Abstract
Objectives:
The detection of autism spectrum disorder (ASD) is based on behavioral observations. To build a more objective datadriven method for screening and diagnosing ASD, many studies have attempted to incorporate artificial intelligence (AI) technologies. Therefore, the purpose of this literature review is to summarize the studies that used AI in the assessment process and examine whether other behavioral data could potentially be used to distinguish ASD characteristics.
Methods:
Based on our search and exclusion criteria, we reviewed 13 studies.
Results:
To improve the accuracy of outcomes, AI algorithms have been used to identify items in assessment instruments that are most predictive of ASD. Creating a smaller subset and therefore reducing the lengthy evaluation process, studies have tested the efficiency of identifying individuals with ASD from those without. Other studies have examined the feasibility of using other behavioral observational features as potential supportive data.
Conclusion:
While previous studies have shown high accuracy, sensitivity, and specificity in classifying ASD and non-ASD individuals, there remain many challenges regarding feasibility in the real-world that need to be resolved before AI methods can be fully integrated into the healthcare system as clinical decision support systems.
Keywords: Autism spectrum disorder, Artificial intelligence, Diagnosis, Screening
INTRODUCTION
Autism spectrum disorder (ASD) is a neurodevelopmental disorder characterized by difficulties in social communication and interaction with restricted or repetitive patterns of behavior, interest, or activities [1]. In the absence of clear identifiable biomarkers [2], the current gold standard in diagnostic criteria relies on behavioral observations administered by healthcare professionals [3]. The reliability and validity of these results come into question when accounting for subjectivity [4], which can stem from differences in professional training and experiences [5], lack of resources [6], or cultural adaptability of the assessments [7]. Such limitations to the current diagnostic system call for the need to develop a novel method that can provide quick, accurate evaluations while affording a well-rounded understanding of the heterogeneous phenotype in each individual with ASD.
Recently, artificial intelligence (AI) has risen as a promising alternative. Built based on the biological networks of the human brain [8], AI covers a wide range of technologies that are capable of performing cognitive functions by mimicking human intelligence [9]. While promising results in other fields (e.g., engineering, business, and everyday applications) have been shown, increasing efforts are being made to incorporate AI into healthcare settings [10,11]. Previous studies have applied AI in recognition of symptoms [12], classification [13], diagnosis [9,10], and prediction of outcome based on structured or unstructured data [9,10,14]. Equipped to improve accuracy through trials, AI can also reduce the likelihood of introducing inevitable human error [10]. For instance, AI is capable of capturing data that may not be visible to the human eye during behavioral observations, which can lead to precise data-fication [15]. With an increasing interest in AI, there have been movements in making such programs accessible to the general public. For instance, by searching ‘autism’ and ‘AI’ in a search engine, one can easily find a phone application that advertises the use AI for detection of autistic traits. However, with-out enough evidence to support their validity and reliability, such programs may provide inaccurate information and cause unnecessary delays in provision of care.
One of the most commonly used subfields of AI in research is machine learning (ML). Machine learning can take a supervised approach by educating itself with a labeled dataset and constructing the best fitting algorithm to forecast an outcome of interest, or an unsupervised approach that analyzes the input features by deducing patterns without pre-existing knowledge [16]. By extracting useful information and building complex models that surpass human performance in analyzing large datasets [11,17], ML can enhance our understanding of ASD and may further help build a stronger foundation for better screening and diagnosis.
To developa more objective methodindetecting ASDthrough assessment of significant features linked to the disorder, previous studies have attempted using a range of data modalities with AI. For instance, as ASD is most likely associated with the combination of the interplay between variants of several genetic biomarkers [18], genetic research has been applied with several AI methods to explore and optimize ASD risk-associated gene candidates [19]. Limitations persist as the current combination of known ASD-associated genes is only capable of explaining a small portion of cases [20]. Additionally, neuroimaging techniques have been used in combination with several AI approaches to study different brain regions and network-wide connectivity that may be unique to individuals with ASD [21]. Unfortunately, based on the study populations and models used, predictive neuroanatomical findings have been inconsistent [22].
Despite studies reporting on ways in which AI can be used with biomarkers to establish a data-driven approach in ASD classification, the current system relies heavily on behavioral observation data. However, in collecting information based upon actions or subtle responses to social situations and their interpretation by the administrator, behavioral observational data face numerous challenges. Unlike genetics and neuroimaging scans that have a well-established streamlined protocol for collection and analysis, there is no objectified system to capture the constant changes in the behavior of an individual. As ASD assessments rely on observational data and efforts are being made to use AI to independently perceive information from the environment [23], the combination of these two elements can help overcome limitations of data collection during the screening and diagnostic process.
While review studies such as Hyde et al. [24] and Thabtah [25] reported on ASD studies focusing on a single AI method, to our knowledge, no literature review has been conducted on the broad use of AI technology to distinguish individuals with ASD through an emphasis on behavioral aspects. Therefore, the aim of this study is to summarize findings on how AI can be implemented into the current evaluation process and explore other potential behavioral aspects that can be used to enhance efficiency in the detection of ASD.
METHODS
A literature review of studies using AI technology in relation to those with ASD was conducted on published peer-reviewed journal articles listed in PubMed from January 1, 2009, to July 31, 2019. Studies were included and excluded by following the practices of Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) (Fig. 1) [26].
Fig. 1.
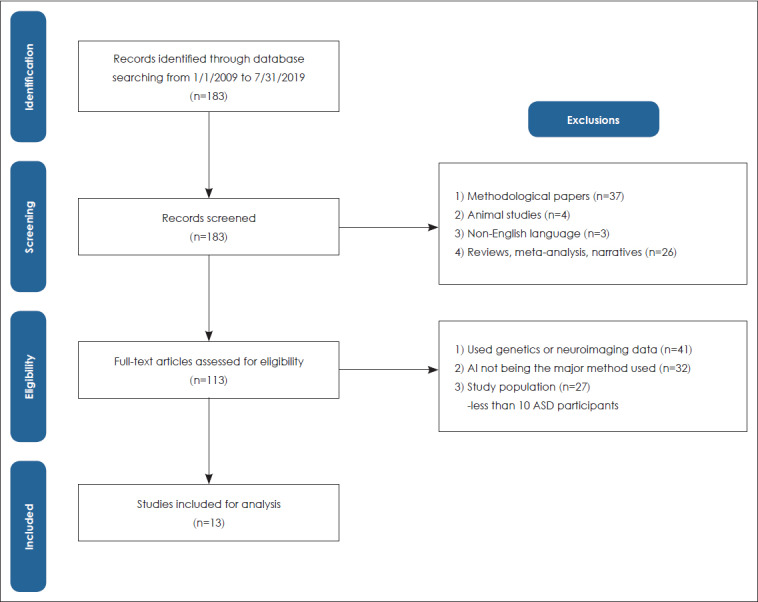
Search strategy and article selection process. ASD: autism spectrum disorder.
Search and exclusion criteria
For a comprehensive search, the keywords included Medical Subject Headings (MeSH) terms ‘autism spectrum disorder’ and ‘artificial intelligence.’ A total of 183 studies were identified through the initial search. Studies were excluded if they were: 1) methodological studies mainly focusing on AI technology; 2) animal studies; 3) studies that were not published (full text) in English; and 4) reviews, meta-analyses, narratives, or editorials. Based on the title and abstract screening, 70 articles were excluded as they met any of the exclusion criteria. The remaining 113 articles were selected for a full-text review and removed for further analysis if: 1) genetics or neuroimaging scans were used as the main source of data; 2) AI technology was not the major method employed in the study; and 3) they included fewer than 10 ASD participants. After full-text review exclusions, 13 studies were finally included in the analysis.
RESULTS
We describe our findings by introducing how AI can be utilized to complement existing ASD assessment tools and introduce new behavioral components with the potential to be incorporated for screening or diagnosis.
Use of AI with existing ASD assessments
To facilitate screening that is sensitive and specific, studies have used diverse AI methods on the existing battery of assessments to build models that can be used to classify individuals with ASD (Table 1).
Table 1.
Summary of studies using Al technology with existing ASD assessments
| Author | Sample size | Mean age | Date type | Method | AUC (%) | Sensitivity (%) | Specificity (%) | Accuracy (%) |
|---|---|---|---|---|---|---|---|---|
| Bone et al. [35] | 1264 (ASD) 462 (non-ASD) |
6.7-15.9 yrs 6.6-17.2 yrs |
ADI-R SRS |
SVM | - | 89.2 86.7 | 59 53.4 | - |
| Bussu et al. [28] | 32(ASD) 43 (atypical) 86 (typical) |
8.1 m (visiti) 14.5 m (visit2) 25.4 m (visit3) 38.4 m (visit4) |
MSEL VABS AOSI |
SVM | 69.2 (8 m) 70.8 (14 m) |
68.8 (8 m) 60.7 (14 m) |
64.4 (8 m) 67.5 (14 m) |
66.4 (8 m) 64.4 (14 m) |
| Duda et al. [36] | 2775 (ASD) 150 (ADHD) |
8.1 yrs (ASD) 11.3 yrs (ADHD) |
SRS | SVC, LDA, CL, LR, RF, DT | 93.3-96.5 | - | - | - |
| Duda et al. [37] | 248(ASD) 174 (ADHD) |
8.2 yrs (ASD) 10.4 yrs (ADHD) |
SRS | SVC, CL, LR, LDA | 82-89 | - | - | - |
| Kosmicki et al. [33] | 1451 ASD (M2) 348 non-ASD (M2) 2434 ASD (M 3) 307 non-ASD (M 3) |
68 m ASD (M 2) 37 m non-ASD (M2) 111 m ASD (M3) 108 m non-ASD (M 3) |
ADOS (M2, M3) | SVM, ADTree, FT, LR, NBT, RF | 96.7-99.7 (M2) 96.1-100 (M3) | 96.5-98.6 (M2) 87.1-98.9 (M3) | ||
| Levy et al. [32] | 1319 ASD (M 2) 70 non-ASD (M 2) 2870 ASD (M 3) 273 non-ASD (M 3) |
83 m ASD (M 2) 60 m non-ASD (M 2) 116 m ASD (M 3) 109 m non-ASD (M 3) |
ADOS (M2, M3) | LR, LDA, SVM | 93 (M2) 95 (M3) |
98 (M2) 95 (M3) |
58 (M2) 87 (M3) |
78 (M2) 90 (M3) |
| Thabtah et al. [49] | 707(ASD) 393 (non-ASD) |
6.3 yrs 14.1 yrs 29.7 yrs |
AQ | CI | 80-87.3 80.95-82.54 |
80 80 90 | ||
| Wall et al. [34] | 2867 (ASD) 92 (non-ASD) |
8.06-8.75 yrs (ASD) 9.24-9.75 yrs (non-ASD) |
ADI-R | ADTree | - | - | 93.8-99 | 99.9-100 |
ADHD: attention-deficit/hyperactivity disorder, ADI-R: Autism Diagnositc Interview-Revised, ADOS: Autism Diagnostic Observational Schedule, ADTree: alternating decision tree, AI: artificial intelligence, AOSI: Autism Observational Scale for Infants, AQ: Autism Spectrum Quotient, ASD: autism spectrum disorder, AUC: area under the curve, CI: computational intelligence, CL: categorical lasso, DT: decision tree, FT: functional tree, LDA: linear discriminant analysis, LR: logistic regression, m: months, MSEL: Mullen Scales of Early Learning, M2: module 2, M3: module 3, NBT: naïve Bayes tree, RF: random forest, SRS: Social Responsiveness Scale, SVC: support vector classification, SVM: support vector machine, VABS: Vineland Adaptable Behavior Scale, yrs: years
Increasing early predictive outcomes
While reliable diagnosis of ASD is usually made around 3 years of age [27], AI methods have been utilized to predict diagnostic outcome using developmental evaluations before the age of 3 years and enable more accurate predictions. For instance, Bussu et al. [28] used support vector machine (SVM), a type of supervised ML algorithm that is used to classify features by assigning binary labels [10], to predict ASD diagnosis at around 3 years of age, based on previous developmental evaluations such as the Mullen Scales of Early Learning (MSEL) and Vineland Adaptable Behavior Scale (VABS) during infancy [28]. The predictive diagnostic outcome at 3 years using SVM was compared to the clinical judgments made by researchers based upon review of the Autism Diagnostic Observation Schedule (ADOS) and Autism Diagnostic Interview Revised (ADI-R). Showing high predictive accuracy at 3 years based on the data obtained from 14 months of age, this study proved how combining information such as symptoms and adaptive functioning from multiple assessment measures could improve classification of atypical development.
Reducing the number of items in assessments
With the initial screening taking around 60 to 90 minutes and an average wait of 13 months before diagnosis [29], efforts are being made to use AI in reducing items to shorten the time in administering lengthy evaluations. Researchers have used the gold-standard diagnostic tests, ADOS [30] and ADI-R [31], to identify a minimal set of items that are most distinguished by ASD characteristics and test whether the subset of features can uphold high sensitivity, specificity, and accuracy in diagnosis. After using classifier algorithms to identify optimal features that contribute to determining the diagnosis, models were trained using the reduced set of items and its performance was tested using a new dataset [32-34]. Of the 28 features in ADOS, studies by Levy et al. [32] and Kosmicki et al. [33] were able to uphold high accuracy while reducing the number of activities to five and nine items in module 2 and 10, and 12 items in module 3, respectively. The alternative decision tree (ADTree), a method combining features to build an accurate predictor, was used in a study by Wall et al. [34] and drastically lowered the number of questions in the ADI-R by 92%.
Classification between different neurodevelopmental disorders
Other studies have also expanded to using assessment tools with AI to differentiate between common neurodevelopmental disorders [35]. Duda et al. [36] used diverse ML algorithms to find the best classifying features using the Social Responsiveness Scale (SRS) to distinguish ASD and attention deficit hyperactivity disorder (ADHD). Of the 65 items on the SRS, they were able to identify five features while maintaining high accuracy (above 90%). Extending from their previous study, Duda et al. [37] applied 15 SRS-derived questions to a crowdsourced dataset and created a novel classification algorithm to reflect real-world data as a source to validate its performance.
Use of AI with novel observational data
To develop a more objective method in identifying ASD, researchers have investigated the feasibility of using AI to capture different types of behavioral features to use as valuable information in detecting characteristics that are unique to individuals with the disorder (Table 2).
Table 2.
Summary of studies using AI technology with novel observational data
| Author | Sample size | Mean age | Date type | Method | AUC (%) | Sensitivity (%) | Specificity (%) | Accuracy (%) |
|---|---|---|---|---|---|---|---|---|
| Tariq et al. [48] | 116 (ASD) 46 (TD) |
4.10yrs (ASD) 2.11yrs (TD) |
Behavioral features | ADTree, SVM, LR, RK, LİSVM Sparse 5 feature LR classifier | 89-92 | 90-100 | 1.13-100 | 94-100 |
| Liu et al. [39] | 29(ASD) 29 (TD) |
7.9 yrs (ASD) 7.86 yrs (TD) |
Eye-tracking | SVM | 89.63 | 93.1 | 86.21 | 88.51 |
| Li et al. [43] | 14(ASD) 16 (TD) |
32 yrs (ASD) 29.31 yrs (TD) |
Hand movement | NB, SVM, RF, DT | - | 57.1-85.7 | 68.8-87.5 | 66.7-86.7 |
| Anzulewicz et al. [44] | 37(ASD) 45 (TD) |
4.5 yrs (ASD) 4.7 yrs (TD) |
Hand movement | RF, RGF | 88.1-93.2 | 76-83 | 67-88 | - |
| Crippa et al. [45] | 15(ASD) 15 (TD) |
3.5yrs (ASD) 2.6yrs (TD) |
Upper-limb movement | SVM | - | 82.2-100 | 89.1-93.8 | 84.9-96.7 |
ADTree: alternating decision tree, AI: artificial intelligence, ASD: autism spectrum disorder, AUC: area under the curve, DT: decision tree, LİSVM: linear support vector machine, LR: logistic regression, NB: naïve Bayes, RF: random forest, RGF: regularized greedy forest, RK: radial kernel, SVM: support vector machine, TD: typically developing, yrs: years
Facial expressions
Many individuals with ASD report difficulty in recognition and expression of emotions [38]. Liu et al. [39] had attempted to use the difference in face scanning patterns between ASD and non-ASD participants as indicators of classification. First, participants were shown six faces to remember. Then, they were shown 18 faces and asked to choose the faces that they had been asked to remember. Eye-tracking was recorded to gain information on eye movement and fixation duration when viewing the faces. Support vector machine was then used to classify the boundary that differentiated between ASD and TD groups. With an overall accuracy of 88.51%, results showed the most distinguishable characteristic was that the TD group spent more time looking at the right eye while the ASD group spent more time on the left eye.
Motor movements
In a study by Hilton et al. [40], it was reported that 83% of individuals with ASD had lower motor composite scores than non-ASD individuals. Therefore, researchers have attempted to capture differences in movement patterns to use as a distinctive characteristic of ASD [41,42]. Studies by Li et al. [43] and Anzulewicz et al. [44], each used imitation based on observation and gesture patterns using smart tablet devices to detect kinematic parameters to use for classifying between ASD and non-ASD.
Crippa et al. [45] investigated whether SVM could be used with the reach, grasp, and drop movement in the upper-limb to identify children with ASD. Trials were designed to observe those motor movements because they are important milestones in the developmental trajectory. Each action was divided into sub-movements and analyzed. Using SVM, a total of 17 kinematic measurements were chosen as classifiers to distinguish preschool children with ASD and their typically developing peers. Tasks that were related to transporting an object to the target was where the two groups showed substantial differences, suggesting that differences in goal-oriented movements may be a strong identifier of ASD.
DISCUSSION
The purpose of this study was to review literature that has applied AI technologies to the current assessment instruments for ASD and to assess whether other behavioral characteristics could potentially be used as identification of observable markers for diagnosis. A total of 13 articles were reviewed with a majority of the studies using supervised ML methods such as SVM to distinguish between individuals with and without ASD. Findings demonstrate that algorithms were used to identify features that were most representative of ASD characteristics and were able to exclude duplicate items to reduce the amount of time and effort required in the assessment process. Other studies have also tried to use other behavioral aspects with AI to analyze whether it could be used to distinguish individuals.
With constant development in the field of AI, its use has rapidly spread to the healthcare arena [10,11]. Being relatively easy to input data, the most advanced areas with AI technology are diagnostic imaging, followed by genetics [10]. Due to the exponential growth in medical image analyses and pipelines that extract features to be used as valuable decision supporting data, a new practice termed radiomics has emerged [46]. Unfortunately, this trend has been focused on the fields of cancer or diseases related to the cardiovascular or nervous system [10]. According to Jiang et al. [10], based on the literature in PubMed, the leading disease areas using AI technology are: neoplasms, nervous, cardiovascular, urogenital, pregnancy, digestive, respiratory, skin, endocrine, and nutritional. These 10 areas have approximately 9000 papers published since 2013. Yet, despite ASD having evolved into a public health issue with one in 59 children diagnosed [47], only 119 studies were published during the same time.
This may largely be due to the challenges that need to be resolved before AI methods can be applied in research and clinical settings. As ML requires big data, the majority of the studies in this analysis used collected data from data repositories [32-37]. Therefore, there was a large imbalance between individuals with and without ASD. To adjust for such limitations, different approaches were undertaken by researchers in deciding who to include and exclude. Whether this has any effect on results would need to be further investigated through replication studies. Second, we may need to consider if we are oversimplifying the assessments by only choosing a few items. A majority of the studies in this analysis reduced features by more than 50% [25,32-34,36,37]. However, a wide range of autistic symptoms with different levels of severity may not have been captured with the reduced number of items. There could also be individuals who do not meet the cut-off threshold but still have some sort of developmental delay. Therefore, simple dichotomous results may not be the most appropriate method to interpret the output data. Additionally, there has yet to be a study that examines how the accuracy, sensitivity, or specificity would be affected if one or more of the items were left unanswered. Third, there remains a lack of clear understanding of the technique that is being used. A number of studies used multiple algorithm approaches and report on the highest predictive value [32,33,36,37,43,44,48,49]. Before arguing on the best algorithm to use, it would be important to understand why there are such differences in the results and the reason as to which approach would be most appropriate depending on the characteristics of the dataset and what sort of an output one is trying to achieve. To enable this, advancement with a theoretical background rather than being strictly data-driven would be needed.
In addition to the limitations from previous studies, implementing AI in the general healthcare system still faces numerous obstacles. While machine learning algorithms heavily depend on the training dataset, there has not yet been any extensive research assessing how the quality of the input data affects the accuracy or targeting to establish a protocol on data collection and cleaning. Requiring vast amounts of data, the ethical challenge around data privacy is also another topic that is under debate [9]. Additionally, while complex disorders like ASD influence both the brain and behavior, there is a lack of reports on current AI technology integrating multiple modalities for a more comprehensive understanding of an individual [11]. Lastly, the majority of current advancements in AI technology have been based on retrospective data. Validation and feasibility studies of such techniques in the realworld are still needed [10,11]. Being able to overcome these challenges to incorporate AI in clinical settings will not only enhance our understanding of ASD, but also enable healthcare professionals to use this technique as a clinical decision support system that can objectively intervene throughout the screening, diagnosis, and treatment process.
CONCLUSION
Without a definite biomarker, ASD screening and diagnosis depend on behavioral observations. To overcome administrator bias during assessments, many have attempted to use AI technology to improve the frequency of accurate detection. In this literature review, we found that studies have attempted to classify items from assessment instruments that are most predicative of the diagnosis to make the process less time-consuming. Other studies have experimented with other behavioral characteristics that may be unique to individuals with ASD to use as markers for classification. However, as research in ASD and AI are both still relatively new, there are numerous obstacles that need to be resolved before applying these methods in research or clinical settings.
Acknowledgments
This work was supported by an Institute for Information & Communications Technology Promotion (ITTP) grant funded by the Korean government (MSIT) (No.2019-0-00330, Development of AI Technology for Early Screening of Infant/Child Autism Spectrum Disorders based on Cognition of the Psychological Behavior and Response).
Footnotes
Conflicts of Interest
The authors have no financial conflicts of interest.
Author Contributions
Conceptualization: Hee Jeong Yoo. Data curation: Da-Yea Song, So Yoon Kim. Formal analysis: Da-Yea Song, So Yoon Kim. Funding acquisition: Hee Jeong Yoo. Investigation: Da-Yea Song, So Yoon Kim. Methodology: Da-Yea Song, So Yoon Kim. Project administration: Da-Yea Song, So Yoon Kim, Guiyoung Bong, Jong Myeong Kim. Supervision: Hee Jeong Yoo. Validation: Guiyoung Bong, Jong Myeong Kim, Hee Jeong Yoo. Writing—original draft: Da-Yea Song, So Yoon Kim. Writing—review & editing: Guiyoung Bong, Jong Myeong Kim, Hee Jeong Yoo.
ORCID iDs
Da-Yea Song https://orcid.org/0000-0002-7144-4739
So Yoon Kim https://orcid.org/0000-0003-1349-8031
Guiyoung Bong https://orcid.org/0000-0001-8630-9399
Jong Myeong Kim https://orcid.org/0000-0002-6917-9358
Hee Jeong Yoo https://orcid.org/0000-0003-0521-2718
REFERENCES
- 1).American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorder-5. 5th ed. Washington DC: American Psychiatric publishing; 2013. pp. 50–59. [Google Scholar]
- 2).Goldani AA, Downs SR, Widjaja F, Lawton B, Hendren RL. Biomarkers in autism. Front Psychiatry. 2014;5:100. doi: 10.3389/fpsyt.2014.00100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3).Falkmer T, Anderson K, Falkmer M, Horlin C. Diagnostic procedures in autism spectrum disorders:a systematic literature review. Eur Child Adolesc Psychiatry. 2013;22:329–340. doi: 10.1007/s00787-013-0375-0. [DOI] [PubMed] [Google Scholar]
- 4).Taylor LJ, Eapen V, Maybery M, Midford S, Paynter J, Quarmby L, et al. Brief report:an exploratory study of the diagnostic reliability for autism spectrum disorder. J Autism Dev Disord. 2017;47:1551–1558. doi: 10.1007/s10803-017-3054-z. [DOI] [PubMed] [Google Scholar]
- 5).Randall M, Albein-Urios N, Brignell A, Gulenc A, Hennel S, Coates C, et al. Diagnosing autism:Australian paediatric research network surveys. J Paediatr Child Health. 2016;52:11–17. doi: 10.1111/jpc.13029. [DOI] [PubMed] [Google Scholar]
- 6).Wallace S, Fein D, Rosanoff M, Dawson G, Hossain S, Brennan L, et al. A global public health strategy for autism spectrum disorders. Autism Res. 2012;5:211–217. doi: 10.1002/aur.1236. [DOI] [PubMed] [Google Scholar]
- 7).Al Maskari TS, Melville CA, Willis DS. Systematic review:cultural adaptation and feasibility of screening for autism in non-English speaking countries. Int J Ment Health Syst. 2018;12:22. doi: 10.1186/s13033-018-0200-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8).Hassabis D, Kumaran D, Summerfield C, Botvinick M. Neuroscience-inspired artificial intelligence. Neuron. 2017;95:245–258. doi: 10.1016/j.neuron.2017.06.011. [DOI] [PubMed] [Google Scholar]
- 9).Noorbakhsh-Sabet N, Zand R, Zhang Y, Abedi V. Artificial intel-ligence transforms the future of health care. Am J Med. 2019;132:795–801. doi: 10.1016/j.amjmed.2019.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10).Jiang F, Jiang Y, Zhi H, Dong Y, Li H, Ma S, et al. Artificial intelli-gence in healthcare:past, present and future. Stroke Vasc Neurol. 2017;2:230–243. doi: 10.1136/svn-2017-000101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11).Yu KH, Beam AL, Kohane IS. Artificial intelligence in healthcare. Nat Biomed Eng. 2018;2:719–731. doi: 10.1038/s41551-018-0305-z. [DOI] [PubMed] [Google Scholar]
- 12).Blomberg SN, Folke F, Ersbøll AK, Christensen HC, Torp-Pedersen C, Sayre MR, et al. Machine learning as a supportive tool to recognize cardiac arrest in emergency calls. Resuscitation. 2019;138:322–329. doi: 10.1016/j.resuscitation.2019.01.015. [DOI] [PubMed] [Google Scholar]
- 13).Belić M, Bobić V, Badža M, Šolaja N, Đurić-Jovičić M, Kostić VS. Artificial intelligence for assisting diagnostics and assessment of Parkinson's disease—a review. Clin Neurol Neurosurg. 2019;184:105442. doi: 10.1016/j.clineuro.2019.105442. [DOI] [PubMed] [Google Scholar]
- 14).Sheth D, Giger ML. Artificial intelligence in the interpretation of breast cancer on MRI. J Magn Reson Imaging. In press. 2019 doi: 10.1002/jmri.26878. [DOI] [PubMed] [Google Scholar]
- 15).Strauß S. From big data to deep learning:a leap towards strong AI or “intelligentia obscura”? Big Data Cogn Comput. 2018;2:16. doi: 10.3390/bdcc2030016. [DOI] [Google Scholar]
- 16).Deo RC. Machine learning in medicine. Circulation. 2015;132:1920–1930. doi: 10.1161/CIRCULATIONAHA.115.001593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17).Baştanlar Y, Ozuysal M. Introduction to machine learning. Methods Mol Biol. 2014;1107:105–128. doi: 10.1007/978-1-62703-748-8_7. [DOI] [PubMed] [Google Scholar]
- 18).Yoo H. Genetics of autism spectrum disorder:current status and possible clinical applications. Exp Neurobiol. 2015;24:257–272. doi: 10.5607/en.2015.24.4.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19).Engchuan W, Dhindsa K, Lionel AC, Scherer SW, Chan JH, Merico D. Performance of case-control rare copy number variation annotation in classification of autism. BMC Med Genomics. 2015;8(Suppl 1):S7. doi: 10.1186/1755-8794-8-S1-S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20).Zhou J, Park CY, Theesfeld CL, Wong AK, Yuan Y, Scheckel C, et al. Whole-genome deep-learning analysis identifies contribution of noncoding mutations to autism risk. Nat Genet. 2019;51:973–980. doi: 10.1038/s41588-019-0420-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21).Mahajan R, Mostofsky SH. Neuroimaging endophenotypes in autism spectrum disorder. CNS Spectr. 2015;20:412–426. doi: 10.1017/S1092852915000371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22).Xiao X, Fang H, Wu J, Xiao C, Xiao T, Qian L, et al. Diagnostic model generated by MRI-derived brain features in toddlers with autism spectrum disorder. Autism Res. 2017;10:620–630. doi: 10.1002/aur.1711. [DOI] [PubMed] [Google Scholar]
- 23).Gavrila DM. The visual analysis of human movement:a survey. Comput Vis Image Und. 1999;73:82–98. doi: 10.1006/cviu.1998.0716. [DOI] [Google Scholar]
- 24).Hyde KK, Novack MN, LaHaye N, Parlett-Pelleriti C, Anden R, Dixon DR, et al. Applications of supervised machine learning in autism spectrum disorder review:a review. Rev J Autism Dev Disord. 2019;6:128–146. doi: 10.1007/s40489-019-00158-x. [DOI] [Google Scholar]
- 25).Thabtah F. Machine learning in autistic spectrum disorder behavioral research:a review and ways forward. Inform Health Soc Care. 2019;44:278–297. doi: 10.1080/17538157.2017.1399132. [DOI] [PubMed] [Google Scholar]
- 26).Moher D, Liberati A, Tetzlaff J, Altman DG PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses:the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27).Steiner AM, Goldsmith TR, Snow AV, Chawarska K. Practitioner's guide to assessment of autism spectrum disorders in infants and toddlers. J Autism Dev Disord. 2012;42:1183–1196. doi: 10.1007/s10803-011-1376-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28).Bussu G, Jones EJH, Charman T, Johnson MH, Buitelaar JK BASIS Team. Prediction of autism at 3 years from behavioural and developmental measures in high-risk infants:a longitudinal cross-domain classifier analysis. J Autism Dev Disord. 2018;48:2418–2433. doi: 10.1007/s10803-018-3509-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29).Wiggins LD, Baio J, Rice C. Examination of the time between first evaluation and first autism spectrum diagnosis in a population-based sample. J Dev Behav Pediatr. 2006;27:S79–S87. doi: 10.1097/00004703-200604002-00005. [DOI] [PubMed] [Google Scholar]
- 30).Lord C, Rutter M, DiLavore PC, Risi S. Autism Diagnostic Observation Schedule (ADOS) Los Angeles: Western Psychological Services; 2000. [DOI] [Google Scholar]
- 31).Rutter M, Le Couteur A, Lord C. Autism Diagnostic Interview-Revised (ADI-R) Los Angeles: Western Psychological Services; 2003. [Google Scholar]
- 32).Levy S, Duda M, Haber N, Wall DP. Sparsifying machine learning models identify stable subsets of predictive features for behavioral detection of autism. Mol Autism. 2017;8:65. doi: 10.1186/s13229-017-0180-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33).Kosmicki JA, Sochat V, Duda M, Wall DP. Searching for a minimal set of behaviors for autism detection through feature selection-based machine learning. Transl Psychiatry. 2015;5:e514. doi: 10.1038/tp.2015.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34).Wall DP, Dally R, Luyster R, Jung JY, Deluca TF. Use of artificial intelligence to shorten the behavioral diagnosis of autism. PLoS One. 2012;7:e43855. doi: 10.1371/journal.pone.0043855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35).Bone D, Bishop SL, Black MP, Goodwin MS, Lord C, Narayanan SS. Use of machine learning to improve autism screening and diagnostic instruments:effectiveness, efficiency, and multi-instrument fusion. J Child Psychol Psychiatry. 2016;57:927–937. doi: 10.1111/jcpp.12559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36).Duda M, Ma R, Haber N, Wall DP. Use of machine learning for behavioral distinction of autism and ADHD. Transl Psychiatry. 2016;6:e732. doi: 10.1038/tp.2015.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37).Duda M, Haber N, Daniels J, Wall DP. Crowdsourced validation of a machine-learning classification system for autism and ADHD. Transl Psychiatry. 2017;7:e1133. doi: 10.1038/tp.2017.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38).Loth E, Garrido L, Ahmad J, Watson E, Duff A, Duchaine B. Facial expression recognition as a candidate marker for autism spectrum disorder:how frequent and severe are deficits? Mol Autism. 2018;9:7. doi: 10.1186/s13229-018-0187-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39).Liu W, Li M, Yi L. Identifying children with autism spectrum disorder based on their face processing abnormality:a machine learning framework. Autism Res. 2016;9:888–898. doi: 10.1002/aur.1615. [DOI] [PubMed] [Google Scholar]
- 40).Hilton CL, Zhang Y, Whilte MR, Klohr CL, Constantino J. Motor impairment in sibling pairs concordant and discordant for autism spectrum disorders. Autism. 2012;16:430–441. doi: 10.1177/1362361311423018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41).Sadouk L, Gadi T, Essoufi EH. A novel deep learning approach for recognizing stereotypical motor movements within and across subjects on the autism spectrum disorder. Comput Intell Neurosci. In press. 2018 doi: 10.1155/2018/7186762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42).Perego P, Forti S, Crippa A, Valli A, Reni G. Proceedings of the 31st Annual International Conference of the IEEE EMBS;2009 Sep 2-6. Minneapolis, MN, USA. Minneapolis: IEEE; 2009. Reach and throw movement analysis with Support Vector Machines in early diagnosis of autism. IEEE, editor; pp. 2555–2558. [DOI] [PubMed] [Google Scholar]
- 43).Li B, Sharma A, Meng J, Purushwalkam S, Gowen E. Applying machine learning to identify autistic adults using imitation:an exploratory study. PLoS One. 2017;12:e0182652. doi: 10.1371/journal.pone.0182652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44).Anzulewicz A, Sobota K, Delafield-Butt JT. Toward the autism motor signature:gesture patterns during smart tablet gameplay identify children with autism. Sci Rep. 2016;6:31107. doi: 10.1038/srep31107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45).Crippa A, Salvatore C, Perego P, Forti S, Nobile M, Molteni M, et al. Use of machine learning to identify children with autism and their motor abnormalities. J Autism Dev Disord. 2015;45:2146–2156. doi: 10.1007/s10803-015-2379-8. [DOI] [PubMed] [Google Scholar]
- 46).Arimura H, Soufi M, Kamezawa H, Ninomiya K, Yamada M. Radiomics with artificial intelligence for precision medicine in radiation therapy. J Radiat Res. 2019;60:150–157. doi: 10.1093/jrr/rry077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47).Baio J, Wiggins L, Christensen DL, Maenner MJ, Daniels J, Warren Z, et al. Prevalence of autism spectrum disorder among children aged 8 years - autism and developmental disabilities monitoring network, 11 sites, United States, 2014. MMWR Surveill Summ. 2018;67:1–23. doi: 10.15585/mmwr.ss6706a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48).Tariq Q, Daniels J, Schwartz JN, Washington P, Kalantarian H, Wall DP. Mobile detection of autism through machine learning on home video:a development and prospective validation study. PLoS Med. 2018;15:e1002705. doi: 10.1371/journal.pmed.1002705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49).Thabtah F, Kamalov F, Rajab K. A new computational intelligence approach to detect autistic features for autism screening. Int J Med Inform. 2018;117:112–124. doi: 10.1016/j.ijmedinf.2018.06.009. [DOI] [PubMed] [Google Scholar]


