Abstract
Sleep has been implicated in learning processes that appear to underlie recovery from posttraumatic stress disorder (PTSD). The importance of quality and timing of sleep following exposure-based therapies has been suggested. The present study evaluated relationships between sleep and adaptive emotional processing following written narrative exposure (WNE) to memories of traumatic events experienced by participants with clinically significant PTSD symptoms. Participants included 21 urban-residing nontreatment-seeking adults with full or subthreshold symptoms of PTSD who completed 4 sessions of 30-minutes WNE with the first session either in the evening or the morning. There was a significant reduction of PTSD symptom severity after WNE sessions (partial η2 = .65), but there was no interaction between group assignment based on the initial session’s proximity to sleep and initial reduction of PTSD symptom severity (partial η2 = .01). Polysomnography following evening WNE revealed increased duration of total sleep and N2 percent, reduced N3 percent, and increased eye movement density during rapid-eye-movement (REM) sleep compared with baseline recordings (dz = 0.65 to 1.15). Reduced N3 percent and increased REM density were associated with less improvement of PTSD symptoms (r = .58 & −.63). These findings suggest a relationship between preservation of diminished arousal during sleep and adaptive trauma memory processing.
Sleep and Processing of Trauma Memories
There has been emerging interest in sleep’s effect on posttraumatic stress disorder (PTSD) treatment outcome. Processes that underlie recovery such as extinction retention and generalization are enhanced by sleep (Pace-Schott, Germain, & Milad, 2015). Rapid-eye-movement (REM) sleep (REMS) during naps increased formation of new semantic associations (Cai, Mednick, Harrison, Kanady, & Mednick, 2009). This could be an analogue of adaptive integration of new information into trauma memories, which has been posited to be central to recovery from PTSD (Foa, Hembree, & Rothbaum, 2007). Findings from a longitudinal (Bryant, Creamer, O’Donnell, Silove, & McFarlane, 2010) and a treatment study (Lommen et al., 2015) suggest that sleep has a role in preventing as well as hastening the treatment response of PTSD. In a recent review, Pace-Schott et al. (2015) suggested the importance of sleep quality when implementing exposure-based therapies as well as strategically timing sessions in proximity to sleep.
In addition to sleep quality and timing, clarification of the role of sleep stages in processing trauma memories could inform intervention strategies. REMS eye movement density negatively predicted response to interpersonal therapy for depression (Buysse, Kupfer, Frank, Monk, & Ritenour, 1992). Studies have implicated REMS in emotional memory learning and there has been emerging evidence for a role for slow wave sleep (SWS; (Pace-Schott et al., 2015).
We designed a study to enhance ecological validity compared with prior experimental research with human subjects which relied on analogues of trauma exposures (e.g., Porcheret, Holmes, Goodwin, Foster, & Wulff, 2015) by recruiting a population with posttraumatic distress and utilized written narrative exposure (WNE) as a study probe. WNE has been shown to have therapeutic benefits related to adaptive restructuring of trauma narratives (Smyth, 1998). The WNE sessions were scheduled to evaluate the role of sleep timing, and polysomnography (PSG) was used to evaluate relationships between sleep characteristics and PTSD symptom reduction. WNE sessions were paired from evening to morning with intervening sleep versus morning to evening with intervening wake. We tested the following hypotheses: (1a) that proximity of sleep to WNE would influence intersession habituation, by comparing changes in peak distress ratings from the first to the second session, and (1b) that proximity of sleep to WNE would influence PTSD symptom reduction, by comparing severity scores from pre-WNE to the first post-WNE evaluation. Second, we evaluated the effect of WNE on sleep characteristics by comparing measures from sleep recordings following evening WNE to measures from baseline recordings. Third, we explored correlative relationships between the effects of WNE on sleep and the degree of PTSD symptom reduction.
Method
Participants
Participants included a nonclinical sample of healthy adults who met criteria for current full or subthreshold PTSD [Diagnostic and Statistical Manual of Mental Disorders – Fourth Edition (DSM-IV) Criterion A for trauma, symptom cluster B and one or both of clusters C and D], with index traumas within the previous 5 years recruited from the Washington DC metropolitan area through flyers and referrals from prior participants. Potential participants were excluded for a body mass index ≥ 40; chronic medical conditions and medication use; bipolar disorder, recurrent depression that preceded the onset of PTSD or alcohol dependence; consumption of more caffeine than five cups of coffee per day equivalents; smoking more than 20 cigarettes per day; regular night shift work or unusual sleep-wake schedules; prior exposure-based therapy; inability to remember most details of the trauma; not having graduated high school; positive urine toxicology; and diagnosis of a sleep disorder other than insomnia including sleep apnea (screened by the first night PSG). The study received IRB approval from Howard University and participants signed consent forms after they were given time for review and questions.
There were 62 participants who consented and were evaluated; 47 met the criteria for full or subthreshold PTSD, 11 were disqualified, and 7 were lost to follow-up before the first WNE session. There were 29 participants who completed the first pair of WNE sessions, and 21 completed the entire protocol. The mean age of the 29 participants was 28.07 years (SD = 10.99), 55.2 % were female and all were Black. There were no significant differences in gender and age between the 15 who were lost to follow-up at various points and those who completed the protocol, and CAPS scores were similar (M = 61.40, SD = 22.02 vs. M = 61.00, SD = 18.22). Among the 29, there were 26 who met full PTSD criteria; the 3 with subthreshold criteria had CAPS scores of 44, 41, and 36, which were within the range of the scores of those meeting full criteria for the PTSD diagnosis. Only one participant had a current psychiatric comorbid diagnosis, which was of generalized anxiety disorder.
Procedure
Participants were evaluated at the Howard University Clinical Research Unit with clinical interviews, a physical examination, and urine drug screening. Within a week of a baseline sleep recording, participants were randomly assigned (with stratification by gender) to complete either two 30-minute WNE sessions at 8 PM and 8 AM with intervening sleep and an overnight PSG recording (Group 1), or 8 AM and 8 PM with intervening wake (Group 2). Participants were instructed not to sleep during the wake period. Participants were assigned to the alternative condition 1 week later.
For WNE, the participant sat at a desk in a private room with a facilitator who provided instructions to write in as much detail as possible about their index trauma starting from several minutes leading up to the event until its aftermath, including what happened, what they perceived, thought, and felt, and to use the entire 30 minutes. The facilitator provided the subjective units of distress scale (SUDS) which participants rated orally at baseline, every 10 minutes, and at the end of the writing period. Participants remained on the unit until their distress level became minimal.
Measures
The Clinician Administered PTSD Scale (CAPS: Blake et al., 1990) was administered to assess PTSD diagnosis and severity, initially in conjunction with the Life Event Checklist (LEC: Blake et al., 1990) to indicate potentially traumatic events. Cronbach’s alpha for the current CAPS score was .91. The CAPS was re-administered before the first (α = .91) and third (α = .82), and one week after the fourth (α = .71) WNE session to assess symptom severity over the preceding week.
The Structured Clinical Interview for DSM-IV (SCID: First, Spitzer, Gibbon, & Williams, 2002) was administered to assess current and lifetime psychiatric diagnoses and confirm inclusion criteria. All clinical interviews were conducted by trained staff members supervised by a licensed psychologist (IK), and a board certified psychiatrist (TAM) reviewed all cases.
The SUDS (Foa et al., 2007) ranges from 0 = no distress to 100 = extreme distress and was designed to measure the level of distress during exposure therapy. The maximum SUDS score in each WNE session was used for analysis.
PSG utilized an Embla (Denver, CO) Titanium portable unit and included bilateral frontal, central, and occipital electroencephalographic leads, 2 electrooculograms, 2 chin electromyograms, and 2 limb electromyograms and respiratory monitors on the first night only. Study staff visually scored sleep records on a computer monitor applying the American Academy of Sleep Medicine Manual, Version 2.0 (American Academy of Sleep Medicine, 2012) scoring rules. Eye movements during REM sleep were determined by criteria of conjugate excursions of electrooculogram traces at least 25μv within 500 milliseconds (rise time) (Mellman, Bustamante, Fins, Pigeon, & Nolan, 2002). REM density was calculated by dividing the number of eye movements by minutes of REM sleep. All scorers had demonstrated > 90% concordance for scoring epochs with reference records. For the screening and the baseline recordings, participants were instructed to go to bed and arise close to their habitual bedtime and rise time.
Data Analysis
SPSS 22 was used to conduct data analysis. Distributions of data were examined and wake after sleep onset was log transformed to normalize its distribution. To test Hypothesis 1a, a 2 (Group) × 2 (Sessions: the maximum SUDS scores at the first and second WNE sessions) split-plot ANOVA was performed. To test hypothesis 1b, a 2(Group) × 2 (Time: CAPS scores at baseline and after the second WNE session) split-plot ANOVA was performed. Partial η2s were computed to evaluate sizes of the main and interaction effects. Paired-sample t tests were conducted to examine changes in PSG measured sleep parameters from the baseline to post-WNE. An effect size, dz, was computed for each t test. Pearson correlation coefficients were computed to examine associations between the reduction of 1-week CAPS scores from the baseline to the final assessment (subtracting final CAPS scores from baseline scores) and changes in sleep parameters from the baseline to post-WNE (subtracting baseline sleep parameters from sleep parameters following the first WNE session for Group 1 and following the third session for Group 2). Only participants with complete data for each analysis were included.
Results
There was a significant main effect of session, F(1, 27) = 5.12, p = .032, partial η2 = .16, indicating a reduction of maximum SUDS scores from the first to second session (M = 44.59, SD = 29.83; M = 37.14, SD = 28.00). Neither the main effect of group nor the interaction was significant, F = 0.87, p = .352, partial η2 = .03, and F = 0.08, p = .782, partial η2 = .003, respectively. Figure 1 displays average 1-week CAPS scores at the three time points for each group. Among the 21 participants who completed the protocol, 19 achieved remission. There was a significant main effect of time, F(1, 23) = 41.99, p < .001, partial η2 = .65, indicating a reduction of CAPS scores from the baseline to after the second WNE, and a significant main effect of group, F = 6.06, p = .022, partial η2 = .21, indicating higher CAPS scores in Group 2, however, the interaction was not significant, F = 0.30, p = .587, partial η2 = .01.
Figure.
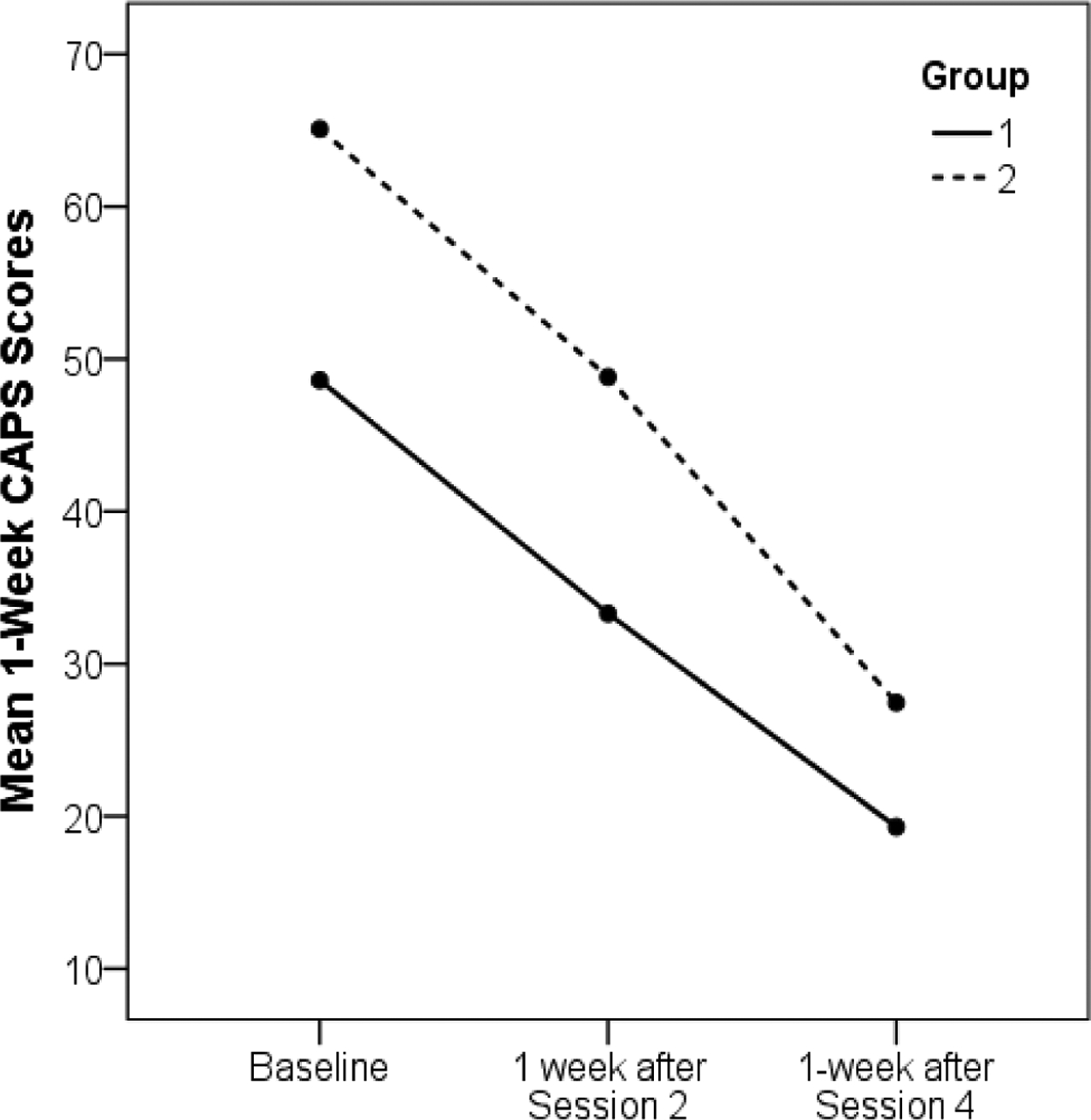
Mean 1-week CAPS scores by group. CAPS = Clinician Administered PTSD Scale; Group 1 completed WNE sessions with intervening sleep first and then sessions with intervening wake. Group 2 completed WNE sessions in the reverse order.
Table 1 presents sleep measures at baseline and after the first evening WNE session (first session for Group 1, third session for Group 2). Due to small ns we combined data from the groups. Participants had more total sleep time after WNE compared with the baseline. N2 percentage of total sleep time and REM density increased, and percent N3 decreased after WNE. Effects were similar for the two groups except for REM latency which decreased after WNE in Group 1 (113.00 vs. 77.91 min, p = .008, Wilcoxon rank tests), but did not significantly change in Group 2 (p = .116).
Table 1:
Sleep Characteristics at Baseline and After Written Narrative Exposure
| Baseline | Post-WNE | Corr. | |||||
|---|---|---|---|---|---|---|---|
| Variable | M | SD | M | SD | t(16) | dz | r |
| Total sleep time (min) | 319.68 | 79.97 | 385.71 | 81.77 | −3.14** | −0.76 | .06 |
| Sleep onset latency (min) | 30.73 | 31.73 | 42.96 | 51.39 | −1.36 | −0.33 | .27 |
| WASO (min) | 18.81 | 15.79 | 17.94 | 11.81 | 0.21 | 0.05 | −.04 |
| N1 (%) | 1.34 | 1.27 | 1.02 | 0.94 | 1.23 | 0.30 | .06 |
| N2 (%) | 46.23 | 9.27 | 55.27 | 8.89 | −4.39*** | −1.06 | −.41 |
| N3 (%) | 35.28 | 11.59 | 25.95 | 7.89 | 3.75** | 0.91 | .58* |
| REM sleep (%) | 17.21 | 8.28 | 17.18 | 5.30 | 0.02 | <0.01 | −.28 |
| REM latency (min) | 99.91 | 49.26 | 95.21 | 44.09 | 0.31 | 0.07 | .17 |
| REM density | 1.17 | 0.87 | 2.11 | 1.63 | −2.73* | −0.66 | −.63* |
Note. WNE = written narrative exposure; REM = rapid eye movement; Corr. = Pearson correlations of CAPS symptom reduction with sleep changes after WNE.
p < .05.
p < .01.
p < .001.
Pearson correlations between CAPS symptoms reduction and post-WNE sleep changes are also presented in Table 1. Change in percent N3 was positively correlated with CAPS reduction, indicating that participants who had less reduction of N3 following WNE had greater PTSD symptom reduction. Change in REM density was negatively correlated with CAPS reduction, indicating that participants who had less increase in REM density after WNE had greater PTSD symptom reduction. Analyses with CAPS scores excluding the two sleep-related items yielded the same relationships.
Discussion
The findings of this study did not support the hypothesis that proximity of WNE to sleep influences its effectiveness. Thus emotional habituation promoted by WNE did not appear to be affected by whether sleep occurs within approximately 2 versus 14 hours after the session. These findings do, however, suggest a relationship between sleep characteristics and PTSD symptom reduction. Sleep following evening WNE had increased sleep duration (likely influenced by earlier bedtimes) percent N2, reduced percent N3, and increased REM density compared with baseline. The latter two of these changes were associated with less improvement of PTSD. This negative relationship to increased REM density is similar to Buysse et al.’s finding (1992) with interpersonal therapy for depression. As REMS eye movements have been linked to alerting/startle responses (Sanford, Morrison, Ball, Ross, & Mann, 1993) and N3 is sleep’s deepest stage, these findings suggest that greater arousal during sleep can impede emotional processing.
Our study is limited by statistical power, which necessitated combining groups for some analyses, as well as sample attrition, and not controlling Type 1 error. Relying on SUDS score changes as the test of overnight memory processing and possible effects of not controlling for time in bed also represent study limitations. Our study is unique, however, to the sleep, trauma adaptation experimental literature for evaluating participants with PTSD or significant PTSD symptoms. Further research on whether behavioral and/or pharmacological approaches to maintaining diminished arousal during sleep enhance the benefit of exposure-based treatments is indicated.
Acknowledgments
This study was supported by NIH grant R21MH094815 to Dr. Thomas A. Mellman and UL1TR000101 from the National Center for Advancing Translational Sciences (NCATS), National Institutes of Health, through the Clinical and Translational Science Awards Program (CTSA), a trademark of DHHS, part of the Roadmap Initiative, “Re-Engineering the Clinical Research Enterprise”. The authors wish to acknowledge Latesha McLaughlin for her contribution to data collection and Bryonna Wilson for her contribution to sleep data scoring, all of whom were compensated. The authors also thank the staff of the Howard University Clinical Research Unit for technical support. Ihori Kobayashi and Thomas A. Mellman contributed equally to the manuscript.
References
- American Academy of Sleep Medicine. (2012). The AASM manual for the scoring of sleep and associated events: Rules, terminology and technical specifications version 2.0 American Academy of Sleep Medicine. [Google Scholar]
- Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Klauminzer G, Charney DS, & Keane TM (1990). A clinician rating scale for assessing current and lifetime PTSD: The CAPS-1. The Behavior Therapist, 13, 187–188. [Google Scholar]
- Bryant RA, Creamer M, O’Donnell M, Silove D, & McFarlane AC (2010). Sleep disturbance immediately prior to trauma predicts subsequent psychiatric disorder. Sleep, 33, 69–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buysse DJ, Kupfer DJ, Frank E, Monk TH, & Ritenour A (1992). Electroencephalographic sleep studies in depressed outpatients treated with interpersonal psychotherapy: II. Longitudinal studies at baseline and recovery. Psychiatry Research, 42, 27–40. doi: 10.1016/0165-1781(92)90036-3 [DOI] [PubMed] [Google Scholar]
- Cai DJ, Mednick SA, Harrison EM, Kanady JC, & Mednick SC (2009). REM, not incubation, improves creativity by priming associative networks. Proceedings of the National Academy of Sciences, 106, 10130–10134. doi: 10.1073/pnas.0900271106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, & Williams JBW (2002). Structured clinical interview for DSM-IV-TR axis I disorders-patient edition (with psychotic screen). New York: Biometrics Research Department. [Google Scholar]
- Foa E, Hembree E, & Rothbaum BO (2007). Prolonged exposure therapy for PTSD: Emotional processing of traumatic experiences therapist guide. New York: Oxford University Press. [Google Scholar]
- Lommen MJJ, Grey N, Clark DM, Wild J, Stott R, & Ehlers A (2015). Sleep and treatment outcome in posttraumatic stress disorder: Results from an effectiveness study. Depression and Anxiety, 33, 575–583. doi: 10.1002/da.22420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellman TA, Bustamante V, Fins AI, Pigeon WR, & Nolan B (2002). REM sleep and the early development of posttraumatic stress disorder. American Journal of Psychiatry, 159, 1696–1701. doi: 10.1176/appi.ajp.159.10.1696 [DOI] [PubMed] [Google Scholar]
- Pace-Schott E, Germain A, & Milad MR (2015). Effects of sleep on memory for conditioned fear and fear extinction. Psychological Bulletin, 141, 835–857. doi: 10.1037/bul0000014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porcheret K, Holmes EA, Goodwin GM, Foster RG, & Wulff K (2015). Psychological effect of an analogue traumatic event reduced by sleep deprivation. Sleep, 38, 1017–1025. doi: 10.5665/sleep.4802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanford LD, Morrison AR, Ball WA, Ross RJ, & Mann GL (1993). The amplitude of elicited PGO waves: A correlate of orienting. Electroencephalography and Clinical Neurophysiology, 86, 438–445. doi: 10.1016/0013-4694(93)90139-M [DOI] [PubMed] [Google Scholar]
- Smyth JM (1998). Written emotional expression: Effect sizes, outcome types, and moderating variables. Journal of Consulting and Clinical Psychology, 66, 174–184. doi: 10.1037/0022-006X.66.1.174 [DOI] [PubMed] [Google Scholar]


