Abstract
Background
Non Helicobacter pylori gastric Helicobacters (NHPGHs) are associated with a range of upper gastrointestinal symptoms, histologic and endoscopic findings. For the first time in Iran, we performed a cross-sectional study in order to determine the prevalence of five species of NHPGHs in patients presenting with dyspepsia.
Methods
The participants were divided into H. pylori-infected and NHPGH-infected groups, based on the rapid urease test, histological analysis of biopsies, and PCR assay of ureA, ureB, and ureAB genes. The study included 428 gastric biopsies form dyspeptic patients, who did not receive any treatment for H. pylori. The samples were collected and sent to the laboratory within two years. H. pylori was identified in 368 samples, which were excluded from the study. Finally, a total of 60 non-H. pylori samples were studied for NHPGH species.
Results
The overall frequency of NHPGH species was 10 for H. suis (three duodenal ulcer, three gastritis, and four gastric ulcer samples), 10 for H. felis (one gastritis, three duodenal ulcer, and six gastric ulcer samples), 20 for H. salomonis (four duodenal ulcer, five gastritis, and 11 gastric ulcer samples), 13 for H. heilmannii (three gastritis, five duodenal ulcer, and five gastric ulcer samples), and 7 for H. bizzozeronii (zero gastric ulcer, two duodenal ulcer, and five gastritis samples).
Conclusions
Given our evidence about the possibility of involvement of NHPGHs in patients suffering from gastritis and nonexistence of mixed H. pylori infection, bacteriological testing of subjects negative for H. pylori becomes clinically relevant and important. Our findings suggest H. salomonis has the highest rate among the NHPGH species in Iranian dyspeptic patients.
Keywords: Dyspepsia, Gastric, Non-Helicobacter pylori gastric Helicobacter
Background
The number of discovered Helicobacter species has increased rapidly in the last decade. Up to now, more than 30 species have been characterized and well-recognized by microbiologists. H. pylori is the most recognized bacterium associated with dyspepsia in humans [1–4]. However, NHPGH species with typical spiral morphology has the ability to colonize the stomach of humans and animals. NHPGHs, by neutralizing the gastric acid, provide a suitable environment for their survival [5–7]. Studies have shown that NHPGHs are involved in gastritis among humans [8–10]. Gastritis may progress to gastric atrophy, intestinal metaplasia, and gastric cancer over time and result in precancerous lesions (similar to monoclonal lymphocytic proliferation), development of lymphoid follicles, and even primary gastric lymphoma, which develops only in some patients with gastritis. The incidence of these conditions varies relative to the multifactorial influence of host virulence and bacterial factors, which are dissimilar in different social and racial groups [11].
NHPGHs can affect the stomach environment through different mechanisms [1, 11, 12]. H. suis is capable of escaping the host’s immune system and shows a long-term presence in the stomach [13]. These bacteria contain genes, essential for their survival in the stomach environment. Also, NHPGH species, such as H. bizzozeronii, can be distinguished from H. pylori considering their higher metabolic flexibility in terms of energy sources and chain of electron transport [14].
H. felis species promote mucosal cytokines, which play a role in the development of gastric cancer [15]. Previous research has reported co-infections with two NHPGH species, i.e., H. salomonis and H. heilmannii, in dyspeptic patients [16, 17]. On the other hand, H. heilmannii may be more involved in the formation of gastric MALT lymphomas, compared to H. pylori. To our knowledge, H. pylori mainly covers the mucosal layer, whereas H. heilmannii invades deeply into the antral glands [18].
The lack of information about the topic of current research was frequently mentioned in national congresses and health ministry priority research list. There is no information about the prevalence of NHPGH species in Iran. In the present study, we report, for the first time, the frequency of NHPGH species in dyspeptic patients in Iran. In addition, the possible relationship between NHPGH species and histological findings was examined.
Methods
Patients and sampling
In this study, between March 2017 and February 2018, 428 dyspeptic patients, scheduled for upper gastrointestinal endoscopy (Mehrad Hospital, Tehran, Iran), were examined for Helicobacter infections using the Rapid Urease Test (RUT) and histological examination of stomach biopsies. According to the result of these tests, the samples were categorized into NHPGHs mono-infected (n, 60) and H. pylori mono-infected (n, 368) groups. The NHPGH mono-infected group comprised of 60 dyspeptic patients, who were studied for the prevalence of NHPGH species, while the H. pylori group included 368 dyspeptic patients, who were excluded from the study. A standard clinical pro forma was used to collect the demographic and clinical characteristics of NHPGH mono-infected patients via interviews. The study’s exclusion criteria included I) receiving treatment for H. pylori, concurrent or recent antibiotic use such as metronidazole, clarithromycin, amoxicillin, tetracycline, doxycycline and other cephalosporin, II) histamine-2 receptor blocker or proton pump inhibitor (PPI) therapy and bismuth compounds in the last four weeks; III) patients with regular use of NSAID; IV) patients with severe concomitant disease and V) patients with upper GI surgery. The participants signed the informed consent forms, and the Ethics Committee of Clinical Research approved the study protocol. The flow diagram of this study is shown in Fig. 1.
Fig. 1.
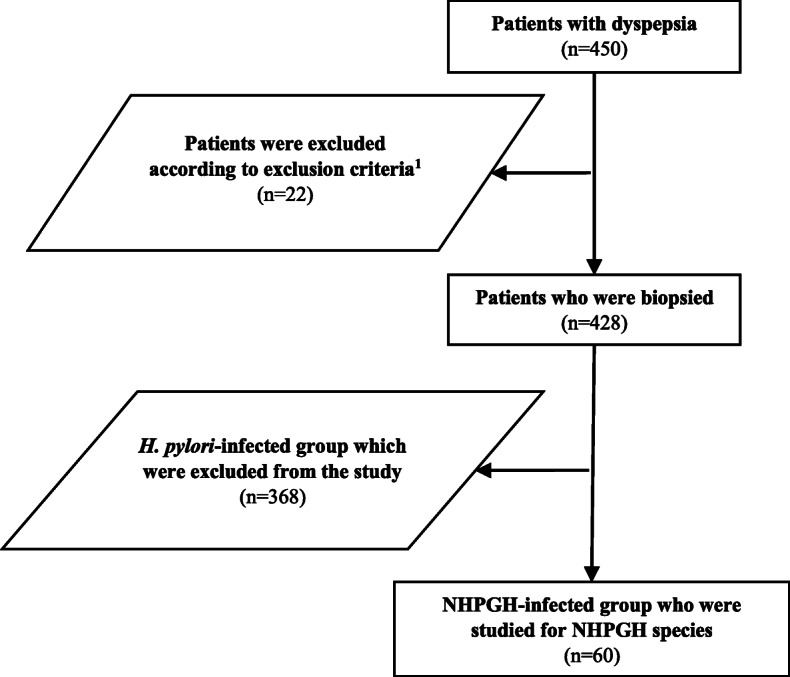
Flow chart of the study. 1The study’s exclusion criteria included I) receiving treatment for H. pylori, concurrent or recent antibiotic use such as metronidazole, clarithromycin, amoxicillin, tetracycline, doxycycline and other cephalosporin, II) histamine-2 receptor blocker or proton pump inhibitor therapy and bismuth compounds in the last four weeks; III) patients with regular use of NSAID; IV) patients with severe concomitant disease and V) patients with upper GI surgery
Histological examination
The biopsy sections were embedded in 10% buffered formalin. Next, hematoxylin and eosin staining was applied to assess gastritis, while Giemsa staining was used to detect Helicobacter species. The histological patterns were classified as gastric ulcer, duodenal ulcer, and gastritis, using the updated Sydney system.
DNA isolation
A Qiagen Genomic DNA Extraction Kit (BioFlux, USA) was used to isolate DNA from the stomach biopsies. Thereafter, DNA was resuspended in distilled water free of RNase/DNase (UltraPure).
Molecular detection of NHPGH species
PCR assay was performed to detect NHPGH species, including H. salomonis, H. bizzozeronii, H. heilmannii, H. felis, and H. suis [19, 20]. Table 1 shows the primer sequences for NHPGH species. The amplification reactions were performed using 1X Reaction Buffer (0.2% gelatin, 16 mM of ammonium sulfate, 67 mM of tris/HCl, and 0.45% triton X-100), Taq DNA polymerase (one unit; Biotech International), deoxynucleotide triphosphates (200 mM each), 2 mM of MgCl2, oligonucleotide primers (10 pmol each), and 1 μL diluted DNA (typically a 1:10 dilution of the original sample at nearly 20–100 ng/μl); with a final volume of 50 μL. For every specific reaction, the amplification parameters are described below. A thermocycler (Perkin Elmer PE2400) was used to perform the reactions. In addition, Agarose mini-gel in TAE buffer (1 mM EDTA and 40 mM tris-acetate) was used to separate the PCR products. The products were then imaged under UV transillumination following ethidium bromide staining.
Table 1.
PCR primers for NHPGH species genes amplification (in both Conversional and Real time PCR)
| Species | Target Gene | Primer sequence | PCR Product Size (bp) | Reference |
|---|---|---|---|---|
| H.suis | ureA |
FW (5′-CAC CAC CCC GGG GAA GTG ATC TTG-3′) RV (5′-CTA CAT CAA TCA AAT GCA CGG TTT TTT CTT CG-3′) |
253 | [20] |
| H. bizzozeronii | ureA |
FW (5′-CGCTTT CAC CCC GGG GAA GTG ATC TTG-3′) RV (5′ TATCGCAACCGCAATTCACAACA-3′) |
172 | [19] |
| H. felis | ureB |
FW (5′-TCCCACTACCGGGGATCGTG-3′) RV (5′ CAGCGGTTACAATCAAGCCCTCA-3′) |
350 | [19] |
| H. salomonis | ureAB |
FW (5′-CTTTGGGTCTGTGCCTGCCTG-3′) RV (5′ CATCGCGGATAGTCTTACCGCCT-3′) |
219 | [19] |
| H. heilmannii | ureA |
FW (5′-CTTTCTCCTGGTGAAGTGATTCTC′) RV (5′ CAGTTGATGGTGCCAAAG-3′) |
368 | [19] |
For urease I reactions, the cycling conditions included three minutes of denaturation at 94 °C for 4 min; then 35 cycles at 94 °C for 10 s, at 52 °C for 20 s, and at 72 °C for 90 s; and a five-minute extension at 72 °C. In urease II reactions, the conditions were similar to those of urease I reactions with some modifications, i.e., 30 s of annealing and two minutes of extension at 42 °C and 72 °C, respectively. By analyzing the urease sequences from the strains and isolates, ureA gene regions, which were dissimilar in H. felis, H. bizzozeronii, and H. salomonis species, could be identified. Also, in Type-I PCR assay, the cycling conditions included five minutes of denaturation at 94 °C, followed by 35 cycles of amplification at 94 °C for 10 s, at 55 °C for 30 s, at 72 °C for one minute, and at 72 °C for four minutes.
Real time PCR
Real-time PCR was performed using a Light Cycler 480 (Roche – Germany) detection system with the SYBR green I fluorophore. Reactions were performed in 20 μl (total volume) mixtures which included 5 μM SYBR green I PCR master mix 5 μl of each primer at a concentration of 5 μM, and 1 μl of the template DNA. Analyses were performed with a Light Cycler 480. The following protocol was used for 50 cycles consisting of 95 °C for 15 s, 55 °C for 15 s, and 72 °C for 30 s [21]. A melting curve analysis was performed following every run to ensure that there was a single amplified product for every reaction (Table 1). We used the Real time PCR alone as a conformity test for PCR (not a quantitative test). In other words, the main purpose of our experiment at the Real-Time PCR was to confirm our findings as we confirmed our positive ones. Thus, it was not aimed to distinguish the various species.
Statistical analysis
Data were analyzed in SPSS v. 16.0 (SPSS Inc., Chicago, IL, USA). The Chi-square test was used to calculate the association between the presences of five non- pylori Helicobacter species in NHPGH-infected group. A P-value of < 0.05 was considered as statistically significant. Results are expressed as mean ± standard deviation for continuous variables (e.g., age) and number (percentage) for categorical data (e.g., gender).
Results
Demographic and clinical characteristics
Genomic DNA was collected from 60 NHPGH mono-infected patients. DNA was analyzed in all subjects. The NHPGH mono-infected patients’ demographic characteristics are presented in Table 2. Based on the findings, there was no significant difference (p > 0.05) among histological groups (i.e., duodenal ulcer, gastric ulcer, and gastritis) with respect to age and gender distribution. Among the 60 NHPGH mono-infected patients (which were negative for H. pylori) there was not co-infection with different species of NHPGH. In other words, was not observed more than one species of non-H. pylori in NHPGH mono-infected group. Agarose gel electrophoresis of the PCR products are shown in Fig. 2.
Table 2.
Demographic data of NHPGH mono-infected patients enrolled
| Duodenal ulcer | Gastric ulcer | Gastritis | |
|---|---|---|---|
|
Total: Female/Male (%) |
25: 10/15 (41%: 16%/25%) |
15: 3/12 (25%: 5%/20%) |
20: 9/11 (33%: 15%/18%) |
| Age year (Mean ± SD) | 37.3 | 29.4 | 42.4 |
Fig. 2.
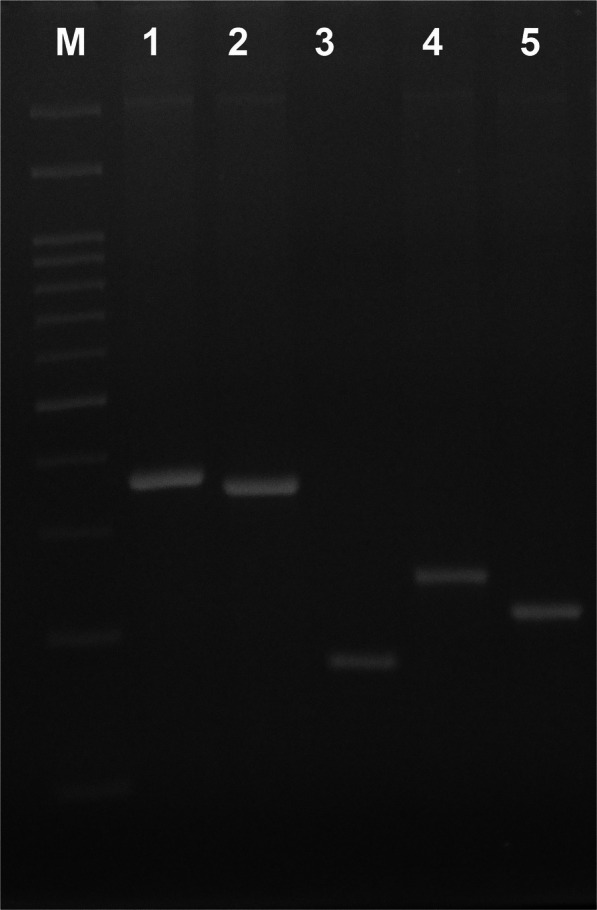
Agarose gel electrophoresis of the PCR products. Lane M is 100 bp (100 bp–3000 bp) DNA ladder (CinnaGen Co.). Lane 1 is DNA from representative NHPGH-infected patients for ureA positive H. heilmannii (368 bp). Lane 2 is DNA from representative NHPGH-infected patients for ureB positive H. felis (350 bp). Lane 3 is DNA from representative NHPGH-infected patients for ureA positive H. bizzozeronii (172 bp). Lane 4 is DNA from representative NHPGH-infected patients for ureA positive H. suis (253 bp). Lane 5 is DNA from representative NHPGH-infected patients for ureAB positive H. salomonis (219 bp)
Prevalence of non-H. pylori species in NHPGH mono-infected group
For comparative studies, NHPGH species were evaluated in histological groups, including duodenal ulcer (n, 25), gastric ulcer (n, 15), and gastritis (n, 20). The frequency of H. suis (n, 10), H. bizzozeronii (n, 7), H. felis (n, 10), H. salomonis (n, 20), and H. heilmannii-infected (n, 13) samples were compared with NHPGH-infected biopsies. Current findings showed that H. salomonis had the highest frequency rate, while the rate for H. bizzozeronii was the lowest. Based on the findings, none of the NHPGH species were associated with histological patterns (duodenal ulcer, gastric ulcer, and gastritis) (Table 3).
Table 3.
Distribution of the five NHPGH species among the 60 pylori-negative patients
| NHPGH species | Duodenal ulcer (n = 25) |
Gastric ulcer (n = 15) |
Gastritis (n = 20) |
Total (n = 60) |
|---|---|---|---|---|
| H. suis | 3 | 4 | 3 | 10 |
| H. bizzozeronii | 2 | 0 | 5 | 7 |
| H. felis | 3 | 6 | 1 | 10 |
| H. salomonis | 4 | 11 | 5 | 20 |
| H. heilmannii | 5 | 5 | 3 | 13 |
| Total | 17 | 26 | 17 | 60 |
Discussion
Introduction of NHGPH species has provided researchers with an opportunity to determine the relationship between these species, which can colonize the animal and human guts, in order to better understand their effects on the host [22]. Within a short period of time after discovery of H. pylori by Marshall in 1983, scientists had understood that we have other members in this spiral type of bacteria causing the inflammation in human gastrointestinal route. The prevalence of NHPGH species in the gastric mucosa of humans and animals is diverse around the world [11, 23–26]. The main advantage of current research was to investigate in such naïve population with no information about prevalence and likely significant association between those strains and severe gastroduodenal diseases. Likely, in close future, current data can be a starting point for similar studies. In the present study, we applied the PCR assay to evaluate the frequency of NHPGH species in Iranian dyspeptic patients. In the literature, H. suis has been introduced as the most prevalent NHPGH species, colonizing the stomach of dyspeptic patients [13, 27]. According to the recent study by Nakagawa et al, H. suis was the main cause of chronic gastritis in individuals without H. pylori infection [28, 29]. According to previous studies, these species have a pathogenic potential due to the presence of gamma-glutamyl transpeptidase (ggt), their immune-suppressing properties, as well as outer membrane vesicles [13, 30]. Although pig farming, which is recognized as an important source of infection [31], is not permitted in Iran, there has been a relative increase in the frequency of H. suis (n, 10, 16%) among NHPGH species. Previous studies have shown that H. suis is a cause of acute inflammation in colonized patients in comparison with H. pylori and non-pylori Helicobacters, but such findings were not repeated in our examination [29, 32]. Similar to findings that De Cooman et al. released about pork meat consumption and the high risk of contaminated [20], we assume that this may happen in our population too, although technical and experimental errors may have been the source of our observation. Nevertheless, this rate of H. suis seems high among the Iranian individuals since pork is not in the regular dairy list. Indeed, the lack of knowledge about required duration time for transmitting the infection is still in place and we hope to have better insights in to this within the foreseeable future. On the other hand, H. bizzozeronii is the predominant NHPGH species in the canine stomach [11, 33]. There are multi potential factors involved in the virulence of H. bizzozeronii, including greater metabolic flexibility, genome plasticity, and harboring multiple methyl-accepting chemotaxis proteins [14, 16]. In addition, this species has been associated with severe dyspeptic symptoms [14]. In our study, we found seven dyspeptic patients infected with H. bizzozeroni, who claimed they were not in contact with dogs (as pets) since pet keeping is not common among Iranians. Therefore, H. bizzozeroni had the lowest prevalence among NHPGH species in our study population. However, further studies are needed to confirm this finding. H. felis infection is associated with reduced levels of interleukin-1β and tumor necrosis factor-α and increased level of interleukin-10, leading to the expression of key gastric mucosal cytokines and possibly gastric cancer [15]. The frequency of H. felis was 10 out of 60 (16%), thus we were unable to report any association between H. felis infection and histological report (P > 0.05). H. salomonis has been isolated from gastric biopsies of healthy dogs and humans. It has been also isolated from individuals infected with H. heilmannii [16, 17]. In the past, many studies reported that H. heilmannii infection is an example of zoonosis and we may have worrying report out of it in humans, but our results are quite contradictory [34]. In this study, H. salomonis was the most frequent NHPGH species (n, 20; 33%), while there is no similar study from the same location in Iran. In our study, the frequency of H. heilmannii was 13 out of 60 (21%). Since the transmission pathways for H. salomonis are unclear, we need to determine the probable source and route of transmission for this NHPGH species. On the other hand, H. heilmannii has been linked to gastritis, gastric ulcers and duodenal ulcers in humans [35]. We did not find significant association between presence of H. heilmannii infection and histological findings (P > 0.05). Indeed, this species can be the cause of apoptosis and angiogenesis in gastric MALT lymphoma [36]. Further studies with emphasis on molecular experiments are necessary to explain reports of such results.
Limitations and future prospects
To the best of our knowledge, ours is the first cross-sectional study representing an Iranian population sample investigated for the clinical relevance of the five tested non-pylori Helicobacter species. Importantly, we found no mixed Helicobacter infections among NHPGH mono-infected group. We tried to make such big sample size in order to draw a good conclusion regardless significant finding or not. We think that our survey has good results, and it can be a reference study in future surveys in this country. However, our study had two limitations. The first limitation of our study was among H. pylori infected group (368 patients who were excluded from the study) that the possible co-infection with NHPGH different species was not investigated. This was due to the limited budget of our project. In this regard, we are starting the new project based on current panel of non-pylori helicobacters co-infection among H.pylori positive group. The second limitation was the need to establish a clear causative association between non-pylori helicobacters colonization and gastritis. Also, the significance of infection with NHPGH in terms of disease development could not be determined. The implications of this study are that non-H. pylori helicobacter species infection occurs in patients with abdominal pain or discomfort similar to H. pylori infection.
Conclusion
Due to the difficulty associated with identification of non-pylori Helicobacters within routine laboratory tests, increased awareness of general health care and infectious diseases experts should be on the priority for decision-makers in hygiene and health in Iran. We conclude that infections with non-pylori Helicobacter species are candidates for further microbiological testing for targeted and improved clinical management. Nowadays the only fact we are confident of is that NHPGH species induce superficial inflammation in the gastric mucosa of colonized patients. The exact mechanism is not yet understood. However, further research is also necessary to clarify the epidemiology and pathogenesis of these mysterious bacteria.
Acknowledgements
We thank the study participants and staff of Mehrad Hospital, Tehran, Iran, for their immense contribution.
Abbreviations
- H. pylori
Helicobacter pylori
- NHPGH
Non-H. pylori gastric Helicobacter
- PCR
Polymerase chain reaction
- H. suis
Helicobacter suis
- H. bizzozeronii
Helicobacter bizzozeronii
- H. felis
Helicobacter felis
- H. salomonis
Helicobacter salomonis
- H. heilmannii
Helicobacter heilmannii
- NSAID
Nonsteroidal anti-inflammatory drugs
- GI
Gastrointestinal
Authors’ contributions
SS, HK and FPG conceived and designed the experiment; SS conducted the study and collected the samples. SS, HK and FPG performed the experiments and analyzed the data. All authors contributed to paper writing. The authors have read and approved the final manuscript.
Funding
This study was supported by the Islamic Azad University, Ayatollah Amoli Branch, Amol, Mazandaran province, Iran (with registration and grant number 23930507962001). The funding body had no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Availability of data and materials
All original data and materials are available upon request from the corresponding author.
Ethics approval and consent to participate
This study was approved by the Research Ethics Committee of Ayatollah Amoli Branch, Islamic Azad University (Approved date: 2017-04-03 and approved ID: 23930507962001) and performed in accordance with the Declaration of Helsinki. The participants signed the informed consent forms from each patient, and the Research Ethics Committee of Ayatollah Amoli Branch, Islamic Azad University approved the study protocol.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no conflicts of interest relevant to this study.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Shakiba Shafaie, Email: shakiibashafaei1224@gmail.com.
Hami Kaboosi, Email: h.kaboosi@iauamol.ac.ir.
Fatemeh Peyravii Ghadikolaii, Email: fpeyravii@gmail.com.
References
- 1.Fox J. The non-H pylori helicobacters: their expanding role in gastrointestinal and systemic diseases. Gut. 2002;50(2):273–283. doi: 10.1136/gut.50.2.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ghalehnoei H, Ahmadzadeh A, Farzi N, Alebouyeh M, Aghdaei HA, Azimzadeh P, Molaei M, Zali MR. Relationship between ureB sequence diversity, urease activity and genotypic variations of different Helicobacter pylori strains in patients with gastric disorders. Pol J Microbiol. 2016;65(2):153–159. doi: 10.5604/17331331.1204761. [DOI] [PubMed] [Google Scholar]
- 3.Šebunova N, Štšepetova J, Sillakivi T, Mändar T. The prevalence of Helicobacter pylori in Estonian bariatric surgery patients. Int J Mol Sci. 2018;19(2):338. doi: 10.3390/ijms19020338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peters C, Schablon A, Harling M, Wohlert C, Costa JT, Nienhaus A. The occupational risk of Helicobacter pylori infection among gastroenterologists and their assistants. BMC Infect Dis. 2011;11(1):154. doi: 10.1186/1471-2334-11-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O'rourke J, Grehan M, Lee A. Non-pylori helicobacter species in humans. Gut. 2001;49(5):601–606. doi: 10.1136/gut.49.5.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leemann C, Gambillara E, Prod'hom G, Jaton K, Panizzon R, Bille J, et al. First case of bacteremia and multifocal cellulitis due to Helicobacter canis in an immunocompetent patient. J Clin Microbiol. 2006;44(12):4598–4600. doi: 10.1128/JCM.01453-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fox JG, Dewhirst FE, Shen Z, Feng Y, Taylor NS, Paster BJ, et al. Hepatic Helicobacter species identified in bile and gallbladder tissue from Chileans with chronic cholecystitis. Gastroenterology. 1998;114(4):755–763. doi: 10.1016/S0016-5085(98)70589-X. [DOI] [PubMed] [Google Scholar]
- 8.Debongnie JC, Donnay M, Mairesse J. Gastrospirillum hominis ("Helicobacter heilmanii"): a cause of gastritis, sometimes transient, better diagnosed by touch cytology?. Am J Gastroenterol. 1995;90(3):411–6. [PubMed]
- 9.Debongnie JC, Donnay M, Mairesse J, Lamy V, Dekoninck X, Ramdani B. Gastric ulcers and Helicobacter heilmannii. Eur J Gastroenterol Hepatol. 1998;10(3):251–254. doi: 10.1097/00042737-199803000-00011. [DOI] [PubMed] [Google Scholar]
- 10.Eschen F, Heyerhoff M, Morgner H, Vogt J. The concentration-depth profile at the surface of a solution of tetrabutylammonium iodide in formamide, based on angle-resolved photoelectron spectroscopy. J Phys Condens Matter. 1995;7(10):1961. doi: 10.1088/0953-8984/7/10/006. [DOI] [Google Scholar]
- 11.Van den Bulck K, Decostere A, Baele M, Driessen A, Debongnie JC, Burette A, et al. Identification of non-Helicobacter pylori spiral organisms in gastric samples from humans, dogs, and cats. J Clin Microbiol. 2005;43(5):2256–2260. doi: 10.1128/JCM.43.5.2256-2260.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sanduleanu S, Jonkers D, De Bruine A, Hameeteman W, Stockbrügger R. Non-Helicobacter pylori bacterial flora during acid-suppressive therapy: differential findings in gastric juice and gastric mucosa. Aliment Pharmacol Ther. 2001;15(3):379–388. doi: 10.1046/j.1365-2036.2001.00888.x. [DOI] [PubMed] [Google Scholar]
- 13.Vermoote M, Flahou B, Pasmans F, Ducatelle R, Haesebrouck F. Protective efficacy of vaccines based on the Helicobacter suis urease subunit B and γ-glutamyl transpeptidase. Vaccine. 2013;31(32):3250–3256. doi: 10.1016/j.vaccine.2013.05.047. [DOI] [PubMed] [Google Scholar]
- 14.Schott T, Kondadi PK, Hänninen ML, Rossi M. Comparative genomics of Helicobacter pylori and the human-derived Helicobacter bizzozeronii CIII-1 strain reveal the molecular basis of the zoonotic nature of non-pylori gastric Helicobacter infections in humans. BMC Genomics. 2011;12(1):534. doi: 10.1186/1471-2164-12-534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stoicov C, Fan X, Liu JH, Bowen G, Whary M, Kurt-Jones E, et al. T-bet knockout prevents Helicobacter felis-induced gastric cancer. J Immunol. 2009;183(1):642–649. doi: 10.4049/jimmunol.0900511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Bock M, Decostere A, Van den Bulck K, Baele M, Duchateau L, Haesebrouck F, et al. The inflammatory response in the mouse stomach to Helicobacter bizzozeronii, Helicobacter salomonis and two Helicobacter felis strains. J Comp Pathol. 2005;133(2–3):83–91. doi: 10.1016/j.jcpa.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 17.Hänninen M-L, Happonen I, Jalava K. Transmission of canine gastric Helicobacter salomonis infection from dam to offspring and between puppies. Vet Microbiol. 1998;62(1):47–58. doi: 10.1016/S0378-1135(98)00198-9. [DOI] [PubMed] [Google Scholar]
- 18.Nishikawa K, Nakamura M, Takahashi S, Matsui H, Murayama SY, Matsumoto T, et al. Increased apoptosis and angiogenesis in gastric low-grade mucosa-associated lymphoid tissue-type lymphoma by Helicobacter heilmannii infection in C57/BL6 mice. FEMS Immunol Med Microbiol. 2007;50(2):268–272. doi: 10.1111/j.1574-695X.2007.00252.x. [DOI] [PubMed] [Google Scholar]
- 19.O'Rourke JL, Solnick JV, Neilan BA, Seidel K, Hayter R, Hansen LM, et al. Description of ‘Candidatus Helicobacter heilmannii’based on DNA sequence analysis of 16S rRNA and urease genes. Int J Syst Evol Microbiol. 2004;54(6):2203–2211. doi: 10.1099/ijs.0.63117-0. [DOI] [PubMed] [Google Scholar]
- 20.De Cooman L, Flahou B, Houf K, Smet A, Ducatelle R, Pasmans F, et al. Survival of Helicobacter suis bacteria in retail pig meat. Int J Food Microbiol. 2013;166(1):164–167. doi: 10.1016/j.ijfoodmicro.2013.05.020. [DOI] [PubMed] [Google Scholar]
- 21.Nakamura M, Murayama SY, Serizawa H, Sekiya Y, Eguchi M, Takahashi S, et al. “Candidatus Helicobacter heilmannii” from a cynomolgus monkey induces gastric mucosa-associated lymphoid tissue lymphomas in C57BL/6 mice. Infect Immun. 2007;75(3):1214–1222. doi: 10.1128/IAI.01459-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mladenova-Hristova I, Grekova O, Patel A. Zoonotic potential of Helicobacter spp. J Microbiol Immunol Infect. 2017;50(3):265–269. doi: 10.1016/j.jmii.2016.11.003. [DOI] [PubMed] [Google Scholar]
- 23.Diker KS, Haziroğlu R, Akan M, Çelik S, Kabakçi N. The prevalence, colonization sites and pathological effects of gastric helicobacters in dogs. Turk J Vet Anim Sci. 2002;26(2):345–351. [Google Scholar]
- 24.Eaton K, Dewhirst F, Paster B, Tzellas N, Coleman B, Paola J, et al. Prevalence and varieties of Helicobacter species in dogs from random sources and pet dogs: animal and public health implications. J Clin Microbiol. 1996;34(12):3165–3170. doi: 10.1128/JCM.34.12.3165-3170.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Happonen I, Saari S, Castren L, Tyni O, Hänninen ML, Westermarck E. Occurrence and topographical mapping of gastric Helicobacter-like organisms and their association with histological changes in apparently healthy dogs and cats. Transbound Emerg Dis. 1996;43(1–10):305–315. doi: 10.1111/j.1439-0442.1996.tb00457.x. [DOI] [PubMed] [Google Scholar]
- 26.Baele M, Pasmans F, Flahou B, Chiers K, Ducatelle R, Haesebrouck F. Non-Helicobacter pylori helicobacters detected in the stomach of humans comprise several naturally occurring helicobacter species in animals. FEMS Immunol Med Microbiol. 2009;55(3):306–313. doi: 10.1111/j.1574-695X.2009.00535.x. [DOI] [PubMed] [Google Scholar]
- 27.Baele M, Decostere A, Vandamme P, Ceelen L, Hellemans A, Mast J, et al. Isolation and characterization of Helicobacter suis sp. nov. from pig stomachs. Int J Syst Evol Microbiol. 2008;58(6):1350–1358. doi: 10.1099/ijs.0.65133-0. [DOI] [PubMed] [Google Scholar]
- 28.Nakagawa S, Shimoyama T, Nakamura M, Chiba D, Kikuchi H, Sawaya M, et al. The resolution of Helicobacter suis-associated gastric lesions after eradication therapy. Intern Med. 2018;57(2):203–207. doi: 10.2169/internalmedicine.8971-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ali B, Chloë W, Mehmet A, Sofie B, Annemieke S, Gökhan T, et al. Presence of gastric Helicobacter species in children suffering from gastric disorders in southern Turkey. Helicobacter. 2018;23(5):e12511. doi: 10.1111/hel.12511. [DOI] [PubMed] [Google Scholar]
- 30.Zhang G, Ducatelle R, Pasmans F, D’Herde K, Huang L, Smet A, et al. Effects of Helicobacter suis γ-glutamyl transpeptidase on lymphocytes: modulation by glutamine and glutathione supplementation and outer membrane vesicles as a putative delivery route of the enzyme. PLoS One. 2013;8(10):e77966. doi: 10.1371/journal.pone.0077966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liang J, Ducatelle R, Pasmans F, Smet A, Haesebrouck F, Flahou B. Multilocus sequence typing of the porcine and human gastric pathogen Helicobacter suis. J Clin Microbiol. 2013;51(3):920–926. doi: 10.1128/JCM.02399-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu J, He L, Haesebrouck F, Gong Y, Flahou B, Cao Q, et al. Prevalence of coinfection with gastric non-Helicobacter pylori helicobacter (NHPH) species in Helicobacter pylori-infected patients suffering from gastric disease in Beijing, China. Helicobacter. 2015;20(4):284–290. doi: 10.1111/hel.12201. [DOI] [PubMed] [Google Scholar]
- 33.Priestnall SL, Wiinberg B, Spohr A, Neuhaus B, Kuffer M, Wiedmann M, et al. Evaluation of “Helicobacter heilmannii” subtypes in the gastric mucosas of cats and dogs. J Clin Microbiol. 2004;42(5):2144–2151. doi: 10.1128/JCM.42.5.2144-2151.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dieterich C, Wiesel P, Neiger R, Blum A, Corthésy-Theulaz I. Presence of multiple “Helicobacter heilmannii” strains in an individual suffering from ulcers and in his two cats. J Clin Microbiol. 1998;36(5):1366–1370. doi: 10.1128/JCM.36.5.1366-1370.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nobutani K, Yoshida M, Nishiumi S, Nishitani Y, Takagawa T, Tanaka H, et al. Helicobacter heilmannii can induce gastric lymphoid follicles in mice via a Peyer's patch-independent pathway. FEMS Immunol Med Microbiol. 2010;60(2):156–164. doi: 10.1111/j.1574-695X.2010.00731.x. [DOI] [PubMed] [Google Scholar]
- 36.Morgner A, Lehn N, Andersen LP, Thiede C, Bennedsen M, Trebesius K, et al. Helicobacter heilmannii–associated primary gastric low-grade MALT lymphoma: complete remission after curing the infection. Gastroenterology. 2000;118(5):821–828. doi: 10.1016/S0016-5085(00)70167-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All original data and materials are available upon request from the corresponding author.


