enter 1 if present, 0 if absent; Bloodtrans=Blood transfusion, enter 1 if present, 0 if absent; Druguse=Drug use during pregnancy, enter 1 if present, 0 if absent;momLOS=Maternal length of stay, enter continuous LOS value (in days).
The predictive strength of our model from Table 4 is visually represented by way of the ROC curve in Figure 4. The AUC (95% CI)/C-statistic associated with this model/ROC curve is 0.791 (0.740 – 0.841) and the Hosmer-Lemeshow test p-value=0.5823, again indicating strong classification and calibration of this iteration of the predictive model.
Figure 4.
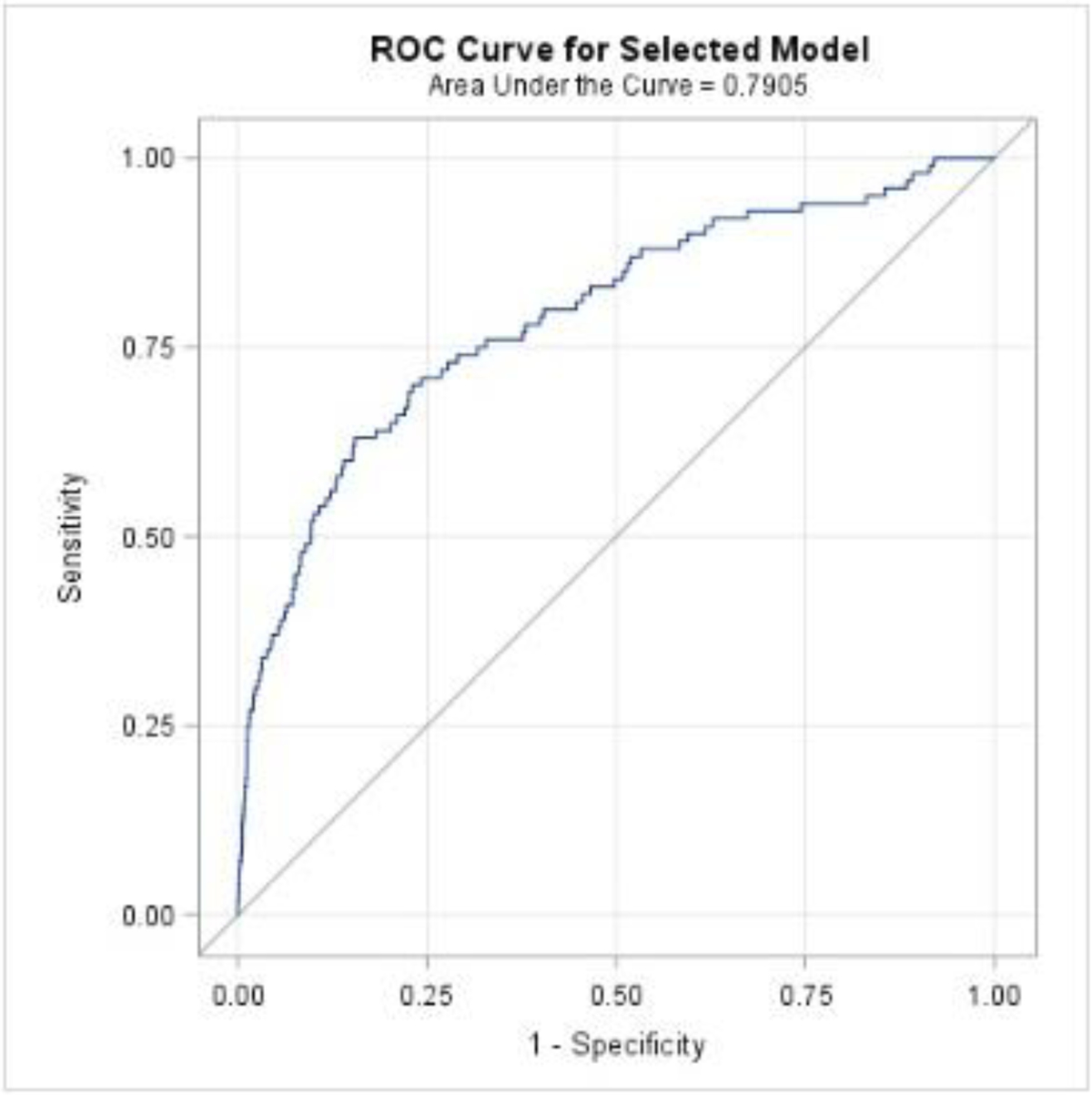
Receiver operating characteristic curve for prediction model of venous thromboembolism in obese pregnant women without antepartum thromboembolic disorder (Table 4)
Model Validation
Bootstrap internal validation of our prediction model using the model from Table 3 that is inclusive of history of thromboembolic disorder is shown in Table 5. AUC (95% CI) of our validation model was 0.828 (0.756 – 0.899), and Pearson’s correlation coefficient ρ=0.999 with corresponding p-value<0.0001, indicating strong validation of our model when comparing the empirical validation model to the original prediction model.
Table 5.
Multivariable Predictive Model (white lines) and Internal Validation Model (gray lines) using Table 3 (inclusive of thromboembolic disorder)
| Predictor Variable | Adjusted OR (95% CI) | p-value |
|---|---|---|
| Mode of delivery (cesarean vs vaginal delivery) | 1.90 (1.23 – 2.95) | 0.004* |
| 1.91 (1.84 – 1.99) | <0.001* | |
| BMI (admission) per 5 unit increase | 1.25 (1.08 – 1.43) | 0.002* |
| 1.23 (1.21 – 1.24) | <0.001* | |
| Pre-gestational diabetes | 3.37 (1.75 – 6.48) | <0.001* |
| 3.41 (3.21 – 3.61) | <0.001* | |
| Chronic heart disease | 3.27 (1.45 – 7.37) | 0.004* |
| 3.25 (3.02 – 3.49) | <0.001* | |
| Preeclampsia | 2.03 (1.21 – 3.40) | 0.011* |
| 1.99 (1.90 – 2.09) | <0.001* | |
| Blood transfusion (intrapartum or postpartum) | 3.29 (1.74 – 6.22) | <0.001* |
| 3.30 (3.12 – 3.50) | <0.001* | |
| Prenatal history of thromboembolic disorder | 140.00 (85.20 – 232.00) | <0.001* |
| 141.45 (135.26 – 147.91) | <0.001* | |
| Postpartum maternal length of stay, per 1 day increase | 1.06 (1.04 – 1.09) | <0.001* |
| 1.06 (1.06 – 1.07) | <0.001* |
p<.05, statistically significant
Bootstrap internal validation of our prediction model using the model from Table 4 that is not inclusive of history of thromboembolic disorder is shown in Table 6. AUC (95% CI) of our validation model was 0.794 (0.724 – 0.864), and Pearson’s correlation coefficient ρ=0.994 with corresponding p-value<0.0001, indicating strong validation of our model when comparing the empirical validation model to the original prediction model.
Table 6.
Multivariable Predictive Model (white lines) and Internal Validation Model (gray lines) using Table 4 (not inclusive of thromboembolic disorder)
| Predictor Variable | Adjusted OR (95% CI) | p-value |
|---|---|---|
| Mode of delivery (cesarean vs vaginal delivery) | 1.86 (1.21 – 2.85) | <0.001* |
| 1.86 (1.79 – 1.94) | <0.001* | |
| BMI (admission) per 5 unit increase | 1.25 (1.09 – 1.42) | <0.001* |
| 1.22 (1.21 – 1.24) | <0.001* | |
| Gestational age, per 1 week increase | 0.93 (0.87 – 0.99) | 0.014* |
| 0.93 (0.92 – 0.93) | <0.001* | |
| Pre-gestational diabetes | 2.31 (1.21 – 4.42) | 0.011* |
| 2.36 (2.22 – 2.50) | <0.001* | |
| Chronic heart disease | 3.87 (1.86 – 8.06) | <0.001* |
| 3.82 (3.58 – 4.09) | <0.001* | |
| Preeclampsia | 1.71 (1.01 – 2.90) | 0.046* |
| 1.70 (1.62 – 1.79) | <0.001* | |
| Blood transfusion (intrapartum or postpartum) | 3.18 (1.74 – 5.83) | <0.001* |
| 3.25 (3.08 – 3.43) | <0.001* | |
| Drug use during pregnancy | 2.87 (1.29 – 6.42) | 0.010* |
| 2.95 (2.75 – 3.17) | <0.001* | |
| Postpartum maternal length of stay, per 1 day increase | 1.07 (1.05 – 1.09) | <0.001* |
| 1.07 (1.06 – 1.07) | <0.001* |
p<.05, statistically significant
Model Calibration
Figures 5 and 6 respectively show observed VTE events by predicted probabilities of VTE by decile in the overall sample and by decile only within the top decile of predicted probabilities.
Figure 5.
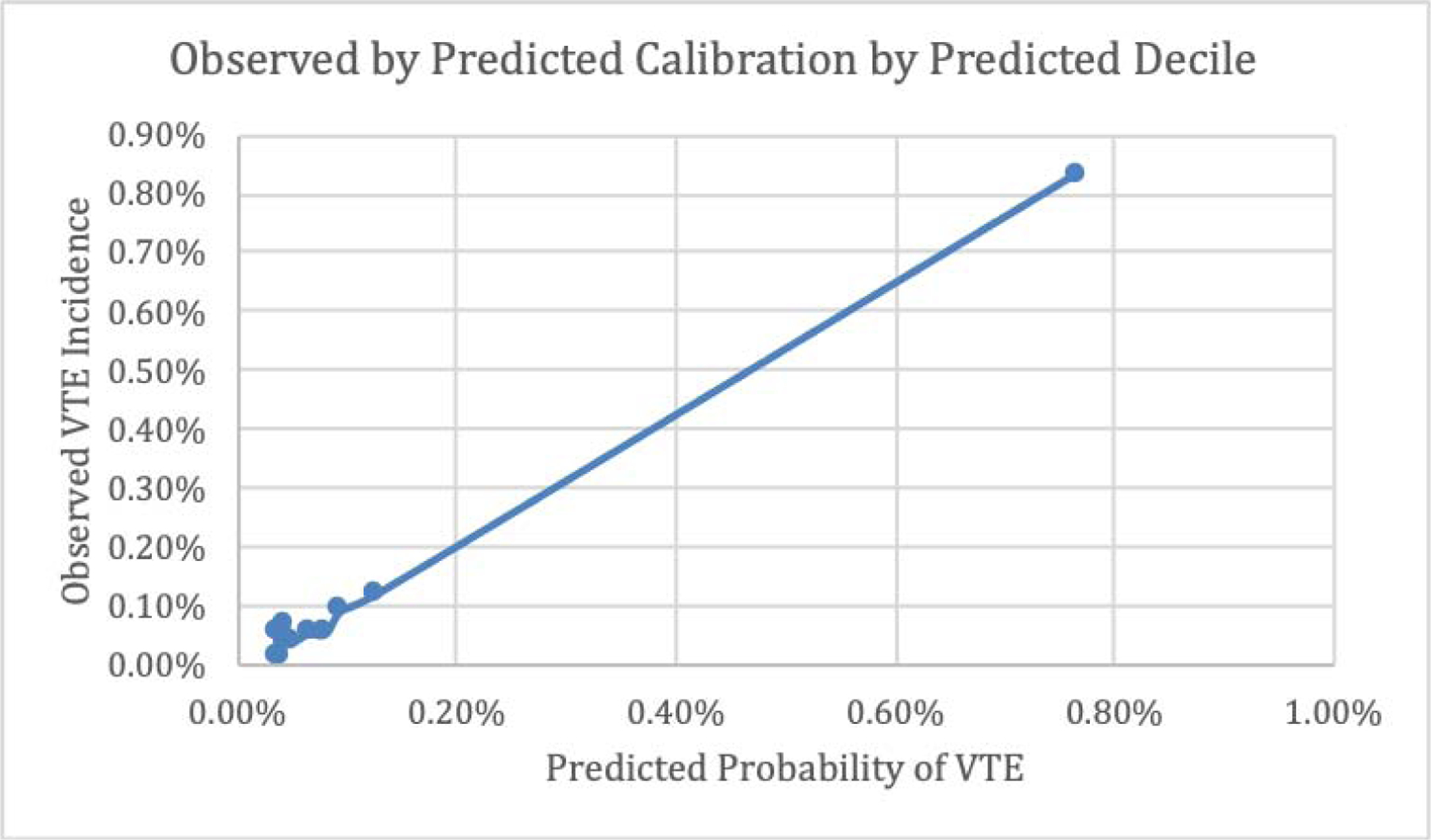
Observed by Predicted Calibration by Predicted Decile, Overall
Figure 6.
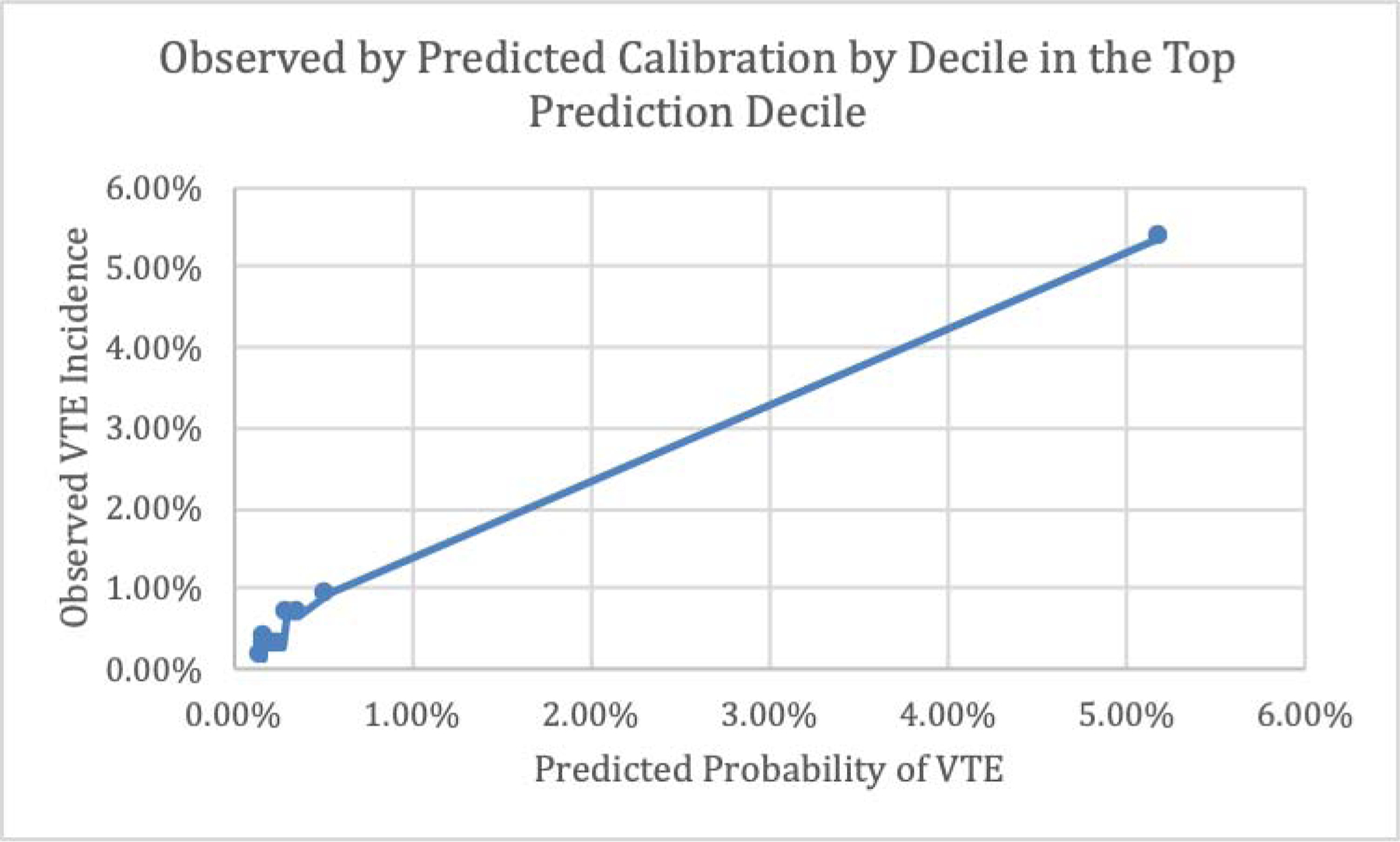
Observed by Predicted Calibration by Decile, Top Prediction Decile
The prediction model predicted probabilities compare extremely well to actual VTE observed occurrence as illustrated in Figures 5 and 6. These figures also highlight the discrepancy between the majority low-risk VTE patients and the relatively small proportion of high-risk VTE patients with an extensive range of predicted probabilities clustered independent of those who are low-risk.
High versus Low Risk Populations
Using the results from Table 3 (inclusive of thromboembolic disorder), the corresponding point weights per predictor variable are outlined in Table 7.
Table 7.
Predictor Variables and Corresponding Point Weights using Table 3
| Predictor Variable | Points |
|---|---|
| Cesarean Delivery | 2 |
| BMI | |
| Pre-gestational Diabetes | 3 |
| Chronic Heart Disease | 3 |
| Preeclampsia | 2 |
| Blood Transfusion | 3 |
| Prenatal History of Thromboembolic Disorder | 5 |
| Postpartum Maternal Length of Stay ≥ 5 days | 1 |
The distribution of the sum of weighted points (variable = “sumpts”) by VTE cohort are shown in Table 8.
Table 8.
Distribution of “sumpts” by VTE cohort
| Sum of Weighted Points (sumpts) | No-VTE (n=83391) | VTE (n=109) | Total (n=83500) | Predicted Probability of VTE |
|---|---|---|---|---|
| 0 | 41237 (49.4%) | 15 (13.8%) | 41252 | 0.019% |
| 1 | 6308 (7.6%) | 4 (3.7%) | 6312 | 0.040% |
| 2 | 19167 (23.0%) | 13 (11.9%) | 19180 | 0.084% |
| 3 | 8737 (10.5%) | 9 (8.3%) | 8746 | 0.177% |
| 4 | 3195 (3.8%) | 9 (8.3%) | 3204 | 0.370% |
| 5 | 2635 (3.2%) | 8 (7.3%) | 2643 | 0.772% |
| 6 or more | 2112 (2.5%) | 51 (46.8%) | 2163 | 1.606% |
Table 9 shows the association between risk group and VTE outcome with corresponding diagnostics and 95% confidence intervals of this high vs low risk classification using Tables 3, 7, and 8.
Table 9.
High vs Low Risk by VTE by no-VTE
| Total Sample | VTE | No-VTE | Total |
|---|---|---|---|
| High Risk, sumpts>3 (predicted positive) | True Positives | False Positives | 8010 |
| Low Risk (predicted negative) | False Negatives | True Negatives | 75490 |
| Total | 109 | 83391 | 83500 |
| Diagnostics with 95% Confidence Intervals | |||
| Sensitivity | 0.62 (0.53 – 0.71) | ||
| Specificity | 0.90 (0.90 – 0.91) | ||
| PPV | 0.01 (0.01 – 0.01) | ||
| NPV | 0.99 (0.99 – 0.99) | ||
| Diagnostic Odds Ratio | 15.75 (10.68 – 23.22) | ||
| AUC, “sumpts” × VTE | 0.817 (0.768 – 0.865) | ||
| Hosmer-Lemeshow p | p-value=0.297 | ||
The odds of a high risk classification if a subject truly has VTE occurrence are 15.75 times higher (95% CI: 10.68 – 23.22) than the odds of a high risk classification if the subject does not have VTE occurrence. With an AUC (95% CI) of 0.817 (0.768 – 0.865) and corresponding Hosmer-Lemeshow p-value=0.297, the sum of weighted points (sumpts) provides strong classification and calibration in relation to true VTE occurrence.
Tables 10 through 12 correspond to Tables 7 through 9 using Table 4 (not-inclusive of thromboembolic disorder) rather than Table 3.
Table 10.
Predictor Variables and Corresponding Point Weights using Table 4
| Predictor Variable | Points |
|---|---|
| Cesarean Delivery | 2 |
| BMI | |
| Gestational Age | |
| Pre-gestational Diabetes | 2 |
| Chronic Heart Disease | 4 |
| Preeclampsia | 2 |
| Blood Transfusion | 3 |
| Drug use during Pregnancy | 3 |
| Postpartum Maternal Length of Stay ≥ 5 days | 1 |
Table 12.
High vs Low Risk by VTE by no-VTE
| Total Sample | VTE | No-VTE | Total |
|---|---|---|---|
| High Risk, sumpts>3 (predicted positive) | True Positives | False Positives | 9989 |
| Low Risk (predicted negative) | False Negatives | True Negatives | 73511 |
| Total | 109 | 83391 | 83500 |
| Diagnostics with 95% Confidence Intervals | |||
| Sensitivity | 0.58 (0.48 – 0.67) | ||
| Specificity | 0.88 (0.88 – 0.88) | ||
| PPV | 0.01 (0.00 – 0.01) | ||
| NPV | 0.99 (0.99 – 0.99) | ||
| Diagnostic Odds Ratio | 10.14 (6.93 – 14.83) | ||
| AUC, “sumpts” × VTE | 0.778 (0.729 – 0.826) | ||
| Hosmer-Lemeshow p | p-value=0.114 | ||
The odds of a high risk classification if a subject truly has VTE occurrence are 10.14 times higher (95% CI: 6.93 – 14.83) than the odds of a high risk classification if the subject does not have VTE occurrence. With an AUC (95% CI) of 0.778 (0.729 – 0.826) and corresponding Hosmer-Lemeshow p-value=0.114, the sum of weighted points (sumpts) provides strong classification and calibration in relation to true VTE occurrence.
Discussion
Using a large, contemporary database, we identified variables associated with VTE events in obese (BMI >30 kg/m2) patients during pregnancy and postpartum through a multivariate regression analysis. Patients who had a high risk of postpartum VTE had predictive factors which included: cesarean delivery, increased BMI, pregestational diabetes, chronic heart disease, preeclampsia, blood transfusion (intrapartum or postpartum), prenatal history of thromboembolic disorder, and an increased postpartum maternal length of stay.
Prenatal thromboembolic disorder had a significant contribution to this predictive model and made us question whether this particular variable was driving the strong c statistic of our model. Therefore, we performed a second multivariate regression analysis with the exclusion of prenatal thromboembolic disorder (Figure 1b). In this model, all predictor variables remained the same, with the addition of gestational age and drug use during pregnancy, and without significant decline in predictive strength. This demonstrates the validity and importance of our other predictor variables.
Previous studies have established the relationship between BMI and increased risk of VTE, which is in part why we chose to make our risk model on this known high risk group. Butwick et al performed a large cohort study investigating pre-pregnancy BMI and VTE, finding that the prevalence of antepartum and postpartum VTE increased with increasing BMI.11 Most importantly, this study found that the connection between maternal BMI with antepartum and postpartum VTE had a dose-response effect. In addition, Blondon et al reported findings with this in a population-based, case-control study. They found that gradation of maternal BMI is a risk factor for postpartum VTE. Both pre-pregnancy and delivery BMI were independent risk factors for VTE. Importantly, they defend the RCOG guidelines that class III obesity, (BMI ≥ 40 kg/m2) should be viewed as a major risk factor for VTE.12
The most effective type of VTE prophylaxis during pregnancy has not yet been established and anticoagulation duration and regimens postpartum are debated.13 A 2014 Cochrane Database systematic review attempted to determine the effects of thromboprophylaxis during pregnancy and the early postpartum period in women at increased risk of VTE. Overall, there was not sufficient evidence upon which to base recommendations for pharmacologic prophylaxis during pregnancy and the immediate postpartum period. Providers are therefore reliant upon consensus-derived clinical practice guidelines, since there is no protocol driven by the findings of high-quality randomized control trials.14 The most recent ACOG Practice Bulletin on Thromboembolism in Pregnancy explains that the evidence for thromboprophylaxis in pregnancy and postpartum period is derived from what has been studied in non-pregnant patients.4
Sultan et al assessed the effect of patients’ preexisting and pregnancy related factors in both antepartum and postpartum periods to determine population-level absolute risk (AR) and relative risk for VTE, using a large primary care database in the UK between 2005 and 2009. Specifically in the postpartum period, there was a 4-fold increase for VTE in women with a BMI of 30 kg/m2 or higher (Incidence Rate Ratio (IRR), 3.75; 95% confidence interval, 2.76–5.08),15 which is supportive of our findings of BMI > 30 kg/m2 as a predictor variable in both multivariable models.
Our predictive model indicates that obese pregnant patients may benefit from pharmacologic prophylaxis, beyond what ACOG currently recommends. This raises the question of potential over prophylaxis and, if so, what these consequences may be. Low Molecular Weight Heparins (LMWH) may be used as prophylaxis for VTE during pregnancy because they do not cross the placenta and are, therefore, safe for the fetus. However, treatment with LMWH also may be associated with an increased risk of postpartum hemorrhage (PPH).16 More recently, in 2018, Kotsaka et al critically examined the recommendation of LMWH for postpartum women indicated by prediction models and international guidelines, asserting that only RCTs can measure the absolute risk ratio (ARR), number needed to treat (NNT) and number needed to harm (NNH).17 Since large-scale placebo-controlled RCTs do not exist for LMWHs in the pregnant population, these values are may only be extrapolated from the existing data. Attention is called to the fact that few studies assess the potential harm, such as hemorrhage, in postpartum patients receiving LMWH.
In 2016, the National Partnership for Maternal Safety (NPMS) released new recommendations for routine thromboembolism risk assessment and expanded use of pharmacological thromboprophylaxis for VTE prevention.18 In response to the NPMS bundle, certain Maternal Fetal Medicine specialists have voiced legitimate concern that this broader application is not substantiated by existing evidence. They posit that these recommendations do not factor in the potential adverse outcomes that could occur in the prospective large number of patients receiving thromboprophylaxis.19 In the absence of sufficient evidence from large scale RCTs in the US, these recommendations were based on two main factors. First, despite current strategies in the US, the incidence of maternal death from VTE has remained constant.3, 20 Second, in the UK, which follows RCOG guidelines, maternal deaths from thromboembolism decreased by more than 50% from 2003–2005 to 2006–2008.21 Now, unlike in the US, in the United Kingdom, VTE is not a leading cause of maternal mortality.7
In using the CSL database, we generated two models which each had strong classification capabilities for predicted risk of VTE. Additional large cohorts could be used to validate this model externally for added generalizability. Future studies could incorporate the identified predictors to shape inclusion criteria for a large-scale randomized trial to see if prophylactic pharmacological use immediately after delivery would reduce risk for VTE in the postpartum period.
Use of a retrospective cohort design, as opposed to case control design, is one of the strengths of our investigation. Although VTE is a rare occurrence, it was more powerful from an analytic standpoint to use a cohort instead of a case-control study since the adverse event of VTE and all possible exposures were included in the CSL database. Furthermore, the CSL database includes a large and contemporary population of women, increasing the generalizability of our findings to contemporary practice.
A major limitation of our study is that the CSL database did not provide a variable that coded for patients treated with pharmacologic and/or mechanical thromboprophylaxis (or both). Use of these therapeutic interventions may have decreased incidence of VTE. Patients who received any intervention were not able to be identified and analyzed as a separate group. The ACOG Practice Bulletin from 2001 on Thromboembolism in Pregnancy are the guidelines that would have been followed at the time of the CSL database.22 In this bulletin, the recommendations were Level C, meaning that they were based primarily on consensus and expert opinion. This is in contrast to the ACOG Practice Bulletin on Thromboembolism in Pregnancy from 2018, which includes both Level A and Level B recommendations.4 It is plausible that since the recommendations regarding thromboprophylaxis had much less of a validated evidence base to support them during 2002–2008, that fewer patients than the current day would have received thromboprophylaxis during pregnancy and postpartum. Another limitation for working within the CSL dataset was that the individual electronic medical records were not available to confirm each case. We also could not confirm whether it was a PE or DVT that occurred or the exact timing of the event. Due to the fact that we cannot comment on the timing of the VTE, we cannot formulate a specific risk assessment for the postpartum risk alone. Lastly, our predictive model still requires external validation.
Conclusions
While current guidelines by professional organizations regarding VTE prophylaxis have considerable variation, and RCTs that can fully evaluate the benefit and harm of LMWH for VTE prophylaxis do not exist, the fact remains that providers must still make clinical decisions to optimize the health of their obese pregnant and postpartum patients. In the immediate time, providers may appreciate the knowledge of our prediction model, which is constructed on the most significant risk factors for VTE in the obese pregnant population. If external validation of our model was achieved, then it has the potential to assist clinicians who are trying to decide which patients should receive thromboprophylaxis.
Supplementary Material
Table 11.
Distribution of “sumpts” by VTE cohort
| Sum of Weighted Points (sumpts) | No-VTE (n=83391) | VTE (n=109) | Total (n=83500) | Predicted Probability of VTE |
|---|---|---|---|---|
| 0 | 37744 (45.3%) | 14 (12.8%) | 37758 | 0.032% |
| 1 | 8291 (9.9%) | 7 (6.4%) | 8298 | 0.056% |
| 2 | 17984 (21.6%) | 17 (15.6%) | 18001 | 0.100% |
| 3 | 9446 (11.3%) | 8 (7.3%) | 9454 | 0.176% |
| 4 | 4246 (5.1%) | 21 (19.3%) | 4267 | 0.311% |
| 5 | 2570 (3.1%) | 5 (4.6%) | 2575 | 0.548% |
| 6 or more | 3110 (3.7%) | 37 (33.9%) | 3147 | 0.964% |
Highlights.
History of Thromboembolic disease and length of stay increase risk of VTE.
This predictive model is strong in detecting risk of VTE in obese patients.
Patients undergoing cesarean had 1.9 times odds of VTE compared to vaginal delivery.
Obstetrical thromboprophylaxis in obese patients is important.
Funding sources:
This work was supported by the K23 NIH award [K23HL141640].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
These results were presented at the 39th Annual Pregnancy Meeting, Society for Maternal Fetal Medicine, Las Vegas Nevada, February 11–16, 2019.
Conflict of Interest:
The authors report no conflict of interest.
References
- 1.Friedman AM, Ananth CV. Obstetrical venous thromboembolism: Epidemiology and strategies for prophylaxis. Seminars in Perinatology. 2016;40(2):81–86. doi: 10.1053/j.semperi.2015.11.011 [DOI] [PubMed] [Google Scholar]
- 2.Khan KS, Wojdyla D, Say L, Gülmezoglu AM, Van Look PF. WHO analysis of causes of maternal death: a systematic review. The Lancet. 2006;367(9516):1066–1074. doi: 10.1016/S0140-6736(06)68397-9 [DOI] [PubMed] [Google Scholar]
- 3.Creanga AA, Syverson C, Seed K, Callaghan WM. Pregnancy-Related Mortality in the United States, 2011–2013: Obstetrics & Gynecology. 2017;130(2):366–373. doi: 10.1097/AOG.0000000000002114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.ACOG Practice Bulletin No. 196: Thromboembolism in Pregnancy. Obstetrics & Gynecology. 2018;132(1):e1. doi: 10.1097/AOG.0000000000002706 [DOI] [PubMed] [Google Scholar]
- 5.Vahratian A. Prevalence of Overweight and Obesity among Women of Childbearing Age. Matern Child Health J. 2009;13(2):268–273. doi: 10.1007/s10995-008-0340-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Larsen TB, Sørensen HT, Gislum M, Johnsen SP. Maternal smoking, obesity, and risk of venous thromboembolism during pregnancy and the puerperium: A population-based nested case-control study. Thrombosis Research. 2007;120(4):505–509. doi: 10.1016/j.thromres.2006.12.003 [DOI] [PubMed] [Google Scholar]
- 7.Palmerola KL, D’Alton ME, Brock CO, Friedman AM. A comparison of recommendations for pharmacologic thromboembolism prophylaxis after caesarean delivery from three major guidelines. BJOG: An International Journal of Obstetrics & Gynaecology. 123(13):2157–2162. doi: 10.1111/1471-0528.13706 [DOI] [PubMed] [Google Scholar]
- 8.EB Research: Consortium on Safe Labor (CSL). http://www.nichd.nih.gov/. http://www.nichd.nih.gov/about/org/diphr/officebranch/eb/safe-labor. Accessed June 9, 2018.
- 9.Kawakita T, Reddy UM, Landy HJ, Iqbal SN, Huang C-C, Grantz KL. Indications for primary cesarean delivery relative to body mass index. American Journal of Obstetrics and Gynecology. 2016;215(4):515.e1–515.e9. doi: 10.1016/j.ajog.2016.05.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang J, Troendle J, Reddy UM, et al. Contemporary cesarean delivery practice in the United States. American Journal of Obstetrics and Gynecology. 2010;203(4):326.e1–326.e10. doi: 10.1016/j.ajog.2010.06.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Butwick AJ, Bentley J, Leonard SA, et al. Prepregnancy maternal body mass index and venous thromboembolism: a population-based cohort study. BJOG: An International Journal of Obstetrics & Gynaecology. 2019;126(5):581–588. doi: 10.1111/1471-0528.15567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blondon M, Casini A, Hoppe KK, Boehlen F, Righini M, Smith NL. Risks of Venous Thromboembolism After Cesarean Sections: A Meta-Analysis. Chest. 2016;150(3):572–596. doi: 10.1016/j.chest.2016.05.021 [DOI] [PubMed] [Google Scholar]
- 13.Bates SM, Middeldorp S, Rodger M, James AH, Greer I. Guidance for the treatment and prevention of obstetric-associated venous thromboembolism. Journal of Thrombosis and Thrombolysis. 2016;41(1):92–128. doi: 10.1007/s11239-015-1309-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bain E, Wilson A, Tooher R, Gates S, Davis L-J, Middleton P. Prophylaxis for venous thromboembolic disease in pregnancy and the early postnatal period. 2014. doi: 10.1002/14651858.CD001689.pub3 [DOI] [PubMed] [Google Scholar]
- 15.Sultan AA, Tata LJ, West J, et al. Risk factors for first venous thromboembolism around pregnancy: a population-based cohort study from the United Kingdom. Blood. 2013;121(19):3953–3961. doi: 10.1182/blood-2012-11-469551 [DOI] [PubMed] [Google Scholar]
- 16.Sirico A, Saccone G, Maruotti GM, et al. Low molecular weight heparin use during pregnancy and risk of postpartum hemorrhage: a systematic review and meta-analysis. The Journal of Maternal-Fetal & Neonatal Medicine. January 2018:1–8. doi: 10.1080/14767058.2017.1419179 [DOI] [PubMed] [Google Scholar]
- 17.Kotaska A. Postpartum venous thromboembolism prophylaxis may cause more harm than benefit: a critical analysis of international guidelines through an evidence-based lens. BJOG. 2018;125(9):1109–1116. doi: 10.1111/1471-0528.15150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.D’Alton ME, Friedman AM, Smiley RM, et al. National Partnership for Maternal Safety: Consensus Bundle on Venous Thromboembolism. Anesth Analg. 2016;123(4):942–949. doi: 10.1213/ANE.0000000000001569 [DOI] [PubMed] [Google Scholar]
- 19.Sibai BM, Rouse DJ. Pharmacologic Thromboprophylaxis in Obstetrics: Broader Use Demands Better Data. Obstet Gynecol. 2016;128(4):681–684. doi: 10.1097/AOG.0000000000001656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Callaghan WM. Overview of Maternal Mortality in the United States. Seminars in Perinatology. 2012;36(1):2–6. doi: 10.1053/j.semperi.2011.09.002 [DOI] [PubMed] [Google Scholar]
- 21.Saving Mothers’ Lives: Reviewing maternal deaths to make motherhood safer: 2006–2008. BJOG: An International Journal of Obstetrics & Gynaecology. 118(s1):1–203. doi: 10.1111/j.1471-0528.2010.02847.x [DOI] [PubMed] [Google Scholar]
- 22.ACOG practice bulletin. International Journal of Gynecology & Obstetrics. 2001;75(2):203–212. doi: 10.1016/S0020-7292(01)00535-5 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


