Abstract
Three-dimensional (3D) culturing models, replicating in vivo tissue microenvironments that incorporate native extracellular matrix (ECM), have revolutionized the cell biology field. Fibroblastic cells generate lattices of interstitial ECM proteins. Cell interactions with ECMs and with molecules sequestered/stored within these, are crucial for tissue development and homeostasis maintenance. Hence, ECMs provide cells with biochemical and biomechanical ques to support and locally control cell function. Further, dynamic changes in ECMs, and in cell-ECM interactions, partake in growth, development, and temporary occurrences such as acute wound healing. Notably, dysregulation in ECMs and fibroblasts could be important triggers and modulators of pathological events such as developmental defects, and diseases associated with fibrosis and chronic inflammation such as cancer. Studying the type of fibroblastic cells producing these matrices and how alterations to these cells enable changes in ECMs are of paramount importance.
This chapter provides a step-by-step method for producing multilayered (e.g. 3D) fibroblastic cell-derived matrices (fCDM). Methods also include means to assess ECM topography and other cellular traits, indicative of fibroblastic functional statuses, like naïve/normal vs. inflammatory and/or myofibroblastic. For these, protocols include indications for isolating normal and diseased fibroblasts (i.e., cancer associated fibroblasts known as CAFs). Protocols also include means for conducting microscopy assessments, querying whether fibroblasts present with fCDM-dependent normal or CAF phenotypes. These are supported by discrete semi-quantitative digital imaging analyses, providing some imaging processing advice. Additionally, protocols include descriptions for effective fCDM decellularization, which renders cellular debris-free patho/physiological in vivo-like scaffolds, suitable as 3D substrates for subsequent cell culturing.
Keywords: Extracellular matrix, three-dimensional cell culture, cell-derived extracellular matrix, primary fibroblasts, cancer-associated fibroblasts, tissue microenvironment, cell-matrix interactions
1. INTRODUCTION
It is well accepted that culturing cells on two-dimensional (2D) surfaces (e.g. flat culture dishes) seldom simulates natural conditions, rendering cells with artificial phenotypes and functions that are often difficult to interpret. This predicament becomes significant when studying microenvironment-directed effects on cellular function, such as cell differentiation, growth, motility, invasion, etc. (Erdogan & Webb, 2017) as well as microenvironmental-regulated signaling (Edna Cukierman, Pankov, & Yamada, 2002). Fibroblasts, a primary cell type component of interstitial connective tissue, constitute a highly plastic cell population, reactive to the physicochemical characteristics of their immediate microenvironment (von Ahrens, Bhagat, Nagrath, Maitra, & Verma, 2017). Importantly, since self-produced extracellular matrices (ECMs) constitute the fibroblastic natural microenvironmental scaffold, culturing these cells in classic 2D conditions was shown ineffective for the in vivo mimicry of these cells’ morphology, adhesion and motility. In fact, it was only when fibroblasts were cultured within natural three-dimensional (3D) environments, using fibroblastic cell-derived ECMs (fCDMs), that the in vivo-like 3D-matrix adhesion structures were first identified (E. Cukierman, Pankov, Stevens, & Yamada, 2001). These fCDMs and their variations have been used to study fibroblastic biology, fCDMs composition, ECM-cell interactions and ECM-induced cellular responses ever since (Gardiner, 2020; Socovich & Naba, 2019; Yamada & Sixt, 2019). Needless to say, fibroblasts produce, secrete and organize ECM components providing a biological scaffold that supports adhesion, proliferation, migration, and differentiation for themselves, as well as for other cells (Theocharis, Skandalis, Gialeli, & Karamanos, 2016; Yamada et al., 2019). Thus for the effective study of microenvironmental influences of connective tissue on fibroblastic cells and other cells’ biology, it is recommended to use the below described fCDM system, that has proven to accurately mimic physiologic (E. Cukierman et al., 2001; Edna Cukierman et al., 2002) as well as pathologic (Amatangelo, Bassi, Klein-Szanto, & Cukierman, 2005; Franco-Barraza et al., 2017; Goetz et al., 2011; Gupta et al., 2011; Malik et al., 2019; Quiros et al., 2008) in vivo interstitial ECM.
Cell interactions with ECM’s fibrous components, such as collagen and fibronectin, are crucial for several cell functions involved in a broad spectrum of physiological processes and specific changes in these ECM-interactions are associated with pathological conditions such as fibrotic diseases, immunological alterations, and neoplastic disorders (Beacham & Cukierman, 2005; Bonnans, Chou, & Werb, 2014; Chandler, Liu, Buckanovich, & Coffman, 2019; Mack, 2018; Rybinski, Franco-Barraza, & Cukierman, 2014). Additionally, cell-ECM interactions are even known to affect the manner cancer cells respond to drug treatments (C. C. Park et al., 2006; Serebriiskii, Castello-Cros, Lamb, Golemis, & Cukierman, 2008).
This chapter provides a step by step method description needed to produce fCDMs, either using immortalized fibroblastic cell lines (e.g. NIH-3T3 cells) or freshly isolated primary fibroblasts, obtained from murine or human surgical tissues. The method is supported by key protocols needed to culture and manipulate these cells during ECM production. Additionally, the chapter includes methods for characterization of both, fibroblasts and ECMs (e.g. phenotype, quality, etc.). A provided macros-based tool allows a quick evaluation of ECM fiber topography, indicative of fibroblastic activation status (Amatangelo et al., 2005; Conklin et al., 2011; Goetz et al., 2011), via indirect immunofluorescence and digital analysis of microscopy generated images. Data obtained using this analysis is suggestive of the pathological ECM architecture that is evident in vivo, initially described by the late P. Keely, and shown to predict cancer patient outcomes (Bredfeldt et al., 2014; Conklin et al., 2011; Provenzano et al., 2006). The use of fCDMs allows the in vitro study of patho-physiologically relevant cell-matrix interactions, which are evident only when cells are studied within 3D in-vivo-mimicking systems (Avery et al., 2018; Cao et al., 2015; E. Cukierman et al., 2001; Damianova, Stefanova, Cukierman, Momchilova, & Pankov, 2008; Franco-Barraza, Beacham, Amatangelo, & Cukierman, 2016; Jones et al., 2019).
2. PROTOCOLS FOR SYNTHESIS OF 3D FIBROBLASTIC CELL-DERIVED ECMs (fCDMs)
The natural existence of mesenchymal cells from all organs, offers a vast source of fibroblastic cells apt for this protocol. Since a key feature for producing fCDMs relies on the ability of fibroblasts to buildup multilayered cultures, any type of fibroblastic cells that are insensitive to contact inhibition of growth (Ribatti, 2017) are suitable for this protocol. Although, this protocol was originally designed for fetal bovine serum-preconditioned (Note 2.2.3.a) normal murine fibroblasts, NIH-3T3 cells (E. Cukierman et al., 2001), other murine cells, such as embryonic mouse fibroblasts (ATCC, CRL-2908), skin fibroblasts (Kaur et al., 2018), mammary gland fibroblasts (Jones et al., 2019), have shown similar efficiency in producing 3D matrices. Additionally, normal human fibroblastic cells from diverse origins, such as skin (ATCC, PCS-201–012) and lung (ATCC, PCS-201–013), have been shown suitable for this method. Moreover, using cancer-associated fibroblasts (CAFs) from diverse types of tumors, such as esophageal cancer (Okawa et al., 2007), ovarian carcinoma (Quiros et al., 2008), renal carcinoma (Franco-Barraza et al., 2017; Gupta et al., 2011), pancreatic carcinoma (Franco-Barraza et al., 2016; Franco-Barraza et al., 2017; Malik et al., 2019; Roy et al., 2017), prostatic carcinoma (Erdogan et al., 2017), and lung carcinoma (Rangarajan et al., 2018), researchers have successfully mimicked many of the ECM characteristics of the in vivo tumor microenvironment. Hence, the protocols presented here offer means to produce 3D fCDM in vitro, using established fibroblastic cell lines, as well as primary cells, which in many cases recapitulate valuable phenotypic traits that are patho/physiologically relevant recapitulating particular microenvironments (e.g. desmoplastic stroma associated with numerous solid cancers).
Important notice for all the following protocols:
-
Proper institutional biosafety procedures are urged to be followed when harvesting primary human cells from surgical tissue, as well as for working with any unfixed human material.
-
To avoid contamination, all solutions and equipment in contact with living cells must be sterile and manipulated using proper aseptic techniques.
-
Unless otherwise stated, all cell culturing must be performed at 37°C in a humidified incubator with 5% CO2 atmosphere (hypoxic conditions are yet to be tested).
-
Prepare all the solutions that contain hazardous chemicals inside the chemical fume hood and wear proper personal protection equipment.
2.1. Protocols for Isolation and Culturing Primary Fibroblastic Cells from Fresh Tissue
Using established (commercially available) fibroblastic cell lines offers consistency during 3D culturing (e.g. conditioned NIH-3T3 produce reliable matrices with thickness of 10μm or more (Edna Cukierman, 2002)), however some of the ECM characteristics, distinctive of particular microenvironments, can be missed. The following protocols are designed to isolate fibroblastic cells from either murine or human fresh surgical tissue, and can also be adapted to other type of mammalian tissues (e.g. bovine specimens (Elyasi Gorji et al., 2017)). Following these protocols facilitates the harvest of normal/naïve fibroblasts as well as of fibrotic/desmoplastic fibroblasts (e.g. CAFs), depending on the type of tissue used.
Two main methods are presented in this section; each offers distinctive features and advantages. The first method (Basic Protocol 1 in subheadings 2.1.2) relies on the enzymatic disaggregation of the tissue mass; the procedure is faster and produces a higher yield of fibroblasts. This approach renders a heterogeneous fibroblastic cells population, encompassing the diversity from the original stroma, either from a healthy or diseased organ (e.g. diverse CAF populations (Ohlund et al., 2017)). Since the cell recovery is less selective with this approach, epithelial and other cells could contaminate the culture, and although many of these non-fibroblastic cells will be in disadvantage during future culturing passages, it is critical to characterize the purity of the recovered fibroblastic population as early as possible. The second method (Basic Protocol 2 in subheadings 2.1.3) takes advantage of the motile behavior of fibroblastic cells in wounds, allowing the more fit cells to migrate out of the tissue mass. This method yields a more homogeneous population. In both cases, the phenotype of the recovered cells needs to be vetted to authenticate the fibroblastic origin of the harvested cells, mostly to avoid the presence of epithelial-to-mesenchymal transduced cells. An immunofluorescence protocol for fibroblasts characterization of isolated cells is provided (see Basic protocol 3 in subheading 2.2.1), which can be supported by cell lysis followed by Western Blotting assessments as previously reported (Castello-Cros & Cukierman, 2009).
2.1.1. Basic Material, Equipment and Reagents
Biological Material
Tumor or Normal (far from the tumor core) fresh tissue maintained ice-cold in Tissue Transport Medium (see below), until further processing. For a better yield of fibroblasts, the collected tissue has to be at least 1.0 cm3 (Note 2.1.1a.) and must be processed as soon as possible.
General Equipment
Biosafety level 2 cabinet (e.g. Thermo Scientific).
Laboratory chemical fume hood (e.g. Hamilton Safeaire)
Cell Culture Incubator (37°C) with 5% (v/v) humidified CO2.
37°C Waterbath (e.g. Polyscience®)
Inverted phase-contrast microscope.
Benchtop swinging bucket centrifuge for 50 ml tubes (up to ~3,400 r.p.m.) (e.g. Thermo IEC Centra CL2).
General Material
Scalpels -one for each tissue mass- (e.g. Bard-Parker ® #10 protected disposable scalpels).
Dissection scissors and tweezers -one set per tissue mass- (e.g. Fisher Scientific).
100-mm tissue culture polystyrene dishes (e.g. Corning)
75 cm2 (T-75) tissue culture flasks with vented cap -at least three for every time that the isolated cells are sub-cultured- (e.g. Fisher Scientific).
Stericup® Vacuum Filter Units: 0.2 μm PES membrane (e.g. Millipore).
35-mm tissue culture polystyrene dishes (e.g. Corning).
Hematocytometer (e.g. Hausser Scientific).
General Reagents and Solutions
Penicillin/Streptomycin stock solution: 10,000 U/ml Penicillin and 10,000 μg/ml Streptomycin. (e.g. Mediatech, Inc.).
Amphotericin B stock solution: 250 μg/mL stock (e.g. Gibco).
Ciprofloxacin (e.g. Bioworld): 10 mg/mL stock.
Bovine serum albumin (BSA) Fraction V (e.g. Sigma-Aldrich).
Dulbecco’s Phosphate-Buffered Saline solution (DPBS+) 1X solution: Add 8 g of NaCl, 0.2 g of KCl, 1.44 g of Na2HPO4•6H2O, 0.25 g of KH2PO4, 0.13g of CaCl2•6H2O and 0.1g of MgCl2•6H2O to a final volume of 1L of distilled H2O and dissolve. Adjust pH using 1M HCl and/or 1M NaOH until obtaining a stable pH of 7.4. Sterilize by filtering through a 0.22-μm filter unit (see above) following manufacturer’s instructions. Store at 4° C for up to 3 months. Verify the lack of phosphate precipitates (crystals) prior to usage.
DPBS (DPBS+) 10X solution: increase all the components from DPBS+ 1X solution (see above) 10 times more. Sterilize by autoclaving for 20 min, 121°C, in liquid cycle. Store at 4° C for up to 3 months. Verify the lack of phosphate precipitates (crystals) prior to usage. Add 100 mL of DPBS+ 10x to 900 mL of double distilled H2O to obtain 1 L of DPBS+ 1 X solution. Adjust pH and sterilize the DPBS+ 1X solution as stated above.
DPBS Ca and Mg free (DPBS−) 1X solution: Add 8 g of NaCl, 0.2 g of KCl, 1.44 g of Na2HPO4•6H2O, and 0.25 g of KH2PO4 to a final volume of 1L of distilled H2O and dissolve. Adjust pH using 1M HCl and/or 1M NaOH until obtaining a stable pH of 7.4. Filter through a 0.22-μm filter unit (see above) following manufacturer’s instructions. Store at 4° C for up to 3 months. Verify the lack of phosphate precipitates (crystals) prior to usage.
Tissue Transport Medium: To a volume of DPBS− add 100 U/mL of penicillin and 100 μg/mL of streptomycin solution (e.g. already mixed solution from Mediatech, Inc.), amphotericin B 2.50 μg/mL, and ciprofloxacin 10 μg/mL. Filter solution through a 0.22-μm filter unit (see above) following manufacturer’s instructions. Store at 4°C for up to 1 week. Use it cold.
High-glucose Dulbecco’s modified Eagle medium (DMEM) (e.g. Mediatech, Inc.).
Fibroblasts Medium: DMEM supplemented with 10% Premium Grade Fetal Bovine Serum (FBS) (e.g. VWR Life Science Seradigm), 100 U/ml penicillin, and 100 μg/ml of streptomycin (e.g. mixed solution from Mediatech, Inc.). Sterilize solution by filtration through a 0.22- μm filter unit (see above) and store up to 1 month at 4° C. Add fresh L-Glutamine (e.g. Corning) 2mM (1% of 200mM stock) prior to use.
DMEM-P/S-Glut-3%BSA: Add 100 U/ml penicillin and 100 μg /ml streptomycin, and 3g of BSA to 100 mL of cold DMEM, mixed gently (avoid foam formation) until BSA is completely dissolved. Maintain the solution cold during the BSA incorporation. Filter the solution through a 0.22-μm filter unit (see above) following manufacturer’s instructions. Store at 4°C for up to 1 week. Add fresh L-Glutamine (e.g. Corning) 2mM prior to use.
2.1.2. Basic Protocol 1: Fibroblasts isolation by Enzymatic Tissue Digestion
Additional Material
50 mL polypropylene tubes (e.g. Corning).
Nylon mesh: 500 μm (Sefar America Inc.) nylon mesh. The mesh should be sterilized by immersion in 100% ethanol and evaporate/dry inside the cell-culture hood or by autoclave.
Cell strainers: 100 μm and 40 μm nylon mesh inserts (e.g. BD Falcon).
Additional Reagents and Solutions
Collagenase, Type 3 (e.g. Worthington) 10X solution: add 150 mg of collagenase-3 to 10 mL DMEM (serum free). Filter solution through a 0.22-μm filter unit (see above) following manufacturer’s instructions. Aliquot and store at −20°C for up to 1 month.
Procedure
-
1)
Collect fresh surgical tissue and place each specimen in a 50 mL polypropylene tube containing 20–30 mL of cold Tissue Transportation Medium. Always keep the tubes on ice. Process the tissues as quickly as possible after surgery.
-
2)
Bring the tubes containing the specimens inside the biosafety cabinet for processing (one by one, while the others remain on ice). Carefully aspirate the volume and rinse the specimen three times with 10–20 mL cold Tissue Transportation Medium. Induce mild turbulence during rinsing to remove blood clots or other debris.
-
3)
Using sterile tweezers transfer each tissue mass to a 100-mm dish containing 5 mL of DMEM-P/S-Glut-3%BSA at room temperature. With aid of sterile tweezers and scissors cut the tissue in smaller pieces. Continue mincing the tissue masses using a sterile scalpel until obtaining even smaller pieces (~1 mm3).
-
4)
Mix 2 mL of 10X Collagenase-3 with 3 mL of fresh DMEM-P/S-Glut-3%BSA (total volume 5 mL). Warm up this mix to 37°C and add it into the 100-mm dish containing the tissue pieces from step 3 (total volume 10 mL). Gently, stir the volume with the plastic pipette to evenly distribute the tissue pieces.
-
5)
Incubate overnight at 37°C inside the incubator [5% (v/v) humidified CO2].
-
6)
After enzymatic digestion, mechanically disrupt the remaining tissue pieces by pipetting the suspension (up and down) several times against the dish bottom.
-
7)
Collect the digested tissue suspension and transfer into a 50-mL polypropylene tube. Centrifuge for 10 min at 200 × g, room temperature.
-
8)
Discard the supernatant and gently break the pellet by adding 2 mL of fibroblasts medium, pipette the suspension for a couple of times and add another 8 mL of fibroblast medium (total volume 10 mL). Finally, pipette gently for a couple of times more.
-
9)
Collect the cell suspension and filter it through a sterile 500-μm pore nylon mesh, attached to a 50 mL polypropylene tube. This step will remove remaining chunks of non-digested tissue from the cell suspension.
-
10)
Proceed to filtrate two additional times, first through a 100-μm pore nylon mesh cell strainer and then through another one of 40-μm pore size.
-
11)
Transfer the final filtrate into a T-75 tissue culture flask (or a smaller flask size if the tissue size processed was small) and allow cells to adhere by placing the flask in the incubator for 2 hr. Do not incubate for a longer time to avoid a strong attachment of other non-fibroblastic cells.
-
12)
Very carefully aspirate the medium, removing all non-adherent cells and debris.
-
13)
Provide the adhered cells with 7 mL of fibroblasts medium and incubate them overnight inside the incubator.
-
14)
Refresh the fibroblast medium (use 10 mL) and avoid changing media while continue culturing them cells until they reach ~35–50% confluence. Monitor the cells daily (Note 2.1.2.a-b). Once cells reach ~35–50% confluence cells will be at log growth rate and media can be refreshed every other day by removing only half (or less depending on confluence; more cells more media refreshed needed) of the volume and restoring it with fresh fibroblasts medium avoiding media to become orange/yellow.
-
15)
Split the cell population into two new T-75 flasks (see Support Protocol 1 in subheading 2.1.4). One flask will be used to freeze primary cells for long-term storage in a cell bank (see Support Protocol 2 in subheading 2.1.5); the other flask will be used for fibroblastic characterization and immortalization (Notes 2.1.2c-g. and Protocols in subheading 2.2).
-
16)
Once cells are vetted, they can be used for fCDM production (see Protocols in subheading 2.3).
2.1.3. Basic Protocol 2: Fibroblasts Isolation by Non-Enzymatic Tissue Digestion
Additional Material
12-well tissue culture flat bottom polystyrene plates -one plate per tissue sample- (e.g. Corning).
25 cm2 (T-25) tissue culture flasks with vented cap (e.g. Fisher Scientific).
Procedure
-
1)
Open the 12-well tissue culture plate inside the biosafety cabinet, to prevent any contamination, and using sterile dissection scissors or scalpel, scratch several times the bottom of each well sketching an asterisk or star shape. Tissue pieces will be placed onto these scratches.
-
2)
Rinse each well a couple of times with 1–2 mL of sterile fibroblasts medium, DMEM or DPBS+ inducing turbulence to remove plastic-debris and let the wells dry inside the cabinet.
-
3)
Collect the fresh surgical tissue as in Basic Protocol 1 in subheading 2.1.2. Proceed with steps 1–3.
-
4)
Using sterile tweezers transfer the minced tissue into the 12-well tissue culture plate. Place one piece of tissue per well, making sure that each piece is located on the center of the scratched/rinsed surface (Note 2.1.3.a.).
-
5)
Let the tissue samples sit for a period of 5–8 minutes with the lid open. This process will ensure adherence of the tissue samples to the scratched bottom of the dishes; careful not to hover on top of the open plates to avoid contamination.
-
6)
Cautiously, provide 1mL of fibroblasts medium to each well, slowly dispensing the volume down the wall of the well to avoid detaching the small tissue masses (Note 2.1.3.b.). Place the plate inside the incubator.
-
7)
Observe the bottom of each well under the microscope (use objectives 4X and 10 X) daily searching for cells that have migrated out. This process varies depending on the tissue from about 3 days to 8 weeks.
-
8)
Once a significant number of cells have been detected to have migrated out, replace half of the fibroblast medium with a similar volume (fresh), every other day.
-
9)
Monitor daily (Note 2.1.2.b) and continue culturing cells until reaching over 50% confluence (Note 2.1.3.c.).
-
10)
Once cells reach the desired confluence, remove the tissue mass using sterile tweezers. The tissue can be transferred to a new well/plate (previously scratched) to recuperate more fibroblasts (repeat steps 4–10 in this protocol). Remaining fibroblasts should be cultured until reaching ~85% confluence.
-
11)
Dissociate attached fibroblasts (follow Support Protocol 1 in subheading 2.1.4.) and transfer them into a T-25 tissue culture flask to further expand the population.
-
12)
Culture the cells with 5 mL of fibroblasts medium until they reach ~85% confluence. Avoid media changes until cells clearly show growth. Once cells achieve a log rate of growth, refresh the culture medium every other day by removing only half (or a quarter, depending on confluence) of the volume and restoring it with fresh fibroblasts medium. Avoid media getting yellow.
-
13)
Expand the cell population for further long-term storage, cell characterization and ECM production by repeating steps 15 and 16 from Basic Protocol 1 in subheading 2.1.2.
2.1.4. Support Protocol 1: Dissociation of Fibroblastic Cell Cultures by Enzymatic Treatment.
Additional Reagents and Solutions
Trypsin solution: 0.25% Trypsin- 0.02% ethylene diaminetetra-acetic acid (EDTA) solution: 2.5 g of trypsin, 0.2 g EDTA, 8 g NaCl, 0.4 g of KCl, 1g of glucose, 0.35 g of NaHCO3, and 0.01 g phenol red dissolved into H2O to a final volume of a 1L. Sterilize solution by filtration through a 0.22-μm a vacuum filter unit (see above), aliquot and store up to 3 months at −20°C. Alternatively, this solution can be found commercially available, sterile and ready to use (e.g. Mediatech, Inc.)
Procedure
-
1)
Once fibroblasts have reached the desired confluence in a T-75 flask, remove growth medium and rinse cells twice with 10 mL of to DPBS−.
-
2)
Aspirate DPBS− and provide 1 mL of Trypsin-EDTA solution at 37°C (Note 2.1.4.a.), allow the volume to cover the entire surface and quickly remove it by aspiration.
-
3)
Dispense 0.8 mL of fresh Trypsin-EDTA solution at 37°C (Note 2.1.4.a.). Incubate for 1–3 min and tap gently and often while monitoring cells using an inverted phase contrast microscope.
-
4)
When 90% of the cells are clearly detached and appear to be floating (with a round morphology, stop trypsin activity by adding 9 mL of fibroblast medium and pipette gently but firmly the suspension (up and down) a couple of times to disaggregate remaining cell clumps. The FBS present in the growth medium contains molecules that hinder trypsin’s activity.
-
5)
Transfer the cells in suspension (10 mL) into a 50 mL polypropylene. Count cells and transfer them to other vessels as required.
2.2. Protocols for Primary Fibroblastic Cells Characterization
Although most contaminant cells (e.g. epithelial cells) found after fibroblastic isolation (especially when using Basic Protocol 1, subheading 2.1.2), often perish after a few passages, due to lack of especial nutritional requirements, it is crucial to verify the fibroblastic lineage of the harvested fibroblasts. The following protocol is designed to assess the expression of specific mesenchymal cell markers using immunofluorescence (for doing this using immunoblotting please refer to (Castello-Cros & Cukierman, 2009)). Under a regular light microscope, cultured fibroblastic cells depict large, flat and, sometimes elongated cell bodies, with characteristically irregular and non-polarized membrane protrusions. Amongst other markers, fibroblasts can be recognized by the expression of vimentin (Castello-Cros & Cukierman, 2009; Franke, Schmid, Osborn, & Weber, 1978). Additional markers have been associated with fibroblasts but also with CAFs such as podoplanin (Yamanashi et al., 2009), fibroblastic activation protein (FAP) (Avery et al., 2018; Lee et al., 2011), alpha-smooth muscle actin (α-SMA) (Amatangelo et al., 2005; Avery et al., 2018; Franco-Barraza et al., 2017; Quiros et al., 2008), palladin (Goicoechea et al., 2010; Gupta et al., 2011), extra domain A (EDA) spliced isoform of fibronectin (Gupta et al., 2011), etc. Assessment of expression levels of the latter type of markers (e.g. FAP, α-SMA, EDA) can be used to define the activation status of fibroblasts, making them useful to sort normal (naïve) fibroblasts from activated/myofibroblasts, including CAFs (Avery et al., 2018; Franco-Barraza et al., 2017; Gupta et al., 2011; Quiros et al., 2008). Additional markers can identify particular populations of fibroblasts, such as lipid droplets in the cytoplasm of hepatic and pancreatic normal stellate cells (one of the local liver and pancreas fibroblastic cell types), which are lost when these cells become activated and are absent in fibrotic tissues (Auciello et al., 2019; Blaner et al., 2009; Kim et al., 2009). In contrast, fibroblasts lack the expression of epithelial markers, such as cytokeratin, which can be used as negative markers for their characterization (Castello-Cros & Cukierman, 2009). The following protocols describe means to assess the purity and uniformity of isolated cells by imaging of fibroblastic markers using indirect immunofluorescence.
Except for the steps involving cell culturing, the following protocols do not require sterile conditions. Instead, preserve samples in optimal conditions for processing and analysis.
2.2.1. Basic Protocol 3: Characterization of Primary Fibroblasts by Indirect Immunofluorescence.
Additional Biological Material
Bona fide Epithelial or EMT control cell lines (e.g. PANC-1 from ATCC®: CRL-1469™), cultured in corresponding growth media (see below). For fibroblastic positive controls, use characterized cell lines (e.g. human HFF-1 (ATCC® SCRC-1041™) or murine NIH-3T3 (ATCC® CRL-1658)).
Additional Equipment
Dark humidified chamber (i.e., any opaque black plastic box with wet paper towels inside to provide moisture)
Epifluorescence microscope supplied with 40X and/or 62X objectives, suitable to excite and collect at least three different fluorescent emissions (e.g. ~488, ~568 and ~647 nm). The microscope should be complemented with CCD camera and image acquisition software (e.g. NIS-Elements Software, Nikon) for image acquisition (at least 8–16-bit). Alternatively (not necessarily for this assay), confocal microscope (e.g. Nikon A1 spectral confocal system, Nikon), with similar capabilities, should serve the same purpose.
Image analysis/edition software: ImageJ-Fiji (Schindelin et al., 2012) (https://fiji.sc/).
Additional Material
Fine pointed tweezers -sterile- (e.g. Dumont #4).
Paper towels
Parafilm® strips
24-well tissue culture flat bottom polystyrene plates (e.g. Corning)
12-mm #1.0 circular high-quality glass coverslips (e.g. Carolina Biological Supply)
Glass microscope slides.
50-mL polypropylene conical tubes (e.g. Corning).
Light-duty tissue paper (wiper) (e.g. VWR).
Additional Reagents and Solutions
D-Sucrose (e.g. Fisher-Scientific).
Paraformaldehyde 16% (w/v) solution (EM-grade from Electron Microscopy Sciences).
Triton X-100 (e.g. Sigma-Aldrich).
Tween-20 (Fisher-Scientific).
Odyssey® Blocking Buffer (PBS) (LI-COR® Biosciences P/N 927–70001).
Donkey serum (e.g. Jackson ImmunoResearch Laboratories).
SYBR™ Green, nucleic acid stain, 10,000 X concentrate (Invitrogen, cat. no. S7567)
Glycerol anhydrous (e.g. Fluka).
Dimethyl sulfoxide (DMSO) (e.g. Sigma-Aldrich).
N-propyl gallate (NPG) (e.g. Sigma-Aldrich). Stock solution: 20% (w/v) diluted in DMSO.
Clear nail polish or a commercially available coverslip sealant (e.g. CoverGrip™, Biotium).
Ethyl alcohol 95% (e.g. PHARMCO-AAPER).
If using PANC-1 cells as control: PANC-1 medium: RPMI-1640 (e.g. Mediatech, Inc.), supplemented with 10% Premium Grade Fetal Bovine Serum (FBS) (e.g. VWR Life Science Seradigm), 100 U/mL penicillin, and 100 μg/ml of streptomycin (e.g. mixed solution from Mediatech, Inc.), and 2 mM L-Glutamine (e.g. Corning). Sterilize the solution by filtration through a 0.22- μm filter unit (see above) and store up to 1 month at 4° C.
DPBS (DPBS+) 1X solution: Add 8 g of NaCl, 0.2 g of KCl, 1.44 g of Na2HPO4•6H2O, 0.25 g of KH2PO4, 0.13g of CaCl2•6H2O and 0.1g of MgCl2•6H2O to a final volume of 1L of distilled H2O and dissolve. Adjust pH using 1M HCl and/or 1M NaOH until obtaining a stable pH of 7.4. Filter through a 0.22-μm filter unit (see above) following manufacturer’s instructions. Store at room temperature and verify the lack of phosphate precipitates (crystals) prior to usage.
DPBS+/Tween 0.05% solution: Add 50 μL of Tween-20 (e.g. Sigma-Aldrich) to 100 mL of DPBS+. This solution can be stored at 4° C. Verify the lack of phosphate precipitates (crystals) prior to usage.
Fixing solution: Dissolve 2 g of D-Sucrose with 10 mL of 16% paraformaldehyde (Note 2.2.1.a) solution in a 50-mL polypropylene conical tube and adjust the volume to 40 mL using DPBS+. If sterile, store this solution in the dark at room temperature for up to 1 week. Otherwise store at 4°C.
Fixing/Permeabilization solution: Add 100 μL of Triton X-100 to 20 mL of fixing solution (Note 2.2.1.a), to obtain a 0.5% solution. Store this solution as the fixing solution.
Odyssey® blocking buffer with donkey serum 1% (Blocking buffer) solution: Add to 100 μL of donkey serum to 10 mL commercially available blocking buffer. Store this solution in the dark at 4° C for up to 1 month.
SYBR™ Green I nucleic acid stain (Nuclear Stain) 1X solution: Dilute 1 μL of the reagent in 10 mL of DPBS+/Tween solution (Notes 2.2.1.b-c.). Store this solution in the dark at −4° C for up to 1 week.
Anti-fade mounting media: Dilute 1 part of DPBS+ 10X in 9 parts of Glycerol and add 0.1 parts of NPG 20% stock solution (adding drop wise) while mixing the components while stirring (Note 2.2.1.d.).
Ethanol 70% solution: Add 26.3 mL of distilled water to 73.6 mL of Ethanol to obtain a final volume of 100 mL. Mix by inversion and store at 4°C.
Antibodies
Primary antibodies: anti-α-smooth muscle actin (anti-α-SMA), mouse antibody (Sigma-Aldrich, cat. no. A2547) [18.6 μg/mL or 1:300]; anti-palladin (anti-Pall), rabbit antibody (Proteintech, cat. no. 10853–1-AP) [1.0 μg/mL or 1:200]; anti-vimentin (anti-Vim), rabbit antibody (Abcam, cat. no. 92547) [1.32 μg/mL or 1:200]; anti-pan-cytokeratin A1/ (anti-PanKer), mouse antibody (Dako, cat. no. ab8068) [5.0 μg/mL or 1:40].
Secondary antibody: Rhodamine Red™-X donkey anti-Mouse IgG (H+L) (anti-Ms/RR); and Cy™5 donkey anti-Rabbit IgG (H+L) (anti-Rb/Cy5) [5.0 μg/mL or 1:100, each]. Both from Jackson ImmunoResearch Laboratories, cat. no. 715–295-151 and 711–175-152, respectively.
Procedure
Make sure cells meet the culturing criteria needed for step 1 in this Protocol.
Immunofluorescence
-
1)
Starting from a cell culture (~50–85% confluence making sure cells are still in log growth rate), dissociate the cells (see Protocol 3.1.4.), count cells and obtain a cell suspension, by diluting, of 4×103 cells/mL.
-
2)
Seed 2×103 cells (using 500 μL) onto coverslips in a 24 well-plate. Prepare four coverslips per batch of isolated fibroblasts and incubate them overnight at 37 °C.
-
3)
Remove the fibroblasts medium wash the specimens 2X with 500 μL DPBS+, 5 min each at room temperature.
-
4)
Aspirate DPBS+ and provide 350 μL of Fixing/Permeabilization solution, incubate for 3 min at room temperature. Remove the volume and provide 350 μL of Fixing solution, incubate for 20 min at room temperature.
-
5)
Remove the last solution and wash the specimens 2X with 500 μL DPBS+/Tween, 5 min each at room temperature.
-
6)
Aspirate the volume and incubate the specimens with 350 μL of blocking buffer for one hour at room temperature. Incubation can be performed on a rocker using the slower speed.
-
7)
Set up a humidified chamber; attaching a Parafilm® square at the center of the opaque black box, surrounded by wet paper towels. Maintain the Parafilm® square dry and draw a grid resembling the coverslips distribution in the 24 well-plate.
-
8)
In separate tubes, dilute each primary antibody using Blocking buffer as dilution buffer as follows: tube 1) anti-α-SMA; tube 2) anti-Pall; tube 3) anti-Vim; and tube 4) anti-PanCKs. Calculate the total volume for each antibody dilution considering ~10–25 μL per coverslip.
-
9)
On the surface of Parafilm® sheet dispense a drop (15–25 μL with no bubbles) of each primary antibody dilution. For each coverslip use the designated squares in the drawn grid (step 7).
-
10)
Using tweezers transfer each coverslip from the plate (step 6) to the humidified chamber placing them place them onto, phase/sample down, the corresponding antibody dilution drop. Use an angle approach touching the drop slowly as the coverslip lowers. It is recommended to get rid of excess liquid, prior to placing the coverslip onto the antibody drop, by carefully touching on a dry wiper paper with the edge of the coverslip letting liquid decant slowly via capillarity. Incubate for 60 min at room temperature.
-
11)
After incubation, transfer each coverslip back to a 24 well-plate, decanting the excess of antibody via carefully touching the coverslip edge onto a dry wiper paper first (as done in the previous step). Make sure that samples are phasing up when placing coverslips into the 24 well-plate (make sure to keep an orderly way to do this so not to mix the samples). Wash specimens 3X with 500–700 μL of DPBS+/Tween, 5 min each at room temperature. Tilting motion rockers can be used in this step.
-
12)
Prepare the corresponding secondary antibodies in Blocking buffer (see above), considering 15–25 μL for each coverslip to calculate the final volume for each antibody dilution.
-
13)
Transfer coverslips from the plate to the humidified chamber (as in step 10) placing them on top of the corresponding antibody dilution drop (15–25 μL), as follows: For coverslips previously incubated with anti-α-SMA or anti-PanCKs, use secondary antibody anti-MsIgG/RR; for coverslips incubated with anti-Pall or anti-Vim, use anti-RbIgG/Cy5. Incubate for 60 min at room temperature.
-
14)
Repeat step 11.
-
15)
Aspirate DPBS+/Tween and dispense 350 μL of Nuclear Stain Solution. Incubate for 7–10 min at room temperature.
-
16)
Aspirate the volume and wash the specimen, 3X with 750 μL of DPBS+/Tween, 5 min each at room temperature.
-
17)
Before mounting the coverslips onto microscope slides, clean the slides by dipping it in cold 70% ethanol and wipe the liquid off with a light-duty tissue paper. Deposit a 5μL drop of anti-fade mounting medium.
The following steps should be performed rapidly to prevent both the specimens and mounting media, from becoming dry.
-
18)
Using tweezers collect each coverslip and remove the excess of liquid by gently dabbing the edge onto a wiper paper.
-
19)
Flip the coverslip (sample phase down) and place the specimen in contact with the mounting media (as done for the antibody incubations before). Allow the reagent to spread through the entire specimen. Do this by slowly lowering the phasedown coverslip/sample onto the drop while maintaining contact with the mounting media, to avoid the presence of bubbles which can cause imaging inconsistencies.
-
20)
Seal the edge of the coverslip with clear nail polish to prevent the specimen from sliding, as well as to prevent the mounting media from drying.
-
21)
Let the slides settle overnight at room temperature in a dark and dry place. Note 2.2.1.e.
-
22)
The next day, assess the mesenchymal phenotype of isolated fibroblasts by imaging the specimens using an epifluorescence microscope.
Image acquisition
-
23)
Within the acquisition software of choice (e.g. Nikon’s NIS-Elements), select the fluorescent channels to be scanned. For this analysis, chose FITC channel (~488nm), TRITC channel (~568nm) and Cy5 channel (~647nm). Note 2.2.1.c.
-
24)
Aided with a 62X objective, locate an area near the center of the specimen and optimize the imaging settings for each fluorochrome (e.g. fluorescence detector gain, laser power, offset).
-
25)
Acquire images, at least 5 fields per coverslip, of both control cells lines for each marker and experimental samples; the control ones will serve as reference for evaluating the freshly-isolated fibroblastic cells. Note 2.2.1.f.
-
26)
Save the acquired monochromatic, 16-bit Tiff formatted, images representative of each color/stain to serve as raw data images.
-
27)Examine the pixel intensity of the fibroblastic markers in the acquired images by using Fiji software as follows. Alternatively, other image analysis software can be used to analyze the pixels’ intensity from monochromatic images (e.g. MetaMorph™).
-
a)Start Fiji software to conduct a pixel intensity (also known as “gray values”) assessment.
-
b)Determine the desired type of analysis (e.g. Minimum and Maximum gray values, Integrated Density, Mean gray value, or Median). To do this, choose Set Measurements (Analyze>Set Measurements) and select the desired options. Make sure to select the option Display label for easy correlation: image name with corresponding results when data is exported to a spreadsheet.
-
c)Open the 16-bit TIF image(s) of interest.
-
d)From Fiji’s Analyze menu choose the Measure tool (Analyze>Measure; or Ctrl+M).
-
e)Analyze as many images as needed (see Statistics section). Data from each image will be added consecutively after the other in the Results table.
-
f)Export the results by selecting all data (Ctrl+A), copying (Ctrl+C) and pasting it (Ctrl+V) into a new Excel spreadsheet.
-
g)Normalize the fluorescence levels of each marker to the total number of cells within the image. To do this, divide the intensity data by total number of nuclei per image. Notes 2.2.1.f-h.
-
a)
-
28)
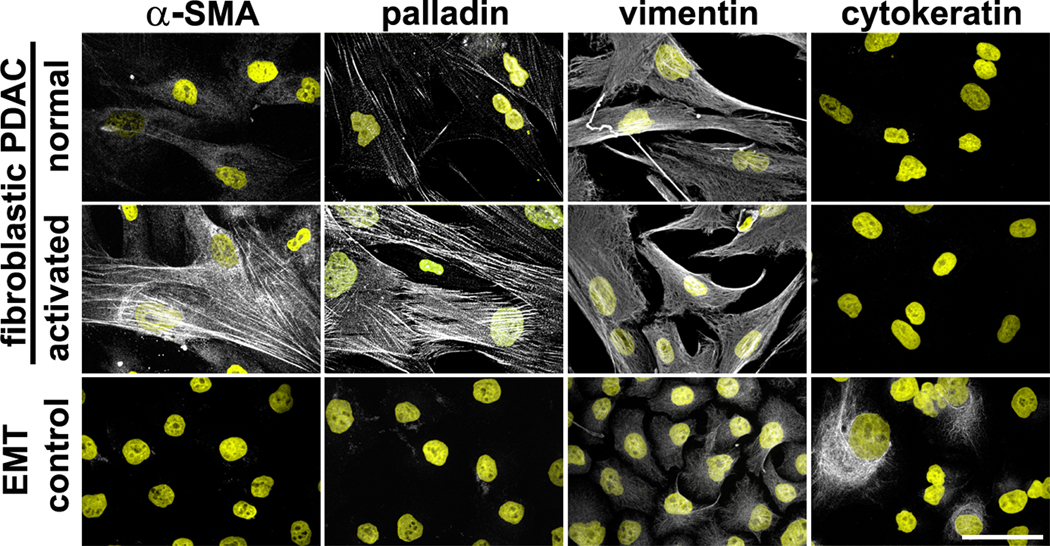
Based on the positive control cell line settings used for this acquisition (exposure time, etc.), establish a cut-off for positive signal (for each marker); to distinguish clearly signal from noise. Then evaluate the presence or absence of the markers and differences in detected levels for those when applicable (i.e., for detecting fibroblastic cells from EMT and comparing normal fibroblasts vs. CAFs, see example in Figure 1). Notes 2.2.1.i-j.
Figure 1. Characterization of human fibroblastic cells isolated from pancreatic ductal adenocarcinoma (PDAC) surgical samples.
Example of pancreatic cancer fibroblastic cells, isolated from human normal/adjacent or tumoral surgical specimens, showing normal vs. activated phenotypes, respectively. Images show indirect immunofluorescent characterization of vimentin-positive and pan-cytokeratin-negative fibroblasts. Additionally, harvested cells were probed for desmoplastic markers α-SMA and palladin. Pancreatic cancer cell line, Panc1, was used as an epithelial-to-mesenchymal transduced (EMT) control, known to express both epithelial and mesenchymal (double-positive) markers. Described markers are shown in white while counterstained nuclei are shown in yellow. Bar represents 50 μm. Figure was adapted from Franco-Barraza, J., et al., Matrix-regulated integrin αvβ5 maintains α5β1-dependent desmoplastic traits prognostic of neoplastic recurrence. eLife, 2017 (Franco-Barraza et al., 2017). Copyright © eLife, under CC BY 4.0 License https://creativecommons.org/licenses/by/4.0/
2.3. MAIN PROTOCOLS for the Production of 3D fibroblast-derived ECMs (fCDM).
This method relies on the ability of fibroblastic cells to overcome contact inhibition of growth in culture. This feature facilitates the production of thick and multi-layered fibroblastic cultures, which is the basis of the three-dimensionality of the system. Importantly, by providing fibroblasts with co-factors (i.e., ascorbic acid) to license collagen polymerization, the method facilitates the maintenance of the multi-dimensional synthesis and secretion of ECM proteins, supported by robust collagen and fibronectin fibers lattices. Hence, if using immortalized NIH-3T3s, cells will require to be pre-adapted to grow in fetal bovine serum-complemented medium (Note 2.3.1.a) prior to ECM production. These fDCM are consistently thick and representative of a normal murine ECM, suitable for large-scale matrix production. If primary fibroblasts are preferred, use these between passages 2 and 6 (older passages usage will depend on the cell type). In this case, expanding the cell population to ensure a high yield of fibroblasts to be frozen, will be important as a reliable and reproducible cell bank. If utilizing immortalized cells, obtained from tissue-isolated primary fibroblasts, these can be used for longer passages. Nonetheless, it is recommended to evaluate their phenotype (both fibroblasts and fCDMs on a regular basis; see Protocols in subheading 2.4.). When separation, away from an artificially stiff underlying substrate (i.e., culturing plastic or coverslip) is needed, the use of physiologic and/or pathologic stiffness fine-tuned acrylamide gels is highly recommended (Malik et al., 2019).
2.3.1. Basic Material, Equipment and Reagents
Biological material
Human or murine fibroblasts isolated from fresh surgical tissue (as described in Basic Protocols 1 and 2 in subheadings 2.1.2. and 2.1.3) or fibroblasts that have been immortalized (i.e., using hTERT (Counter et al., 1998; Franco-Barraza et al., 2017)).
General Material
75 cm2 (T-75) tissue culture flasks with vented cap (e.g. Fisher Scientific).
Syringe filter units: 0.22 μm PES membrane (e.g. RPI).
Stericup® Vacuum Filter Units: 0.2 μm PES membrane (e.g. Millipore).
Rapid-Flow™ Top Filter Units: 0.2 μm PES membrane attached to 50 mL polystyrene conical tube (e.g. Nalgene)
Fine pointed tweezers -sterile- (e.g. Dumont #4).
Parafilm® strips.
General Reagents and Solutions
High-glucose Dulbecco’s modified Eagle medium (DMEM) (e.g. Mediatech, Inc.).
Fibroblasts medium: DMEM (see above) supplemented with 10% Premium Grade Fetal Bovine Serum (FBS) (e.g. VWR Life Science Seradigm) (Note 2.3.1.a), 100 U/ml penicillin, and 100 μg/ml of streptomycin (e.g. mixed solution from Mediatech, Inc.), and 2 mM L-Glutamine (e.g. Corning). Sterilize solution by filtration through a 0.22- μm filter unit (see above) and store up to 1 month.
DPBS (DPBS+) 1X solution: Add 8 g of NaCl, 0.2 g of KCl, 1.44 g of Na2HPO4•6H2O, 0.25 g of KH2PO4, 0.13g of CaCl2•6H2O and 0.1g of MgCl2•6H2O to a final volume of 1L of distilled H2O and dissolve. Adjust pH using 1M HCl and/or 1M NaOH until obtaining a stable pH of 7.4. Sterilize by filtering through a 0.22-μm filter unit (see above) following manufacturer’s instructions. Store at room temperature and verify the lack of phosphate precipitates (crystals) prior to usage.
DPBS, Ca and Mg free (DPBS−) 1X solution: Add 8 g of NaCl, 0.2 g of KCl, 1.44 g of Na2HPO4•6H2O, and 0.25 g of KH2PO4 to a final volume of 1L of distilled H2O and dissolve. Adjust pH using 1M HCl and/or 1M NaOH until obtaining a stable pH of 7.4. Filter through a 0.22-μm filter unit (see above) following manufacturer’s instructions. Store at room temperature and verify the lack of phosphate precipitates (crystals) prior to usage.
Penicillin/Streptomycin stock solution (e.g. Mediatech, Inc.): 10,000 U/mL Penicillin and 10,000 μg/mL Streptomycin.
DPBS+ with antibiotics: To a volume of DPBS+ add 100 U/ml penicillin and 100 μg/ml of streptomycin solution.
2.3.2. Basic Protocol 4: Underlying Surface Preparation for 3D fCDM Deposition
Although not required, both underlying surface preparation (gelatin coating) and cross-linking of it, stabilize the anchoring of fCDMs to their substratum and this can critically improve final yield (especially if cell extraction is planned). However, the authors have observed that the resulting fCDM may be thinner than those obtained without gelatin cross-linking. Therefore, ECM thickness should be determined for each fibroblastic cell type and batch, and a decision to follow the optional steps should be made for each cell type and fCDM need.
Additional Material
24-well tissue culture flat bottom polystyrene plates (e.g. Corning) or 35-mm tissue culture polystyrene dishes (e.g. Corning).
Round glass coverslips (#1). For 24-well plates use 12 mm diameter coverslips (e.g. Carolina biological supplies.)
Additional Reagents and Solutions
Gelatin 0.2% solution: Dissolve 1 g of granular laboratory grade Gelatin type B (e.g. Fisher-Scientific) in 500 mL of DPBS+ and autoclave the solution. Once the solution is cool, filter it through a 0.22 μm Stericup® unit (see above). Store the solution for up to 6 months at 4°C.
Anhydrous absolute Ethanol (e.g. PHARMCO-AAPER)
Glutaraldehyde 1% solution: Dilute 1 mL of Glutaraldehyde grade II 25% stock solution (e.g. Sigma-Aldrich) in 24 mL DPBS+ and sterilize by filtration through a 0.2 μm top filter unit (see above). Prepare fresh prior usage. Note 2.3.2.a.
Ethanolamine 1M solution: Dilute 62 μL of Ethanolamine 99% stock solution (e.g. Acros-Organics) in 1 mL of distilled H2O and sterilize by filtration through a 0.2 μm top filter unit (see above). Prepare fresh prior usage. Note 2.3.2.a.
Procedure
-
1)Coat with gelatin 0.2% solution all cell culture surfaces that will support the deposition of fdCEM as follows:
-
a)If using coverslips for imaging: Sterilize coverslips by dipping them in anhydrous ethanol (absolute), flaming them (prevent cracking the coverslips by quickly stopping the ethanol flame) and place one coverslip per well of a 24 multi-well plate (or use sterile coverslips obtained by other means). Rinse coverslips with 500 μL of DPBS+ and proceed to coat them with same volume of 0.2% gelatin solution for 1 hr. at 37°C, or overnight at 4°C. Notes 2.3.2.b-c.
-
b)If using tissue culture dishes: Add 2 ml as opposed to 500 μL and proceed as in a). Note 2.3.2.d.
-
a)
-
c)
Remove gelatin by aspiration and wash the surfaces with DPBS+ (500 μL/wells of a 24 multi-well plate, or 2 mL for 35-mm dishes) 3 × 5 min each.
-
d)
Crosslink the gelatin coat using 1% glutaraldehyde solution (500 μL/wells of a 24 multi-well plate, or 2 mL for 35-mm dishes) and incubate 30 min at room temperature.
-
e)
Remove glutaraldehyde, wash the surfaces with DPBS+ (500 μL/wells of a 24 multi-well plate, or 2 mL for 35-mm dishes) 3 × 5 min each, and quench aldehyde residues using 1 M ethanolamine solution (500 μL/wells of a 24 multi-well plate, or 2 mL for 35-mm dishes), incubate 30 min at room temperature.
-
f)
Wash the surfaces with DPBS+ (500 μL/well of a 24 multi-well plate, or 2 mL for 35-mm dishes) 3 × 5 min each.
-
g)
Verify that surfaces are free of chemical residues by testing them with DMEM medium. Rinse the wells of dishes with a volume (300 μL/wells of a 24 multi-well plate, or 1 mL for 35-mm dishes), of DMEM, if the medium turns color from red to magenta (indicative of basic pH), repeat step 5 and verify again. Aspirate the last volume prior cells seeding. Note 2.3.2.e.
2.3.3. Basic Protocol 6: Deposition of fCDM on Pre-Coated Surfaces
Although this protocol was originally developed for NIH-3T3 cells (Edna Cukierman, 2002; E. Cukierman et al., 2001), its principle is suitable for using other fibroblastic cell lines, rendering similar efficiency and quality of deposited 3D fCDM. As mentioned, the following steps are applicable for either murine or human fibroblastic cells (primary or immortalized cell lines), comprising phenotypes ranging from naïve to activated fibroblasts, including CAFs.
Additional Material
0.2% gelatin pre-coated 24-well tissue culture flat bottom polystyrene plates with coverslips (e.g. Corning) or 35-mm tissue culture polystyrene dishes (e.g. Corning). See Basic Protocol 5 in subheading 2.3.2.
1.5 mL sterile polypropylene microcentrifuge tubes (e.g. VWR).
Additional Reagents and Solutions
L-Ascorbic acid (LAA) solution: prepare stock tubes with 25–75 μg of L-Ascorbic acid sodium salt (e.g. Sigma-Aldrich) in 1.5 mL polystyrene tubes. Prior usage, add 1 mL of DPBS+ and mix the solution with a pipette until the salt is completely dissolved. Filter this solution through a syringe filter unit (see above) and maintain the collecting tube protected from light. The stock concentration of each tube will be the amount of LAA dry (X) in 1 mL (X μg/mL). Since once LAA is re-suspended it is no longer stable, LAA solution should be used fresh, not longer than 60 min after prepared.
Procedure (see scheme in Figure 2)
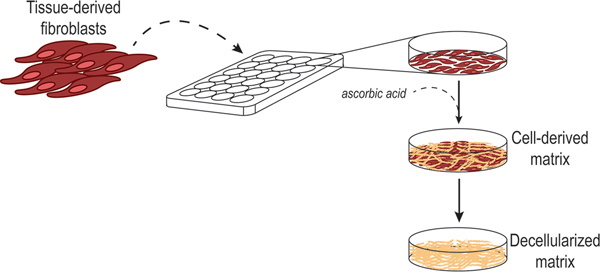
Figure 2. Fibroblastic cell-derived 3D ECM (fdECM) production scheme.
Cartoon depicting the procedure for 3D culturing of fibroblastic cells under stimulation of L-ascorbic acid (ascorbic acid) to sustain a matured polymerization of ECM fibers, followed by the decellularization of the matrices to recover the extracted 3D fCDM substrates. Graphics courtesy of Dr. J. Gardiner.
-
1)
Obtain fibroblasts in suspension by dissociating cell cultures (see Support Protocol 1 in subheading 2.1.2), estimate the total amount of cells and dilute to a final concentration of 3.5 × 105 cells/mL using fibroblasts medium. Note 2.3.3.a.
-
2)
From this cell suspension seed cells in desired well/culturing plate (500 μL/wells of a 24 multi-well plate, or 2 mL for 35-mm dishes). Spread cells evenly and allow cells to settle at room temperature (~5 min.) before placing in the incubator.
-
3)
Incubate cells overnight (16–18 h.) and assure these are 100% confluent the next morning. If these are not confluent, repeat steps 1–3.
-
4)
Aspirate the medium and provide fibroblast medium with fresh LAA 75 μg/mL (use 500 μL/well of a 24 multi-well plate, or 2 mL for 35-mm dishes). Incubate the cells for 24 h. Note 2.3.3.b.
-
5)
Remove half the volume mL (250 μL/well of a 24 multi-well plate, or 1 mL for 35-mm dishes), and replenish that volume with fibroblast medium with fresh LAA 150 μg/mL. Note 2.3.3.c.
-
6)
Repeat step 5 for three additional days achieving a 5-day LAA treatment. Note 2.3.3.d.
-
7)
Incubate the cells for an extra two days (day 7), without removing the fibroblast medium with LAA from day 5.
-
8)
Next day (day 8) the 3D fCDM are ready to be characterized (see Protocols in section 2.4) or extracted (see Basic Protocol 7).
2.3.4. Basic Protocol 7: fCDM Extraction via Alkaline Detergent Decellularization of fibroblastic 3D (multi-layered) Cultures.
Additional Reagents and Solutions
ECM extraction buffer: Prepare a base solution of 0.5% Triton X-100 diluted in DPBS− (this base solution can be stored in the dark at 4°C for up to 1 month). Add fresh NH4OH to a final concentration of 20nM prior usage. Filter this solution through a 0.2 μm top filter unit (see above).
Procedure (see scheme in Figure 2)
-
1)
Remove fibroblasts medium by gentle (very careful) aspiration using a manual pipette. Avoid using excessive vacuum strength to prevent damaging the multilayered cell culture containing fCDMs.
-
2)
Gently rinse the culture 2X using DPBS− (500 μL/well of a 24 multi-well plate, or 2 mL for 35-mm dishes).
-
3)
Incubate the culture with fresh DPBS− for 5–10 min at room temperature.
-
4)
Remove DPBS− and slowly (i.e., very gently) supply/treat with ECM extraction buffer pre-heated to 37° C (500 μL /wells in a 24-well plate, or 2 mL for 35-mm dishes).
From this point forward, fCDMs are extremely fragile, therefore avoid any turbulence when manipulating or transporting.
-
5)
Incubate inside the incubator (37° C) for ~5 min. Monitor the cellular lysis process under the microscope and either proceed or placed back in the incubator. Some samples might require as long as 10 or 15 min.
-
6)
When no cell bodies evident, due to effective lysis (compare images A and C versus B and D in Figure 3), add an equal volume of DPBS− at room temperature for a 1:1 final dilution (500 μL /wells in a 24-well plate, or 2 mL for 35-mm dishes).
-
7)
Let fCDMs stabilize overnight by placing dishes at 4° C overnight.
-
8)
Return the plate or dish inside the biosafety cabinet and gently and slowly remove half the volume. Replenish it carefully (to avoid turbulence) with fresh DPBS− mL (500 μL for wells of 24 multi-well plate, or 2 mL for 35-mm dishes) and let the matrix settle for ~5 min.
-
9)
Aspirate the entire volume with a manual pipette and gently wash the fCDMs three times with DPBS− (500 μL for wells of 24 multi-well plate, or 2 mL for 35-mm dishes).
-
10)
Remove DPBS− and provide a similar volume of DPBS+ and wash two additional times.
-
11)
Store freshly extracted fCDM by sealing the plate or dish with Parafilm® strips and store them at 4° C for up to three months. Notes 2.3.4.a-d.
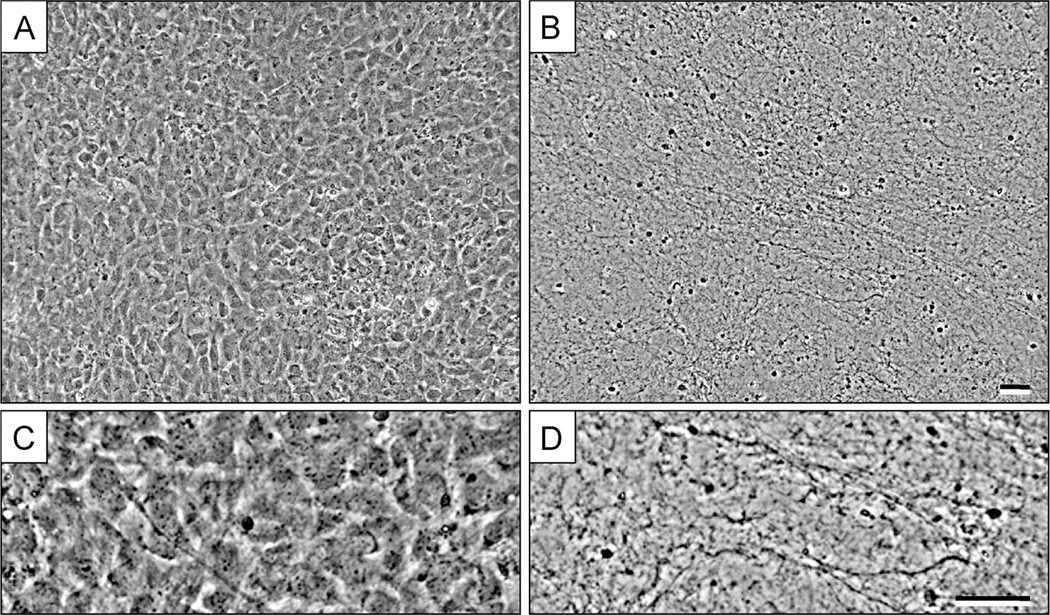
Figure 3. Fibroblastic cell-derived 3D ECM (fCDM) before and after extraction process.
A, shows a representative image of fibroblasts (NIH-3T3) in 3D culture at day 5, prior to matrix extraction. B, show the resulting 3D fCDM fibers after the decellularization process. Panels C and D are magnified areas from images A and B, respectively. Note the fibrous pattern and the absence of cell bodies and debris in D. Bars represent 50 μm. Image was adapted from Cukierman E., Preparation of Extracellular Matrices Produced by Cultured Fibroblasts. Curr Protoc Cell Biol, 2002 (Edna Cukierman, 2002). Copyright © 2002 by John Wiley & Sons, Inc.
2.3.5. Support Protocol 4: DNA-debris cleanse from extracted 3D fCDM.
During decellularization of matrices, the bursting of cells and their nuclei produce a sticky mesh of spilled DNA. The contaminant DNA-debris covers the surface of the matrices and when this is excessive, it could interfere with attachment and spreading of the re-plated cells during functional assays (e.g. adhesion, migration, differentiation, etc.), especially when natural DNA shearing is limited by attempting to use the fCDMs too soon following cell extraction. The following protocol aims to remove this DNA-debris by enzymatic degradation, taking advantage of the activity of DNAse I. Notice that this matrix treatment is also recommended to be performed before long-term storage (Notes 2.3.4.e-d)
Additional Reagents and Solutions
DNAse I (e.g. Thermo Scientific, #EN0521)
DNAse reaction buffer 10X solution: Prepare 500 mL solution of distilled H2O containing 100mM Tris HCl (pH 7.5), 25mM MgCl2 and1mM CaCl2. Sterilize by filtering through a 0.22-μm filter unit (see above) following manufacturer’s instructions. Store at 4° C and verify the lack of precipitates (crystals) prior to usage. Use at 37°C.
DNAse inactivation buffer 10X solution: Prepare 500 mL solution of distilled H2O containing 100mM Tris HCl (pH 7.5), 25mM MgCl2 and1mM CaCl2. Sterilize by filtering through a 0.22-μm filter unit (see above) following manufacturer’s instructions. Store at 4° C and verify the lack of precipitates (crystals) prior to usage. Use at room temperature (~25° C)
Procedure
-
1)
Gently and slowly remove the DPBS+ from extracted matrices, previously stored at 4° C, using a manual pipette.
-
2)
Carefully rinse the fCDMs with DPBS+ (500 μL/well of 24 multi-well plate, or 2 mL for 35-mm dishes). Avoid turbulence to assure matrix stability.
-
3)
Dilute DNAse I to 10 U/mL in pre-warmed 1X solution of DNAse reaction buffer.
-
4)
Incubate matrices for 30 min at 37° C.
-
5)
Gently aspirate the DNAse solution and provide a similar volume (500 μL/well of a 24 multi-well plate, or 2 mL for 35-mm dishes) of 1X DNAse inactivation buffer. Incubate for 15 min at room temperature.
-
6)
Wash the matrices two more time with a similar volume of 1X DNAse inactivation buffer for 5 min each at room temperature.
-
7)
Slowly remove the volume and maintain the matrices in DPBS+ (use at least 500 μL/well of a 24-well plate, or 2 mL for 35-mm dishes). Matrices can now be used for functional assays or stored for further use (see step 11 from Basic Protocol 7 in subheading 2.3.4).
2.4. Protocols for Characterization of fCDM by Imaging of Unextracted 3D Cultures.
The significant fibrotic reaction that accompanies a vast variety of invasive solid cancers, known as desmosplasia, contains, amongst other type of cells, a significant population of activated local and/or recruited fibroblasts (Alexander & Cukierman, 2016; Kalluri, 2016; Thomas & Radhakrishnan, 2019). Selected subpopulations of these tumor or cancer-associated fibroblasts, respectively known as TAFs or CAFs, can be identified by the elevated expression of fibrotic markers, such as α-SMA, high constitutive levels of Y397-phosphorylated FAK (P-FAK) (Franco-Barraza et al., 2017; Gupta et al., 2011; Jiang et al., 2019; Quiros et al., 2008) and more (Sherman, 2018). By studying the phenotype of 3D-cultured fibroblasts and their produced ECMs (e.g. morphology of their cell body and nucleus (Cao et al., 2015; Castello-Cros & Cukierman, 2009; Franco-Barraza et al., 2016), expression and organization of α-SMA (Franco-Barraza et al., 2017; Gupta et al., 2011; Quiros et al., 2008), levels of P-FAK (E. Cukierman et al., 2001; Franco-Barraza et al., 2017; Jones et al., 2019; Quiros et al., 2008), as well as the orientation of ECM fibers (Amatangelo et al., 2005; Franco-Barraza et al., 2016; Franco-Barraza et al., 2017; Kaur et al., 2018; Lee et al., 2011; Malik et al., 2019; Quiros et al., 2008), it has been possible to categorize fCDM as normal/primed or fibrotic/cancer-associated (for more details refer to Quiros et al., 2009 (Quiros et al., 2008)) and more recently, as tumor-supportive or tumor-restrictive (e.g. C2 or C1 CAF-derived ECM (Franco-Barraza et al., 2017; Gardiner, 2020; Malik et al., 2019)). Distinct 3D fCDM phenotypes trigger diverse cell responses (Castello-Cros, Khan, Simons, Valianou, & Cukierman, 2009; Kaur et al., 2018). Hence, the understanding of these matrix-induced cell behaviors could be translated into means to predict the progression and outcome of cancer patients (Franco-Barraza et al., 2017; Gupta et al., 2011; Kaur et al., 2018; Shafi et al., 2018).
The sorting of fCDM based on the expression of their phenotypic traits can be accomplished by indirect immunofluorescent detection. The following protocols are aimed to interrogate native 3D fibroblastic cultures and their derived ECM, prepared on coverslips (see Basic Protocols 5–6 in subheadings 2.3.2–3.), through a simultaneous multi-channel indirect immunofluorescence approach, supported by confocal microscopy and digital imaging analysis.
The principle of the following immunofluorescence and image acquisition protocols is similar to Basic Protocol 3 in subheading 2.2.1. Only specific/relevant steps and considerations are presented for the current protocols, for basic details (e.g. equipment, material, reagents and solutions) refer to mentioned protocol.
2.4.1. Material, Equipment and Reagents
Biological material
Unextracted native 3D fCDM cultures grown on glass coverslips (use 12 mm coverslips for 24 well-plates; see Basic Protocol 6 in subheading 2.3.3.).
Equipment
Confocal microscope supplied with 40X and/or 62X objectives and equipped with a Krypton/Argon laser unit suitable to excite and collect at least three different fluorescent emissions (e.g. ~488, ~568 and ~647 nm) (e.g. Nikon A1 spectral confocal system, Nikon). The microscope should be complemented with a CCD camera and image acquisition software (e.g. Nikon’s NIS-Elements Software) capable to record 8–16-bit images.
Image analysis/edition software: ImageJ-Fiji (Schindelin et al., 2012) (https://fiji.sc/), including the OrientationJ plugin (Rezakhaniha et al., 2012) (http://bigwww.epfl.ch/demo/orientation/#soft).
Excel software (Microsoft).
Optional image analysis/edition software: MetaMorph™ (Molecular Devices); Photoshop (Adobe).
Reagents and solutions
Anti-fade mounting media: For mounting of 3D cultures or decellularized fCDM, it is particularly important to use an aqueous mounting medium to preserve the matrix’s tridimensional features. For recipe refer to Reagents and Solutions in Basic Protocol 3, subheading 2.2.1. Alternatively, commercially available aqueous mounting media can be used (e.g. Abcam Aqueous Mounting Medium, ab128982).
Antibodies
Primary antibodies: anti-α-smooth muscle actin (anti-α-SMA), mouse antibody (e.g. Sigma-Aldrich, cat. no. A2547) [18.6 μg/mL or 1:300]; anti-fibronectin (anti-FN), rabbit antibody (e.g. for murine samples: Abcam, cat. no. ab23750 [2.0 μg/mL or 1:200]; for human samples: Sigma-Aldrich, cat. no. F3648) [2.5 μg/mL or 1:200]; anti-Y397-Phospho-FAK (anti-P-FAK), rabbit antibody (e.g. Thermo Fisher Scientific, cat. no. 44–624G) [5 μg/mL or 1:200].
Secondary antibodies: Rhodamine Red™-X donkey anti-Mouse IgG (H+L) (anti-Ms/RR); and Cy™5 donkey anti-Rabbit IgG (H+L) (anti-Rb/Cy5) [5.0 μg/mL or 1:100, each] (e.g. both from Jackson ImmunoResearch Laboratories, cat. no. 715–295-151 and 711–175-152, respectively).
2.4.2. Basic Protocol 8: Immunofluorescence of 3D fCDM within Native Culture.
The following protocol requires careful manipulation of specimens to avoid damaging or detaching of the fibroblastic 3D cultures. Ideally, the coverslips with deposited matrices should already be inside the wells of a 24-well plate (see Basic Protocol 6 in subheading 2.3.3.). If the matrices were produced on coverslips inside a 35 mm dish, gently transfer these coverslips using fine tweezers into a 24-well plate (place one coverslip per well). Consider two specimens (unextracted native 3D fCDM cultures on coverslips) per batch of matrices to be assessed.
-
1)
Proceed as in steps 1–7 from Basic Protocol 3 in subheading 2.2.1.
-
2)
Prepare Primary Antibodies Cocktails diluted in Blocking buffer as follows: Tube 1) Primary Cocktail 1: anti-α-SMA with anti-FN. Tube 2) Primary Cocktail 2: anti-P-FAK. Calculate the total volume for each cocktail considering using ~10–25 μL per coverslip.
-
3)
Continue as in steps 9–11 from Basic Protocol 3 in subheading 2.2.1.
-
4)
Prepare Secondary Antibodies Cocktails using the listed antibodies as follows: Secondary Cocktail 1: anti-Ms/RR mixed with anti-Rb/Cy5 (each at 1:100) diluted in blocking buffer. Secondary Cocktail 2: anti-Rb/Cy5 (each at 1:100) diluted in blocking buffer. For each cocktail, calculate the total volume to prepare considering ~10–25 μL of each cocktail per coverslip.
-
5)
Repeat step 3 using secondary cocktails as follows: Secondary Cocktail 1 for samples incubated with Primary Cocktail 1; and complementary Secondary Cocktail 2 for samples incubated with Primary Cocktail 2.
-
6)
Proceed with steps 14–22 from Basic Protocol 3 in subheading 2.2.1.
2.4.3. Basic Protocol 9: Image Acquisition of 3D fCDM within Native Culture
For this Protocol, a confocal microscope equipped with precise z-control capabilities is recommended; however, an epifluorescence microscope upgraded with a motorized stage for z-axis and aided with deconvolution software can also suffice. The use of appropriate excitation and emission filters is crucial for this Protocol (Note 2.2.1.a.)
-
1)
Proceed with steps 23–24 from Basic Protocol 3 in subheading 2.2.1.
-
2)
In the acquisition software, set a distance of 0.5 μm between z-steps when acquiring images (this number was selected according to the resolution of the objective proposed to be used). Note 2.4.3.a.
-
3)
Establish the specimen’s spatial frontiers (top and bottom requested by the software, prior the z-scanning/acquisition of 3D specimens) by providing the above and below limits of the actual sample (consider the specimen span of about one third of total z-stack). Use the fluorescent channel for fibronectin (e.g. Cy5 channel), travel up and down the specimen and locate the specimen’s lower focal plane and move further down to assure no signal is detected (this will span about a similar distance to the sample thickness). This z-plane will correspond to the “bottom” limit. Proceed identically to find the top of the specimen by traveling higher until no signal is detected again and set this z-plane as “top”. This way, the specimen imaged will be included between the top and bottom’s actual borders of the sample. The number of positive fluorescent signal z-planes, divided by two, will correspond to the fCDM thickness measured in microns. (Notes 2.4.3.a).
-
4)
By conducting step 1, some bleaching of the specimen fluorescence will be generated. Hence, displace and locate a new field for imaging (e.g. unexposed region), this will be area 1 of a minimum of five needed z-scanned acquisition areas per coverslip.
-
5)
Obtain the above-metioned z-scans instructing the software to acquire every excitation wavelenght using the preset values from the positive controls (see Note 2.2.1.e.),
-
6)
Export the multi-file images (stacks) for future analysis as 16-bit data files; ideally these should be organized by corresponding fluorescent channels acquired (e.g. all images from FITC, or TRITC, or Cy5 channels). Save images as monochromatic 16-bit TIF format. Note 2.4.3.b.
-
7)Open the image stack files (e.g. LIM files generated by NIS-Elements) using Fiji software, obtain the Z projection of the stack (combination of all z-frame images per channel, resulting in a single 2D image) (Note: 2.4.3.c.) as follows:
- Using the Fiji software, open the file from its corresponding folder using the open file function from File menu (or CTRL+O).
- From the Bio-Formats Import Options chose: Stack viewing>Hyperstack; Color options>Grayscale; Split into separate windows>Split channels. The resulting image stacks will appear separated, named “C0, C1 and C2”, corresponding to the order of acquisition (in this case: C0 = FITC, C1 = TRITC and C2 = Cy5)
- Obtain a single 16-bit image by combining all z-frame images from each stack. Use the Z Projection tool (Image>Stacks>Z Projection). Merge all the fames (from 1 to n) choosing the option Sum to preserve the fluorescence intensity for further analyses.
- Save the resultant image as 16-bit TIF files for further analyses (verify the bit size from the general information displayed on top of each image). Notes 2.4.3.d-f.
2.4.4. Basic Protocol 10: Phenotypic Evaluation of Fibroblastic Cells and Derived ECM (fCDMs).
The last four protocols in this chapter are detailed methods to assess the above-mentioned phenotypic characteristics of native 3D fibroblastic cultures and their derived ECM. These are based on unbiased digital analysis of confocal-imaged specimens, representative of each batch of fCDM produced. The phenotypic evaluation is comprised by the combination of fibroblastic activation markers analysis, complemented with nuclear morphology assessment and an additional survey ECM fibers orientation patterns. The last one is supported by a macros-based tool for easy assessment of data generated by digital ECM images analysis (See Supplemental Material).
Of note, 3D cultures of myofibroblastic CAF within their desmoplastic ECM, present elevated and homogenous expression of α-SMA which localize to actin stress fibers, together with high levels of P-FAK (Franco-Barraza et al., 2017). Cells present an elongated/elliptical nuclear morphology (Cao et al., 2015), embedded within a thick ECM with a parallel-patterned topography of fibronectin fibers (Amatangelo et al., 2005; Franco-Barraza et al., 2017; Lee et al., 2011). Then again, matrices produced by normal or solely primed fibroblasts present less organized or completely disorganized ECM fibers, relatively rounded nuclei and either lack α-SMA expression or express at relatively low levels, with diminished levels of P-FAK (see Figure 4 and refer to Franco-Barraza et al., 2017 (Franco-Barraza et al., 2017) for more illustrations). For added details on how to sort fCDM in normal, primed or cancer-associated are needed, refer to (Amatangelo et al., 2005; Quiros et al., 2008). Please note that as the field progresses, additional markers other than the ones proposed herein may be more suitable and thus α-SMA expression and P-FAK levels solely constitute examples of the types of assays that could be conducted for unextracted fCDM characterization.
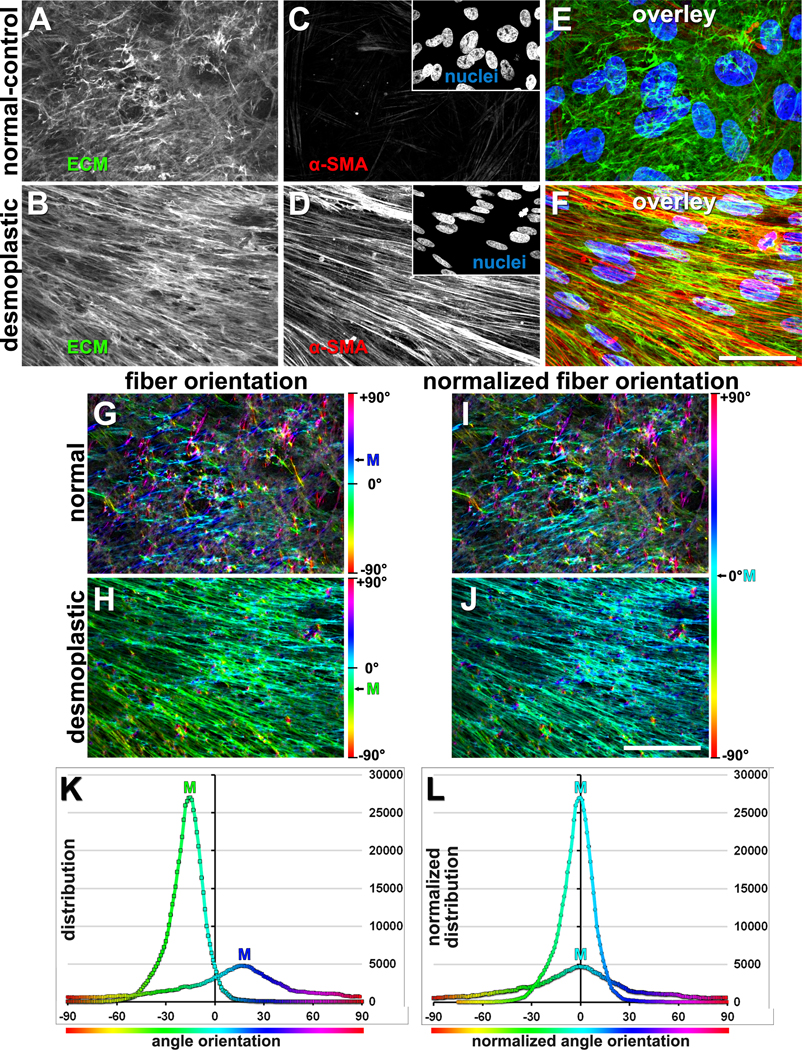
Figure 4. Unextracted fibroblastic cell-derived 3D ECM (fCDM) phenotypes.
(A-F) Confocal indirect immunofluorescence reconstructed images, representative of assorted 3D unextracted human pancreatic primary fCDM cultures. Compare normal vs. desmoplastic (i.e., myofibroblastic activated) phenotypic characterization including monochromatic matrix (i.e., fibronectin; A-B) and α-SMA (C-D) images, with corresponding nuclei images in the inserts. Matching color overlaid images are shown in panels E and F; green=fibronectin, red=αSMA and blue=nuclei. G and H panels exemplify OrientationJ software’s visual outputs obtained from the analysis of fibronectin fibers in A and B. Color gradient bars to the right represent the orientation angles assessed by the software, while the corresponding mode angle color is shown for each image. I and J are the resultant Hue-corrected images displaying mode angle in Cyan representing normalized image/color (i.e., 0°). Note how color variations are diminished in the desmoplastic example where the majority of fibers are parallel oriented. Bars represent 50 μm. Graphs in K represent numeral outputs obtain from G and H, while L corresponds to the normalized distributions (similar to approach in I and J). Image was adapted from Franco-Barraza, J., et al., Preparation of Extracellular Matrices Produced by Cultured and Primary Fibroblasts. Curr Protoc Cell Biol, 2016. (Franco-Barraza et al., 2016). Copyright © 2016 John Wiley & Sons, Inc.
2.4.4.1. Assessment of α-SMA Expression and P-FAK Levels
This protocol is aimed to evaluate (semi-quantitative estimation) levels of α-SMA and Y397-Phopho FAK in fibroblastic cells that generated fCDMs. This evaluation can help to characterize the corresponding in vivo simulated microenvironment. Keep in mind that additional biomarkers could also be assessed while the proposed ones solely serve as proposed examples. The following steps provide a guide to assess monochromatic fluorescence images using Fiji software; other image analysis software can be used to analyze the pixels’ intensity from monochromatic images (e.g. MetaMorph™).
-
1)
Start Fiji software to conduct a pixel intensity (also known as “gray values”) assessment.
-
2)
Determine the desired type of analysis (e.g. Minimum and Maximum gray values, Integrated Density, Mean gray value, or Median). For that, choose Set Measurements (Analyze>Set Measurements) and select the options of interest. Make sure to select the option Display label for easy correlation: image name with corresponding results when data is exported to a spreadsheet.
-
3)
Open the 16-bit TIF image(s) of interest.
-
4)
From Fiji’s Analyze menu choose the Measure tool (Analyze>Measure; or Ctrl+M).
-
5)
Analyze as many images as needed (see Statistics section). Data from each image will be added consecutively after the other in the Results table.
-
6)
Export the results by selecting all data (Ctrl+A), copying (Ctrl+C) and pasting it (Ctrl+V) into a new Excel spreadsheet.
-
7)
Normalize the α-SMA fluorescence levels to total number of cells within the image. Divide the intensity results by total number of nuclei per image. Note 2.2.1g.
-
8)
Evaluate and compare α-SMA fluorescence levels between different types of fCDM (e.g. ECM secreted by normal fibroblasts versus CAFs). Notes 2.2.1.i. and 2.4.4.1.a.
2.4.4.2. Nuclear Morphology Analysis
This protocol will assess the characteristics of the cell nuclei within 3D fCDM. By evaluating the Elliptical Form Factor (EFF) is possible to determine if the nuclei present elongated (elliptical) or more circular shapes. EFF relates to the nuclear axial ratio, calculated by dividing the length (distance of the longest axis) and the breadth (width) of each nucleus.
-
1)
Using Fiji, open the desired nuclei image obtained from step 7 in Basic Protocol 9 in subheading 2.4.3.
-
2)
From the main menu choose the *Straight* tool (icon) and draw a track covering the length of the nucleus. Note 2.4.4.2.a.
-
3)
From the Analyze menu choose the Measure tool (Analyze>Measure; or Ctrl+M). Note 2.4.4.2.b.
-
4)
Similarly, draw another line tracking the width of the nuclear body and repeat step 3.
-
5)
After measurements are taken, export the results by selecting all data (Ctrl+A), copying (Ctrl+C) and pasting it (Ctrl+V) into a new Excel spreadsheet.
-
6)
Calculate the nuclear axial ratio: dividing the length axis by the breadth axis readouts. An EFF greater than 1.0 describes a nuclear body elongated (larger length that width), while values close to 1.0 correspond to circular shapes. Notes 2.4.4.2.c-d.
2.4.4.3. Assessment of 3D fCDM thickness.
Although estimation 3D cultures’ thickness by assessing the ECM limits (top and bottom edges) could represent a subjective evaluation (Note 2.4.3.c.), it is a rather informative and quick way to assess the quality of deposited matrices. By correlating the fibronectin-positive z-planes with the preset distance between each z plane (e.g. 0.5 μm) is possible to obtain a semi-quantitative estimation of 3D matrix’s thickness per area imaged. The later can be used to calculate the thickness average for each specimen. The evaluated fCDMs can be considered of “acceptable quality” when specimens present a minimum averaged thickness of 7 μm (~14 fibronectin-positive z-planes; if fixed gelatin is used averages will be lower, ~5 μm). Complementary to this assessment, the evaluation of nuclei-positive z-planes should be included. Good quality 3D cultures should have cut-off of at least three layers of nuclei.
2.4.4.4. Comprehensive Orientation Analysis (COA) of fCDM Fibers.
The COA protocol is aimed to evaluate the topographic characteristics (e.g. orientation), of fCDM fibers by computational assessment of fibronectin immunofluorescence images obtained from confocal imaging. The protocol relies on the Fiji software analyses aided with the plugin OrientationJ (available from the Biomedical Imaging Group, Switzerland; http://bigwww.epfl.ch/demo/orientation/). This software uses a hue, saturation, lightness (HSL) color distribution system to visualize the orientation of fibrillar objects (e.g. ECM fibers). Analysis of fibronectin images will render a color-coded output, in which each ECM fiber shows a unique color correlation to its orientation angle (from −90° to +90°). These values can be used to graph the total distribution of ECM fibers based on angle of orientation.
Comprehensive Orientation Analysis (COA)
-
1)
Once downloaded, deposit OrientationJ’s executable Jar file (version 2.0.4) in the plugins folder of Fiji.app.
-
2)
Open the desired 16-bit monochromatic fibronectin (3D reconstituted from a z-stack) image from its corresponding folder (see step 8 from Basic Protocol 9 in subheading 2.4.3); use the open function from File menu (or Ctrl+O).
-
3)
To calculate the angle distribution of ECM fibers, select the OrientationJ from the plugins tab and choose the Distribution tool (Plugins>OrientationJ>OrientationJ_Distribution):
-
4)Set the following parameters:
-
a)In the Processing tab, locate Structure Tensor section: Set 3 pixels in Local window σ and Gaussian (distribution) in Gradient. Keep unchecked the Energy, Orientation and Coherency features.
-
b)At the Selection section: Choose the Table feature to obtain the data organized by angle (Orientation column) and distribution (Slice1 column).Optional: Select the Histogram feature to visualize the Gaussian distribution graphed as x = Orientation in Degrees and y = Distribution of Orientation. This type of graph can also be generated from Excel in order to color code the results for results for a comprehensive figure, see below.
- Run the analysis to obtain the data.
-
a)
-
5)Conduct a comprehensive orientation analysis (COA) of ECM fibers using the Excel file provided (download the Supplementary_COA_tool.xlsx file from Supplemental Material). This is a “macros-like” spread sheet developed for COA of ECM fibers. The file includes eight tabs in which data is arranged as follows:
- Tabs 1–5. Images Data (one tab per image analyzed for a total of five images per specimen/coverslip)
- Tab 6. Compiled Data
- Tab 7. Distribution Graph
- Tab 8. Example Data
-
a)Open the provided Supplementary_COA_tool.xlsx
-
b)Raw data from the OrientationJ table for COA will include Orientation Angles and their matched Occurrence Value (slice 1). Import Orientation and Slice 1 values into Columns A and B, respectively.
- Column A/Orientation Value- lists a range of orientation angle values from −89.5° – +89.5° in 0.5 intervals.
-
Column B/ Occurrence Value (slice1)- This lists the relative amount of ECM fibers oriented to the matched degree in column AOnce the data has been input into columns A and B, the subsequent calculations should proceed automatically. Cell P3 will compute the percent alignment of ECM fibers within 15° away the mode angle. That value, as well as the corrected fiber occurrences, and matched angles will automatically populate their respective cells in the “Compiled Data” tab. The following breaks down the functions of each column to achieve the final value outputs.
- Column C/Angle- Presets orientation angles, correcting those from column A to the closest integer. This is done to match the readout FIJI, which MUST sort data from low to high. (−89.5° should always be first listed angle.)
- Column D/MAX Occurrence Value- Selects the Maximum Occurrences (fibers) Value associated with a particular orientation angle (column B). This identifies the highest value for fibers in one orientation degree.
- Column E/Mode Angle- The orientation angle that correlates with the maximum occurrence value (cell D3).
- Column F/Normalized Angles- The mode angle (value in cell E3) is normalized to be 0 degrees. All angles are listed now arranged in reference to the mode angle. Normalization subtracts every orientation angle by the mode angle. Depending on what the mode angle is, this will create values less than/greater than −90° or 90° respectively. This will be corrected in the following columns.
- Column G/Angle Rank- Ranks the linked angle’s value compared to the whole set of angles. This will return a positive integer from 1– 180.
- Column H/Corrected Angles- Corrects the normalized angles to fit within the range −90 ° – +90 °. This has 2 conditional equations to include values less than −90 °, and a second for values greater than +90 °. If the angle in column F is less than −90°, then add 180 ° to that value, if it is greater than +90 °, then subtract 180 from that value, and if it is within −90 ° – +90°, simply return that value.
-
Column I/Angle Occurrence Value- Corresponds to angle occurrences reported in column B (Slice1 from Fiji results).The following 3 columns (K-M) will now sort the normalized and corrected orientation angles and the occurrence values they are associated with. Using the linked values in columns L and M, the distribution and orientation of fibers in the ECM can be graphed (orientation angle vs. Occurrence Value). The normalization ensures a Gaussian distribution, so to help ensure proper analysis has occurred, make sure the lowest and highest angles should correspond to the smallest occurrence values.
- Column K/Desired Rank- this is a housekeeping column used to sort the values using a formula as opposed to Excel’s sorting command. This lists ascending integers from 1– 180.
- Column L/Sorted Angles- sorts the corrected angles (Col. H) low to high (−90° – 90°). Data is ready to use in a graph together with linked values in Column M. This column returns the corrected angle value in Column H whose rank (Col. G) is equals the value defined in K. K3 will always be listed as “1” so L3 will return the corrected angle that is ranked “1” which should be −90° or −89°.
-
Column M- organizes the Occurrence Values in relationship to their corresponding orientation angles (column L).The ECM Fiber Distribution is now ready to be graphed. The following columns (N-P) will be used to calculate the percentage of ECM fibers that are oriented within (+/−) 15° of the mode. In most cases, ECM is determined to be “aligned” if greater than 55% of fibers are oriented within this 30° range (this number was et empirically and it is arbitrary).
- Column N- calculates the Sum of Angle Occurrence Values (Col M). This value is used to calculate the percentage of occurrence values per angle.
- Column O- calculates the Percentage of Angle Occurrence. The occurrence value (Col. M) for each angle is divided by the sum of all occurrence values
-
Column P- returns the Percentage of Fibers Aligned Within 15° around the Mode Angle. This function sums the percentage values given in Column O within the range of angles −15° – +15°. Due to slight differences in readouts this function requires a conditional formula.Based on previous ECM orientation analyses (Franco-Barraza et al., 2016; Franco-Barraza et al., 2017), the cell has been conditionally formatted such that if the returned value is (≥55%) it will appear in green and can be considered associated with the CAF-fCDMs. Values below the threshold of 55% return a red cell, suggesting an ECM phenotype related to normal fibroblasts.
-
6)Organize this data for graphs and statistical analysis.
-
a)The fiber distribution and percent alignments for each acquired image will automatically populate in the Sheet titled “Compiled Data.” Because of this auto-populate function it is very important that one does not change the names of the individual picture sheets/tabs. On the compiled sheet, there will be directions in text boxes for how to graph and/or analyze both data sets.
-
b)Fiber Occurrence Distribution- The first table (Col. A-I) returns the fiber occurrence values for their respective angles for each picture. This data can be graphed to show fiber distribution around the mode. If using statistical software, the individual picture replicate values for each angle can be transferred directly to be analyzed and graphed as an XY scatter plot. If doing analysis in Excel, the average occurrence value for each angle has been averaged (Col. B), paired with its standard deviation (Col. C), and Standard Error of the mean (Col. D)
-
c)A slight variability will arise depending on whether the mode angle is positive or negative, resulting in the min/max angle being −90/+89° or −89/+90°, respectively. To account for this, −90° and +90° are removed from the distribution range, allowing for more consistency without affecting neither the calculation of significance nor the resulting distribution.
-
d)The x-axis should be degrees of alignment −89° – +89°, and the y-axis will display the fiber values for each degree. For added visual clarity, it is recommended to create a lightly shaded range on the x-axis between −15° and +15° to help distinguish alignment between two conditions being graphed on the same axes.
-
d)Percent Fiber Alignment- The second table (Col. K-L) compiles the percentage of fibers within 15° of the mode (cell “P3” for each picture tab). These values can be used for statistical analysis to determine significance of percent fiber alignment between all pictures taken. The Average and Standard Deviation for the set of pictures is also calculated for graphing in Excel.
Color-coded Visualization of Angles distribution
-
a)
-
7)To visualize the angle distribution of ECM fibers in color-coded images, call OrientationJ plugin and choose the Analysis tool (Plugins>OrientationJ>OrientationJ_Analysis):
-
a)Locate the mode angle value calculated above (see Step 5b from this protocol, value in column E)
-
b)Using the mode angle as (x), adjust its value as follows: (x)•(−2) = −2x.
-
c)Open the representative image in Photoshop® software. From the Image menu, choose Adjustments, Hue/Saturation (or Ctrl+U). Modify the Hue rate using the value obtained from previous step (−2x).
-
d)Save image as 8-bit RGB TIF image. Note 2.4.4.4.a.
-
a)
-
8)
An auto-populating Gaussian distribution graph using the orientation data has been included in a separate tab labeled “Distribution Graph.” A description of the criteria used to design the graph is included in the “Compiled Data” Tab.
-
9)To represent the percentage of fibers oriented within 15° around the mode angle, use Excel software as follows. These Values Can Be Used for Calculating Statistical Significance of Alignment
-
a)Open the cumulative worksheet that will house all the analysis for all conditions.
-
b)Import the values in cells “L2 (Average percent)” & “O4 (Standard Error)” in the “Compiled Data” tab for each condition. Make sure to include headings for each condition and data set.
-
c)Select the average percent alignment values (L2), and insert a bar graph. The percent values should be graphed on the Y-axis, while sample conditions are grouped on the X-axis.
-
d)Manually enter the standard error calculated for each data series (L4). Note 2.4.4.4.b.
-
a)
3. PRECURSOR TECHNIQUES
The protocols presented here were initially based on the steps of a method described by I. Vlodavsky in 1999 (Vlodavsky, 1999), using bovine endothelial cells grown in 3D cultures. That method resulted as an improved version of an earlier approach described by Gospodarowicz, Delgado & Vlodavsky in 1980 (Gospodarowicz, Delgado, & Vlodavsky, 1980). Based on Vlodavsky’s approach, the method was modified and adapted to obtain 3D fCDMs using murine cells (e.g. NIH-3T3) by E. Cukierman (Edna Cukierman, 2002; E. Cukierman et al., 2001). Further work helped to standardize this method to produce 3D fCDMs from human fibroblasts isolated from healthy or diseased fresh tissue (Beacham, Amatangelo, & Cukierman, 2007; Castello-Cros & Cukierman, 2009; Franco-Barraza et al., 2016). Additionally, the Cukierman’s core protocol has been complemented with means to evaluate not only the quality of the matrix production, but also to characterize their phenotype (e.g. C2 or C1, Note 2.4.4.1.b.) (Amatangelo et al., 2005; Castello-Cros & Cukierman, 2009; Franco-Barraza et al., 2016). Other additions have included protocols to evaluate ECM-induced responses in cells cultured within these substrates (e.g. morphological changes, myofibroblastic activation, etc.) (Beacham et al., 2007; Castello-Cros & Cukierman, 2009; Franco-Barraza et al., 2016; Goetz et al., 2011).
4. ANALYSIS AND STATISTICS
Most of the protocols presented here are meant to guide the production of fCDMs and qualitative analysis should suffice. However, the protocols 2.4.4.1–4, contained within the Basic Protocol 10, require a quantitative analysis to validate the quality of the produced matrices.
-
a)
For Protocol 2.4.4.1. Assessment of Expression of α-SMA and levels of Y397-Phopho FAK:
A minimum of 5 images (see Basic Protocol 9 in subheading 2.4.3.), are needed for accurate statistical analyses (nevertheless, many more would be better) comparing between different cell types, specimens, (e.g. quiescent fibroblasts compared to activated/myofibroblasts, or normal fibroblasts compared to CAFs).
-
a)
For Protocol 2.4.4.2. Nuclear Morphology Analysis:
-
b)
A minimum of 5 images, containing at least 15 cells each, are recommended for accurate statistical analyses.
-
c)
For Protocol 2.4.4.3. Assessment of 3D fCDM thickness:
-
d)
A minimum of 5 images, are needed for accurate statistical analyses comparing between different cell types, specimens, (e.g. quiescent fibroblasts compared to activated/myofibroblasts, or normal fibroblasts compared to CAFs).
-
e)
For Protocol 2.4.4.4. Comprehensive Orientation Analysis (COA) of fCDM fibers: A minimum of 5 images (ideally 8 images as proposed for the supplied ECM-fibers-orientation tool. See Supplemental Material), are needed for accurate statistical analyses.
Each specimen condition (fibroblastic 3D culture) should be evaluated at least three times (different coverslip each time). Ideally, the mean of each specimen type should be compared with the mean of a control condition (e.g. bona fide CAFs). If comparing only two conditions, evaluate the readouts using Welch corrected t-test. The unpaired t-test assumes that the two populations have the same variances. Since the variance equals the standard deviation squared, this means that the populations have the same standard deviation. A modification of the t-test (developed by Welch) can be used when you are unwilling to make that assumption. If more than two specimen types are evaluated, use One-Way ANOVA test, analyzing each dataset as a block of non-paired readouts to compare to other specimen type’s dataset block. Choose to analyze these blocks without assuming a Gaussian distribution and again, compare each dataset block to each other. These values can be calculated using any statistical software (e.g. GraphPad Instat).
To point significant biological differences between fCDM produced by diverse cell populations in the above listed protocols, consider the following for interpretation of the calculated p values.
A p value greater than 0.05 should be interpreted as not biologically different.
A p value between 0.05 and 0.001 reveals differences which are statistically significant.
A p value between 0.001 and 0.0001 reflects differences with a greater significance. These can be stated as “very” significant,
A p value lower than 0.0001 reflects the greatest significance and the biological differences should be pointed as “extremely” significant.
5. LIMITATIONS OF THE PROTOCOL
The major limitation of the current protocol is the fact that cells at the bottom layer of the 3D culture are attached to a 2D surface (glass or plastic). This underlying substrate stiffness could potentially alter the phenotype of these cells especially when culturing normal fibroblasts, which could become pre-activated by the substrate’s stiffness, producing altered matrices (Calvo et al., 2013). Recently, our team has provided a solution to this predicament by culturing fibroblasts attached onto an underlying polyacrylamide gel, pre-coated with fibronectin or collagen, to physically isolate the cells from the original 2D stiff substrate (Malik et al., 2019). This modification has rendered promising benefits and more importantly, has offered the option to modulate the rigidity of the gels simulating physiological or pathological underlying stiffness in vitro.
6. ALTERNATIVE METHODS
Currently, several protocols based on the original method proposed by Vlodavsky (Vlodavsky, 1999) and later fibroblasts-adapted by Cukierman (Edna Cukierman, 2002), aiming to synthesize fibroblasts-derived ECM in vitro are available (Harris, Raitman, & Schwarzbauer, 2018; Hellewell, Rosini, & Adams, 2017; Kaukonen, Jacquemet, Hamidi, & Ivaska, 2017; Ng et al., 2019; Riis et al., 2019). These alternative options present some variations or adaptations made according to specific needs, based on the model of study. These approaches highlight the flexibility of the method presented here. Additionally, other options for 3D culturing have been reported. These include decellularized ECM from sectioned tissue or even whole organs, useful as bioscaffolds for cell repopulation (Akbarzadeh et al., 2019; Campbell, Cukierman, & Artym, 2014; Napierala et al., 2017), collagen or other ECM proteins-rich lattices and structures or gels (Catoira, Fusaro, Di Francesco, Ramella, & Boccafoschi, 2019; J. Park et al., 2019; Qiu et al., 2019), as well as fibrillar matrices composed by synthetic polymers (Abazari et al., 2019; Chen et al., 2019; Ma et al., 2016), have been successfully used as scaffolds for 3D culturing of a diversity of cells, aimed not only for cell research but as promising avenue for tissue regeneration strategies.
7. TROUBLESHOOTING & OPTIMIZATION
See Table 1.
Table 1.
Troubleshooting and Optimization of fCDM production
| Problem | Solution |
| Isolated fibroblasts yield very low |
|
| Cultured fibroblasts are not detached after using trypsin solution |
|
| Fibroblasts seeded for ECM deposition are not confluent after 18 h post seeding |
|
| Fibroblasts seeded for ECM deposition are detached or dead after 18 h post seeding |
|
| Fibroblastic culture is not multi-layered after 5 days of LAA treatment |
|
| 3D fCDM are detached during the decellularization process |
|
| 3D fCDM are detached after removing extraction buffer |
|
| 3D fCDM appear thin (less than 5 μm thick) |
|
| fCDM specimens are detached during immunofluorescence procedure |
|
| Unextracted 3D fCDM cultures from normal fibroblasts show comparable levels of αSMA or P-FAK as fibrotic/CAFs. |
|
| ECM fibers of unextracted 3D fCDM cultures from normal fibroblasts appear very organized as matrices produced by CAFs. |
|
8. SUMMARY
The methods presented here to obtain 3D fCDM provide a detailed guide to produce 3D matrices from fibroblastic cells, exploding the ability of using fibroblasts of diverse phenotypes. This flexibility offers the possibility to obtain 3D matrices with relevant characteristics for mimicking particular in vivo microenvironments (e.g. normal vs. desmoplastic or fibrous).
Since these matrices recapitulate several important characteristics (e.g. architecture and biochemical composition) of their tissue of origin, they constitute a relevant 3D culture system to study ECM-cells interactions (especially when including the shock absorbing acrylamide gels (Malik et al., 2019)). Moreover, fCDMs can be used to scrutinize co-cultures of different cell types (tumor, stroma, immune neural etc.) that co-exist in the interstitial microenvironment of diverse tissues. Furthermore, the use of this 3D system has shown to recapitulate numerous characteristics of the cancer-associated stroma, offering means to study relevant stimuli affecting the progression of diverse malignancies, including resistance to drugs, influenced by tumor-associated components. Lastly, fCDM have shown to serve as a useful platform to establish pre-clinical studies which later could serve as basis for the design of translational/clinical interventions.
Supplementary Material
9. ACKNOWLEDGMENTS
This chapter is dedicated to the memory of the late P. Keely, who continues to inspire our work. We apologize to all investigators whose studies we could not cite and thank C. O’Donnell for assertive proofreading. The team’s work is supported in part by gifts donated to the memory of Judy Costin, funds from The Martin and Concetta Greenberg Pancreatic Cancer Institute, Pennsylvania’s DOH Health Research Formula Funds, the Greenfield Foundation, the 5th AHEPA Cancer Research Foundation, Inc., Fox Chase In Vino Vita Institutional Pilot Award, as well as NIH/NCI grants R21-CA231252 and R01-CA232256, Core Grant CA06927 in support to Fox Chase Cancer Center’s facilities including: Bio Sample Repository, Light Microscopy, Biostatistics and Bioinformatics, Cell Culture, Histopathology, Immune Monitoring and Talbot Library.
Notes:
If the tissue sample is large enough, it can be divided into two pieces. Process one half for fibroblasts isolation and freeze the other half for further analysis (e.g. immunohistochemistry or immunofluorescence for markers of interest). Freezing tissue specimen: inside a plastic cryomold, embed the fresh tissue specimen using a glycols and resins solution for freezing (e.g. Tissue-Tek® O.C.T. compound). Next, take the cryomold with the preparation and freeze it by flotation on liquid N2 (avoid directly contact of the sample with liquid N2). Once the preparation is frozen, store at −80a° C.
Since primary fibroblasts proliferate better when in close proximity to other fibroblasts, cells should be maintained in culture until they reach ~85% of confluence prior to splitting in ratios 1:3
Start counting passages once the fibroblasts are initially transferred into a new vessel after the first cell expansion.
The fibroblasts are stable by morphological and biochemical criteria at least until passage 10.
Morphological evaluation (cell body and nuclear shapes), as well as immuno-profiling of fibroblastic markers are necessary. See Basic Protocols 4 for characterization by immunofluorescence; for supporting immunoblotting refer to (Castello-Cros & Cukierman, 2009).
Genetic characterization of fibroblastic cells is highly recommended, especially when isolating CAFs, for detection and exclusion of contaminating cancer cells (e.g. epithelial-to-mesenchymal transduced cells). Assessment of gene mutations distinctive of the tumor tissue used (e.g. KRAS, CDKN2A, TP53, and SMAD4 mutations in pancreatic cancer (Pelosi, Castelli, & Testa, 2017)), is commended. Exclusion in expression of epithelial markers such as cytokeratin and EpCAM may be needed as well. These are recommended to be done via qPCR.
Optional: to extend the lifespan of the cell population, fibroblasts can be immortalized (e.g. overexpressing hTERT (Counter et al., 1998; Franco-Barraza et al., 2017)).
Always Maintain frozen stocks of primary cells to be used as reference of the original phenotype, as well as for other cell-type authentication purposes (e.g. Short tandem repeat (STR) analysis. Additional information can be found at the ATCC® online site: https://www.atcc.org/Services/Testing%20Services/Cell%20Authentication%20Testing%20Service/Cell%20Line%20Authentication%20Test%20Recommendations.aspx)
The rough surfaces, made by the scratches, will facilitate the small tissue mass adhesion; these will also provide channels or pathways for fibroblastic cells migrating out of the tissue mass.
If the tissue mass is detached, simply repeat the steps 4–6 placing the tissue mass onto a new scratched dish and continue with the next steps.
The authors have observed that this process varies for each type of tissue, e.g. ovarian tissues can take about 2 weeks, while pancreas tissue could take up to 8 weeks. Other factors can also modulate this process, e.g. whether tissue was obtained from treatment naïve or intensely treated patients (i.e., neoadjuvant chemotherapy treatment and/or irradiated prior to surgery, etc.).
The following volumes of Trypsin-EDTA solution are suggested for different size cell-culture flask/dishes: for T-175 flask: use 1.5 mL; for T-25 flask: use 0.5 mL; for 100 mm dish: use 1.0 mL; for 60 mm dish: use 0.5 mL; for 35 mm dish: use 0.3 mL; for 6-well plate: use 0.3 mL per well; for 12-well plate: use 0.2 mL per well; for 24-well plate: use 0.1 mL per well. The less trypsin volume used the better; hence, trypsin needs to be fresh so avoid freeze and thawing by maintaining aliquoted stocks in the freezer and thawing only once for effective usage.
Paraformaldehyde is an irritant for eyes, respiratory tract and skin. Handle it properly by using gloves and safety eyewear, and always prepare solutions inside the chemical hood. For more information visit: https://www.fishersci.com/store/msds?partNumber=O4042500&productDescription=PARAFORMALDEHYDE+R+500G&vendorId=VN00033897&countryCode=US&language=en
Depending on the capabilities of the epifluorescence microscope, alternative fluorescent reagents can be used to detect cellular nuclei (DNA): Hoechst 33342 [Ex.: ~350 nm; Emm. Max: ~461 nm], DAPI [Ex.: ~358 nm, Emm. Max.: ~461 nm]. Alternatively, other fluorescent dyes away from the green-blue spectrum can also be used (e.g. Propidium Iodine [Ex.: ~535 nm, Emm. Max.: ~617 nm] or DRAQ5™ [Ex.: ~646 nm, Emm. Max.: ~681–697 nm]). If any alternate reagent is used, the proposed set of pre-conjugated fluorescent secondary antibodies for step 13 should be customized to avoid overlapping of emitted fluorescent signals.
The use of appropriate excitation/emission filters is crucial for this protocol (for more information see Herman, 2001(Herman, 2001) and Lichtman & Conchello, 2005 (Lichtman & Conchello, 2005)).
This recipe was borrowed from Rebecca Williams (Cornell Imaging Facility). Alternatively, commercially available mounting media with hardening properties can also be used for 2D cultures (e.g. Thermo Fisher: ProLong™ antifade mountants). If a hardening mounting medium is used, step 20 in this protocol can be omitted.
For long term storage and fluorescence preservation, after drying the sealing nail polish, place the slides in a container protected from light and humidity and store them at −80 °C. Before imaging the specimens let them to return to room temperature and dry any moisture condensation before image acquisition.
When comparing several experimental conditions, the acquisition settings should be stablished based on the positive control specimens. For accurate image analysis, these settings must be consistent along all the acquisitions (set each biomarker independently).
Alternatively, normalize each marker’s fluorescence to the percentage (%) of cellularity in each image. This is reflected by the % of area covered by nuclei within the entire image (taking the full image as 100% of the area). This parameter can be calculated using the plugin NII built for a comprehensive Nuclear Morphometric Analysis (NMA) using ImageJ and it is suitable for Fiji software by Filippi-Chiela et al [4]. Read the reference for more details.
More useful information and suggestions for the analysis of fluorescence images using Fiji can be found in Peter Bankhead’s online handbook (2014): Analyzing fluorescence microscopy images with ImageJ. https://sydney.edu.au/medicine/bosch/facilities/advanced-microscopy/user-support/ImageJ_FL_Image_Analysis.pdf
Ideally the profile of the fibroblastic markers evaluated in the isolated primary fibroblasts should be corroborated by other techniques (e.g. western blot analysis). For details and protocols, refer to the previous versions of these protocols (Castello-Cros & Cukierman, 2009).
If using pancreatic or hepatic fibroblasts (also known as stellate cells), another phenotypic marker that could be used are lipid-rich droplets in the cytoplasm of these cells (Blaner et al., 2009; Kim et al., 2009). Besides identifying the type of cell, this marker can also be used to sort normal (quiescent) cells from activated fibroblasts, based on the presence or absence of lipid droplets, respectively (Auciello et al., 2019). A simple qualitative analysis based on the detection of fluorescent Nile red-positive droplets can be executed (e.g. Cayman Chemical, Lipid Droplets Fluorescence Assay Kit.)
The optimal conditions for fibroblasts culturing and fCDM production rely heavily on the quality of FBS used. It is highly recommended testing the quality of every new lot (and brand) of FBS to be used before switching to a different serum source. For that, evaluate the growth rate of fibroblasts in culture, as well as the quality of the fCDM produced (see Protocols in subheading 2.4). Compare new serum batches with currently used that has proven to render good quality matrices.
Glutaraldehyde and Ethanolamine are potent irritants for eyes, respiratory tract and skin. Handle them properly by using gloves and safety eyewear, and always prepare solutions inside the chemical hood. For more information visit: https://www.osha.gov/Publications/glutaraldehyde.pdf, https://www.sigmaaldrich.com/MSDS/MSDS/DisplayMSDSPage.do?country=US&language=en&productNumber=G6257&brand=SIAL&PageToGoToURL=https%3A%2F%2Fwww.sigmaaldrich.com%2Fcatalog%2Fproduct%2Fsial%2Fg6257%3Flang%3Den, and https://www.fishersci.ca/store/msds?partNumber=AC451760010&productDescription=ethanolamine-acros-organics-3&language=en&countryCode=CA
If preferred, coverslips can be sterilized by microwaving them 3 X for 30 seconds each while inside the multi-well plate. Alternatively, for large amount of coverslips, sterilize all of them in a glass Petri dish by autoclaving for 20 min, 121°C, in gravity cycle. For both alternatives, after the sterilization rinse the coverslips with sterile DPBS+ and proceed with gelatin coating.
As an alternative, few sterile coverslips can be placed inside Petri dishes for bacterial cultures, forcing the fCDM deposition to be only on coverslips. Bacterial dishes are recommended because fibroblasts do not adhere well to their surface; therefore, cells are encouraged to attach mainly to pre- treated glass coverslips. If tissue culture dishes are used, fCDM deposition can be achieved on both types of surfaces (treated glass and plastic). Coverslips can be later recuperated for imaging, while the remaining matrix in the dish can be lysed for further analysis.
This protocol is ideal for 35-, 60-, or 100-mm dishes. If 60- or 100-mm dishes are used, scale up the volumes of all reagents from 2 mL to 4 or 8 mL, respectively.
Pre-coated plates or dishes can be stored by maintaining them at 4°C with DPBS+ with antibiotics (500 μL/well of a 24 multi-well plate, or 2 mL for 35-mm dishes) for up to 1 month (avoid coated surface desiccation by replenishing DPBS+ with antibiotics as needed).
If using NIH-3T3 cells, they must be routinely cultured in high-glucose Dulbecco’s modified Eagle medium supplemented with 10% calf serum, 100 U/mL penicillin, and 100 μg/mL streptomycin unless otherwise specified. Never allow cultured calf serum cultured NIH-3T3 cells to become confluent. However, prior plating for matrix production, NIH-3T3 cells should be adapted (i.e., preconditioned). This adaptation is needed for NIH-3T3s to overcome growth inhibition by contact. Cells should be adapted for a minimum of 20 passages (adapted cells can be frozen and thawed when needed for fCDM production) to grow in 10% fetal bovine serum rather than calf serum.
It is possible to achieve an increase in ECM thickness, for some cells, during fCDM production if using 500 μg/mL of LAA on the first day of treatment. Note that this high concentration can be toxic for some fibroblasts, like NIH-3T3 cells. The optimal concentration of LAA for each fibroblastic cell type should and could be determined experimentally. This is recommended for avid fCDM users.
The main goal of this step is to maintain constant the effective concentration of LAA (e.g. 75 μg/mL), without removing completely the fibroblast-secreted growth factors present in the conditioned medium.
The LAA concentrations and treatment frequency here had proven to be optimal for human pancreatic cancer-derived fibroblasts; nevertheless, these must be optimized for each fibroblastic cell type according to the user needs. For example, when using NIH-3T3s LAA needs to be changes every other day as opposed to daily.
It is recommended to let the matrices settle for at least 2 days before using them in de novo cell re-plating experiments, especially for functional assays.
Avoid matrix desiccation by assuring a substantial volume of DPBS+ with antibiotics is present during storage (replenish as needed).
Optional: fCDMs can be treated to remove contaminant DNA-debris prior to long-term storage (see Support Protocol 4 in subheading 2.3.5.)
If matrices need to be stored at 4° C for longer periods (up to 3 months), exchange DPBS+ for DPBS+ with antibiotics (at least 500 μL/well of 24 multi-well plate, or 2 mL for 35-mm dishes), sealing the vessels with Parafilm® strips.
It is crucial for the acquisition software to be properly calibrated (correlation of pixel size in μm) for the objective used to image the specimen.
If NIS-Elements Software is used for image acquisition, export original nd2 files as LIM file, containing original 16-bit z-stacks images, organized by channel acquisition order (frequently from 488–647, unless otherwise).
Be aware that if using Metamorph™ software, instead of Z projection the nomenclature used for the reconstitution of z-stacks is 3D Reconstruction, which renders a maximal projection (2D image) of those.
For a quick visual evaluation (e.g. gross categorization), reconstitution of z-stacks into 8-bit format images should suffice. Nonetheless, 16-bit monochromatic z-stacks constitute the original intact raw data; therefore, the corresponding z-projections (see previous note) will suffice for accurate software analyses (i.e., using ImageJ).
For convenience assign the name of the corresponding protein or cellular component imaged, as file’s name (in this case, Nuclei or αSMA or P-FAK or Fibronectin) followed by a consecutive numbering.
Notice that the specimen processed to detect P-FAK and Nuclei should have a blank stack corresponding to TRITC channel. There is no need to process this blank stack.
When comparing fibroblasts cultured within their own 3D ECM, differences in expression of α-SMA and detected levels of P-FAK are noticeable lower in fibroblasts with normal/quiescent phenotype, than myofibroblastic cells (activated fibroblasts) or even CAFs (E. Cukierman et al., 2001; Franco-Barraza et al., 2017; Gupta et al., 2011; Quiros et al., 2008). In particular to CAFs, these markers, together with others (some of which are used in this protocols compilation), have helped researchers to sort fibroblastic 3D cultures and their secreted ECMs according to their characteristics as normal or desmoplastic (See Figure 4 for examples), and amongst desmoplastic ECMs, if they present tumor-promoting vs., tumor-restrictive properties (e.g. C2 vs., C1 CAFs, respectively) (Gardiner, 2020).
For accurate measurements, the images assessed should be calibrated to a known pixel-to-μm scale.
Make sure to select the option Display label from the Set Measurements menu for to easily correlate an image’s name with its corresponding value reflected in the length column of the Results table.
Alternatively, for a multi-nuclei analysis per image or to conduct batch processing, use the plugin NII built for a comprehensive Nuclear Morphometric Analysis (NMA). This plugin was developed by Filippi-Chiela, et al (Filippi-Chiela et al., 2012) for ImageJ and it is suitable for Fiji software. This useful plugin automatically estimates several morphology parameters from a nuclei image. For this protocol’s purpose utilize the data calculated within the Aspect Equiv Ellipse column from the Results spreadsheet generated by NII plugin. The plugin, as well as proper instructions on how to install and use it can be found online: http://www.ufrgs.br/labsinal/NMA/
Spindle nuclear shape morphology reflected by EFF should be larger in matrices produced by activated fibroblasts, including CAFs, when compared to normal fibroblasts (e.g. NIH-3T3 or primary normal cells) (Amatangelo et al., 2005).
Intensity and saturation levels of images with normalized-hue images can be fine-tuned for publication purposes. For this, Photoshop® or similar software can be used, as long as these tuning is applied with identical levels throughout all the images employed to generate the figure (e.g. distinct experimental conditions). See figure 4G-J.
If more than 5 replicates/images are analyzed, the ranges used to calculate the standard deviation (L3) and standard error of the mean (O4) will need to be adjusted to include the whole series of replicates.
10. REFERENCES
- Abazari MF, Soleimanifar F, Amini Faskhodi M, Mansour RN, Amini Mahabadi J, Sadeghi S, . . . Zare Karizi S (2019). Improved osteogenic differentiation of human induced pluripotent stem cells cultured on polyvinylidene fluoride/collagen/platelet-rich plasma composite nanofibers. J Cell Physiol. doi: 10.1002/jcp.29029 [DOI] [PubMed] [Google Scholar]
- Akbarzadeh A, Khorramirouz R, Ghorbani F, Hossein Beigi RS, Hashemi J, & Kajbafzadeh AM (2019). Preparation and characterization of human size whole heart for organ engineering: scaffold microangiographic imaging. Regen Med. doi: 10.2217/rme-2018-0111 [DOI] [PubMed] [Google Scholar]
- Alexander J, & Cukierman E (2016). Stromal dynamic reciprocity in cancer: intricacies of fibroblastic-ECM interactions. Curr Opin Cell Biol, 42, 80–93. doi: 10.1016/j.ceb.2016.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amatangelo MD, Bassi DE, Klein-Szanto AJ, & Cukierman E (2005). Stroma-derived three-dimensional matrices are necessary and sufficient to promote desmoplastic differentiation of normal fibroblasts. Am J Pathol, 167(2), 475–488. doi: 10.1016/S0002-9440(10)62991-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auciello FR, Bulusu V, Oon C, Tait-Mulder J, Berry M, Bhattacharyya S, . . . Sherman, M. H. (2019). A Stromal Lysolipid-Autotaxin Signaling Axis Promotes Pancreatic Tumor Progression. Cancer Discov, 9(5), 617–627. doi: 10.1158/2159-8290.CD-18-1212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avery D, Govindaraju P, Jacob M, Todd L, Monslow J, & Pure E (2018). Extracellular matrix directs phenotypic heterogeneity of activated fibroblasts. Matrix Biol, 67, 90–106. doi: 10.1016/j.matbio.2017.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beacham DA, Amatangelo MD, & Cukierman E (2007). Preparation of extracellular matrices produced by cultured and primary fibroblasts. Curr Protoc Cell Biol, Chapter 10, Unit 10 19. doi: 10.1002/0471143030.cb1009s33 [DOI] [PubMed] [Google Scholar]
- Beacham DA, & Cukierman E (2005). Stromagenesis: the changing face of fibroblastic microenvironments during tumor progression. Semin Cancer Biol, 15(5), 329–341. doi: 10.1016/j.semcancer.2005.05.003 [DOI] [PubMed] [Google Scholar]
- Blaner WS, O’Byrne SM, Wongsiriroj N, Kluwe J, D’Ambrosio DM, Jiang H, . . . Libien (2009). Hepatic stellate cell lipid droplets: a specialized lipid droplet for retinoid storage. Biochim Biophys Acta, 1791(6), 467–473. doi: 10.1016/j.bbalip.2008.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnans C, Chou J, & Werb Z (2014). Remodelling the extracellular matrix in development and disease. Nat Rev Mol Cell Biol, 15(12), 786–801. doi: 10.1038/nrm3904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bredfeldt JS, Liu Y, Conklin MW, Keely PJ, Mackie TR, & Eliceiri KW (2014). Automated quantification of aligned collagen for human breast carcinoma prognosis. J Pathol Inform, 5, 28. doi: 10.4103/2153-3539.139707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvo F, Ege N, Grande-Garcia A, Hooper S, Jenkins RP, Chaudhry SI, . . . Sahai, E. (2013). Mechanotransduction and YAP-dependent matrix remodelling is required for the generation and maintenance of cancer-associated fibroblasts. Nat Cell Biol, 15(6), 637–646. doi: 10.1038/ncb2756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell CB, Cukierman E, & Artym VV (2014). 3-D extracellular matrix from sectioned human tissues. Curr Protoc Cell Biol, 62, Unit 19 16 11–20. doi: 10.1002/0471143030.cb1916s62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao X, Lin Y, Driscoll TP, Franco-Barraza J, Cukierman E, Mauck RL, & Shenoy VB (2015). A Chemomechanical Model of Matrix and Nuclear Rigidity Regulation of Focal Adhesion Size. Biophys J, 109(9), 1807–1817. doi: 10.1016/j.bpj.2015.08.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castello-Cros R, & Cukierman E (2009). Stromagenesis during tumorigenesis: characterization of tumor-associated fibroblasts and stroma-derived 3D matrices. Methods Mol Biol, 522, 275–305. doi: 10.1007/978-1-59745-413-1_19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castello-Cros R, Khan DR, Simons J, Valianou M, & Cukierman E (2009). Staged stromal extracellular 3D matrices differentially regulate breast cancer cell responses through PI3K and beta1-integrins. BMC Cancer, 9, 94. doi: 10.1186/1471-2407-9-94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catoira MC, Fusaro L, Di Francesco D, Ramella M, & Boccafoschi F (2019). Overview of natural hydrogels for regenerative medicine applications. J Mater Sci Mater Med, 30(10), 115. doi: 10.1007/s10856-019-6318-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler C, Liu T, Buckanovich R, & Coffman LG (2019). The double edge sword of fibrosis in cancer. Transl Res, 209, 55–67. doi: 10.1016/j.trsl.2019.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YW, Chen CC, Ng HY, Lou CW, Chen YS, & Shie MY (2019). Additive Manufacturing of Nerve Decellularized Extracellular Matrix-Contained Polyurethane Conduits for Peripheral Nerve Regeneration. Polymers (Basel), 11(10). doi: 10.3390/polym11101612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conklin MW, Eickhoff JC, Riching KM, Pehlke CA, Eliceiri KW, Provenzano PP, . . . Keely PJ (2011). Aligned Collagen Is a Prognostic Signature for Survival in Human Breast Carcinoma. Am J Pathol, 178(3), 1221–1232. doi: 10.1016/j.ajpath.2010.11.076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Counter CM, Hahn WC, Wei W, Caddle SD, Beijersbergen RL, Lansdorp PM, . . . Weinberg RA (1998). Dissociation among in vitro telomerase activity, telomere maintenance, and cellular immortalization. Proc Natl Acad Sci U S A, 95(25), 14723–14728. doi: 10.1073/pnas.95.25.14723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cukierman E (2002). Preparation of Extracellular Matrices Produced by Cultured Fibroblasts. Current Protocols in Cell Biology, 16(1), 10.19.11–10.19.15. doi:doi: 10.1002/0471143030.cb1009s16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cukierman E, Pankov R, Stevens DR, & Yamada KM (2001). Taking cell-matrix adhesions to the third dimension. Science, 294(5547), 1708–1712. doi: 10.1126/science.1064829 [DOI] [PubMed] [Google Scholar]
- Cukierman E, Pankov R, & Yamada KM (2002). Cell interactions with three-dimensional matrices. Curr Opin Cell Biol, 14(5), 633–639. doi: 10.1016/s0955-0674(02)00364-2 [DOI] [PubMed] [Google Scholar]
- Damianova R, Stefanova N, Cukierman E, Momchilova A, & Pankov R (2008). Three-dimensional matrix induces sustained activation of ERK1/2 via Src/Ras/Raf signaling pathway. Cell Biol Int, 32(2), 229–234. doi: 10.1016/j.cellbi.2007.08.029 [DOI] [PubMed] [Google Scholar]
- Elyasi Gorji Z, Khaledi J, Daneshvar K, Amoli A, Ganjibakhsh M, Nasimian A, Gohari NS, . . . Farzaneh P (2017). Establishment and characteristics of Iranian Sistani cattle fibroblast bank: a way to genetic conservation. Conservation Genetics Resources, 9(2), 305–312. doi: 10.1007/s12686-016-0640-x [DOI] [Google Scholar]
- Erdogan B, Ao M, White LM, Means AL, Brewer BM, Yang L, . . . Webb DJ (2017). Cancer-associated fibroblasts promote directional cancer cell migration by aligning fibronectin. J Cell Biol, 216(11), 3799–3816. doi: 10.1083/jcb.201704053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdogan B, & Webb DJ (2017). Cancer-associated fibroblasts modulate growth factor signaling and extracellular matrix remodeling to regulate tumor metastasis. Biochem Soc Trans, 45(1), 229–236. doi: 10.1042/BST20160387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippi-Chiela EC, Oliveira MM, Jurkovski B, Callegari-Jacques SM, da Silva VD, & Lenz G (2012). Nuclear morphometric analysis (NMA): screening of senescence, apoptosis and nuclear irregularities. PLoS One, 7(8), e42522. doi: 10.1371/journal.pone.0042522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco-Barraza J, Beacham DA, Amatangelo MD, & Cukierman E (2016). Preparation of Extracellular Matrices Produced by Cultured and Primary Fibroblasts. Curr Protoc Cell Biol, 71, 10 19 11–10 19 34. doi: 10.1002/cpcb.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco-Barraza J, Francescone R, Luong T, Shah N, Madhani R, Cukierman G, . . . Cukierman E (2017). Matrix-regulated integrin alphavbeta5 maintains alpha5beta1-dependent desmoplastic traits prognostic of neoplastic recurrence. Elife, 6. doi: 10.7554/eLife.20600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke WW, Schmid E, Osborn M, & Weber K (1978). Different intermediate-sized filaments distinguished by immunofluorescence microscopy. Proc Natl Acad Sci U S A, 75(10), 5034–5038. doi: 10.1073/pnas.75.10.5034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardiner JCR, K. S.; Alexander JI; Franco-Barraza J; Cukierman E (2020). Fibroblastic Cell-Derived Extracellular Matrices: Cell Culturing Systems Mimicking Key Aspects of the Tumor Microenvironment. In Decellularized extracellular matrix: characterization, fabrication and applications. Royal Society of Chemistry, Chapter 16(Biomaterials Science Series No. 6), 305–327. [Google Scholar]
- Goetz JG, Minguet S, Navarro-Lerida I, R, J. J. L, Samaniego R, Calvo E, . . . Pozo MAD (2011). Biomechanical remodeling of the microenvironment by stromal caveolin-1 favors tumor invasion and metastasis. Cell, 146(1), 148–163. doi:doi: 10.1016/j.cell.2011.05.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goicoechea SM, Bednarski B, Stack C, Cowan DW, Volmar K, Thorne L, . . . Otey CA (2010). Isoform-specific upregulation of palladin in human and murine pancreas tumors. PLoS One, 5(4), e10347. doi: 10.1371/journal.pone.0010347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gospodarowicz D, Delgado D, & Vlodavsky I (1980). Permissive effect of the extracellular matrix on cell proliferation in vitro. Proc Natl Acad Sci U S A, 77(7), 4094–4098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta V, Bassi DE, Simons JD, Devarajan K, Al-Saleem T, Uzzo RG, & Cukierman E (2011). Elevated expression of stromal palladin predicts poor clinical outcome in renal cell carcinoma. PLoS One, 6(6), e21494. doi: 10.1371/journal.pone.0021494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris GM, Raitman I, & Schwarzbauer JE (2018). Cell-derived decellularized extracellular matrices. Methods Cell Biol, 143, 97–114. doi: 10.1016/bs.mcb.2017.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellewell AL, Rosini S, & Adams JC (2017). A Rapid, Scalable Method for the Isolation, Functional Study, and Analysis of Cell-derived Extracellular Matrix. J Vis Exp(119). doi: 10.3791/55051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman B (2001). Absorption and emission maxima for common fluorophores. Curr Protoc Cell Biol, Appendix 1, Appendix 1E. doi: 10.1002/0471143030.cba01es00 [DOI] [PubMed] [Google Scholar]
- Jiang H, Liu X, Knolhoff BL, Hegde S, Lee KB, Jiang H, . . . DeNardo DG (2019). Development of resistance to FAK inhibition in pancreatic cancer is linked to stromal depletion. Gut. doi: 10.1136/gutjnl-2018-317424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones CE, Hammer AM, Cho Y, Sizemore GM, Cukierman E, Yee LD, . . . Leight JL (2019). Stromal PTEN Regulates Extracellular Matrix Organization in the Mammary Gland. Neoplasia, 21(1), 132–145. doi: 10.1016/j.neo.2018.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalluri R (2016). The biology and function of fibroblasts in cancer. Nat Rev Cancer, 16(9), 582–598. doi: 10.1038/nrc.2016.73 [DOI] [PubMed] [Google Scholar]
- Kaukonen R, Jacquemet G, Hamidi H, & Ivaska J (2017). Cell-derived matrices for studying cell proliferation and directional migration in a complex 3D microenvironment. Nat Protoc, 12(11), 2376–2390. doi: 10.1038/nprot.2017.107 [DOI] [PubMed] [Google Scholar]
- Kaur A, Ecker BL, Douglass SM, Kugel CH 3rd, Webster MR, Almeida FV, . . . Weeraratna AT. (2018). Remodeling of the Collagen Matrix in Aging Skin Promotes Melanoma Metastasis and Affects Immune Cell Motility. Cancer Discov. doi: 10.1158/2159-8290.CD-18-0193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim N, Yoo W, Lee J, Kim H, Lee H, Kim YS, . . . Oh, J. (2009). Formation of vitamin A lipid droplets in pancreatic stellate cells requires albumin. Gut, 58(10), 1382–1390. doi: 10.1136/gut.2008.170233 [DOI] [PubMed] [Google Scholar]
- Lee HO, Mullins SR, Franco-Barraza J, Valianou M, Cukierman E, & Cheng JD (2011). FAP-overexpressing fibroblasts produce an extracellular matrix that enhances invasive velocity and directionality of pancreatic cancer cells. BMC Cancer, 11, 245. doi: 10.1186/1471-2407-11-245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtman JW, & Conchello JA (2005). Fluorescence microscopy. Nat Methods, 2(12), 910–919. doi: 10.1038/nmeth817 [DOI] [PubMed] [Google Scholar]
- Ma Q, Yang L, Jiang Z, Song Q, Xiao M, Zhang D, . . . Cheng G (2016). Three-Dimensional Stiff Graphene Scaffold on Neural Stem Cells Behavior. ACS Appl Mater Interfaces, 8(50), 34227–34233. doi: 10.1021/acsami.6b12305 [DOI] [PubMed] [Google Scholar]
- Mack M (2018). Inflammation and fibrosis. Matrix Biol, 68-69, 106–121. doi: 10.1016/j.matbio.2017.11.010 [DOI] [PubMed] [Google Scholar]
- Malik R, Luong T, Cao X, Han B, Shah N, Franco-Barraza J, . . . Cukierman E (2019). Rigidity controls human desmoplastic matrix anisotropy to enable pancreatic cancer cell spread via extracellular signal-regulated kinase 2. Matrix Biol, 81, 50–69. doi: 10.1016/j.matbio.2018.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napierala H, Hillebrandt KH, Haep N, Tang P, Tintemann M, Gassner J, . . . Struecker B (2017). Engineering an endocrine Neo-Pancreas by repopulation of a decellularized rat pancreas with islets of Langerhans. Sci Rep, 7, 41777. doi: 10.1038/srep41777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng WH, Ramasamy R, Yong YK, Ngalim SH, Lim V, Shaharuddin B, & Tan JJ (2019). Extracellular matrix from decellularized mesenchymal stem cells improves cardiac gene expressions and oxidative resistance in cardiac C-kit cells. Regen Ther, 11, 8–16. doi: 10.1016/j.reth.2019.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlund D, Handly-Santana A, Biffi G, Elyada E, Almeida AS, Ponz-Sarvise M, . . . Tuveson DA (2017). Distinct populations of inflammatory fibroblasts and myofibroblasts in pancreatic cancer. J Exp Med, 214(3), 579–596. doi: 10.1084/jem.20162024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okawa T, Michaylira CZ, Kalabis J, Stairs DB, Nakagawa H, Andl CD, . . . Rustgi AK (2007). The functional interplay between EGFR overexpression, hTERT activation, and p53 mutation in esophageal epithelial cells with activation of stromal fibroblasts induces tumor development, invasion, and differentiation. Genes Dev, 21(21), 2788–2803. doi: 10.1101/gad.1544507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park CC, Zhang H, Pallavicini M, Gray JW, Baehner F, Park CJ, & Bissell MJ (2006). Beta1 integrin inhibitory antibody induces apoptosis of breast cancer cells, inhibits growth, and distinguishes malignant from normal phenotype in three dimensional cultures and in vivo. Cancer Res, 66(3), 1526–1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J, Lee KP, Kim H, Park S, Wijesinghe RE, Lee J, . . . Kim, J. (2019). Biocompatibility evaluation of bioprinted decellularized collagen sheet implanted in vivo cornea using swept-source optical coherence tomography. J Biophotonics, e201900098. doi: 10.1002/jbio.201900098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelosi E, Castelli G, & Testa U (2017). Pancreatic Cancer: Molecular Characterization, Clonal Evolution and Cancer Stem Cells. Biomedicines, 5(4). doi: 10.3390/biomedicines5040065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provenzano PP, Eliceiri KW, Campbell JM, Inman DR, White JG, & Keely PJ (2006). Collagen reorganization at the tumor-stromal interface facilitates local invasion. BMC Med, 4(1), 38. doi:1741–7015-4–38 [pii] 10.1186/1741-7015-4-38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu YL, Chen X, Hou YL, Hou YJ, Tian SB, Chen YH, . . . Liu XQ (2019). Characterization of different biodegradable scaffolds in tissue engineering. Mol Med Rep, 19(5), 4043–4056. doi: 10.3892/mmr.2019.10066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quiros RM, Valianou M, Kwon Y, Brown KM, Godwin AK, & Cukierman E (2008). Ovarian normal and tumor-associated fibroblasts retain in vivo stromal characteristics in a 3-D matrix-dependent manner. Gynecol Oncol, 110(1), 99–109. doi: 10.1016/j.ygyno.2008.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangarajan S, Bone NB, Zmijewska AA, Jiang S, Park DW, Bernard K, . . . Zmijewski JW (2018). Metformin reverses established lung fibrosis in a bleomycin model. Nat Med, 24(8), 1121–1127. doi: 10.1038/s41591-018-0087-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezakhaniha R, Agianniotis A, Schrauwen JT, Griffa A, Sage D, Bouten CV, . . . Stergiopulos N (2012). Experimental investigation of collagen waviness and orientation in the arterial adventitia using confocal laser scanning microscopy. Biomech Model Mechanobiol, 11(3–4), 461–473. doi: 10.1007/s10237-011-0325-z [DOI] [PubMed] [Google Scholar]
- Ribatti D (2017). A revisited concept: Contact inhibition of growth. From cell biology to malignancy. Exp Cell Res, 359(1), 17–19. doi: 10.1016/j.yexcr.2017.06.012 [DOI] [PubMed] [Google Scholar]
- Riis S, Hansen AC, Johansen L, Lund K, Pedersen C, Pitsa A, . . . Pennisi CP (2019). Fabrication and characterization of extracellular matrix scaffolds obtained from adipose-derived stem cells. Methods. doi: 10.1016/j.ymeth.2019.07.004 [DOI] [PubMed] [Google Scholar]
- Roy I, Boyle KA, Vonderhaar EP, Zimmerman NP, Gorse E, Mackinnon AC, . . . Dwinell MB (2017). Cancer cell chemokines direct chemotaxis of activated stellate cells in pancreatic ductal adenocarcinoma. Lab Invest, 97(3), 302–317. doi: 10.1038/labinvest.2016.146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rybinski B, Franco-Barraza J, & Cukierman E (2014). The wound healing, chronic fibrosis, and cancer progression triad. Physiol Genomics, 46(7), 223–244. doi: 10.1152/physiolgenomics.00158.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, . . . Cardona A (2012). Fiji: an open-source platform for biological-image analysis. Nat Methods, 9(7), 676–682. doi: 10.1038/nmeth.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serebriiskii I, Castello-Cros R, Lamb A, Golemis EA, & Cukierman E (2008). Fibroblast-derived 3D matrix differentially regulates the growth and drug-responsiveness of human cancer cells. Matrix Biol, 27(6), 573–585. doi: 10.1016/j.matbio.2008.02.008 PMC2603546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafi AA, Schiewer MJ, de Leeuw R, Dylgjeri E, McCue PA, Shah N, . . . Knudsen. (2018). Patient-derived Models Reveal Impact of the Tumor Microenvironment on Therapeutic Response. Eur Urol Oncol, 1(4), 325–337. doi: 10.1016/j.euo.2018.04.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman MH (2018). Stellate Cells in Tissue Repair, Inflammation, and Cancer. Annu Rev Cell Dev Biol, 34, 333–355. doi: 10.1146/annurev-cellbio-100617-062855 [DOI] [PubMed] [Google Scholar]
- Socovich AM, & Naba A (2019). The cancer matrisome: From comprehensive characterization to biomarker discovery. Semin Cell Dev Biol, 89, 157–166. doi: 10.1016/j.semcdb.2018.06.005 [DOI] [PubMed] [Google Scholar]
- Theocharis AD, Skandalis SS, Gialeli C, & Karamanos NK (2016). Extracellular matrix structure. Adv Drug Deliv Rev, 97, 4–27. doi: 10.1016/j.addr.2015.11.001 [DOI] [PubMed] [Google Scholar]
- Thomas D, & Radhakrishnan P (2019). Tumor-stromal crosstalk in pancreatic cancer and tissue fibrosis. Mol Cancer, 18(1), 14. doi: 10.1186/s12943-018-0927-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlodavsky I (1999). Preparation of Extracellular Matrices Produced by Cultured Corneal Endothelial and PF-HR9 Endodermal Cells. Current Protocols in Cell Biology, 1(1), 10.14.11–10.14.14. doi:doi: 10.1002/0471143030.cb1004s01 [DOI] [PubMed] [Google Scholar]
- von Ahrens D, Bhagat TD, Nagrath D, Maitra A, & Verma A (2017). The role of stromal cancer-associated fibroblasts in pancreatic cancer. J Hematol Oncol, 10(1), 76. doi: 10.1186/s13045-017-0448-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada KM, Collins JW, Cruz Walma DA, Doyle AD, Morales SG, Lu J, . . . Wang S (2019). Extracellular matrix dynamics in cell migration, invasion and tissue morphogenesis. Int J Exp Pathol, 100(3), 144–152. doi: 10.1111/iep.12329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada KM, & Sixt M (2019). Mechanisms of 3D cell migration. Nat Rev Mol Cell Biol. doi: 10.1038/s41580-019-0172-9 [DOI] [PubMed] [Google Scholar]
- Yamanashi T, Nakanishi Y, Fujii G, Akishima-Fukasawa Y, Moriya Y, Kanai Y, . . . Hirohashi S (2009). Podoplanin expression identified in stromal fibroblasts as a favorable prognostic marker in patients with colorectal carcinoma. Oncology, 77(1), 53–62. doi: 10.1159/000226112 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.